- 1Biostatistics Research Group, Department of Health Sciences, University of Leicester, Leicester, United Kingdom
- 2University Hospital Leicester National Health Service (NHS) Trust, Leicester Royal Infirmary, Leicester, United Kingdom
- 3Centre for Health Economics, University of York, York, United Kingdom
- 4Leicester Cancer Research Centre, University of Leicester, Leicester, United Kingdom
- 5Department of Statistics, University of Warwick, Coventry, United Kingdom
- 6Medical School, Swansea University, Swansea, United Kingdom
Breast cancer is the fifth leading cause of cancer-related deaths worldwide. The randomized controlled trials (RCTs) of targeted therapies in human epidermal receptor 2 (HER2)–positive advanced breast cancer (ABC) have provided an evidence base for regulatory and reimbursement agencies to appraise the use of cancer therapies in clinical practice. However, a subset of these patients harbor additional biomarkers, for example, a positive hormone receptor status that may be more amenable to therapy and improve overall survival (OS). This review seeks to explore the reporting of evidence for treatment effects by the hormone receptor status using the RCT evidence of targeted therapies for HER2-positive ABC patients. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed to identify published RCTs. Extracted data were synthesized using network meta-analysis to obtain the relative effects of HER2-positive-targeted therapies. We identified a gap in the reporting of the effectiveness of therapies by the hormone receptor status as only 15 out of 42 identified RCTs reported hormone receptor subgroup analyses; the majority of which reported progression-free survival but not OS or the overall response rate. In conclusion, we recommend that future trials in ABC should report the effect of cancer therapies in hormone receptor subgroups for all outcomes.
Introduction
Breast cancer is the most commonly diagnosed cancer and the fifth leading cause of cancer-related deaths worldwide (1). Advances in breast cancer screening and radiological and surgical techniques have helped to improve overall survival (OS) rates. Additionally, a deeper understanding of the underlying molecular drivers of breast cancer pathogenesis has led to the development of a range of targeted treatments, for example, to hormone receptors, human epidermal receptor 2 (HER2) receptors, or programmed death receptor ligand 1, allowing an era of personalized medicine to be realized (2). When considering HER2-positive breast cancer, the examples of targeted therapies include trastuzumab, lapatinib, trastuzumab emtansine, trastuzumab deruxtecan, and neratinib (3). The efficacy of these therapies has been demonstrated in randomized controlled trials (RCTs), leading to their market access approval by regulatory agencies, such as the European Medicines Agency and Food and Drug Administration in the US. These have been subsequently appraised by reimbursement agencies such as the National Institute for Health and Care Excellence (NICE) in the UK for use in routine clinical practice. The NICE determines clinical and cost-effectiveness (or value for money) for the population covered in the full market authorization. However, they may consider the use of subgroups (such as subgroups defined by the hormone-receptor biomarker status) if evidence shows an unclear value for money within one of the groups or in subgroups where patients are known to have improved prognosis. For example, the NICE appraisal of lapatinib or trastuzumab in combination with an aromatase inhibitor (AI) is recommended as the first-line treatment of HER2-positive advanced breast cancer (ABC), in the hormone-receptor-positive (HR+ve) population only (TA257; https://www.nice.org.uk/guidance/ta257). This review was undertaken to ascertain if there is available RCT evidence on the hormone-receptor status in HER2-positive ABC, as to whether the hormone-receptor status has a bearing on the clinical outcomes of individuals being treated for HER2-positive ABC. Specifically, we investigated the level of reporting of RCT results by the hormone-receptor status and explore whether the effectiveness of therapies in HER2-positive ABC patients varies according to the hormone-receptor status (i.e., estrogen and or progesterone biomarker status). Hormone-receptor subgroups were established as the HR+ve subgroup, which includes patients with a positive estrogen and/or progesterone receptor status, and the hormone-receptor-negative (HR-ve) subgroup, which includes patients whose status for both estrogen and progesterone were negative. Evidence from the identified trials was synthesized to estimate the effect of treatments on progression-free survival (PFS) in HR+ve or HR-ve subgroups. The next section in this paper discusses the methods used in this review, the results are discussed in section three, and section four concludes with a summary of the findings, recommendations, limitations, and further research.
Methodology
Literature review
RCTs were identified following a systematic approach, with a review of reviews carried out first followed by a search of more recent RCTs. The first step identified all the trials used as evidence in technology appraisals by the NICE for targeted therapies in HER2-positive ABC patients. This was followed by identifying reviews, systematic reviews, meta-analysis, and network meta-analysis published in peer-reviewed journals that included the RCTs of women with HER2-positive ABC (4–23, 25–29). This approach was employed to utilize comprehensive systematic reviews and network meta-analyses that included the RCTs of targeted therapies for HER2-positive ABC patients. The final step was an additional search for more recent RCTs evaluating targeted therapies among HER2-positive ABC patients. The eligibility criteria for the selection of RCTs and search terms are listed below.
Eligible criteria of selecting randomized controlled trials
The eligibility of the RCTs for inclusion in this study was defined by the following criteria for the population, interventions, comparators, and outcomes (PICOs):
● Phase two and three RCTs focusing primarily on female patients with HER2-positive ABC
● All treatments (interventions and comparators) targeted at HER2-positive ABC
● RCTs that reported at least one of the following outcomes: OS, PFS, and overall response rate (ORR)
RCTs excluded were:
● Studies reporting only outcomes with adverse effect or patients
● Studies focusing on treatment dose escalation and the biosimilar studies of trastuzumab
● Single-arm studies
● Studies involving only postmenopausal women, patients with brain metastasis, leptomeningeal meningitis, or central nervous system metastases to ensure the homogeneity of the trial populations across treatments
Search strategies
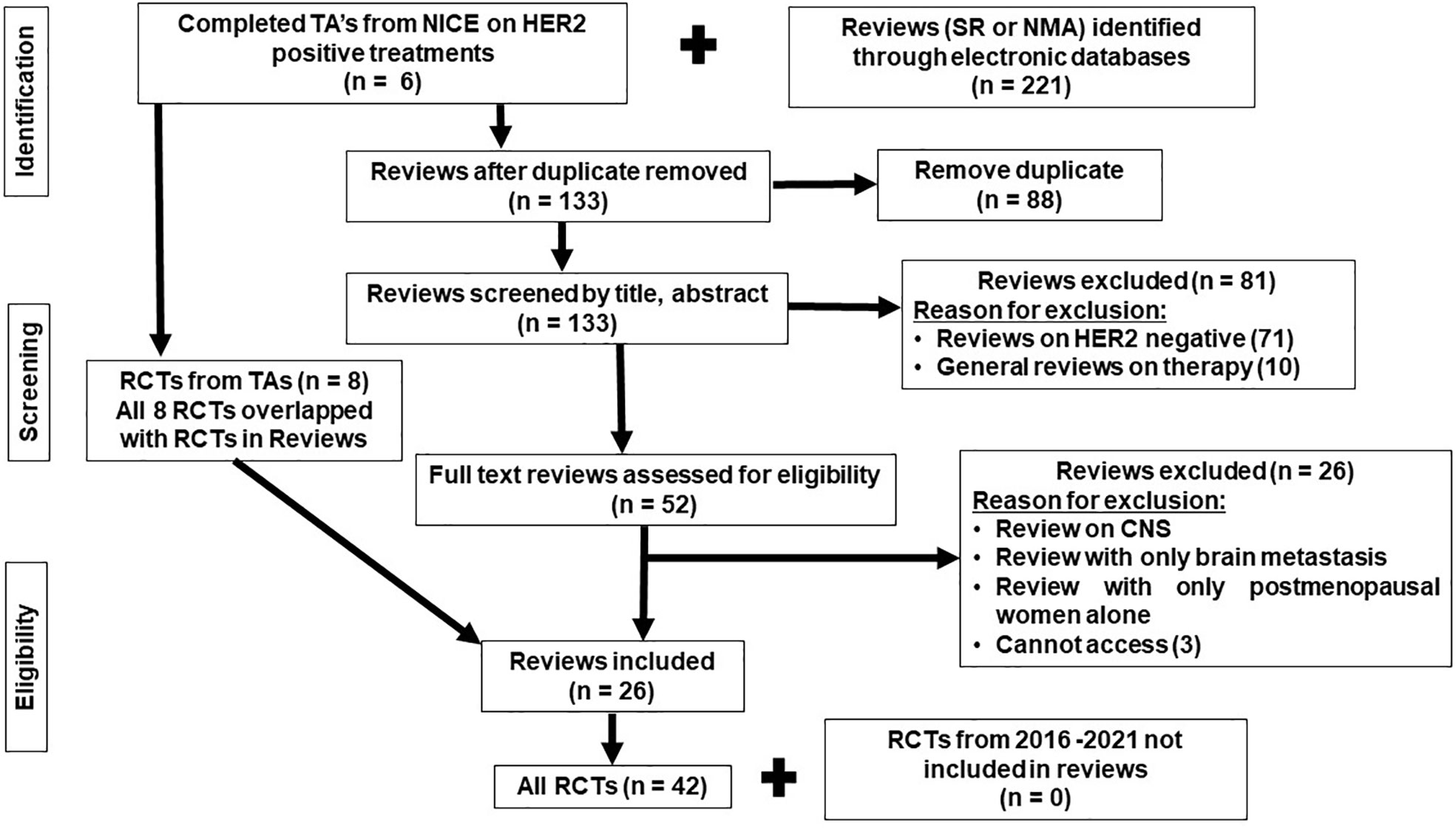
The search of the systematic reviews covered NICE guidelines, PubMed, Cochrane Library, and Scopus, with the search covering the period from the inception of the databases to 20 March 2022. More recent RCTs were then searched for within Scopus and PubMed, published in the last 6 years (2016–2022) to ensure that more recent RCTs were included. The PRISMA flow chart presenting all stages of study selection is shown in Figure 1. The search terms are included in the Supplementary File 1.
Statistical methods
Network meta-analyses (NMAs) were carried out to assess the efficacy of treatments identified in the review. Firstly, NMA was conducted using all the identified RCTs that formed a connected network (i.e., the trial had at least one treatment arm in common with another trial in the network) irrespective of whether the trial reported subgroups analyses or not. Secondly, NMA was conducted using information reported for hormone-receptor subgroups. The experimental treatments and comparators of the identified RCTs included in the NMAs are different, and thus, in order to make comparisons across treatments, a reference treatment comparator needed to be identified. The reference treatment comparator was selected as the most commonly evaluated treatment in the connected networks, or where there were multiple common treatment comparisons; then, the most efficacious treatment was selected (30). The efficacy of the treatments in the network including all HER2-positive patients were assessed based on PFS, OS, and the ORR. Treatment effects on PFS and OS were measured using hazard ratios (HRs), and the effects on ORR were measured using odds ratios (ORs). The comparative efficacy of cancer therapies by hormone-receptor subgroups was based on PFS, which was the most commonly reported outcome in the identified RCTs. A random-effects (31, 32) NMA in a Bayesian framework was used to synthesize evidence from the identified trials. The analyses were performed using the WinBUGS 1.4.3 software. The effectiveness estimates were reported as means and corresponding 95% credible intervals (Crls). Non-informative prior distributions were used with the full WinBUGS code provided in the Technical Support Document (33).
Results
All randomized controlled trial network results
Forty-two published RCTs focusing on treatments administered to HER2-positive ABC patients were identified from 26 reviews and four NICE technology appraisals (TAs) (34–80). The eight RCTs identified from the TAs overlapped with the RCTs identified in the reviews. There were no additional RCTs identified from the additional search (of RCTs published between 2006 and 2022) that have not been included in the reviews (Figure 1). All RCTs meeting the eligibility criteria and included in the review were phase II and phase III.
A network diagram of all 42 trials (reporting PFS) is displayed in Figure 2, similarly as in Cope et al. (81). Figure 2 included three networks of trials (with at least one arm common with another trial, thus forming a network) disconnected from each other due to a lack of a common comparator. In the plot (Figure 2), different colors in the circles indicate the proportion of patients in each RCT that are HR+ve (orange), HR-ve (green), unknown (blue), and not reported (gray). The trials reporting subgroup analyses by the hormone-receptor status are highlighted with a purple circle in the middle of a colored circle. Six RCTs recruited HR+ve patients, and of the 36 RCTs recruiting the mixed populations of HR+ve and HR-ve patients, only 15 RCTs reported separate hormone receptor subgroup analyses. The identified RCTs do not all form a connected network for the broader population; hence, three connected networks were investigated. These connected networks are the trastuzumab–taxane (HX)–connected network (Figure 2A), AI-connected network (Figure 2B), and the trastuzumab–chemotherapy (HChem)–connected network (Figure 2C). Paclitaxel and docetaxel, which inhibit microtubule dynamics, were classified as a taxane. Letrozole and anastrozole, which are non-steroid third-generational AIs that interfere with the production of estrogen, were classified as AIs (30, 82–85). NMAs were carried out to compare treatments that form each of the smaller connected networks. A list of all included RCTs is provided in the Supplementary File 2.
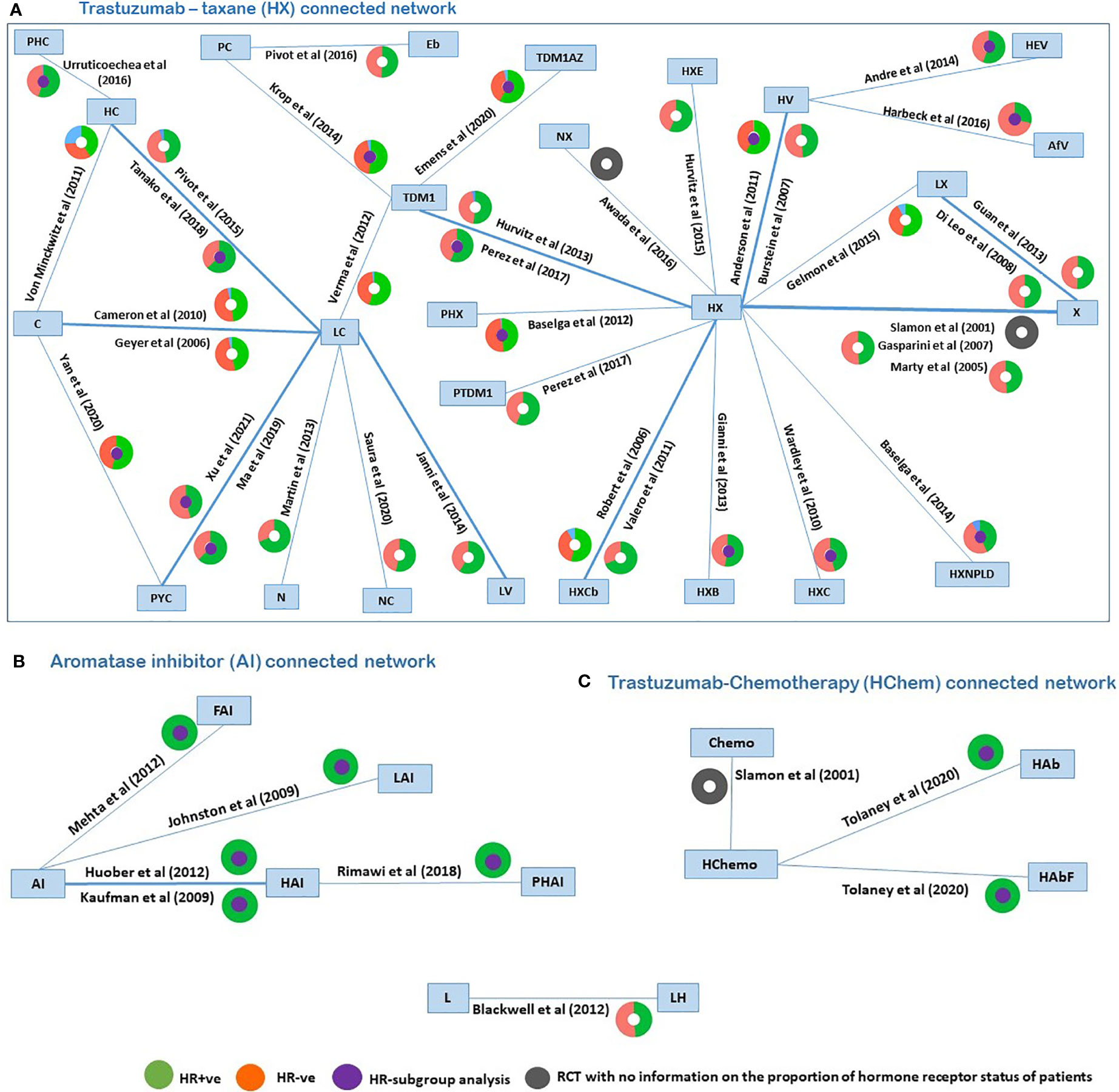
Figure 2 Network plots of identified trials (reporting PFS), with colors in the circles representing the proportion of patients in each RCT that are HR+ve (orange), HR-ve (green), unknown (blue), not reported (grey), and the middle purple circle indicated RCTs reporting subgroup analyses. PHC, pertuzumab + trastuzumab + capecitabine; PC, physician choice; LC, lapatinib + capecitabine; TDM1, trastuzumab emtansine; C, capecitabine; PYC, pyrotinib + capecitabine; LV, lapatinib + vinorelbine; HC, trastuzumab + capecitabine; N, neratinib; TDM1AZ, trastuzumab emtansine + atezolizumab; NX, neratinib + taxane; X, taxane (paclitaxel or docetaxel);NC, neratinib + capecitabine; HX, trastuzumab + taxane; HXB, trastuzumab + taxane + bevacizumab; LX, lapatinib + taxane; HV, trastuzumab + vinorelbine; HXE, trastuzumab + taxane + everolimus; PHX, pertuzumab + trastuzumab + taxane; HXC, trastuzumab + capecitabine + taxane; AfV, afatinib + vinorelbine; HEV, trastuzumab + everolimus + vinorelbine; HXCb, trastuzumab + taxane + carboplatin; PTDM1, pertuzumab + trastuzumab emtansine; Chemo, standard chemotherapy; LH, lapatinib + trastuzumab; L, lapatinib; AI, aromatase inhibitors (letrozole or anastrozole); LAI, lapatinib + AI; FAI, fulverstrant + AI; HAI, trastuzumab +AI; PHAI, pertuzumab + trastuzumab +AI; HAb, trastuzumab + abemaciclib; HAbF, trastuzumab + abemaciclib + fulverstrant; HXNPLD, trastuzumab + taxane + NPLD; NPLD, non-pegylated liposomal doxorubicin; HChemo, trastuzumab + chemotherapy; Eb, eribulin.
For the network of treatment comparisons for the total population (Figure 2), HX was the most commonly evaluated intervention and was thus used as the reference treatment comparator. The treatment effect estimates and corresponding 95% Crls for PFS in this population for each connected network are provided in Figure 3. In the overall NMA, taxane showed an important increase in the risk of disease progression compared to HX with a hazard ratio of 2.21 (95% Crl: 1.61, 2.91); pyrotinib + capecitabine (PYC) showed an important reduction in the risk of progression compared to HX with a hazard ratio of 0.44 (0.20, 0.82); and capecitabine appeared to show a meaningful increase in the risk of progression compared to HX with a hazard ratio of 2.22 (1.00, 3.86). Other treatments evaluated using HX as the reference treatment did not show a meaningful difference in effect as their 95% CrI spans the point of no difference (1). The relative treatment effects (for all treatment comparisons in the network) for PFS, OS, and ORR are reported in the Supplementary File 3. For example, HER2-positive-targeted therapies combined with taxane—such as lapatinib with taxane (LX), neratinib with taxane (NX), trastuzumab with taxane and bevacizumab (HXB), trastuzumab with taxane and carboplatin (HXCb), trastuzumab with taxane and capecitabine (HC), trastuzumab with taxane and pertuzumab (PHX), trastuzumab with everolimus and taxane (HXE), and trastuzumab with taxane and non-pegylated liposomal doxorubicin (HXNPLD)—and some targeted therapies like trastuzumab emtansine (TDM1) and neratinib with capecitabine all had an important decreased risk of disease progression compared to taxane alone. In addition, TDM1 (using the point estimates) showed to prolong overall survival when compared to other HER2-positive-targeted therapies like HX, HC, LC, taxane, and LX (see Supplementary File 3). Pertuzumab with TDM1 (PTDM1) showed a meaningful decreased risk in disease progression compared to LC, capecitabine, taxane, and neratinib. The relative treatment effects of all treatments evaluated in the mixed and hormone receptor subgroup population are reported in the Supplementary File 3. PYC showed a meaningful decreased risk in disease progression compared to some targeted therapies such as HX, TDM1, LX, and trastuzumab with capecitabine. The meaningful treatment effects showed by PYC could be associated with the fact that pyrotinib is an irreversible inhibitor of the ERBB family including HER1, HER2, and HER4; therefore, potentially allowing wider HER2 inhibition compared to other anti-HER2 therapies. In addition, PYC was evaluated only as a second line of therapy, which may have had an impact on the results from the NMA as we discuss in more detail in the Discussion section. For the AI-connected network (Figure 2B), only HR+ve patients were included as the AI therapies are only used in the HR+ve breast cancer setting (84).
Results of subgroup analyses
Among the 15 RCTs that recruited the mixed populations of hormone-receptor status patients and reported their subgroup analyses; 13 RCTs reported results for HR+ve patients and 14 RCTs reported results for HR-ve patients. The number of treatment regimens evaluated in the hormone-receptor subgroups (16) was smaller than the treatment regimens evaluated in the overall NMA (26). These do not include treatment regimens in the AI- and HChem-connected network, as RCTs in both connected networks have primarily HR+ve participants. The network plots of RCTs within the hormone-receptor subgroups are displayed in Figure 4. The RCTs that reported results for the hormone-receptor subgroups formed two disconnected networks in the subgroup analysis: HX-connected network, and capecitabine-connected network. Figures 5, 6 shows the summary forest plots of treatment effects for PFS in the hormone-receptor subgroups, respectively, for the HX-connected network and capecitabine-connected network. The treatment effects from the HR+ve subgroup and HR-ve subgroup are depicted with red and blue bar plots, respectively. The green bar plots show the estimated treatment effects for the mixed patients using only RCTs that reported subgroup analysis, and the gray bar plots depict the treatment effects extracted from the overall NMA including all RCTs (Figure 3). In the subgroup analysis, PYC showed a meaningful reduction in the risk of disease progression compared to lapatinib with capecitabine (LC) in the HR-ve subgroup analysis with a hazard ratio of 0.31 (95%Crl: 0.12, 0.70). Other treatment regimens evaluated in the capecitabine- or HX-connected network did not show a meaningful effect as the 95% CrIs included the point of no difference (value of 1).
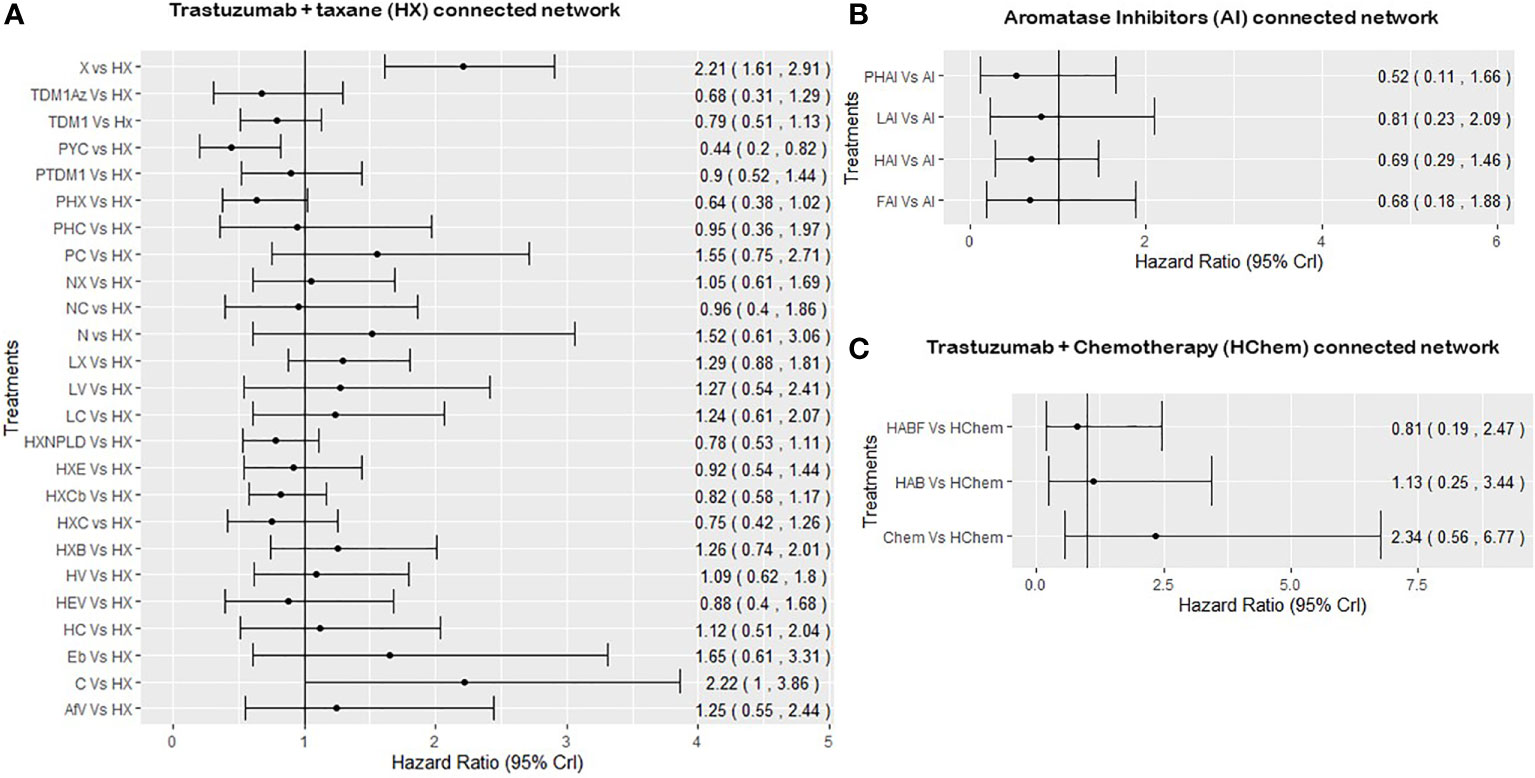
Figure 3 Summary forest plots obtained from the NMA including all RCTs for PFS. PHC, pertuzumab + trastuzumab + capecitabine; PC, physician choice; LC, lapatinib + capecitabine; TDM1, trastuzumab emtansine; C, capecitabine; PYC, pyrotinib + capecitabine; LV, lapatinib + vinorelbine; HC, trastuzumab + capecitabine; N, neratinib; TDM1AZ, trastuzumab emtansine + atezolizumab; NX, neratinib + taxane; X, taxane (paclitaxel or docetaxel);NC, neratinib + capecitabine; HX, trastuzumab + taxane; HXB, trastuzumab + taxane + bevacizumab; LX, lapatinib + taxane; HV, trastuzumab + vinorelbine; HXE, trastuzumab + taxane + everolimus; PHX, pertuzumab + trastuzumab + taxane; HXC, trastuzumab + capecitabine + taxane; AfV, afatinib + vinorelbine; HEV, trastuzumab + everolimus + vinorelbine; HXCb, trastuzumab + taxane + carboplatin; PTDM1, pertuzumab + trastuzumab emtansine; Chemo, standard chemotherapy; LH, lapatinib + trastuzumab; L, lapatinib; AI, aromatase inhibitors (letrozole or anastrozole); LAI, lapatinib + AI; FAI, fulverstrant + AI; HAI, trastuzumab +AI; PHAI, pertuzumab + trastuzumab +AI; HAb, trastuzumab + abemaciclib; HAbF, trastuzumab + abemaciclib + fulverstrant; HXNPLD, trastuzumab + taxane + NPLD; NPLD, non-pegylated liposomal doxorubicin; HChemo, trastuzumab + chemotherapy; Eb, eribulin. Treatment effects are considered to be statistically significance if the 95% credible interval does not include the point of no difference which, 1.
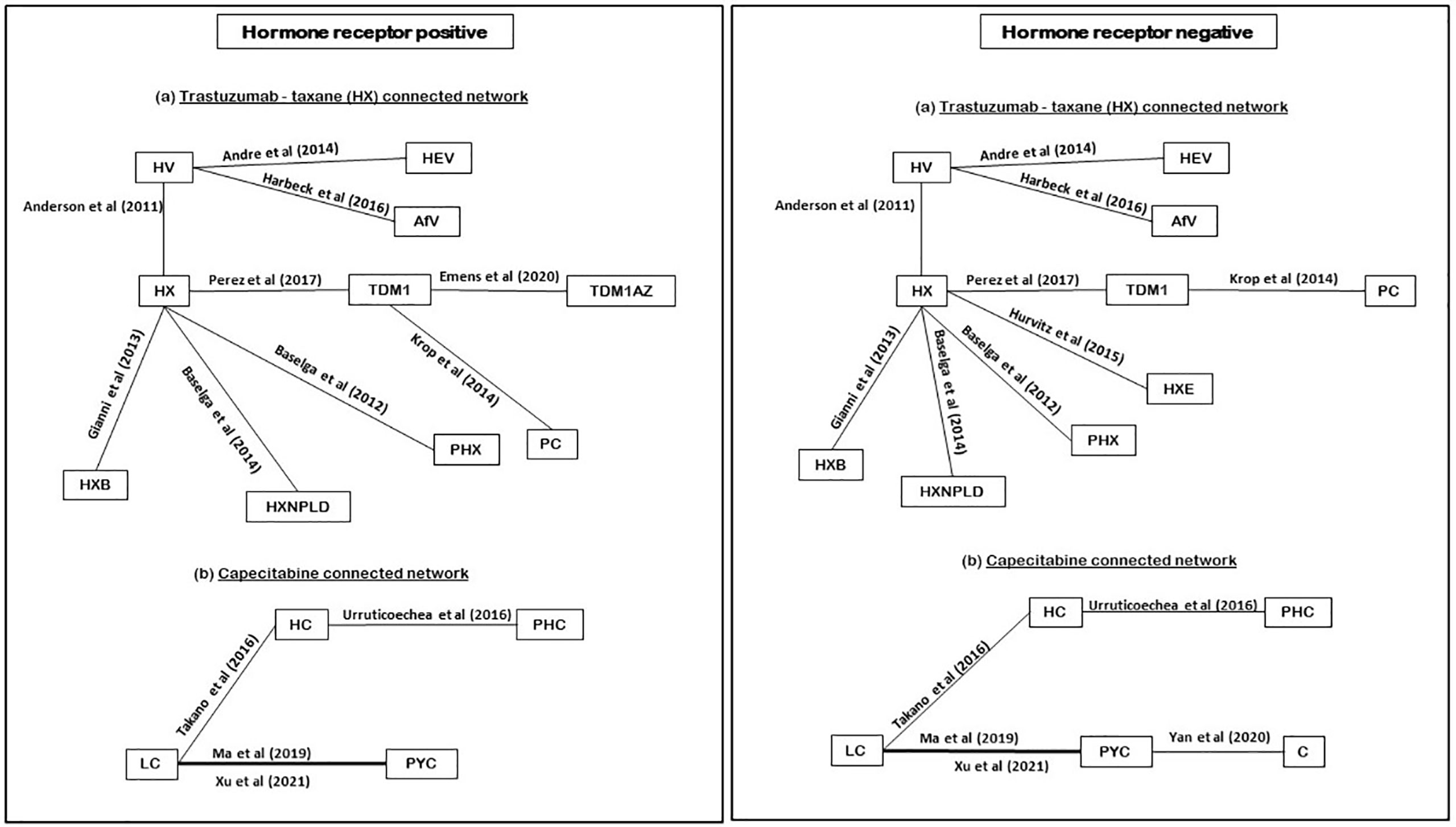
Figure 4 Network plot of hormone receptors subgroup RCTs (reporting PFS). PHC, pertuzumab + trastuzumab + capecitabine; PC, physician choice; LC, lapatinib + capecitabine; TDM1, trastuzumab emtansine; C, capecitabine; PYC, pyrotinib + capecitabine; HC, trastuzumab + capecitabine; TDM1AZ, trastuzumab emtansine + atezolizumab; HX, trastuzumab + taxane; HXB, trastuzumab + taxane + bevacizumab; LX, lapatinib + taxane; HV, trastuzumab + vinorelbine; HXE, trastuzumab + taxane + everolimus; PHX, pertuzumab + trastuzumab + taxane; AfV, afatinib + vinorelbine; HEV, trastuzumab + everolimus + vinorelbine; HXNPLD, trastuzumab + taxane + NPLD; NPLD, non-pegylated liposomal doxorubicin.
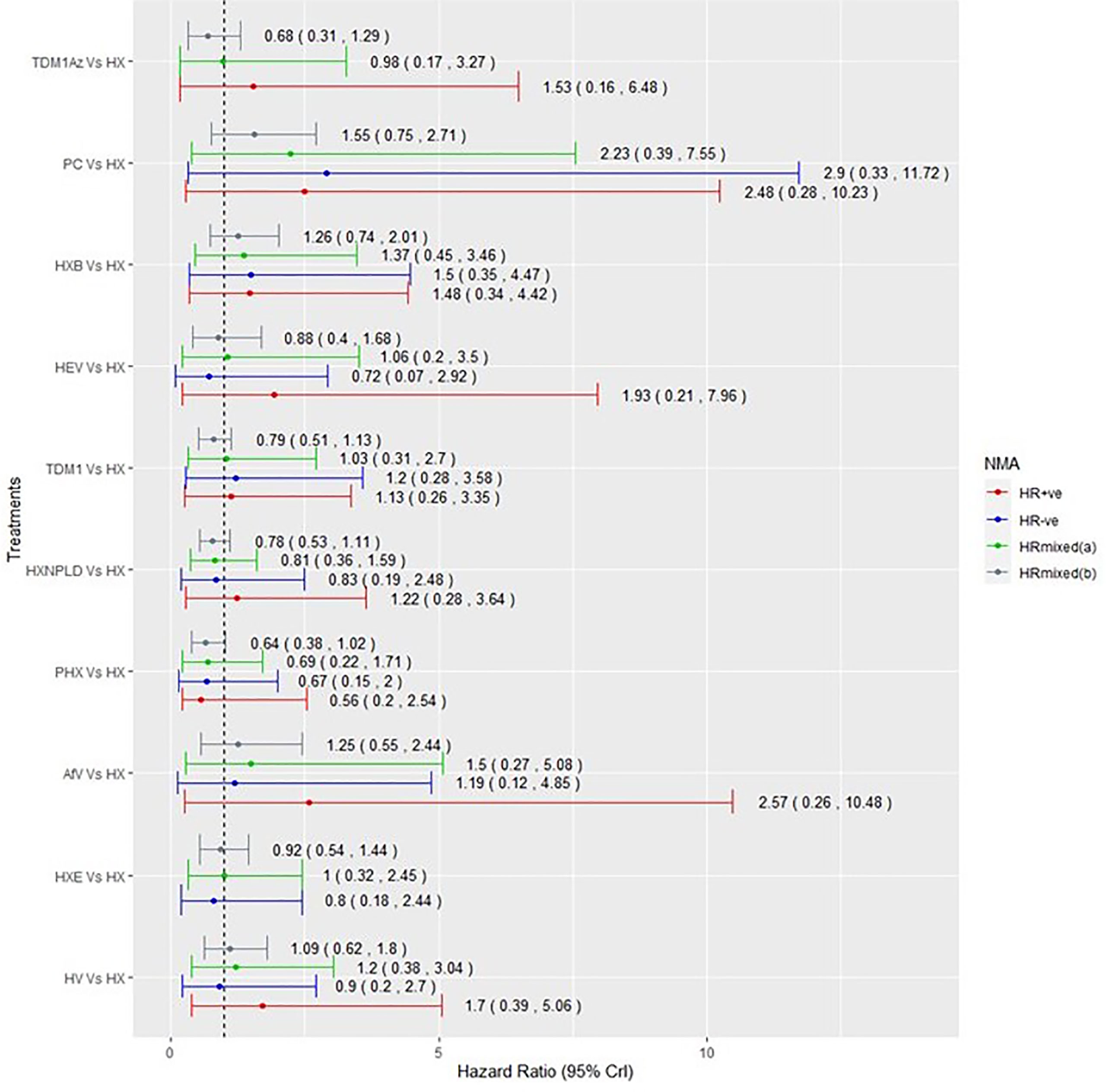
Figure 5 Comparative summary forest plots of treatment effects obtained from the HX connected network for PFS. PC, physician choice; LC, lapatinib + capecitabine; TDM1, trastuzumab emtansine; TDM1AZ, trastuzumab emtansine + atezolizumab; HX, trastuzumab + taxane; HXB, trastuzumab + taxane + bevacizumab; LX, lapatinib + taxane; HV, trastuzumab + vinorelbine; HXE, trastuzumab + taxane + everolimus; PHX, pertuzumab + trastuzumab + taxane; AfV, afatinib + vinorelbine; HEV, trastuzumab + everolimus + vinorelbine; HXNPLD, trastuzumab + taxane + NPLD; NPLD, non-pegylated liposomal doxorubicin.
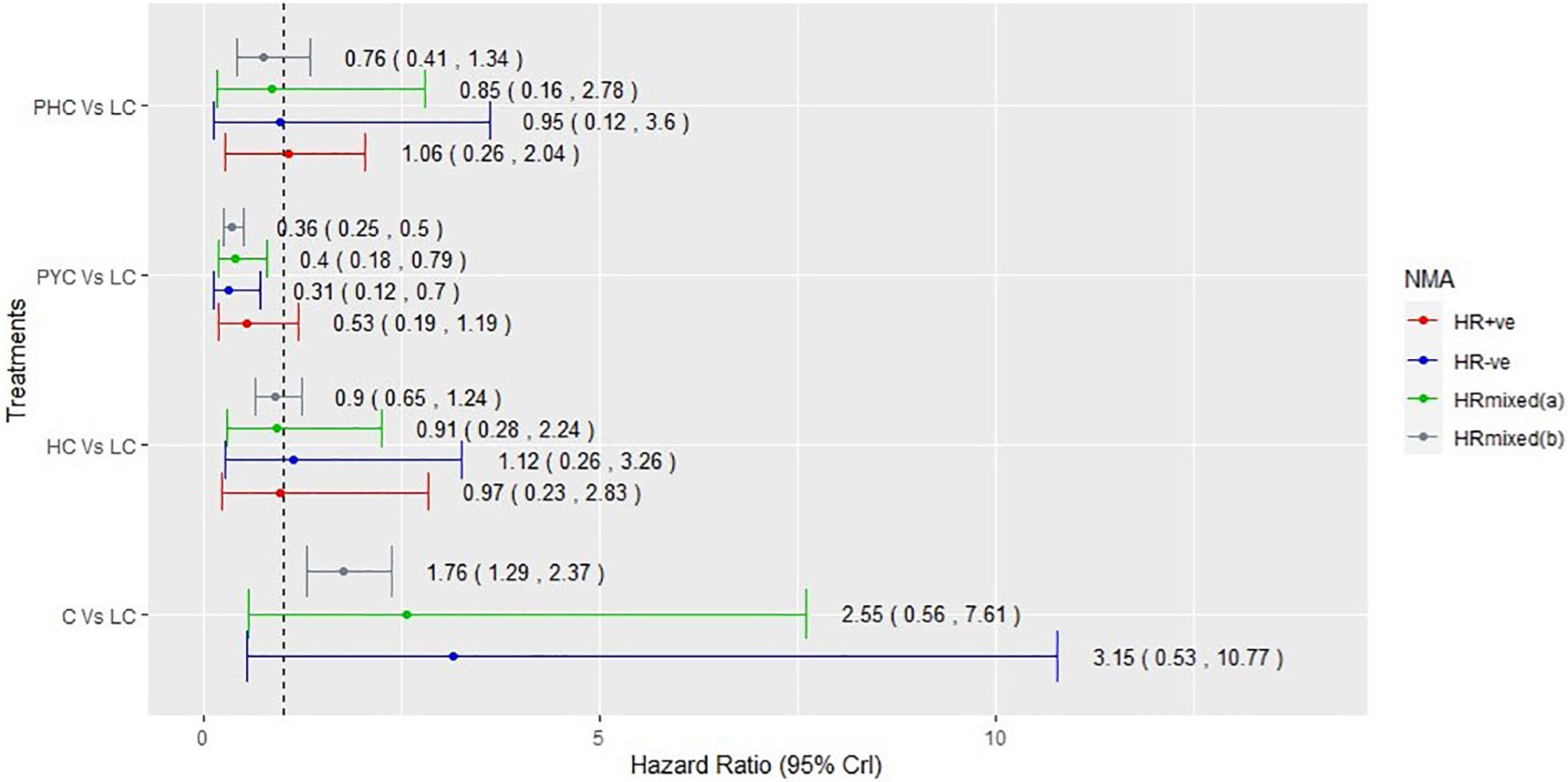
Figure 6 Comparative summary forest plots of treatment effects obtained from capecitabine connected network for PFS. PHC, pertuzumab + trastuzumab + capecitabine; LC, lapatinib + capecitabine; C, capecitabine; PYC, pyrotinib + capecitabine; HC, trastuzumab + capecitabine.
Discussion and conclusion
We have conducted the first review of RCTs involving HER2-positive ABC, specifically focusing on the reporting of treatment effects by the hormone receptor status. We found that the RCTs that reported subgroup analyses reported PFS, not OS or ORR. We would like to note that despite PFS being the primary endpoint of these RCTs, the evidence of its surrogacy for OS in HER2-positive ABC is limited (86).
Our results show that, regardless of the hormone-receptor status of the patients, taxane-only therapies were associated with an important increased risk of disease progression compared to HX as well as to other targeted therapies combined with a taxane (as shown in Supplementary File 3). This supports the findings from the wider literatures (7, 45, 48, 59, 66). PYC showed a meaningful effect over HX with a hazard ratio of 0.44 (95% Crl: 0.20, 0.82). In the subgroup analyses, PYC showed a meaningful effect over LC in the HR-ve subgroup analysis with a hazard ratio of 0.31 (95% Crl: 0.12, 0.70) and the mixed patients’ analysis with a hazard ratio of 0.40 (95% Crl: 0.18, 0.79).
In addition, our results indicate that the point estimates of HER2 treatments in combination with an AI show a meaningful effect over AI alone, which support the findings by Kawalec et al. (13).
One of the limitations of the review, from the point of view of the clinical interpretation, was the fact that our NMA for both the overall population and the hormone-receptor subgroups included all RCTs that evaluated targeted therapies in HER2-positive patients irrespective of their line of treatments. We chose this approach to capture accumulating relevant evidence available in the reporting of hormone receptor subgroup analysis in the RCTs, as the primary aim of this review was to assess the level of reporting of the effectiveness of therapies in the biomarker subgroups and the impact of under-reporting on the results of NMA. The non-homogeneity of the included RCTs in terms of the treatment line could have played a significant role in the results obtained from the NMA. For example, as mentioned in the Results section, the three RCTs that evaluated PYC in comparison to either LC or capecitabine recruited HER2-positive ABC patients whose disease has progressed after receiving HX, which could have resulted in a meaningful and relatively large treatment difference between PYC and HX. The conclusions drawn from these results are not specific to the line of therapy, and, therefore, the clinical interpretation of these results is limited. Moreover, the sparse and almost-star-shaped geometry of the network as well as the lack of a direct evidence of PYC with other HER2-targeted therapies, such as TDM1, pertuzumab, or HX, mean that there are further limitations of the results in terms of their reliability for the clinical interpretation.
Our review did not identify important differences in treatment effectiveness across hormone-receptor subgroups.
The treatment effect estimates for the subgroup analyses were estimated with increased uncertainty (compared to the mixed population), not only due to the reduced sample size in the subgroups but also due to the limited reporting of the subgroup analyses of the RCTs. However, across treatments, the HR-ve subgroup often presents with a lower estimated hazard ratio than HR+ve patients for PFS. This may therefore warrant a further RCT, powered to investigate the efficacy of HER2-targeted therapies among hormone-receptor subgroups and extending the outcomes assessed by the subgroups to include not only PFS but also OS and ORR. This is because, while PFS may be an attractive primary endpoint as it is available earlier than OS, and is not influenced by subsequent treatments, questions regarding whether PFS is a valid surrogate for OS remain (87–89). Alternatively, an RCT could also be complemented with an analysis of electronic health records (EHRs) to explore if these HER2-targeted therapies are more effective in HR+ve patients compared to HR-ve patients.
Our work serves as an example of exploring the support of a broad evidence base (across treatments) for subgroup effects. It illustrates the evidential and methodological challenges in formally considering subgroup effects using extended networks, which arise due to the limited reporting of subgroup results not only across trials but also across outcomes. This work is still important to inform the value and uncertainty over restricted use in decisions at the national level, such as those facilitated by the NICE in the UK. This is particularly important where the clinical and economic value of a treatment in a particular subgroup is unclear, and therefore, the value of wide adoption is also unclear. In this case, drawing on such an extended evidence base can inform further research recommendations, particularly in considering whether subgroup effects may be generalized across treatments. Our review could be further extended to include data that target the wider HER2 treatment pathway or to include outcomes such as adverse events, the quality or life, or time to progression.
Author contributions
Conceptualization and design: CMU-C, SB. Data collection: CMU-C, SB. Statistical analysis: CMU-C. Clinical expertise: OA, SK. Manuscript: CMU-C. Critical revision of the manuscript: CMU-C, MS, OA, RO, SK, KA, SB. Supervision: MS, RO, KA, SB. Funding acquisition: SB, KA, RO. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Medical Research Council, Methodology Research Panel grant [MR/T025166/1].
Conflict of interest
SB is a member of the NICE Decision Support Unit. She served as a paid consultant, providing unrelated methodological advice to the NICE, pharmaceutical industry and consultancy companies. She received payments for educational events from Roche and has received research funding from European Federation of Pharmaceutical Industries and Association (EFPAI) and Johnson and Johnson. RO is a member of the National Institute for health and Care Excellence (NICE) Technology Appraisal committee, member of the NICE Decision Support Unit (DSU), and associate member of the NICE Technical Support Unit (TSU). She has served as a paid consultant to the pharmaceutical industry, providing unrelated methodological advice. She reports teaching fees from the Association of British Pharmaceutical Industry (ABPI) and the University of Bristol. KA is a member of the National Institute for Health and Care Excellence (NICE) Diagnostics Advisory Committee and is a National Institute for Health and Care Research (NIHR) Senior Investigator Emeritus. He has acted as a paid consultant, providing unrelated methodological and strategic advice, to the pharmaceutical and life sciences industry generally, as well as to UK Department of Health and Social Care (DHSC)/NICE, and has received unrelated research funding from; Association of the British Pharmaceutical Industry (ABPI), European Federation of Pharmaceutical Industries and Associations (EFPIA), Pfizer, Sanofi and Swiss Precision Diagnostics. He has also received course fees from ABPI and is a Partner/Director of Visible Analytics Limited. SK is supported by a NIHR academic Clinical Lecturer award and has no conflicts of interest to declare. MS is a member of a research funding panel for the National Institute for Health and Care Research (NIHR), and collaborates with the NICE Decision Support Unit (DSU). She has served as a paid consultant to the pharmaceutical and life sciences industry generally, as well as to DHSC/NICE, providing unrelated methodological advice.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.943154/full#supplementary-material
Supplementary file 1 | Name: Searchterms.docx. Description: List of the search terms used in the review
Supplementary file 2 | Name: IncludedRCTs.docx. Description: List of RCTs included in the review
Supplementary file 3 | Name: PFS treatment effects.xls. Description: Relative treatment effects of the targeted therapies evaluated
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Park Y, Senkus-Konefka E, Im S-A, Pentheroudakis G, Saji S, Gupta S, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with early breast cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol (2020) 31(4):451–69. doi: 10.1016/j.annonc.2020.01.008
3. Cesca MG, Vian L, Cristóvão-Ferreira S, Pondé N, de Azambuja E. HER2-positive advanced breast cancer treatment in 2020. Cancer Treat Rev (2020) 88:102033. doi: 10.1016/j.ctrv.2020.102033
4. Wilcken N, Zdenkowski N, White M, Snyder R, Pittman K, Mainwaring P, et al. Systemic treatment of HER2-positive metastatic breast cancer: A systematic review. Asia Pac J Clin Oncol (2014) 10 Suppl S4:1–14. doi: 10.1111/ajco.12206
5. Bai X, Lin X, Song J, Chang JH, Han LL, Fan C. Incidence of central nervous system metastases in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer treated with trastuzumab: A meta-analysis. Clinics (Sao Paulo) (2021) 76:e2653. doi: 10.6061/clinics/2021/e2653
6. Chen IC, Hu FC, Lin CH, Huang SM, Chang DY, Cheng AL, et al. Anti-HER2 antibody prolongs overall survival disproportionally more than progression-free survival in HER2-positive metastatic breast cancer patients. Breast (2021) 59:211–20. doi: 10.1016/j.breast.2021.07.006
7. Xie BJ, Zhu LN, Ma C, Li JB, Dong L, Zhu ZN, et al. A network meta-analysis on the efficacy of HER2-targeted agents in combination with taxane-containing regimens for treatment of HER2-positive metastatic breast cancer. Breast Cancer (2020) 27(2):186–96. doi: 10.1007/s12282-019-01007-9
8. Yu Q, Zhu Z, Liu Y, Zhang J, Li K. Efficacy and safety of HER2-targeted agents for breast cancer with HER2-overexpression: A network meta-analysis. PLoS One (2015) 10(5):e0127404. doi: 10.1371/journal.pone.0127404
9. Paracha N, Reyes A, Diéras V, Krop I, Pivot X, Urruticoechea A. Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: A systematic review and network meta-analysis. Breast Cancer Res Treat (2020) 180(3):597–609. doi: 10.1007/s10549-020-05577-7
10. Koleva-Kolarova RG, Oktora MP, Robijn AL, Greuter MJW, Reyners AKL, Buskens E, et al. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat Rev (2017) 55:16–25. doi: 10.1016/j.ctrv.2017.01.001
11. Leung HWC, Leung JH, Chan ALF. Efficacy and safety of a combination of HER2-targeted agents as first-line treatment for metastatic HER2-positive breast cancer: a network meta-analysis. Expert Opin Drug Saf (2018) 17(1):1–7.
12. Zhang J, Huang Y, Wang C, He Y, Zheng S, Wu K. Efficacy and safety of endocrine monotherapy as first-line treatment for hormone-sensitive advanced breast cancer: A network meta-analysis. Med (Baltimore) (2017) 96(33):e7846. doi: 10.1097/MD.0000000000007846
13. Kawalec P, Łopuch S, Mikrut A. Effectiveness of targeted therapy in patients with previously untreated metastatic breast cancer: A systematic review and meta-analysis. Clin Breast Cancer (2015) 15(2):90–100.e1. doi: 10.1016/j.clbc.2014.10.006
14. Mendes D, Alves C, Afonso N, Cardoso F, Passos-Coelho JL, Costa L, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer–a systematic review. Breast Cancer Res (2015) 17:140. doi: 10.1186/s13058-015-0648-2
15. Chen F, Chen N, Lv Z, Li L, Cui J. Efficacy of second-line treatments for patients with advanced human epidermal growth factor receptor 2 positive breast cancer after trastuzumab-based treatment: A systematic review and bayesian network analysis. J Cancer (2021) 12(6):1687. doi: 10.7150/jca.51845
16. Erickson AW, Ghodrati F, Habbous S, Jerzak KJ, Sahgal A, Ahluwalia MS, et al. HER2-targeted therapy prolongs survival in patients with HER2-positive breast cancer and intracranial metastatic disease: A systematic review and meta-analysis. Neuro-oncol Adv (2020) 2(1):vdaa136. doi: 10.1093/noajnl/vdaa136
17. Yu YF, Wang Y, Fu TP, Chen K, Liu JQ, Yao HR. Trastuzumab combined with doublet or single-agent chemotherapy as first-line therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat (2018) 168(2):337–48. doi: 10.1007/s10549-017-4592-y
18. Zhang T, Feng F, Zhao W, Yao Y, Tian J, Zhou C, et al. Comparative efficacy of different targeted therapies plus fulvestrant for advanced breast cancer following progression on prior endocrine therapy: A network meta-analysis. Cancer Manag Res (2018) 10:5869–80. doi: 10.2147/CMAR.S176172
19. Niraula S, Ocana A. Mechanism of drug resistance in relation to site of metastasis: Meta-analyses of randomized controlled trials in advanced breast cancer according to anticancer strategy. Cancer Treat Rev (2016) 50:168–74. doi: 10.1016/j.ctrv.2016.09.011
20. Squires H, Stevenson M, Simpson E, Harvey R, Stevens J. Trastuzumab emtansine for treating HER2-positive, unresectable, locally advanced or metastatic breast cancer after treatment with trastuzumab and a taxane: An evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics (2016) 34(7):673–80. doi: 10.1007/s40273-016-0386-z
21. Ibrahim EM, Kazkaz GA, Al-Mansour MM, Al-Foheidi ME. The predictive and prognostic role of phosphatase phosphoinositol-3 (PI3) kinase (PIK3CA) mutation in HER2-positive breast cancer receiving HER2-targeted therapy: A meta-analysis. Breast Cancer Res Treat (2015) 152(3):463–76. doi: 10.1007/s10549-015-3480-6
22. Petrelli F, Barni S. Surrogate endpoints in metastatic breast cancer treated with targeted therapies: An analysis of the first-line phase III trials. Med Oncol (2014) 31(1):776. doi: 10.1007/s12032-013-0776-4
23. Balduzzi S, Mantarro S, Guarneri V, Tagliabue L, Pistotti V, Moja L, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev (2014) 2014(6):Cd006242.
24. Wilcken N, Zdenkowski N, White M, Snyder R, Pittman K, Mainwaring P, et al. Systemic treatment of HER 2-positive metastatic breast cancer: A systematic review? Asia-Pacific Journal of Clinical Oncology (2014) 10:1–14
25. Zhu ZL, Zhang J, Chen ML, Li K. Efficacy and safety of trastuzumab added to standard treatments for HER2-positive metastatic breast cancer patients. Asian Pac J Cancer Prev (2013) 14(12):7111–6. doi: 10.7314/APJCP.2013.14.12.7111
26. Riemsma R, Forbes CA, Amonkar MM, Lykopoulos K, Diaz JR, Kleijnen J, et al. Systematic review of lapatinib in combination with letrozole compared with other first-line treatments for hormone receptor positive(HR+) and HER2+ advanced or metastatic breast cancer(MBC). Curr Med Res Opin (2012) 28(8):1263–79. doi: 10.1185/03007995.2012.707643
27. Fleeman N, Bagust A, Boland A, Dickson R, Dundar Y, Moonan M, et al. Lapatinib and trastuzumab in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone receptor-positive breast cancer which over-expresses human epidermal growth factor 2 (HER2): A systematic review and economic analysis. Health Technol Assess (2011) 15(42):1–93, iii-iv. doi: 10.3310/hta15420
28. Amir E, Ocaña A, Seruga B, Freedman O, Clemons M. Lapatinib and HER2 status: results of a meta-analysis of randomized phase III trials in metastatic breast cancer. Cancer Treat Rev (2010) 36(5):410–5. doi: 10.1016/j.ctrv.2009.12.012
29. Tan PS, Haaland B, Montero AJ, Lopes G. A meta-analysis of anastrozole in combination with fulvestrant in the first line treatment of hormone receptor positive advanced breast cancer. Breast Cancer Res Treat (2013) 138(3):961–5. doi: 10.1007/s10549-013-2495-0
30. Ter Veer E, Van Oijen MG, Van Laarhoven HW. The use of (network) meta-analysis in clinical oncology. Front Oncol (2019) 9:822. doi: 10.3389/fonc.2019.00822
31. Lu G, Ades A. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med (2004) 23(20):3105–24. doi: 10.1002/sim.1875
32. Caldwell DM, Ades A, Higgins J. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj (2005) 331(7521):897–900. doi: 10.1136/bmj.331.7521.897
33. Dias S, Welton NJ, Sutton AJ, Ades A. NICE DSU technical support document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. (2011). Explore Bristol Research, University of Bristol, 2011.
34. Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2–positive breast cancer: the HERNATA study. J Clin Oncol (2011) 29(3):264–71. doi: 10.1200/JCO.2010.30.8213
35. André F, O'Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol (2014) 15(6):580–91. doi: 10.1016/S1470-2045(14)70138-X
36. Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: The NEfERT-T randomized clinical trial. JAMA Oncol (2016) 2(12):1557–64. doi: 10.1001/jamaoncol.2016.0237
37. Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. New Engl J Med (2012) 366(6):520–9. doi: 10.1056/NEJMoa1109653
38. Baselga J, Manikhas A, Cortés J, Llombart A, Roman L, Semiglazov V, et al. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Ann Oncol (2014) 25(3):592–8. doi: 10.1093/annonc/mdt543
39. Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol (2010) 28(7):1124–30. doi: 10.1200/JCO.2008.21.4437
40. Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: The trastuzumab and vinorelbine or taxane study. Cancer (2007) 110(5):965–72. doi: 10.1002/cncr.22885
41. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat (2008) 112(3):533–43. doi: 10.1007/s10549-007-9885-0
42. Gelmon KA, Boyle F, Kaufman B, Huntsman D, Manikhas A, Di Leo A, et al. Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA. 31/GSK EGF 108919. Am Soc Clin Oncol (2012). doi: 10.1200/jco.2012.30.18_suppl.lba671
43. Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim S-B, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol (2020) 21(10):1283–95. doi: 10.1016/S1470-2045(20)30465-4
44. Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, et al. Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with her-2 positive advanced breast cancer. Breast Cancer Res Treat (2007) 101(3):355–65. doi: 10.1007/s10549-006-9306-9
45. Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol (2008) 26(34):5544.
46. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New Engl J Med (2006) 355(26):2733–43. doi: 10.1056/NEJMoa064320
47. Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, et al. AVEREL: A randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol (2013) 31(14):1719–25. doi: 10.1200/JCO.2012.44.7912
48. Guan Z, Xu B, DeSilvio ML, Shen Z, Arpornwirat W, Tong Z, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2–overexpressing metastatic breast cancer. J Clin Oncol (2013) 31(16):1947–53. doi: 10.1200/JCO.2011.40.5241
49. Harbeck N, Huang C-S, Hurvitz S, Yeh D-C, Shao Z, Im S-A, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-breast 1): An open-label, randomised, phase 3 trial. Lancet Oncol (2016) 17(3):357–66. doi: 10.1016/S1470-2045(15)00540-9
50. Huober J, Fasching P, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer–results of the eLEcTRA trial. Breast (2012) 21(1):27–33. doi: 10.1016/j.breast.2011.07.006
51. Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol (2015) 16(7):816–29. doi: 10.1016/S1470-2045(15)00051-0
52. Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol (2013) 31(9):1157–63. doi: 10.1200/JCO.2012.44.9694
53. Janni W, Sarosiek T, Karaszewska B, Pikiel J, Staroslawska E, Potemski P, et al. Randomized, multicenter study evaluating the combination of lapatinib and vinorelbine in women with ErbB2 overexpressing metastatic breast cancer. Breast Cancer Res Treat (2014) 143(3):493–505. doi: 10.1007/s10549-013-2828-z
54. Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol (2009) 27(33):5538–46. doi: 10.1200/JCO.2009.23.3734
55. Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2–positive, hormone receptor–positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol (2009) 27(33):5529–37. doi: 10.1200/JCO.2008.20.6847
56. Krop IE, Kim S-B, González-Martín A, LoRusso PM, Ferrero J-M, Smitt M, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(7):689–99. doi: 10.1016/S1470-2045(14)70178-0
57. Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. New Engl J Med (2012) 367(5):435–44. doi: 10.1056/NEJMoa1201622
58. Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, et al. Pyrotinib or lapatinib combined with capecitabine in HER2–positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: A randomized, phase II study. J Clin Oncol (2019) 37(29):2610–9. doi: 10.1200/JCO.19.00108
59. Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol (2005) 23(19):4265–74. doi: 10.1200/JCO.2005.04.173
60. Martin M, Bonneterre J, Geyer CE Jr., Ito Y, Ro J, Lang I, et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer (2013) 49(18):3763–72. doi: 10.1016/j.ejca.2013.07.142
61. Perez EA, Barrios C, Eiermann W, Toi M, Im Y-H, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2–positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol (2017) 35(2):141. doi: 10.1200/JCO.2016.67.4887
62. Pivot X, Marmé F, Koenigsberg R, Guo M, Berrak E, Wolfer A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol (2016) 27(8):1525–31. doi: 10.1093/annonc/mdw203
63. Rimawi M, Ferrero J-M, de la Haba-Rodriguez J, Poole C, De Placido S, Osborne CK, et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2–positive and hormone receptor–positive metastatic or locally advanced breast cancer (PERTAIN): A randomized, open-label phase II trial. J Clin Oncol (2018) 36(28):2826–35. doi: 10.1200/JCO.2017.76.7863
64. Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2–overexpressing metastatic breast cancer. J Clin Oncol (2006) 24(18):2786–92. doi: 10.1200/JCO.2005.04.1764
65. Saura C, Oliveira M, Feng Y-H, Dai M-S, Chen S-W, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol (2020) 38(27):3138.
66. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med (2001) 344(11):783–92. doi: 10.1056/NEJM200103153441101
67. Takano T, Tsurutani J, Takahashi M, Yamanaka T, Sakai K, Ito Y, et al. A randomized phase II trial of trastuzumab plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxanes: WJOG6110B/ELTOP. Breast (2018) 40:67–75. doi: 10.1016/j.breast.2018.04.010
68. Urruticoechea A, Rizwanullah M, Im S-A, Ruiz ACS, Láng I, Tomasello G, et al. Randomized phase III trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therapy. J Clin Oncol (2017) 35(26):3030–8. doi: 10.1200/JCO.2016.70.6267
69. Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, Von Minckwitz G, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): Two highly active therapeutic regimens. J Clin Oncol (2011) 29(2):149–56. doi: 10.1200/JCO.2010.28.6450
70. Von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New Engl J Med (2019) 380(7):617–28. doi: 10.1056/NEJMoa1814017
71. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. New Engl J Med (2012) 367(19):1783–91. doi: 10.1056/NEJMoa1209124
72. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22(3):351–60. doi: 10.1016/S1470-2045(20)30702-6
73. Yan M, Bian L, Hu X. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): A randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res (2020) 1:13. doi: 10.21037/tbcr-20-25
74. Wardley AM, Pivot X, Morales-Vasquez F, Zetina LM, de Fátima Dias Gaui M, Reyes DO, et al. Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol (2010) 28(6):976–83. doi: 10.1200/JCO.2008.21.6531
75. De La Pena L, Cortes J, Manikhas A, Roman L, Semiglazov V, Biakhov MY, et al. Phase III trial of non-pegylated liposomal doxorubicin (M) in combination with trastuzumab (T) and paclitaxel (P) in HER2+ metastatic breast cancer (MBC). Am Soc Clin Oncol (2013). doi: 10.1200/jco.2013.31.15_suppl.517
76. Pivot X, Manikhas A, Żurawski B, Chmielowska E, Karaszewska B, Allerton R, et al. CEREBEL (EGF111438): A phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J Clin Oncol (2015) 33(14):1564–73. doi: 10.1200/JCO.2014.57.1794
77. Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet (2011) 377(9769):914–23. doi: 10.1016/S0140-6736(11)60070-6
78. Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol (2015) 33(6):594. doi: 10.1200/jco.2015.33.28_suppl.59
79. Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. New Engl J Med (2012) 366(2):109–19. doi: 10.1056/NEJMoa1113216
80. Swain SM, Kim S-B, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2013) 14(6):461–71. doi: 10.1016/S1470-2045(13)70130-X
81. Cope S, Zhang J, Saletan S, Smiechowski B, Jansen JP, Schmid P. A process for assessing the feasibility of a network meta-analysis: A case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med (2014) 12(1):1–17. doi: 10.1186/1741-7015-12-93
82. Gradishar W. Taxanes for the treatment of metastatic breast cancer. Breast Cancer: Basic Clin Res (2012) 6:BCBCR. S8205. doi: 10.4137/BCBCR.S8205
83. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. New Engl J Med (2003) 348(24):2431–42. doi: 10.1056/NEJMra023246
84. Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol (2011) 125(1-2):13–22. doi: 10.1016/j.jsbmb.2011.02.001
85. Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocrine Rev (2005) 26(3):331–45. doi: 10.1210/er.2004-0015
86. Michiels S, Pugliano L, Marguet S, Grun D, Barinoff J, Cameron D, et al. Progression-free survival as surrogate end point for overall survival in clinical trials of HER2-targeted agents in HER2-positive metastatic breast cancer. Ann Oncol (2016) 27(6):1029–34. doi: 10.1093/annonc/mdw132
87. Saad E, Katz A, Hoff P, Buyse M. Progression-free survival as surrogate and as true end point: Insights from the breast and colorectal cancer literature. Ann Oncol (2010) 21(1):7–12. doi: 10.1093/annonc/mdp523
88. Belin L, Tan A, De Rycke Y, Dechartres A. Progression-free survival as a surrogate for overall survival in oncology trials: A methodological systematic review. Br J Cancer (2020) 122(11):1707–14. doi: 10.1038/s41416-020-0805-y
Keywords: Advanced breast cancer, hormone receptor, HER2 positive, metastatic breast cancer, targeted therapies, network meta-analysis, subgroup analysis
Citation: Umemneku-Chikere CM, Ayodele O, Soares M, Khan S, Abrams K, Owen R and Bujkiewicz S (2022) Comparative review of pharmacological therapies in individuals with HER2-positive advanced breast cancer with focus on hormone receptor subgroups. Front. Oncol. 12:943154. doi: 10.3389/fonc.2022.943154
Received: 13 May 2022; Accepted: 22 July 2022;
Published: 18 August 2022.
Edited by:
Dirk Geerts, University of Amsterdam, NetherlandsReviewed by:
Edmund Ui-Hang Sim, Universiti Malaysia Sarawak, MalaysiaNathaniel Parker, Yale University, United States
Gábor Rubovszky, National Institute of Oncology (NIO), Hungary
Copyright © 2022 Umemneku-Chikere, Ayodele, Soares, Khan, Abrams, Owen and Bujkiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chinyereugo M. Umemneku-Chikere, Y211YzFAbGVpY2VzdGVyLmFjLnVr
 Chinyereugo M. Umemneku-Chikere
Chinyereugo M. Umemneku-Chikere Olubukola Ayodele2
Olubukola Ayodele2 Keith Abrams
Keith Abrams Rhiannon Owen
Rhiannon Owen Sylwia Bujkiewicz
Sylwia Bujkiewicz