
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 November 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.943294
This article is part of the Research Topic 365 Days of Progress In Gastrointestinal Cancers View all 11 articles
 Shuai Xiong1,2†
Shuai Xiong1,2† Ke Liu1,2†
Ke Liu1,2† Fei Yang1,2†
Fei Yang1,2† Yuanwei Dong2†
Yuanwei Dong2† Hongcai Zhang2†
Hongcai Zhang2† Pengning Wu1
Pengning Wu1 Yu Zhou1
Yu Zhou1 Lu Zhang1
Lu Zhang1 Qin Wu3
Qin Wu3 Xiaojing Zhao4
Xiaojing Zhao4 Wei Li1
Wei Li1 Lingling Yuan1
Lingling Yuan1 Biao Huang5*
Biao Huang5* Rensong Yue2*
Rensong Yue2* Li Feng2*
Li Feng2* Jing Chen2*
Jing Chen2* Yi Zhang2*
Yi Zhang2*Inflammatory bowel disease (IBD) is a chronic non-specific inflammatory disease of intestinal tract and a common digestive system disease. Current studies have shown that IBD significantly increases the incidence of colorectal cancer (CRC), and is positively correlated with the degree and extent of inflammation of IBD. The relationship between IBD and CRC has attracted extensive attention. However, the relationship between IBD and CRC has not been systematically studied by bibliometrics and visual analysis. This study conducted bibliometric analysis based on 3528 publications from the Core Collection of Web of Science to determine the research status, research hotspots and frontiers of this field. The results show that the number of publications has increased significantly over the past 10 years. The cooperative network analysis shows that the United States, Mayo Clin and Bo Shen are the country, institution and author with the most publications respectively. Belgium, Icahn Sch Med Mt Sinai and Erik Mooiweer are the most collaborative country, institution and author respectively. Analysis of keywords and references showed that inflammation, intestinal flora, and obesity were hot topics in this field. Analysis of keyword outbreaks shows that the gut microbiome and metabolism will be an emerging new research area and a potential hot spot for future research. This study is the first to visually examine the association between IBD and CRC using bibliometrics and visual analysis, and to predict potential future research trends.
Inflammatory bowel disease (IBD) is a chronic autoimmune disease affecting the gastrointestinal tract (GI). It includes Crohn’s disease (CD) and ulcerative colitis (UC), characterized by repeated bouts of mucosal inflammation (1, 2). In long-standing extensive ulcerative colitis, the severity of colonic inflammation is an important determinant of the risk of colorectal neoplasia (3, 4). Longstanding UC correlates with an increased risk of developing colitis associated cancer(CAC)through cumulative inflammatory burden (5), especially when they are male, have generalized colitis and are young when diagnosed with UC (6). Based on long-term epidemiological data, IBD patients are at high risk for colorectal cancer (CRC), especially in patients with extensive UC (4). IBD-CRC is one of the major and serious complications of IBD, and although only 1% to 2% of patients with IBD develop IBD-CRC, it is responsible for approximately 15% of IBD-related mortality (7).
Many common factors influence the onset and progression of IBD and CRC, including intestinal microbiome dysregulation, changes in interleukin pathways, and tumor necrosis factor. On the other hand, the patient’s age, race, genetics, family history, dietary composition, obesity and vitamin and mineral levels also contribute to the progression (8). These multiple factors contribute to the higher incidence of CRC in IBD patients.
However, recent population-based studies have shown that with advances in CRC screening and surveillance and improved IBD treatment, the incidence of CRC in patients with UC and CD has declined (9, 10). The 3-year post-colonoscopy colorectal cancer (PCCRC) rate in IBD patients is 24.3%, which is much higher than 7.5% in the general population. This means that of all IBD patients diagnosed with CRC, 24.3 percent had a colonoscopy and had a chance to diagnose or prevent CRC at an early stage (11). The relationship between UC and CAC has influenced the development of clinical practice guidelines, with increased endoscopic surveillance recommended among UC patients starting 8 years after initial UC diagnosis. These recommendations have led to successful reductions in CAC morbidity and mortality (12).
Among the many potential factors known to increase the risk of IBD-CRC, perhaps chronic persistent inflammation is the most important (4). Therefore, prevention of inflammation may be a necessary measure to reduce IBD-CRC in clinical practice. According to the “common ground hypothesis”, microbial dysbiosis and intestinal barrier impairment are at the core of the chronic inflammatory process associated with IBD-CRC. The persistent inflammation in the colon results in increased proliferation of cells necessary for repair but this also increases the risk of dysplastic changes due to chromosomal and microsatellite instability (4). Although inflammation has been identified as a risk factor for the development of UC into CRC, the specific mechanisms need to be further investigated. The characterization of key microbial communities and their influence on the pathogenesis of UC and CAC may provide opportunities to modulate intestinal inflammation through microbial-targeted therapy. Despite recent advances in sequencing technology, further research is needed to elucidate the causal role of the gut microbiome in regulating UC inflammation (1).
Publications have demonstrated the link between IBD and CRC. However, as far as we know, no scholar has systematically analyzed the topic of this field through bibliometric analysis. Therefore, this study aims to comprehensively analyze the status and development trend of IBD and CRC from 2012 to 2021 through bibliometrics. Previous reviews only rely on individuals to study through literature review and extraction, which cannot fully reflect the spatial and temporal distribution of researchers, institutions and countries. In addition, it is difficult to visualize the internal structure of the knowledge base and research focus, and systematic, comprehensive and visual studies are rarely found.
Bibliometrics refers to the application of mathematical and statistical methods to objectively analyze the nature of the spatial distribution of scientific literature in a certain period in a specific field (13). Bibliometrics can evaluate research trends qualitatively and quantitatively according to the characteristics of literature database and bibliometrics. It can not only help scholars keep track of trends in a particular research field, but also evaluate the contributions of journals, institutions and countries in a particular research field. In addition, for the medical field, it can provide a basis for the development of clinical guidelines (14). This paper aims to discuss specific key areas that IBD and CRC may be related to future research, summarize the research status in this field, grasp the research direction and hot spots, and provide some reference for future research direction.
We selected the Web of Science Core Collection (WOSCC) from Clarivate Analytics as the retrieval database for this study. WOSCC is a highly authoritative citation index database, which is widely used in scientific research and bibliometrics research at present. We use the combination of subject words and free words for line search. The subject words include: inflammatory bowel diseases and colorectal neoplasms. Free words mainly include:inflammatory bowel disease, colorectal neoplasms, colorectal neoplasm, colorectal tumors, colorectal cancer, colorectal carcinoma. We set the search language as “English”. The search document type is set to “Article or Review”. We set the publication date condition of the retrieved literature as “2011-01-01 to 2021-12-31”. The retrieval process was carried out independently by two researchers and the retrieval date was May 5, 2022. Detailed retrieval results are shown in Figure 1.
The software used for data analysis in this study are Microsoft Office Excel 2021, Citespace.5.8.R3 and Vosviewer1.6.17. Among them, Microsoft Office Excel 2021 is used for the statistics of literature years and the production of related tables; Citespace.5.8.R3 is used for the analysis of the number of published countries, institutions and authors in the data and the cooperation relationship, and the production of keyword co-occurrence map, keyword burst map. Vosviewer1.6.17 is used to analyze highly cited and co-cited references in the data. The analysis in this study uses WOSCC to analyze the publication output, web of science subject category, publication year, author and other functions. After that, the selected documents were imported into Cite Space, and time span was 2010–2020. Select the node based on the type of analysis need to perform. The specific parameter settings in Cite Space are as follows: Time slicing: January 2012–December 2021, Term source: Title, Abstract, Author Keywords and Keyword Plus, Node type: Author, Institution, Country and Keyword. Link strength: Cosine. Selection Criteria: Top N =50. In the generated map, the color of the circular nodes represents the time when the article was published. The thickness of the node is positively related to the frequency. the thickness of the line describes the strength between the projects, and the color of the line describes the year when the two projects first collaborated. The nodes in the map represent elements such as author, country/region, or institution. The link lines between the nodes indicate the collaboration relationship. The larger the circle, the more articles published. The wider the line, the stronger the relationship. The outermost purple ring represents the Centrality. The centrality value represents the cooperation intensity, and the higher the value, the stronger the cooperation. Use VOS viewer software to statistically analyze highly cited and co-cited documents. First, import the results obtained through WOSs into the software. Then set number of terms to be selected:10. Finally, import the results to excel for sorting.
We retrieved a total of 4214 relevant literatures through the web of Science Core Collection (WOSCC) database, and 3528 were screened. The specific screening process is shown in Figure 1.
As shown in Figure 2, the number of articles published in 2012 was at least 262, and the number of articles published in 2021 was at most 434. From 2012 to 2021, the number of articles published in joint studies on IBD and CRC showed a linear growth trend, satisfying the functional relationship “Y = 17.224x + 258.07 R² = 0.928”.
A total of 106 countries/regions published papers on IBD and CRC. The top 10 countries/regions in terms of publication volume and centrality are shown in Table 1. The top 10 countries published 3,261 papers, accounting for 92.43% of the total. The United States had the largest number of articles with 1,110, accounting for 34% of the top 10 countries, followed by China (550, 17%) and Italy (275, 8%). However, the national centrality of the top ten publications is not high. In contrast, Belgium, Saudi Arabia and the United Arab Emirates have high centrality, but they post very little. Countries/regions with more than 20 articles are visualized in Figure 3. It can be seen that the United States cooperated more with Switzerland, Italy and France cooperated more with Belgium.
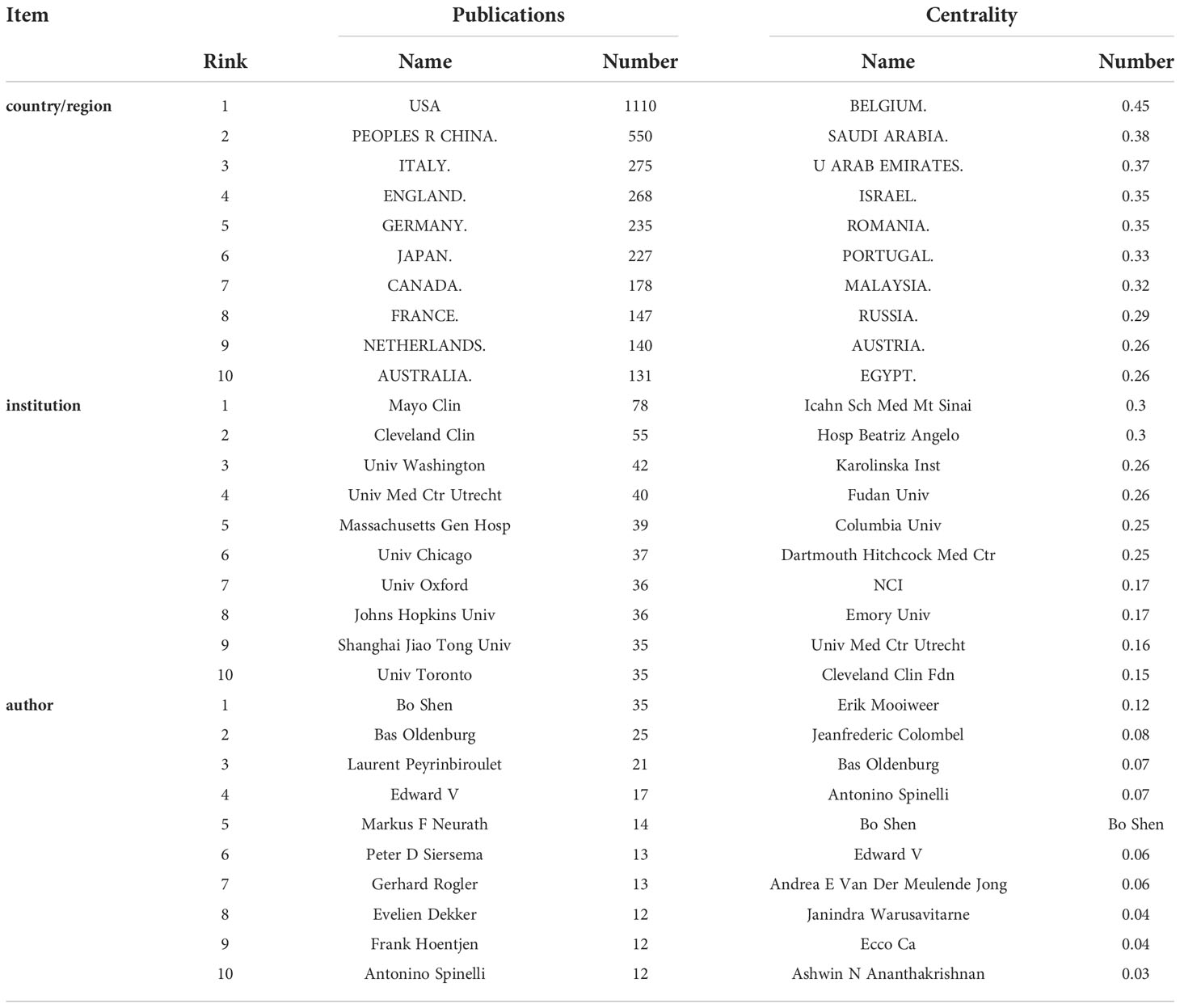
Table 1 Top 10 countries/regions, institutions, and authors in terms of publications and centrality.
As shown in the Table 1, a total of 382 institutions published papers in this field, with Mayo Clin (78 papers) leading the way, followed by Cleveland Clin (55 papers) and Univ Washington (42 papers). The institution with the largest centrality was Icahn Sch Med Mt Sinai, followed by Hosp Beatriz Angelo and Karolinska Inst. Based on the analysis of publications and centrality, Univ Med Ctr Utrecht (Publications: 40, centrality: 0.16) and Cleveland Clin Fdn (publications: 55, centrality: 0.15) were the leading institutions in the field. According to Figure 4, the cooperation relationship between institutions is not very close. Mayo Clin, the largest institution in the node, has less cooperation with other institutions. On the contrary, Massachusetts Gen Hosp has many collaborations with Harvard Univ and Harvard Med Sch, and has many publications among them.
A total of 512 authors published papers on the relationship between IBD and CRC from 2012 to 2021, and the top 10 authors in terms of publication volume and centrality are shown in Table 1. Three authors with more than 10 publications are Bo Shen (35 papers), Bas Oldenburg (25 papers) and Laurent Peyrinbiroulet (21 papers). The more central authors are Erik Mooiweer, followed by Jeanfrederic Colombel and Bas Oldenburg. It can be seen that Bo Shen, Bas Oldenburg, Edward V, Antonino Spinelli have an important influence on the joint research of IBD and CRC. The authors with more than 5 papers are visualized in Figure 5. The author with the largest node, Bo Shen, has a close cooperative relationship with Edward V, Charl Es N Bernstbn, Feza H Remzi and other authors. It is worth mentioning that in recent years, Jonas F Ludvigsson, Olaolen, Henrik Toft Sorensen, Claudiamescoli and Anders Ekbom have formed a close cooperation network.
Co-occurrence keywords can be used to analyze and discover research hotspots. There are 618 keywords in this study, and Table 2 lists the top 20 keywords based on co-occurring frequency. Figure 6 shows the visualization of keywords with keyword frequency more than 50. The higher the frequency, the more hotter it is. The connection between the keywords indicates that they are jointly studied. The top 4 are “inflammatory bowel disease”, “colorectal cancer”, “Ulcerative Colitis”, and “Crohn’s disease”. Obviously, the frequency of UC was significantly higher than that of CD. In addition, from 2012 to 2021, the risk factors, mechanism, diagnosis and treatment of the relationship between IBD and CRC were also studied.
Keyword burst refers to a sudden increase in research content at a given moment, which can be used to predict the potential development trend of the research field. Figure 7 shows 2012 - 2021 IBD and CRC common area 25 keywords from the initial research focus on “mutation”, “tumor progress”, “to oxygen dose bear bile acid”, “to the current research of clostridium” nuclear “and” bacteria “, “consensus”, “fat”, “maintenance treatment”, “experimental colitis”, “random”, “metabolism”, “probiotic” and “popular”, “task Changes in research. It can be seen that in recent years, the research focus has shifted from mechanism to diagnosis and treatment, among which fusobacterium nucleatum, Microbiota, probiotics, Obesity, and metabolism, and “maintenance therapy” may be the research trend.
Keyword clustering can clearly understand the different research directions in the research field and classify and summarize the research topics. In this study, LLR algorithm was used to cluster IBD and CRC-related keywords from 2012 to 2021. There were 21 clusters in total, and the cluster value of modules was Q=0.7888 (>0.3), clustering average contour value S=0.9213 (>0.7), indicating that the clustering effect is reasonable. The first 10 clusters are shown in Table 3. It can be seen that there are many studies on the mechanism of the relationship between inflammatory bowel disease and colorectal cancer comorbidity. Now more and more studies have been transferred from mechanism research to clinical research. How to reduce the transformation of IBD into CRC is the topic of current research, such as colorectal resection, activity marker monitoring, dietary intervention, intestinal flora adjustment, etc.
Highly cited literature refers to the publications cited frequently and with a wide range of influence. As shown in Table 4, the two literatures “Gastrointestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota” (1216 times) and “The Gut Microbiota and Host Health:A New Clinical Frontier” (1089 times) were cited for more than 1000 times, and both of them were studies on intestinal microbes, indicating that the current research hotspot is intestinal microbes.
Co-cited references are the basis and basis of later research. The more times of co-citation, the greater the contribution of the literature to future research. As shown in Table 3, the meta-analysis published in 2001 by Eaden JA et al., which cited the most, demonstrated the relationship between UC and CRC, indicating that UC has a high risk of developing into CRC.
In recent 10 years, the research on inflammatory bowel disease and colorectal cancer has maintained a high level, and the joint research on inflammatory bowel disease and colorectal cancer has also been a hot spot in the field of digestion and tumor research.
It is worth noting that Italy, with a centrality of 0.15, ranks 14th, but ranks 3rd in the number of publications. This shows that Italy is not only publishing more, but also cooperating more. China ranked second in the total number of published articles, but its centrality was only 0.06, reflecting the lack of international collaborative research in China. It is expected that China will strengthen international cooperation in the study of IBD and CRC in the near future.
Co-cited references are the basis of subsequent research, while highly cited references reflect the research trend. According to Table 4, the basis of the research focuses on the following aspects:the relationship between IBD and CRC, the role of inflammation in the development of ulcerative colitis into colorectal cancer, epidemiological investigations related to IBD and CRC; and guidelines for colorectal cancer surveillance. We can find that IBD increases the risk of CRC, and inflammation plays an important role in this process, providing a basis for further mechanism studies. Studies (15) showed that the estimated overall prevalence of CRC in any UC patient was 3.7%. But in a population-based cohort, UC increased the risk of CRC by 2.4 times. The severity of colon inflammation is an important determinant of the risk of CRC (3). The longer and wider the inflammation, the greater the risk of developing CRC.
Studies have found that Clostridium, particularly Fusobacterium nucleatus(F. nucleatum), has become a potential cause of CRC susceptibility. As far as we know, F. nucleatum, a Gram-negative bacterium present in the oral cavity, is the primary causative agent of chronic periodontitis (16). It has attracted interest in the past decade because of its association with CAC. Although studies have found that it may play an important role in the progression of CRC (17), its exact role and related mechanisms in the progression of IBD-CRC remain unclear. A recent study found that F. nucleatum significantly increased the malignancy of azomethane (AOM)/DSS induced CRC. And compared with untreated CRC cells, F. nucleatum synergistically increased the invasiveness and epithelial-mesenchymal transformation (EMT) characteristics of DSS-treated mouse CRC cells. In conclusion, Clostridium nucleatus promotes the occurrence of IBD-CRC (18). It was also found that AOM/DSS-treated ctP-knockout mice had increased tumor load, suggesting that CTPS are essential for maintaining intestinal homeostasis (19). This field is in its infancy and further work is needed to determine whether bacterial and/or defensive peptides play an important role in the development of IBD-CRC.
On the other hand, as a recognized risk factor, obesity causing about 15-20% of cancers (20). It is noteworthy that CRC is also a type of cancer closely related to obesity (21, 22). In Europe, around 11% of CRC cases have been attributed to overweight and obesity (23). Visceral fat or abdominal obesity seems to be of greater concern than subcutaneous fat obesity, and any 1 kg/m2 increase in body mass index confers more risk (24). Diet-induced obesity accelerates CAC in mice by increasing inflammation and immune cell recruitment. Dietary fat increases taurocholic acid production in the colon, leading to dilation of Bilophila wadsworthia and colitis in interleukin-10 deficient mice (25). In addition, high fat diet drives colorectal tumorigenesis through inducing gut microbial dysbiosis, metabolomic dysregulation with elevated lysophosphatidic acid, and gut barrier dysfunction in mice (26). Meanwhile, CRC has been linked to a sedentary lifestyle and obesity. Obesity might increase the likelihood of recurrence or mortality of the primary cancer and may affect initial management, including accurate staging (24). This suggests that obesity treatment strategies that reduce inflammation can be easily implemented in patients through diet and lifestyle interventions (27). But it is unclear whether bariatric surgery can help reduce the risk of CRC (24). The research on how obesity increases the risk of colorectal cancer is undoubtedly a research hotspot, but the direct link between obesity-induced dysbiosis and CRC remains to be further studied (28). Further understanding of the molecular and cellular mechanisms associated with obesity and inflammation and colorectal cancer is needed to develop potential new therapies (29).
Although inflammation has been identified as a risk factor for the progression of UC to CRC, the exact mechanism is unclear. On this basis, the latest research trends are suggested by highly cited references. Highly cited literatures suggest that the mechanism of UC developing into CRC is related to intestinal flora. Microbiome and host form a complex “superorganism”, and the two have a symbiotic relationship (28). When the environment changes (infection, diet, or lifestyle) or the microbiome changes, it can disrupt this symbiosis and promote disease. More and more evidences indicate that microbiome plays a key role in carcinogenesis (27, 30).
99% of the microbiota is located in the gastrointestinal tract, so the gastrointestinal microbiota has been studied the most (28). A large number of studies in patients and mice have linked microbiota to the occurrence of colorectal cancer (31). On the one hand, several studies have shown that gut microbiota is an essential factor in driving inflammation in the colon, and this inflammatory environment is related to CRC development (32). On the other hand, CRC patients with obesity (OB-CRC) display a specific gut microbiota profile characterized by a reduction in butyrate-producing bacteria and an overabundance of opportunistic pathogens, which in turn could be responsible, at least in part, for the higher levels of proinflammatory cytokine IL-1β, the deleterious bacterial metabolite TMAO, and gut permeability found in these patients (33). Furthermore, intestinal inflammation and genotoxin-induced DNA damage of intestinal cells have been proposed as the possible mechanisms responsible for the role of microbial dysbiosis in carcinogenesis. The enrichment of a variety of bacterial species in the intestinal tract, including Fusobacterium nucleatum, anaerobic digestion streptococcus and enterotoxic Bacteroides fragilis, has been proved to contribute to the occurrence of colorectal cancer by inducing tumor proliferation, promoting inflammation, leading to DNA damage and protecting tumors from immune attacks (34). In a word, Microbiota regulates various mechanisms of carcinogenesis, including inflammation, metabolism and genotoxicity (28). This suggests that the above aspects may provide microbiome targeted prevention strategies for cancer. Microbium-based cancer prevention strategies may be the trend of future research.
Associations between microbial dysbiosis, chronic inflammation, autoimmunity, and tumorigenesis are well established (1). Because inflammation alters the composition of gut microbes and promotes IBD to become CRC, microbiome related treatments have been extensively studied in clinical trials. Gut microbiota modulation, with the aim to reverse established microbial dysbiosis, is a novel strategy for prevention and treatment of CRC (34). In recent years, the use of probiotics to treat CRC is becoming increasingly popular, as they have achieved positive and favorable results in many in vitro, in vivo and clinical studies, and it has become a popular candidate for the prevention and treatment of CRC (35). Probiotics, such as Lachnospiraceae species, Bifidobacterium animalis and Streptococcus thermophilus, are found to be depleted in CRC patients. These bacteria are suggested to exert a protective effect against CRC (36). Probiotics, or other commensal microbiota, confer colonization resistance by competing for nutrients and adhering surface on epithelial cells or mucus (37), or alternatively by antagonizing pathogen colonization through aggregation with pathogens, so robiotic administration is suggested to restore microbial dysbiosis and maintain intestinal microbial balance by occupying host tissue and preventing colonization of pathogenic bacteria. Furthermore, probiotics can produce metabolites such as lactic and acetic acid, or bacteriocins, which inhibit pathogen growth by lowering luminal Ph (38). The mechanisms of probiotics in the treatment of CRC are mainly as follows: establishing symbiosis, improving intestinal barrier function, regulating intestinal immune system, producing anticancer compounds and degrading carcinogenic compounds in the intestinal environment (39, 40). It is worth noting that probiotics may have adverse effects on immunocompromised patients. And there has also been some safety concerns regarding probiotic use in cancer patients, including the risk of bacterial translocation and systemic invasion, as well as the potential transmission of resistant genes to resident microbiota and the rise of antimicrobial resistance (34). Therefore, inactivated probiotics can be used as a substitute for live bacteria in these patients (41). Probiotics prevention and treatment of CRC may continue to be a focus of future research due to their high safety and low adverse reactions. In addition, given the potential role of the gut microbiome in the pathogenesis of CAC, the targeted regulation of gut bacteria by prebiotics, homologous genes, antibiotics and FMT may also be a trend of research (1).
Similarly, metabolic syndrome (MetS) is a group of metabolic risk factors including abdominal obesity, hypertension, hyperglycemia, and dyslipidemia (42). Prevalence of MetS ranges from 34.8% to 41.9% in the US and from 18% to 46% in Europe (14, 43). Studies on intestinal microbiome dysregulation and the resulting inflammatory state help us understand the underlying pathogenesis (44). A nested case-control study (21) found that the association between MetS and CRC was caused by abdominal obesity and abnormal glucose metabolism. A recent meta-analysis (45) also found that MetS are associated with an increased risk of CRC incidence and cancer-specific mortality, but further research is needed to clarify the mechanism. Predictably, the interaction between IBD, CRC, intestinal microflora, obesity and metabolic syndrome may be a research hotspot.
Through Cite Space and VOS viewer software, this study carried out bibliometric and visual analysis in the field of IBD and CRC, intuitively displayed the current research status of research countries/regions, institutions and authors, and provided references for scholars in related fields for further research. Through this study, it can be found that at present, intestinal flora disorder, inflammation, obesity and metabolic disorders may be important influencing factors for IBD to develop into CRC. It can be predicted that adjusting intestinal flora, controlling intestinal inflammation and regulating metabolism will become the research trend in the future. Our study may promote the development of this field and laying foundation for future research.
This is the first study to assess and quantify the global research productivity associated with IBD and CRC, presenting the overall picture of the topic and exploring future research directions. Since 2012, the volume of literature on IBD and CRC has continued to grow. The US, China, Italy and England are the most productive regions. Currently, the main hot topics related to both IBD and CRC are the following: the intestinal microflora associated with the development of IBD into CRC, microbiome based cancer prevention strategies, obesity and metabolic syndrome in relation to colorectal cancer.
The study had some limitations. First of all, our data only come from the WOSCC and only contain literature in English, which may lead to incomplete data and certain deviations. Secondly, some authors or institutions have different name formats in the WOSCC, and the research count may be scattered. As a result, their names may not appear in the results. Finally, due to publication bias, all conclusions of this study are from published studies, but many other studies (mainly negative results) may never be published, so there may be publication bias. However, we still consider that the findings of our analysis were adequate to characterize accurately the state of IBD and CRC research at the global level.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
SX and YiZ jointly determined the theme, SX wrote the draft, and PW, LZ, HZ, FY, BH, LF and YD supplemented and revised the manuscript. KL, YuZ, QW, XZ, JC, RY, LY and WL reviewed the manuscript. All of them contributed to the manuscript and agreed to submit the final version of the manuscript.
This research was funded by National Natural Science Foundation of China (81973821), National Natural Science Foundation of China (8227152900), The Xinglin Scholars Program (MPRC2022014), Hospital of Chengdu University of Traditional Chinese Medicine (19PJ04), Sichuan Administration of Traditional Chinese Medicine (CKY2021106), Jiangxi Provincial Natural Science Foundation Youth Fund (20202BAL216065), Jiangxi Provincial Education Department Science Program (GJJ201259), Jiangxi Provincial Science and Technology Department (20212BAG70037), Sichuan Provincial Department of Science and Technology (2021YFS0268), Sichuan Provincial Department of Science and Technology (2022JDKP0082), Sichuan Provincial Department of Finance (CJJ2022055).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dulai PS, Sandborn WJ, Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: A review of disease epidemiology, pathophysiology, and management. Cancer Prev Res (Philadelphia Pa.) (2016) 9(12):887–94. doi: 10.1158/1940-6207
2. Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol (2020) 64:51–60. doi: 10.1016/j.semcancer.2019.05.001
3. Popov J, Caputi V, Nandeesha N, Rodriguez DA, Pai N. Microbiota-immune interactions in ulcerative colitis and colitis associated cancer and emerging microbiota-based therapies. Int J Mol Sci (2021) 22(21):11365. doi: 10.3390/ijms222111365
4. Majumder S, Shivaji UN, Kasturi R, Sigamani A, Ghosh S, Iacucci M. Inflammatory bowel disease-related colorectal cancer: Past, present and future perspectives. World J gastrointestinal Oncol (2022) 14(3):547–67. doi: 10.4251/wjgo.v14.i3.547
5. Kao TW, Huang CC. Recent progress in metabolic syndrome research and therapeutics. Int J Mol Sci (2021) 22(13):6862. doi: 10.3390/ijms22136862
6. Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology (2004) 126(2):451–9. doi: 10.1053/j.gastro.2003.11.010
7. Choi CR, Al Bakir I, Ding NJ, Lee GH, Askari A, Warusavitarne J, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut (2019) 68(3):414–22. doi: 10.1136/gutjnl-2017-314190
8. Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, et al. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: An updated overview. Am J Gastroenterol (2015) 110(7):1022–34. doi: 10.1038/ajg.2015.65
9. Zhu Z, Mei Z, Guo Y, Wang G, Wu T, Cui X, et al. Reduced risk of inflammatory bowel disease-associated colorectal neoplasia with use of thiopurines: a systematic review and meta-analysis. J Crohn’s colitis (2018) 12(5):546–58. doi: 10.1093/ecco-jcc/jjy006
10. Burr NE. Interpreting post-colonoscopy colorectal cancer rates in inflammatory bowel disease. Endoscopy (2021) 53(10):1034–6. doi: 10.1055/a-1395-7536
11. Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet (London England) (2020) 395(10218):123–31. doi: 10.1016/s0140-6736(19)32545-0
12. Han F, Wu G, Zhang S, Zhang J, Zhao Y, Xu J. The association of metabolic syndrome and its components with the incidence and survival of colorectal cancer: A systematic review and meta-analysis. Int J Biol Sci (2021) 17(2):487–97. doi: 10.7150/ijbs.52452
13. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol hepatol: Off Clin Pract J Am Gastroenterological Assoc (2012) 10(6):639–45. doi: 10.1016/j.cgh.2012.01.010
14. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet (London England) (2005) 365(9468):1415–28. doi: 10.1016/s0140-6736(05)66378-7
15. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut (2001) 48(4):526–35. doi: 10.1136/gut.48.4.526
16. Cai Z, Zhu T, Liu F, Zhuang Z, Zhao L. Co-Pathogens in periodontitis and inflammatory bowel disease. Front Med (2021) 8:723719. doi: 10.3389/fmed.2021.723719
17. Yu MR, Kim HJ, Park HR. Fusobacterium nucleatum accelerates the progression of colitis-associated colorectal cancer by promoting EMT. Cancers (2020) 12(10):2728. doi: 10.3390/cancers12102728
18. Yoshimura T, Mclean MH, Dzutsev AK, Yao X, Chen K, Huang J, et al. The antimicrobial peptide CRAMP is essential for colon homeostasis by maintaining microbiota balance. J Immunol (Baltimore Md.: 1950) (2018) 200(6):2174–85. doi: 10.4049/jimmunol.1602073
19. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer (2004) 4(8):579–91. doi: 10.1038/nrc1408
20. Wunderlich CM, Ackermann PJ, Ostermann AL, Adams-Quack P, Vogt MC, Tran ML, et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat Commun (2018) 9(1):1646. doi: 10.1038/s41467-018-03773-0
21. Chassaing B, Gewirtz AT. Pathobiont hypnotises enterocytes to promote tumour development. Gut (2014) 63(12):1837–8. doi: 10.1136/gutjnl-2014-306890
22. Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, et al. Colonic mucosa-associated diffusely adherent afaC+ escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut (2014) 63(5):761–70. doi: 10.1136/gutjnl-2013-304739
23. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut (2013) 62(6):933–47. doi: 10.1136/gutjnl-2013-304701
24. Bardou M, Rouland A, Martel M, Loffroy R, Barkun AN, Chapelle N. Review article: obesity and colorectal cancer. Aliment Pharmacol Ther (2022) 56(3):407–18. doi: 10.1111/apt.17045
25. Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin immunopathol (2013) 35(2):229–44. doi: 10.1007/s00281-012-0352-6
26. Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology (2022) 162(1):135–49.e2. doi: 10.1053/j.gastro.2021.08.041
27. Dang Q, Luo Z, Ouyang C, Wang L. First systematic review on health communication using the CiteSpace software in China: Exploring its research hotspots and frontiers. Int J Environ Res Public Health (2021) 18(24):13008. doi: 10.3390/ijerph182413008
28. Tuominen H, Rautava J. Oral microbiota and cancer development. Pathobiology: J immunopathol Mol Cell Biol (2021) 88(2):116–26. doi: 10.1159/000510979
29. Molska M, Reguła J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients (2019) 11(10):2453. doi: 10.3390/nu11102453
30. Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: A bibliometric and visualized study. Front Oncol (2020) 10:627891. doi: 10.3389/fonc.2020.627891
31. Ranjbar M, Salehi R, Haghjooy Javanmard S, Rafiee L, Faraji H, Jafarpor S, et al. The dysbiosis signature of fusobacterium nucleatum in colorectal cancer-cause or consequences? a systematic review. Cancer Cell Int (2021) 21(1):194. doi: 10.1186/s12935-021-01886-z
32. Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, et al. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers (2020) 12(6):1406. doi: 10.3390/cancers12061406
33. Sánchez-Alcoholado L, Ordóñez R, Otero A, Plaza-Andrade I, Laborda-Illanes A, Medina JA, et al. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int J Mol Sci (2020) 21(18):6782. doi: 10.3390/ijms21186782
34. Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene (2020) 39(26):4925–43. doi: 10.1038/s41388-020-1341-1
35. Aleksandrova K, Boeing H, Jenab M, Bas Bueno-De-Mesquita H, Jansen E, Van Duijnhoven FJ, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Philadelphia Pa.) (2011) 4(11):1873–83. doi: 10.1158/1940-6207.Capr-11-0218
36. Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome (2018) 6(1):70. doi: 10.1186/s40168-018-0451-2
37. Tuomola EM, Ouwehand AC, Salminen SJ. The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol Med Microbiol (1999) 26(2):137–42. doi: 10.1111/j.1574-695X.1999.tb01381.x
38. Fayol-Messaoudi D, Berger CN, Coconnier-Polter MH, Lievin-Le Moal V, Servin AL. pH-, lactic acid-, and non-lactic acid-dependent activities of probiotic lactobacilli against salmonella enterica serovar typhimurium. Appl Environ Microbiol (2005) 71(10):6008–13. doi: 10.1128/AEM.71.10.6008-6013
39. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature (2012) 487(7405):104–8. doi: 10.1038/nature11225
40. Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer (2013) 13(11):800–12. doi: 10.1038/nrc3610
41. Minehira K, Tappy L. Dietary and lifestyle interventions in the management of the metabolic syndrome: present status and future perspective. Eur J Clin Nutr (2002) 56(12):7. doi: 10.1038/sj.ejcn.1601645
42. Tripathy A, Dash J, Kancharla S, Kolli P, Mahajan D, Senapati S, et al. Probiotics: A promising candidate for management of colorectal cancer. Cancers (2021) 13(13):3178. doi: 10.3390/cancers13133178
43. Shamekhi S, Abdolalizadeh J, Ostadrahimi A, Mohammadi SA, Barzegari A, Lotfi H, et al. Apoptotic effect of saccharomyces cerevisiae on human colon cancer SW480 cells by regulation of Akt/NF-ĸB signaling pathway. Probiotics antimicrobial Proteins (2020) 12(1):311–9. doi: 10.1007/s12602-019-09528-7
44. Grundy SM. Metabolic syndrome pandemic. Arteriosclerosis thrombosis Vasc Biol (2008) 28(4):629–36. doi: 10.1161/atvbaha.107.151092
Keywords: inflammatory bowel diseases, colorectal cancer, gut microbiome, global research trends, bibliometric
Citation: Xiong S, Liu K, Yang F, Dong Y, Zhang H, Wu P, Zhou Y, Zhang L, Wu Q, Zhao X, Li W, Yuan L, Huang B, Yue R, Feng L, Chen J and Zhang Y (2022) Global research trends on inflammatory bowel diseases and colorectal cancer: A bibliometric and visualized study from 2012 to 2021. Front. Oncol. 12:943294. doi: 10.3389/fonc.2022.943294
Received: 13 May 2022; Accepted: 25 October 2022;
Published: 29 November 2022.
Edited by:
Yun Dai, First Hospital, Peking University, ChinaReviewed by:
Javier P Gisbert, Princess University Hospital, SpainCopyright © 2022 Xiong, Liu, Yang, Dong, Zhang, Wu, Zhou, Zhang, Wu, Zhao, Li, Yuan, Huang, Yue, Feng, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Huang, ODYzNjUzNzc4QHFxLmNvbQ==; Li Feng, MzM1Mzg0ODUzQHFxLmNvbQ==; Rensong Yue, eXJzMTg0MjgzMDMxNTZAMTI2LmNvbQ==; Jing Chen, amluZ2NoZW4yMDIyMTEgQDE2My5jb20=; Yi Zhang, emhhbmd5aTIyODhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.