- 1Department of Thoracic Oncology and State Key Laboratory of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2West China school of medicine, Sichuan University, Chengdu, China
BRAF gene has been identified as an oncogenic driver and a potential target in various malignancies. BRAF fusions are one subtype of BRAF alterations with a rare frequency. Here, we first report a previously treated advanced lung adenocarcinoma patient with de novo SND1-BRAF fusion who achieves partial response to the MAK inhibitor trametinib. We also provide a literature review on targeted therapies for BRAF fusions.
Introduction
BRAF gene encodes the RAF kinase and activates the MAPK pathway. It has emerged as an oncogenic driver and a potential therapeutic target in a wide variety of solid tumors (1, 2). Based on signaling mechanism, kinase activity, and sensitivity to inhibitors, BRAF mutations have been categorized into three functional classes: RAS-independent kinase-activating V600 monomers (class I), RAS-independent kinase-activating dimers (class II), and RAS-dependent kinase-inactivating heterodimers (class III) (3, 4). BRAF alterations occur in 4.4% of non-small cell lung cancer (NSCLC) patients (1). The most prevalent variant is the BRAF V600 mutation, accounting for 1%–2% of BRAF-mutated NSCLC patients (5). However, BRAF fusions, one subtype of BRAF class II mutations, are only identified in 0.2% of NSCLC samples (1). The Food and Drug Administration (FDA) has approved dabrafenib plus trametinib to treat NSCLC patients with BRAF V600 mutation (6). In contrast, BRAF fusions are not yet eligible for targeted therapies (4).
Unlike BRAF mutation, BRAF gene fusions activate the MAPK signaling pathway by inducing the removal of the auto-inhibitory N-terminal moiety (7). BRAF fusions exist in numerous solid tumors, including melanoma, glioma, thyroid cancer, pancreatic carcinoma, NSCLC, and colorectal cancer (1, 8). BRAF fusions can take on various forms. Ross et al. and Zehir et al. have reported 44 distinct types of BRAF fusions in multiple solid tumors, especially in NSCLC, such as EPS15-BRAF, NUP214-BRAF, ARMC10-BRAF, BTF3L4-BRAF, AGK-BRAF, GHR-BRAF, ZC3HAV1-BRAF, TRIM24-BRAF, GK-BRAF, PJA2-BRAF, SND1-BRAF, MRPS33-BRAF, and PARP12-BRAF (1, 9). Unfortunately, reports on anti-BRAF therapies for NSCLC with de novo BRAF gene fusion are scarce.
The study shows a rapid response to trametinib monotherapy in the advanced lung adenocarcinoma patient with de novo SND1-BRAF fusion for the first time.
Case report
A 60-year-old man was admitted to West China Hospital, Sichuan University in February 2017 with symptoms of expectoration and shortness of breath for 1 month. He was a current smoker (30 packs/years) with no family history of cancer. High concentration levels of serum carcinoembryonic antigen (CEA) (63.92 ng/ml) and neuron-specific enolase (NSE) (23.35 ng/ml) were revealed by blood tests. Right lung masses and solid nodules were visible on a chest computed tomography (CT) scan, which also revealed enlargement of the right hilar and mediastinal lymph nodes, focal thickening of the right pleura, and right pleural effusions. He underwent a CT-guided biopsy of the right lung mass, and the lesion was pathologically diagnosed as adenocarcinoma. Immunohistochemical examination of the right pleural effusions showed positivity of CK7, Napsin A, P63, and TTF-1 and negativity of CK5/6, ALK-V, and ROS-1, PD-L1 tumor proportion score (TPS) of 30% , which confirms that the metastatic adenocarcinoma originated from the lung. Next-generation sequencing (NGS) (Burning Rock, Guangzhou, China) with a panel of 295 cancer-related genes was conducted to examine the tumor tissue. The outcome was positive for SND1-BRAF (S10:B9) fusion (abundance 3.8%), whereas EGFR, ALK, ROS1, and other sensitive genes were all negative.
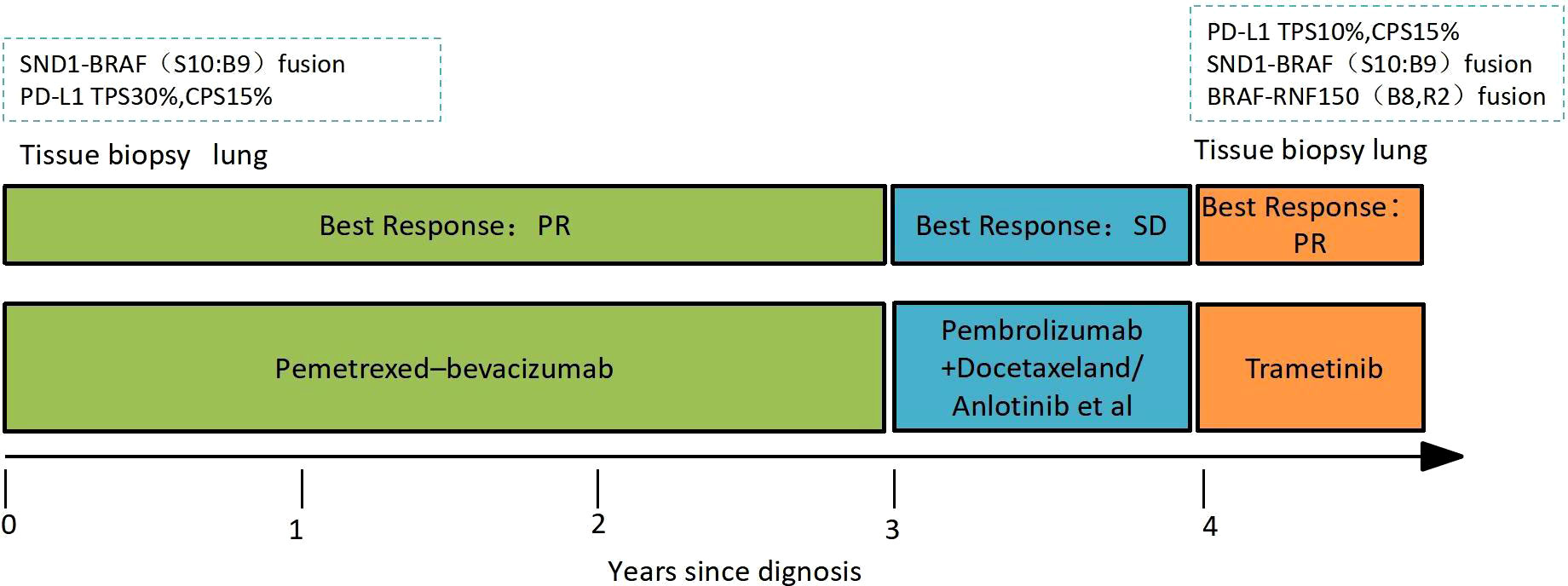
According to the Eighth Edition of the TNM Classification for Lung Cancer, the patient was classified as stage cT4N3M1a (cIVA) lung cancer. Therefore, the patient underwent four cycles of cisplatin/pemetrexed combined with bevacizumab as the first-line therapy. Then, the patient received maintenance treatment of pemetrexed/bevacizumab until the disease spread to his right thoracic cavity and left the adrenal gland. The disease progression was revealed in March 2020 through the chest and abdomen CT. The best response was partial response (PR) with a PFS of 36 months during first-line therapy. The major treatment-associated adverse events were Grade 1 gingival bleeding and Grade 2 leukopenia and neutropenia. Pembrolizumab (200 mg, Q3W) was administered to the patient as the second-line therapy. However, the patient’s right thoracic solid nodules continued to grow. Several treatment lines failed, including pembrolizumab (200 mg, Q3W) plus docetaxel (110 mg, Q3W) and pembrolizumab (200 mg, Q3W) plus anlotinib (10 mg D1–14, Q3W).
Laboratory results revealed an increased NSE level of 24.5 ng/ml in July 2021, and multiple lesions on the CT scan suggested disease progression. It should be noted that the NSE concentration remained normal throughout last year. Furthermore, a percutaneous right lung biopsy guided by CT confirmed the patient’s adenocarcinoma diagnosis. Whole-exome sequencing (WES) (Berry Oncology Corporation, Beijing, China) was conducted to analyze the biopsy specimen. The result showed the positivity of SND1-BRAF (S10:B9) and BRAF-RNF150(B8:R2)fusions with a PD-L1 TPS of 10% in August 2021. The patient received the MEK inhibitor trametinib (2 mg daily) from 1 September 2021. His cough was significantly relieved following 1-week trametinib treatment. The only adverse effect, which appeared 3 weeks after taking trametinib, was scattered acne with itching over the head and face (CTCAE 1 grade). After 4 months, the CT scan confirmed the patient with PR with a dramatic tumor shrinkage of 57% (Figure 1). He is now treated with trametinib. The overall timeline of diagnosis and treatment is displayed in Figure 2.
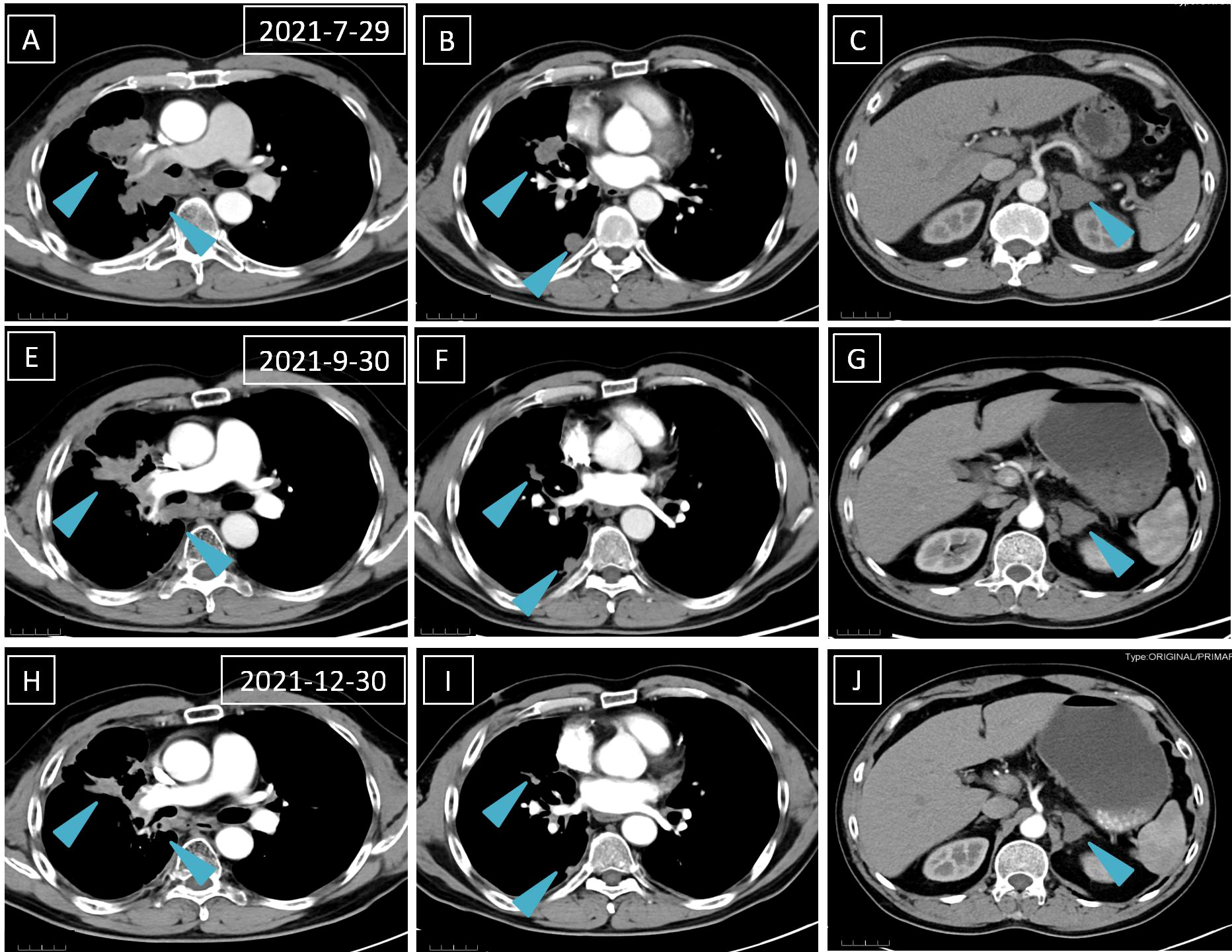
Figure 1 A computed tomography scan is performed before (A–C), 1 month after (D–F), and 4 months after (H–J) trametinib treatment. The blue arrows mean lung and left adrenal gland nodules.
Discussion
BRAF fusions occur at a rare frequency (1, 10). Moreover, there are no anti-BRAF treatment guidelines for lung cancer patients with BRAF fusions. The situation is further worsened by the dearth of case reports on anti-BRAF targeted therapy. Our patient is the first reported NSCLC case with de novo SND1-BRAF fusion responding to the MEK inhibitor trametinib.
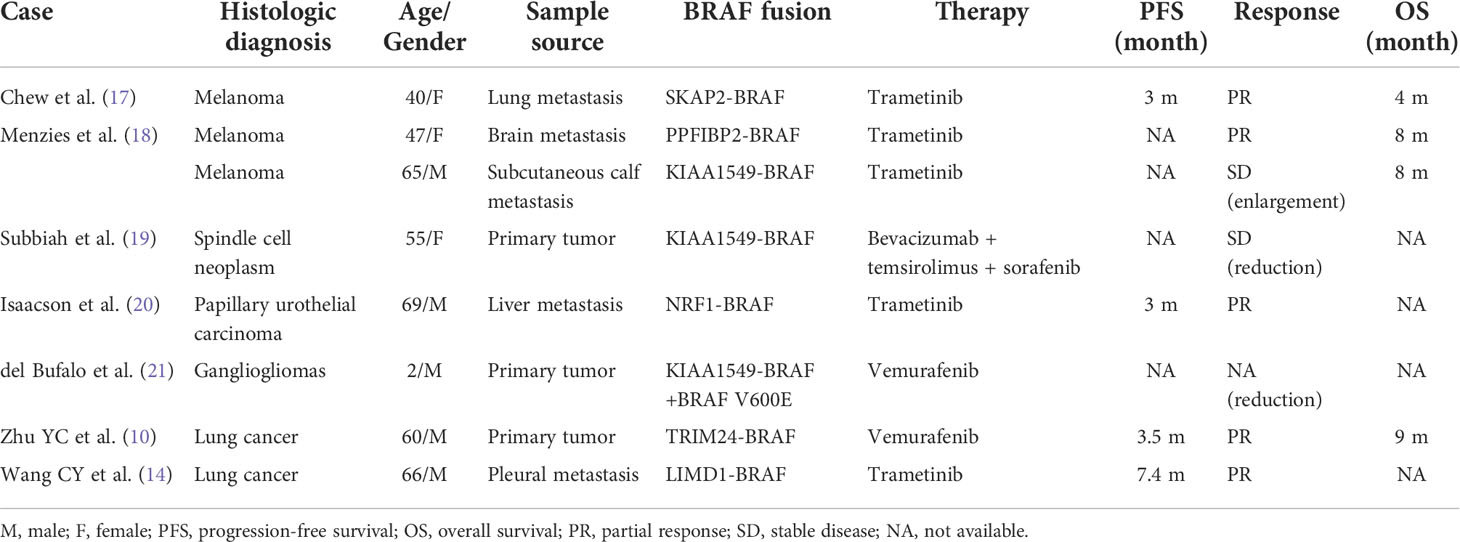
Despite the rarity of BRAF gene fusions, the alterations can be found in several solid tumors, including melanoma, glioma, thyroid cancer, pancreatic carcinoma, NSCLC, and colorectal cancer (1, 11). In contrast to BRAF monomer mutations, which often result in the mutation of the kinase domain sand, BRAF fusion proteins retain the normal kinase domain sand while inducing the loss of the auto-inhibitory N-terminal moiety (7) (Figure 3). Moreover, BRAF fusions exhibit variability due to their distinct partners, which have been documented in several papers (1, 9). However, there are no literature reports on BRAF-RNF150. Its role and mechanism in tumorigenesis and tumor development are still being investigated. SND1-BRAF fusion has been found in pancreatic cancer and lung adenocarcinoma. Nevertheless, no clinical investigation has shown any instances of SND1-BRAF fusion in solid malignancies. Preclinical research on pancreatic acinar cell carcinoma revealed that trametinib could significantly inhibit the growth of SND1-BRAF transformed cells. At the same time, TAK-632 exhibited a weak suppressive effect, and sorafenib showed no inhibitory effect (12). Hutchinson et al. studied PAPSS1-BRAF or KIAA1549-BRAF transfected 293H cells treated with vemurafenib or trametinib in vitro (13). The finding revealed that the mutant-specific BRAF inhibitor trametinib, rather than vemurafenib, might block the downstream signaling induced by the PAPSS1-BRAF or KIAA1549-BRAF fusion. Botton et al. found that six melanoma cell lines harboring BRAF fusions were resistant to first- and second-generation RAF inhibitors (14). By contrast, next-generation αC-IN/DFG-OUT RAF inhibitors blunted the activation of all cell lines, and showed synergistic effects when combined with the MEK inhibitors. Similarly, Usta et al. screened MAPK inhibitors using the KIAA1549-BRAF transfected glioma cell line (15) The result revealed that MEK inhibitors inhibited the MAPK signaling pathway with the lowest IC50s, followed by ERK and next-generation RAF inhibitors. A synergistic effect was observed in the combination of RAF and MEK inhibitors. In addition, Vojnic et al. identified four individuals with EGFR-mutated lung cancers that acquired BRAF rearrangements and showed secondary resistance to anti-EGFR therapy (16). The study further induced the AGK-BRAF fusion in H1975 (L858R+T790M), PC9 (ex19del), and HCC827 (ex19del) cell lines, and the cells also displayed osimertinib resistance. Furthermore, trametinib could synergistically suppress the proliferation of these cell lines when combined with osimertinib (16). Clinical references for our regimen included various studies describing anti-BRAF targeted therapies on different forms of BRAF fusions (Table 1). Pan-RAF inhibitors were an effective agent for targeting the BRAF fusions as demonstrated both in animal models and in vitro. Yao et al. reported that a dual RAF inhibitor BGB659 could inactivate RAF fusion proteins by blocking the ERK signaling pathway (22). Similarly, Peng et al. showed that LY3009120, a pan-RAF and RAF dimer inhibitor, could inhibit RAF isoforms (ARAF, BRAF, and CRAF) and occupy protomers in RAF dimers (23). Another BRAF-specific dimer breaker called PLX8394 was discovered by Yao et al. It showed a superior safety profile in clinical practice and preferentially suppressed the ERK signaling pathway in tumors driven by dimeric BRAF mutants while sparing RAF function in normal cells (24).
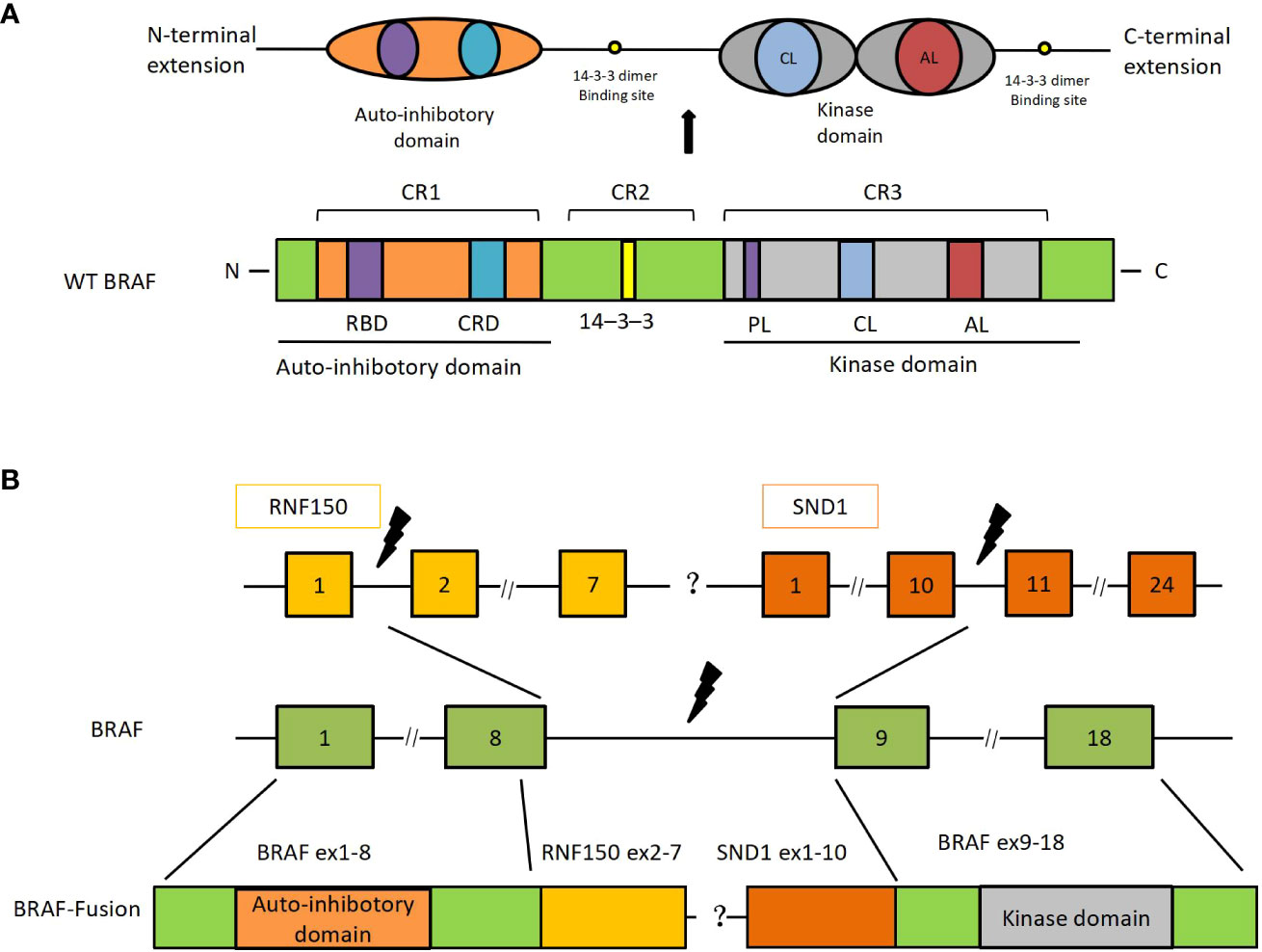
Figure 3 SND1-BRAF transcript fusion in metastatic lung adenocarcinoma. (A) The primary structure of BRAF and its functional domains. (B) BRAF-RNF150 and SND1-BRAF fusions. RBD, ras-binding domain; CRD, cysteine-rich domain; PL, phosphate-binding loop; CL, catalytic loop; AL, activation loop; SND, staphylococcal nuclease domain; Ex, exon.
In conclusion, drugs targeting the MAPK pathway, particularly MEK inhibitors whose antitumor benefits have been shown in preclinical research and clinical case reports, may effectively treat BRAF fusion-related cancers (15). In addition, ERK and next-generation RAF inhibitors may have a synergistic antitumor impact across distinct classes of MAPK inhibitors. In this research, the NSCLC patient with primary SDN1-BRAF fusion receives continuous trametinib monotherapy, and it is encouraging to observe PR. Further research is required to understand the biochemical and oncogenic mechanisms and identify the targeted strategy in NSCLC patients harboring BRAF fusions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MH, Corresponding author, contributed to the conception of the study and helped perform the analysis with constructive discussions. YY contributed significantly to manage and treat these patients, analysis and wrote the manuscript. MY, YL, XZ, TT, YD, ZT helped manage and treat this patient. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank TopEdit (www.topeditsci.com) for English language editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ross JS, Wang K, Chmielecki J, Gay L, Johnson A, Chudnovsky J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer (2016) 138(4):881–90. doi: 10.1002/ijc.29825
2. Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology (2013) 45(4):346–56. doi: 10.1097/PAT.0b013e328360b61d
3. Fois SS, Paliogiannis P, Zinellu A, Fois AG, Cossu A, Palmieri G. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int J Mol Sci (2021) 22(2):612. doi: 10.3390/ijms22020612
4. Lin Q, Zhang H, Ding H, Qian J, Lizaso A, Lin J, et al. The association between BRAF mutation class and clinical features in BRAF-mutant Chinese non-small cell lung cancer patients. J Transl Med (2019) 17(1):298. doi: 10.1186/s12967-019-2036-7
5. Bracht JWP, Karachaliou N, Bivona T, Lanman RB, Faull I, Nagy RJ, et al. II, and III in NSCLC Patients Included in the SLLIP Trial: The Need for a New Pre-Clinical Treatment Rationale. Cancers (Basel) (2019) 11(9):1381. doi: 10.3390/cancers11091381
6. Planchard D, Besse B, Groen HJM, Hashemi SMS, Mazieres J, Kim TM, et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J Thorac Oncol (2022) 17(1):103–15. doi: 10.1016/j.jtho.2021.08.011
7. Weinberg F, Griffin R, Fröhlich M, Heining C, Braun S, Spohr C, et al. Identification and characterization of a BRAF fusion oncoprotein with retained autoinhibitory domains. Oncogene (2020) 39(4):814–32. doi: 10.1038/s41388-019-1021-1
8. Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med (2010) 16(7):793–8. doi: 10.1038/nm.2166
9. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med (2017) 23(6):703–13. doi: 10.1038/nm.4333
10. Zhu YC, Wang WX, Xu CW, Zhuang W, Du KQ, Chen G, et al. A Patient With Lung Adenocarcinoma With BRAF Gene Fusion and Response to Vemurafenib. Clin Lung Cancer (2019) 20(3):e224–8. doi: 10.1016/j.cllc.2019.02.020
11. Wang CY, Hsia JY, Li CH, Ho CC, Chao WR, Wu MF. Lung Adenocarcinoma With Primary LIMD1-BRAF Fusion Treated With MEK Inhibitor: A Case Report. Clin Lung Cancer (2021) 22(6):e878–80. doi: 10.1016/j.cllc.2021.05.003
12. Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discovery (2014) 4(12):1398–405. doi: 10.1158/2159-8290.CD-14-0617
13. Hutchinson KE, Lipson D, Stephens PJ, Otto G, Lehmann BD, Lyle PL, et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin Cancer Res (2013) 19(24):6696–702. doi: 10.1158/1078-0432.CCR-13-1746
14. Botton T, Talevich E, Mishra VK, Zhang T, Shain AH, Berquet C, et al. Genetic Heterogeneity of BRAF Fusion Kinases in Melanoma Affects Drug Responses. Cell Rep (2019) 29(3):573–588.e7. doi: 10.1016/j.celrep.2019.09.009
15. Usta D, Sigaud R, Buhl JL, Selt F, Marquardt V, Pauck D, et al. A Cell-Based MAPK Reporter Assay Reveals Synergistic MAPK Pathway Activity Suppression by MAPK Inhibitor Combination in BRAF-Driven Pediatric Low-Grade Glioma Cells. Mol Cancer Ther (2020) 19(8):1736–50. doi: 10.1158/1535-7163
16. Vojnic M, Kubota D, Kurzatkowski C, Offin M, Suzawa K, Benayed R, et al. Acquired BRAF Rearrangements Induce Secondary Resistance to EGFR therapy in EGFR-Mutated Lung Cancers. J Thorac Oncol (2019) 14(5):802–15. doi: 10.1016/j.jtho.2018.12.038
17. Chew SM, Lucas M, Brady M, Kelly CM. SKAP2-BRAF fusion and response to an MEK inhibitor in a patient with metastatic melanoma resistant to immunotherapy. BMJ Case Rep (2021) 14(6):e238494. doi: 10.1136/bcr-2020-238494
18. Menzies AM, Yeh I, Botton T, Bastian BC, Scolyer RA, Long GV. Clinical activity of the MEK inhibitor trametinib in metastatic melanoma containing BRAF kinase fusion. Pigment Cell Melanoma Res (2015) 28(5):607–10. doi: 10.1111/pcmr.12388
19. Subbiah V, Westin SN, Wang K, Araujo D, Wang WL, Miller VA, et al. Targeted therapy by combined inhibition of the RAF and mTOR kinases in malignant spindle cell neoplasm harboring the KIAA1549-BRAF fusion protein. J Hematol Oncol (2014) 7:8. doi: 10.1186/1756-8722-7-8
20. Isaacson AL, Guseva NV, Bossler AD, Ma D. Urothelial carcinoma with an NRF1-BRAF rearrangement and response to targeted therapy. Cold Spring Harb Mol Case Stud (2019) 5(3):a003848. doi: 10.1101/mcs.a003848
21. del Bufalo F, Carai A, Figà-Talamanca L, Pettorini B, Mallucci C, Giangaspero F, et al. Response of recurrent BRAFV600E mutated ganglioglioma to Vemurafenib as single agent. J Transl Med (2014) 12:356. doi: 10.1186/s12967-014-0356-1
22. Yao Z, Torres NM, Tao A, Gao Y, Luo L, Li Q, et al. BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms that Determine Their Sensitivity to Pharmacologic Inhibition. Cancer Cell (2015) 28(3):370–83. doi: 10.1016/j.ccell.2015.08.001
23. Peng SB, Henry JR, Kaufman MD, Lu WP, Smith BD, et al. Inhibition of RAF Isoforms and Active Dimers by LY3009120 Leads to Anti-tumor Activities in RAS or BRAF Mutant Cancers. Cancer Cell (2015) 28(3):384–98. doi: 10.1016/j.ccell.2015.08.002
Keywords: BRAF fusion, SDN1-BRAF, lung adenocarcinoma, trametinib, MAPK pathway
Citation: Yu Y, Yu M, Li Y, Zhou X, Tian T, Du Y, Tu Z and Huang M (2022) Rapid response to monotherapy with MEK inhibitor trametinib for a lung adenocarcinoma patient harboring primary SDN1-BRAF fusion: A case report and literature review. Front. Oncol. 12:945620. doi: 10.3389/fonc.2022.945620
Received: 16 May 2022; Accepted: 26 July 2022;
Published: 19 August 2022.
Edited by:
Giuseppe Palmieri, University of Sassari, ItalyReviewed by:
Panagiotis Paliogiannis, University of Sassari, ItalyTilman Brummer, University of Freiburg, Germany
Copyright © 2022 Yu, Yu, Li, Zhou, Tian, Du, Tu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meijuan Huang, aG1qMTA3QDE2My5jb20=
 Yang Yu1
Yang Yu1 Min Yu
Min Yu Tian Tian
Tian Tian Zegui Tu
Zegui Tu Meijuan Huang
Meijuan Huang