
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 10 August 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.945166
This article is part of the Research TopicThe Ever-Changing Scenario of First Line Treatment of Metastatic Renal Cell CarcinomaView all 7 articles
Background: So far, whether positive surgical margin(PSM) has adverse effects on the prognosis of patients is still controversial, so we designed this study to systematically evaluate the effect of PSM on the prognosis of patients with renal cell carcinoma (RCC) after partial nephrectomy (PN).
Methods: On the basis of three electronic databases (PubMed, Embase and the Cochrane Library) up to May 2022, all case–control studies (CCSs) comparing the effects of PSM and negative surgical margin (NSM) after PN on the oncological results of RCC patients were included. Two evaluators independently conducted a systematic literature search and extracted the data we needed. The methodological quality of all studies was evaluated by the modified Newcastle–Ottawa scale. The odds ratio (OR) was used to describe the results for dichotomous variables, and the meta-analysis was conducted using Cochrane Review Manager 5.2 and Stata 14.2.
Results: A total of 39 studies involving 21461 patients were included in our meta-analysis. The pooled results showed that the rates of tumor recurrence (OR 3.93, 95% CI 2.95-5.24; p < 0.00001) and metastasis (OR 4.63, 95% CI 3.11-6.88; p < 0.00001) in the PSM group were significantly higher than those in the NSM group. However, there were no significant differences in the rates of all-cause death (OR 1.35, 95% CI 0.92-1.99; p = 0.13) or cancer-specific death (OR 0.99, 95% CI 0.51-1.94; p = 0.99) between the two groups. In addition, subgroup analyses were carried out according to different average follow-ups, which revealed similar results.
Conclusion: Insignificant differences in survival between the PSM and NSM groups were observed, although significant differences in recurrence and metastasis in the PSM group were reported. Our study supported that close monitoring might be another effective choice for patients with PSM after PN. Considering the possible limitations, we recommended cautious interpretation of our results.
Partial nephrectomy (PN) is one of the standard treatments for renal cell carcinoma (RCC) (1), and it has been shown to be as safe and effective as radical nephrectomy (RN) for selected patients (2, 3). According to the surgeons’ preference along with the feasibility of technology, a robot-assisted, pure laparoscopic or open method can be chosen for PN. Theoretically, the purpose of the operation is to completely remove the tumor and provide a negative surgical margin (NSM) while preserving as much normal renal parenchyma as possible (4). However, positive surgical margins (PSM) occasionally occur, with an incidence of 2-8% (5), which is defined as the presence of cancer cells at the parenchymal inked margin of resection.
Dozens of studies have shown that PSM is an independent predictor for progression-free survival (PFS) due to significantly more long-term recurrences (6). However, other studies have shown a negative correlation between PSM and disease progression or death (7, 8). To date, whether PSM has an adverse effect on the prognosis of patients is still controversial (9). Therefore, we carried out a systematic review and meta-analysis to explore the effects of PSM on patients with RCC after PN.
Two reviewers independently and systematically searched the literature published in three electronic databases (PubMed, Embase and the Cochrane Library) prior to May 2022. The search terms included recurrence/death/metastasis/oncologic outcome/survival AND surgical margin AND nephron-sparing surgery/partial nephrectomy. In addition, the reference lists from identified publications were also searched.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Because of the nature of our study, randomized controlled trials (RCT) and prospective cohort studies were unethical. Therefore, all case–control studies (CCSs) comparing the effects of PSM and NSM after partial nephrectomy on the oncological results in RCC patients were included. When articles were not written in English, they were translated for data extraction if possible.
After reviewing the title and abstract, we reviewed the full text to more fully assess whether it met the inclusion criteria: the population we included in the study comprised patients who underwent partial nephrectomy with all surgical methods (open surgery, laparoscopy, robot-assisted) and whose pathological diagnosis was renal cell carcinoma (including any histological subtype). We excluded studies that approved the treatment of renal cell carcinoma through ablation therapy and excluded studies on the pathological diagnosis of benign tumors after partial nephrectomy. Studies without available data (such as comments and letters) were excluded. We manually reviewed the references included in each study to identify other relevant studies.
The quality of all included studies was evaluated using the Newcastle–Ottawa Scale (NOS), in which a score of 1-9 stars was allocated. The more stars a study acquired, the higher quality it was.
The relevant data about oncological results was extracted from the included study, including local recurrence, metastasis, all-cause death, and cancer-specific death. Recurrence was defined as ipsilateral local renal or retroperitoneal recurrence according to imaging tests, including lymph nodes, resection bed and renal scar.
The meta-analysis was conducted using Review Manager Version 5.3 and Stata 14.2. Continuous variables were presented as weighted mean differences (WMDs) with 95% confidence intervals (CIs), and dichotomous variables were presented as odds ratios (ORs) with 95% CIs. If no significant heterogeneity between two groups was found, the fixed-effect model was used. Otherwise, the random-effect model was used. Furthermore, subgroup analyses were carried out to exclude the bias of different average follow-ups, which were divided into three subgroups: less than 3 years, between 3 and 6 years, and more than 6 years.
For studies reported as a median follow-up, it will be converted to an average according to the conversion formula (10). Heterogeneity among the studies was calculated using the χ2 test and I2 statistics. A p value < 0.10 and an I2 value >50% were considered to be significant. To evaluate publication bias, Egger’s test was performed.
A total of 452 publications were searched after eliminating repetition, and 47 studies passed title screening. After identifying the abstracts and full texts according to the inclusion criteria, 8 studies were excluded (1 study had no control group, and 7 studies had no available results). The PRISMA flow diagram was presented in Figure 1.
Finally, 39 CCSs (4, 6–8, 11–45), with a total of 21461 patients were included, from which 1278 patients were assigned to the PSM group and 20183 cases to the NSM group. In addition, these studies were carried out from 13 countries in Europe, North America, South America, and Asia. The methods of operation included open, laparoscopic and robotic-assisted PN. The characteristics and quality of the included studies are summarized in Table 1. There were 9 studies (6, 16, 24, 27, 31–34, 44) explaining the specific quantitative differences between the two groups in different tumor stages. After statistical analysis, it was found that there were significant differences in patients with different stages between the two groups (p < 0.001) (Table 2).
We extracted data on local recurrence in two groups from 33 studies (4, 6, 11–15, 17–23, 25–30, 32–38, 40–45) including 13473 patients (PSM: 785, NSM: 12688). In 8 studies (13, 14, 18–21, 36, 38), the OR value could not be calculated because the number of local recurrence was zero in both the PSM and NSM groups. The pooled results showed that the rate of local recurrence in the PSM group was significantly higher than that in the NSM group (OR 3.93, 95% CI 2.95-5.24; p < 0.00001). Egger’s test showed no published bias (P = 0.266). The results of subgroup analysis showed that the results of each subgroup were similar to the total results (Figure 2).
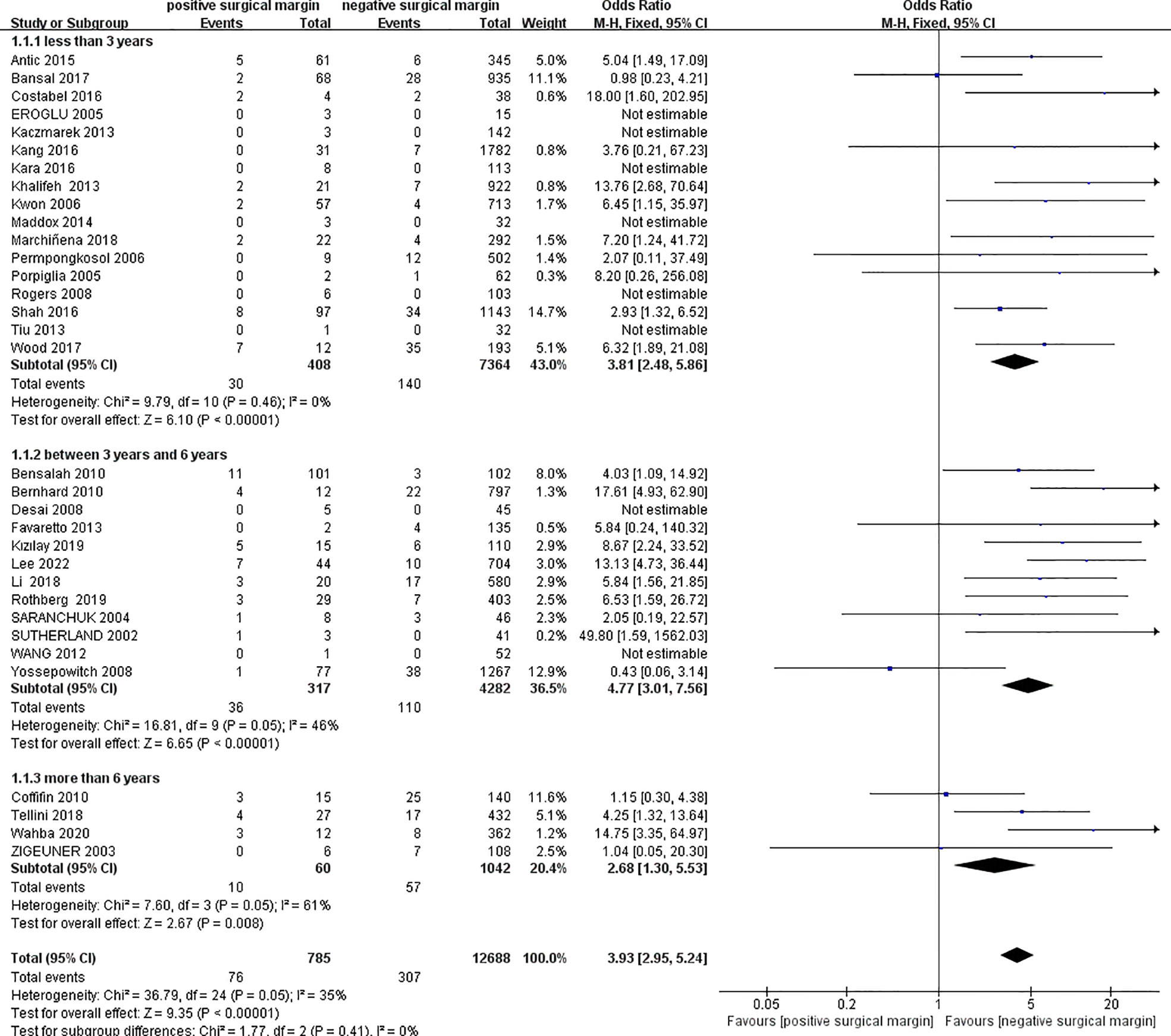
Figure 2 Comparison of local recurrence between positive surgical margin (PSM) and negative surgical margin (NSM) groups.
Twenty studies provided comparative data on tumor metastasis between the two groups (4, 6, 13–15, 17, 18, 20, 25, 27, 30, 33–36, 38, 40–43), including 434 patients in the PSM group and 8298 patients in the NSM group. In 4 studies (13, 14, 35, 36), the number of metastatic cases in the two groups was zero, and the OR value could not be calculated. Pooled data showed a significantly higher rate of metastasis in the PSM group (OR 4.63, 95% CI 3.11-6.88; p < 0.00001), and similar results could be observed in subgroup analyses (Figure 3). Egger’s test showed insignificant published bias (P = 0.145).
Twelve studies including 10155 patients reported data on all-cause death in two groups (4, 7, 8, 16–18, 24, 25, 31, 32, 39, 44), from which a significantly higher rate of all-cause death in the PSM group could be found than that in the NSM group (OR 1.51, 95% CI 1.19-1.93; p = 0.0008; Figure 4). However, subgroup analyses showed insignificant results between the two groups for those less than 3 years (OR 2.08, 95% CI 0.62-7.01; p = 0.24) and those more than 6 years (OR 1.26, 95% CI 0.73-2.17; p = 0.40) (Figure 4).
Maurice et al. (39) performed a data analysis based on the National Cancer Database, which accounted for too much weight (57.6%). Therefore, a sensitivity analysis excluding this study to avoid possible duplication in the population was performed to test the credibility of the results. The final results after excluding Maurice’s study showed that there were no significant differences in the rate of all-cause death between the two groups in the subgroup analyses and pooled analysis (OR 1.35, 95% CI 0.92-1.99; p = 0.13) (Figure 5). Egger’s test showed no significant published bias (P = 0.209).
Seven studies provided data on cancer-specific death in the two groups (8, 11, 18, 24, 27, 32, 41), which showed an insignificant difference in the incidence of cancer-specific death between the two groups (less than 3 years: OR 1.94, 95% CI 0.10-38.03; p = 0.66; between 3 years and 6 years: OR 0.86, 95% CI 0.30-2.50; p = 0.79; more than 6 years: OR 1.05, 95% CI 0.43-2.54; p = 0.92; and totally: OR 0.99, 95% CI 0.51-1.94; p = 0.99;, respectively) (Figure 6).
RCC is a common malignancy in the urinary system, and its incidence is increasing year by year (46). PN is an internationally recognized standard treatment for localized renal tumors according to the EAU and AUA guidelines (1, 47). Although it can prevent the loss of renal function to some extent compared to radical nephrectomy, there is also a risk of incomplete resection of tumors, leading to PSM. It has been confirmed that NSM is very important in reducing the risk of tumor recurrence in many cancers, such as prostate cancer, bladder cancer, breast cancer, colon cancer, and pancreatic cancer (48–50). However, the effect of PSM of RCC on oncological outcomes after PN is still controversial.
The results of this study showed that there was a significant correlation between PSM and local recurrence and metastasis after PN, which was consistent with the results from Ficarra et al., in which a systematic review including 14 studies was conducted (51). Our study included more literature and carried out subgroup analyses, which might provide more support for these results. Moreover, Khalifeh et al. found that only PSM was statistically significant in tumor recurrence and metastasis (15). In a study by Memorial-Sloan Kettering Cancer Center, it was reported that renal tumors with high malignant potential were more likely to relapse when they were PSM (33). When PSM patients were subdivided into a low-risk group and a high-risk group according to the pathological stage and grade, it was found that PSM accompanied by high-risk pathology could significantly increase the risk of 5-year recurrence (34). In addition, other studies suggested recurrence is usually associated with patients at risk of multifocal tumors, in which recurrence might be due to new primary tumors rather than PSM (31). We speculated that the follow-up of patients with PSM might be more rigorous, which would also affect their prognosis.
Our study demonstrated that the rate of PSM could be calculated to be 6.36%, which was similar to 2%-8% (5) in previous publications. Multiple risk factors might have contributed to PSM, including age of patients (52), tumor location (53), tumor size[26 (24),], tumor stage (53), tumor grade (32, 52), tumor infiltration into perirenal fat (53), preoperative renal function (24), surgery volume of surgeons (54), surgical approach (9), etc.
The debate on how to further manage patients with PSM after PN has not been eliminated. Several measures/interventions could be adopted, including radical nephrectomy, tumor bed reresection, marginal energy ablation and observation. However, our results showed that there was an insignificant relationship between the two groups in all-cause death and cancer-specific death, which demonstrated that vigilant and close monitoring might be an effective strategy in lieu of active intervention (55).
It should be carefully noted that the 5-year survival of localized RCC was up to 75% but less than 10% for metastatic RCC (56). In our study, PSM had a more than fourfold impact on the metastasis of RCC, but an impact on the survival of patients was not found. This might be related to insufficient follow-up duration. Although subgroup analyses were carried out, the follow-up of most studies was less than 5 years. In addition, according to the latest cancer report in 2021 (57), it was mentioned that there were some differences in the survival for patients with different pathological types of RCC in different populations, which was not separately analyzed in our study. In this study, it was found that there was a significant difference in tumor stage between the PSM group and the NSM group, although the data of this analysis only came from 9 studies. This still reminded us that tumor stage might also have an impact on the results of the study. Meanwhile, a few studies (8, 11, 12, 15, 18, 22, 25, 26, 30, 31, 36, 38) have mentioned the use of frozen section assessment (FSA) in surgery. However, Miyamoto’s (58) study suggested that the diagnostic accuracy of FSA in partial nephrectomy and its impact on the status of the incision margin had not been determined. They did not believe that FSA will benefit patients. In their opinion, there remains limitations in the FSA diagnosis on margin specimens, such as inadequate sampling and suboptimal tissue preparation due to histologic frozen artifact or cautery artifact, any of which may result in an indeterminate or inaccurate interpretation. Indeed, the diagnostic accuracy of FSA at the surgical margins is not necessarily high, while it is dependent on the pathologist’s knowledge and experience. This issue may require further RCT to determine the usefulness of FSA on surgical margins.
Above all, several potential limitations of this meta-analysis must be taken into consideration. Because all the included studies were retrospective CCSs, the quality of this study was limited. In addition, the heterogeneity among the studies could be easily affected by different urologists’ experience, surgical technique, patient selection, tumor size, stage, grade and location, and incompletely uniform follow-up, which could cause possible biases. Moreover, different methods and their combinations, including open, laparoscopy and robot-assisted laparoscopy, were used in these studies, which made it difficult to carry out subgroup analyses.
By comparing the effects of different surgical margin states on the development of RCC after PN, we found that PSM was closely related to postoperative tumor recurrence and metastasis. However, there was no significant difference in all-cause death or cancer-specific death between the PSM and NSM groups. Therefore, for patients with PSM after PN, close monitoring might be another effective choice apart from active treatments. Considering some limitations in our study, we strongly suggest that our results be carefully cautioned and further validated.
RB, LG, JW and QJ conceived and designed the study. RB and LG performed the analysis, prepared the figures and tables, and wrote the main manuscript. All of the authors reviewed the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European Association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol. (2019) 75(5):799–810. doi: 10.1016/j.eururo.2019.02.011
2. Crépel M, Jeldres C, Sun M, Lughezzani G, Isbarn H, Alasker A, et al. A population-based comparison of cancer-control rates between radical and partial nephrectomy for T1A renal cell carcinoma. Urology (2010) 76(4):883–8. doi: 10.1016/j.urology.2009.08.028
3. Deklaj T, Lifshitz DA, Shikanov SA, Katz MH, Zorn KC, Shalhav AL. Laparoscopic radical versus laparoscopic partial nephrectomy for clinical T1b N0 M0 renal tumors: Comparison of perioperative, pathological, and functional outcomes. J Endourol. (2010) 24(10):1603–7. doi: 10.1089/end.2009.0312
4. Kizilay F, Eskidemir U, Bahceci T, Simsir A, Ozdemir H, Sarsik B, et al. The effect of surgical margin on cancer-specific survival in patients treated with nephron-sparing surgery. Niger J Clin Pract (2019) 22(10):1396. doi: 10.4103/njcp.njcp_267_18
5. Choi JE, You JH, Kim DK, Rha KH, Lee SH. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: A systematic review and meta-analysis. Eur Urol. (2015) 67(5):891–901. doi: 10.1016/j.eururo.2014.12.028
6. Tellini R, Antonelli A, Tardanico R, Fisogni S, Veccia A, Furlan MC, et al. Positive surgical margins predict progression-free survival after nephron-sparing surgery for renal cell carcinoma: Results from a single center cohort of 459 cases with a minimum follow-up of 5 years. Clin Genitourin Canc. (2019) 17(1):e26–31. doi: 10.1016/j.clgc.2018.08.004
7. López-Costea MÁ, Bonet X, Pérez-Reggeti J, Etcheverry B, Vigués F. Oncological outcomes and prognostic factors after nephron-sparing surgery in renal cell carcinoma. Int UROL Nephrol. (2016) 48(5):681–6. doi: 10.1007/s11255-016-1217-z
8. Raz O, Mendlovic S, Shilo Y, Leibovici D, Sandbank J, Lindner A, et al. Positive surgical margins with renal cell carcinoma have a limited influence on long-term oncological outcomes of nephron sparing surgery. Urology. (2010) 75(2):277–80. doi: 10.1016/j.urology.2009.06.110
9. Tabayoyong W, Abouassaly R, Kiechle JE, Cherullo EE, Meropol NJ, Shah ND, et al. Variation in surgical margin status by surgical approach among patients undergoing partial nephrectomy for small renal masses. J Urol (2015) 194(6):1548–53. doi: 10.1016/j.juro.2015.06.076
10. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5(1):5–13. doi: 10.1186/1471-2288-5-13
11. Favaretto RL, Sanchez-Salas R, Benoist N, Ercolani M, Forgues A, Galiano M, et al. Oncologic outcomes after laparoscopic partial nephrectomy: Mid-term results. J Endourol. (2013) 27(1):52–7. doi: 10.1089/end.2012.0132
12. Costabel JI, Marchiñena PG, Tirapegui F, Dantur A, Jurado A, Gueglio G. Functional and oncologic outcomes after nephron-sparing surgery in a solitary kidney: 10 years of experience. Int Braz J Urol. (2016) 42(2):253–61. doi: 10.1590/S1677-5538.IBJU.2014.0463
13. Wang J, Qi L, Zu X, Chen M. Application of retroperitoneal laparoscopic partial nephrectomy for renal cell carcinoma of the early stage. Zhong Nan Da Xue Xue Bao. Yi Xue Ban = J Of Cent South University. Med Sci (2012) 37(5):485. doi: 10.3969/j.issn.1672-7347.2012.05.010
14. Kara O, Maurice MJ, Malkoc E, Ramirez D, Nelson RJ, Caputo PA, et al. Comparison of robot-assisted and open partial nephrectomy for completely endophytic renal tumours: a single centre experience. Bju Int (2016) 118(6):946–51. doi: 10.1111/bju.13572
15. Khalifeh A, Kaouk JH, Bhayani S, Rogers C, Stifelman M, Tanagho YS, et al. Positive surgical margins in robot-assisted partial nephrectomy: A multi-institutional analysis of oncologic outcomes (Leave no tumor behind). J Urol (2013) 190(5):1674–9. doi: 10.1016/j.juro.2013.05.110
16. Rothberg MB, Paulucci DJ, Okhawere KE, Reynolds CR, Badani KK, Abaza R, et al. A multi-institutional analysis of the effect of positive surgical margins following robot-assisted partial nephrectomy on oncologic outcomes. J ENDOUROL. (2020) 34(3):304–11. doi: 10.1089/end.2019.0506
17. Rothberg MB, Peak TC, Reynolds CR, Hemal AK. Long-term oncologic outcomes of positive surgical margins following robot-assisted partial nephrectomy. Trans Androl Urol (2020) 9(2):879–86. doi: 10.21037/tau.2019.11.21
18. Tiu A, Shin TY, Kim KH, Lim SK, Han WK, Rha KH. Robotic laparoendoscopic single-site transumbilical partial nephrectomy: Functional and oncologic outcomes at 2 years. Urology. (2013) 82(3):595–9. doi: 10.1016/j.urology.2013.05.010
19. Rogers CG, Menon M, Weise ES, Gettman MT, Frank I, Shephard DL, et al. Robotic partial nephrectomy: a multi-institutional analysis. J Robotic Surg (2008) 2(3):141–3. doi: 10.1007/s11701-008-0098-2
20. Maddox M, Mandava S, Liu J, Boonjindasup A, Lee BR. Robotic partial nephrectomy for clinical stage T1b tumors: Intermediate oncologic and functional outcomes. Clin Genitourin Canc. (2015) 13(1):94–9. doi: 10.1016/j.clgc.2014.07.011
21. Kaczmarek BF, Sukumar S, Kumar RK, Desa N, Jost K, Diaz M, et al. Comparison of robotic and laparoscopic ultrasound probes for robotic partial nephrectomy. J Endourol. (2013) 27(9):1137–40. doi: 10.1089/end.2012.0528
22. Porpiglia F, Fiori C, Terrone C, Bollito E, Fontana D, Scarpa Rm. Assessment of surgical margins in renal cell carcinoma after nephron sparing: A comparative study. J Urol (2005) 173(4):1098–101. doi: 10.1097/01.ju.0000148360.47191.5e
23. Bernhard J, Pantuck AJ, Wallerand H, Crepel M, Ferrière J, Bellec L, et al. Predictive factors for ipsilateral recurrence after nephron-sparing surgery in renal cell carcinoma. Eur Urol. (2010) 57(6):1080–6. doi: 10.1016/j.eururo.2010.02.019
24. Ani I, Finelli A, Alibhai SMH, Timilshina N, Fleshner N, Abouassaly R. Prevalence and impact on survival of positive surgical margins in partial nephrectomy for renal cell carcinoma: a population-based study. Bju Int (2013) 111(8):E300–5. doi: 10.1111/j.1464-410X.2012.11675.x
25. Permpongkosol S, Colombo JR, Gill IS, Kavoussi LR. Positive surgical parenchymal margin after laparoscopic partial nephrectomy for renal cell carcinoma: Oncological outcomes. J Urol (2006) 176(6):2401–4. doi: 10.1016/j.juro.2006.08.008
26. Yossepowitch O, Thompson RH, Leibovich BC, Eggener SE, Pettus JA, Kwon ED, et al. Positive surgical margins at partial nephrectomy: Predictors and oncological outcomes. J Urol (2008) 179(6):2158–63. doi: 10.1016/j.juro.2008.01.100
27. Bansal RK, Tanguay S, Finelli A, Rendon R, Moore RB, Breau RH, et al. Positive surgical margins during partial nephrectomy for renal cell carcinoma: Results from Canadian kidney cancer information system (Ckcis) collaborative. Can Urolog Assoc J (2017) 11(6):182–7. doi: 10.5489/cuaj.4264
28. Wood EL, Adibi M, Qiao W, Brandt J, Zhang M, Tamboli P, et al. Local tumor bed recurrence following partial nephrectomy in patients with small renal masses. J Urol (2018) 199(2):393–400. doi: 10.1016/j.juro.2017.09.072
29. Antic T. Taxy JB. partial nephrectomy for renal tumors. Am J Clin Pathol (2015) 143(5):645–51. doi: 10.1309/AJCP7LKLZ8JSJQRG
30. Kang HW, Lee SK, Kim WT, Yun SJ, Lee S, Kim W, et al. Surgical margin does not influence recurrence rate in Pt1 clear cell renal cell carcinoma after partial nephrectomy: A multicenter study. J Surg Oncol (2016) 114(1):70–4. doi: 10.1002/jso.24259
31. Petros FG, Metcalfe MJ, Yu K, Keskin SK, Fellman BM, Chang CM, et al. Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: a 25-year single institution experience. World J Urol. (2018) 36(7):1093–101. doi: 10.1007/s00345-018-2241-7
32. Bensalah K, Pantuck AJ, Rioux-Leclercq N, Thuret R, Montorsi F, Karakiewicz PI, et al. Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol. (2010) 57(3):466–73. doi: 10.1016/j.eururo.2009.03.048
33. Kwon EO, Carver BS, Snyder ME, Russo P. Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. Bju Int (2007) 99(2):286–9. doi: 10.1111/j.1464-410X.2006.06623.x
34. Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA, et al. Positive surgical margins increase risk of recurrence after partial nephrectomy for high risk renal tumors. J Urol (2016) 196(2):327–34. doi: 10.1016/j.juro.2016.02.075
35. Li G, Zhu D, Lang Z, Wang A, Li Y, Zhang R, et al. Classification of positive surgical margins and tumor recurrence after nephron-sparing surgery for small renal masses. (2018) 10:6591–8. doi: 10.2147/CMAR.S181843
36. Eroğlu M, Ünsal A, Bakirtaş H, Tekdoğan ÜT, Ataoğlu Ö, Balbay MD. Routine frozen-section biopsy from the surgical bed should be performed during nephron-sparing surgery for renal cell carcinoma. Scandinavian J Urol Nephrol (2009) 39(3):222–5. doi: 10.1080/00365590510007757
37. Coffin G, Hupertan V, Taksin L, Vaessen C, Chartier-Kastler E, Bitker M, et al. Impact of elective versus imperative indications on oncologic outcomes after open nephron-sparing surgery for the treatment of sporadic renal cell carcinomas. Ann Surg Oncol (2011) 18(4):1151–7. doi: 10.1245/s10434-010-1457-6
38. Desai PJ, Andrews PE, Ferrigni RG, Castle EP. Laparoscopic partial nephrectomy at the Mayo clinic Arizona: Follow-up surveillance of positive margin disease. Urology. (2008) 71(2):283–6. doi: 10.1016/j.urology.2007.08.050
39. Maurice MJ, Zhu H, Kim SP, Abouassaly R. Reexamining the association between positive surgical margins and survival after partial nephrectomy in a Large American cohort. J Endourol. (2016) 30(6):698–703. doi: 10.1089/end.2016.0031
40. Se S, Mi R, Gt M, Hb G. Does the size of the surgical margin in partial nephrectomy for renal cell cancer really matter? J Urol (2002) 167(1):61–4. doi: 10.1016/S0022-5347(05)65383-9
41. Saranchuk JW, Touijer AK, Hakimian P, Snyder ME, Russo P. Partial nephrectomy for patients with a solitary kidney: The memorial Sloan-Kettering experience. Bju Int (2004) 94(9):1323–8. doi: 10.1111/j.1464-410X.2004.05165.x
42. Zigeuner R, Quehenberger F, Pummer K, Petritsch P, Hubmer G. Long-term results of nephron-sparing surgery for renal cell carcinoma in 114 patients: Risk factors for progressive disease. Bju Int (2003) 92(6):567–71. doi: 10.1046/j.1464-410X.2003.04414.x
43. Marchiñena PG, Tirapegui S, Gonzalez IT, Jurado A, Gueglio G. Positive surgical margins are predictors of local recurrence in conservative kidney surgery for Pt1 tumors. Int Braz J Urol. (2018) 44(3):475–82. doi: 10.1590/s1677-5538.ibju.2017.0039
44. Wahba BM, Chow AK, Du K, Sands KG, Paradis AG, Vetter JM, et al. Positive surgical margins after robot-assisted partial nephrectomy predict long-term oncologic outcomes for clinically localized renal masses. J Endourol (2021). doi: 10.1089/end.2020.0707
45. Lee J, Kim J, Kim JC, Ham WS, Han WK, Rha KH, et al. Evaluation of the surgical margin threshold for avoiding recurrence after partial nephrectomy in patients with renal cell carcinoma. Yonsei Med J (2022) 63(2):173–8. doi: 10.3349/ymj.2022.63.2.173
46. King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United states 2001 to 2010. J Urol (2014) 191(6):1665–70. doi: 10.1016/j.juro.2013.12.046
47. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol (2017) 198(3):520–9. doi: 10.1016/j.juro.2017.04.100
48. Eastham Ja, Kattan Mw, Riedel E, Begg Cb, Wheeler Tm, Gerigk C, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol (2003) 170(6):2292–5. doi: 10.1097/01.ju.0000091100.83725.51
49. Tseng JF, Pisters PWT, Lee JE, Wang H, Gomez HF, Sun CC, et al. The learning curve in pancreatic surgery. Surgery. (2007) 141(4):456–63. doi: 10.1016/j.surg.2006.09.013
50. Swallow CJ, Catton CN. Local management of adult soft tissue sarcomas. Semin Oncol (2007) 34(3):256–69. doi: 10.1053/j.seminoncol.2007.03.008
51. Ficarra V, Crestani A, Inferrera A, Novara G, Rossanese M, Subba E, et al. Positive surgical margins after partial nephrectomy: A systematic review and meta-analysis of comparative studies. Kidney Cancer. (2018) 2(2):133–45. doi: 10.3233/KCA-180037
52. Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: Possible role and prognostic implications in cancer patients. Eur J CANCER. (1997) 33(6):873–7. doi: 10.1016/S0959-8049(97)00002-6
53. Schiavina R, Serni S, Mari A, Antonelli A, Bertolo R, Bianchi G, et al. A prospective, multicenter evaluation of predictive factors for positive surgical margins after nephron-sparing surgery for renal cell carcinoma: The RECORd1 Italian project. Clin Genitourin Canc. (2015) 13(2):165–70. doi: 10.1016/j.clgc.2014.08.008
54. Malkoc E, Maurice MJ, Kara O, Ramirez D, Nelson RJ, Dagenais J, et al. Predictors of positive surgical margins in patients undergoing partial nephrectomy: A Large single-center experience. Türk Üroloji Dergisi/Turkish J Urol (2019) 45(1):17–21. doi: 10.5152/tud.2018.57767
55. Borghesi M, Brunocilla E, Schiavina R, Martorana G. Positive surgical margins after nephron-sparing surgery for renal cell carcinoma: Incidence, clinical impact, and management. Clin Genitourin Canc. (2013) 11(1):5–9. doi: 10.1016/j.clgc.2012.09.010
56. Zhang H, Zhu G. Predictive biomarkers and updated targets of current guidance in treatment of metastatic renal cell carcinoma. Curr Med Chem (2020) 28:31–8. doi: 10.1097/CAD.0000000000000931
57. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
Keywords: partial nephrectomy, positive surgical margin, prognosis, renal cell carcinoma, survival
Citation: Bai R, Gao L, Wang J and Jiang Q (2022) Positive surgical margins may not affect the survival of patients with renal cell carcinoma after partial nephrectomy: A meta-analysis based on 39 studies. Front. Oncol. 12:945166. doi: 10.3389/fonc.2022.945166
Received: 16 May 2022; Accepted: 22 July 2022;
Published: 10 August 2022.
Edited by:
Adam R. Metwalli, Howard University Hospital, United StatesReviewed by:
Nikhil Gopal, National Cancer Institute (NIH), United StatesCopyright © 2022 Bai, Gao, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Jiang, amlhbmdxaW5nMDY2QDE2My5jb20=
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.