- 1National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Institute of Blood and Marrow Transplantation, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, China
- 3Key Laboratory of Thrombosis and Hemostasis of Ministry of Health, Suzhou, China
- 4State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, China
Prolonged isolated thrombocytopenia (PT) is a common complication affecting the outcome of stem cell transplantation. In this study, we undertook a real-world study of 303 myelodysplastic syndrome (MDS) patients who received allogeneic hematopoietic stem cell transplantation (HSCT) between December 2007 and June 2018. 28.4% of MDS patients suffered from PT after HSCT. Survival analysis indicated that PT was associated with worse overall survival (OS) in MDS patients. The 2-year and 5-year OS in MDS patients with PT after HSCT were 49% and 47%, significantly worse than that of 68% and 60% in patients without PT (P=0.005). For RFS, patients with PT did not have an increased risk of disease relapse (P=0.964). After multivariate adjustment, PT was proved to be the independent risk factor associated with the worse OS (HR 1.49, 95% CI 1.00-2.21, P =0.048). We further analyzed risk factors associated with the occurrence of PT in MDS patients. Multiple logistic regression identified grade II-IV aGVHD, extensive chronic GVHD, hemorrhagic cystitis, and CMV activation as significant risk factors for developing PT. Among these variables, the Odds Ratio (OR) of grade II-IV aGVHD was the highest (P =0.001, OR: 2.65, 95% CI: 1.51-4.64). These data indicated the prognostic value of PT in MDS after HSCT. The identification of risk factors for PT may help improve patient management and lead to the design of effective treatment strategies.
Introduction
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is the only curative treatment for patients with myelodysplastic syndrome (MDS). Prolonged isolated thrombocytopenia (PT) is a frequent complication after transplantation, includes primary poor platelet graft function (PPGF) and secondary failure of platelet recovery (SFPR) (1, 2). PT has been suggested to be associated with an increased platelet transfusion requirement and poor overall survival following allo-HSCT (2–6).
The mechanisms underlying development of PT after HSCT are complex, and are usually categorized into impaired platelet production and increased platelet destruction (7). Several potential risk factors for PT after HSCT have been suggested, including graft-versus-host disease (GVHD), doses of infused CD34+ cells, disease status, cytomegalovirus (CMV) infection, and donor-specific antibodies (6, 8–11). Reports from Kong Y and her colleagues demonstrated that impaired bone marrow vascular microenvironment and aberrant T cell responses in immune microenvironment may contribute to the occurrence of PT after HSCT (12–14). They also proposed that disease type, especially diagnosed as MDS, was an independent risk factor for SFPR (12).
We undertook a single-center real-world study in the Chinese population, focusing on characteristics of PT in MDS patients. The purpose of present study was to evaluate the prognostic impact and defined the potential risk factors for PT in MDS patients after HSCT.
Materials and Methods
Patients and Study Design
303 consecutive MDS patients who received allo-HSCT in the First Affiliated Hospital of Soochow University between December 2007 and June 2018 were included in our study. Patients’ age, gender, WHO classification, IPSS and IPSS-R risk, donor type, conditioning regimen, stem cell source, disease status, HLA typing, ABO blood group, GVHD prophylaxis, and transplant related complications were recorded. Informed consent was obtained from all patients or from their immediate family before data was collected. All protocols conformed to the guidelines of the ethics committee of Soochow University and the Declaration of Helsinki. All patients were followed until September 2019 or death.
Conditioning Regimens in Allo-HSCT
Myeloablative conditioning (MAC) regimens were applied in most cases, while all other patients received reduced intensive conditioning (RIC) regimens. For HLA matched sibling donor transplant (MSDT), MAC regimens comprised administration of semustine (250mg/m2, day −10), cytarabine (2g/m2/d, days −9 to −8), busulfan (3.2 mg/kg/d, days −7 to −5), and cyclophosphamide (1.8g/m2/d, days −4 to −3). For HLA matched unrelated donors transplant (MUDT) and haploidentical donors transplant (HIDT), patients received a MAC regimen identical to the MSDT regimen except for receiving a higher dose of cytarabine (4 g/m2/d, days −9 to −8). Patients receiving MUDT also received hydroxycarbamide (80mg/kg, day −10). The RIC comprised fludarabine (30 mg/m2/d, days −10 to −6), cytarabine (1.5 g/m2/d, days −10 to −9), busulfan (3.2 mg/kg/d, days −8 to −6), cyclophosphamide (1.0 g/m2/day, days −5 to −4), and semustine (250 mg/m2/day, day -3). Additionally, Rabbit ATG (Genzyme Polyclonals S.A.S, Lyon, France), ATG-F (Fresenius Biotech GmbH, Munich, Germany), or porcrine ALG (Wuhan Institute of Biological Products Co., Ltd., Wuhan, Hubei, China) was given to patients receiving MUDT and HIDT for GVHD prophylaxis. The regimens were: ATG 2.5mg/kg/day, for four days; ATG-F 5mg/kg/day, for four days; ALG 15mg/kg/day, for four days. For a small number of patients who received MSDT, a lower dose of ATG (2.5mg/kg/day, for two days) or ATG-F (5mg/kg/day, for two days) was used.
Definitions
Prolonged isolated thrombocytopenia (PT) includes primary poor platelet graft function (PPGF) and secondary failure of platelet recovery (SFPR) (1, 2). Patients with primary PPGF were defined as those who did not achieve initial platelet reconstitution, with persistent platelet counts below 20×109/L or depended on PLT transfusions for more than 90 days after HSCT (1). SFPR was defined as a decline of platelet count to <50×109/L for more than 7 consecutive days after initial platelet reconstitution (2). Patients with thrombocytopenia due to graft rejection or disease recurrence were not defined as PPGF, in accordance with the definition from a previous study (2). The date of platelet engraftment was defined as the first of 7 consecutive days with a platelet count of ≥20×109/L, without transfusion support. Overall survival (OS) was defined from the time of transplant until death from any cause, or until the date of last follow-up. Relapse free survival (RFS) was defined from the time of transplant until disease relapse, or death from any cause, or until the date of last follow-up.
Statistical Analysis
Categorical variables are shown as percentages and compared using the χ2 test. Continuous variables are presented as medians with interquartile ranges, and compared using Mann-Whitney U tests. Missing data were replaced using Random Forests in the ‘mice’ package of R, version 3.6.0 (http://www.r-project.org/). Cumulative incidence was visualized using Kaplan-Meier curves and compared using the log-rank test. Univariate and multivariate survival analyses for OS and RFS were undertaken by Cox proportional hazard models. The importance of individual variables was visualized using forest plots. Univariate analyses of risk factors were performed with univariate logistic regression. Risk factors with values of P <0.05 in the univariate analyses were chosen for further evaluation by multivariate logistic regression.
Results
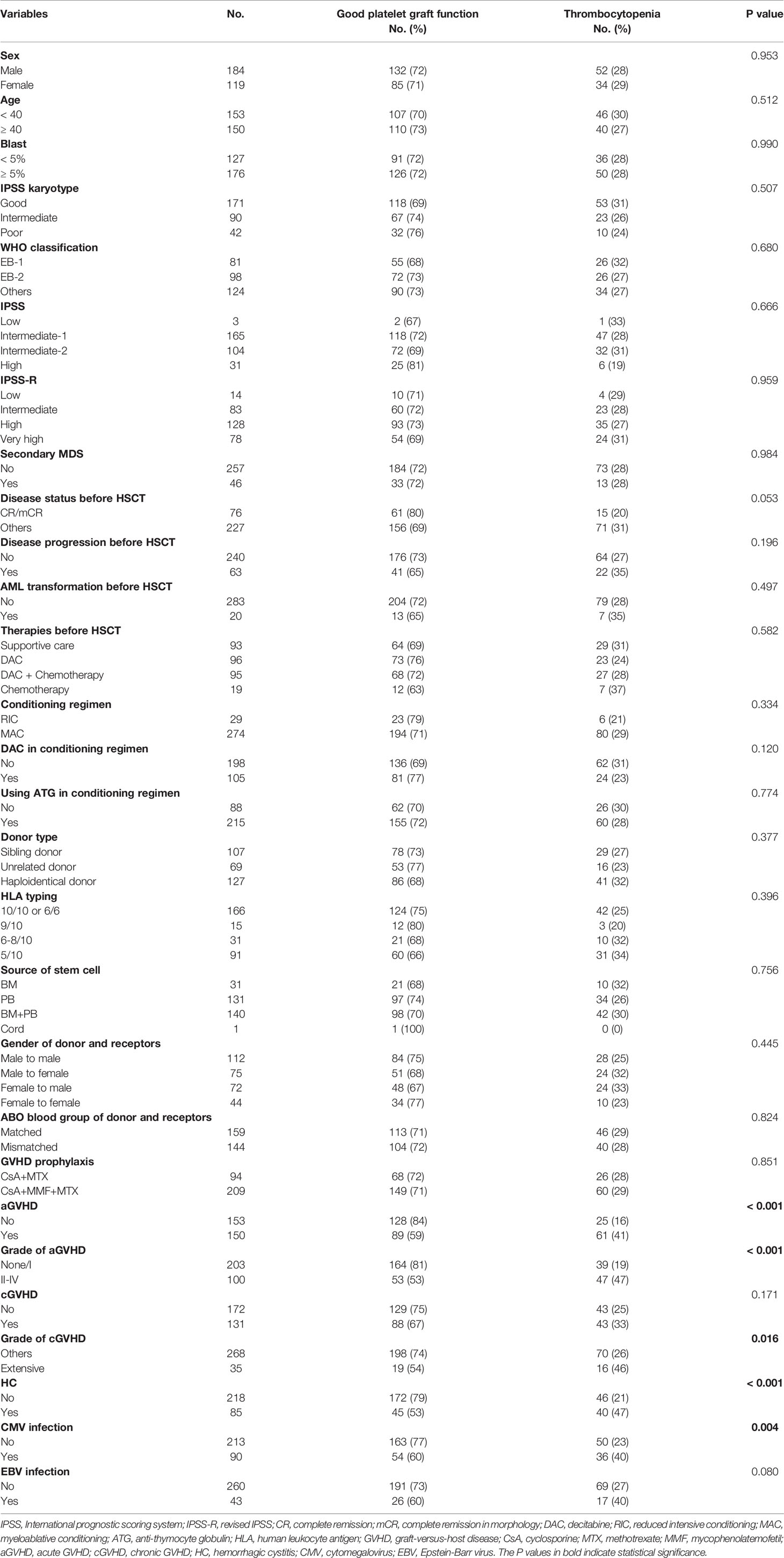
303 MDS patients who received allo-HSCT were included in our study. 184 (60.7%) were male and 119 (39.3%) were female. The median age of the cohort was 39 years (IQR 28-46). 107 (35.3%) patients received HLA matched sibling donors transplant, 69 (22.8%) received unrelated donors transplant, and 127 (41.9%) received haploidentical donors transplant. Before transplant, 76 (25.1%) achieved morphology-complete remission or complete remission. Most patients received MAC conditioning regimen (274 cases, 90.4%). After transplantation, 35 patients (11.6%) experienced primary PPGF, and 51 patients (16.8%) had SFPR. Acute GVHD (aGVHD) occurred in 150 (49.5%) patients. 100 patients (33.0%) had grade II–IV aGVHD and 35 patients (11.6%) extensive chronic GVHD (cGVHD). Cytomegalovirus (CMV) viremia was detected in 90 patients (29.7%) and Epstein-Barr virus (EBV) was identified in 43 patients (14.2%). Hemorrhagic cystitis (HC) occurred in 85 patients (28.1%) (Table 1).
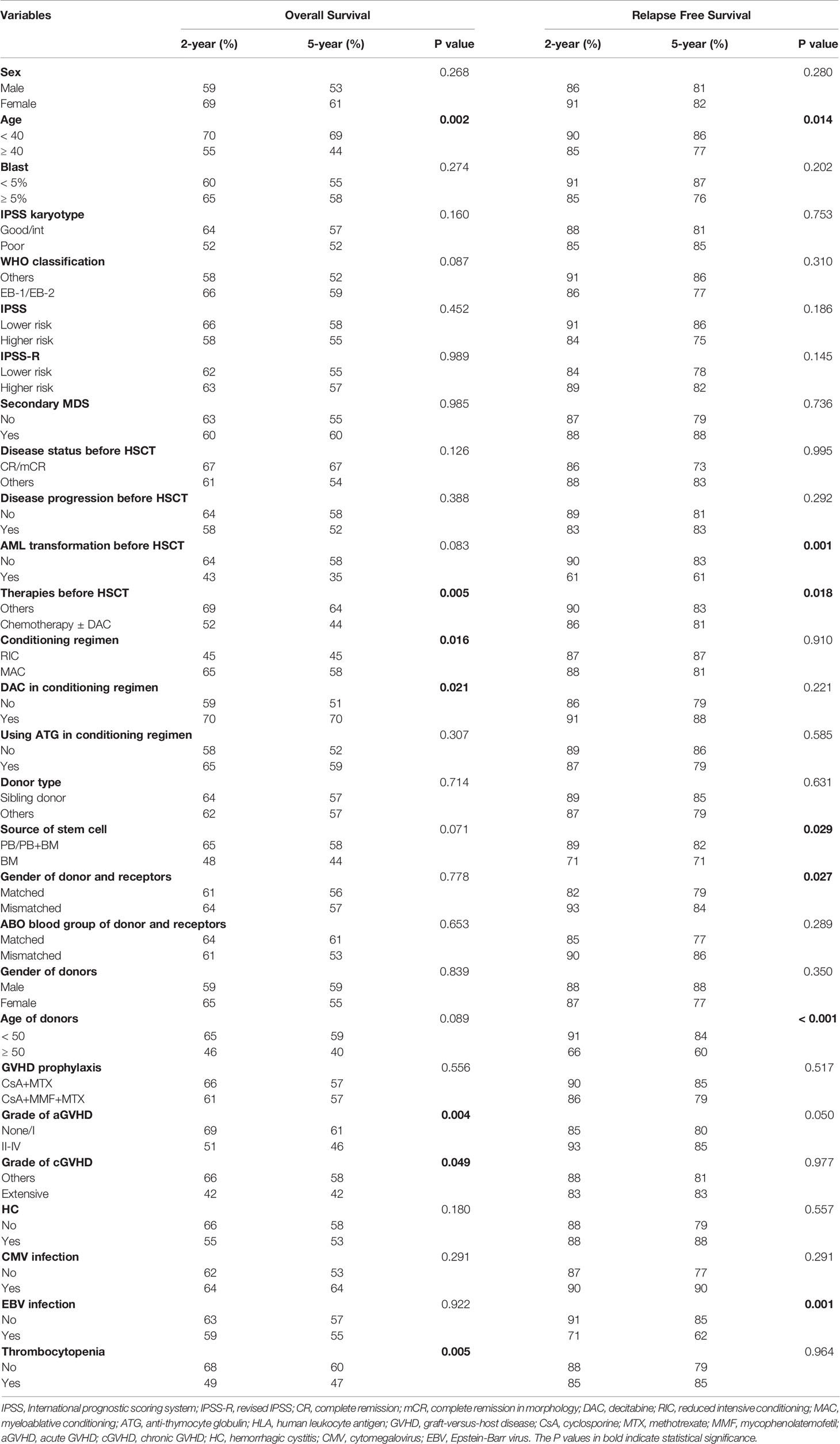
Our analyses showed that OS of patients without PT was significantly better than that of patients with either primary PPGF (P = 0.033) or SFPR (P = 0.003), while no significant difference in OS was observed between the patients with primary PPGF and SFPR (P = 0.903) (Supplementary Figure S1A). However, for RFS, patients with primary PPGF or SFPR did not impact RFS in MDS patients after transplantation (Supplementary Figure S1B). Univariate analysis of risk factors affecting OS and RFS are listed in Table 2. Apart from older age, receiving chemotherapy before HSCT, receiving the RIC conditioning regimen, receiving a conditioning regimen without decitabine, grade II-IV aGVHD, and extensive cGVHD, PT was also a significant predictor of poor OS (Figure 1). The 2-year and 5-year OS in MDS patients with PT after HSCT were 49% and 47%, significantly worse than that of 68% and 60% in patients without PT (Figure 1A, P =0.005). However, for RFS, patients with PT did not have an increased risk of disease relapse, as shown in Figure 1B (P=0.964). After multivariate adjustment, PT was proved to be the independent risk factor associated with the worse OS (HR 1.49, 95% CI 1.00-2.21, P =0.048) (Figure 1C).
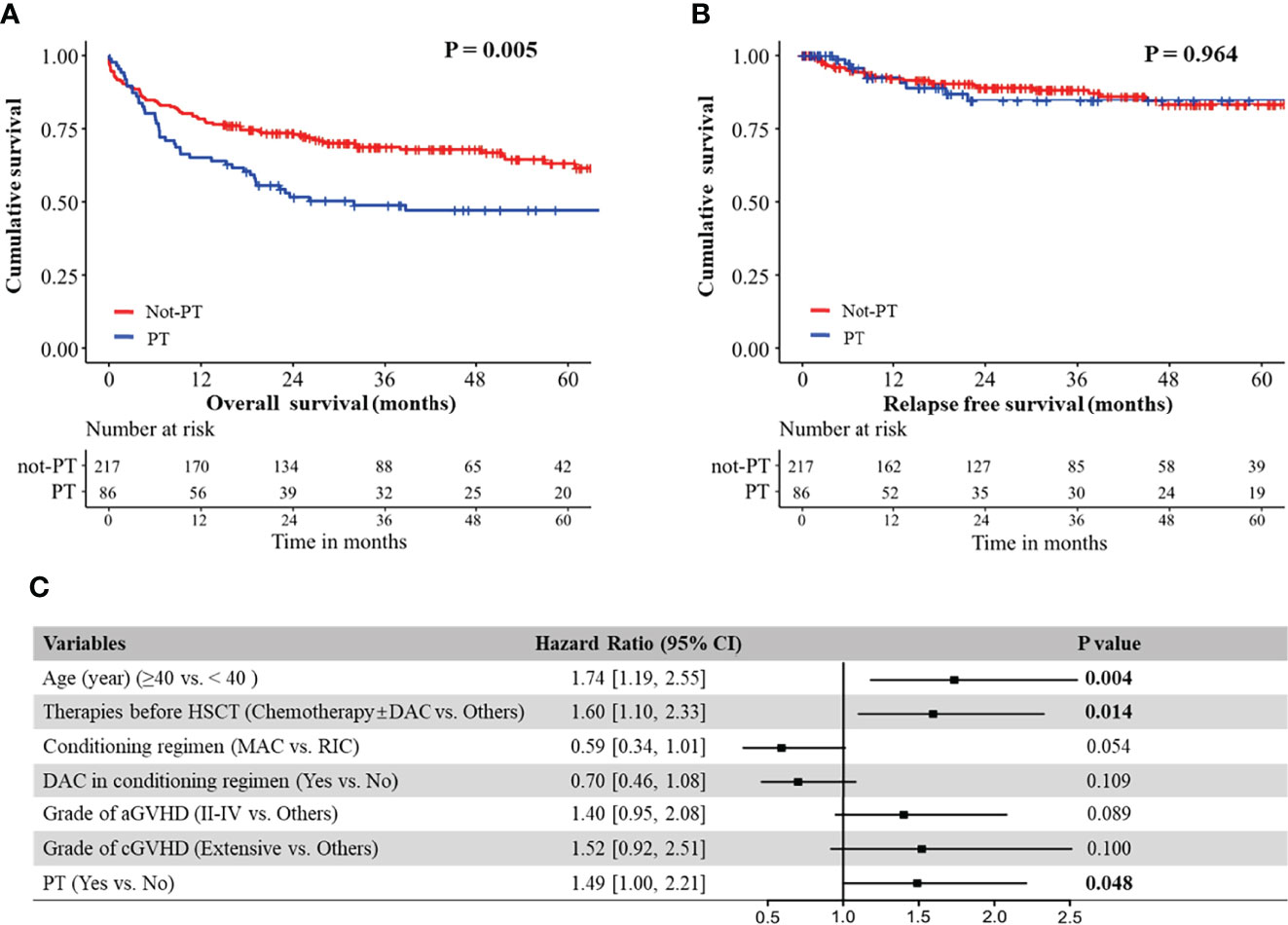
Figure 1 (A) Overall survival of MDS patients with and without PT; (B) Relapse free survival of MDS patients with and without PT. (C) Forest plot for hazard ratio of different risk factors on overall survival in MDS patients, as established using a Cox regression model.
Being a significant complication after HSCT, patients with PT had inferior survival. We further analyzed risk factors associated with the occurrence of PT in MDS patients. Univariate analysis identified grade II-IV aGVHD, extensive cGVHD, HC, and CMV activation as risk factors for developing PT (Table 3). Including these variables in a multivariate logistic regression, the result showed that these four variables were the independent risk factors associated with the occurrence of PT in MDS after HSCT (Figure 2A). Of these, the OR of grade II-IV aGVHD was the greatest (P =0.001, OR: 2.65, 95% CI: 1.51-4.64).
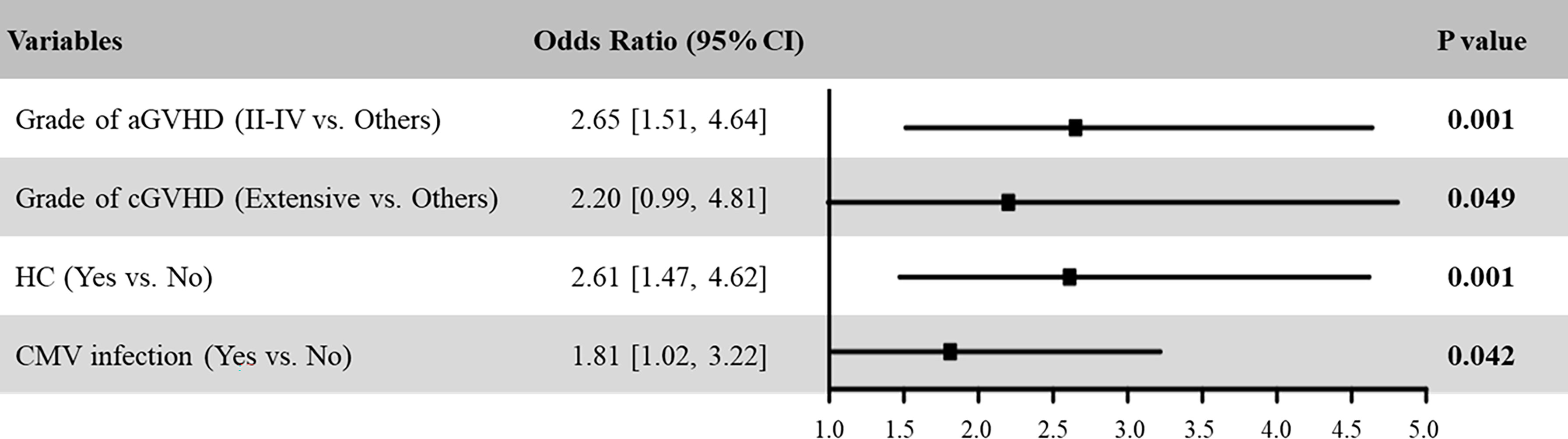
Figure 2 Multivariable logistic regression model assessing risk factors for PT in MDS patients after HSCT.
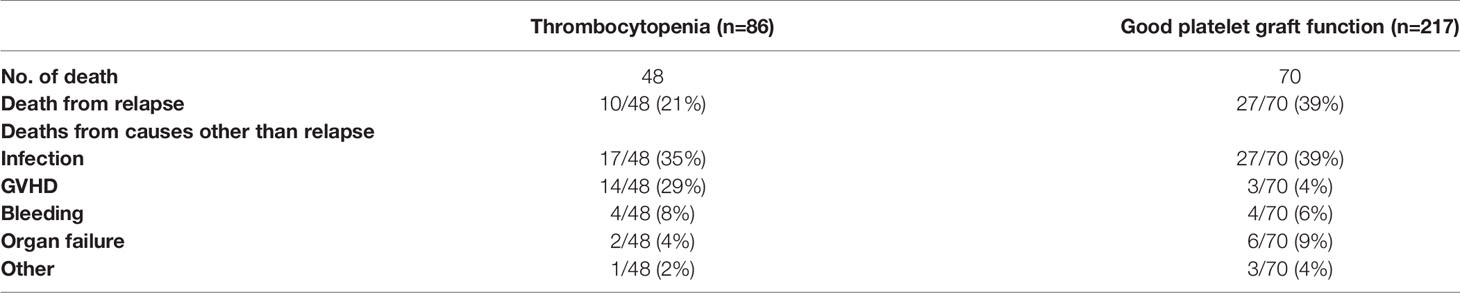
Until last follow-up, 48 cases in PT group and 70 in non-PT group have died. Among these, 37(31%) were due to disease relapse. Of the 81 non-relapse deaths, infection and GVHD were either the main or contributing causes in patients with PT. And infection was the main cause of death in patients without PT. The causes of death in MDS patients after transplantation were shown in Table 4.
Discussion
PT is a serious complication post HSCT with a poor prognosis. Its reported incidence ranged from 3 to 50% following HSCT (1, 2, 4, 5, 7, 13). In our real-world study, we found an incidence of 28.4% for PT in MDS patients post HSCT. This variation may arise from the heterogeneity of criteria used to define PT (1–3, 12, 15, 16). Moreover, different patient selection in different centers may also contribute to differences in reported incidences. Most previous studies, including a report from our center, included several types of hematologic malignancy, including acute myeloid leukemia, acute lymphocytic leukemia, MDS, aplastic anemia, lymphoma and other disease type (1, 4, 5, 13, 16).
In this study, both grade II-IV aGVHD and extensive cGVHD were independent risk factors associated with the occurrence of PT in MDS patients post HSCT. Several previous studies reported correlations between aGVHD and platelet recovery after HSCT (2, 16, 17). Similar to the work of Kim et al., grade III-IV aGVHD was shown to be an independent risk factor for developing PT (16). The key mechanism involved in its development is thought to be GVHD-related autoimmune destruction (8). Platelet autoantibodies have been observed in patients after both autologous HSCT and allo-HSCT (7, 8, 18). According to the report from Anasetti C et al., platelet autoantibodies were only seen patients with GVHD, whereas in patients without GVHD, autoantibodies were not observed (8). Yamazaki R et al. indicated that in addition to antiplatelet antibody, reticuloendothelial system, which was damaged by GVHD, was also implicated in the development of PT (7).
CMV infection is another common complication, causing morbidity and mortality after HSCT. Consistent with previous studies, CMV infection has been suggested to be correlated with PT after HSCT (2, 4, 19). The role of CMV infection in the pathophysiology of PT is not fully understood. Several in vitro studies have shown that early hematopoietic progenitors are more susceptible to CMV infection, resulting in the inhibition of their proliferative function (20, 21). Apart from the direct cytotoxicity of CMV in hematopoietic progenitor cells, CMV-related impairment of stromal function, abnormal gene expression, and the indirect immune destruction of CMV infected hematopoietic cells have all been suggested as pathological mechanisms underlying PT development (22–25). In addition, Crapnell et al. have shown that differentiated megakaryocytes and their precursors are targets of CMV infection in vitro, contributing to thrombocytopenia (26).
Our study supported HC as an independent risk factor predictive of PT development of PT post HSCT. The correlation of platelet recovery and HC has been evaluated in several studies, and the results are controversial (27–29). Lunde et al. observed that HC resolution is associated with raised platelet counts (27). However, other studies suggest platelet counts are maintained > 50 × 109/L in patients with active HC (30–32). Because acute or chronic GVHD and HC may exist or that immunosuppressive therapies used to treat GVHD increase can the probability of opportunistic infections which subsequently cause HC (33, 34). Other studies have suggested an association between CMV reactivation and HC (35, 36), as DNA viruses may induce BK virus Replication (37, 38). The involvement in PT of both GVHD and CMV have been suggested, with different mechanisms as discussed above. A complex relationship exists amongst HC, GVHD, and CMV infection, and it is reasonable that HC was identified as a risk factor for PT in MDS patients post HSCT.
In conclusion, our results indicate that PT predicts poor OS in MDS patients after HSCT. The identification of risk factors for PT may help clinicians to more accurately assess the prognosis and design new treatment strategies.
Data Availability Statement
The original data presented in this study is available on request from the corresponding author aGFueXVlQHN1ZGEuZWR1LmNu.
Ethics Statement
This study was reviewed and approved by Ethics Committee of the First affiliated Hospital of Soochow University. Participants provided their written informed consent to participate in this study.
Author Contributions
HW, JQ, XL, TC: contribution of patients, acquisition of data, analysis and interpretation of data. YH, DW and HW: Design of study, acquisition of funding contribution of patients, interpretation of data, supervision of the study, and revision of the manuscript. HQ, CF, XT and CR: contribution of patients and revision of the manuscript. HW, JQ and XL wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (81100342, 81873432 and 82070143), grants from the Jiangsu Province of China (BE2021645), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.940320/full#supplementary-material
References
1. Bielski M, Yomtovian R, Lazarus HM, Rosenthal N. Prolonged Isolated Thrombocytopenia After Hematopoietic Stem Cell Transplantation: Morphologic Correlation. Bone Marrow Transplant (1998) 22(11):1071–6. doi: 10.1038/sj.bmt.1701499
2. Bruno B, Gooley T, Sullivan KM, Davis C, Bensinger WI, Storb R, et al. Secondary Failure of Platelet Recovery After Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2001) 7(3):154–62. doi: 10.1053/bbmt.2001.v7.pm11302549
3. First LR, Smith BR, Lipton J, Nathan DG, Parkman R, Rappeport JM. Isolated Thrombocytopenia After Allogeneic Bone Marrow Transplantation: Existence of Transient and Chronic Thrombocytopenic Syndromes. Blood (1985) 65(2):368–74. doi: 10.1182/blood.V65.2.368.368
4. Wang H, Huang M, Zhao Y, Qi JQ, Chen C, Tang YQ, et al. Recombinant Human Thrombopoietin Promotes Platelet Engraftment and Improves Prognosis of Patients With Myelodysplastic Syndromes and Aplastic Anemia After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2017) 23(10):1678–84. doi: 10.1016/j.bbmt.2017.06.010
5. Sun YQ, He GL, Chang YJ, Xu LP, Zhang XH, Han W, et al. The Incidence, Risk Factors, and Outcomes of Primary Poor Graft Function After Unmanipulated Haploidentical Stem Cell Transplantation. Ann Hematol (2015) 94(10):1699–705. doi: 10.1007/s00277-015-2440-x
6. Zhao X, Zhao X, Huo M, Fan Q, Pei X, Wang Y, et al. Donor-Specific Anti-Human Leukocyte Antigen Antibodies Predict Prolonged Isolated Thrombocytopenia and Inferior Outcomes of Haploidentical Hematopoietic Stem Cell Transplantation. J Immunol Res (2017) 2017:1043836. doi: 10.1155/2017/1043836
7. Yamazaki R, Kuwana M, Mori T, Okazaki Y, Kawakami Y, Ikeda Y, et al. Prolonged Thrombocytopenia After Allogeneic Hematopoietic Stem Cell Transplantation: Associations With Impaired Platelet Production and Increased Platelet Turnover. Bone Marrow Transplant (2006) 38(5):377–84. doi: 10.1038/sj.bmt.1705444
8. Anasetti C, Rybka W, Sullivan KM, Banaji M, Slichter SJ. Graft-V-Host Disease is Associated With Autoimmune-Like Thrombocytopenia. Blood (1989) 73(4):1054–8. doi: 10.1182/blood.V73.4.1054.1054
9. Chang YJ, Xu LP, Liu DH, Liu KY, Han W, Chen YH, et al. Platelet Engraftment in Patients With Hematologic Malignancies Following Unmanipulated Haploidentical Blood and Marrow Transplantation: Effects of CD34+ Cell Dose and Disease Status. Biol Blood Marrow Transplant (2009) 15(5):632–8. doi: 10.1016/j.bbmt.2009.02.001
10. Nakamae H, Storer B, Sandmaier BM, Maloney DG, Davis C, Corey L, et al. Cytopenias After Day 28 in Allogeneic Hematopoietic Cell Transplantation: Impact of Recipient/Donor Factors, Transplant Conditions and Myelotoxic Drugs. Haematologica (2011) 96(12):1838–45. doi: 10.3324/haematol.2011.044966
11. Verdonck LF, de Gast GC, van Heugten HG, Nieuwenhuis HK, Dekker AW. Cytomegalovirus Infection Causes Delayed Platelet Recovery After Bone Marrow Transplantation. Blood (1991) 78(3):844–8. doi: 10.1182/blood.V78.3.844.844
12. Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, et al. Association of an Impaired Bone Marrow Microenvironment With Secondary Poor Graft Function After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2013) 19(10):1465–73. doi: 10.1016/j.bbmt.2013.07.014
13. Kong Y, Hu Y, Zhang XH, Wang YZ, Mo XD, Zhang YY, et al. Association Between an Impaired Bone Marrow Vascular Microenvironment and Prolonged Isolated Thrombocytopenia After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2014) 20(8):1190–7. doi: 10.1016/j.bbmt.2014.04.015
14. Song Y, Shi MM, Zhang YY, Mo XD, Wang Y, Zhang XH, et al. Abnormalities of the Bone Marrow Immune Microenvironment in Patients With Prolonged Isolated Thrombocytopenia After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2017) 23(6):906–12. doi: 10.1016/j.bbmt.2017.02.021
15. Zhang X, Fu H, Xu L, Liu D, Wang J, Liu K, et al. Prolonged Thrombocytopenia Following Allogeneic Hematopoietic Stem Cell Transplantation and its Association With a Reduction in Ploidy and an Immaturation of Megakaryocytes. Biol Blood Marrow Transplant (2011) 17(2):274–80. doi: 10.1016/j.bbmt.2010.09.007
16. Kim DH, Sohn SK, Jeon SB, Baek JH, Kim JG, Lee NY, et al. Prognostic Significance of Platelet Recovery Pattern After Allogeneic HLA-Identical Sibling Transplantation and its Association With Severe Acute GVHD. Bone Marrow Transplant (2006) 37(1):101–8. doi: 10.1038/sj.bmt.1705203
17. Dominietto A, Raiola AM, van Lint MT, Lamparelli T, Gualandi F, Berisso G, et al. Factors Influencing Haematological Recovery After Allogeneic Haemopoietic Stem Cell Transplants: Graft-Versus-Host Disease, Donor Type, Cytomegalovirus Infections and Cell Dose. Br J Haematol (2001) 112(1):219–27. doi: 10.1046/j.1365-2141.2001.02468.x
18. Minchinton RM, Waters AH. Autoimmune Thrombocytopenia and Neutropenia After Bone Marrow Transplantation. Blood (1985) 66(3):752.
19. Torok-Storb B, Boeckh M, Hoy C, Leisenring W, Myerson D, Gooley T. Association of Specific Cytomegalovirus Genotypes With Death From Myelosuppression After Marrow Transplantation. Blood (1997) 90(5):2097–102. doi: 10.1182/blood.V90.5.2097
20. Movassagh M, Gozlan J, Senechal B, Baillou C, Petit JC, Lemoine FM. Direct Infection of CD34+ Progenitor Cells by Human Cytomegalovirus: Evidence for Inhibition of Hematopoiesis and Viral Replication. Blood (1996) 88(4):1277–83. doi: 10.1182/blood.V88.4.1277.bloodjournal8841277
21. Zhuravskaya T, Maciejewski JP, Netski DM, Bruening E, Mackintosh FR, St Jeor S. Spread of Human Cytomegalovirus (HCMV) After Infection of Human Hematopoietic Progenitor Cells: Model of HCMV Latency. Blood (1997) 90(6):2482–91. doi: 10.1182/blood.V90.6.2482
22. Sing GK, Ruscetti FW. Preferential Suppression of Myelopoiesis in Normal Human Bone Marrow Cells After In Vitro Challenge With Human Cytomegalovirus. Blood (1990) 75(10):1965–73. doi: 10.1182/blood.V75.10.1965.1965
23. Duncombe AS, Grundy JE, Prentice HG, Brenner MK. IL2 Activated Killer Cells may Contribute to Cytomegalovirus Induced Marrow Hypoplasia After Bone Marrow Transplantation. Bone Marrow Transplant (1991) 7(2):81–7.
24. Maciejewski JP, Bruening EE, Donahue RE, Mocarski ES, Young NS, St Jeor SC. Infection of Hematopoietic Progenitor Cells by Human Cytomegalovirus. Blood (1992) 80(1):170–8. doi: 10.1182/blood.V80.1.170.bloodjournal801170
25. Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons PJ, et al. The Effect of Cytomegalovirus on Hemopoiesis: In Vitro Evidence for Selective Infection of Marrow Stromal Cells. Exp Hematol (1989) 17(1):38–45.
26. Crapnell K, Zanjani ED, Chaudhuri A, Ascensao JL, St Jeor S, Maciejewski JP. In Vitro Infection of Megakaryocytes and Their Precursors by Human Cytomegalovirus. Blood (2000) 95(2):487–93. doi: 10.1182/blood.V95.2.487
27. Lunde LE, Dasaraju S, Cao Q, Cohn CS, Reding M, Bejanyan N, et al. Hemorrhagic Cystitis After Allogeneic Hematopoietic Cell Transplantation: Risk Factors, Graft Source and Survival. Bone Marrow Transplant (2015) 50(11):1432–7. doi: 10.1038/bmt.2015.162
28. El-Zimaity M, Saliba R, Chan K, Shahjahan M, Carrasco A, Khorshid O, et al. Hemorrhagic Cystitis After Allogeneic Hematopoietic Stem Cell Transplantation: Donor Type Matters. Blood (2004) 103(12):4674–80. doi: 10.1182/blood-2003-08-2815
29. Hale GA, Rochester RJ, Heslop HE, Krance RA, Gingrich JR, Benaim E, et al. Hemorrhagic Cystitis After Allogeneic Bone Marrow Transplantation in Children: Clinical Characteristics and Outcome. Biol Blood Marrow Transplant (2003) 9(11):698–705. doi: 10.1016/s1083-8791(03)00269-6
30. Dropulic LK, Jones RJ. Polyomavirus BK Infection in Blood and Marrow Transplant Recipients. Bone Marrow Transplant (2008) 41(1):11–8. doi: 10.1038/sj.bmt.1705886
31. Brugieres L, Hartmann O, Travagli JP, Benhamou E, Pico JL, Valteau D, et al. Hemorrhagic Cystitis Following High-Dose Chemotherapy and Bone Marrow Transplantation in Children With Malignancies: Incidence, Clinical Course, and Outcome. J Clin Oncol (1989) 7(2):194–9. doi: 10.1200/JCO.1989.7.2.194
32. Cheuk DK, Lee TL, Chiang AK, Ha SY, Lau YL, Chan GC. Risk Factors and Treatment of Hemorrhagic Cystitis in Children Who Underwent Hematopoietic Stem Cell Transplantation. Transpl Int (2007) 20(1):73–81. doi: 10.1111/j.1432-2277.2006.00404.x
33. Echavarria M, Herrera F, Solimano J, Villamea L, Riera L, de Klerk EP, et al. Cyclic Recovery of Adenovirus in a Stem Cell Transplant Recipient: An Inverse Association With Graft-Versus-Host Disease. Bone Marrow Transplant (2003) 31(4):301–3. doi: 10.1038/sj.bmt.1703814
34. Ruutu T, Ruutu M, Volin L, Leskinen R. Severe Cystitis as a Manifestation of Chronic Graft-Versus-Host Disease After Bone Marrow Transplantation. Br J Urol (1988) 62(6):612–3. doi: 10.1111/j.1464-410x.1988.tb04439.x
35. Uhm J, Hamad N, Michelis FV, Shanavas M, Kuruvilla J, Gupta V, et al. The Risk of Polyomavirus BK-Associated Hemorrhagic Cystitis After Allogeneic Hematopoietic SCT is Associated With Myeloablative Conditioning, CMV Viremia and Severe Acute GVHD. Bone Marrow Transplant (2014) 49(12):1528–34. doi: 10.1038/bmt.2014.181
36. Arai Y, Maeda T, Sugiura H, Matsui H, Jo T, Ueda T, et al. Risk Factors for and Prognosis of Hemorrhagic Cystitis After Allogeneic Stem Cell Transplantation: Retrospective Analysis in a Single Institution. Hematology (2012) 17(4):207–14. doi: 10.1179/1607845412Y.0000000010
37. Pari GS, St Jeor SC. Human Cytomegalovirus Major Immediate Early Gene Product can Induce SV40 DNA Replication in Human Embryonic Lung Cells. Virology (1990) 179(2):785–94. doi: 10.1016/0042-6822(90)90146-i
Keywords: hematopoietic stem cell transplantation, myelodysplastic syndrome, prognosis, thrombocytopenia, risk factor
Citation: Wang H, Qi J, Li X, Chu T, Qiu H, Fu C, Tang X, Ruan C, Wu D and Han Y (2022) Prognostic Value of Thrombocytopenia in Myelodysplastic Syndromes After Hematopoietic Stem Cell Transplantation. Front. Oncol. 12:940320. doi: 10.3389/fonc.2022.940320
Received: 10 May 2022; Accepted: 21 June 2022;
Published: 11 July 2022.
Edited by:
Gang Zheng, Mayo Clinic, United StatesReviewed by:
Yujun Dong, First Hospital, Peking University, ChinaZimu Gong, Houston Methodist Hospital, United States
Copyright © 2022 Wang, Qi, Li, Chu, Qiu, Fu, Tang, Ruan, Wu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Han, aGFueXVlQHN1ZGEuZWR1LmNu; Depei Wu, d3VkZXBlaXN6QDE2My5jb20=
†These authors have contributed equally to this work
 Hong Wang1,2,3†
Hong Wang1,2,3† Yue Han
Yue Han