- 1Department of Surgery, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, South Korea
- 2Division of Breast Surgery, Department of General Surgery, Changi General Hospital, Singapore, Singapore
- 3Singhealth Duke-NUS Breast Centre, Singapore Health Services Pte Ltd, Singapore, Singapore
- 4Department of Plastic and Reconstructive Surgery, School of Medicine, Kyungpook National University, Daegu, South Korea
Aim: While many studies reported the oncological outcomes of oncoplastic breast-conserving surgery (OBCS), there were inherent differences in the study population, surgeons’ expertise, and classifications of techniques used. There were also limited studies with long term follow up oncological outcomes beyond 5 years. This current study aimed to compare long-term oncological outcomes of ipsilateral breast tumor recurrence (IBTR) disease-free survival (DFS) and overall survival (OS) following conventional and oncoplastic breast-conserving surgery using volume displacement and replacement techniques.
Methods: Between 2009 and 2013, 539 consecutive patients who underwent breast conservation surgery including 174 oncoplastic and 376 conventional procedures were analysed. A systematic review of studies with at least five years of median follow up were performed to compare long term oncological outcomes.
Results: At a median follow-up of 82.4 months, there were 23 (4.2%) locoregional recurrences, 17 (3.2%) metachronous contralateral breast cancer, 26 (4.8%) distant metastases, and 13 (2.4%) deaths. The hazard ratio of OBCS for IBTR, DFS and OS were 0.78 (95% confidence interval [CI] 0.21–2.94, p=0.78), 1.59 (95% CI, 0.88 to 2.87, p=0.12), and 2.1 (95% CI, 0.72 to 5.9, p=0.17) respectively. The 10-year IBTR-free, DFS and OS rate were 97.8%, 86.2%, and 95.7% respectively.
Conclusion: There remained a dearth in well-balanced comparative studies with sufficient long-term follow-up, and our study reported long-term oncological outcomes for OBCS which were favourable of either VD or replacement techniques.
Introduction
Historical data have shown that breast-conserving surgery followed by radiotherapy has equivalent oncological outcomes to those of mastectomy in early breast cancer (1, 2). As long-term survival after breast cancer treatment has become commonplace, more attention has been given to develop oncoplastic techniques to provide better patient and aesthetic satisfaction (3, 4). The primary role of oncoplastic breast-conserving surgery (OBCS) is to achieve oncological safety while minimizing the risk of unacceptable local deformity by allowing reconstruction of the defect and preventing the need for mastectomy (5, 6).
Following the inception of tumor-specific immediate breast reconstruction more than 20 years ago, Werner Audretsch coined the term oncoplastic surgery, and many international experts contributed to the burgeoning field of OBCS (5, 7–11). Despite the similarity in rationale behind various oncoplastic techniques, there remained differences across geographical locations in terms of surgeons’ perspectives and practices in defining OBCS (12–14). Clough described a classification based on tumor volume, location, and glandular density, while Hoffmann and Wallweiner divided breast cancer surgery into two broad types with six tiers, each of increasing complexity (13, 14). A notable consensus definition came from the American Society of Breast Surgeons, which stated that OBCS incorporated oncologic partial mastectomy with ipsilateral defect repair using volume displacement (VD) and volume replacement (VR) techniques, with contralateral symmetry surgery as appropriate (11). For small-to-moderate breast volumes, however, there was also a difference in technical considerations compared with those for larger breast volumes, which require significantly more VR techniques (6, 15–17).
Korea had been an early adopter of oncoplastic surgery but long-term follow up data remained limited. As with any surgical procedures, long-term follow up was necessary to establish safety parameters of surgical techniques. Furthermore, locoregional recurrences after breast conservation surgery could occur later than mastectomy, perhaps due to the differences in biology or presentations that led to a decision for mastectomy (18). Having previously examined the short-term oncological safety and patient-reported outcomes of various OBCS techniques (17–20), this current study aimed to compare long-term oncological safety following conventional BCS (CBCS) and OBCS, focusing on overall survival (OS), ipsilateral breast tumor recurrence (IBTR) rates, and disease-free survival (DFS). We also report the rate of positive margins (PMR) detected in intraoperative frozen sections and eventual rate of conversion to mastectomy (CMR) following BCS during a 10-year follow-up period. A literature review was performed to discuss the available data on long-term oncological outcomes with at least five years median follow up duration reported to date and how our results compared with those of other centers.
Methods
We analyzed prospectively collected data from 539 consecutive breast cancer patients at Kyungpook National University Chilgok Hospital who underwent breast conservation surgery performed by four breast surgeons between January 2009 and December 2013. Treatment strategy was coordinated at multidisciplinary board discussions, which included breast surgeons, plastic surgeons, radiologists, pathologists, and medical and radiation oncologists. All breast conservation surgeries were performed by the breast surgeons, with oncoplastic techniques performed by either a breast or plastic surgeon.
A literature review was performed to summarise suitable studies for comparison of definitions and reported oncological outcomes (Tables 6, 7) (21–40). A search was conducted through the MEDLINE database using PubMed in March 2022. Our search terms included ‘oncoplastic’ [All Fields] AND (‘breast’ [MeSH Terms] OR ‘breast’ [All Fields]) AND (‘surgery’ [Subheading] OR ‘surgery’ [All Fields] OR ‘surgical procedures, operative’ [MeSH Terms]) AND (‘oncological’ [All Fields] OR ‘outcomes’ [All Fields]). A manual search of bibliographies of relevant articles was performed.
We included single center studies reporting on various oncoplastic breast conserving surgery to ensure consistency in the reported surgical procedures. Studies with cohort size less than 50 were deemed too small; similarly, a follow up period less than 60 months inadequate to capture late recurrences and death events and hence excluded. Case series or cohort studies reporting on particular surgical techniques were also excluded as they were not generalisable to all oncoplastic breast conserving surgery. A PRISMA flowchart is available as supplementary material.
Definition of conventional and oncoplastic BCS techniques
CBCS involved a direct skin incision, including use of a parallelogram incision overlying the index tumor to allow direct parenchymal closure. Following excision of primary breast tumors with gross margins, a frozen section of the circumferential margins was processed. The defect was closed primarily without further mobilization. When tumor cells were detected on the frozen section, more extensive resection was performed until negative frozen section results were achieved or no further surgical margins were deemed necessary. A final paraffin block of the surgical margins was examined by pathologists for the presence of tumor cells, and the presence of no stained tumor cell was defined as a negative resection margin.
OBCS was performed as described previously in detail based on general principles of oncoplastic breast surgery in small-to-moderate-sized breasts (15–17).
The procedures were divided into VD and replacement techniques. VD techniques included dual-plane glandular flap mobilization-closure, purse string suture closure of central defect, roundblock mastopexy, tennis racket incision, batwing mastopexy, rotating flap, and reduction mammoplasty (Table 1) (15).
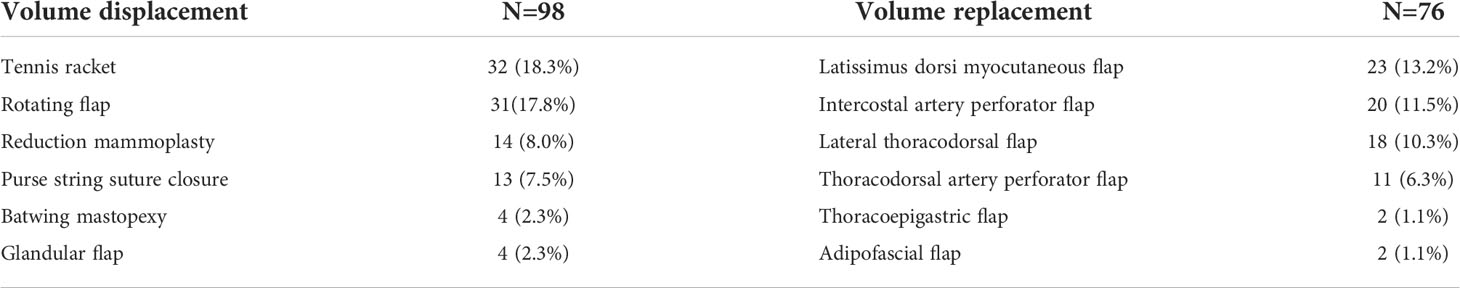
Table 1 Oncoplastic procedures divided into volume displacement and volume replacement techniques (N=174).
In cases of anticipated significant breast volume loss, VR techniques were individualized according to the excised breast volume and tumor location with planned use of either adipofascial flap, lateral thoracodorsal flap (LTD), intercostal artery perforators (ICAP), thoracodorsal artery perforator (TDAP), thoracoepigastric (TE), or latissimus dorsi (LD). LD myocutaneous flaps were preferred for excised specimen >150 g (16, 17).
Patient and tumor characteristics
Patient demographics, surgical details, clinicopathological characteristics, including clinical tumor size, specimen weight, tumor type, pathological tumor size, pathological tumor, nodal stage, receptor status, grade, presence of neoadjuvant and adjuvant therapy, metachronous contralateral breast cancer, locoregional and distant disease recurrences, and death were recorded (Table 2).
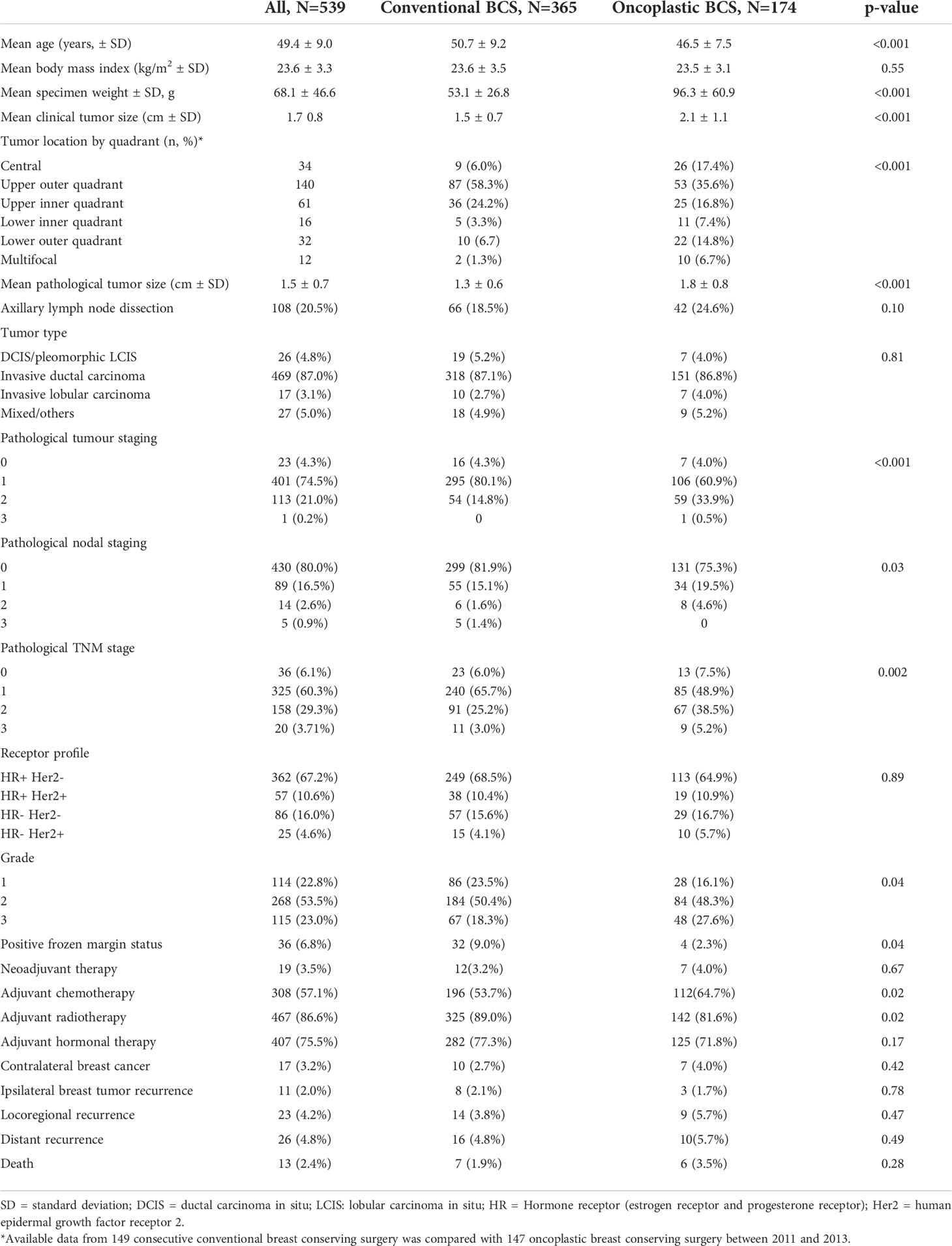
Table 2 Baseline characteristics of patients who underwent conventional and oncoplastic breast-conserving surgery (BCS).
Follow-up
Patients were followed up after surgery using a standardized protocol. After completing adjuvant treatments, frequency of follow-up was biannually for the first 2 years and annually for 5 to 10 years. Locoregional recurrence or distant metastasis was evaluated with clinical examination, blood tests including tumor markers, mammography, breast ultrasonography, with or without magnetic resonance imaging, bone scans, and positron emission tomography/computed tomography.
Oncological outcomes
The oncological outcomes assessed include OS, DFS with disease events defined as local or regional recurrences, distant recurrences, and metachronous contralateral breast cancer.
Statistical analysis
Statistical analysis was performed in March 2022 using Stata software, v17.0 (StataCorp); a statistically significant difference was concluded when p<0.05. Categorical variables were analyzed using the chi-square or Fisher exact test. Continuous variables were analyzed using the Student’s t-test. The Kaplan–Meier method was used to estimate survival function, and the log-rank test was used to compare survival functions. The univariate Cox proportional hazard regression model was also used to examine the correlation of clinically relevant covariates that were likely to affect oncological outcomes. These included patient age, tumor grade, hormonal profile, pathological tumor stage, nodal disease, and adjuvant therapy received. A multivariate analysis was performed with variables with significant p-values in the univariate model.
The study was approved by the Institutional Review Board of Kyungpook National University (2015-05-205) and conducted in compliance with the principles of the Declaration of Helsinki.
Results
Of the 539 patients who were analyzed, 365 (67.7%) patients underwent CBCS while 174 (32.3%) underwent OBCS. Of the 174 cases of OBCS, VR techniques were utilized in 98 (56.3%) cases, while VD techniques were utilized in 76 (43.7%) cases. Table 1 shows the breakdown of oncoplastic procedures in descending order. The most commonly employed techniques among the oncoplastic procedures were tennis racket incision (32), rotating flap (31), and LD myocutaneous flap (23). Five patients who defaulted further clinical visits or transferred care to other hospitals were considered lost to follow up.
Patient characteristics
Patients who underwent CBCS were older (50.7 vs. 46.5 years old), had smaller clinical tumor size (1.5 cm vs. 2.1cm), smaller specimen weight (53.1 g vs. 96.3 g), and smaller pathological tumor size (1.3 cm vs. 1.8 cm) compared to those who underwent OBCS. In terms of tumor characteristics, patients who underwent CBCS had earlier pathological T and N stage compared to those who underwent OBCS, while there was no statistically significant difference in histology subtype, grade, or hormone profile (Table 2) among the two groups.
Tumor location
OBCS was performed on a higher proportion of central (17.4%), lower outer quadrant (14.8%), lower inner quadrant (7.4%), and multifocal tumors (6.7%) than CBCS. The majority of all CBCS was performed on upper outer quadrant tumors (58.5%).
Intraoperatively detected involved margins on frozen section
The rate of intraoperatively detected involved margins on frozen section was higher in the CBCS than in the oncoplastic group, and further margins were excised intraoperatively. Three patients required completion mastectomy for close or involved final margins.
Disease recurrence, overall survival, and success of breast conservation surgery at 10 years
At a median follow-up of 82.4 months, (range, 1.4–156.7 months) there were 23 (4.2%) locoregional recurrences of which 11 had ipsilateral breast tumor recurrences, 17 (3.2%) metachronous contralateral breast cancer, 26 (4.8%) distant metastases, and 13 (2.4%) deaths. The hazard ratio of OBCS for IBTR, DFS and OS were 0.78 (95% confidence interval [CI] 0.21–2.94, p=0.78), 1.59 (95% CI, 0.88 to 2.87, p=0.12), and 2.1 (95% CI, 0.72 to 5.9, p=0.17) respectively. The 10-year IBTR-free, DFS and OS rate were 97.8%, 86.2%, and 95.7% respectively. Overall, five patients underwent mastectomy either from involved margins or disease recurrence, giving a successful BCS rate of 99.1%.
Statistical analysis of oncological outcomes
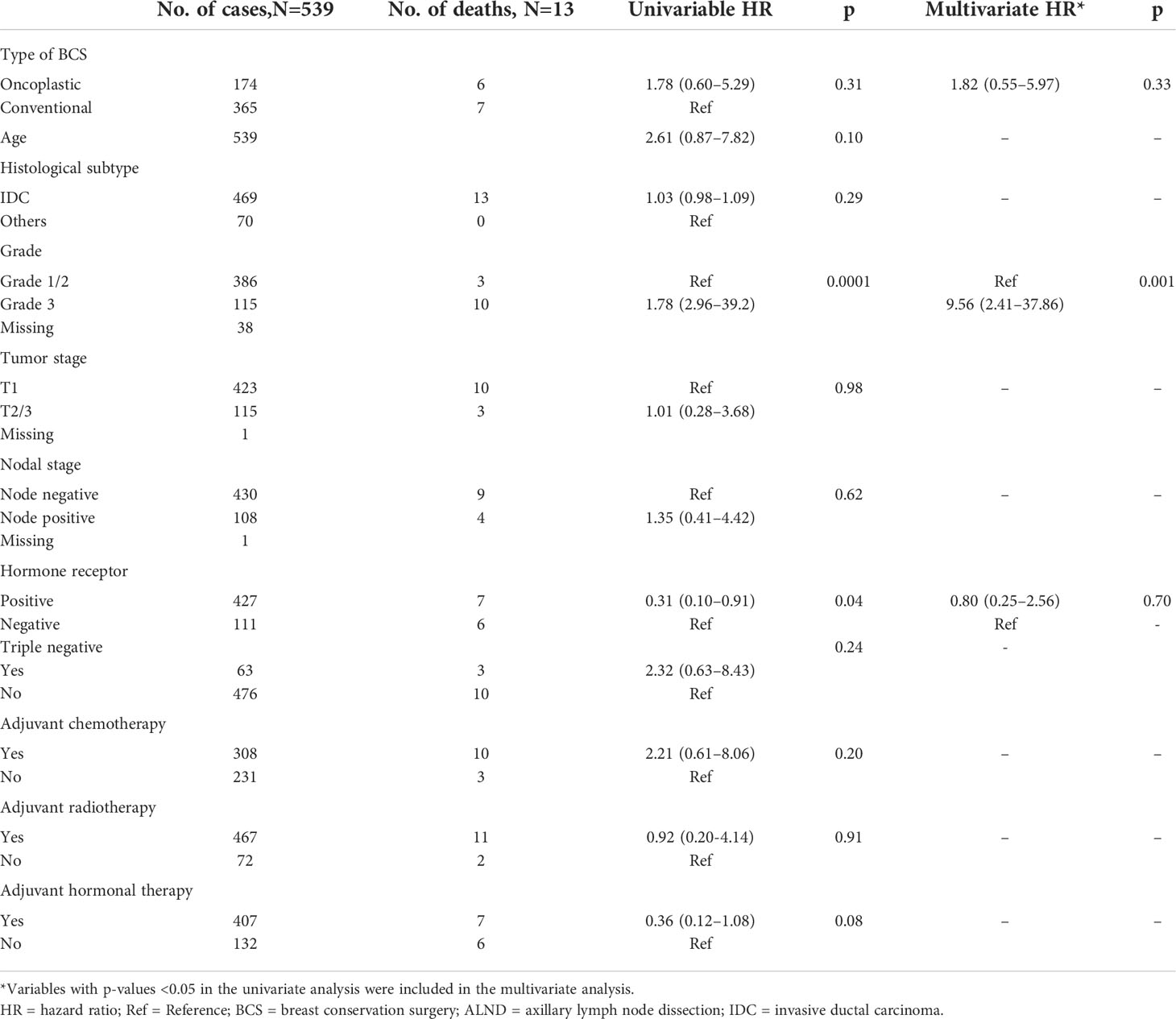
The use of oncoplastic surgery was not associated with a higher likelihood of IBTR or death in the Cox regression model analysis (Tables 3, 4, 5). Patients who underwent adjuvant chemotherapy had significantly lower IBTR rates, with a hazard ratio of 0.25 (95% CI, 0.07 to 0.98). Regarding OS, higher histological grade was significantly associated with higher risk of death, with a hazard ratio of 9.56 (95% CI, 2.41 to 37.86) (Tables 3 and 5). Univariate analysis was performed using the log-rank method stratified by tumor histological grade, pathological tumor staging, nodal disease, and hormone receptor profile. There was no difference in IBTR-free survival when performing OBCS after stratifying by high-grade tumors; larger tumors (T2/3); and node positive, hormone receptor-positive, or triple negative breast tumors (Figures 1, 2, 3).
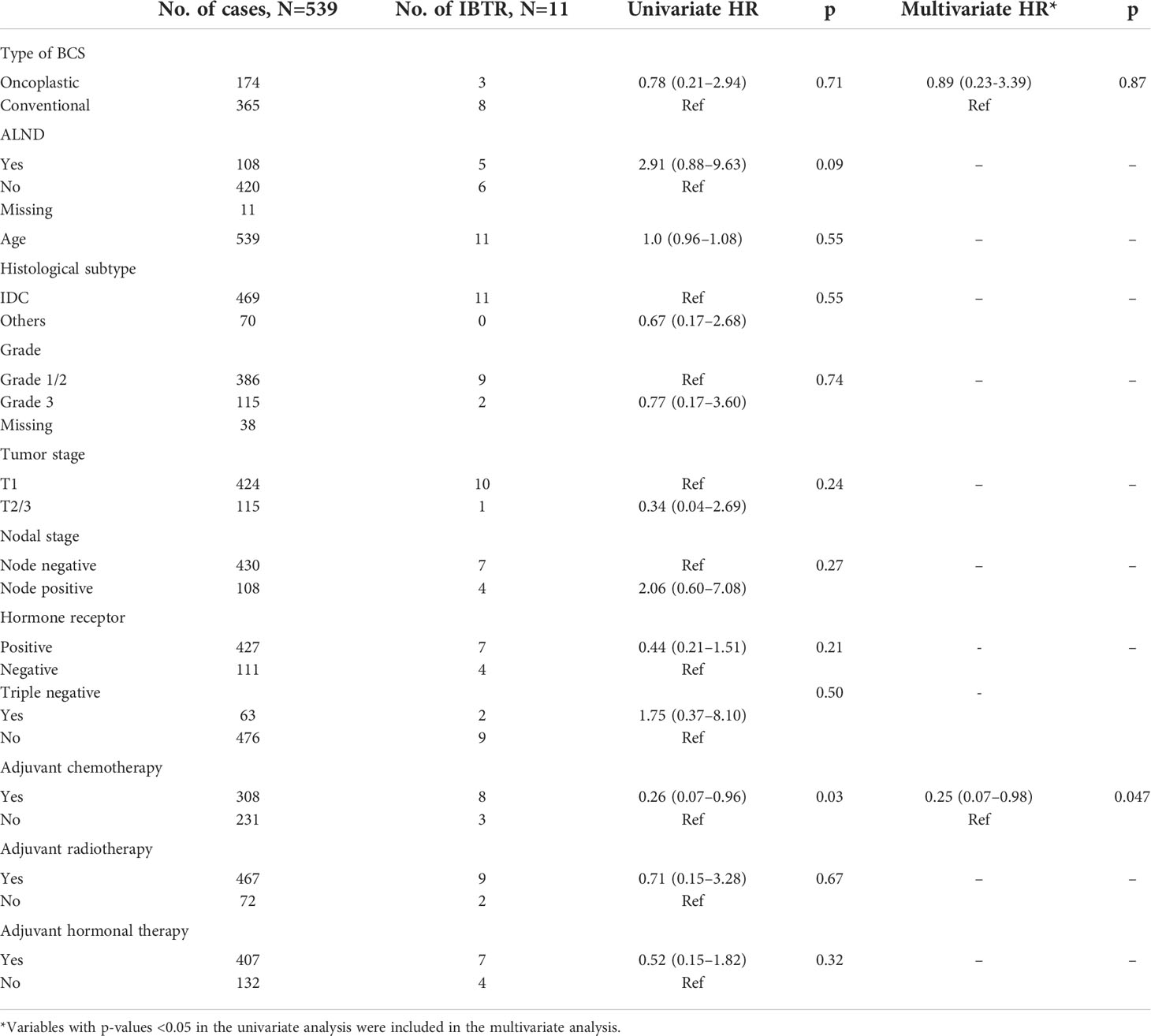
Table 3 Univariate and multivariate Cox regression analysis with ipsilateral breast tumor recurrence-free survival as an endpoint.
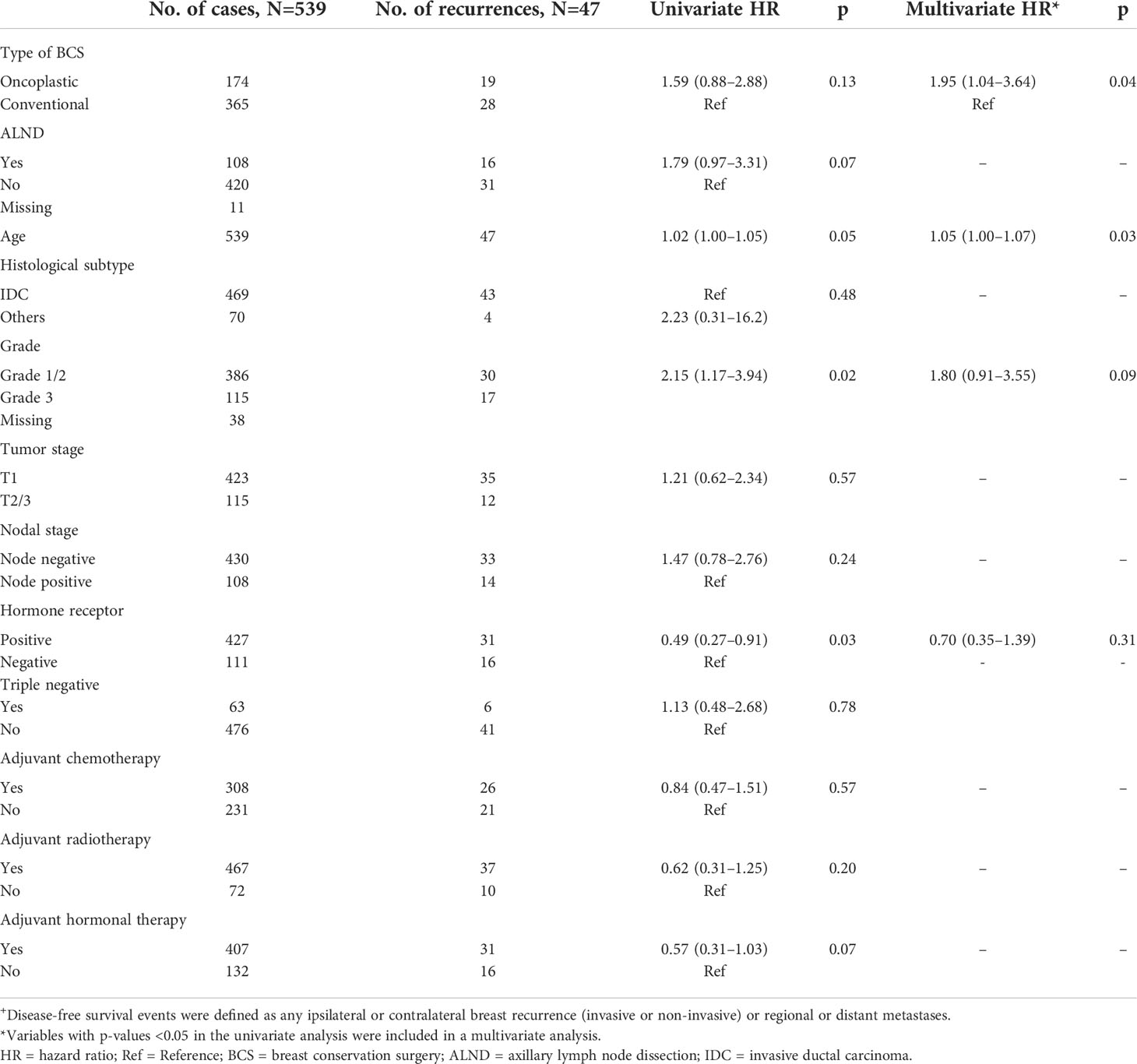
Table 4 Univariate and multivariate Cox regression analysis with disease-free survival+ as an endpoint.
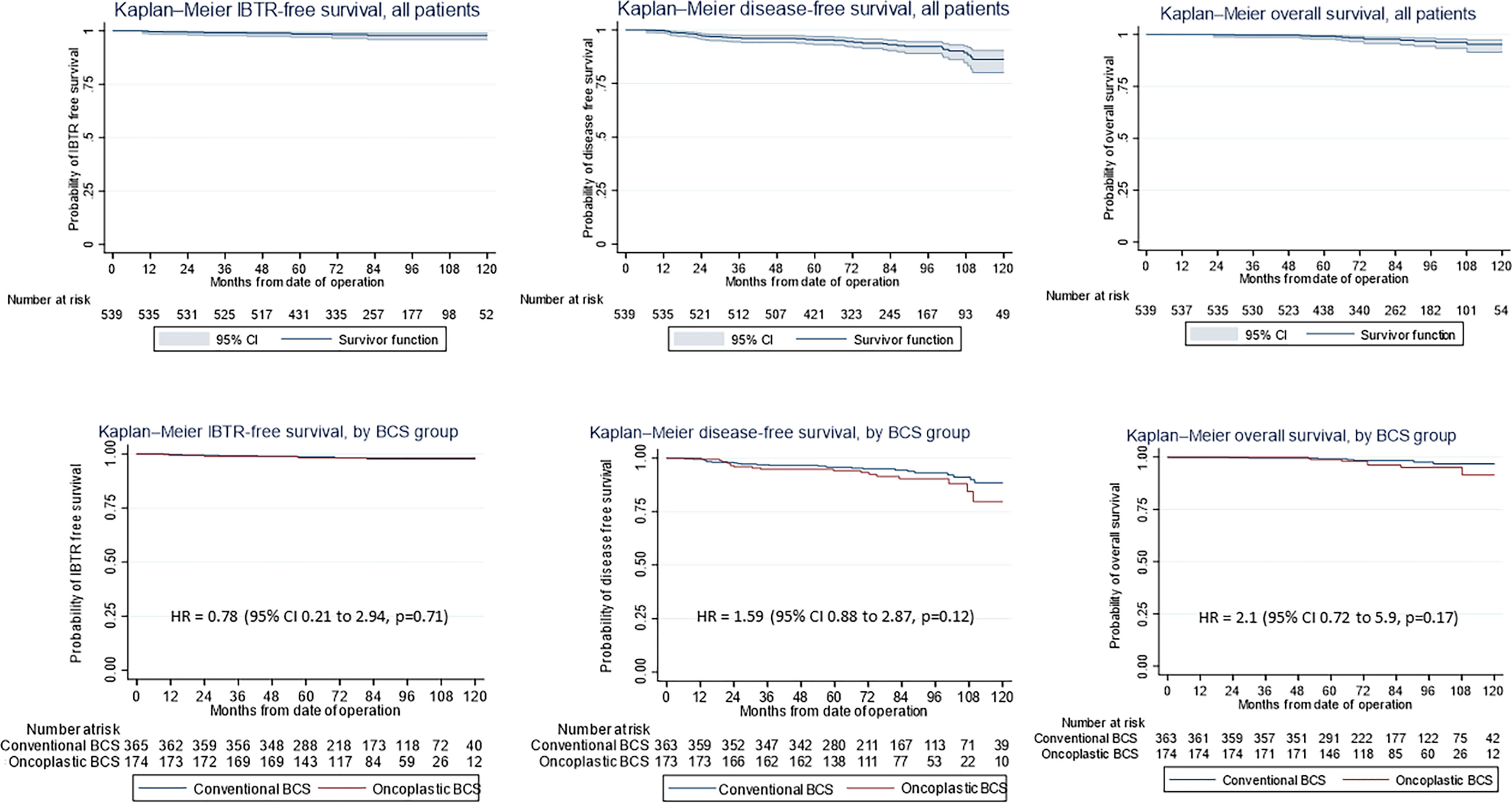
Figure 1 (First row) Kaplan–Meier estimates of (Left) ipsilateral breast tumor recurrence (IBTR)-free survival, (Middle) disease-free survival (DFS), and (Right) overall survival (OS) curves (shown with 95% confidence level) for all patients undergoing breast-conserving surgery (BCS) and (second row) by conventional (CBCS) versus oncoplastic breast-conserving surgery (OBCS) group.
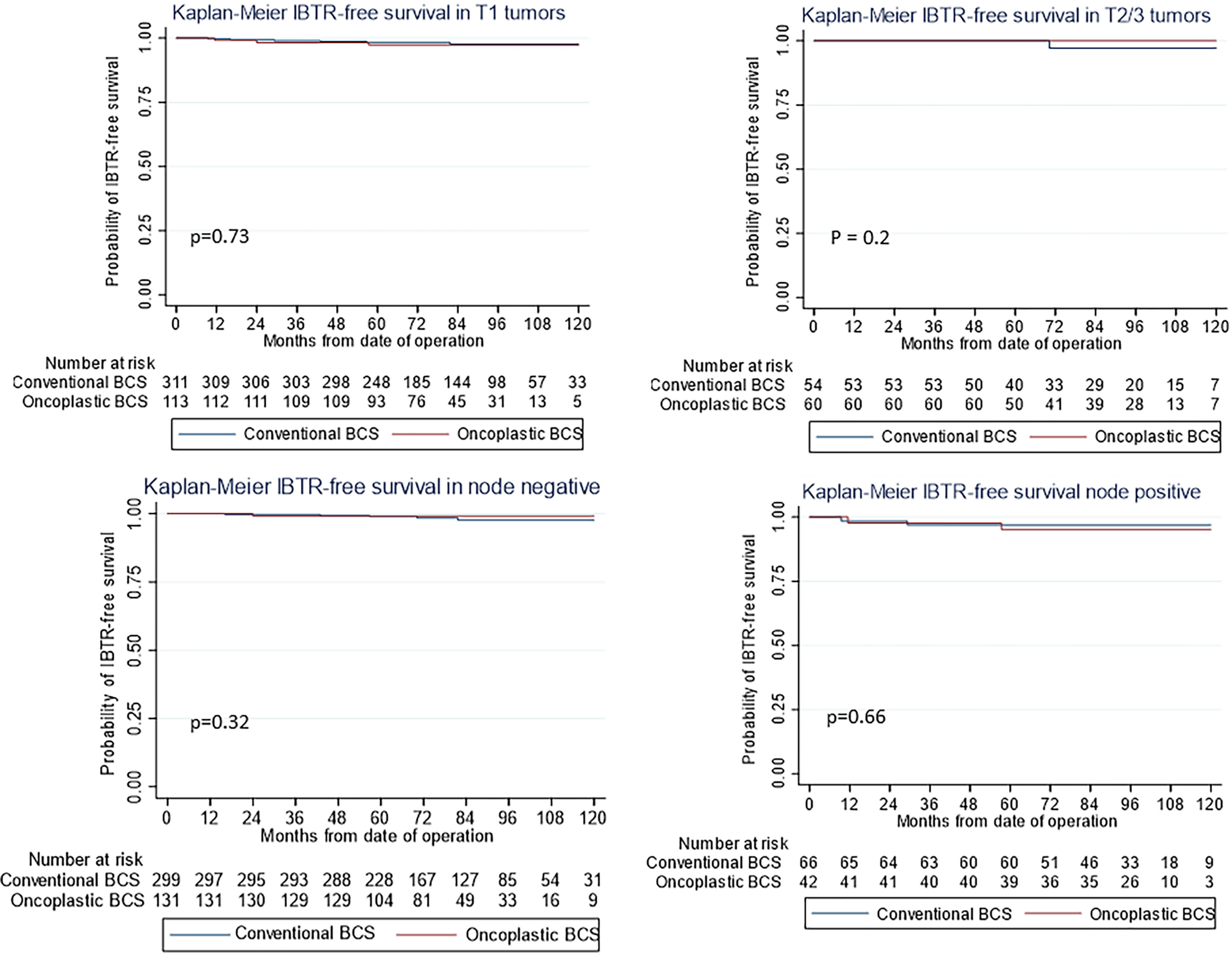
Figure 2 Kaplan–Meier estimates for ipsilateral breast tumor recurrence (IBTR)-free survival by (First Row) pathological tumor stage (first row) and (Second Row) nodal stage (second row) showing no difference between oncoplastic and conventional breast-conserving surgery (BCS).
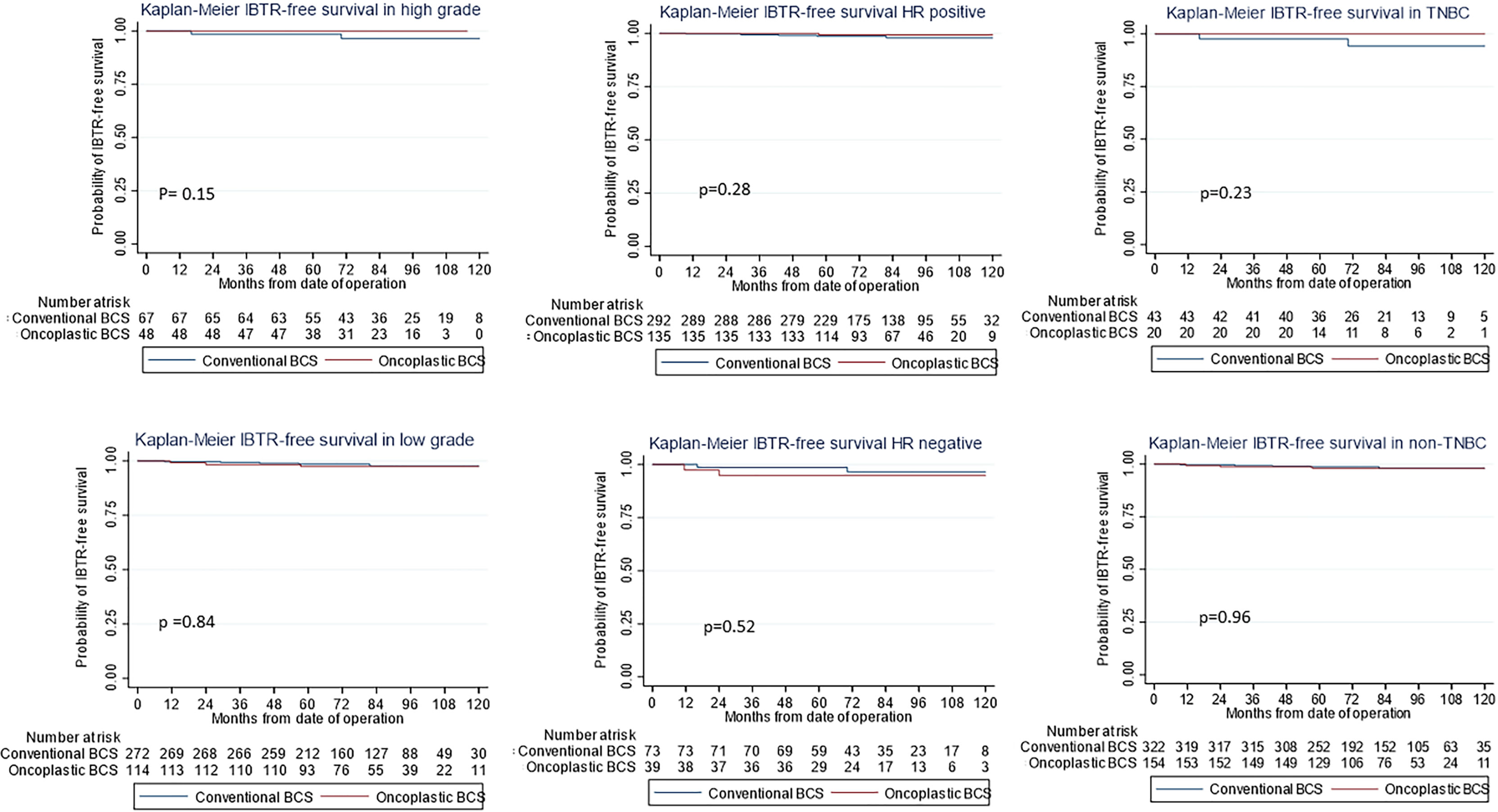
Figure 3 Kaplan–Meier estimates for ipsilateral breast tumor recurrence (IBTR)-free survival stratified by (Left) high-grade, (Middle) hormone-positive tumors, and (Right) triple negative breast cancer subtypes (first row) and others (second row) showing no difference between oncoplastic and conventional breast-conserving surgery (BCS).
Comparison of our current study with other similar studies reporting long-term oncological outcomes are summarized in Tables 6 and 7.
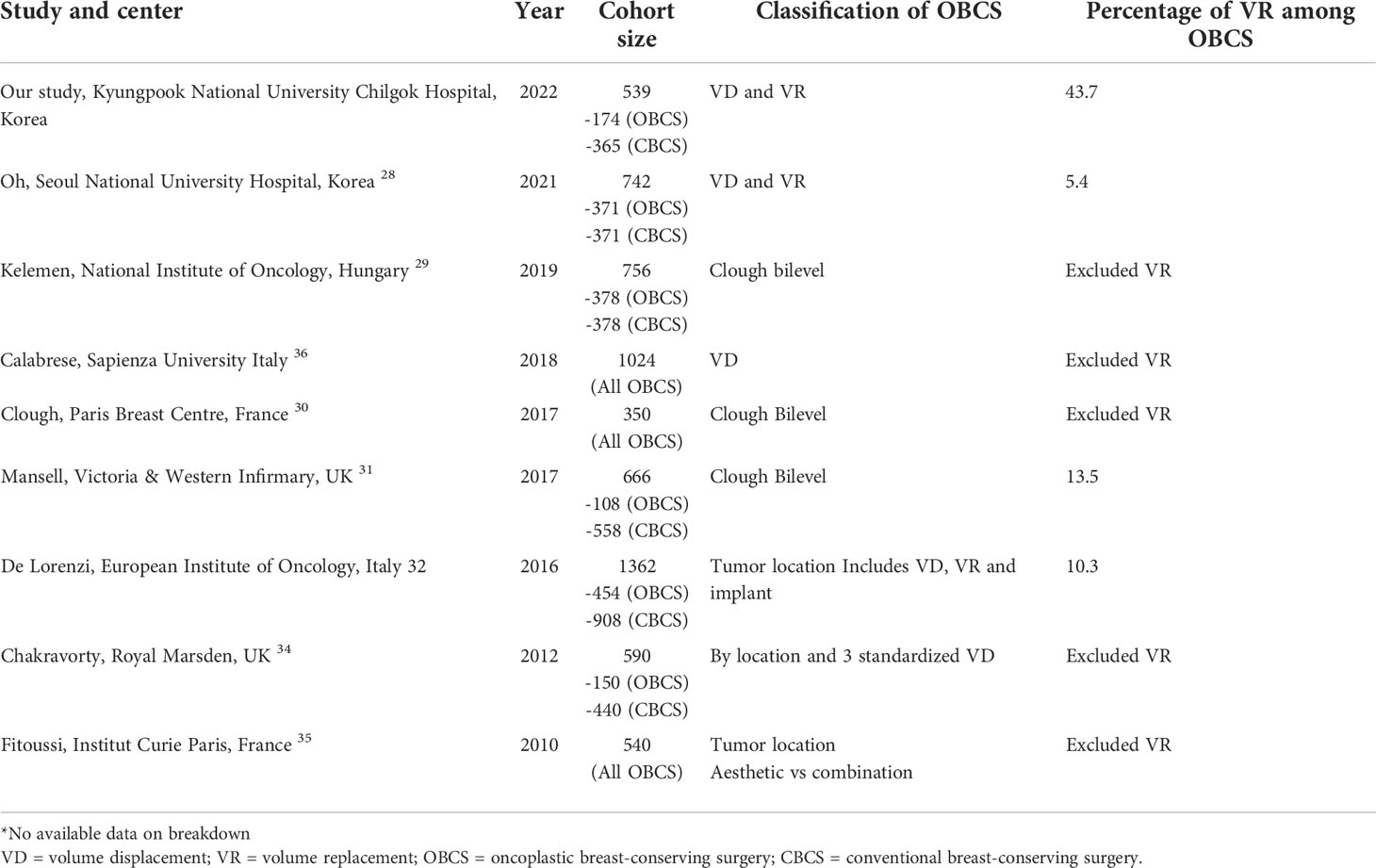
Table 6 Retrospective studies showing single center studies with large cohort and long term follow up, comparing definitions of OBCS and breakdown of oncoplastic procedures by year of published study.
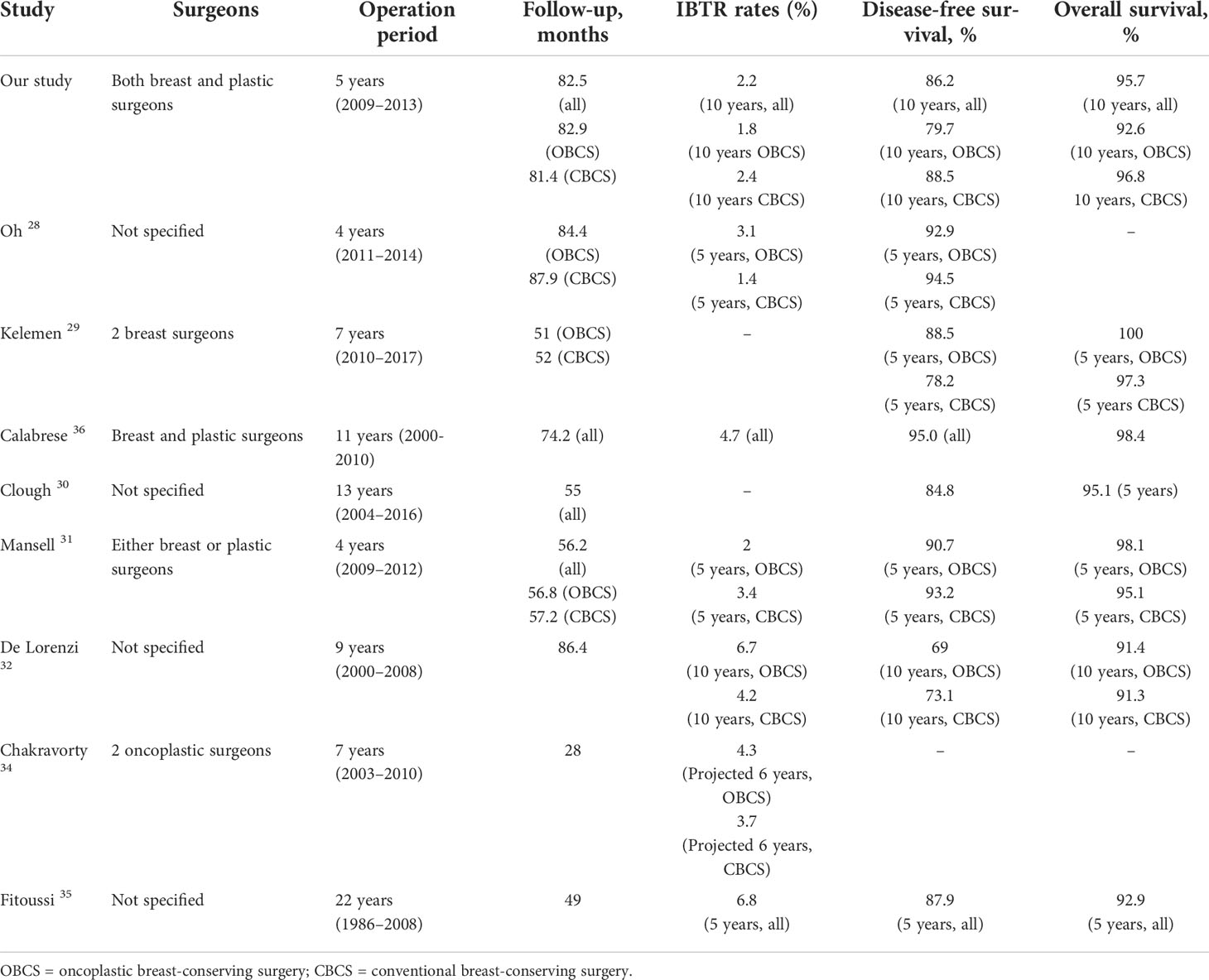
Table 7 Retrospective studies showing oncologic outcomes of oncoplastic breast conservation surgery according to surgeons, operation period, and follow-up interval to show directly reported results for local recurrence, disease-free survival, and overall survival.
Discussion
Over the last two decades, oncoplastic breast surgery quickly gained widespread acceptance as a standard of care option that balanced oncological and aesthetic outcomes of oncological resection in breast cancer management (21–27). The main findings of this study were that there was an overall low rate of IBTR (2.2%) and death (4.3%) observed in this cohort of 539 patients after a median follow-up of 82.4 months. This study had one of the largest single center cohorts with a long follow-up period (Table 7). Like other studies, IBTR rates were estimated to be between 1.4% and 14.6%, and 10-year OS rates were approximately 90.2–100%. Stratified analysis did not reveal any associated difference in survival outcomes in larger tumors, higher grade disease, or disease with a nodal burden. The observed outcomes could be the result of other factors, such as younger age (mean age <50 year), earlier disease stage (majority stage 1 and 2), favorable histological subtype, and generally high uptake rates of adjuvant therapies such as chemotherapy, radiotherapy, and hormonal therapy, when indicated. The proportion of cases with neoadjuvant chemotherapy was lower than expected in current practice; this might be because of the trend of favoring upfront surgery 10 years ago. However, as this was a retrospective cohort analysis, the cumulative incidence of events could also be underestimated because of a loss to follow-up or selection bias.
We also observed a similar trend that oncoplastic techniques allowed higher resection volumes for larger tumors and reduced intraoperative positive margin rates. Large systematic reviews showed that oncoplastic surgery was more frequently performed in younger patients who required greater breast volume removal for larger tumors (23, 25, 27). While this may not translate to any survival benefit, there could be improvement in patients’ satisfaction rates given the lower rate of reoperation and conversion to mastectomy (28).
Our literature review showed that there were several registry studies and meta-analyses published on oncological outcomes of oncoplastic breast surgery (21–42). However, we must caution that conclusions drawn from such meta-analyses or registries have inherent limitations. Many studies have difficulties pooling study subjects together due to the heterogeneity of the study population, surgeons’ expertise, and techniques and classifications used (21–26, 42). Therefore, we analyzed the different definitions and breakdowns of oncoplastic techniques used across various studies (Table 5). We noted that majority of the studies were small observational studies on specific techniques, limiting their generalizability and had to be excluded from the meta-analysis. Most had a limited cohort size or a barely sufficient follow-up duration to fully capture recurrence or death events. As it would be impossible to conduct any randomized control trial studying conventional and oncoplastic techniques because of ethical considerations, large cohort studies with long-term follow-up could be regarded as the highest level of evidence.
This study generated fresh data on long-term outcomes so as to compare with the reported standards over the last decade. First, the main strength of this study was the clear definition of procedures performed with balanced representations of both VD and replacement techniques. Second, consistency in surgical standards was maintained in the procedures performed by a dedicated oncoplastic team made up of both breast and plastic surgeons. Third, these 539 patients were followed up for more than a median of 80 months to allow for more valid capture of long-term outcomes.
Next, we examined the most commonly used definition of the Clough classification in the literature. The Clough classification of oncoplastic techniques primarily considers the excision volume ratio, requirement of skin excision for reshaping or mammoplasty, and tumor location. However, VR techniques were notably excluded because of their primary use in smaller breasts (13). Similarly, we found that many comparative studies with long-term outcomes reported a disproportionately low number of VD techniques, mainly level 2 oncoplastic mammoplasty with little or no representation of VR techniques. In our and many other East Asian populations, we adopted similar principles of deciding the type of oncoplastic procedures such as breast-to-tumor volume ratio, and tumor locations, but it was also proposed that an absolute value of tumor volume excised in itself could be an indication for VR techniques in small-to-moderate-sized breasts. These may be due to inherent differences in the patients’ morphometric characteristics or influenced by different cultural beliefs and resource settings (15, 41). In a smaller native breast with less space for VD maneuvers, a different threshold for VR techniques may apply. Evidence also shows that patients are more accepting of VR options and have good functional outcomes regardless of the VR technique (17).
As a result, our percentage of VR performed among OBCS was the highest among the selected studies, with VR techniques accounting for 43% of all oncoplastic procedures. Most of the other studies either had less than 10% of procedures represented by VR, or did not specify the type of reconstruction techniques at all. Our cohort also showed that the LD myocutaneous flap was the most commonly used VR techniques followed by chest wall perforator flaps. This was concordant to our finding that the LD flap was the largest and the most commonly reported VR technique as a single cohort series in the literature (20, 25, 37–42). However, we did not report and compare the oncological results from these studies that only focused on singular technique such as LD flap or omental flap reconstruction because they would have limited generalizability to other oncoplastic techniques and patient selection (40, 41).
We maintained that both VD and VR techniques formed the fundamentals of oncoplastic techniques and would not need to be separately studied from each other. Hence, it remained vital to establish comparable oncological outcomes of various oncoplastic techniques to reassure patients that oncoplastic breast surgery would not compromise on oncological safety in the long run, and that both aesthetic outcomes and patient satisfaction were equally important performance indicators in the treatment of breast cancer.
Limitations
The main limitations of the study were largely in its retrospective nature, which could lead to underestimated incidence rates due to the nature of selection bias and loss to follow-up. The surgical teams involved a dedicated oncoplastic team including both breast and plastic surgeons; consequently, these findings may not be logistically reproducible in all centers. We acknowledged that there were many confounding factors that could affect oncological outcomes and tried to address these by adjusting for the variables in the statistical analysis. However, considering the limitations of cohort size and event rates, it would be prudent to avoid generating too many hypotheses regarding secondary analysis findings but rather appreciate the general theme of oncological safety established across various tumor characteristics and adjuvant therapies provided in our study population. We also noted there was a low percentage of patients treated with neoadjuvant chemotherapy in our cohort. Neoadjuvant chemotherapy has gained much traction in its role in increasing rates of breast conservation; therefore, future research should be directed to study its influence on long-term oncological outcomes (43).
Conclusion
Our review of existing literature on the oncological outcomes of OBCS highlighted the dearth in well-balanced comparative studies with sufficient long-term follow-up, and reported our center’s own long-term oncological outcomes for OBCS to support the use of either VD or replacement techniques.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Kyungpook National University Chilgok Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.944589/full#supplementary-material
References
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med (2002) 347:1227–32. doi: 10.1056/NEJMoa020989
3. Keelan S, Flanagan M, Hill ADK. Evolving trends in surgical management of breast cancer: An analysis of 30 years of practice changing papers. Front Oncol (2021) 11:622621. doi: 10.3389/fonc.2021.622621
4. Kaufman CS. Increasing role of oncoplastic surgery for breast cancer. Curr Oncol Rep (2019) 21(12):111. doi: 10.1007/s11912-019-0860-9
5. Audretsch WP, Rezai M, Kolotas C. Tumor-specific immediate reconstruction in breast cancer patients. Perspect Plast Surg (1998) 11:71–100. doi: 10.1055/s-2008-1080243
6. Rainsbury RM. Surgery insight: oncoplastic breast-conserving reconstruction–indications, benefits, choices and outcomes. Nat Clin Pract Oncol (2007) 4:657–64. doi: 10.1038/ncponc0957
7. Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg (1999) 104:409–20. doi: 10.1097/00006534-199908000-00014
8. Petit JY, Rietjens M, Garusi C. Breast reconstructive techniques in cancer patients: which one, when to apply, which immediate and long-term risks? Crit Rev Oncol Hematol (2001) 38:231–9. doi: 10.1016/S1040-8428(00)00137-2
9. Silverstein MJ, Mai T, Savalia N, Vaince F, Guerra L. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol (2014) 110:82–9. doi: 10.1002/jso.23641
10. Macmillan RD, James R, Gale KL, McCulley SJ. Therapeutic mammaplasty. J Surg Oncol (2014) 110:90–5. doi: 10.1002/jso.23659
11. Chatterjee A, Gass J, Patel K, Holmes D, Kopkash K, Peiris L, et al. A consensus definition and classification system of oncoplastic surgery developed by the american society of breast surgeons. Ann Surg Oncol (2019) 26:3436–44. doi: 10.1245/s10434-019-07345-4
12. Lebovic G. Oncoplastic surgery: a creative approach to breast cancer management. Surg Oncol Clin N Am (2010) 19:567–80. doi: 10.1016/j.soc.2010.04.003
13. Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol (2010) 17:1375–91. doi: 10.1245/s10434-009-0792-y
14. Hoffmann J, Wallwiener D. Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. MC Cancer (2009) 9:108. doi: 10.1186/1471-2407-9-108
15. Yang JD, Lee JW, Cho YK, Kim WW, Hwang SO, Jung JH, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 1): volume displacement. J Breast Cancer (2012) 15:1–6. doi: 10.4048/jbc.2012.15.1.1
16. Yang JD, Lee JW, Cho YK, Kim WW, Hwang SO, Jung JH, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 2): volume replacement. J Breast Cancer (2012) 15:7–14. doi: 10.4048/jbc.2012.15.1.7
17. Lee JW, Kim MC, Park HY, Yang JD. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small- to moderate-sized breasts. Gland Surg (2014) 3:14–21. doi: 10.3978/j.issn.2227-684X.2014.02.02
18. Sabel MS, Hirsch A, Bellon JR. Clinical manifestations and evaluation of locoregional recurrences of breast cancer (2021). Available at: https://www.uptodate.com/contents/clinical-manifestations-and-evaluation-of-locoregional-recurrences-of-breast-cancer.
19. Lee J, Jung JH, Kim WW, Hwang SO, Chae YS, Lee SJ, et al. Five-year oncologic outcomes of volume displacement procedures after partial mastectomy for breast cancer. Clin Breast Cancer (2017) 17:70–5. doi: 10.1016/j.clbc.2016.06.018
20. Lee J, Jung JH, Kim WW, Hwang SO, Kang JG, Baek J, et al. Oncologic outcomes of volume replacement technique after partial mastectomy for breast cancer: A single center analysis. Surg Oncol (2015) 24:35–40. doi: 10.1016/j.suronc.2014.12.001
21. Kosasih S, Tayeh S, Mokbel K, Kasem A. Is oncoplastic breast conserving surgery oncologically safe? a meta-analysis of 18,103 patients. Am J Surg (2020) 220:385–92. doi: 10.1016/j.amjsurg.2019.12.019
22. Raufdeen F, Murphy J, Ahluwalia M, Coroneos CJ, Thoma A. Outcomes in volume replacement and volume displacement techniques in oncoplastic breast conserving surgery: A systematic review. J Plast Reconstr Aesthet Surg (2021) 74:2846–55. doi: 10.1016/j.bjps.2021.06.004
23. Haloua MH, Krekel NM, Winters HA, Rietveld DH, Meijer S, Bloemers FW, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg (2013) 257:609–20. doi: 10.1097/SLA.0b013e3182888782
24. Chen JY, Huang YJ, Zhang LL, Yang CQ, Wang K. Comparison of oncoplastic breast-conserving surgery and breast-conserving surgery alone: A meta-analysis. J Breast Cancer (2018) 21:321–9. doi: 10.4048/jbc.2018.21.e36
25. Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg (2014) 72:145–9. doi: 10.1097/SAP.0b013e3182605598
26. Rutherford CL, Barker S, Romics L. A systematic review of oncoplastic volume replacement breast surgery: oncological safety and cosmetic outcome. Ann R Coll Surg Engl (2022) 104:5–17. doi: 10.1308/rcsann.2021.0012
27. De La Cruz L, Blankenship SA, Chatterjee A, Geha R, Nocera N, Czerniecki BJ, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: A systematic literature review. Ann Surg Oncol (2016) 23:3247–58. doi: 10.1245/s10434-016-5313-1
28. Gulcelik MA, Dogan L, Karaman N, Bahcecitapar M, Ozaslan C. Oncoplastic level II surgical techniques for breast cancer treatment: Long-term outcomes. Breast Care (Basel) (2022) 17:24–30. doi: 10.1159/000514468
29. Oh MY, Kim Y, Kim J, Cheun JH, Jung JG, Kim HK, et al. Comparison of long-term oncological outcomes in oncoplastic breast surgery and conventional breast-conserving surgery for breast cancer: A propensity score-matched analysis. J Breast Cancer (2021) 24:520–30. doi: 10.4048/jbc.2021.24.e52
30. Kelemen P, Pukancsik D, Újhelyi M, Sávolt Á, Kovács E, Ivády G, et al. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: A single-centre retrospective study. Eur J Surg Oncol (2019) 45:118–24. doi: 10.1016/j.ejso.2018.09.006
31. Clough KB, van la Parra RFD, Thygesen HH, Levy E, Russ E, Halabi NM, et al. Long-term results after oncoplastic surgery for breast cancer: A 10-year follow-up. Ann Surg (2018) 268:165–71. doi: 10.1097/SLA.0000000000002255
32. Mansell J, Weiler-Mithoff E, Stallard S, Doughty JC, Mallon E, Romics L. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast (2017) 32:179–85. doi: 10.1016/j.breast.2017.02.006
33. De Lorenzi F, Hubner G, Rotmensz N, Bagnardi V, Loschi P, Maisonneuve P, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: A matched-cohort analysis. Eur J Surg Oncol (2016) 42:71–7. doi: 10.1016/j.ejso.2015.08.160
34. Carter SA, Lyons GR, Kuerer HM, Bassett RL Jr, Oates S, Thompson A, et al. Operative and oncologic outcomes in 9861 patients with operable breast cancer: Single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol (2016) 23:3190–8. doi: 10.1245/s10434-016-5407-9
35. Chakravorty A, Shrestha AK, Sanmugalingam N, Rapisarda F, Roche N, Querci Della Rovere G, et al. How safe is oncoplastic breast conservation? comparative analysis with standard breast conserving surgery. Eur J Surg Oncol (2012) 38:395–8. doi: 10.1016/j.ejso.2012.02.186
36. Fitoussi AD, Berry MG, Fama F, Falcou MC, Curnier A, Couturaud B, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases. Plast Reconstr Surg (2010) 125:454–62. doi: 10.1097/PRS.0b013e3181c82d3e
37. Calabrese C, Casella D, Di Taranto G, Marcasciano M, Kothari A, Sordi S, et al. Oncoplastic conservative surgery for breast cancer: long-term outcomes of our first ten years experience. Eur Rev Med Pharmacol Sci (2018) 22(21):7333–42. doi: 10.26355/eurrev_201811_16270
38. Teoh LY, Lai LL, Hanim Aa A, Teh MS, Jamaris S, Yahya A, et al. Oncological safety and postoperative complications in oncoplastic breast surgery among Asian women: A single institutional review. Breast J (2020) 26(11):2208–12. doi: 10.1111/tbj.14060
39. Rietjens M, Urban CA, Rey PC, Mazzarol G, Maisonneuve P, Garusi C, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast (2007) 16:387–95. doi: 10.1016/j.breast.2007.01.008
40. Rusby JE, Paramanathan N, Laws SAM, Rainsbury RM. Immediate latissimus dorsi miniflap volume replacement for partial mastectomy: use of intra-operative frozen sections to confirm negative margins. Am J Surg (2008) 196:512–18. doi: 10.1016/j.amjsurg.2008.06.026
41. Zaha H, Abe N, Sagawa N, Unesoko M. Oncoplastic surgery with omental flap reconstruction: a study of 200 cases. Breast Cancer Res Treat (2017) 162:267–74. doi: 10.1007/s10549-017-4124-9
42. André C, Holsti C, Svenner A, Oikonomou I, Appelgren M, Johansson A, et al. Recurrence and survival after standard versus oncoplastic breast-conserving surgery for breast cancer. BJS Open (2021) 5(1):zraa013. doi: 10.1093/bjsopen/zraa013
Keywords: oncoplastic, breast-conserving surgery, oncological outcomes, volume displacement, volume replacement
Citation: Hing JX, Kang BJ, Keum HJ, Lee J, Jung JH, Kim WW, Yang JD, Lee JS and Park HY (2022) Long-term oncological outcomes of oncoplastic breast-conserving surgery after a 10-year follow-up – a single center experience and systematic literature review. Front. Oncol. 12:944589. doi: 10.3389/fonc.2022.944589
Received: 15 May 2022; Accepted: 07 July 2022;
Published: 09 August 2022.
Edited by:
René Aloisio Da Costa Vieira, Barretos Cancer Hospital, BrazilReviewed by:
Idam Oliveira Junior, Barretos Cancer Hospital, BrazilFábio Bagnoli, Santa Casa of Sao Paulo, Brazil
Copyright © 2022 Hing, Kang, Keum, Lee, Jung, Kim, Yang, Lee and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho Yong Park, cGh5MTIzQGtudS5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
 Jun Xian Hing
Jun Xian Hing Byeong Ju Kang
Byeong Ju Kang Hee Jung Keum
Hee Jung Keum Jeeyeon Lee
Jeeyeon Lee Jin Hyang Jung1
Jin Hyang Jung1