
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 15 August 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.940428
This article is part of the Research TopicPerioperative Management and Cancer OutcomeView all 11 articles
 Meilin Weng1,2,3†
Meilin Weng1,2,3† Miaomiao Guo1,2†
Miaomiao Guo1,2† Ting Li1,2†
Ting Li1,2† Changming Zhou4
Changming Zhou4 Caihong Sun1,2
Caihong Sun1,2 Ying Yue1,2
Ying Yue1,2 Qingwu Liao1,2
Qingwu Liao1,2 Sanjun Cai5
Sanjun Cai5 Xihua Lu6*
Xihua Lu6* Di Zhou1,2*
Di Zhou1,2* Changhong Miao1,2*
Changhong Miao1,2*Background: Perioperative anemia and transfusion are intertwined with each other, and both have adverse impacts on the survival of colorectal cancer (CRC) patients. But the treatment of anemia still relies on transfusion in several countries, which leads us to question the effects of anemia tolerance and transfusion on the long-term outcomes of CRC patients. We investigated the combined effect of preoperative anemia and postoperative anemia and of preoperative anemia and blood transfusion, which imposes a greater risk to survival, to compare the effects of anemia tolerance and transfusion on overall survival (OS) and disease-free survival (DFS) in patients undergoing CRC surgery.
Methods: A retrospective propensity-score-matched analysis included patients with CRC undergoing elective surgery between January 1, 2008, and December 31, 2014. After propensity-score matching, Kaplan–Meier survival analysis and univariable and multivariable Cox proportional hazards models were used to study the prognostic factors for survivals. In univariate and multivariate Cox regression analysis, two novel models were built.
Results: Of the 8,121 patients with CRC, 1,975 (24.3%) and 6,146 (75.7%) patients presented with and without preoperative anemia, respectively. After matching, 1,690 patients remained in each group. In the preoperative anemia and postoperative anemia model, preoperative anemia and postoperative anemia was independent risk factor for OS (HR, 1.202; 95% CI, 1.043–1.385; P=0.011) and DFS (HR, 1.210; 95% CI, 1.050–1.395; P=0.008). In the preoperative anemia and transfusion model, preoperative anemia and transfused was the most dangerous independent prognostic factor for OS (HR, 1.791; 95% CI, 1.339–2.397; P<0.001) and DFS (HR, 1.857; 95% CI, 1.389–2.483; P<0.001). In patients with preoperative anemia, the OS and DFS of patients with transfusion were worse than those of patients without transfusion (P=0.026 in OS; P=0.037 in DFS).
Conclusions: Preoperative anemia and blood transfusion imposed a greater risk to OS and DFS in patients undergoing CRC surgery, indicating that the harm associated with blood transfusion was greater than that associated with postoperative anemia. These findings should encourage clinicians to be vigilant for the timely prevention and treatment of anemia, by appropriately promoting toleration of anemia and restricting the use of blood transfusion in patients with CRC.
Among all the types of cancers, colorectal cancer (CRC) has the third and second highest morbidity and mortality rates worldwide, respectively (1, 2). Although CRC is the fifth leading cause of cancer death among men and women in China, the death rate from CRC has been on the rise during the past few decades (3, 4). Currently, the most common treatment for CRC is radical resection; although progress has been made in diagnosis and treatment strategies, approximately half of the patients relapse within 3 years post-operation (5). Therefore, there is an urgent need to find prognostic factors capable of predicting patient prognosis in CRC, especially if it is possible to act on them and modify them accordingly.
A considerable number of patients with colon or rectal cancer suffer from anemia (38%–50% and 18%–50%, respectively) (6, 7). The possibility that anemia can affect the prognosis of cancer has aroused a widespread concern. Preoperative anemia in patients with cancer is usually the result of blood loss caused by advanced cancer progression or myelosuppression (8). Accumulating evidence has revealed that preoperative anemia is associated with worse outcomes in patients undergoing CRC surgery (6, 9–11). Surgical resection of tumors aggravates anemia (postoperative anemia), which is markedly common but is typically neglected after surgery (12–15). As pre- and postoperative anemia may be used as prognostic factors in patients with CRC, it is reasonable to further investigate which of the two is most influential, and whether their combined relationship could be informative for improving the prediction of patients’ survival. However, this association has not been confirmed in a clinical study. Perioperative anemia and transfusion are always related; although anemia can be traditionally treated with transfusion, it is not a desirable treatment option. Indeed, transfusion may cause more harm than benefits to patients (14–16), which leads us to question the effects of anemia tolerance and transfusion on the long-term outcomes of cancer patients.
Currently, anesthesiologists and surgeons are paying increasing attention to both short- and long-term prognoses of cancer patients (17, 18). Enhanced recovery after surgery also focuses on perioperative anemia and its associated morbidity and mortality (19, 20). Therefore, we conducted this retrospective study to investigate the combined effect of preoperative and postoperative anemia, and preoperative anemia and blood transfusion, to determine which of these factors impose a greater risk to overall survival (OS) and disease-free survival (DFS) in patients undergoing colorectal surgery and to investigate the effects of anemia tolerance and transfusion on the long-term outcomes of CRC patients. Though two other studies investigated the combined effect of preoperative anemia and blood transfusion on complications and 30-day death rate in patients undergoing colectomy (21, 22), our study further evaluated the combined effect of preoperative anemia and blood transfusion on the long-term outcomes (longer median follow-up period) after CRC surgery. To the best of our knowledge, the association between anemia tolerance and transfusion on the long-term outcomes of CRC patients has not been reported. First, we built two novel models to evaluate which of the two combined factors imposed a greater risk to OS and DFS in patients undergoing CRC surgery. Second, we aimed to guide physicians on treatment implementation and modification for anemia in this subset of patients.
This retrospective study was performed at Shanghai Cancer Center, Fudan University, Shanghai, China and was approved by the appropriate ethics committee (IRB2105235-6). Informed consent was obtained from all subjects involved in the study. This study was conducted according to the Declaration of Helsinki and was consistent with the STROBE criteria.
Among individuals (n = 13,721) who underwent CRC surgery at Shanghai Cancer Center from January 1, 2008, to December 31, 2014, 8121 were enrolled in this study. The inclusion criteria were as follows: histologically confirmed CRC, elective radical surgery for CRC, and older than 20 years of age. The exclusion criteria were as follows: incomplete data in medical records, benign tumor/carcinoma in situ, emergency operation and a previous history of cancer (Figure 1). Ultimately, 8,121 patients were included in this study. According to the diagnostic criteria in China (23), anemia is defined as serum hemoglobin (Hb) levels < 120 g/L for men or < 110 g/L for women, which is different from the criteria indicated by the World Health Organization criteria (24) (Hb < 130 g/L for men or Hb < 120 g/L for women); importantly, this biological reference interval is more suitable for Chinese individuals (25). Patients were divided into either the preoperative anemia group or not preoperative anemia group, according to their Hb levels before surgery.
The data were retrieved from Shanghai Cancer Center’s electronic clinical information system. The patients’ baseline characteristics included sex, age, American Society of Anesthesiology (ASA) score, preoperative Hb concentrations, preoperative hematocrit (HCT), preoperative adjuvant chemotherapy, tumor histology, tumor differentiation, vascular tumor thrombus, surgical margin positive, Pathologic Tumor Node Metastasis/Union for International Cancer Control (pTNM/UICC) stage, infiltrating lymph nodes > 12, number of cancer nodules > 1, and clinical conditions. Perioperative outcomes included intraoperative blood transfusion, intraoperative blood loss, postoperative Hb, postoperative anemia, reoperation within 30 days, duration of intensive care unit stay, and death.
The primary outcomes were OS and DFS. OS was defined as the time from the date of first treatment to the date of death due to any reason. DFS was defined as the time from the date of first treatment to the date of recurrence or metastasis or secondary primary tumor or death. The follow-up ended on December 31, 2019, ranging from 5 to 11 years (median: 69.6 months).
SPSS (Version 25; IBM, Armonk, NY, USA) and R software (version 3.4.4, R Foundation for Statistical Computing, Austria) were used to analyze the data. Patients’ baseline characteristics were presented as n and percent (%) for categorical variables and mean ± standard deviation (SD) for continuous variables. We compared the association between preoperative anemia and malignant clinicopathological features by t-test and Chi-squared test. Spearman analysis was used to assess the correlation between preoperative anemia and postoperative anemia.
We used propensity score matching to reduce any potential confounding factors related to baseline differences between the two groups. The key confounders including sex, age, preoperative adjuvant chemotherapy, tumor histology, pTNM/UICC stage, and lymph node invasion > 12 were matched. The nearest neighbor method was employed, and 0.05 SD was used as the caliper with 1:1 matching. The balanced distribution of matched patients in each group was evaluated by standardized mean difference (SMD). SMD < 0.10 meant a balanced distribution between the two groups. We used the R package “MatchIt” for propensity score matching. After matching, 1,690 patients remained in each group (Table 1).
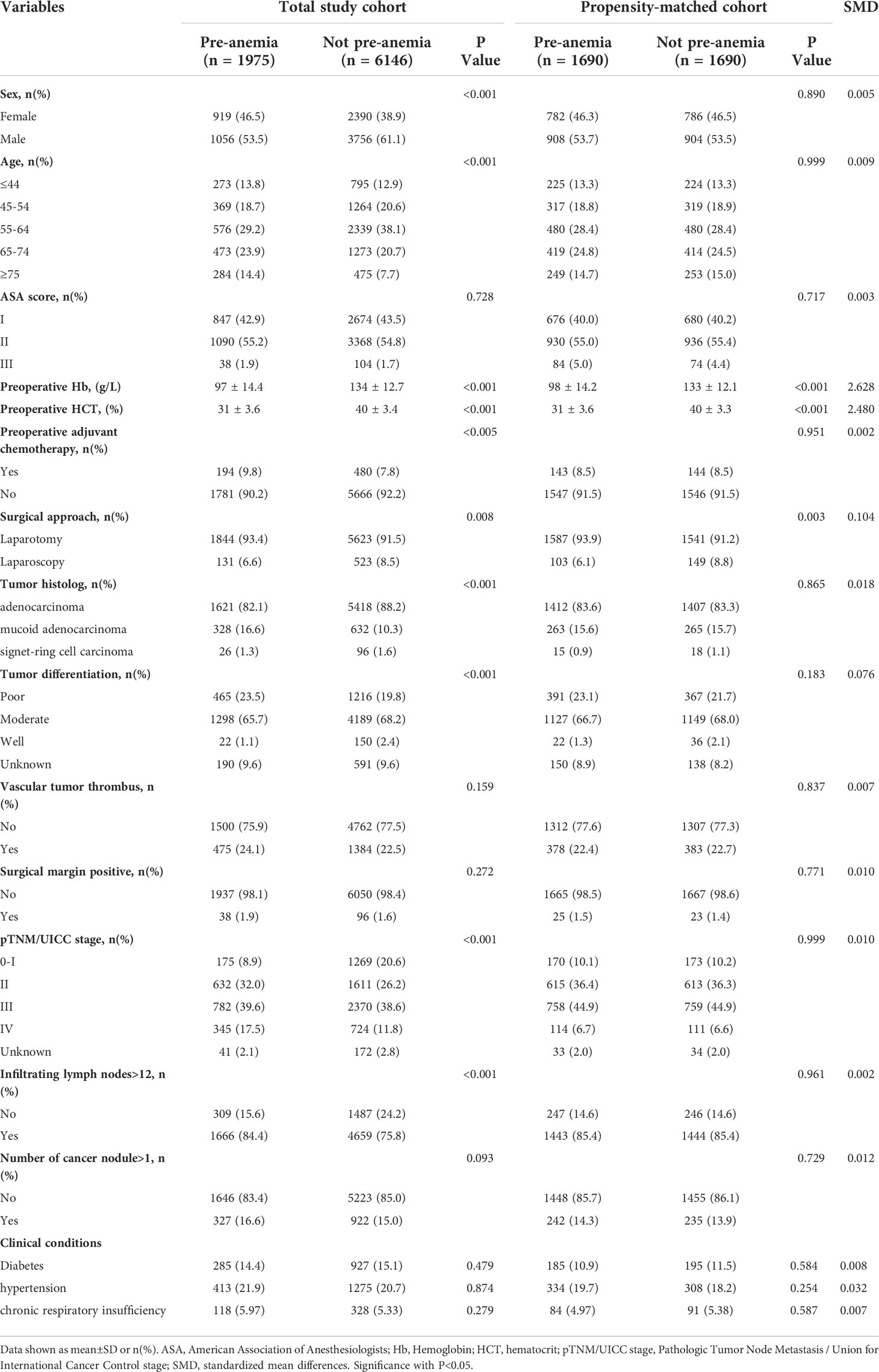
Table 1 Patient baseline characteristics in the total study cohort and the Propensity score matched.
In the propensity-matched cohort, Kaplan–Meier survival analysis was used to compare OS and DFS by log-rank test. We used the Cox proportional hazards model to study the prognostic factors for OS and DFS. The univariate Cox proportional hazards model was used to analyze all variables. Variables with a P-value < 0.05 were included in the multivariate analysis. Multivariate Cox proportional hazards model using the enter method was conducted to select variables. In univariate and multivariate Cox regression analysis, two new models were built. “Preoperative anemia and postoperative anemia” model included patients who were not anemic either before or after surgery, those who had preoperative anemia but not postoperative anemia, those with postoperative anemia but not preoperative anemia, and those with both pre- and postoperative anemia. “Preoperative anemia and transfusion” model included patients who were not anemic preoperatively and not transfused, those who were not anemic before surgery but transfused, those with preoperative anemia only but not transfused, and those with preoperative anemia who were also transfused. The hazard ratio (HR) was not only compared in each model, but also compared between different models to investigate its prediction in cancer prognosis. Multivariate Cox analysis model 1 was designed to estimate preoperative anemia effect on survival. Multivariate Cox analysis model 2 was designed to estimate postoperative anemia effect on survival. Multivariate Cox analysis model 3 was designed to estimate the interaction of preoperative and postoperative anemia effect on survival. Multivariate Cox analysis model 4 was designed to estimate the interaction of preoperative anemia and transfusion effect on survival.
Of the 8,121 patients who met our inclusion criteria, 1,975 (24.3%) patients presented with preoperative anemia and 6,146 (75.7%) did not show preoperative anemia (Figure 1). Patient characteristics are summarized in Table 1. Our median postoperative follow-up period was 69.4 (95% CI [confidence interval]: 68.7–70.0) months for all patients. Because there were significant differences in baseline characteristics that could influence cancer recurrence between the two groups, we used propensity score matching to reduce the imbalance. After matching, 1,690 patients remained in each group. SMD values were less than 0.1 for all characteristics except for surgical approach (Table 1). After matching, no significant differences were found for sex, age, preoperative adjuvant chemotherapy, tumor histology, tumor differentiation, pTNM/UICC stage, and number of infiltrating lymph nodes > 12, which were greatly different between the two groups before matching.
In the propensity-matched cohort, preoperative Hb was markedly higher in the not preoperative anemia group than in the preoperative anemia group (133 ± 12.1 g/L vs. 98 ± 14.2 g/L, P<0.001, n=1690 in each group, Table 1 and Figure 2A). A greater percentage of patients in the preoperative anemia group required blood transfusion (8.2% vs. 0.7%, P<0.001, Table 2) than that in the not preoperative anemia group. The postoperative Hb was markedly higher in the not preoperative anemia group than in the preoperative anemia group (124 ± 13.3 g/L vs. 99 ± 13.1 g/L, P<0.001, n=1690 in each group, Table 2 and Figure 2B). A higher percentage of patients in the preoperative anemia group (90.5% vs. 20.7%, P<0.001, Table 2) than that in the not preoperative anemia group exhibited postoperative anemia. Preoperative Hb values correlated positively with postoperative Hb concentrations (r = 0.843, P < 0.001, Figure 2C). The overall mortality rate was significantly higher in the preoperative anemia group (31.1% vs. 26.7%, P = 0.005) during the extended follow-up (+5 years). Summarizing this propensity-matched cohort, preoperative anemia was associated with more blood transfusion, more postoperative anemia, and higher mortality rate after CRC surgery.
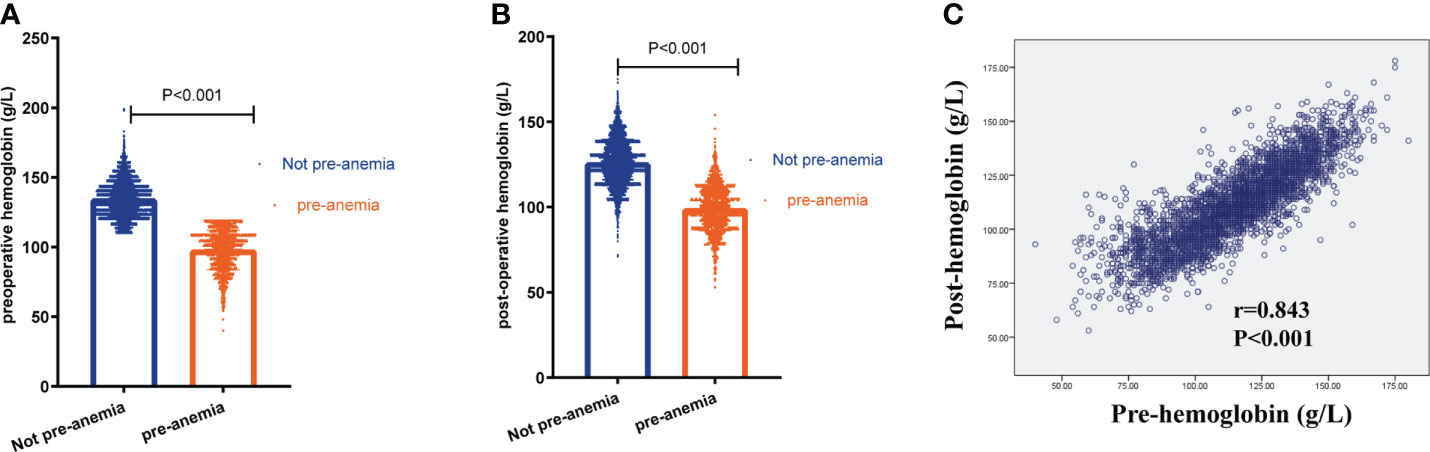
Figure 2 Preoperative anemia was associated with more postoperative anemia. (A) The level of preoperative hemoglobin in patients with and without preoperative anemia (pre-anemia) (133 ± 12.1 g/L vs. 98 ± 14.2 g/L, n=1690 in each group, P<0.001). (B) The level of postoperative hemoglobin in patients with or without pre-anemia (124 ± 13.3 g/L vs. 99 ± 13.1 g/L, P<0.001, n=1690 in each group). (C) The correlation between preoperative hemoglobin (pre-hemoglobin) and postoperative hemoglobin (post-hemoglobin) using Spearman analysis. Significance with P < 0.05.
In the propensity-matched cohort, patients who were not anemic preoperatively demonstrated better OS than those who were anemic before surgery (median survival time 130.9 months vs. 121.5 months; 5-year OS rate 75% vs. 71.5%, P=0.005; Figure 3A). Meanwhile, patients who were not anemic before surgery also exhibited better DFS than those who were anemic preoperatively (median survival time 134.6 months vs. 124.0 months; 5-year DFS rate 73.3% vs. 69.0%; P=0.003; Figure 3B).
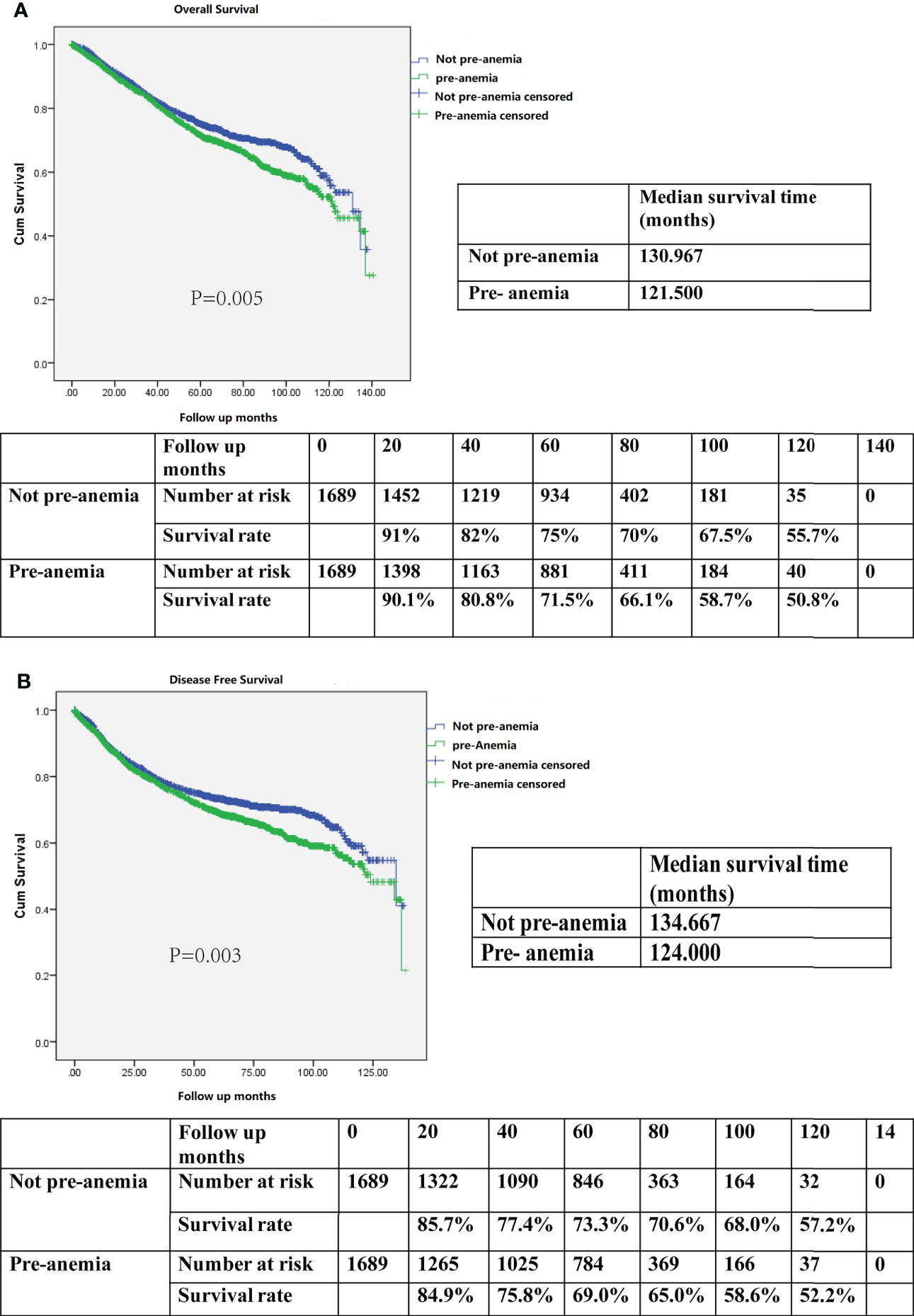
Figure 3 (A) Kaplan–Meier survival curve for overall survival (OS) according to preoperative anemia (pre-anemia) in the propensity score-matched cohort. The OS rates, median survival time, and number at risk are shown. (B) Kaplan–Meier survival curve for disease-free survival (DFS) according to pre-anemia in the propensity score-matched cohort. The DFS rates, median survival time, and number at risk are shown. Significance with P < 0.05.
After multivariate analysis, preoperative anemia remained an independent risk factor for decreased OS (HR, 1.144; 95% CI, 1.005–1.302; P=0.042, Table 3-Multivariate analysis 1) and DFS (HR, 1.166; 95% CI, 1.024–1.327; P=0.020, Table 4-Multivariate analysis 1). Altogether, a diagnosis of preoperative anemia was an independent predictor for worse OS and DFS after CRC surgery.
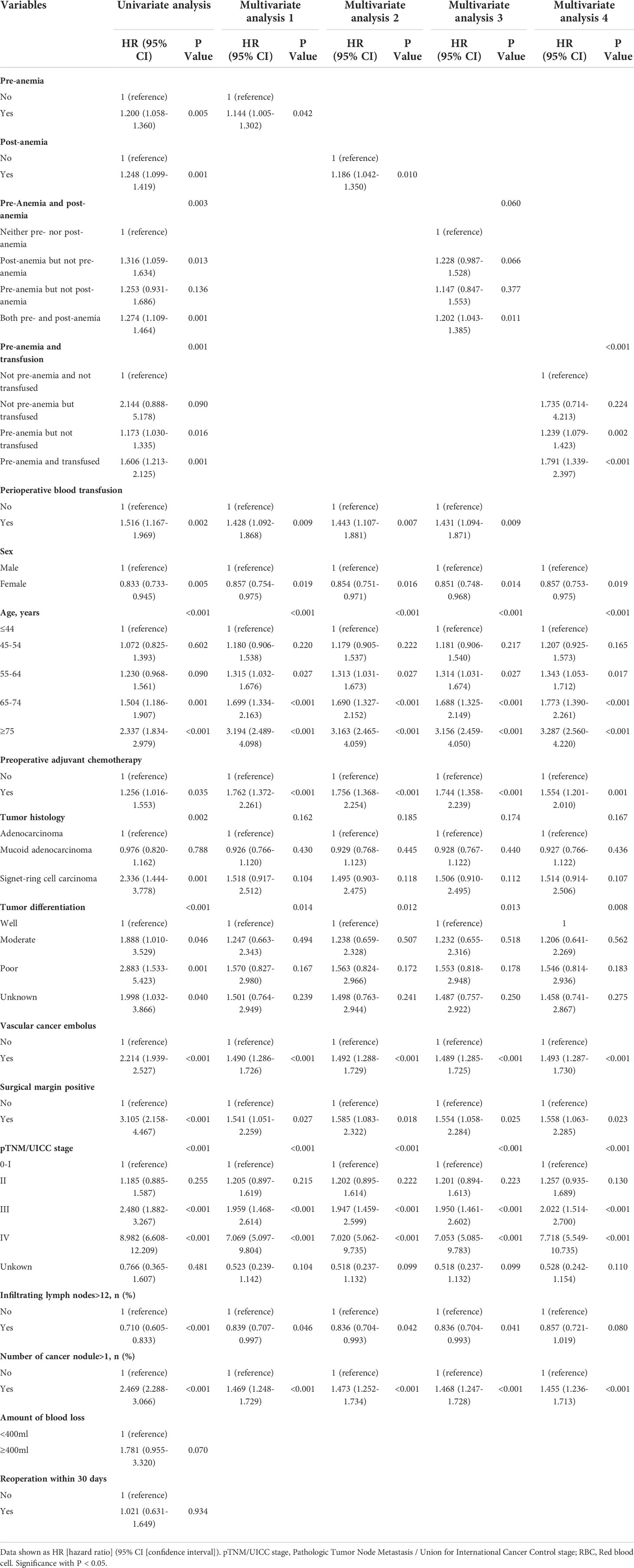
Table 3 Univariate analysis and multivariate Cox regression analysis for overall survival in the Propensity score matched cohort.
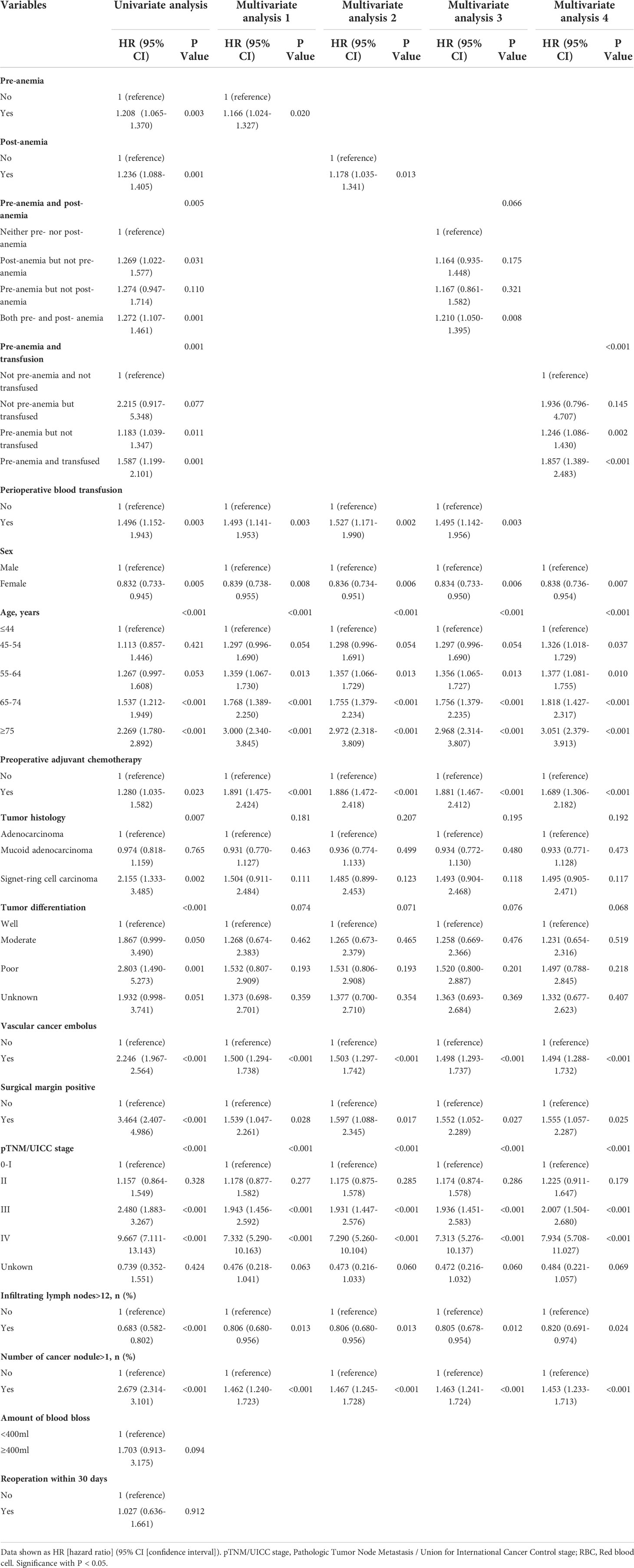
Table 4 Univariate analysis and multivariate Cox regression analysis for disease-free survival in the Propensity score matched cohort.
Similarly, after multivariate analysis, postoperative anemia was also an independent predictor for shorter OS (HR, 1.186; 95% CI, 1.042–1.350; P=0.010; Table 3-Multivariate analysis 2) and DFS (HR, 1.178; 95% CI, 1.035–1.341; P=0.013; Table 4-Multivariate analysis 2) of patients after CRC surgery.
Patients who were not anemic either before or after surgery demonstrated the best OS when compared with those who had preoperative anemia but not postoperative anemia, those with postoperative anemia but not preoperative anemia, and those with both pre- and postoperative anemia (P=0.003, Figure 4A). Patients who did not show perioperative anemia also exhibited the best DFS of all groups of patients that were studied (P = 0.005, Figure 4B). However, patients with preoperative anemia had no difference in OS (P=0.886) and DFS (P=0.989), regardless of whether they presented with postoperative anemia or not.
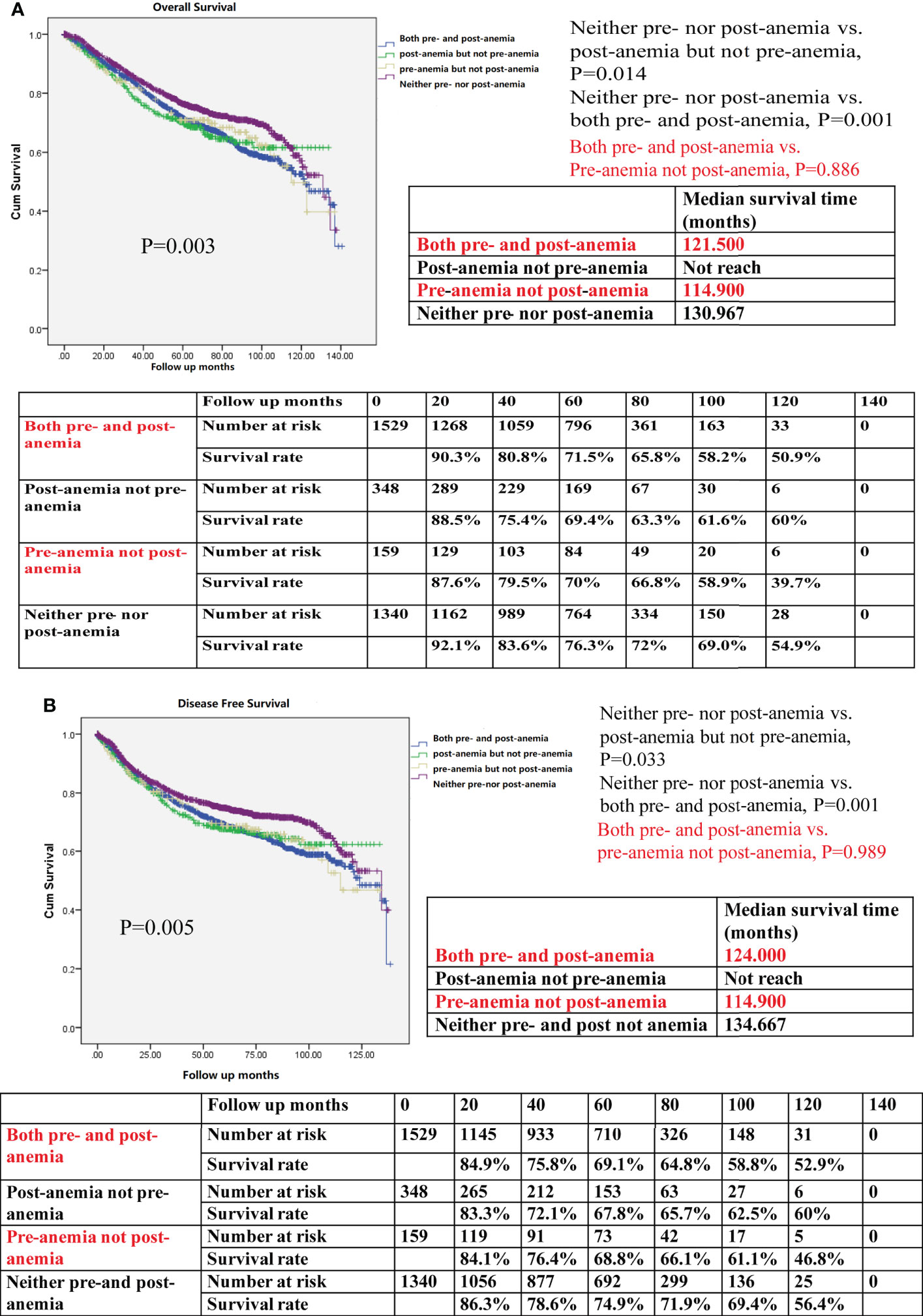
Figure 4 (A) Kaplan–Meier survival curve for overall survival (OS) for both preoperative anemia (pre-anemia) and postoperative anemia (post-anemia), post-anemia but not pre-anemia, pre-anemia but not post-anemia, and neither pre- nor post-anemia in the propensity score-matched cohort. The OS rates, median survival time, and number at risk are shown. (B) Kaplan–Meier survival curve for disease-free survival (DFS) for both pre- and post-anemia, post-anemia but not pre-anemia, pre-anemia but not post-anemia, and neither pre- nor post-anemia in the propensity score-matched cohort. The DFS rates, median survival time, and number at risk are shown. The median survival time refers to the corresponding survival time when the survival rate is 50%. “not reach” means when a line is drawn vertically on the Y axis 0.5, it does not intersect with the survival curve. There is no corresponding survival time here. Significance with P < 0.05.
After the multivariable analysis, the presence of pre- and postoperative anemia remained an independent risk factor for shorter OS (HR, 1.202; 95% CI, 1.043–1.385; P=0.011, Table 3-Multivariate analysis 3) and worse DFS (HR, 1.210; 95% CI, 1.050–1.395; P=0.008, Table 4-Multivariate analysis 3). However, having preoperative but not postoperative anemia, and having postoperative but not preoperative anemia were not independent predictors for worse OS and DFS, indicating that appropriate prevention and treatment of anemia were required. In summary, both pre- and postoperative anemia was an independent predictor for negative OS and DFS of patients after CRC surgery, which experienced the highest mortality risk after CRC surgery in this model.
Patients who were not anemic preoperatively and not transfused showed the best OS when compared with those who were not anemic before surgery but transfused, those with preoperative anemia only but not transfused, and those with preoperative anemia who were also transfused (P=0.001, Figure 5A). Patients who were not preoperatively anemic and not transfused showed the best DFS of all studied groups of patients (P=0.001, Figure 5B). In patients with preoperative anemia, the OS and DFS of patients with transfusion were worse than those of patients without transfusion using Kaplan–Meier survival analysis (P=0.026 in OS; P=0.037 in DFS), indicating that the prognosis associated with intraoperative blood transfusion was worse than that associated with postoperative anemia.
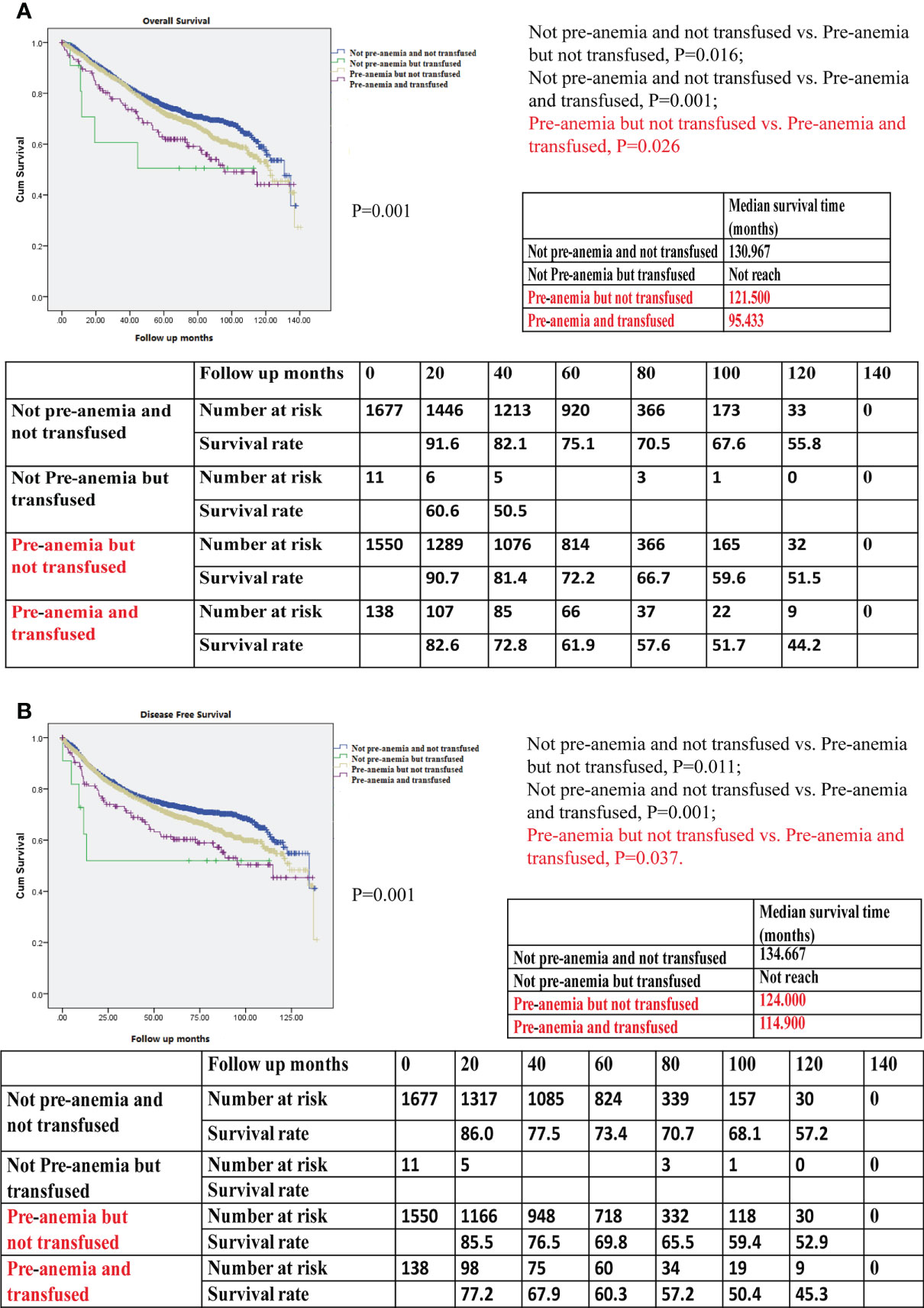
Figure 5 (A) Kaplan–Meier survival curve for overall survival (OS) for not preoperative anemia (pre-anemia) and not transfused, not pre-anemia but transfused, pre-anemia but not transfused, and pre-anemia and transfused in the propensity score-matched cohort. The OS rates, median survival time, and number at risk are shown. (B) Kaplan–Meier survival curve for disease-free survival (DFS) for not pre-anemia and not transfused, not pre-anemia but transfused, pre-anemia but not transfused, and pre-anemia and transfused in the propensity score-matched cohort. The DFS rates, median survival time, and number at risk are shown. The median survival time refers to the corresponding survival time when the survival rate is 50%. “not reach” means when a line is drawn vertically on the Y axis 0.5, it does not intersect with the survival curve. There is no corresponding survival time here. Significance with P < 0.05.
After the multivariable analysis, preoperative anemia without or with transfusion were independent risk factors for OS (HR, 1.239; 95% CI, 1.079–1.423; P=0.002; HR, 1.791; 95% CI, 1.339–2.397; P<0.001, respectively; Table 3-Multivariate analysis 4) and DFS (HR, 1.246, 95% CI, 1.086–1.430; P=0.002; HR, 1.857; 95% CI, 1.389–2.483; P<0.001, respectively; Table 4-Multivariate analysis 4). Owing to the HRs of preoperative anemia with transfusion being higher than those of preoperative anemia without transfusion (HR 1.791 vs. 1.239 in OS in Table 3; HR 1.857 vs. 1.246 in DFS in Table 4), the risks of death and cancer progression in patients preoperatively anemic who were also transfused were the highest in this model. When comparing the most dangerous risk factors between two models, the HRs of preoperative anemia with transfusion were higher than those of preoperative and postoperative anemia (HR 1.791 vs. 1.202 in OS in Table 3; HR 1.857 vs. 1.210 in DFS in Table 4), indicating that the harm associated with blood transfusion was greater than that associated with postoperative anemia.
Our study demonstrated that preoperative anemia and postoperative anemia were independent risk factors for worse OS and DFS after colorectal surgery. Since preoperative anemia is highly associated with the presence of postoperative anemia and the need for blood transfusions, we evaluated two new prognostic models involving these factors. In the preoperative anemia and postoperative anemia model, the presence of both preoperative anemia and postoperative anemia had the highest risk of worse OS and DFS. Patients with preoperative anemia had no difference in OS and DFS, regardless of whether they presented with postoperative anemia or not. In the preoperative anemia and transfusion model, preoperative anemia and transfused was the most dangerous independent prognostic factor for OS and DFS. In patients with preoperative anemia, the OS and DFS of patients with transfusion were worse than those of patients without transfusion.
Our large study indicated that anemia before surgery was present in 24.3% of CRC patients and was strongly associated with worse OS and DFS. However, the mechanisms behind preoperative anemia and poor cancer outcomes were unclear, as some studies reported that low Hb indicates hypoxia, a decrease of oxygen-carrying function, and low tolerance to bleeding (26, 27). Hypoxia is the key initiating factor for tumors. Increasing evidence shows that anemia could lead to hypoxia in the tumor microenvironment, leading to up-regulation of hypoxia-inducible factor-1 α expression. Hypoxia-inducible factor-1 α could inhibit the effect of tumor infiltrating lymphocytes and promote immunosuppressive activity by activating tumor-associated macrophages; these factors further promoted tumor proliferation and revascularization (26, 27). Moreover, preoperative anemia is also a sign of the severity of the underlying disease. In our study, after propensity score matching, SMD values for all characteristics were < 0.1 except for surgical approach. Preoperative anemia was associated with laparotomy. It would be interesting to explore whether laparotomy correlated with more bleeding and greater number of transfusions. There was no difference in bleeding between laparotomy and laparoscopy surgical approaches. A greater percentage of patients in the laparotomy group required blood transfusion (4.7% vs. 2.0%, P < 0.047, Supplementary Table 1). In our study, after matching, preoperative anemia was associated with laparotomy, which is related to immunomodulation as well as greater number of transfusions. This explains the association between preoperative anemia and poor prognosis in patients with CRC.
Furthermore, preoperative anemia correlated positively with postoperative anemia in our study. Single exposure to preoperative anemia or postoperative anemia was a risk factor for worse OS and DFS, yet postoperative anemia but not preoperative anemia and preoperative anemia but not postoperative anemia were no longer risk factors for OS and DFS in our study. This finding is very important for anesthesiologists and surgeons, as it indicates that treatment or intervention for preoperative anemia or postoperative anemia, which benefits cancer patients’ outcomes, should be considered. Several studies concluded that blood management before surgery, according to preoperative anemia status, can effectively improve patients’ safety and reduce medical expenditure, blood transfusion, hospital stay, complications, and mortality (28–32). The management of postoperative anemia includes erythropoiesis, blood loss prevention, and restricted blood transfusion strategies (14, 28, 31). Correct evaluation is crucial, with prevention being the best treatment (14). However, patients with preoperative anemia, regardless of whether they had postoperative anemia or not, presented no difference in OS and DFS. Therefore, anemia should warrant anesthesiologists and surgeons’ attention, as they can use this combined assessment to identify particularly sensitive patients and implement effective strategies to improve their outcomes.
In our study, we showed that preoperative anemia was associated with a greater percentage of patients needing blood transfusions. Similarly, preoperative anemia is strongly correlated with perioperative blood transfusion and increased mortality in patients undergoing elective surgery (29, 33, 34). Anemia, blood loss, and transfusion can be considered “three evils” that adversely affect mortality (8), and are inextricably interrelated (35). One of the main purposes of this study was also to evaluate the interaction between preoperative anemia and intraoperative transfusions. In the preoperative anemia and transfusion model, we found that the combination of preoperative anemia with or without intraoperative blood transfusions were independent risk factors for OS and DFS after multivariate analysis. The HR of the combination of preoperative anemia and transfusion was much higher than the HR of preoperative anemia without transfusion, indicating that preoperatively anemic patients who were transfused had higher risks of death and cancer progression than those of patients who were not transfused. When comparing the most dangerous risk factors between the two models, the HR of the combination of preoperative anemia and transfusion was also higher than the HR of preoperative anemia and postoperative anemia. Our HRs with very narrow 95% CIs showed robust predictive values, indicating that the harm associated with blood transfusion was worse than that associated with postoperative anemia. Concurrently, in patients with preoperative anemia, the OS and DFS of patients with transfusion were significantly worse than those of patients without transfusion, suggesting that treating anemia with intraoperative blood transfusion should be considered carefully, and highlighting a need for strategies targeting anemia tolerance and for appropriately restricting the use of blood transfusion.
Now, is it better to tolerate anemia than to correct anemia with blood transfusion? The perioperative period is a critical window in the recovery of patients with an impaired immune response due to surgical trauma. Blood transfusion is thought to have immunomodulatory effects and may damage tumor immune surveillance and promote tumor growth and spread (36, 37). Moderate to severe anemia (first strike) and transfusion (second strike) may lead to elevated systemic inflammation and immunosuppression accompanied by endothelial dysfunction (6, 36, 38–40). Historically, treatment and management of patients with anemia mostly rely on blood transfusion. However, the fundamental purpose of medical treatment is not to treat “laboratory values,” but to improve patients’ conditions to achieve a better outcome (41). The indication of allogeneic blood transfusion should take into account the patient’s underlying disease (42), laboratory test results, benefits and risks, and whether bleeding is present or absent (43–45). Patient blood management (PBM) has encouraged physicians to treat anemia, optimize hemostasis, minimize blood loss, promote toleration of anemia, and restrict transfusion where appropriate in order to improve patient prognosis (15, 43, 46–49). However, the actual implementations of Patient blood management (PBM) in many countries are not satisfactory (50, 51). Owing to barriers of application of PBM in many medical centers, consideration should be given to education and training to raise awareness of the clinical hazards of anemia and blood transfusion, and the need for alternatives to blood transfusion (31, 41, 49). Therefore, the results of our study may significantly aid health care providers in several countries.
Our study addressed an important topic. The advantage of our study was that our overall sample size (>8000) and allocation (>1000) in each group were much larger than those of previous studies, and the data were obtained from one of the largest cancer centers in China. Another advantage was that our median postoperative follow-up period was more than 5 years (median: 69.6 months), and we focused on CRC patients’ long-term outcomes. Further, we were the first to build two novel models to clarify the effect of anemia tolerance and transfusion on long-term survival after CRC surgery, which to the best of our knowledge, has not been reported previously. Our study adds to the growing body of literature regarding the efficacy of PBM on the identification of anemia, anemia tolerance, and restriction of transfusion use to lead to improved patient outcomes. Due to the poor application of PBM in many countries, our large sample cohort could provide more reference for physicians when they are considering anemia tolerance or blood transfusion for patients with CRC. Although these results contribute important information to the existing literature, our study also has several limitations. For example, this is a retrospective, and not a randomized, study from a singular institution, which cannot avoid the possibility of residual confounding factors.
Another limitation is that the indication for transfusion is unknown. This is inherent to the nature of this study (retrospective observational study). We can’t get the information of the threshold levels for RBC transfusion of every patient. Hb thresholds of 7 to 8 g/dL are used for most hemodynamically stable medical and surgical patients to avoid unnecessary transfusions in our hospital. After matching for preoperative anemia, no significant differences were found for patient baseline characteristics between transfused and not transfused patients, except preoperative adjuvant chemotherapy (Supplementary Table 2).
The combined prognostic value of preoperative anemia and blood transfusion imposed a greater risk to OS and DFS in patients undergoing CRC surgery. These findings should encourage clinicians to be vigilant for the timely prevention and treatment of anemia, by appropriately promoting toleration of anemia and restricting the use of blood transfusion in patients with CRC. Prospective randomized controlled trials are needed to explore perioperative risk and treatment opportunity in patients with CRC to improve their long-term prognosis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This retrospective study was performed at Shanghai Cancer Center, Fudan University, Shanghai, China and was approved by the appropriate ethics committee (IRB2105235-6). The patients/participants provided their written informed consent to participate in this study.
Conceptualization, MW, and CM. Methodology, MW, MG, and TL. Investigation and formal analysis, MW, CZ, CS, QL, SC and YY. Writing-original draft preparation, MW, and DZ. Writing-Review and Editing, MW, DZ and XL. All authors contributed to the article and approved the submitted version.
Our study was supported by the National Natural Science Foundation of China (No.82002538, 82072213); Shanghai Pujiang Talent Plan (No. 2020PJD013); Clinical Research Plan of SHDC (No. SHDC2020CR1005A); National Key Research and Development Program of China (No. 2020YFC2008400), and Wu Jieping Medical Foundation Clinical Research Special funding.
We thank Changming Zhou (Department of Cancer Prevention, Shanghai Cancer Center, Fudan University) for providing statistical analysis and consultation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.940428/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
4. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
5. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA: Cancer J Clin (2019) 69(5):363–85. doi: 10.3322/caac.21565
6. Wilson MJ, van Haaren M, Harlaar JJ, Park HC, Bonjer HJ, Jeekel J, et al. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg Oncol (2017) 26(1):96–104. doi: 10.1016/j.suronc.2017.01.005
7. Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European cancer anaemia survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer (Oxford Engl 1990) (2004) 40(15):2293–306. doi: 10.1016/j.ejca.2004.06.019
8. Cata JP. Perioperative anemia and blood transfusions in patients with cancer: when the problem, the solution, and their combination are each associated with poor outcomes. Anesthesiology (2015) 122(1):3–4. doi: 10.1097/ALN.0000000000000518
9. Mörner ME, Edgren G, Martling A, Gunnarsson U, Egenvall M. Preoperative anaemia and perioperative red blood cell transfusion as prognostic factors for recurrence and mortality in colorectal cancer-a Swedish cohort study. Int J colorectal disease (2017) 32(2):223–32. doi: 10.1007/s00384-016-2678-3
10. Kwon YH, Lim HK, Kim MJ, Park JW, Ryoo SB, Jeong SY, et al. Impacts of anemia and transfusion on oncologic outcomes in patients undergoing surgery for colorectal cancer. Int J colorectal disease (2020) 35(7):1311–20. doi: 10.1007/s00384-020-03601-2
11. Bruns ERJ, Borstlap WA, van Duijvendijk P, van der Zaag-Loonen HJ, Buskens CJ, van Munster BC, et al. The association of preoperative anemia and the postoperative course and oncological outcome in patients undergoing rectal cancer surgery: A multicenter snapshot study. Dis colon rectum (2019) 62(7):823–31. doi: 10.1097/DCR.0000000000001360
12. Kim JH, Bae YJ, Jun KH, Chin HM. The prevalence and clinical significance of postgastrectomy anemia in patients with early-stage gastric cancer: A retrospective cohort study. Int J Surg (London England) (2018) 52:61–6. doi: 10.1016/j.ijsu.2018.02.037
13. Jun JH, Yoo JE, Lee JA, Kim YS, Sunwoo S, Kim BS, et al. Anemia after gastrectomy in long-term survivors of gastric cancer: A retrospective cohort study. Int J Surg (London England) (2016) 28:162–8. doi: 10.1016/j.ijsu.2016.02.084
14. Warner MA, Shore-Lesserson L, Shander A, Patel SY, Perelman SI, Guinn NR. Perioperative anemia: Prevention, diagnosis, and management throughout the spectrum of perioperative care. Anesth Analg (2020) 130(5):1364–80. doi: 10.1213/ANE.0000000000004727
15. Desai N, Schofield N, Richards T. Perioperative patient blood management to improve outcomes. Anesth Analg (2018) 127(5):1211–20. doi: 10.1213/ANE.0000000000002549
16. Johnson DJ, Scott AV, Barodka VM, Park S, Wasey JO, Ness PM, et al. Morbidity and mortality after high-dose transfusion. Anesthesiol (2016) 124(2):387–95. doi: 10.1097/ALN.0000000000000945
17. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med (2018) 16(1):8–. doi: 10.1186/s12967-018-1389-7
18. Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology (2018) 129(5):932–41. doi: 10.1097/ALN.0000000000002357
19. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA surg (2017) 152(3):292–8. doi: 10.1001/jamasurg.2016.4952
20. Hardy PY, Degesve M, Joris J, Coimbra C, Decker E, Hans G. Impact of preoperative anemia on outcomes of enhanced recovery program after colorectal surgery: A monocentric retrospective study. World J surg (2021) 45(8):2326–36. doi: 10.1007/s00268-021-06161-w
21. Liu Z, Luo JJ, Pei KY, Khan SA, Wang XX, Zhao ZX, et al. Joint effect of pre-operative anemia and perioperative blood transfusion on outcomes of colon-cancer patients undergoing colectomy. Gastroenterol Rep (2020) 8(2):151–7. doi: 10.1093/gastro/goz033
22. Loor G, Rajeswaran J, Li L, Sabik JF 3rd, Blackstone EH, McCrae KR, et al. The least of 3 evils: Exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg (2013) 146(6):1480–7.e6. doi: 10.1016/j.jtcvs.2013.06.033
23. Experts Committee on Cancer -Related Anemia, and Chinese Society of Clinical Oncology (CSCO). (2012). Clinical practice guidelines on cancer-related anemia (2012-2013 edition). Chin Clin Oncol (2012) 1(2):18. doi: 10.3978/j.issn.2304-3865.2012.10.01
24. Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J anaesthesia (2011) 107 Suppl 1:i41–59. doi: 10.1093/bja/aer350
25. Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: Does hemoglobin change with aging? Semin Hematol (2015) 52(4):261–9. doi: 10.1053/j.seminhematol.2015.07.006
26. McLaren AT, Marsden PA, Mazer CD, Baker AJ, Stewart DJ, Tsui AK, et al. Increased expression of HIF-1alpha, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regulatory Integr Comp Physiol (2007) 292(1):R403–14. doi: 10.1152/ajpregu.00403.2006
27. Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology (2014) 143(4):512–9. doi: 10.1111/imm.12380
28. Spahn DR, Muñoz M, Klein AA, Levy JH, Zacharowski K. Patient blood management: Effectiveness and future potential. Anesthesiology (2020) 133(1):212–22. doi: 10.1097/ALN.0000000000003198
29. Spahn DR. Anemia and patient blood management in hip and knee surgery: A systematic review of the literature. Anesthesiology (2010) 113(2):482–95. doi: 10.1097/ALN.0b013e3181e08e97
30. Fowler AJ, Ahmad T, Abbott TEF, Torrance HD, Wouters PF, De Hert S, et al. Association of preoperative anaemia with postoperative morbidity and mortality: An observational cohort study in low-, middle-, and high-income countries. Br J anaesthesia (2018) 121(6):1227–35. doi: 10.1016/j.bja.2018.08.026
31. Meybohm P, Westphal S, Ravn HB, Ranucci M, Agarwal S, Choorapoikayil S, et al. Perioperative anemia management as part of PBM in cardiac surgery - a narrative updated review. J cardiothoracic Vasc anesthesia (2020) 34(4):1060–73. doi: 10.1053/j.jvca.2019.06.047
32. Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet (London England) (2013) 381(9880):1855–65. doi: 10.1016/S0140-6736(13)60808-9
33. Kim K, Seo H, Chin JH, Son HJ, Hwang JH, Kim YK. Preoperative hypoalbuminemia and anemia as predictors of transfusion in radical nephrectomy for renal cell carcinoma: A retrospective study. BMC anesthesiol (2015) 15:103. doi: 10.1186/s12871-015-0089-6
34. Klein AA, Collier TJ, Brar MS, Evans C, Hallward G, Fletcher SN, et al. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first association of cardiothoracic anaesthetists national audit. Anaesthesia (2016) 71(6):627–35. doi: 10.1111/anae.13423
35. Frank SM, Cushing MM. Bleeding, anaemia, and transfusion: An ounce of prevention is worth a pound of cure. Br J anaesthesia (2021) 126(1):5–9. doi: 10.1016/j.bja.2020.09.009
36. Vamvakas EC. Allogeneic blood transfusion and cancer recurrence: 20 years later. Transfusion (2014) 54(9):2149–53. doi: 10.1111/trf.12689
37. Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J anaesthesia (2013) 110(5):690–701. doi: 10.1093/bja/aet068
38. Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. New Engl J Med (1993) 328(19):1372–6. doi: 10.1056/NEJM199305133281902
39. Gross I, Shander A, Sweeney J. Patient blood management and outcome, too early or not? Best Pract Res Clin anaesthesiol (2013) 27(1):161–72. doi: 10.1016/j.bpa.2012.12.005
40. Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology (2011) 114(2):283–92. doi: 10.1097/ALN.0b013e3182054d06
41. Butcher A, Richards T. Cornerstones of patient blood management in surgery. Transfusion Med (Oxford England) (2018) 28(2):150–7. doi: 10.1111/tme.12476
42. Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood cell transfusion: A matter of tolerance. Crit Care Med (2006) 34(5 Suppl):S102–8. doi: 10.1097/01.CCM.0000214317.26717.73
43. Hare G., Mazer C. D. (2021). Anemia: Perioperative risk and treatment opportunity. Anesthesiology. 135(3): 520–530. doi: 10.1097/ALN.0000000000003870
44. Kim YW, Bae JM, Park YK, Yang HK, Yu W, Yook JH, et al. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: The FAIRY randomized clinical trial. JAMA (2017) 317(20):2097–104. doi: 10.1001/jama.2017.5703
45. Baker L, Park L, Gilbert R, Ahn H, Martel A, Lenet T, et al. Intraoperative red blood cell transfusion decision-making: A systematic review of guidelines. Ann surg (2021) 274(1):86–96. doi: 10.1097/SLA.0000000000004710
46. Kougias P, Sharath S, Mi Z, Biswas K, Mills JL. Effect of postoperative permissive anemia and cardiovascular risk status on outcomes after major general and vascular surgery operative interventions. Ann surg (2019) 270(4):602–11. doi: 10.1097/SLA.0000000000003525
47. Gani F, Cerullo M, Ejaz A, Gupta PB, Demario VM, Johnston FM, et al. Implementation of a blood management program at a tertiary care hospital: Effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann surg (2019) 269(6):1073–9. doi: 10.1097/SLA.0000000000002585
48. Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Füllenbach C, et al. Multimodal patient blood management program based on a three-pillar strategy: A systematic review and meta-analysis. Ann surg (2019) 269(5):794–804. doi: 10.1097/SLA.0000000000003095
49. Delaforce A, Duff J, Munday J, Hardy J. Overcoming barriers to evidence-based patient blood management: A restricted review. Implementation Sci IS (2020) 15(1):6. doi: 10.1186/s13012-020-0965-4
50. Delaforce A, Duff J, Munday J, Hardy J. Preoperative anemia and iron deficiency screening, evaluation and management: Barrier identification and implementation strategy mapping. J Multidiscip healthcare (2020) 13:1759–70. doi: 10.2147/JMDH.S282308
51. Pearse BL, Keogh S, Rickard CM, Fung YL. Barriers and facilitators to implementing evidence based bleeding management in Australian cardiac surgery units: A qualitative interview study analysed with the theoretical domains framework and COM-b model. BMC Health Serv Res (2021) 21(1):550. doi: 10.1186/s12913-021-06269-8
Keywords: preoperative anemia and postoperative anemia, preoperative anemia and transfusion, long-term outcomes, colorectal cancer, propensity-score-matched analysis, anemia tolerance and blood transfusion
Citation: Weng M, Guo M, Li T, Zhou C, Sun C, Yue Y, Liao Q, Cai S, Lu X, Zhou D and Miao C (2022) Anemia tolerance versus blood transfusion on long-term outcomes after colorectal cancer surgery: A retrospective propensity-score-matched analysis. Front. Oncol. 12:940428. doi: 10.3389/fonc.2022.940428
Received: 10 May 2022; Accepted: 25 July 2022;
Published: 15 August 2022.
Edited by:
Jie Tian, Shanghai Jiao Tong University, ChinaCopyright © 2022 Weng, Guo, Li, Zhou, Sun, Yue, Liao, Cai, Lu, Zhou and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changhong Miao, MTUxMTEyMzAwMzJAZnVkYW4uZWR1LmNu; Di Zhou, anVkeTYxMjU0MkAxNjMuY29t; Xihua Lu, aG5seGg2NkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.