- 1Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Background: Genetic studies previously reported that variants in TERT-CLPTM1L genes were related to susceptibility of cancer and non-cancer diseases. However, conclusions were not always concordant.
Methods: We performed meta-analyses to assess correlations between 23 variants within TERT-CLPTM1L region and susceptibility to 12 cancers and 1 non-cancer disease based on data in 109 papers (involving 139,510 cases and 208,530 controls). Two approaches (false-positive report probability test and Venice criteria) were adopted for assessing the cumulative evidence of significant associations. Current study evaluated the potential role of these variants based on data in Encyclopedia of DNA Elements (ENCODE) Project.
Results: Thirteen variants were statistically associated with susceptibility to 11 cancers and 1 non-cancer disease (p < 0.05). Besides, 12 variants with eight cancers and one non-cancer disease were rated as strong evidence (rs2736098, rs401681, and rs402710 in bladder cancer; rs2736100, rs2853691, and rs401681 in esophageal cancer; rs10069690 in gastric cancer; rs2736100 and rs2853676 in glioma; rs2242652, rs2736098, rs2736100, rs2853677, rs31489, rs401681, rs402710, rs465498, and rs4975616 in lung cancer; rs2736100 in idiopathic pulmonary fibrosis and myeloproliferative neoplasms; and rs401681 in pancreatic and skin cancer). According to data from ENCODE and other public databases, 12 variants with strong evidence might fall within putative functional regions.
Conclusions: This paper demonstrated that common variants of TERT-CLPTM1L genes were related to susceptibility to bladder, esophageal, gastric, lung, pancreatic, and skin cancer, as well as to glioma, myeloproliferative neoplasms, and idiopathic pulmonary fibrosis, and, besides, the crucial function of the TERT-CLPTM1L region in the genetic predisposition to human diseases is elucidated.
Introduction
Cancer as a dominating reason for death is threatening life and health of human being worldwide, with ~19.3 million newly diagnosed tumor patients along with ~10.0 million cancer-associated death cases in 2020 (1). Both environmental and genetic factors lead to cancer occurrence and progression, and 5%– 10% of cancer is resulted from variation in genes (2). Genome-wide association studies (GWAS) and genetic association research have proved multiple single-nucleotide polymorphisms (SNPs) linked with risk of human diseases (3).
The telomerase reverse transcriptase (TERT) gene and cleft lip and palate transmembrane 1–like (CLPTM1L) gene are mapped on chromosome 5p15.33. Of them, TERT gene, encoding the rate-limiting telomerase enzyme for catalysis, exerts a significant influence on maintaining cell immortality, telomere DNA length, and chromosomal stability (4). The protein encoded by CLPTM1L is a membrane protein associated with cisplatin resistance, and the overexpression of CLPTM1L in cisplatin-sensitive cells causes apoptosis (5). In early 1990s, researchers had made attempts to account for the existing relationships of telomeres, telomerase, aging, and cancer risk (6, 7). Wang et al. first uncovered that a novel variant (TERT MNS16A) had an elevated risk of lung cancer in 2003 (8). In 2006, Matsubara et al. first revealed that TERT rs2735940 had an elevated risk of coronary artery disease (non-cancer disease) (9). Since then, numerous genetic association studies were conducted to investigate the associations among SNPs of TERT and CLPTM1L regions with human diseases. In 2008, a GWAS was performed on the Caucasian population, and then it was found that two variants (rs402710 and rs2736100) were featured with a higher susceptibility to lung cancer (10). Subsequently, from a Japanese GWAS, it was seen that rs2736100 increased risk of idiopathic pulmonary fibrosis (11). Apart from idiopathic pulmonary fibrosis, several GWAS showed that rs2736100 could enhance lung cancer and testicular germ cell cancer susceptibility in Caucasians (12, 13) while could decrease the risk of idiopathic interstitial pneumonia and glioma in Caucasians (14, 15) and lung cancer in Asians (16). Moreover, in a GWAS conducted in multiple countries from the European ancestry, rs401681 associated with 75,000 individuals was tested, and then it was discovered that this SNP could elevate susceptibility to lung, urinary bladder, prostate, and basal cell cancer while could decrease cutaneous melanoma susceptibility (17).
Even though numerous genetic association studies investigate the association of variant in TERT and CLPTM1L regions and cancers or non-cancer disease susceptibility, the conclusions are not always consistent and the functional mechanisms remain unclear. Although, in previous published meta-analysis studies, a single SNP (for example, rs2736100 and rs2736098) with risk of individual cancer (18–20) was investigated, the results were still inconsistent. Besides, a comprehensive research synopsis with systematic functional annotation had not been performed to evaluate the epidemiological evidence of genetic correlations between TERT and CLPTM1L genes and susceptibility to cancers or non-cancer disease until now. As a result, we conducted meta-analyses to account for the relationships of SNPs in the TERT-CLPTM1L genes with cancers or non-cancer disease predisposition, provided the epidemiological evidence for variants with significant associations, and assessed the roles of significant SNPs using information from public databases.
Material and Methods
This study was conducted by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement guidelines (Supplementary Table 1) and the Human Genome Epidemiology Network for systematic review of genetic association studies (21, 22).
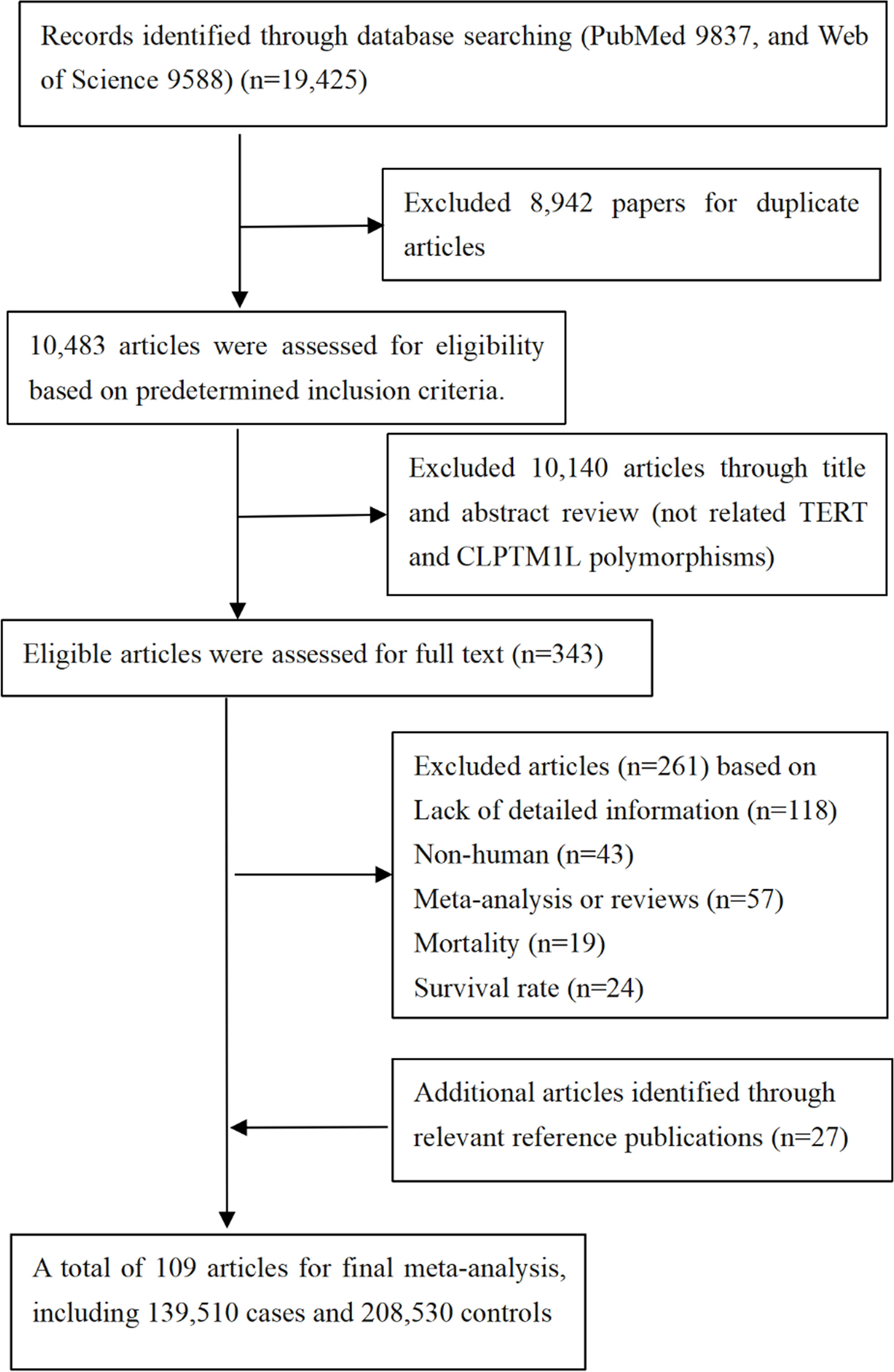
Here, papers from PubMed, Web of science, and Embase before 30 Dec 2021 were screened using “{human telomerase reverse transcriptase} OR {hTERT} OR {TERT} OR {CLPTM1L} OR {cleft and palate transmembrane 1 like} OR {TERT-CLPTM1L region} OR {5p15.33}”, and 19,425 citations were identified. Apart from that, additional articles were also collected through examining the relevant references of publications (reviews, meta-analysis studies, etc.). Finally, 109 papers were included in our study (Figure 1).
The included criteria were as follows: (1) assessing relationships of variations in the TERT-CLPTM1L region with human cancers or non-cancer disease by conducting case-control, cross-sectional, cohort studies, or GWAS; (2) providing details of genotype amount for computing the values of odds ratios (ORs) and 95% confidence intervals (95% CIs); (3) being published or full text in English, whereas the criteria of exclusion are shown as follows: (1) there is no sufficient information (especially genotype amount); (2) the interests is not TERT-CLPTM1L region polymorphism; (3) the study is a letter to editors or conference abstract; (iv) the interests are cancer mortality (but not incidence).
Data Extraction
Two investigators obtained data from eligible papers on their own. Any inconsistencies could be addressed by discussion with the rest of investigators. As for included variants, the information was extracted, as follows: first author, publishing year, the country or region, type of cancer and non-cancer disease, ethnicity, the gene name, the variant, amount of genotype, cases and controls, and minor allele frequency (MAF). Apart from that, ethnicity comprises four parts [Asians (East Asian descent), Caucasians (European descent), Africans (African descent), or others (Indians, Native Hawaiians, Latinos, Hispanics and the mixed, etc.)] following that more than 80% of research subjects were within the abovementioned groups; “overall populations” are composed of two or more. Furthermore, in this study, the data were extracted from publications with the largest individuals and the most comprehensive data if numerous papers had the same or overlapping data.
Statistical Analysis
P < 0.05 (two-sided) indicated the significance threshold, which was computed with the use of Stata, version 12 (Stata, College Station, TX, USA). As for one variant to one cancer or non-cancer disease risk, the additive genetic model with at least three independent datasets and on the basis of the minor allele was established in meta-analyses. Moreover, the analyses performed by ethnicity and histological/pathological/clinical subtypes were also recommended if necessary. This study adopted the I2 statistics and Cochran’s Q test for investigating the heterogeneity between studies, as well as P < 0.1 is the significant level, as recommended (23). The values of I² were divided into three parts: ≤ 25%, 25%–50%, and ≥ 50% (mild heterogeneity heterogeneity, moderate heterogeneity, and large heterogeneity, respectively). We conducted sensitive analyses in order to reveal whether the significant ORs were lost by eliminating one individual study, or the first published study, or articles which have been deviated from the Hardy–Weinberg equilibrium (HWE) among controls. We investigated the probability of an excess of significant findings (P < 0.1 as the significant level) (24). The Begg’s test and the Egger’s test were used to assess potential publication bias and small-study bias, as well as P < 0.1 is the significant level, as recommended (25, 26).
Epidemiological Credibility of Significant Associations
The epidemiological credibility of significant relationships was evaluated combining the Venice guideline (27) and the false-positive report probability (FPRP) (28) (see Supplementary Method).
Functional Annotation
The underlying functional role of variants on 5p15.33 was evaluated with information from the Encyclopedia of DNA Elements (ENCODE) tool HaploReg (v4.1) (29) and the UCSC Genome browser (http://genome.ucsc.edu/). Furthermore, our work explored genome-wide cis-eQTL data in multiple tissues from the Genotype-Tissue Expression Project (30) and the Multiple Tissue Human Expression Resource Project (31) databases in order to reveal whether these genes might demonstrate the observed findings in these loci.
Results
Characteristics of the Included Studies
In our study, available data from 109 papers were extracted in these meta-analyses (Supplementary Table 2), thus further evaluating associations between 23 variants in TERT-CLPTM1L region and 12 cancers and 1 non-cancer disease under an additive genetic model (Figure 1). In addition, the distributions of SNPs (n) with cancer and non-cancer disease were as follows: esophageal (n = 4), gastric (n = 4), glioma (n = 2), breast (n = 11), hepatocellular (n = 1), lung (n = 10), pancreatic (n = 2), skin (melanoma) (n = 1), bladder (n = 4), colorectal (n = 1), thyroid cancer (n = 1), myeloproliferative neoplasms (n = 1), and idiopathic pulmonary fibrosis (n = 1). Of these, analysis was performed with 139,510 cases and 208,530 controls (the publishing year ranged from 2007 to 2020), and then it was found that 13 SNPs were significantly associated with 11 cancers and 1 non-cancer disease risk (Table 1 and Supplementary Table 3).
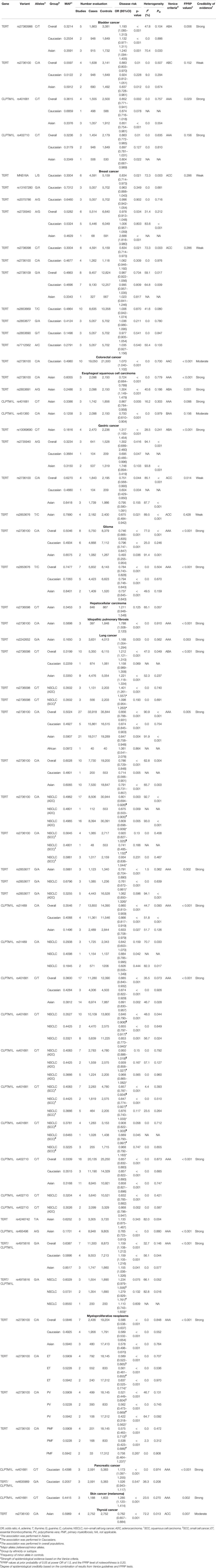
Table 1 Epidemiological evidence for associations between variants in the TERT and CLPTM1L gene with risk of cancer and non-cancerous diseases in additive model.
Associations Between TERT-CLPTM1L Variants and Risk of Cancer and Non-Cancer Diseases
We conducted meta-analyses to assess relationships of 23 variants in TERT-CLPTM1L region with 12 cancers and 1 non-cancer disease under an additive genetic model. Then, it was seen that 13 SNPs (rs10069690, rs2242652, rs2736098, rs2736100, rs2853676, rs2853677, rs2853691, rs31489, rs401681, rs402710, rs451360, rs465498, and rs4975616) had significantly associated with risk of 11 cancers (bladder, breast, colorectal, esophageal, gastric, glioma, lung, pancreatic, skin, thyroid, and myeloproliferative neoplasms) and 1 non-cancer disease (idiopathic pulmonary fibrosis) (Table 1). It is worth noting that the histological/pathological types of esophageal carcinoma and skin cancer were squamous cell carcinoma and melanoma, respectively. Apart from that, significant relationships with susceptibility to bladder cancer could be found for rs2736098 (OR = 1.193), rs2736100 (OR = 0.883), rs401681 (OR = 0.852), and rs402710 (OR = 0.863). Moreover, these associations were further assessed by ethnicity, demonstrating that the four SNPs mentioned above had significant association with bladder cancer predisposition in Asians (rs2736098: OR = 1.240; rs2736100: OR = 0.837; rs401681: OR = 0.851; rs402710: OR = 0.804), rather than the Caucasian population. In addition, the significant relationship with breast cancer predisposition was only presented for rs2736098 (OR = 0.834), whereas rs2736100 could increase colorectal cancer predisposition (OR = 1.070).
Apart from colorectal carcinoma, the A allele of rs2736100 possessed a decreased predisposition of esophageal squamous cell carcinoma (ESCC) among Asian populations (OR = 0.724). Moreover, one SNP (rs2853691) had an enhanced predisposition of ESCC (OR = 1.304) and another two SNPs (rs401681 and rs451360) were featured by the reduced predisposition of ESCC, in Asians (OR = 0.867 and OR = 0.700).
Significant relationships with gastric carcinoma predisposition were exclusively found for rs10069690 (OR = 1.317) and rs2853676 (OR = 0.675) in Asians. Besides, SNP rs2736100 was statistically associated with gastric carcinoma (OR = 0.751). Interestingly, rs2736100 remarkably associates with gastric cancer predisposition in Caucasians (OR = 0.604) but not in Asians. Moreover, two SNPs could reduce the predisposition of glioma (rs2736100: OR = 0.746; rs2853676: OR = 0.784). Noticeably, it was also uncovered that these two SNPs had a reduced susceptibility to glioma both in Asian populations and Caucasian populations.
For lung cancer, it was found that nine SNPs were significantly related to lung cancer predisposition, which also remains true in the subgroup analyses by ethnicity and pathological subtypes. Specifically, three SNPs exclusively appeared in Asians and had significant relationships with lung cancer predisposition (rs2242652: OR = 1.168; rs2853677: OR = 0.791; rs465498: OR = 0.765, respectively). Besides, additional findings from subgroup analyses by pathological subtypes for rs2853677 indicated that this SNP could decrease the predisposition of non–small cell lung cancer (NSCLC) (OR = 0.761). Interestingly, it was found that rs2853677 had no association with NSCLC (adenocarcinoma) risk. In addition, there were noticeable relationships between six SNPs and lung cancer predisposition among different races/pathological subtypes. Among them, rs2736098 had an elevated predisposition of lung cancer (OR = 1.212), which was shown in Asians (OR = 1.221), but not in Caucasians. Besides that, rs2736098 was distinctly associated with predisposition of NSCLC (adenocarcinoma) (OR = 1.401), rather than NSCLC (squamous cell carcinoma), whereas SNP rs2736100 had a close relationship with lung cancer predisposition (OR = 0.856), which appeared in Caucasians (OR = 0.874) and Asians (OR = 0.847) instead of Africans. Then, subgroup analyses were performed by pathological type/race, and it was uncovered that rs2736100 had a decreased risk of NSCLC (OR = 0.786) both in Caucasians (OR = 0.714) and Asians (OR = 0.791). Surprisingly, rs2736100 had a decreased risk of NSCLC (adenocarcinoma) (OR = 0.801), rather than NSCLC (squamous cell carcinoma), whereas SNP rs31489 was closely connected with lung cancer predisposition (OR = 0.860) both in Caucasians (OR = 0.866) and Asians (OR = 0.833). Noticeably, subgroup analyses by pathology type presented that rs31489 was not related to NSCLC predisposition (OR = 0.842). Additionally, SNP rs401681 was featured by the decreased lung cancer incidence (OR = 0.885) both in Caucasians (OR = 0.874) and Asians (OR = 0.891). Furthermore, the analyses also showed that rs401681 had a decreased risk of NSCLC (OR = 0.846), but not associated with small cell carcinoma (OR = 0.908). For NSCLC, it was also known that SNP rs401681 featured a reduced NSCLC (squamous cell carcinoma) incidence (OR = 0.857) but had no relationship with NSCLC (adenocarcinoma) (OR = 0.950). Other than that, SNP rs402710 could decrease predisposition of lung cancer (OR = 0.857) both in Caucasians (OR = 0.857) and Asians (OR = 0.858). Furthermore, from subgroup analyses by pathology type, it was seen that rs401681 could reduce NSCLC predisposition (OR = 0.832), especially in lung adenocarcinoma (OR = 0.868). SNP rs4975616 faced an enhanced risk of lung cancer (OR = 1.159) both in Caucasians (OR = 1.159) and Asians (OR = 1.155), while there was no relationship between rs4975616 and NSCLC predisposition (OR = 1.234).
For myeloproliferative neoplasms, SNP rs2736100 could decrease the risk of myeloproliferative neoplasms (OR = 0.586) both in Caucasians (OR = 0.589) and in Asians (OR = 0.578). Subgroup analyses by clinical subtypes indicated that rs2736100 could reduce the risk of essential thrombocythemia (OR = 0.589), polycythemia vera (OR = 0.521), and primary myelofibrosis (OR = 0.575).
Besides myeloproliferative neoplasms, SNP rs2736100 could reduce thyroid carcinoma predisposition in Asians (OR = 0.762). Moreover, SNP rs401681 could increase risk of pancreatic cancer (OR = 1.173) and skin cancer (melanoma) (OR = 1.285) in Caucasians.
In terms of non-cancer disease, it was found that rs2736100 had an increased risk of idiopathic pulmonary fibrosis in Asian populations (OR = 1.788).
Furthermore, 13 SNPs (TERT MNS16A, rs13167280, rs2075786, rs2735940, rs2736100, rs2736109, rs2853669, rs2853677, rs2853690, rs7712562, rs2735940, rs2736098, rs4246742, and rs4635969) were not related to risk of five types of cancer (breast, gastric, hepatocellular, lung, and pancreatic cancer) (Supplementary Results). Of these, eight SNPs (rs13167280, rs2075786, rs2735940, rs2736100, rs2736109, rs2853669, rs2853677, rs2853690, and rs7712562) had no association with breast cancer with at least 10,000 individuals (Table 2). Beyond that, the statistical power was also calculated so as to confirm whether the large-scale sample size confirming these associations is required in the future (Supplementary Table 4).

Table 2 Summary of functional annotations for 12 SNPs in eight cancers and one neoplastic disease (strong epidemiological credibility).
Heterogeneity, Bias, and Sensitivity Analysis
The assessment of heterogeneity was performed for 29 significant correlations with 13 variants and 12 cancers and 1 non-cancer disease (Table 1). Of them, mild heterogeneity could be assigned to 16 (55%) associations, moderate heterogeneity fell into 7 (24%) associations, and high heterogeneity was found in 6 (21%) associations. There existed little evidence of publication bias (p > 0.10), except for rs2853676 with risk of glioma. Furthermore, findings from sensitivity analyses displayed that removal of some key factors did not alter the summary ORs, except for rs2736100 in bladder and colorectal cancer (low OR), rs2736100 in gastric cancer (HWE), and rs2736100 in thyroid cancer (excess of significant findings).
Cumulative Evidence of Association
Epidemiological credibility of totally 29 significant relationships was assessed using the Venice guideline. Specifically, there were 28 grades A in the amount of evidence, 21 grades A in the replication of association, and 23 grades A in the protection from bias, respectively; there were 1, 4, and 0 grades B in these three criteria, respectively; and there were 0, 4, and 6 grades C in these three criteria, respectively (Table 1). Therefore, strong, moderate, and weak evidence of a significant relationship with susceptibility to cancer and non-cancer disease could be found for 18, 5, and 6 associations, respectively. Subsequently, the probability for a true correlation between the 29 significant correlations was evaluated on the basis of FPRP values. Briefly, a FPRP level < 0.05 was found for 11 variants with 10 cancers and 1 non-cancer disease, whereas FPRP 0.05 to 0.2 for five variants with susceptibility to three cancers, and FPRP > 0.2 for three SNPs with risk of two cancers. Finally, strong evidence was assigned to 12 SNPs (TERT: rs10069690, rs2242652, rs2736098, rs2736100, rs2853676, rs2853677, and rs2853691; CLPTM1L: rs31489, rs401681, rs402710, and rs465498; TERT/CLPTM1L: rs4975616) with eight cancers and one non-cancer disease, which is presented in detail below: rs2736098, rs401681, and rs402710 in bladder cancer; rs2736100, rs2853691, and rs401681 in esophageal cancer; rs10069690 in gastric cancer; rs2736100 and rs2853676 in glioma; rs2242652, rs2736098, rs2736100, rs2853677, rs31489, rs401681, rs402710, rs465498, and rs4975616 in lung cancer; rs2736100 in idiopathic pulmonary fibrosis and myeloproliferative neoplasms; and rs401681 in pancreatic and skin cancer, whereas moderate evidence belonged to two SNPs with risk of three cancers (rs2736100 in colorectal and thyroid cancer and rs451360 in esophageal cancer), and the weak one was to three SNPs with risk of three cancers (rs2736100 in bladder cancer, rs2736098 in breast cancer, and rs2736100 and rs2853676 in gastric cancer).
Functional Annotation
The potential function roles for strong associations (12 SNPs associated with eight cancers and one non-cancer disease) were investigated with the use of the ENCODE tool HaploReg v4.1 (Table 2). In terms of functional annotations, 10 variants were mapped to intronic regions and TERT rs2736098 was mapped to synonymous regions. The total 12 SNPs might locate in a region having strong promoter and enhancer activity, DNase I hypersensitivity site, and alteration in regulatory motifs (Table 2). The linkage disequilibrium (LD) plots explained that the regions represented by significant SNPs had distinct genetic structures among European, Asian, and African ancestries (Figure 2). Besides, the Genotype-Tissue Expression Project revealed that rs2736100, rs2853676, rs2853676, rs31489, rs401681, rs402710, rs465498, and rs4975616 are eQTLs for TERT and CLPTM1L. Additionally, rs2736100, rs2853676, rs2853677, and rs4975616 are associated with an increase in TERT and CLPTM1L gene expression, whereas rs31489, rs401681, rs402710, and rs465498 are relevant to a decrease in CLPTM1L gene expression in skin tissues; different from that, rs31489, rs401681, rs402710, and rs465498 relate to a decrease, but rs4975616 to an increase in CLPTM1L gene expression in esophagus tissues; apart from that, rs31489, rs401681, and rs465498 are connected with an increase, but rs4975616 with a decrease in the CLPTM1L gene in stomach tissues (Supplementary Table 5).

Figure 2 Evidence from the Encyclopedia of DNA Elements (ENCODE) data for regulatory function of variants in 5p15.33 using the UCSC Genome Browser. The plot represents 5p15.33 within a 50-kb window centered on TERT-CLPTM1L gene region. Tracks (from top to bottom) in each of the plots are genome base position, chromosome bands, UCSC genes, human messenger RNAs from GenBank, human-expressed sequence tag (ESTs) that have been spliced, ENCODE enhancer and promoter-associated histone mark (H3K4Me1) on 8 Cell Lines, ENCODE promoter-associated histone mark (H3K4Me3) on 9 cell lines, ENCODE digital DNaseI hypersensitivity clusters, ENCODE transcription factor ChIP-seq, ENCODE chromatin state segmentation by hidden Markov model (HMM) from Broad Institute (bright red, active promoter; light red, weak promoter; purple, inactive/poised promoter; orange, strong enhancer; yellow, weak/poised enhancer; blue, insulator; dark green, transcriptional transition/elongation; light green, weak transcribed; gray, polycomb-repressed; light gray, heterochromatin/low signal/repetitive/copy number variation), single-nucleotide polymorphisms (dbSNP build 130), linkage disequilibrium for the Yoruba (YRI) from phased genotypes, linkage disequilibrium for the CEPH (CEU) from phased genotypes and LD for the Han Chinese + Japanese from Tokyo (JPT+CHB) from Phased Genotypes. The scale bar for the LD plot could be found in the data source (ldlink.nci.nih.gov/?tab=ldpair.).
Discussion
Admittedly, the current large-scale research synopsis and meta-analysis comprehensively summarize and update the correlations between variants in TERT-CLPTM1L genes and cancer and non-cancer disease predisposition, which provides precise results for the variants and offers more SNPs and diseases that were never assessed before. To be specific, in this paper, meta-analyses were performed by employing available data from 109 papers with 139,510 cases and 208,530 controls, thus evaluating associations of 23 SNPs with risk of 12 cancers and 1 non-cancer disease; then, it was revealed that, among them, 13 SNPs had significant association with 11 cancers and 1 non-cancer disease predisposition. Besides, the Venice guidelines and FPRP tests were taken for the first time to assess these significant correlations. At last, 12 variants were rated as being strong for cumulative evidence with eight cancers and one non-cancer disease predisposition (22 significant associations: rs2736098, rs401681, and rs402710 in bladder cancer; rs2736100, rs2853691, and rs401681 in esophageal cancer; rs10069690 in gastric cancer; rs2736100 and rs2853676 in glioma; rs2242652, rs2736098, rs2736100, rs2853677, rs31489, rs401681, rs402710, rs465498, and rs4975616 in lung cancer; rs401681 in pancreatic and skin cancer; and rs2736100 in myeloproliferative neoplasms and idiopathic pulmonary fibrosis). Moreover, the study here tended to construct functional annotations for these 12 SNPs with strong evidence using information from the ENCODE Project and other public databases and subsequently revealed that these variants might fall in several putative regulatory regions. Briefly, our research offers comprehensive epidemiological evidence that common variants in the TERT-CLPTM1L region show association with predisposition of glioma, myeloproliferative neoplasms, idiopathic pulmonary fibrosis, esophageal cancer, gastric cancer, skin cancer, bladder cancer, lung cancer, and pancreatic cancer.
The TERT gene (Gene ID: 7015), encoding the enzyme of TERT, plays crucial roles in maintaining the telomere length (32, 33). In the previous research, it was pointed out that telomere length had linked with glioma, ovarian cancer, lung cancer, and melanoma predisposition, rather than breast and prostate cancer (34, 35). Besides, a recent meta-analysis on the GWAS revealed that the variants in TERT-CLPTM1L genes may affect cancer risk through a variety of different biological pathways, and telomere length is the only one of the related mechanisms (36), whereas in our study, strong evidence was given to seven SNPs (rs10069690, rs2242652, rs2736098, rs2736100, rs2853676, rs2853677, and rs2853691) in TERT. Four SNPs (rs2853677, rs2242652, rs2736098, and rs2736100) were related to the predisposition of lung cancer. The phase 3 of the 1000 Genomes Project (37) (Supplementary Table 6) presented that rs2853677 is in moderate LD with rs2736098 (in East Asians: r2 = 0.3070) and rs2736100 (in East Asians: r2 = 0.5789, in Europeans: r2 = 0.4352), is in weak LD with rs2736098 (in Europeans: r2 = 0.1504), and is uncorrelated with rs2736098 and rs2736100 in Africans (r2 < 0.05). Moreover, rs2242652 is in moderate LD with rs2736098 in Europeans (r2 = 0.1504) while is uncorrelated both in East Asians and Africans (r2 < 0.05). According to the above findings, the functional mechanisms of the four variants related to lung cancer risk might be different in various ethnic groups, partially accounting for why some variants are demonstrated to be related to a cancer site in one ethnic group but not in others. In addition, two SNPs (rs2736100 and rs2853676) were associated with glioma risk; however, SNP rs2736100 is in weak LD with rs2853676 in East Asians (r2 = 0.2057), Europeans (r2 = 0.2475), and Africans (r2 = 0.1062). To sum up, these data indicated that there might exist different causal variants and functional mechanisms involved associated with variants in the TERT gene with predisposition of glioma. Apart from that, SNP rs2736100 and SNP rs2853691 are intron variants of the TERT gene, and they are linked with predisposition of esophageal cancer in our study; while in our study, it was found that rs2736100 is unrelated to rs2853691 in Europeans, East Asians, and Africans (r2 < 0.05 for all tests), revealing that there might exist various functional mechanisms for relationships of TERT variants with predisposition of esophageal cancer. Current evidence presents that the rs10069690 T allele can trigger the development of the coproduction of full-length TERT and an alternatively spliced by creating splice donor site in intron 4 of TERT, which may increase gastric cancer risk by reducing telomerase activity and telomere shortening (38). Moreover, rs2242652 allele (G > A) could influence telomere length, which could increase predisposition for lung cancer in Asians (39), whereas SNP rs2736098 could cause the overexpression of TERT and increase telomerase activity, which regulated bladder and lung cancer development by modulating unlimited cell division, and carcinogenesis, and interacted with the activation of the glycolytic pathway (40). Previous study showed that the intron 2 segment including the rs2736100 flanking sequence proved promoter activities in ESCC cell lines and uncovered an elevated association with ESCC predisposition in carriers of rs2736100 G allele (41), which demonstrated the oncogene inherent characteristics of TERT in ESCC. Here, it should be noted that the T allele of rs2736100 could lead to telomere length shortening and then increase lung cancer and non-cancerous disease predisposition (like chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis) (42, 43).
The CLPTM1L gene (Gene ID: 81037), encoding the cleft lip and palate–associated transmembrane 1–like protein, could arouse cell apoptosis and cytokinesis (44, 45). In our study, strong evidence was assigned to four SNPs (rs31489, rs401681, rs402710, and rs465498) in CLPTM1L and one SNP rs4975616 in TERT-CLPTM1L. Of the two variants associated with bladder cancer, rs401681 is in strong LD with rs402710 in East Asians (r2 = 0.9371) while has moderate LD in Europeans (r2 = 0.6624) and Africans (r2 = 0.5961). Moreover, four SNPs (rs31489, rs401681, rs402710, and rs465498) were related to lung cancer, and, in our study, it was found that rs31489 is in strong LD with rs401681 in Europeans (r2 = 0.8145) while is in moderate LD in East Asians (r2 = 0.4785) and Africans (r2 = 0.6131); rs31489 is in strong LD with rs402710 in Africans (r2 = 0.8376) while is in moderate LD in East Asians (r2 = 0.4634) and Europeans (r2 = 0.6624); rs31489 is in strong LD with rs465498 both in East Asians and Europeans (r2 > 0.809) while is in moderate LD in Africans (r2 = 0.6572). Moreover, it was revealed that rs401681 is in strong LD with rs402710 in East Asians (r2 = 0.9371) but shows moderate LD in Europeans (r2 = 0.6624) and Africans (r2 = 0.5961); rs401681 is in strong LD with rs465498 both in Europeans and Africans (r2 > 0.809) while is in moderate LD in East Asians (r2 = 0.4839). Based on the obtained results, the functional mechanisms of the four variants associated with risk of bladder and lung cancer may be different in different ethnic groups and partly account for why some variants are discovered to be related to a cancer site in one ethnic group but not in others. Furthermore, current evidence demonstrates that rs31489, a variant in which C is changed to A in CLPTM1L gene, could influence the telomere length that could decrease the risk of nonsmokers’ lung carcinoma (rather than in smokers), because smoking can counteract the protective role of A allele, shorten telomere length, and enhance telomerase activity (46). Interestingly, rs401681 could affect transcription regulation, result in the over-expression of the CLPTM1L gene, and increase risk of lung and skin carcinoma (47). Moreover, CLPTM1L rs402710 may affect lung tissue tumorigenesis in vitro by blocking DNA damage–induced apoptosis via enhanced accumulation of Bcl-xL, an antiapoptotic Bcl2 family member (48). Beyond that, SNP rs402710 could maintain the telomere length which could decrease the risk of nonsmokers’ lung cancer since the protective role of rs2736100 was counteracted in patients with bladder cancer who currently were smokers (49). In addition, seven variants in the TERT gene are uncorrelated or had weak LD with the four variants in the CLPTM1L gene in European, Asian, and African populations. According to the results, there exist different causal variants and functional mechanisms in relationships of variants in the TERT-CLPTM1L regions with idiopathic pulmonary fibrosis, myeloproliferative neoplasms, and glioma, as well as esophageal, gastric, bladder, lung, pancreatic, and skin cancer predisposition.
In addition, 13 SNPs had no association with five cancer risk in additive model. Of these, eight SNPs (rs13167280, rs2075786, rs2735940, rs2736100, rs2736109, rs2853669, rs2853677, rs2853690, and rs7712562) had no association with breast cancer with at least 5,000 case and 5,000 controls in additive model, which had approximately 98% statistical power to detect an OR of 1.15 for a variant with MAF 0.20 and 86% power with MAF 0.10 (type 1 error 0.05). Therefore, further research with a smaller sample size on these eight SNPs for breast cancer in Caucasians will not be helpful in evaluating effects of those SNPs (Supplementary Result, Table 3 and Supplementary Table 4).
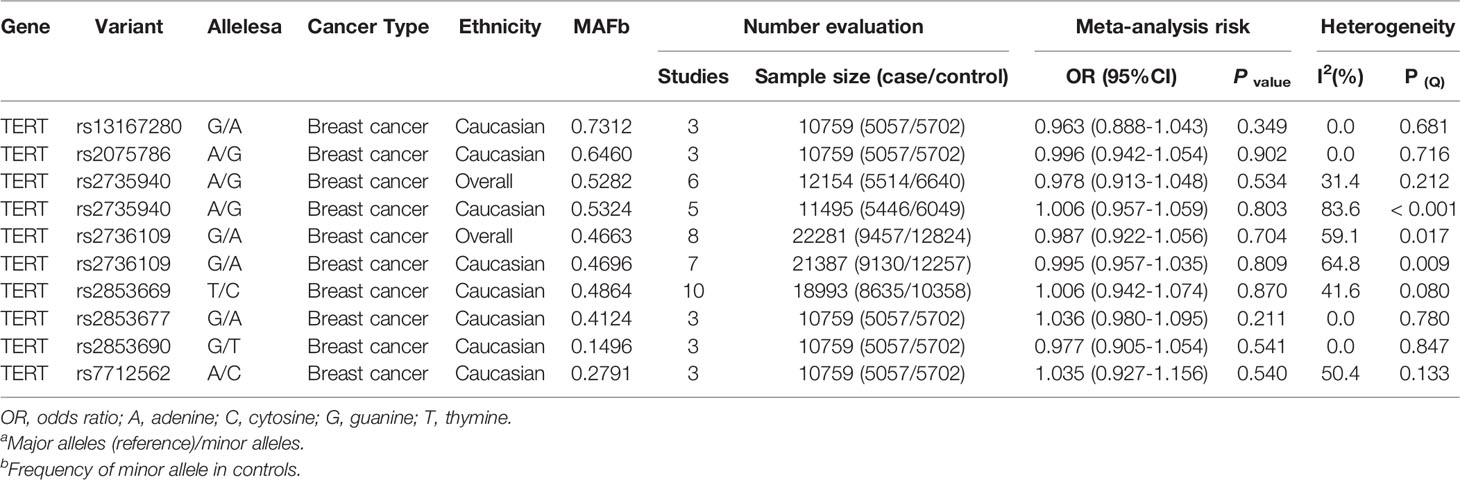
Table 3 Variants in TERT-CLPTM1L showing no relation to breast cancer risk in meta-analyses with at least 5000 cases and 5000 controls in additive model.
In fact, our study has several limitations: (i) although a comprehensive research on databases was conducted, some publications may have been missed, as well as the papers with insufficient data such as the genotype amount, which might result in incomplete assessment of other malignancies (lymphoma, gallbladder cancer, cervical cancer, etc.) and non-cancer disease (chronic hepatitis B, Alzheimer’s disease, diabetes mellitus, etc.); (ii) the potential publication bias might be found due to the usage of the search approach (only search for English papers); (iii) as the subgroup analyses according to ethnicity and partial pathological/clinical subtypes were only performed on lung cancer, idiopathic pulmonary fibrosis and myeloproliferative neoplasms, further analyses based on subgroups such as pathological type, gene-gene or gene-environment associations and interactions, could be required to confirm or refute the correlations with risk of cancers and non-cancer disease; (iv) potential bias for variants with cancers and non-cancer risk could be evaluated by the Venice criteria; however, the unreasonable data, like errors in genotype, could not be evaluated; and (v) meta-analyses were conducted on the basis of the minor allele of a variant; therefore, a protective association for some variants might be found because of the inherent factors in meta-analysis (for example, rs2736100 and rs2853676 could reduce the risk of glioma, myeloproliferative neoplasms, and lung cancer and rs401681 could decrease risk of bladder cancer). Given that, all genetic associations in the current work should be further confirmed and clarified by doing the molecular biology experiments.
To conclude, in our study, it was identified that 12 variants in the TERT-CLPTM1L genes were rated as revealing strong evidence for a significant correlation with eight cancers and one non-cancer disease risk. Moreover, our study offers foundation for further demonstrating that the variants in the TERT-CLPTM1L genes are related to the risk of idiopathic pulmonary fibrosis, myeloproliferative neoplasms, glioma, esophageal cancer, gastric cancer, bladder cancer, lung cancer, pancreatic cancer, and skin cancer. Apart from that, the crucial roles of the TERT-CLPTM1L region in the etiology of human diseases were highlighted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
JT, YW, and GC designed this work. JT and YW integrated and analyzed the data. JT, YW, and GC wrote this manuscript. JT, YW, YD, JC, YMW, SC, and GC finished the related Tables and Figures. JT, YW, and GC edited and revised the manuscript. All authors approved this manuscript.
Funding
This work was supported by the Sichuan Science and Technology Program (grant No. 2020YFS0252).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.946039/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and Heritable Factors in the Causation of Cancer–Analyses of Cohorts of Twins From Sweden, Denmark, and Finland. N Engl J Med (2000) 343:78–85. doi: 10.1056/NEJM200007133430201
3. MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, et al. The New NHGRI-EBI Catalog of Published Genome-Wide Association Studies (GWAS Catalog). Nucleic Acids Res (2017) 45:D896–901. doi: 10.1093/nar/gkw1133
4. Cong YS, Wen J, Bacchetti S. The Human Telomerase Catalytic Subunit hTERT: Organization of the Gene and Characterization of the Promoter. Hum Mol Genet (1999) 8:137–42. doi: 10.1093/hmg/8.1.137
5. Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. A Novel Gene, CRR9, Which Was Up-Regulated in CDDP-Resistant Ovarian Tumor Cell Line, Was Associated With Apoptosis. Biochem Biophys Res Commun (2001) 280:1148–54. doi: 10.1006/bbrc.2001.4250
6. Blasco MA. Telomeres and Human Disease: Ageing, Cancer and Beyond. Nat Rev Genet (2005) 6:611–22. doi: 10.1038/nrg1656
7. Calado RT, Young NS. Telomere Diseases. N Engl J Med (2009) 361:2353–65. doi: 10.1056/NEJMra0903373
8. Wang L, Soria JC, Chang YS, Lee HY, Wei QY, Mao L. Association of a Functional Tandem Repeats in the Downstream of Human Telomerase Gene and Lung Cancer. Oncogene (2003) 22:7123–9. doi: 10.1038/sj.onc.1206852
9. Matsubara K, Murata M, Watanabe K, Saito I, Miyaki K, Omae K, et al. Coronary Artery Disease and a Functional Polymorphism of hTERT. Biochem Biophys Res Commun (2006) 348:669–72. doi: 10.1016/j.bbrc.2006.07.103
10. McKay JD, Hung RY, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, et al. Lung Cancer Susceptibility Locus at 5p15.33. Nat Genet (2008) 40:1404–6. doi: 10.1038/ng.254
11. Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, et al. A Genome-Wide Association Study Identifies an Association of a Common Variant in TERT With Susceptibility to Idiopathic Pulmonary Fibrosis. J Med Genet (2008) 45:654–6. doi: 10.1136/jmg.2008.057356
12. Wang YF, Wei YY, Gaborieau V, Shi JX, Han YH, Timofeeva MN, et al. Deciphering Associations for Lung Cancer Risk Through Imputation and Analysis of 12,316 Cases and 16,831 Controls. Eur J Hum Genet (2015) 23:1723–8. doi: 10.1038/ejhg.2015.48
13. Turnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, et al. Variants Near DMRT1, TERT and ATF7IP Are Associated With Testicular Germ Cell Cancer. Nat Genet (2010) 42:604–7. doi: 10.1038/ng.607
14. Fingerlin TE, Murphy E, Zhang WM, Peljto AL, Brown KK, Steele MP, et al. Genome-Wide Association Study Identifies Multiple Susceptibility Loci for Pulmonary Fibrosis. Nat Genet (2013) 45:613–20. doi: 10.1038/ng.2609
15. Rajaraman P, Melin BS, Wang ZM, McKean-Cowdin R, Michaud DS, Wang SS, et al. Genome-Wide Association Study of Glioma and Meta-Analysis. Hum Genet (2012) 131:1877–88. doi: 10.1007/s00439-012-1212-0
16. Li ZH, Pu ZN, Fan JY, Li N, Zhu M, Zhang JH, et al. Fine Mapping in TERT-CLPTM1L Region Identified Three Independent Lung Cancer Susceptibility Signals: A Large-Scale Multi-Ethnic Population Study. Mol Carcinog (2018) 57:1289–99. doi: 10.1002/mc.22843
17. Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, et al. Sequence Variants at the TERT-CLPTM1L Locus Associate With Many Cancer Types. Nat Genet (2009) 41:221–7. doi: 10.1038/ng.296
18. Zhang XY, Chen Y, Yan DL, Han J, Zhu LB. TERT Gene Rs2736100 and Rs2736098 Polymorphisms are Associated With Increased Cancer Risk: A Meta-Analysis. Biochem Genet (2021) 60:241–66. doi: 10.1007/s10528-021-10097-0
19. Li T, Xian Y, Tian T, Zhuang X, Chu MJ. New Evidence of TERT Rs2736098 Polymorphism and Cancer Risk: An Updated Meta-Analysis. J BUON (2016) 21:491–7.
20. Li H, Xu YY, Mei H, Peng L, Li XJ, Tang JH. The TERT Rs2736100 Polymorphism Increases Cancer Risk: A Meta-Analysis. Oncotarget (2017) 8:38693–705. doi: 10.18632/oncotarget.16309
21. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Sagoo GS, Little J, Higgins-Julian PT. Systematic Reviews of Genetic Association Studies. Hum Genome Epidemiol Network PloS Med (2009) 6:e28. doi: 10.1371/journal.pmed.1000028
23. Higgins-Julian PT, Thompson-Simon G. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
24. Ioannidis JPA, Trikalinos TA. An Exploratory Test for an Excess of Significant Findings. Clin Trials (2007) 4:245–53. doi: 10.1177/1740774507079441
25. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
26. Egger M, Smith GD, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Ioannidis-John PA, Boffetta P, Little J, O'Brien TR, Uitterlinden AG, Vineis P, et al. Assessment of Cumulative Evidence on Genetic Associations: Interim Guidelines. Int J Epidemiol (2008) 37:120–32. doi: 10.1093/ije/dym159
28. Wacholder S, Chanock S, Garcia-Closas M, Ghormli LE, Rothman N. Assessing the Probability That a Positive Report is False: An Approach for Molecular Epidemiology Studies. J Natl Cancer Inst (2004) 96:434–42. doi: 10.1093/jnci/djh075
29. Ward LD, Kellis M. HaploReg V4: Systematic Mining of Putative Causal Variants, Cell Types, Regulators and Target Genes for Human Complex Traits and Disease. Nucleic Acids Res (2016) 44:D877–881. doi: 10.1093/nar/gkv1340
30. Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreserv Biobank (2015) 13:311–9. doi: 10.1089/bio.2015.0032
31. Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, et al. Mapping Cis- and Trans-Regulatory Effects Across Multiple Tissues in Twins. Nat Genet (2012) 44:1084–9. doi: 10.1038/ng.2394
32. Bianchi A, Shore D. How Telomerase Reaches its End: Mechanism of Telomerase Regulation by the Telomeric Complex. Mol Cell (2008) 31:153–65. doi: 10.1016/j.molcel.2008.06.013
33. Osterhage JL, Friedman KL. Chromosome End Maintenance by Telomerase. J Biol Chem (2009) 284:16061–5. doi: 10.1074/jbc.R900011200
34. Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol (2017) 3:636–51. doi: 10.1001/jamaoncol.2016.5945
35. Zhang CN, Doherty JA, Burgess S, Hung RJ, Lindström S, Kraft P, et al. Genetic Determinants of Telomere Length and Risk of Common Cancers: A Mendelian Randomization Study. Hum Mol Genet (2015) 24:5356–66. doi: 10.1093/hmg/ddv252
36. Chen HJ, Majumdar A, Wang L, Kar S, Brown KM, Feng HL, et al. Large-Scale Cross-Cancer Fine-Mapping of the 5p15.33 Region Reveals Multiple Independent Signals. HGG Adv (2021) 2:100041. doi: 10.1016/j.xhgg.2021.100041
37. Machiela MJ, Chanock SJ. LDlink: A Web-Based Application for Exploring Population-Specific Haplotype Structure and Linking Correlated Alleles of Possible Functional Variants. Bioinformatics (2015) 31:3555–7. doi: 10.1093/bioinformatics/btv402
38. Zhang N, Zheng Y, Liu J, Lei TS, Xu TT, Yang M. Genetic Variations Associated With Telomere Length Confer Risk of Gastric Cardia Adenocarcinoma. Gastric Cancer (2019) 22:1089–99. doi: 10.1007/s10120-019-00954-8
39. Gao L, Thakur A, Liang YQ, Zhang S, Wang T, Chen TJ, et al. Polymorphisms in the TERT Gene are Associated With Lung Cancer Risk in the Chinese Han Population. Eur J Cancer Prev (2014) 23:497–501. doi: 10.1097/CEJ.0000000000000086
40. Singh V, Jaiswal PK, Mittal RD. Replicative Study of GWAS TP63C/T, TERTC/T, and SLC14A1C/T With Susceptibility to Bladder Cancer in North Indians. Urol Oncol (2014) 32:1209–14. doi: 10.1016/j.urolonc.2014.05.013
41. Zhou LQ, Fu GB, Wei JY, Shi J, Pan WT, Ren YL, et al. The Identification of Two Regulatory ESCC Susceptibility Genetic Variants in the TERT-CLPTM1L Loci. Oncotarget (2016) 7:5495–506. doi: 10.18632/oncotarget.6747
42. Tuder RM, Kern JA, Miller YE. Senescence in Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc (2012) 9:62–3. doi: 10.1513/pats.201201-012MS
43. Dong J, Cheng Y, Zhu M, Wen Y, Wang C, Wang YZ, et al. Fine Mapping of Chromosome 5p15.33 Identifies Novel Lung Cancer Susceptibility Loci in Han Chinese. Int J Cancer (2017) 141:447–56. doi: 10.1002/ijc.30702
44. James MA, Wen WD, Wang YA, Byers LA, Heymach JV, Coombes KR, et al. Functional Characterization of CLPTM1L as a Lung Cancer Risk Candidate Gene in the 5p15.33 Locus. PLoS One (2012) 7:e36116. doi: 10.1371/journal.pone.0036116
45. Jia JP, Bosley AD, Thompson A, Hoskins JW, Cheuk A, Collins I, et al. CLPTM1L Promotes Growth and Enhances Aneuploidy in Pancreatic Cancer Cells. Cancer Res (2014) 74:2785–95. doi: 10.1158/0008-5472.CAN-13-3176
46. Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, Cigarette Smoking, and Telomere Length in Women. Lancet (2005) 366:662–4. doi: 10.1016/S0140-6736(05)66630-5
47. Yin J, Wang LM, Zheng L, Wang X, Shi YJ, Shao AZ, et al. TERT-CLPTM1L Rs401681 C>T Polymorphism was Associated With a Decreased Risk of Esophageal Cancer in a Chinese Population. PLoS One (2014) 9:e100667. doi: 10.1371/journal.pone.0100667
48. Alkhadhari S, Alsabbrri AO, Mohammad-Ibrahim HA, Atwan AA, Alqudaihi F, Zahid MA. Prevalence of Psychiatric Morbidity in the Primary Health Clinic Attendees in Kuwait. J Affect Disord (2016) 195:15–20. doi: 10.1016/j.jad.2016.01.037
Keywords: TERT, CLPTM1L, genetic variant, disease, susceptibility
Citation: Tian J, Wang Y, Dong Y, Chang J, Wu Y, Chang S and Che G (2022) Cumulative Evidence for Relationships Between Multiple Variants in the TERT and CLPTM1L Region and Risk of Cancer and Non-Cancer Disease. Front. Oncol. 12:946039. doi: 10.3389/fonc.2022.946039
Received: 17 May 2022; Accepted: 30 May 2022;
Published: 30 June 2022.
Edited by:
Xiao Zhu, Guangdong Medical University, ChinaReviewed by:
Youtao Lu, University of Pennsylvania, United StatesTianfang Ma, Tulane University, United States
Copyright © 2022 Tian, Wang, Dong, Chang, Wu, Chang and Che. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guowei Che, Y2hlZ3Vvd2VpeHdAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jie Tian
Jie Tian Yan Wang
Yan Wang Yingxian Dong
Yingxian Dong Junke Chang1
Junke Chang1 Yongming Wu
Yongming Wu Shuai Chang
Shuai Chang Guowei Che
Guowei Che