- 1Department of Intensive Care Unit, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Orthopaedic Surgery, Baodi Clinical College of Tianjin Medical University, Tianjin, China
- 3Sun Yat-Sen Memorial Hospital, Graduate School of Sun Yat-sen University, Guangzhou, China
- 4Graduate School of Tianjin Medical University, Tianjin, China
- 5Clinical College of Neurology, Neurosurgery and Neurorehabilitation, Tianjin Medical University, Tianjin, China
- 6Department of Orthopaedic Surgery, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China
- 7Tianjin Key Laboratory of Cerebrovascular and Neurodegenerative Diseases, Tianjin Neurosurgical Institute, Tianjin Huanhu Hospital, Tianjin, China
In recent years, ferroptosis has become a research hotspot in programmed cell death. Since the concept of ferroptosis was proposed, a growing number of articles have been published on this topic. Nevertheless, to our knowledge, these ferroptosis-related publications that have received a great deal of attention have not been quantitatively evaluated. In this study, we analyzed the top 100 most influential articles over the past decade through a bibliometric method to characterize the research status and trends in this field. Web of Science Core Collection was searched to identify relevant studies. After being manually screened, the top 100 most cited studies with original data were identified and analyzed. Bibliometric software including VOSviewer and R-Bibliometrix were used to perform visualization analysis. The citation frequency for the top 100 selected articles ranged from 135 to 3603 (326.6 citations on average). These articles originated from 25 countries/regions, with more than half originating from the United States and China. The most frequently nominated author was Stockwell BR from the Columbia University, and of the top 100 articles, 19 listed his name. Three core journals were Nature, Cell and Proceedings of the National Academy of Sciences of the United States of America. In addition to term of ferroptosis, these terms or phrases including cell death, cancer cell, GPX4, pathway, inhibitor, mechanism, iron, lipid peroxidation, resistance, erastin, sorafenib, P53, reactive oxygen species, necroptosis, apoptosis, glutathione peroxidase, ACSL4, autophagy, and SLC7A11 appeared more frequently in the top 100 articles. Overall, although much progress has been made, the research on ferroptosis is still at an early stage. The current attention in this field mainly focuses on potential regulatory mechanism and pathways including key ferroptosis-related genes/molecules, oxidant and antioxidant system, ferroptosis-inducing agents or nanomedicine for cancer therapy, as well as the role of ferroptosis in non-neoplastic disorders. Meanwhile, combination therapeutic strategies targeting ferroptosis in radiotherapy or immunotherapy also deserve further attention.
Introduction
Cell death is generally classified into two main distinct categories: accidental cell death (ACD) and regulated cell death (RCD) (1). Unlike ACD, RCD follows multiple subroutines, which could be mediated through a series of molecular cascades and regulatory pathways. Although initial studies on RCD have focused on apoptosis, several other novel forms of non-apoptotic cell death such as ferroptosis, pyroptosis, necroptosis, NETosis, etc., have drawn extensive attention recently (2–4). Among them, ferroptosis, an iron-dependent cell death modality, is characterized by iron overload and lipid reactive oxygen species (ROS) accumulation (5, 6). Meanwhile, it displays unique morphological features such as shrinkage of the mitochondria, reduction or disappearance of mitochondrial ridges, and a ruptured outer mitochondrial membrane. Since the term “ferroptosis” was coined in 2012, a notable amount of works has been devoted to identifying the underlying regulation mechanisms and signaling pathways of ferroptosis (7). In brief, multiple ferroptosis-inducing factors are able to affect glutathione peroxidase directly or indirectly via different pathways, resulting in an imbalance between oxidant and antioxidant ability, and elevated lipid peroxidation in cells, ultimately leading to irreversible oxidative damage and cell death (8, 9). Lipid peroxidation products generated in this process can be pharmacologically inhibited by iron chelators (e.g., deferoxamine) or lipid peroxidation inhibitors (e.g., eugenol), and lipophilic antioxidants (e.g., ferrostatin-1, liproxstatin-1, vitamin E) (10–12). Besides, Glutathione, GSH peroxidase 4 (GPX4), nuclear factor erythroid 2-related factor 2 (Nrf2), heat shock protein beta-1 (HSPB1) exert negative regulatory roles in ferroptosis by limiting the production of ROS or altering cellular iron uptake (13–15).
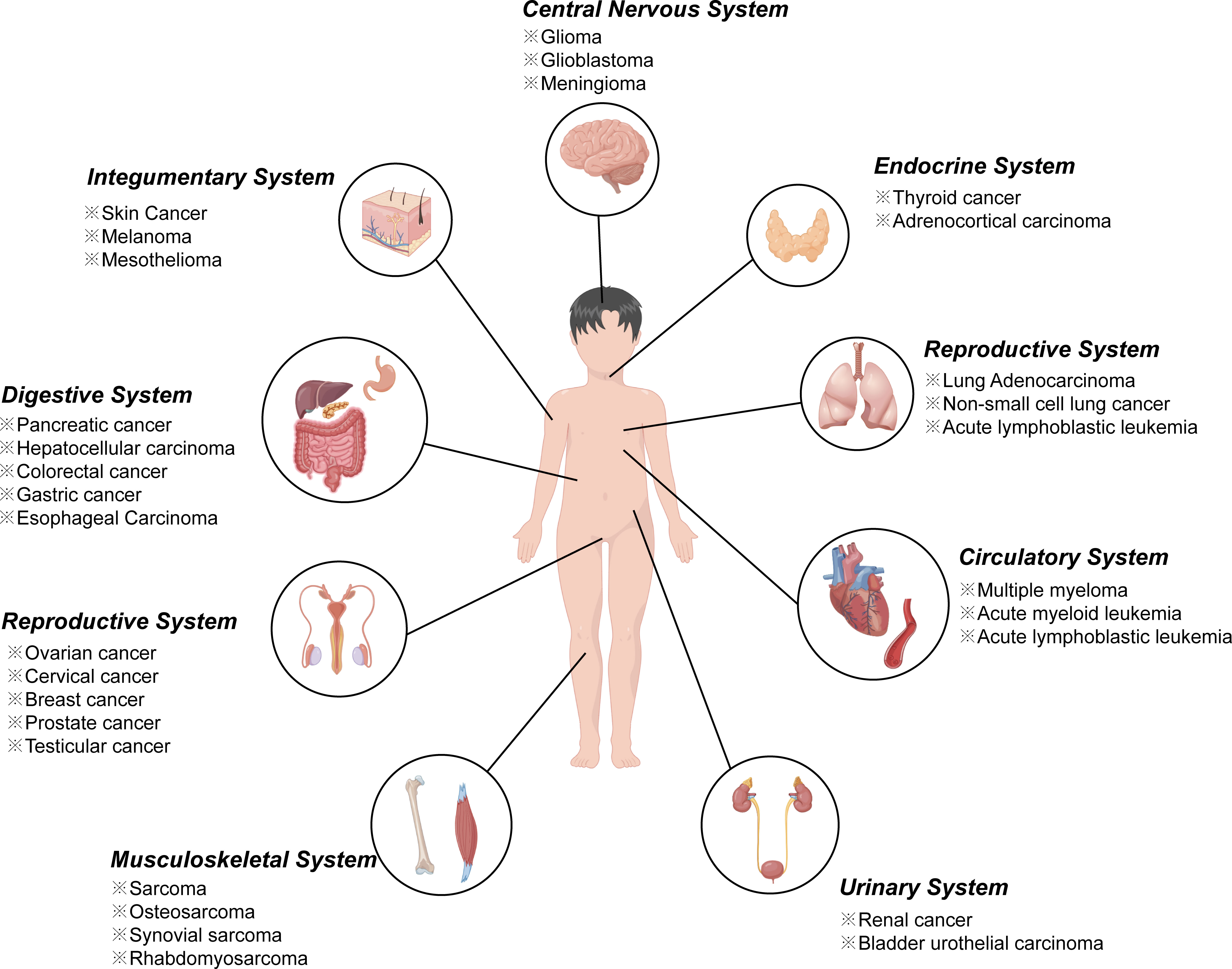
In recent year, an increasing number of studies have found that ferroptosis played an important regulatory role in the initiation and development of various diseases including almost all cancers (16–19) (Figure 1), ischemia reperfusion injury (20), neurological diseases (21–23), digestive disorders (24), hematological system diseases (25), as well as diseases of other systems. Take tumor diseases as examples, ferroptosis inducers such as erastin, sulfasalazine, and sorafenib, several conventional drugs or natural compounds have been extensively examined as novel anti-cancer therapeutics (26–28). In addition, further investigations revealed that erastin combining with traditional chemotherapeutic drugs including temozolomide, cisplatin, and doxorubicin offered remarkable synergistic therapeutic effect on their anti-tumor activity, compared with chemotherapy alone (29, 30).
In view of this, a plethora of work related to ferroptosis have been published and added our understanding to this field. Generally speaking, citation analysis is a widely accepted index for evaluating the impact of scientific articles (31, 32);. These studies with a higher number of citations are often considered as pioneering articles or hot research topics in an area. Thus, highly cited publications in a certain field could provide valuable evidence and information on research trends and scientific progress (33). In previous studies, many special fields have summarized the most-cited studies in their specialty with bibliometric method, such as hepatocellular carcinoma (34), gastric cancer (35), psychosomatic research (36), programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors (37), metabolic disorders (38, 39), and so on. Nevertheless, no work has been published on the top 100 most cited studies regarding ferroptosis.
In this study, we analyzed the characteristics of these top 100 most influential papers in the field of ferroptosis over the past decade. In turn, the data could help researchers better understand the influential works in the evolution of the specialty, as well as provide meaningful insight to conduct further studies. To the best of our knowledge, this work is not the first to evaluate the publications in the field of ferroptosis with bibliometric methods (40), but is the first bibliometric analysis dedicated to ferroptosis related studies with high levels of influence.
Materials and methods
Search strategies
The top 100 most cited articles on ferroptosis were retrieved from Science Citation Index Expanded (SCIE) of Web of Science Core Collection (WoSCC, Clarivate Analytics, Philadelphia, PA, USA) on the same day. Data were collected based on the titles (TI), abstracts (AB) and author keywords (AK) with the following strategy: TI=(ferroptosis OR ferroptotic) OR AK=(ferroptosis OR ferroptotic) OR AB=(ferroptosis OR ferroptotic). The period of the publication for selection was from January 2012 to May 2022, with no limitations on languages.
Data extraction
Firstly, records were placed in descending order based on their frequency of citations in WoSCC. Two researchers (CKM and GQ) independently screened the title, abstract and document type, if necessary, reading the full article for a more detailed assessment, to confirm if it should be included. The inclusion criterion was ferroptosis related studies with original data. Review articles or meta-analysis without original data were excluded. To our knowledge, some reviews articles have been mis-annotated as articles in WoSCC. Discrepancies were resolved via discussion until the two researchers reached an agreement under the verification of senior experts. Then, these top 100 articles were downloaded and exported in plain text format for further analysis. A similar procedure was employed to identify the top 50 highly-cited reviews. Journal impact factors were obtained from 2021 Journal Citation Reports. The citation density of each article was defined as the average citations since published, that is the ratio of total citations and literature age (41).
Statistical analysis
Microsoft Excel 2019 and R software (v 4.1.0) software was used for descriptive statistical analysis and generating diagrams. VOSviewer 1.6.16 (Leiden University, the Netherlands) software were used to perform country/institutional co-authorship analysis, author/journal co-citation analysis, terms co-occurrence analysis. VOSviewer, developed by van Eck and Waltman, is a literature knowledge visualization software for constructing bibliometric networks (42). In the network visualization maps, different nodes represent different elements such as countries, institutions, authors or terms. The links between nodes represent relationships such as co-authorship, co-citation or co-occurrence, and weighted by total link strength (TLS) (43). Co-authorship analysis measures collaboration links between countries or institutions. The relatedness of nodes is determined based on the number of coauthored documents. While co-citation analysis measures the relationship among nodes based on the times they are cited by the same document. As for co-occurrence analysis, the relatedness of nodes is determined according to the number of documents in which they occur together (42). The size of the nodes reflects the number of outputs, citations or occurrences and the color indicates different clusters or average appearing year (AAY) of these elements. The “bibliometrix” package of R software was used for mapping historical direct citation network and cloud map of author keywords (44).
Results
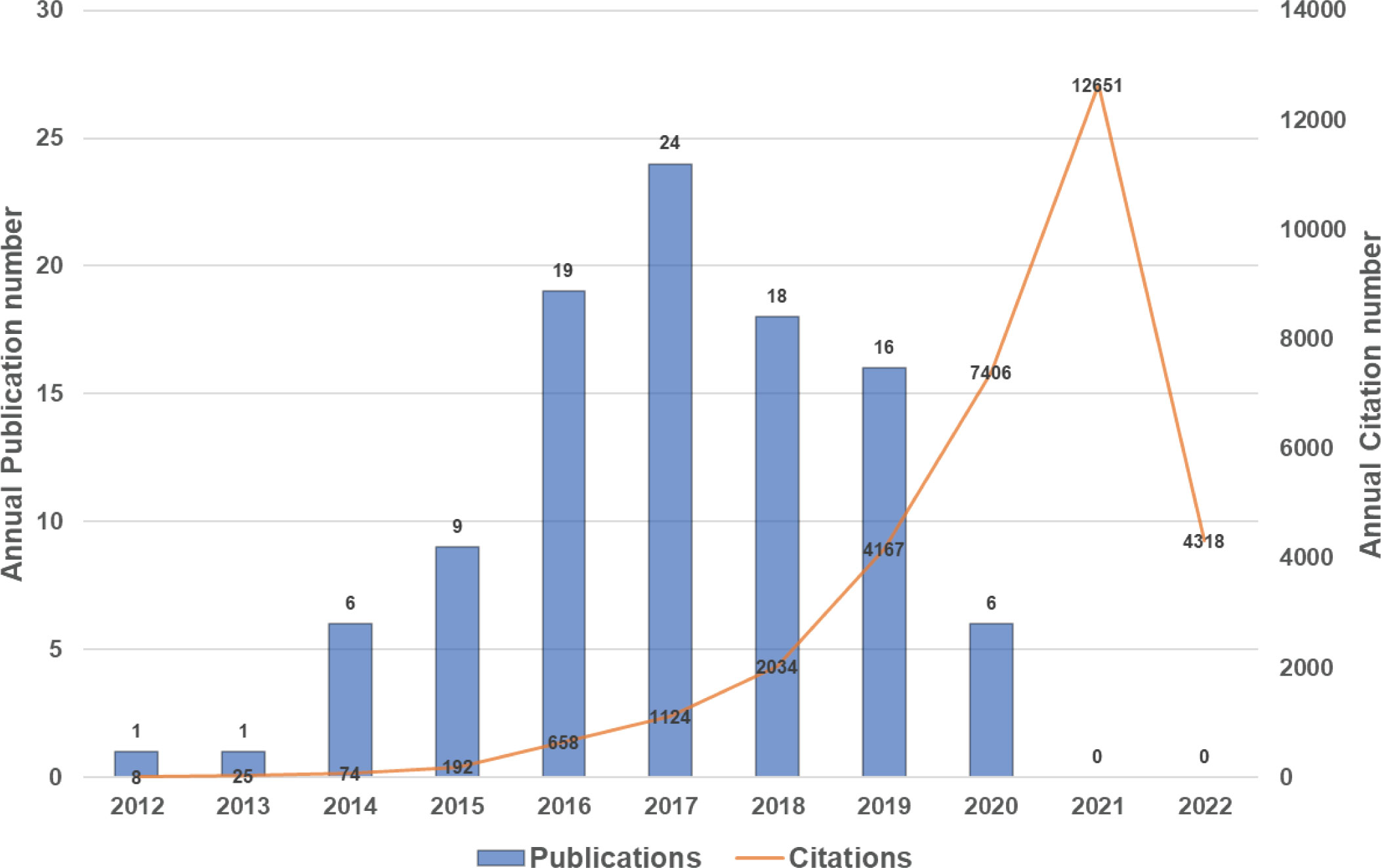
The top 100 highly cited articles are listed in descending order based on their total citation number in Table 1. The citation frequency received by these 100 studies ranged from 135 to 3603 (mean: 326.6). About half of the articles (n=54) received more than 200 citations, and only 3 articles were cited more than 1000 times. The citation density of each article was ranged from 18.56 to 327.55 (mean: 53.1, Supplementary Figure 1). As is apparent, the pioneering study titled “Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death”, by Dixon SJ et al., published in Cell in 2012, has received the most citations either total citation or adjusted citation count over the past 11 years. As shown in Figure 2, the top100 articles were published between 2012 and 2020. The year that yielded the greatest number of high-impact articles was in 2017 (n=24), followed by the year in 2016 (n=19), and 2018 (n=18). Additionally, the top 50 highly cited reviews on ferroptosis were summarized in Supplementary Table1.
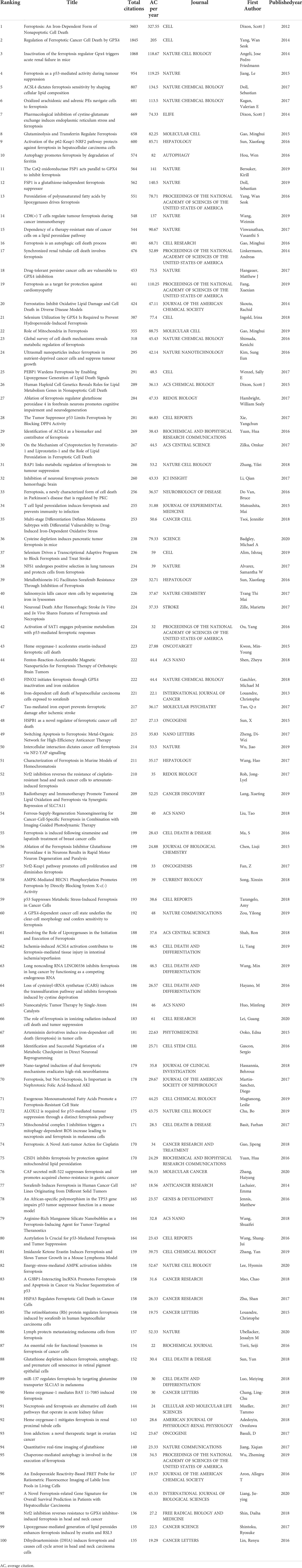
Table 1 Top 100 highly cited articles on ferroptosis ranked according to their total citations counts.
A total of 25 countries/regions worldwide contributed to the top 100 most cited articles, and 12 countries/regions had more than 3 articles, as shown in Figure 3A. Of them, the United States dominated the area with 68 articles and 26087 citations (shown in Figure 3B). China ranked second with 35 articles and 8173 citations, followed by Germany (20 articles and 7358 citations), France (8 articles and 1684 citations), and Japan (7 articles and 2691 citations). Figure 3C depicted the annual number of publications among the top 10 countries with the most outputs from 2012 to 2020. In addition, the network visualization map of co-authorship analysis among countries/regions, which could illustrate the collaboration network of countries/regions was conducted by VOSviewer in Figure 3D.
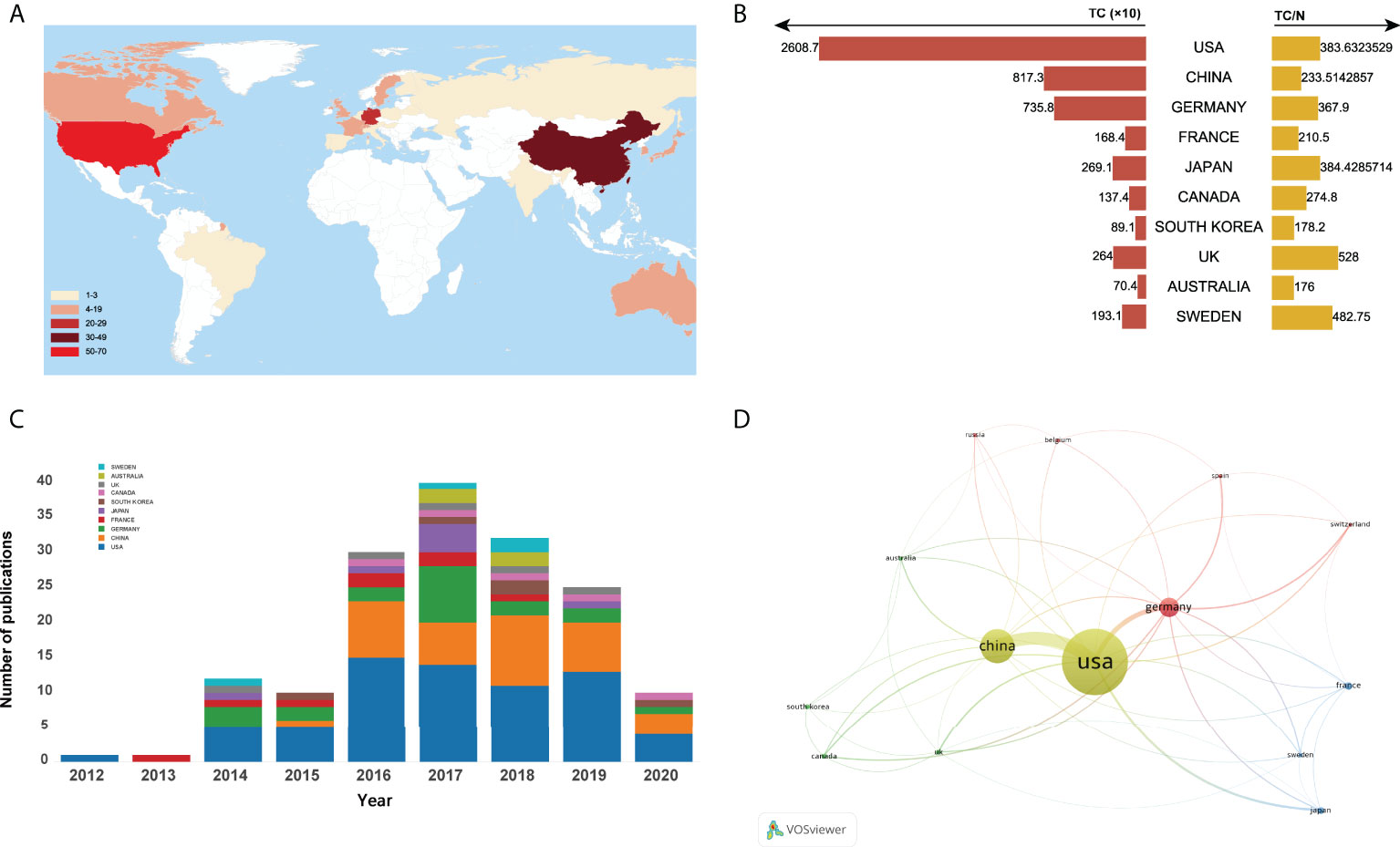
Figure 3 (A) Number of top-cited publications by countries/regions. (B)Total citations and average citations per document of top 10 most prolific countries/regions. (C) The number of annual publications of top 10 countries/regions on ferroptosis research from 2000 to 2020. (D) Network visualization map of country co-authorship analysis.
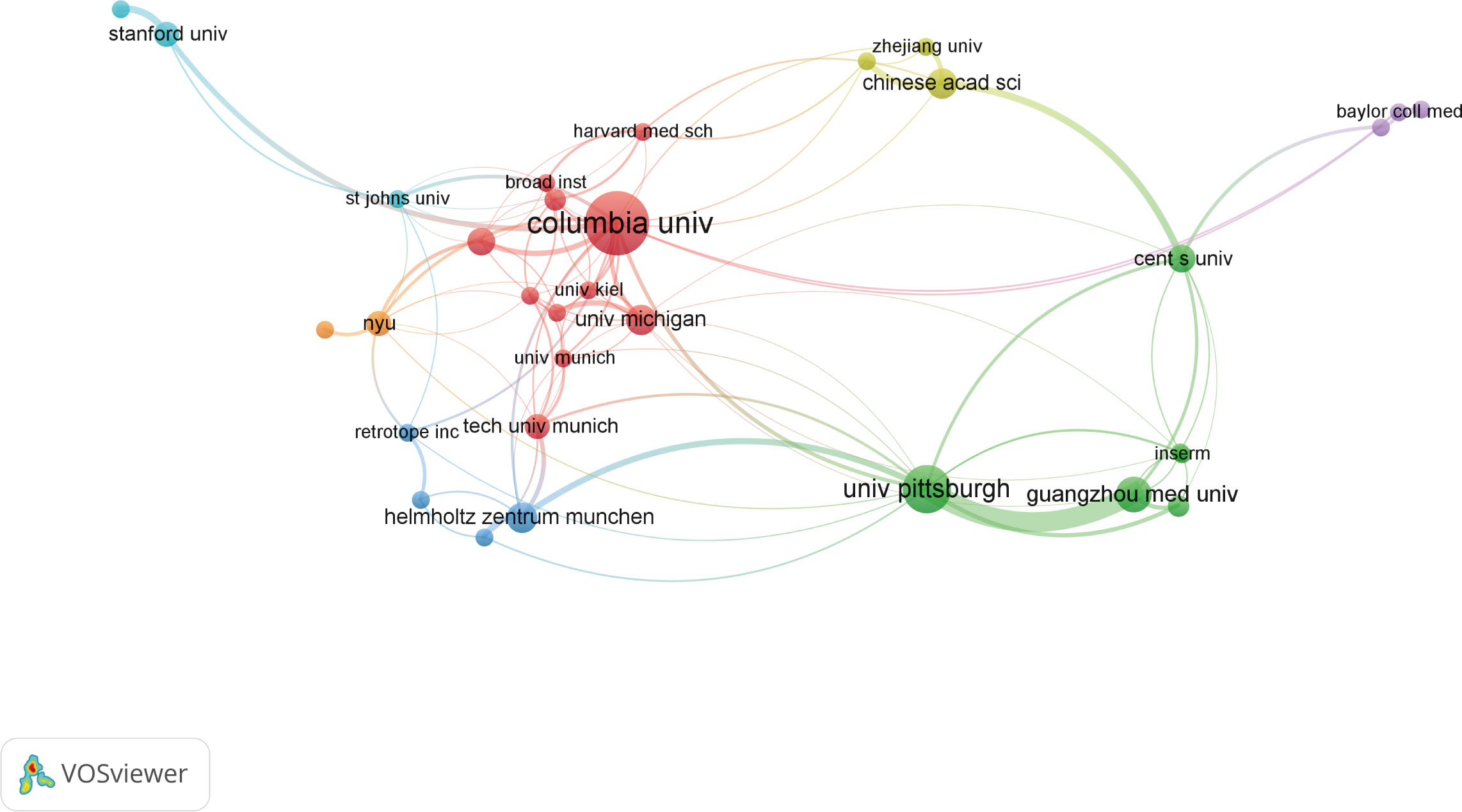
As for institutions, more than 200 institutions contributed to the top 100 research. Of them, Columbia University produced the most top-cited articles (n=25), followed by University of Pittsburgh (n=15). As for institutional co-authorship analysis in Figure 4, only institutions with more than 3 documents were included. Of the 33 institutions met the threshold, Columbia University, University of Pittsburgh, and Guangzhou Medical University were the top 3 institutions with the largest TLS.
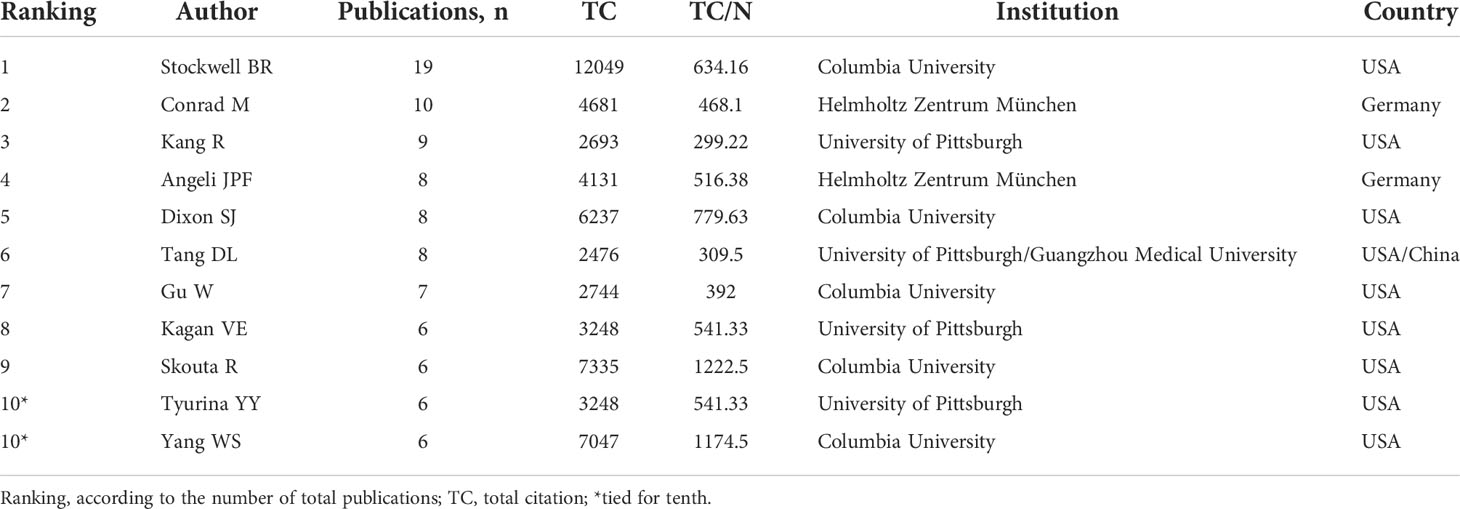
The top 10 authors involved in the top 100 articles were listed in Table 2. The list was led by Stockwell BR from the Columbia University, who participated 19 of the top 100 articles. Meanwhile, four other authors in the list also came from this university. Figure 5A depicted the annual outputs of these top 10 authors between 2012 and 2020. As for author co-citation analysis in Figure 5B, Dixon SJ, Yang WS, and Angeli JPF were the top three authors with the greatest TLS, and occupied the central position in the network map.
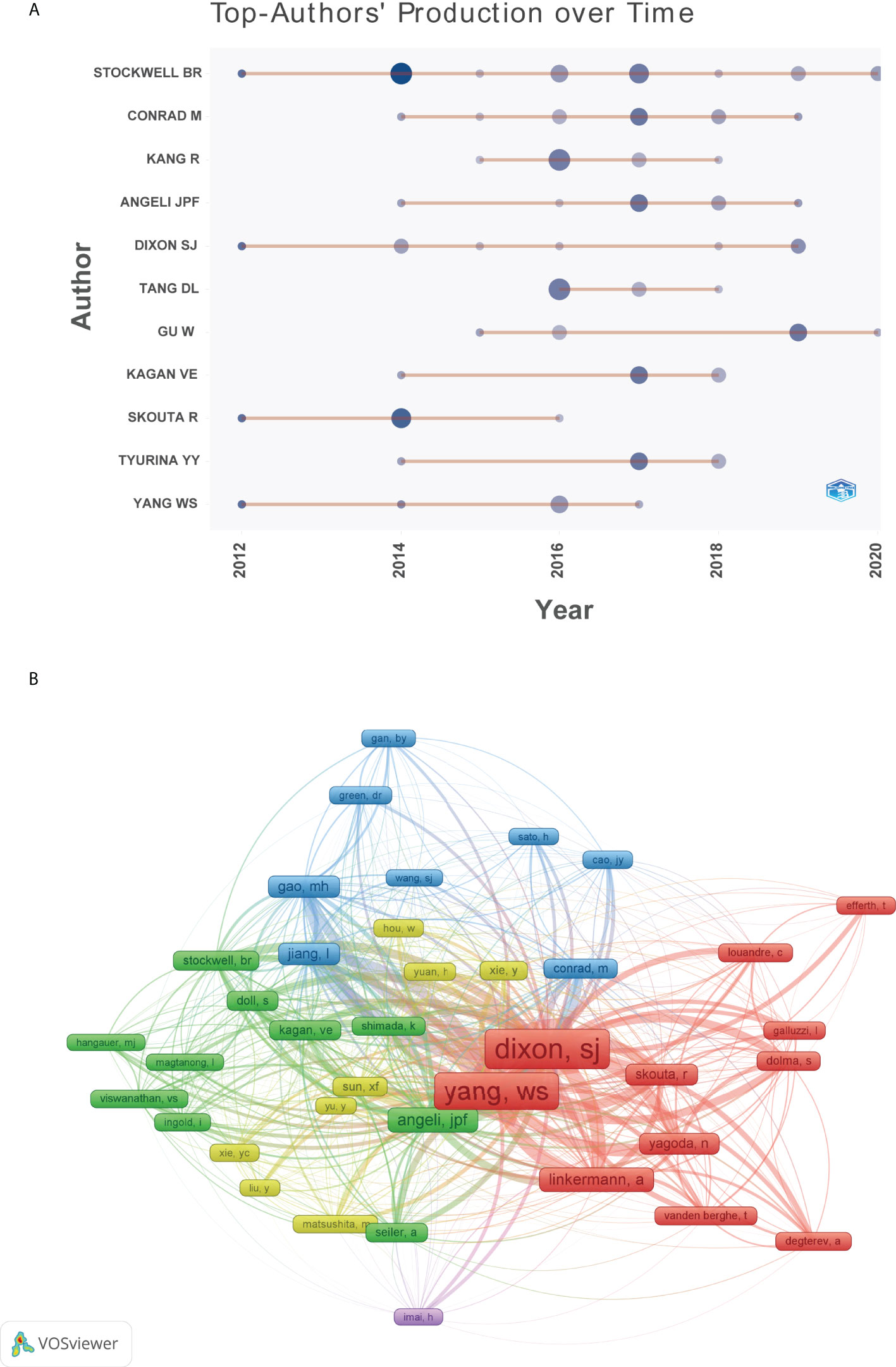
Figure 5 (A) Top 10 authors’ production over time. (B) Network visualization map of author co-citation analysis.
The top 100 articles were published in 54 different journals. Journals with more than 2 publications were listed in Table 3 with their citations and impact factors. Nature published the most (n=9), followed by Cell (n=5) and Proceedings of the National Academy of Sciences of the United States of America (n=5). Journal co-citation analysis was conducted in Figure 6. According to the density visualization map, Nature, Cell, and Proceedings of the National Academy of Sciences of the United States of America were also the top 3 most co-citation journals in this field.
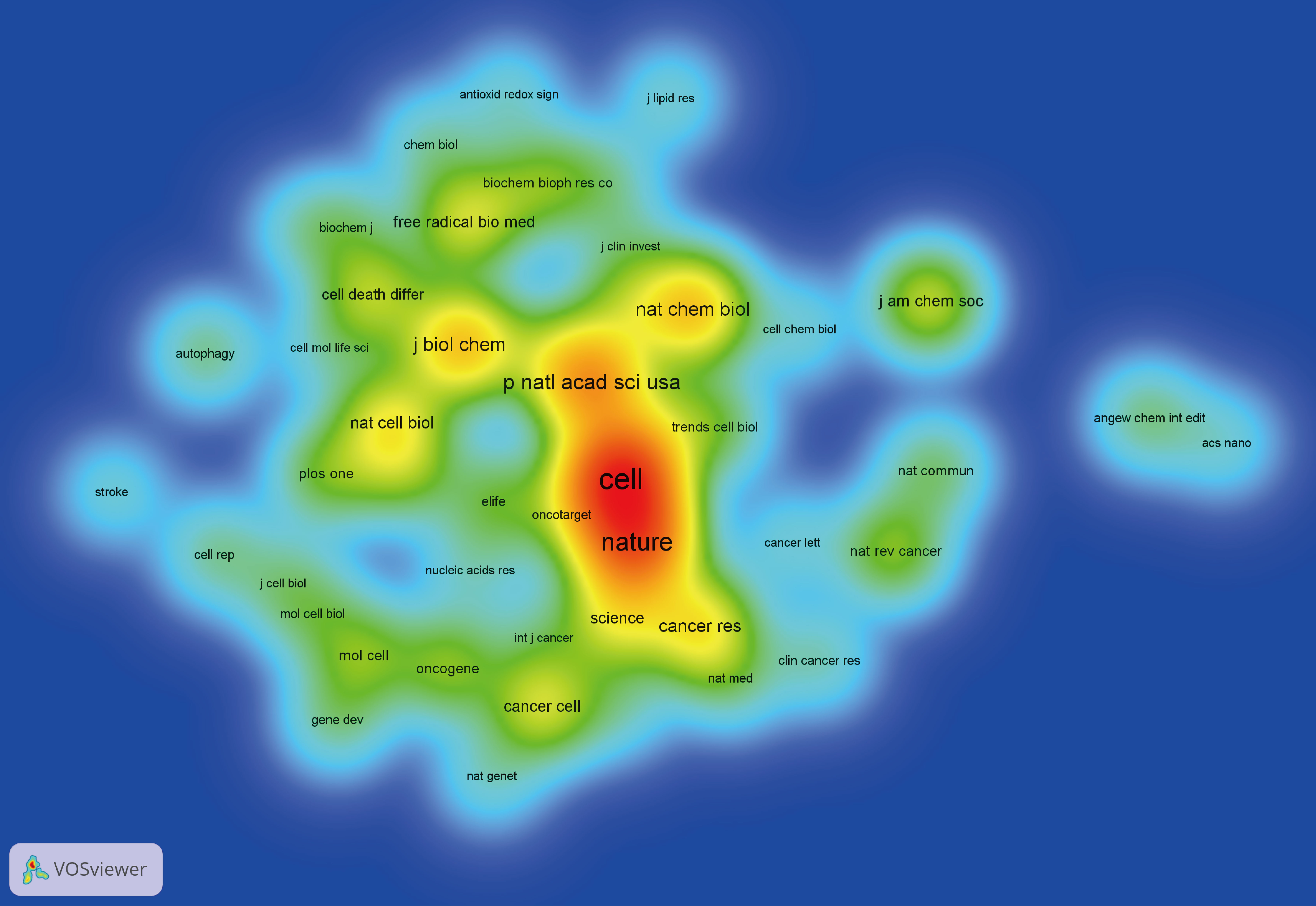
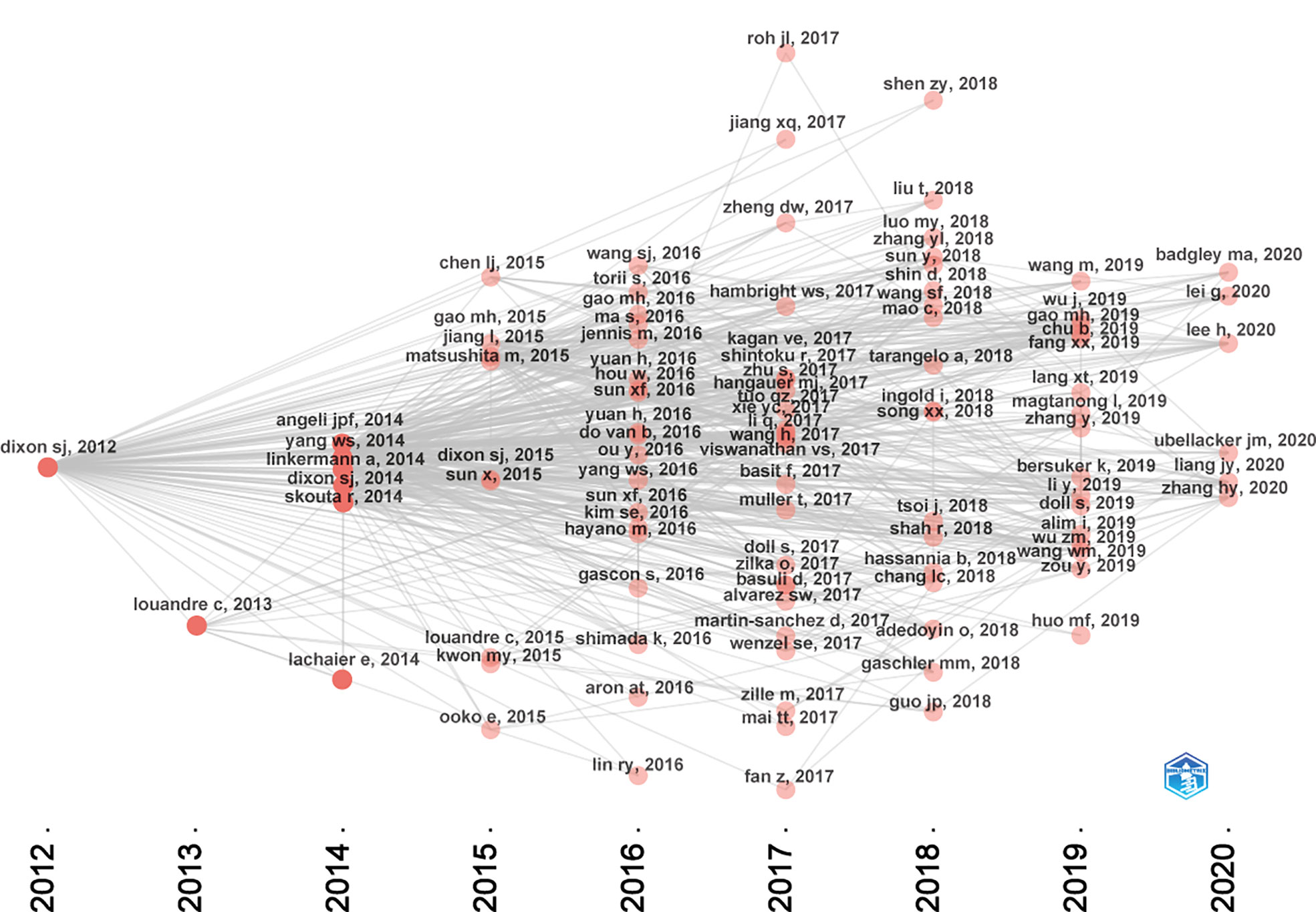
Figure 7 presented the historical direct citation network map of these top 100 articles. It can be seen that there are extensive connections among these studies. In addition, we also analyzed the terms or phrases appeared in the author keywords, titles and abstracts. A cloud map of author keywords is shown in Figure 8, apart from the keywords of ferroptosis (n=27), iron (n=8), reactive oxygen species (n=5), cell death (n=4), erastin (n=4), GPX4 (n=3), autophagy (n=3), hepatocellular carcinoma (n=3), head and neck cancer (n=3), necroptosis (n=3), and sorafenib (n=3) were these keywords with more than 3 times of frequency. As for network visualization map using terms from the titles and abstracts of the 100 most-cited articles, terms or phrases with a minimum of 7 occurrences were included (Figure 9). The 30 most frequent occurrences terms or phrases were listed in Table 4. In addition, all the included terms or phrases were scored according to the average publication year of the publications.
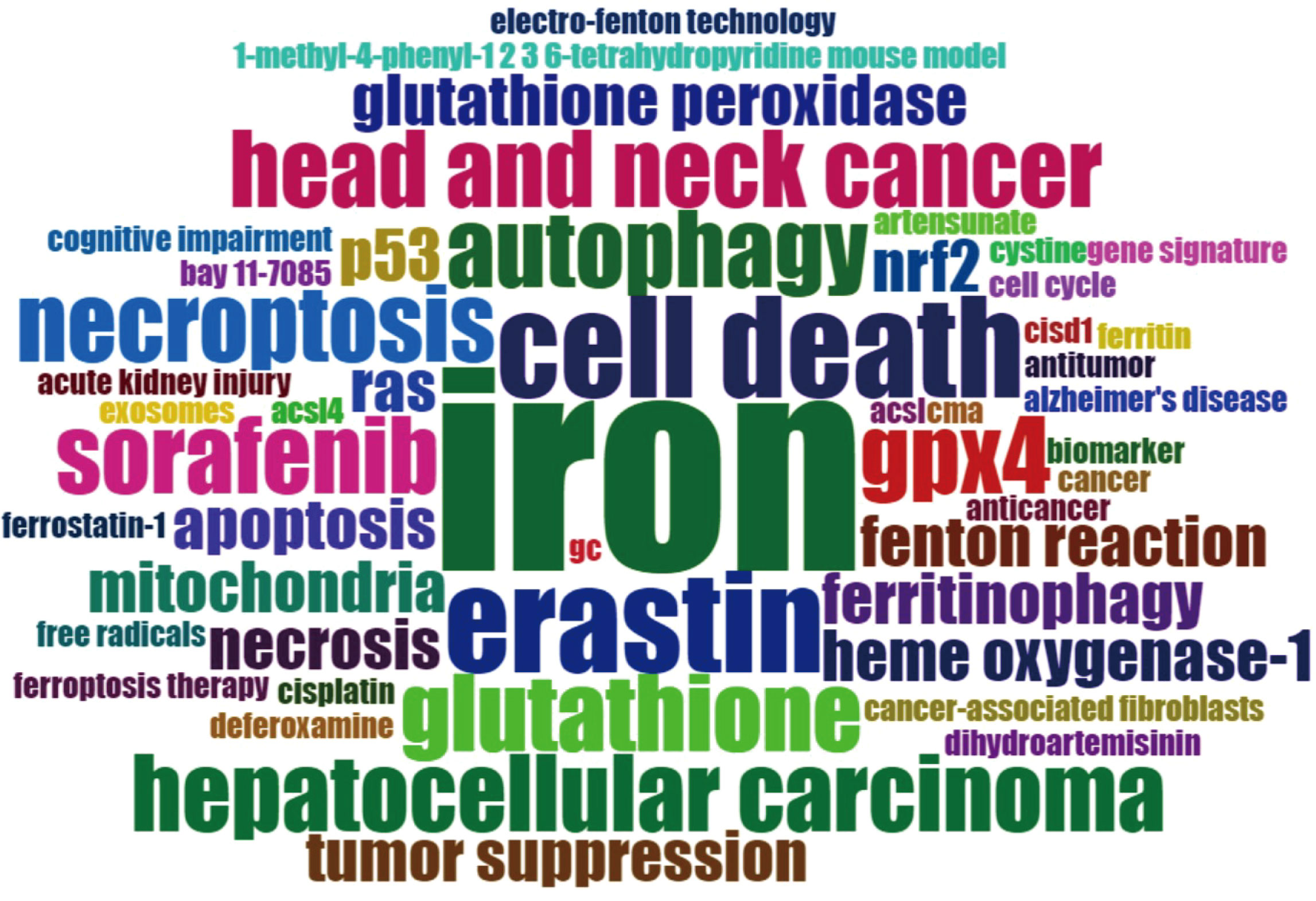
Figure 8 Cloud map of author keywords. Note: the keyword of “ferroptosis” was not included in this map.
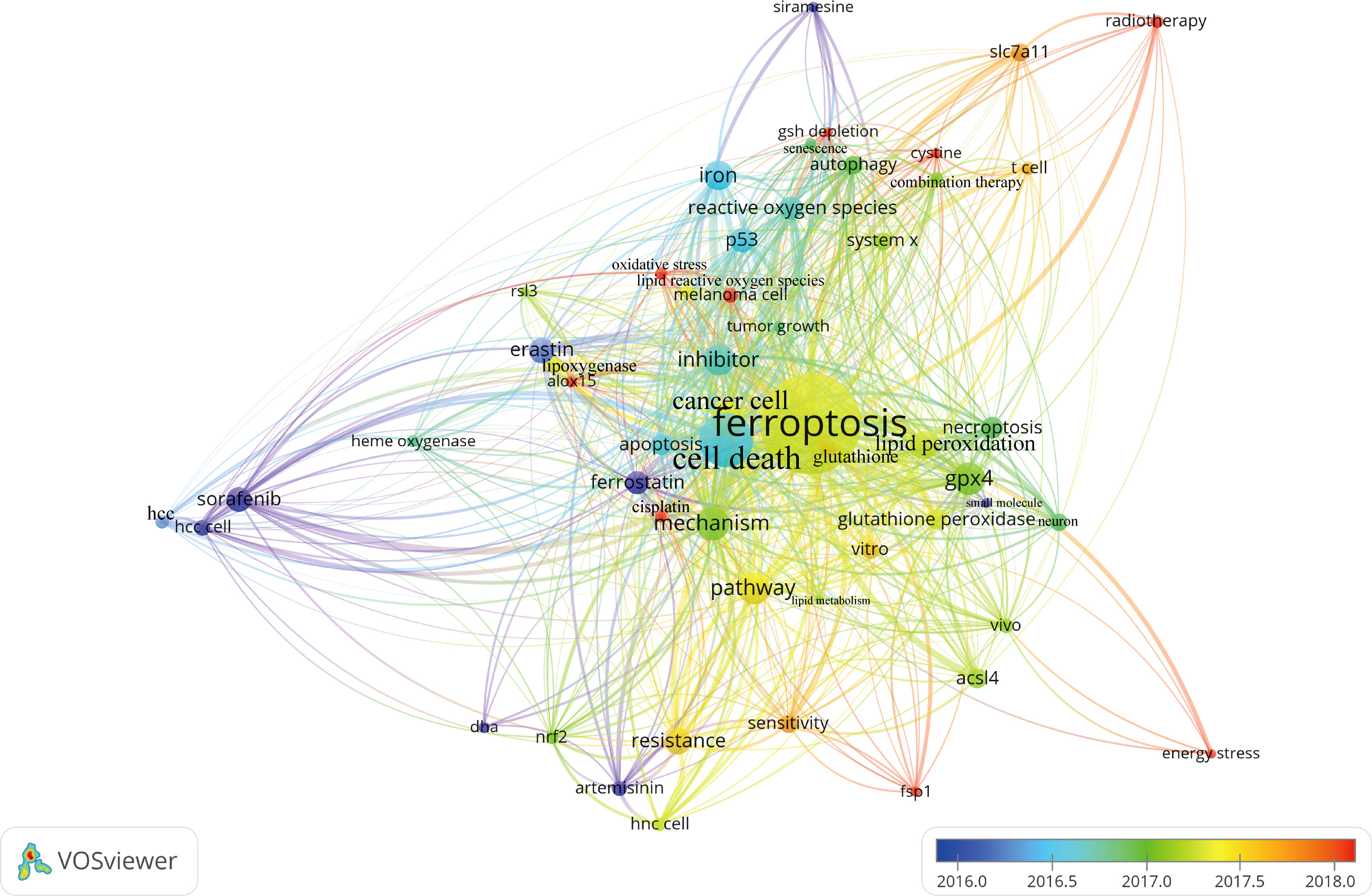
Figure 9 Overlay visualization map of terms generated with words from titles and abstracts by VOSviewer. Each node represents a term or phrase and the node size is proportional to occurrences. Distances between nodes indicates relatedness of words in terms of co-occurrence links. Different terms were given different colors based on their AAY.
Discussion
With rapid development of biomedicine, the amount of literature in biomedical domain is growing exponentially. It is reported that the volume of biomedical publications has exceeded 21 million with an annual average increase of nearly 1 million recently (45). Thus, the massive data poses a significant challenge for researchers to efficiently filter out useful information from it. Bibliometric analysis, a well-established research method using mathematical and statistical approaches, has been commonly used for revealing pervious research efforts. It is also a feasible tool to comprehensively evaluate the research advances of a certain scientific area quantitatively and qualitatively, and could predict the trends and hotspots in a specific field through information visualization (40, 46, 47). In the field of cell biology, mechanism of cell death has always been a hot topic in life science research for decades. Since the concept of ferroptosis was first proposed in 2012, endless efforts have been put into revealing its underlying molecular mechanisms and potential applications (7). In our pervious study, we have conducted a comprehensive bibliometric analysis based on the literature related to ferroptosis from 2012 to 2020 (40). Nevertheless, this study was powered based on all publications regarding ferroptosis, and top highly cited studies were not analyzed in detail. The most−cited studies in a certain field are generally considered landmarks, which have important reference value for further analysis owing to their ground−breaking contributions. In this study, we aimed to provide a comprehensive overview of the top 100 most influential studies with original data in the field of ferroptosis.
From the time distribution of these top100 articles, all of them were published between 2012 and 2020, and the years that yielded the relatively large number of high-impact articles were in 2017, 2016, and 2018. This result is consistent with our previous study. Our findings revealed that the number of publications in this domain has been continuously growing since 2012, and the past five years have witnessed an exponential growth (40). Therefore, these three years could be regarded as the inflection points for explosive growth. In addition, it must be emphasized that the total citation counts of publications in the last three years could be underestimated, considering the fact that the citations after publication usually need at least 3 years to accumulate. In order to balance the citations and temporal factors, we therefore adopted the citation density as another index to assess the average citations per year (41). The results showed that, although the total number of citations for recently published articles was lower than earlier publications, the citation densities of recently published articles were non-inferior and even higher than these early studies. It can be speculated that more and more recently published studies have chances to become highly cited articles over time.
As regards countries, the major part of the top 100 most-cited articles in the ferroptosis field was from the United States, which also owns the overwhelming number of total citation counts, indicating that the United States was the most influential country in this domain. As the largest high-tech power after the Second World War, the United States occupies a leading position in multiple global research areas (40, 48). The development of science and technology cannot be separated from the contribution from these first-class academic institutions and scholars. According to statistics, the United States owns the largest number of famous scholars and leads the tide of ferroptosis research around the world. Our result showed that nearly all of the top 10 highly cited authors were from the United States (9/11). As we all know that the concept of ferroptosis was first proposed by scholars in Columbia University (7). Columbia University and University of Pittsburgh were the institutions with the most top-cited articles among all institutions, reflecting their authority in the field of ferroptosis. In terms of the cooperation of countries or institutions, the United States collaborated most closely with China and Germany. When it comes to an institutional level, University of Pittsburgh and Guangzhou Medical University worked most closely among all institutions. This result might mainly be associated with several common research projects and Professor Tang’s team is an important nexus of them (49–52).
The top-cited studies within the research field were more likely to be published in high impact journals such as Nature, Cell and Proceedings of the National Academy of Sciences of the United States of America, as well as the authoritative journals of cell biology including Cell Death and Differentiation, Nature Cell Biology, Cell Death Disease, and Cell Reports, suggesting that these journals are prone to publishing original research in ferroptosis. Meanwhile, as can be seen that most of these journals have a high Impact factor value. This result also supports the well-known paradigm that high‐quality studies are often published in these journals topping the impact factor list, and in turn, maintain the high impact factor of these journals (53). Additionally, except for the field of cell biology, several studies published in these journals belonging to other fields such as materials science, nanoscience & nanotechnology, gastroenterology & hepatology, suggesting that numerous groundbreaking advances regarding ferroptosis have been made in these research directions. Take these studies published in ACS Nano as examples, Wang et al., reported a ferroptosis-inducing agent based on arginine-rich manganese silicate nanobubbles (AMSNs). They found that AMSNs have highly efficient glutathione depletion capabilities and subsequently leads to ferroptosis by inactivating GPX4, which provide important insights for tumor targeting with nanomedicines (54). Concurrently, Liu and colleagues from Wuhan University have constructed a ferrous-supply-regeneration SRF@FeIIITA nanoparticles. In brief, Fe3+ ion and naturally derived tannic acid formed a network-like corona onto sorafenib nanocrystal. Their results showed that SRF@FeIIITA can serve as an effective ferroptosis-inducing nanotherapeutic through interfering tumorous iron metabolism (55). Two other studies revealed that nanocatalysts such as PEGylated single-atom Fe-containing nanocatalysts (PSAF NCs) and Fenton-reaction-acceleratable magnetic nanoparticles could effectively trigger or accelerate tumor-specific Fenton reaction to generate abundant reactive oxygen species to induce cancer cell death (23, 56). In recent years, as nanotechnology has rapidly developed, many engineered nanomaterials that could induce ferroptosis have been developed for applications in cancer therapy. Although ferroptosis-inducing nanomedicines show some unique advantages such as increasing the active targeting to tumors, prolong the half-life in the blood, and improving antitumor ability with the synergy effect, most of the current research was only based on cell lines or animal models (57). And the biosecurity, specific mechanisms, and potential clinical application of these emerging treatments remain to be further investigated.
As shown in Figure 7, the citation network revealed that the study titled “Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death”, published in Cell in 2012 is the seminal article of this field (7). In this study, they have found that erastin, a small molecule compound, could selectively kill RAS-mutated cancer cells by overwhelming lipid peroxidation. This regulated cell death process depends on iron rather than other metals, and could be suppressed by iron chelators or lipophilic antioxidants like ferrostatin-1. Thus, such iron-dependent form of cell death was termed as “ferroptosis” since then. However, it is worth noting that before ferroptosis was officially named, this nonapoptotic form of cell death has already been observed in vitro. As early as 2003, erastin was first found through synthetic lethal high-throughput screening for tumor therapeutic drugs, and it could induce a nonapoptotic cell death process (58). Subsequently, two small molecules compounds, named RSL3 and RSL5, were found that they could increase lethality in the presence of oncogenic RAS, and activate a similar death mechanism like erastin (59). Although these two studies were not included in the top 100 articles of this field due to the setting of keywords search. They are also worth emphasizing as they have laid important groundwork for future studies. At the same time, Professor Stockwell BR and colleagues have made tremendous contributions to the establishment and progression of this field. In terms of these the top 100 articles, 19 listed his name.
Through the analysis of high-frequency terms and phrases extracted from the titles and abstracts, the top 100 most-cited articles covered a wide range of topics regarding ferroptosis. As evident from Table 4, in addition to term of ferroptosis, these terms or phrases including cell death, cancer cell, GPX4, pathway, inhibitor, mechanism, iron, lipid peroxidation, resistance, erastin, sorafenib, P53, reactive oxygen species, necroptosis, apoptosis, glutathione peroxidase, ACSL4, autophagy, and SLC7A11 appeared more frequently in the top 100 articles. From these terms, we could find that the current attentions of ferroptosis mainly focus on potential regulatory mechanism and pathways, key ferroptosis-related genes/molecules such as GPX4 (60, 61), P53 (62), SLC7A11 (63), ACSL4 (49, 64, 65), NRF2 (16, 66), ALOX15 (67, 68), oxidant and antioxidant system (69, 70), ferroptosis-inducing agents or nanomedicine for cancer therapy (23, 55, 56, 71), as well as the role of ferroptosis in non-neoplastic disorders such as renal failure and tubular necrosis (60, 72, 73), cardiomyopathy (74), hemorrhagic stroke (22, 75), intestinal ischemia/reperfusion (20), and neurodegenerative diseases (21, 76). Meanwhile, as shown in Figure 9, the combination therapeutic strategies targeting ferroptosis in radiotherapy or immunotherapy are gradually being valued (77, 78).
Regarding the potential regulatory mechanism and pathways on ferroptosis, there have been many high quality systematic and narrative reviews in Supplementary Table1 (8, 79), and thus will not be discussed further here. In brief, the key regulatory mechanisms and pathway of ferroptosis includes iron metabolism, lipid metabolism, amino-acid metabolism, as well as other factors such as GSH-dependent pathway, transsulfuration pathway, thioredoxin, peroxiredoxin, and so on (80, 81). With increasing ferroptotic mechanisms were clarified and ferroptosis inducers/inhibitors were found, there is increasing number of studies focusing on the discovery of ferroptosis-inducing agents such as small molecules and nanomaterials to eradicate malignancies (82–84). Ferroptosis inducers such as erastin, could be used with various chemotherapeutic agents including temozolomide, doxorubicin, cisplatin, and cytarabine in different type of cancers (66, 85). However, ferroptosis is also considered to be related to the onset of various diseases (74, 86). Thus, controlling the dosage of ferroptosis inducers and improving the specificity are essential for reducing the adverse events on normal tissues. And it is also of great clinical significance to clarify the key mechanism regulating ferroptotic sensitivity to tumor cells and avoiding the escape of malignancies from anticancer modalities.
Additionally, an increasing number of studies have confirmed that ferroptosis could interact with immune cells and enhance the immunogenicity of cancer cells. A study by Wang and colleagues found that CD8+ T cells activated by immune checkpoint blockade were able to promote ferroptosis-specific lipid peroxidation in tumor cells by secreting interferon gamma (IFNγ). Mechanistically, IFNγ significantly downregulated the expression of SLC3A2 and SLC7A11, and then impaired the uptake of cystine by tumor cells, which resulted in enhanced lipid peroxidation and ferroptosis (77). Meanwhile, ferroptosis can expose tumor antigens and improve the immunogenicity of tumor microenvironment and thereby promoting T-cell activation and facilitating antitumor T-cell immune response (87, 88). A study conducted by Lang et al. found that ferroptosis was a novel point of synergy between radiotherapy and immunotherapy. Immunotherapy sensitizes tumors to radiotherapy by promoting tumor-cell ferroptosis and SLC7A11 was a critical regulator in this process (78). Consequently, ferroptosis plays an essential role in the regulation of T-cell-mediated cellular immunity. Induction of ferroptosis through iron deposition-based combination strategies with direct or indirect ferroptosis inducers emerges to be promising therapeutic approaches to improve anti-PD-1/PD-L1 immunotherapy.
Limitations
This study has several limitations of note. First, we only used WoSCC database as data source, which may miss several related publications in other databases such as Scopus and Pubmed. However, different databases have different ways to count citations, this may be inappropriate to merge data from different databases. Among these databases, WoSCC is the most commonly used for analyzing the highly cited articles in a certain field (34, 35, 38). And it is generally thought that WoSCC is able to represent the condition of most publications in a field and is a reliable source of international peer-reviewed publications (89–91). Second, it should be noted that the frequency of citations in earlier studies should be higher than the recently published ones owing to the time factor, though the academic impact of former ones may be not really stronger than that of later ones (92). Thus, some breakthrough works published recently might be excluded due to lack of sufficient time to accumulate citations. Third, it remains controversial whether citations counts could reflect academic influence, though it is a widely used reference index (93). We are in agreement with the viewpoint that citations might not be fully representative of real academic values and the impact of one study should be evaluated comprehensively. In spite of this, the results of this study still could answer several important questions.
Conclusion
Overall, based on bibliometric analysis of the publications over the past decade, the top 100 most influential articles regarding ferroptosis were identified to provide a comprehensive and quantitative analysis of the key contributions made to drive the evolution of this field. Moreover, the current hotspots were also identified to provide useful insights for scholars. Since the ferroptosis concept was proposed in 2012, it has been noted that the ferroptosis related domain is developing tremendously. The USA could be viewed as the dominant country in terms of the number of high-impact articles, world-class academic institutions, and leading scientists in this research field. Currently, research in the field of ferroptosis is mainly focused on potential regulatory mechanism and pathways, key ferroptosis-related genes/molecules, oxidant and antioxidant system, ferroptosis-inducing agents or nanomedicine for cancer therapy, as well as combination therapeutic strategies. Continued in-depth studies in this area will contribute to a better understanding of the molecular pathophysiological mechanisms of multiple diseases and help to discover more potential therapeutic targets.
Author contributions
KC, ZSh, and HW designed the study. KC, QG, ZSh, and WY collected the data. KC, ZSu, YZ, WY, XY, and HW analyzed the data and drafted the manuscript. KC, QG, ZSh, YZ, WY, SunZ, XY, and HW revised and approved the final version of the manuscript. All authors read and approved the submitted version.
Funding
This research was funded by the Tianjin Health Science and Technology Project (Grant No. QN20016).
Acknowledgments
The authors thank Professor Xiuhua Yao from Tianjin huanhu hospital and “home-for-researchers (www.home-for-researchers.com)” for their effort in polishing the English content of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.948389/full#supplementary-material
References
1. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ (2018) 25(3):486–541. doi: 10.1038/s41418-017-0012-4
2. Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol (2014) 15(2):135–47. doi: 10.1038/nrm3737
3. Yan Y, Liang Q, Xu Z, Huang J, Chen X, Cai Y, et al. Downregulated ferroptosis-related gene STEAP3 as a novel diagnostic and prognostic target for hepatocellular carcinoma and its roles in immune regulation. Front Cell Dev Biol (2021) 9:743046. doi: 10.3389/fcell.2021.743046
4. Chu L, Yi Q, Yan Y, Peng J, Li Z, Jiang F, et al. A prognostic signature consisting of pyroptosis-related genes and SCAF11 for predicting immune response in breast cancer. Front Med (Lausanne) (2022) 9:882763. doi: 10.3389/fmed.2022.882763
5. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol (2014) 10(1):9–17. doi: 10.1038/nchembio.1416
6. Yang WS, Stockwell BR. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol (2016) 26(3):165–76. doi: 10.1016/j.tcb.2015.10.014
7. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell (2012) 149(5):1060–72. doi: 10.1016/j.cell.2012.03.042
8. Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ (2016) 23(3):369–79. doi: 10.1038/cdd.2015.158
9. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res (2019) 29(5):347–64. doi: 10.1038/s41422-019-0164-5
10. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell (2017) 171(2):273–85. doi: 10.1016/j.cell.2017.09.021
11. Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci (2017) 3(3):232–43. doi: 10.1021/acscentsci.7b00028
12. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol (2021) 22(4):266–82. doi: 10.1038/s41580-020-00324-8
13. Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of iron homeostasis: New players in metabolism, cell death, and disease. Trends Biochem Sci (2016) 41(3):274–86. doi: 10.1016/j.tibs.2015.11.012
14. Zou Z, Chang H, Li H, Wang S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis (2017) 22(11):1321–35. doi: 10.1007/s10495-017-1424-9
15. Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol (2019) 23:101107. doi: 10.1016/j.redox.2019.101107
16. Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology (2016) 63(1):173–84. doi: 10.1002/hep.28251
17. Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer (2019) 19(7):405–14. doi: 10.1038/s41568-019-0149-1
18. Xu Z, Peng B, Liang Q, Chen X, Cai Y, Zeng S, et al. Construction of a ferroptosis-related nine-lncRNA signature for predicting prognosis and immune response in hepatocellular carcinoma. Front Immunol (2021) 12:719175. doi: 10.3389/fimmu.2021.719175
19. Xu Z, Chen X, Song L, Yuan F, Yan Y. Matrix remodeling-associated protein 8 as a novel indicator contributing to glioma immune response by regulating ferroptosis. Front Immunol (2022) 13:834595. doi: 10.3389/fimmu.2022.834595
20. Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ (2019) 26(11):2284–99. doi: 10.1038/s41418-019-0299-4
21. Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol (2017) 12:8–17. doi: 10.1016/j.redox.2017.01.021
22. Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight (2017) 2(7):e90777. doi: 10.1172/jci.insight.90777
23. Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W, et al. Fenton-Reaction-Acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano (2018) 12(11):11355–65. doi: 10.1021/acsnano.8b06201
24. Xu C, Liu Z, Xiao J. Ferroptosis: A double-edged sword in gastrointestinal disease. Int J Mol Sci (2021) 22(22):12403. doi: 10.3390/ijms222212403
25. Zhang J, Liu Y, Li Q, Xu A, Hu Y, Sun C. Ferroptosis in hematological malignancies and its potential network with abnormal tumor metabolism. Biomed Pharmacother (2022) 148:112747. doi: 10.1016/j.biopha.2022.112747
26. Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol (2017) 46:65–83. doi: 10.1016/j.semcancer.2017.02.009
27. Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol (2019) 26(5):623–633.e9. doi: 10.1016/j.chembiol.2019.01.008
28. Zhou Y, Wu H, Wang F, Xu L, Yan Y, Tong X, et al. GPX7 is targeted by miR-29b and GPX7 knockdown enhances ferroptosis induced by erastin in glioma. Front Oncol (2022) 11:802124. doi: 10.3389/fonc.2021.802124
29. Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater (2018) 30(12):e1704007. doi: 10.1002/adma.201704007
30. Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol (2019) 12(1):34. doi: 10.1186/s13045-019-0720-y
31. Wang D, Song C, Barabási AL. Quantifying long-term scientific impact. Science (2013) 342(6154):127–32. doi: 10.1126/science.1237825
32. Sinatra R, Wang D, Deville P, Song C, Barabási AL. Quantifying the evolution of individual scientific impact. Science (2016) 354(6312):aaf5239. doi: 10.1126/science.aaf5239
33. Van Noorden R, Maher B, Nuzzo R. The top 100 papers. Nature (2014) 514(7524):550–3. doi: 10.1038/514550a
34. Xu G, Jin B, Xian X, Yang H, Zhao H, Du S, et al. Evolutions in the management of hepatocellular carcinoma over last 4 decades: An analysis from the 100 most influential articles in the field. Liver Cancer (2021) 10(2):137–50. doi: 10.1159/000513412
35. Powell AG, Hughes DL, Wheat JR, Lewis WG. The 100 most influential manuscripts in gastric cancer: A bibliometric analysis. Int J Surg (2016) 28:83–90. doi: 10.1016/j.ijsu.2016.02.028
36. Shah SM, Ahmad T, Chen S, Yuting G, Liu X, Yuan Y. A bibliometric analysis of the one hundred most cited studies in psychosomatic research. Psychother Psychosom (2021) 90(6):425–30. doi: 10.1159/000516185
37. Liu M, Gao Y, Yuan Y, Shi S, Wu J, Tian J, et al. An evidence mapping and scientometric analysis of the top-100 most cited clinical trials of anti-PD-1/PD-L1 drugs to treat cancers. Biomed Pharmacother (2021) 143:112238. doi: 10.1016/j.biopha.2021.112238
38. Zhao X, Guo L, Lin Y, Wang H, Gu C, Zhao L, et al. The top 100 most cited scientific reports focused on diabetes research. Acta Diabetol (2016) 53(1):13–26. doi: 10.1007/s00592-015-0813-1
39. Koh B, Banu R, Nusinovici S, Sabanayagam C. 100 most-cited articles on diabetic retinopathy. Br J Ophthalmol (2021) 105(10):1329–36. doi: 10.1136/bjophthalmol-2020-316609
40. Wu H, Wang Y, Tong L, Yan H, Sun Z. Global research trends of ferroptosis: A rapidly evolving field with enormous potential. Front Cell Dev Biol (2021) 9:646311. doi: 10.3389/fcell.2021.646311
41. Sun M, Li X, Sun J, Wang H, Xie Q, Wang M. The top-cited original articles on the role of microglia in neurodegenerative diseases: A bibliometric and visualized study. Front Aging Neurosci (2022) 14:869964. doi: 10.3389/fnagi.2022.869964
42. Van-Eck NJ, Waltman L. Software survey: VOS viewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
43. Wu H, Sun Z, Tong L, Wang Y, Yan H, Sun Z. Bibliometric analysis of global research trends on male osteoporosis: a neglected field deserves more attention. Arch Osteoporos (2021) 16(1):154. doi: 10.1007/s11657-021-01016-2
44. Aria M, Cuccurullo C. Bibliometrix: An r-tool for comprehensive science mapping analysis. J Informetr (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
45. Hu Y, Li Y, Lin H, Yang Z, Cheng L. Integrating various resources for gene name normalization. PLoS One (2012) 7(9):e43558. doi: 10.1371/journal.pone.0043558
46. Yeung A, Kletecka-Pulker M, Eibensteiner F, Plunger P, Völkl-Kernstock S, Willschke H, et al. Implications of twitter in health-related research: A landscape analysis of the scientific literature. Front Public Health (2021) 9:654481. doi: 10.3389/fpubh.2021.654481
47. Cheng K, Zhou Y, Wu H. Bibliometric analysis of global research trends on monkeypox: Are we ready to face this challenge? J Med Virol (2022). doi: 10.1002/jmv.27892
48. Wang CY, Li BH, Ma LL, Zhao MJ, Deng T, Jin YH, et al. The top-100 highly cited original articles on drug therapy for ventilator-associated pneumonia. Front Pharmacol (2019) 10:108. doi: 10.3389/fphar.2019.00108
49. Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun (2016) 478(2):838–44. doi: 10.1016/j.bbrc.2016.08.034
50. Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep (2017) 20(7):1692–704. doi: 10.1016/j.celrep.2017.07.055
51. Zhu S, Zhang Q, Sun X, Zeh HJ 3rd, Lotze MT, Kang R, et al. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res (2017) 77(8):2064–77. doi: 10.1158/0008-5472.CAN-16-1979
52. Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system xc- activity. Curr Biol (2018) 28(15):2388–2399.e5. doi: 10.1016/j.cub.2018.05.094
53. Garfield E. The history and meaning of the journal impact factor. JAMA (2006) 295(1):90–3. doi: 10.1001/jama.295.1.90
54. Wang S, Li F, Qiao R, Hu X, Liao H, Chen L, et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS Nano (2018) 12(12):12380–92. doi: 10.1021/acsnano.8b06399
55. Liu T, Liu W, Zhang M, Yu W, Gao F, Li C, et al. Ferrous-Supply-Regeneration nanoengineering for cancer-Cell-Specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano (2018) 12):12181–92. doi: 10.1021/acsnano.8b05860
56. Huo M, Wang L, Wang Y, Chen Y, Shi J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano (2019) 13(2):2643–53. doi: 10.1021/acsnano.9b00457
57. Wang Y, Liu T, Li X, Sheng H, Ma X, Hao L. Ferroptosis-inducing nanomedicine for cancer therapy. Front Pharmacol (2021) 12:735965. doi: 10.3389/fphar.2021.735965
58. Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell (2003) 3(3):285–96. doi: 10.1016/s1535-6108(03)00050-3
59. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol (2008) 15(3):234–45. doi: 10.1016/j.chembiol.2008.02.010
60. Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol (2014) 16(12):1180–91. doi: 10.1038/ncb3064
61. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell (2014) 156(1-2):317–31. doi: 10.1016/j.cell.2013.12.010
62. Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature (2015) 520(7545):57–62. doi: 10.1038/nature14344
63. Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) (2018) 38(1):12. doi: 10.1186/s40880-018-0288-x
64. Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun (2016) 478(3):1338–43. doi: 10.1016/j.bbrc.2016.08.124
65. Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol (2017) 13(1):91–8. doi: 10.1038/nchembio.2239
66. Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol (2017) 11:254–62. doi: 10.1016/j.redox.2016.12.010
67. Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A (2016) 113(44):E6806–12. doi: 10.1073/pnas.1607152113
68. Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci (2017) 108(11):2187–94. doi: 10.1111/cas.13380
69. Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol (2017) 13(1):81–90. doi: 10.1038/nchembio.2238
70. Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature (2019) 575(7784):688–92. doi: 10.1038/s41586-019-1705-2
71. Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol (2016) 11(11):977–85. doi: 10.1038/nnano.2016.164
72. Müller T, Dewitz C, Schmitz J, Schröder AS, Bräsen JH, Stockwell BR, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci (2017) 74(19):3631–45. doi: 10.1007/s00018-017-2547-4
73. Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol (2017) 28(1):218–29. doi: 10.1681/ASN.2015121376
74. Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A (2019) 116(7):2672–80. doi: 10.1073/pnas.1821022116
75. Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke In vitro and In vivo shares features of ferroptosis and necroptosis. Stroke (2017) 48(4):1033–43. doi: 10.1161/STROKEAHA.116.015609
76. Do Van B, Gouel F, Jonneaux A, Timmerman K, Gelé P, Pétrault M, et al. Ferroptosis, a newly characterized form of cell death in parkinson's disease that is regulated by PKC. Neurobiol Dis (2016) 94:169–78. doi: 10.1016/j.nbd.2016.05.011
77. Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature (2019) 569(7755):270–4. doi: 10.1038/s41586-019-1170-y
78. Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discovery (2019) 9(12):1673–85. doi: 10.1158/2159-8290.CD-19-0338
79. Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci (2016) 73(11-12):2195–209. doi: 10.1007/s00018-016-2194-1
80. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell (2015) 59(2):298–308. doi: 10.1016/j.molcel.2015.06.011
81. Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy (2016) 12(8):1425–8. doi: 10.1080/15548627.2016.1187366
82. Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene (2015) 34(45):5617–25. doi: 10.1038/onc.2015.32
83. Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature (2017) 551(7682):639–43. doi: 10.1038/nature24637
84. Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest (2018) 128(8):3341–55. doi: 10.1172/JCI99032
85. Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, et al. Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Res Treat (2018) 50(2):445–60. doi: 10.4143/crt.2016.572
86. Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A (2014) 111(47):16836–41. doi: 10.1073/pnas.1415518111
87. Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T Cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med (2015) 212(4):555–68. doi: 10.1084/jem.20140857
88. Sun LL, Linghu DL, Hung MC. Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res (2021) 11(12):5856–63.
89. Cheng K, Guo Q, Yang W, Wang Y, Sun Z, Wu H. Mapping knowledge landscapes and emerging trends of the links between bone metabolism and diabetes mellitus: A bibliometric analysis from 2000 to 2021. Front Public Health (2022) 10:918483. doi: 10.3389/fpubh.2022.918483
90. Li C, Wu H, Sun Z, Chen Z, Trampuz A. Global publication trends and research hotspots of revision hip and knee arthroplasty: A 21-year bibliometric approach. J Arthroplasty (2022) 37(5):974–84. doi: 10.1016/j.arth.2022.01.022
91. Yeung A, Tzvetkov NT, Balacheva AA, Georgieva MG, Gan RY, Jozwik A, et al. Lignans: Quantitative analysis of the research literature. Front Pharmacol (2020) 11:37. doi: 10.3389/fphar.2020.00037
92. Wu H, Zhou Y, Xu L, Tong L, Wang Y, Liu B, et al. Mapping knowledge structure and research frontiers of ultrasound-induced blood-brain barrier opening: A scientometric study. Front Neurosci (2021) 15:706105. doi: 10.3389/fnins.2021.706105
Keywords: ferroptosis, citation, hotspot, bibliometric analysis, cancer
Citation: Cheng K, Guo Q, Shen Z, Yang W, Zhou Y, Sun Z, Yao X and Wu H (2022) Frontiers of ferroptosis research: An analysis from the top 100 most influential articles in the field. Front. Oncol. 12:948389. doi: 10.3389/fonc.2022.948389
Received: 25 May 2022; Accepted: 25 July 2022;
Published: 11 August 2022.
Edited by:
Zhijie Xu, Xiangya Hospital, Central South University, ChinaReviewed by:
Susu Guo, Shanghai Jiao Tong University, ChinaJiao Wu, Fourth Military Medical University, China
Demeng Xia, Shanghai University, China
Fei-Long Wei, Fourth Military Medical University (Air Force Medical University), China
Copyright © 2022 Cheng, Guo, Shen, Yang, Zhou, Sun, Yao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunming Cheng, Y2hlbmdrbTIwMTNAMTYzLmNvbQ==; Xiuhua Yao, eWFvX3hpdWh1YTg3QDE2My5jb20=; Haiyang Wu, d3VoYWl5YW5nMjAyMUB0bXUuZWR1LmNu
†These authors have contributed equally to this work
 Kunming Cheng1*†
Kunming Cheng1*† Qiang Guo
Qiang Guo Zefeng Shen
Zefeng Shen Yan Zhou
Yan Zhou Haiyang Wu
Haiyang Wu