
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 02 August 2022
Sec. Cancer Immunity and Immunotherapy
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.946307
Purpose: Insomnia in cancer patients is a common symptom contributing to poor quality of life and poor functioning. Sleep disturbances have been associated with inflammatory activity, and systemic cancer therapies chemotherapy, hormonal therapy, and immunotherapy may cause insomnia. We have carried out a meta-analysis to estimate the occurrence of insomnia in patients with solid cancer treated with immunotherapy using checkpoint inhibitors (CPI).
Methods: PubMed and ClinicalTrials.gov were searched for phase 3 studies in solid tumours where treatment included a checkpoint inhibitor in the experimental arm. Data on the incidence of insomnia were acquired from the adverse events tables available from clinicaltrials.gov and/or from the full texts. Random effect logistic model was used to compare pooled data. Heterogeneity between studies was assessed using Cochrane Q statistics and I2 statistics.
Results: A total of 54 studies (including six three-arm studies) involving 37,352 patients were included in the analysis. Insomnia was reported in 8.3% of subjects (95% confidence interval [CI] 8.0%-8.7%) treated with immunotherapy. Insomnia was significantly more common in patients receiving immunotherapy compared to those enrolled in study arms with inactive treatment (odds ratio [OR] 1.49, 95% CI 1.13-1.96). The odds for insomnia were similar between the arms for studies comparing CPI versus chemotherapy and CPI versus non-immunologic targeted therapies (OR 1.07, 95% CI 0.94-1.22 and OR 1.40, 95% CI 0.90-2.18, respectively). The OR for insomnia was higher for cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptor inhibitors compared to the inhibitors of programmed death-1 (PD-1) receptor (OR 1.36, 95% CI 1.06 – 1.74).
Conclusion: Cancer immunotherapy using CPI is associated with insomnia but the odds of developing the symptom are not greater with immunotherapy than with other systemic modalities including chemotherapy and non-immunologic targeted therapies.
Insomnia is a common and underestimated problem in cancer patients. Insomnia is an important contributing factor to poor quality of life, chronic fatigue, and impaired cognitive functioning. The aetiology of insomnia in cancer patients is multifactorial. Sleep disturbances may be the cause but also the consequence of chronic fatigue, depression, anxiety, and cognitive impairment. Important causes of insomnia in cancer patients also include pain or physical discomfort, decreased physical activity and changes in sleeping routine, such as occurring during hospitalizations (1).
Sleep disturbances have been linked to increased cancer risk, with evidence pointing to a causal relationship between lack of sleep and, especially, endocrine function-related cancers such as prostate and breast carcinoma (2–4). On the other hand, the chronic inflammatory state associated with conditions such as diabetes, autoimmune disease, and cancer has been shown to trigger disruption in circadian rhythm manifesting as insomnia (5–7). Systemic cancer treatments including chemotherapy, hormonal therapy, and immunotherapy using checkpoint inhibitors (CPI) have been associated with insomnia (8–10). CPIs are a part of standard treatment for many solid and haematological malignancies, radically improving the prognosis of a significant proportion of patients. However, monoclonal antibodies inhibiting the programmed death (PD)-1 receptor, its ligand PD-L1, and the cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptor are also associated with activation of inflammatory processes. Treatment with these agents specifically designed to stimulate antitumour immune responses leads to complex changes in the immune system (11, 12). Due to the strong link between inflammation and sleep disorders, there is a rationale to examine the occurrence of insomnia during therapy with CPIs (13, 14).
The aim of the present meta-analysis was to examine the incidence of insomnia as an adverse event in clinical trials with CPIs in patients with solid cancers, and to compare its occurrence in patients treated with CPIs to those receiving other systemic therapies for solid cancers, including chemotherapy and non-immunologic targeted agents.
The search was carried out in the PubMed and ClinicalTrials.gov databases using terms “cancer” and “ipilimumab or MDX-010”, “nivolumab or MDX-1106”, “avelumab or MSB0010718C”, “durvalumab or MEDI-4736”, “pembrolizumab or MK-3475”, “atezolizumab or MPDL3280A”, “tremelimumab or CP-675,206”, “cemiplimab or REGN2810” (15). The database searches were performed on February 1, 2021. Furthermore, recent systematic studies were screened for further studies missed by the database search (16, 17). The study selection process is shown in Figure 1. The search was limited to phase 3 studies with in extenso publications in English and with tabulated adverse event data in the ClinicalTrials.gov database or in the available article. For all identified studies, the incidence of insomnia was determined from the adverse event tables. Two authors retrieved the data independently. The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (18).
The percentages and confidence intervals of patients with insomnia were reported within each study, as well as an aggregate for the different classes of CPI agents. The odds ratio (OR) and confidence interval (CI) for each study were reported. The types of treatment in the CPI arms were classified as follows: CPI, CPI in combination with chemotherapy, and CPI in combination with non-immunologic targeted therapy. Differences between the individual types of CPI were analysed for the following categories: anti-PD-1 agents, anti-PDL-1 agents, anti-CTLA-4 agents, and combinations of anti-CTLA-4 agents with anti-PD-1/L1 antibodies (anti-PD-1 and anti-PD-L1 agents were analysed jointly in combinations with antiCTLA-4 drugs) (15). If control arm contained the combination of chemotherapy and a non-immunologic targeted agent, it was classified as "chemotherapy" for the meta-analysis.
The random effect model was used to compare pooled data (19). Two-arm and three-arm studies were included in the meta-analysis. A three-arm study with two experimental arms (E1 and E2) and one control arm (C) will generate two study arm pairs (E1 versus C; E2 versus C). Data from three-arm studies included in the meta-analysis were processed according to a method recommended by Rucker et al. (splitting the shared group of multi-arm trials in pairwise meta-analysis) (20).
Cochrane Q statistics and I2 statistics were used to estimate heterogeneity. Certainty of evidence was assessed per Grading of Recommendations, Assessment, Development and Evaluations (GRADE) guidelines (21). I2 values were used to classify heterogeneity as low (<25%), intermediate (25-75%), or high (>75%) (22).
The logistic model with random effect was used to compare different classes of immunotherapy agents, i.e. those targeting PD-1, PD-L1, and CTLA-4, respectively. All statistical analyses were performed using software R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) using the R package meta (23).
A total of 8,632 records of phase 3 studies for cancer were identified in the initial step of the search. Of 93 studies using CPI therapy in the experimental arm, 54 studies (including six three-arm studies) enrolling 37,352 patients with evaluated toxicity were included in the present analysis. The list of the included studies is provided in Supplementary Table 1 (24–77).
The solid cancers treated in the included studies were the following: lung cancer (23 studies), melanoma (six studies), renal cancer (five studies), urothelial cancer (five studies), head and neck carcinoma (four studies), breast cancer (three studies), gastro-oesophageal junction cancer (three studies), mesothelioma (two studies), prostate cancer (two studies), gastric, oesophageal or colorectal cancer, hepatocellular carcinoma (one study each). The pairwise analysis was carried out comparing 60 study arm-pairs: two study pairs were generated for each of the three-arm studies comparing each of the CPI-containing arms with the control arm. Because high-grade (grade 3) insomnia was not reported in the included studies, all-grade insomnia was analysed (78). Summary of the results is shown in Table 1.
The Cochrane risk of bias tool was used for quality assessment. The main source of was performance bias, i.e., the lack of blinding of participants and personnel in some studies (Supplementary Table 1). Because the analysed studies were all randomised phase III trials, there was a low risk of other types of bias including random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting bias. The risk of evidence selection bias was low because insomnia was not the main assessed parameter or the clinically most important toxicity in any of the studies.
Insomnia was reported in 8.3% of subjects (95% CI 8.0%-8.7%) treated with immunotherapy. Across all types of control arms, the OR of insomnia was 1.15 (95% CI 1.05–1.25) (Table 2). The highest OR for insomnia was detected for the combination of antiCTLA-4 and antiPD-1/L1 agents (OR 1.36, 95% CI 1.06 – 1.75 using antiPD-1 agents as reference). The OR was also higher for antiCTLA-4 therapy compared to antiPD1 drugs (OR 1.36, 95% CI 1.05-1.74) and, moderately but statistically significantly also for antiPDL1 agents compared to antiPD1 drugs (OR 1.22, 95% CI 1.00-1.49) (Table 3). The heterogeneity was low for all drug classes except for the comparison of antiCTLA-4 versus antiPD-1 where it was intermediate (Table 4).
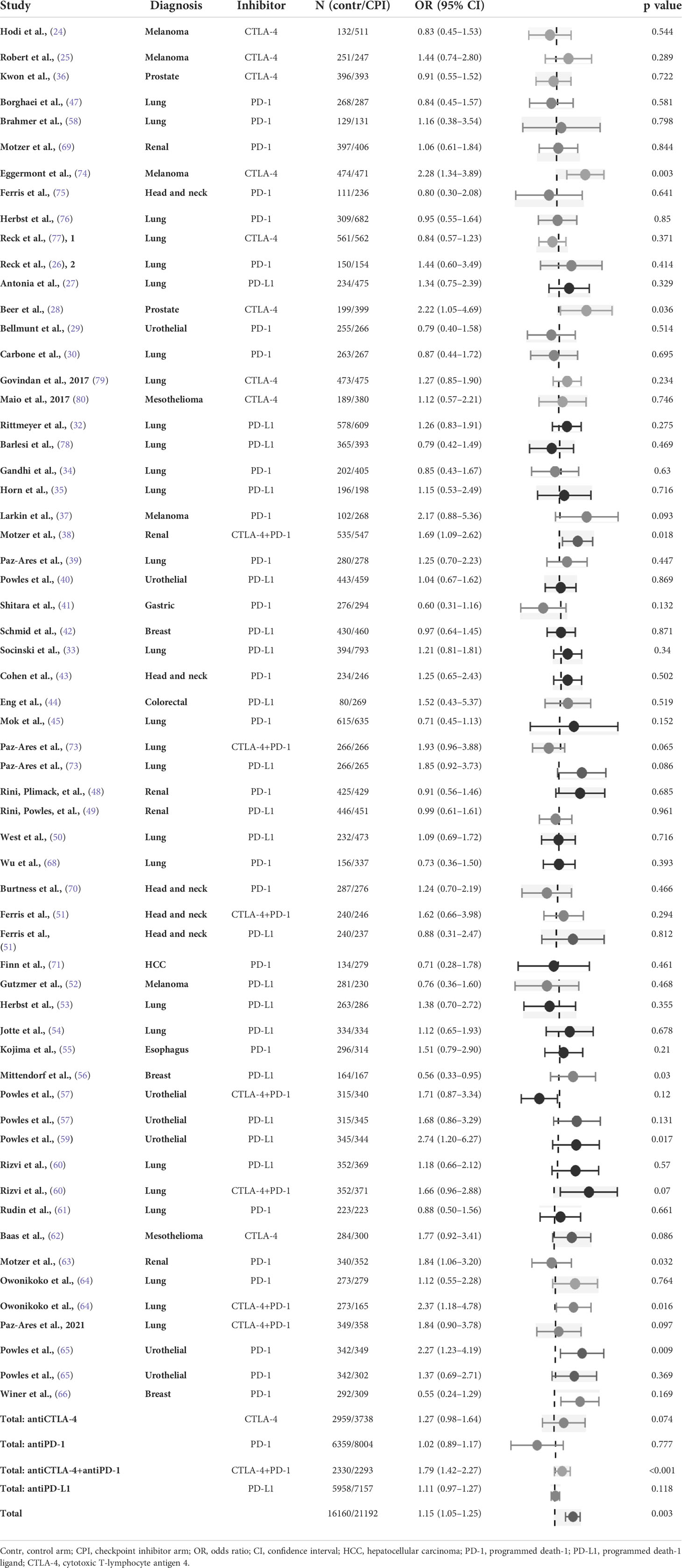
Table 2 Odds ratio of insomnia in randomised studies of checkpoint inhibitors versus all types of control (control arm as reference).
Nine studies were carried out comparing a CPI to inactive treatment, including one three-arm study. In total, 10 study arm pairs were analysed. The control arm was considered inactive if the allocated patients received placebo therapy or best supportive care but not active antineoplastic systemic agents. Insomnia was significantly more common in patients receiving immunotherapy compared to those enrolled in study arms with inactive treatment (OR 1.49, 95% CI 1.13-1.96). There was an intermediate heterogeneity among the studies (Table 5).
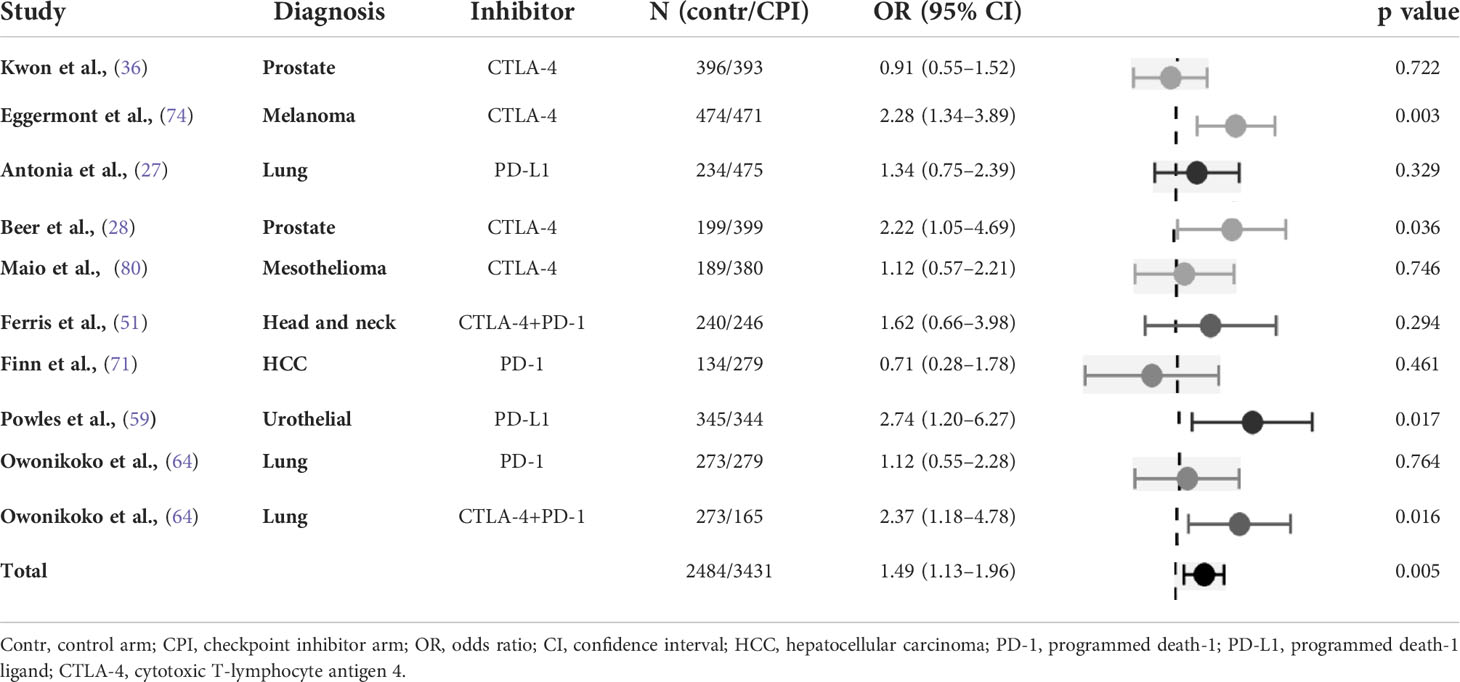
Table 5 Odds ratio of insomnia in randomised studies of checkpoint inhibitors versus inactive treatment (placebo and/or best supportive care), with control arm used as reference.
The meta-analysis was carried out for 24 individual randomised studies including two three-arm studies. The odds for insomnia were similar between the arms (OR 1.07, 95% CI 0.94-1.22). There was a low heterogeneity among the studies (Table 6).
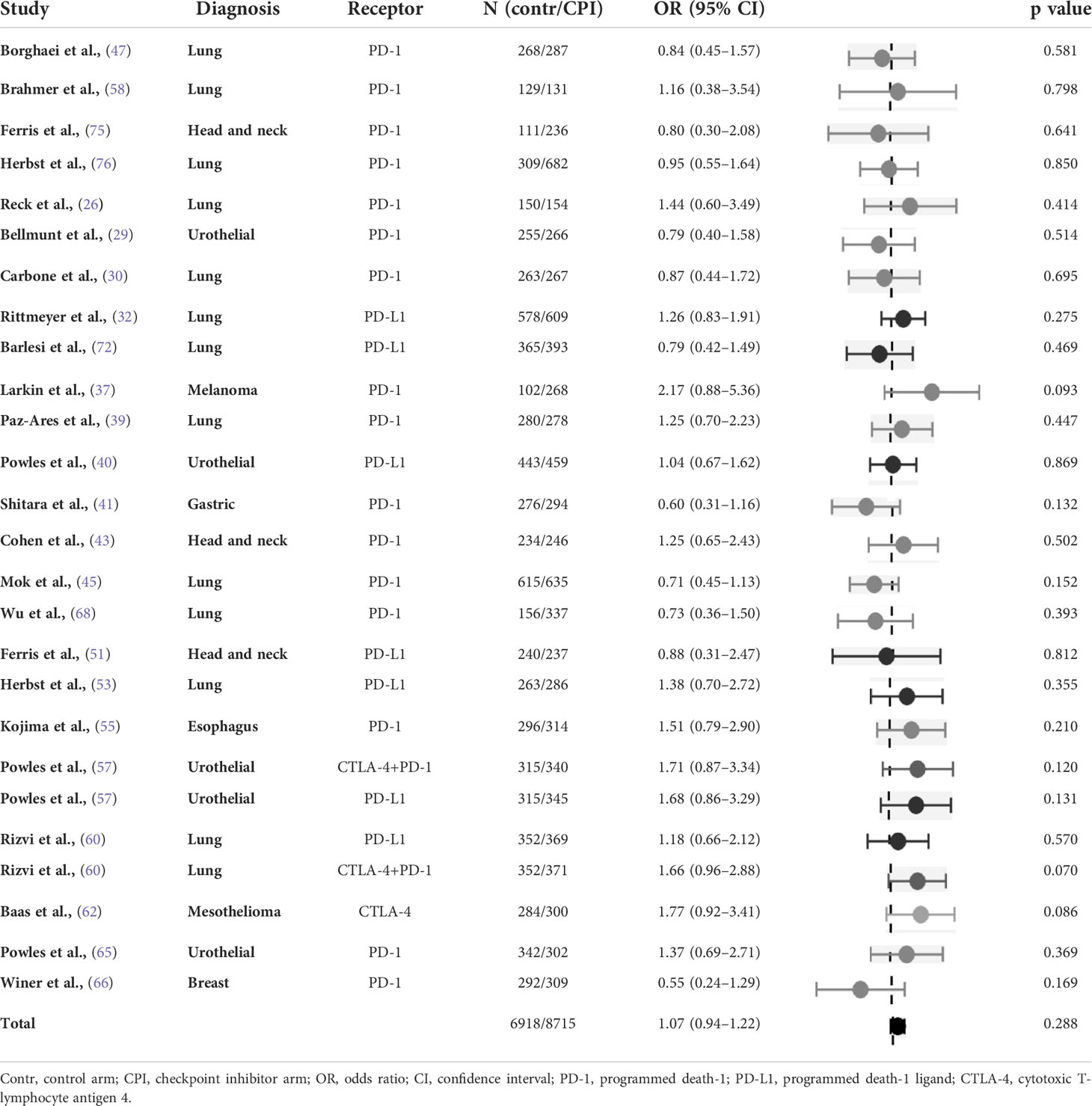
Table 6 Odds ratio of insomnia in randomised studies of checkpoint inhibitors versus chemotherapy (control arm as reference).
All studies (n=3) in this category involved therapy for metastatic renal cell carcinoma. There was a trend to increased occurrence of insomnia in the immunotherapy arms (OR 1.40, 95% CI 0.90-2.18) that however failed to reach statistical significance. There was an intermediate heterogeneity among the studies (Table 7).
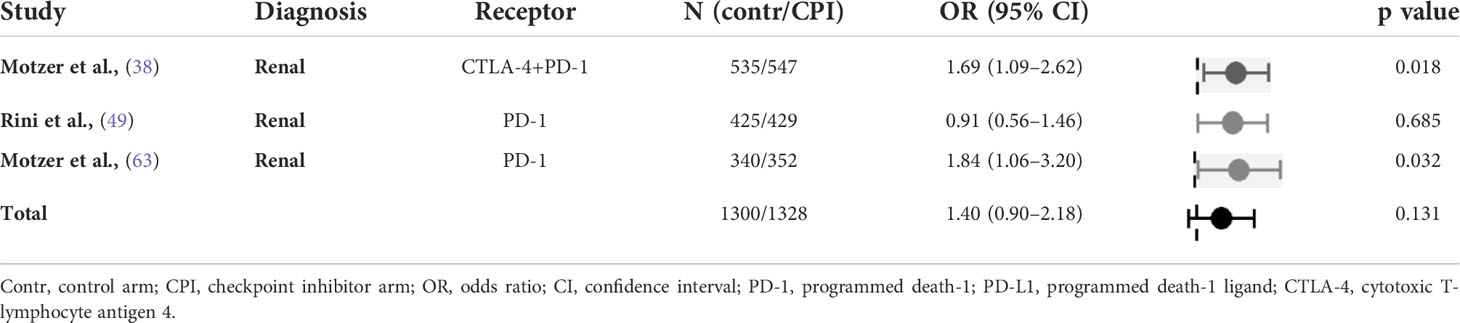
Table 7 Odds ratio of insomnia in randomised studies of checkpoint inhibitors versus non-immunologic targeted therapies (control arm as reference).
Fifteen studies including one three-arm study (i.e. 16 study arm pairs) were included in the analysis, of those 10 (66%) were carried out in lung cancer. There was no significant difference in the risk of insomnia (OR 1.13, 95% CI 0.96-1.33) with an intermediate heterogeneity (Table 8).
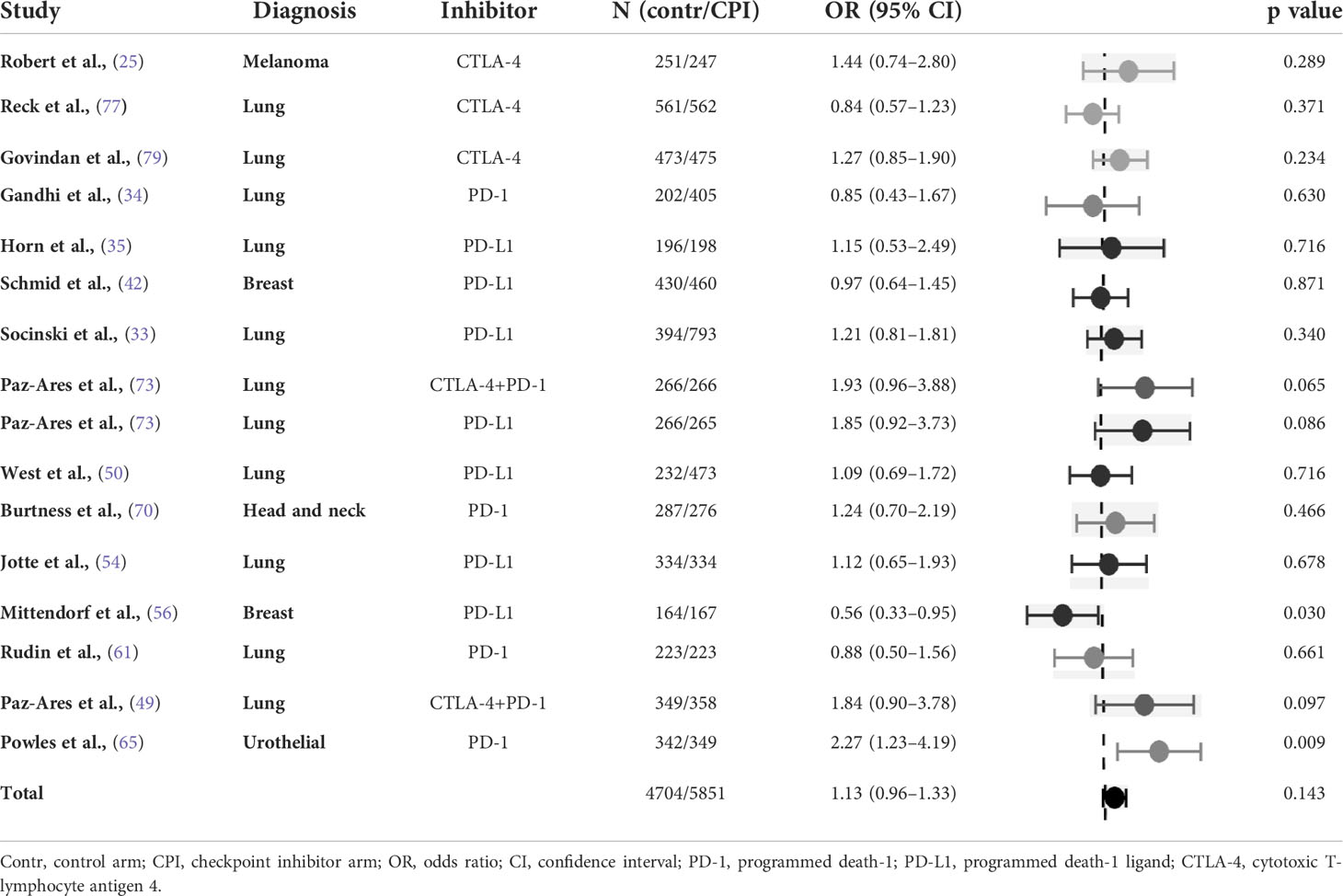
Table 8 Odds ratio of insomnia in randomised studies of checkpoint inhibitors combined with chemotherapy versus chemotherapy (control arm as reference).
The results of the present extensive meta-analysis of phase III trials indicate that treatment with CPI for solid cancers is associated with increased risk of insomnia. However, the odds of insomnia are not increased compared to other systemic antineoplastic modalities such as chemotherapy and non-immunologic targeted therapies. Immune system activation occurring with CPI therapy is the putative causative mechanism linking the treatment with insomnia.
There is a strong, bidirectional link between insomnia and inflammation. Poor sleeping consistency has been associated with increase in inflammatory markers, including interleukin (IL)-6 and C-reactive protein (CRP) as well as serum amyloid-α, tumour necrosis factor-α, and granulocyte-macrophage colony-stimulating factor (13, 14). IL-6 is a proinflammatory cytokine and elevated levels have been detected in advanced cancer as well as during autoimmune adverse events in patients treated with CPI. Indeed, an anti-IL-6 agent, tocilizumab, is used for the treatment of corticosteroid-refractory autoimmune toxicities (81–83). IL-17 has also been associated with CPI efficacy and toxicity but also with sleep restriction (84–86).
Evidence suggests a common link between circadian cycle and cancer mediated by circadian core genes (2–4). An extensive meta-analysis of related to sleep disorders and inflammatory markers confirmed association between disturbed circadian rhythms and inflammatory markers including CRP and IL-6 (13). The circadian rhythm of IL-6 is altered in patients with chronic insomnia, providing a possible link between chronic inflammatory state induced by cancer and/or CPI and insomnia (87). Fatigue is another result of this immune activation, and the question has been addressed in a recent analysis by our group (15).
Recently, in an animal model, overexpression of NF-kB has been identified as the common underlying factor for insomnia and inflammation (88). Circadian clock genes play a complex role in cancer development and anti-cancer immune response, regulating even the formation of tumour-related immune cell infiltrates (88, 89). Thus, there is ample evidence that excessive, chronic inflammatory activation may provide a link between cancer, cancer therapies, and insomnia (5, 90).
Interestingly, on the other side of the spectrum of sleep disorders, narcolepsy type 1 is thought to have autoimmune aetiology and T cells directed against hypocretin/orexin neurons have been identified in some patients (91). A case report has been published of narcolepsy possibly caused by pembrolizumab (92).
A recent pioneering study, the first to look specifically at the population of cancer patients treated with CPI has been published by (10). They did not find any association between the occurrence of insomnia, obstructive sleep apnoea and the number of CPI infusions. However, the study was relatively small and, as our analysis shows, the effect of CPI on insomnia is relatively modest.
Insomnia recorded during a cancer-related clinical trial is self-reported and is a composite endpoint covering sleep inconsistency (night-to-night variability in sleep pattern), short sleep duration relative to patient previous habits or expectations, poor sleep quality (including mid-sleep awakenings), and unrefreshing sleep. Insomnia as an adverse event represents an increase in the severity of the symptom over the study period and the follow-up. Thus, the relatively low incidence of insomnia in the analysed studies does not reflect the pre-existing insomnia which is thought to affect 30-75% of cancer patients, a prevalence approximately three times higher than in the healthy population (1, 10, 93, 94). In a very recent study, Ashraf et al. reported that the prevalence of sleep disturbance reached 67.9% in a population of patients with solid malignancies. The complaint was mostly not addressed by attending oncologists (95). The wide reported incidence range probably reflects different populations and methodology, particularly questionnaires versus symptom reporting (13). Various diagnostic criteria are used, including broadly defined sleep problems per Common Terminology Criteria for Adverse Events (CTCAE), and, at the other end of the spectrum, the very detailed insomnia disorder (i.e. primary insomnia) definition provided by the Diagnostic and Statistical Manual of Mental Disorders (78, 96). Notably, the latter excludes medication-induced insomnia and is consequently less useful for the oncology practice.
There are several limitations of our meta-analysis. There is the possibility of underreporting the very common symptom present at baseline in many patients, and the fact that the severity and type of sleep disturbances may change over the course of cancer and therapy. Longitudinal evolution of insomnia in clinical trials can be assessed using formal quality of life (QoL) analysis using standard QoL questionnaires. Sleep disturbances are more prevalent in women and there is also a stronger association between insomnia and inflammation in females (7) but we have not been able to account for this fact in the present meta-analysis as gender-specific toxicity data were not available from published sources. Insomnia has been reported as an early symptom in autoimmune endocrine abnormalities in patients treated with CPI but we did not test this correlation in the present study (97). Insomnia is also linked to cognitive impairment (98). There are currently few reports assessing the cognitive sequelae of CPI therapy, and the topic remains an interesting research question for the future (99).
Cancer immunotherapy using CPI is clearly associated with insomnia. The risk of insomnia as an adverse event was not significantly higher in patients treated with CPI compared to those receiving chemotherapy. AntiCTLA-4 agents are associated with higher incidence of insomnia compared to PD-1/PD-L1 inhibitors.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
TB had the right to deal with all the data and was responsible for the decision to submit the manuscript for publication. IK, MK and TB had the data of all included clinical trials. TB, BB and KH retrieved the data. MK carried out the statistical analysis. LB extensively revised the manuscript and provided interpretation of the statistical methods and results. TB, IK and BB were responsible for checking and evaluating the quality of the collected data. All authors contributed to the article and approved the submitted version.
Publication fees have been covered by unrestricted grant from Roche, Servier, AstraZeneca, and Bristol Myers Squibb. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
IK has received research support and honoraria from Roche, Bristol Myers Squibb, Merck Sharp Dohme, Merck, and Servier, all unrelated to the present paper. TB has received research support and honoraria from Roche, Bristol Myers Squibb, Merck Sharp Dohme, Merck, and AstraZeneca, all unrelated to the present paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.946307/full#supplementary-material
1. Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol Off J Am Soc Clin Oncol (2001) 19:895–908. doi: 10.1200/JCO.2001.19.3.895
2. Sigurdardottir LG, Valdimarsdottir UA, Fall K, Rider JR, Lockley SW, Schernhammer E, et al. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2012) 21:1002–11. doi: 10.1158/1055-9965.EPI-12-0116
3. Wendeu-Foyet MG, Menegaux F. Circadian disruption and prostate cancer risk: An updated review of epidemiological evidences. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2017) 26:985–91. doi: 10.1158/1055-9965.EPI-16-1030
4. Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C, et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr Relat Cancer (2014) 21:629–38. doi: 10.1530/ERC-14-0121
5. Walker WH 2nd, Borniger JC. Molecular mechanisms of cancer-induced sleep disruption. Int J Mol Sci (2019) 20. doi: 10.3390/ijms20112780
6. Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflammation Res Off J Eur Histamine Res Soc . (2007) 56:51–7. doi: 10.1007/s00011-006-6067-1
7. Dzierzewski JM, Donovan EK, Kay DB, Sannes TS, Bradbrook KE. Sleep inconsistency and markers of inflammation. Front Neurol (2020) 11:1042. doi: 10.3389/fneur.2020.01042
8. Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum (2008) 35:635–42. doi: 10.1188/08.ONF.635-642
9. Barsevick A, Beck SL, Dudley WN, Wong B, Berger AM, Whitmer K, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage (2010) 40:200–16. doi: 10.1016/j.jpainsymman.2009.12.020
10. Sillah A, Peters U, Watson NF, Tykodi SS, Hall ET, Silverman A, et al. Associating sleep problems with advanced cancer diagnosis, and immune checkpoint treatment outcomes: A pilot study. Support Care Cancer Off J Multinatl Assoc Support Care Cancer (2022) 30:3829–38. doi: 10.1007/s00520-022-06825-w
11. Cogdill AP, Andrews MC, Wargo JA. Hallmarks of response to immune checkpoint blockade. Br J Cancer (2017) 117:1–7. doi: 10.1038/bjc.2017.136
12. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
13. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
14. Xia L, Zhang P, Niu J-W, Ge W, Chen J-T, Yang S, et al. Relationships between a range of inflammatory biomarkers and subjective sleep quality in chronic insomnia patients: A clinical study. Nat Sci Sleep (2021) 13:1419–28. doi: 10.2147/NSS.S310698
15. Kiss I, Kuhn M, Hrusak K, Buchler T. Incidence of fatigue associated with immune checkpoint inhibitors in patients with cancer: A meta-analysis. ESMO Open (2022) 7:100474. doi: 10.1016/j.esmoop.2022.100474
16. Yang F, Markovic SN, Molina JR, Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, et al. Association of sex, age, and Eastern cooperative oncology group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: A systematic review and meta-analysis. JAMA Netw Open (2020) 3:e2012534. doi: 10.1001/jamanetworkopen.2020.12534
17. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc (2015) 13:196–207. doi: 10.1097/XEB.0000000000000065
20. Rücker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods (2017) 8:392–403. doi: 10.1002/jrsm.1259
21. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
22. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
23. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with r: A practical tutorial. Evid Based Ment Health (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
24. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
25. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med (2011) 364:2517–26. doi: 10.1056/NEJMoa1104621
26. Reck M, Luft A, Szczesna A, Havel L, Kim S-W, Akerley W, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol (2016) 34:3740–8. doi: 10.1200/JCO.2016.67.6601
27. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-Small-Cell lung cancer. N Engl J Med (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
28. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35:40–7. doi: 10.1200/JCO.2016.69.1584
29. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
30. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-Small-Cell lung cancer. N Engl J Med (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
31. Bersanelli M, Giannarelli D, Castrignanò P, Fornarini G, Panni S, Mazzoni F, et al. INfluenza vaccine indication during therapy with immune checkpoint inhibitors: A transversal challenge. INVIDIa study Immunotherapy (2018) 10:1229–39. doi: 10.2217/imt-2018-0080
32. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet (London England) (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
33. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
34. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
35. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
36. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2014) 15:700–12. doi: 10.1016/S1470-2045(14)70189-5
37. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WHJ, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36:383–90. doi: 10.1200/JCO.2016.71.8023
38. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
39. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
40. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet (London England) (2018) 391:748–57. doi: 10.1016/S0140-6736(17)33297-X
41. Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet (London England) (2018) 392:123–33. doi: 10.1016/S0140-6736(18)31257-1
42. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
43. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet (London England) (2019) 393:156–67. doi: 10.1016/S0140-6736(18)31999-8
44. Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol (2019) 20:849–61. doi: 10.1016/S1470-2045(19)30027-0
45. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet (London England) (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
46. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
47. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
48. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
49. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet (London England) (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
50. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 tri. Lancet Oncol (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
51. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol Off J Eur Soc Med Oncol (2020) 31:942–50. doi: 10.1016/j.annonc.2020.04.001
52. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England) (2020) 395:1835–44. doi: 10.1016/S0140-6736(20)30934-X
53. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
54. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2020) 15:1351–60. doi: 10.1016/j.jtho.2020.03.028
55. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
56. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 tri. Lancet (London England) (2020) 396:1090–100. doi: 10.1016/S0140-6736(20)31953-X
57. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol (2020) 21:1574–88. doi: 10.1016/S1470-2045(20)30541-6
58. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
59. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med (2020) 383:1218–30. doi: 10.1056/NEJMoa2002788
60. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn M-J, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol (2020) 6:661–74. doi: 10.1001/jamaoncol.2020.0237
61. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38:2369–79. doi: 10.1200/JCO.20.00793
62. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet (London England) (2021) 397:375–86. doi: 10.1016/S0140-6736(20)32714-8
63. Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med (2021) 384:1289–300. doi: 10.1056/NEJMoa2035716
64. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39:1349–59. doi: 10.1200/JCO.20.02212
65. Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY-S, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22:931–45. doi: 10.1016/S1470-2045(21)00152-2
66. Winer EP, Lipatov O, Im S-A, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22:499–511. doi: 10.1016/S1470-2045(20)30754-3
67. Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med (2019) 380:720–8. doi: 10.1056/NEJMoa1814630
68. Wu Y-L, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2019) 14:867–75. doi: 10.1016/j.jtho.2019.01.006
69. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
70. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro GJ, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet (London England) (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
71. Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38:193–202. doi: 10.1200/JCO.19.01307
72. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN lung 200): An open-label, randomised, phase 3 study. Lancet Oncol (2018) 19:1468–79. doi: 10.1016/S1470-2045(18)30673-9
73. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet (London England) (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
74. Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med (2016) 375:1845–55. doi: 10.1056/NEJMoa1611299
75. Ferris RL, Blumenschein GJ, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
76. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet (London England) (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
77. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
78. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. (2017). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf [Accessed May 01, 2022]
79. Govindan R, Szczesna A, Ahn M-J, Schneider C-P, Gonzalez Mella PF, Barlesi F, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-Small-Cell lung cancer. J Clin Oncol (2017) 35:34493457. doi: 10.1200/JCO.2016.71.7629
80. Maio M, Scherpereel A, Calabrò L, Aerts J, Perez SC, Bearz A, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol (2017) 18:12611273. doi: 10.1016/S1470-2045(17)30446-1
81. Yang T, Yang Y, Wang D, Li C, Qu Y, Guo J, et al. The clinical value of cytokines in chronic fatigue syndrome. J Transl Med (2019) 17:213. doi: 10.1186/s12967-019-1948-6
82. Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer (2021) 157:214–24. doi: 10.1016/j.ejca.2021.08.031
83. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract (2019) 25:551–7. doi: 10.1177/1078155217745144
84. Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer (2015) 3:39. doi: 10.1186/s40425-015-0081-1
85. Esfahani K, Miller WHJ. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med (2017) 376:1989–91. doi: 10.1056/NEJMc1703047
86. van Leeuwen WMA, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PloS One (2009) 4:e4589. doi: 10.1371/journal.pone.0004589
87. Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin H-M, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism (2002) 51:887–92. doi: 10.1053/meta.2002.33357
88. Hong H-K, Maury E, Ramsey KM, Perelis M, Marcheva B, Omura C, et al. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev (2018) 32:1367–79. doi: 10.1101/gad.319228.118
89. Zhang Z, Zeng P, Gao W, Zhou Q, Feng T, Tian X. Circadian clock: A regulator of the immunity in cancer. Cell Commun Signal (2021) 19:37. doi: 10.1186/s12964-021-00721-2
90. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol Off J Am Soc Clin Oncol (2011) 29:3517–22. doi: 10.1200/JCO.2011.36.1154
91. Kornum BR. Narcolepsy type I as an autoimmune disorder. Handb Clin Neurol (2021) 181:161–72. doi: 10.1016/B978-0-12-820683-6.00012-9
92. Natori Y, Sasaki E, Soeda S, Furukawa S, Azami Y, Tokuda E, et al. Risk of immunotherapy-related narcolepsy in genetically predisposed patients: A case report of narcolepsy after administration of pembrolizumab. J Immunother Cancer (2020) 8. doi: 10.1136/jitc-2020-001164
93. Malone M, Harris AL, Luscombe DK. Assessment of the impact of cancer on work, recreation, home management and sleep using a general health status measure. J R Soc Med (1994) 87:386–9.
94. Berger AM, Parker KP, Young-McCaughan S, Mallory GA, Barsevick AM, Beck SL, et al. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum (2005) 32:E98–126. doi: 10.1188/05.ONF.E98-E126
95. Ashraf S, Jafri MAS, Alsharedi MF. Insomnia in cancer patients. J Clin Oncol (2021) 39:e18651–1. doi: 10.1200/JCO.2021.39.15_suppl.e18651
96. Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc (2013) 21:591–643.
97. Griewing LM, Schweizer C, Schubert P, Rutzner S, Eckstein M, Frey B, et al. Questionnaire-based detection of immune-related adverse events in cancer patients treated with PD-1/PD-L1 immune checkpoint inhibitors. BMC Cancer (2021) 21:314. doi: 10.1186/s12885-021-08006-0
98. Chellappa SL, Morris CJ, Scheer FAJL. Effects of circadian misalignment on cognition in chronic shift workers. Sci Rep (2019) 9:699. doi: 10.1038/s41598-018-36762-w
Keywords: immunotherapy, cancer, checkpoint inhibitors, insomnia, systematic analysis
Citation: Kiss I, Kuhn M, Hrusak K, Buchler B, Boublikova L and Buchler T (2022) Insomnia in patients treated with checkpoint inhibitors for cancer: A meta-analysis. Front. Oncol. 12:946307. doi: 10.3389/fonc.2022.946307
Received: 17 May 2022; Accepted: 04 July 2022;
Published: 02 August 2022.
Edited by:
Mohammad Hojjat-Farsangi, Karolinska Institutet (KI), SwedenReviewed by:
Seockhoon Chung, University of Ulsan College of Meidicine, South KoreaCopyright © 2022 Kiss, Kuhn, Hrusak, Buchler, Boublikova and Buchler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomas Buchler, dG9tYXMuYnVjaGxlckBmdG4uY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.