- Department of General Surgery, Lishui People’s Hospital, Lishui, China
Purpose: Laparoscopic liver resection (LLR) is a widely practiced therapeutic method and holds several advantages over open liver resection (OLR) including less postoperative pain, lower morbidity, and faster recovery. However, the effect of LLR for the treatment of hepatocellular carcinoma (HCC) in elderly patients remains controversial. Therefore, we aimed to perform the first meta-analysis of propensity score-matched (PSM) studies to compare the short- and long-term outcomes of LLR versus OLR for elderly patients with HCC.
Methods: Databases including PubMed, Embase, Scopus, and Cochrane Library were systematically searched until April 2022 for eligible studies that compared LLR and OLR for the treatment of HCC in elderly patients. Short-term outcomes include postoperative complications, blood loss, surgical time, and length of hospital stay. Long-term outcomes include overall survival (OS) rate and disease-free survival (DFS) rate at 1, 3, and 5 years.
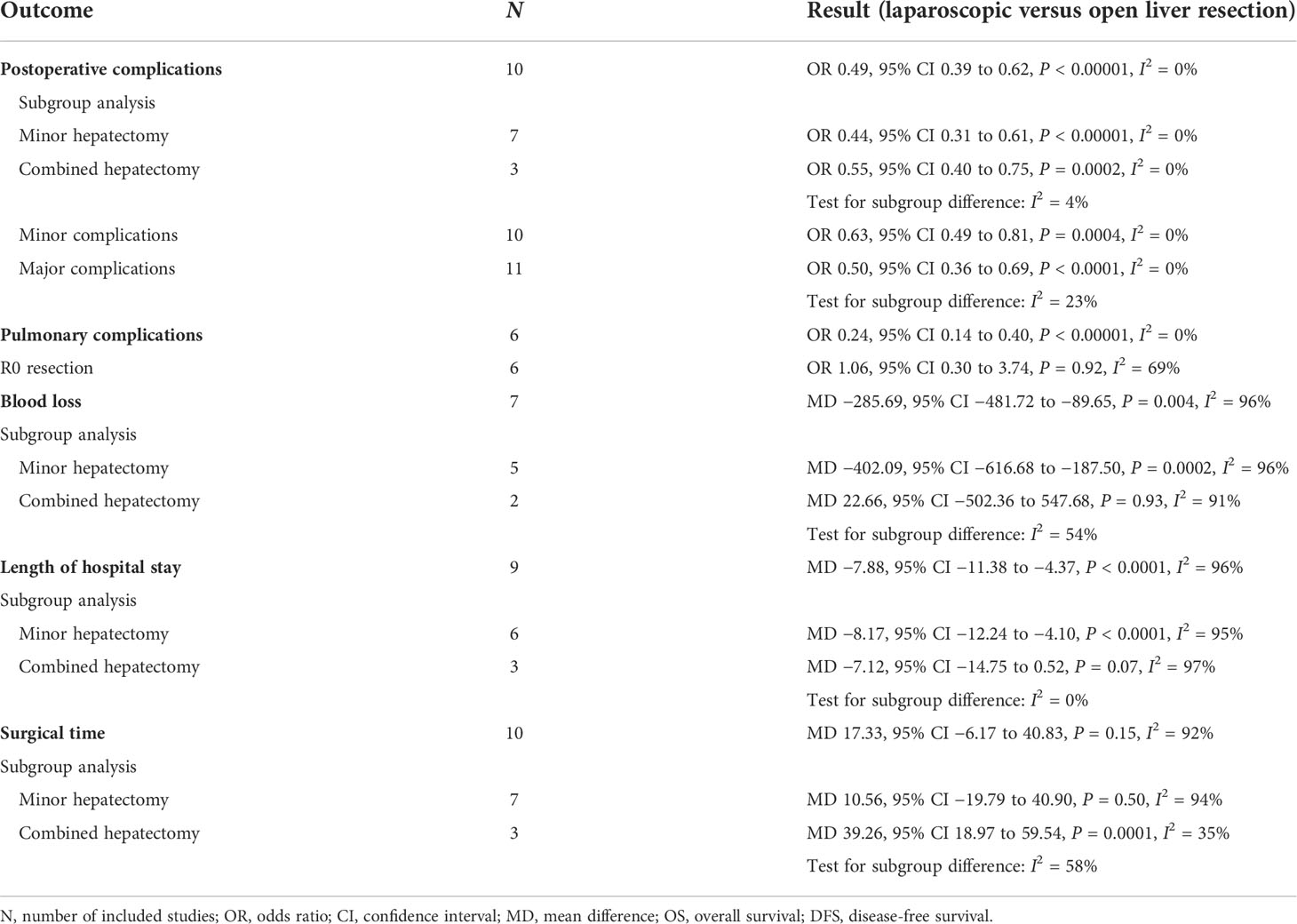
Results: A total of 12 trials involving 1,861 patients (907 in the LLR group, 954 in the OLR group) were included. Compared with OLR, LLR was associated with lower postoperative complications (OR 0.49, 95% CI 0.39 to 0.62, P < 0.00001, I2 = 0%), less blood loss (MD −285.69, 95% CI −481.72 to −89.65, P = 0.004, I2 = 96%), and shorter hospital stay (MD −7.88, 95% CI −11.38 to −4.37, P < 0.0001, I2 = 96%), whereas operation time (MD 17.33, 95% CI −6.17 to 40.83, P = 0.15, I2 = 92%) was insignificantly different. Furthermore, there were no significant differences for the OS and DFS rates at 1, 3, and 5 years.
Conclusions: For elderly patients with HCC, LLR offers better short-term outcomes including a lower incidence of postoperative complications and shorter hospital stays, with comparable long-term outcomes when compared with the open approach. Our results support the implementation of LLR for the treatment of HCC in elderly patients.
Systematic review registration: https://inplasy.com/inplasy-2022-4-0156/, identifier INPLASY202240156.
Introduction
Liver cancer is one of the most common cancers and a major global health challenge (1). According to GLOBOCAN 2020, liver cancer is the fourth leading cause of cancer death, causing an estimated 830,180 deaths in 2020 globally (2). Hepatocellular carcinoma (HCC) represents about 90% of primary liver cancers and constitutes a major health problem worldwide (3). Furthermore, modern advances in healthcare systems have greatly extended life expectancy (4), and the increased incidence of HCC is closely related to the aging of the population.
Surgical resection is one of the most effective treatments of choice for early HCC. Since Reich et al. reported the first laparoscopic liver resection (LLR) in 1991 (5), this minimally invasive technique has advanced continuously. Nowadays, this minimally invasive technique has gained increasing acceptance for some major well-known benefits, including a lower incidence of postoperative complications, shorter hospital stay, faster recovery, and better quality of life (6–8).
However, several factors such as the presence of comorbidities and the age of the patients may have a significant effect on the efficacy and safety of this minimally invasive technique. Age is a challenging feature given the significant heterogeneity of general conditions among individuals of the same age range and the growing number of elderly patients in good clinical condition presenting with HCC (9). Also, elderly patients are infrequently included in the range of randomized clinical trials, resulting in a lack of understanding of the benefits and risks of treatment strategies (10). Due to the factors that are mentioned above, clinicians are required to reconsider the treatment indications of this minimally invasive technique. Moreover, to surmount the existing selection and confounding biases inherent in non-randomized studies, we elected to limit to studies that performed propensity score matching (PSM), because a great number of research (11–14) have shown that PSM studies are comparable to RCTs empirically in terms of their capability of deriving unbiased estimates.
Accordingly, in order to summarize the present high-quality evidence, we performed a meta-analysis of PSM studies to compare the short- and long-term outcomes of LLR versus OLR for the treatment of HCC in elderly patients.
Methods
We conducted our study on the basis of the updated PRISMA statement (15) (Supplementary Material 1), and the protocol was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY 202240156). We systematically searched the PubMed, Embase, Scopus, and Cochrane Library databases for PSM studies up to April 2022. The search used broad search terms containing “HCC”, “liver cancer”, “hepatoma”, “laparoscopic”, “open liver resection”, “hepatectomy”, “elderly”, and “propensity score” (the comprehensive search strategies are listed in Supplementary Material 2).
Eligibility criteria
The inclusion criteria were as follows: 1) population: elderly patients (≥65 years old) with pathology‐confirmed HCC; 2) intervention: LLR; 3) comparison: OLR; 4) outcomes: short-term outcomes including postoperative complications, blood loss, surgical time, and length of hospital stay and long-term outcomes including 1-, 3-, and 5-year overall survival (OS) rates and 1-, 3-, and 5-year disease-free survival (DFS) rates; and 5) design: PSM.
Data extraction and quality assessment
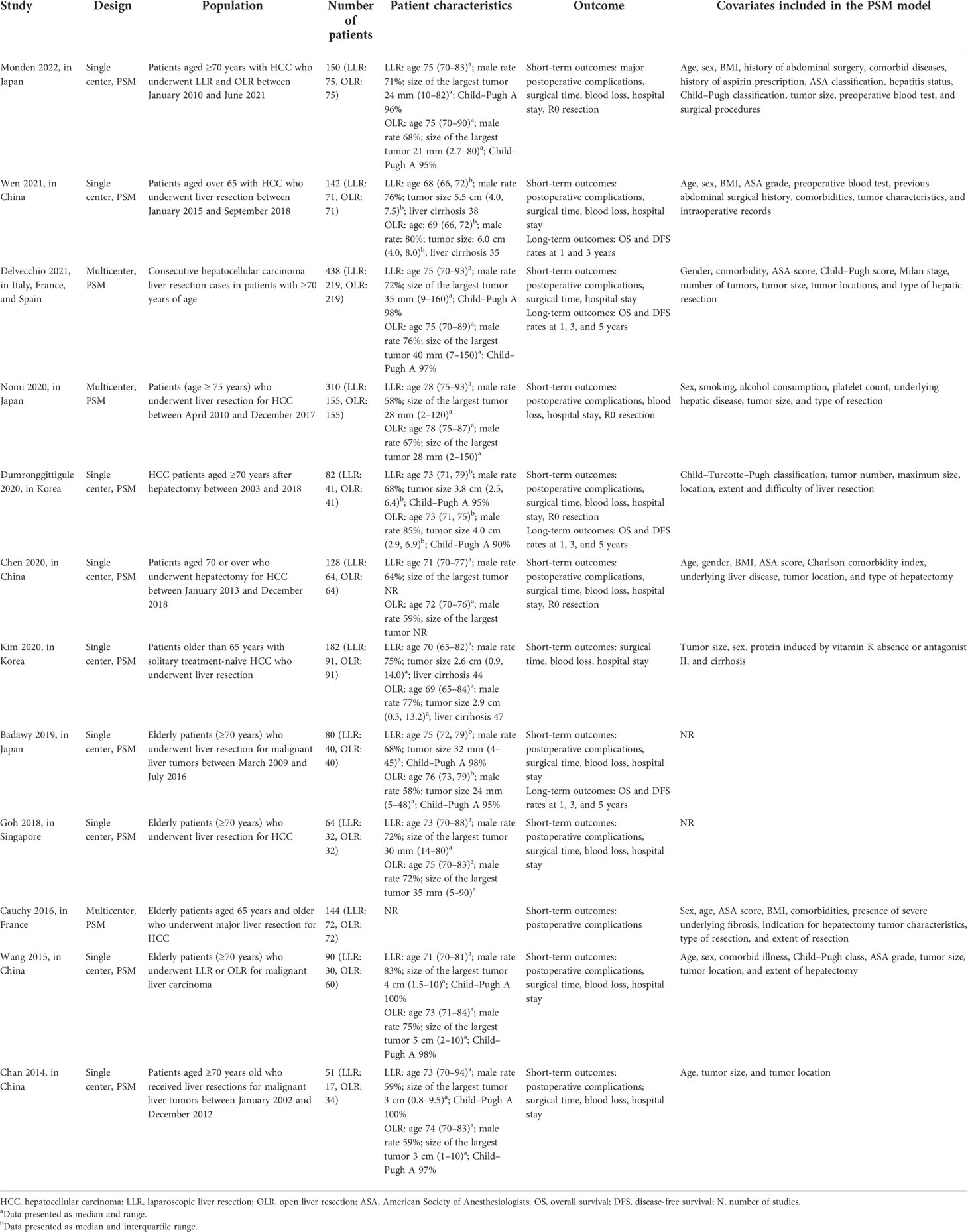
Two authors (SW and HY) independently searched relevant studies and extracted data. The characteristics of the included studies (e.g., author, years of publication, study design, population, number of patients, patient characteristics, outcomes, and covariates included in the PSM model) are recorded in Table 1.
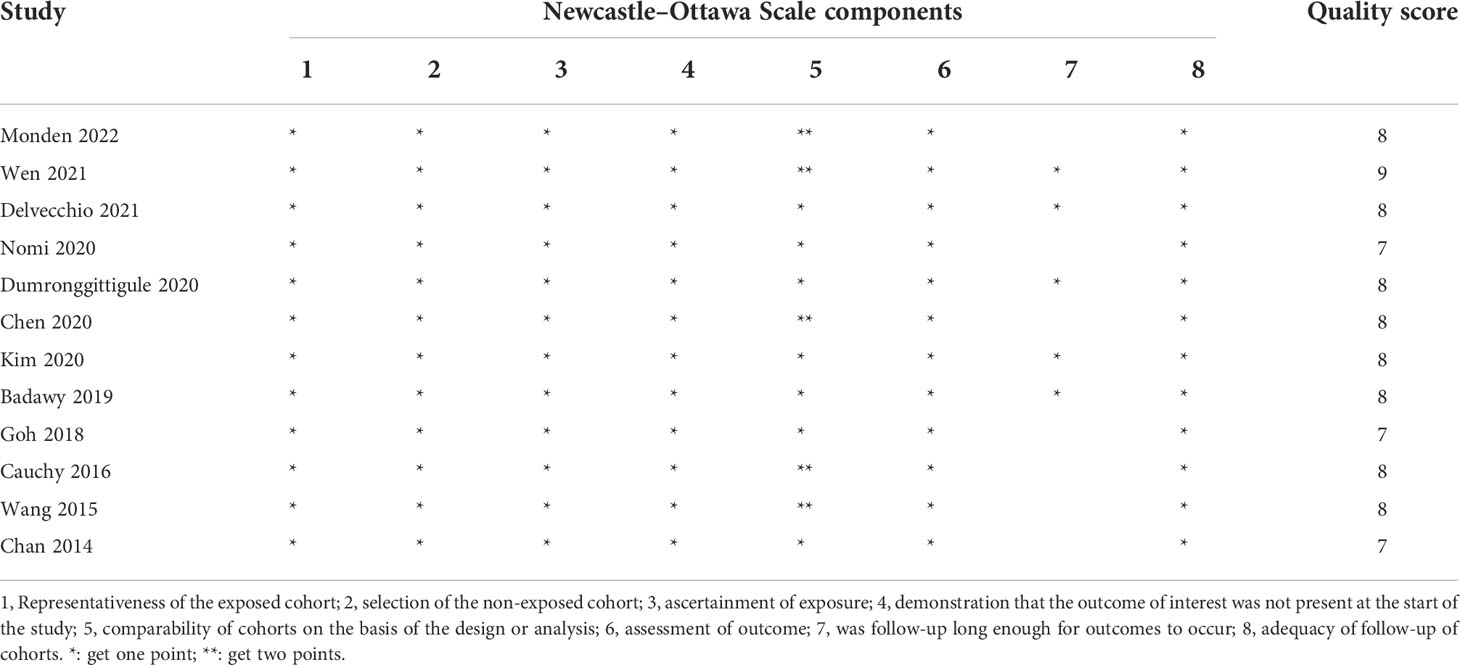
Two authors (GY and SW) independently evaluated the methodological quality of the included studies by using the Newcastle–Ottawa Scale for cohort studies. The Newcastle–Ottawa Scale contains three categories (including eight subcategories), and each study is able to acquire a maximum of 9 stars. The detailed grading standards are as follows: a score of 7 to 9 stars is graded as a high-quality study, a score of 4 to 6 stars is considered an average-quality study, whereas a score of 0 to 3 stars is classified as a low-quality study.
Statistical synthesis and analysis
We computed the pooled odds ratio (OR) with 95% confidence interval (CI) for dichotomous outcomes and the mean difference (MD) with 95% CI for continuous outcomes. For survival data, we used the hazard ratio (HR) with 95% CI reported in the included studies. If the HR data were not reported in the original study, we imputed the HR by digitizing the Kaplan–Meier survival curves (16). The heterogeneity between studies was assessed by the Higgins inconsistency (I2) statistics (17). Substantial heterogeneity was identified when the I2 value >30%, and a random-effects model was employed to perform the analysis; otherwise, a fixed-effects model would be used. Funnel plots were generated to assess the possibility of publication bias, and the Egger regression test was used to measure funnel plot asymmetry (18). We considered P <0.05 to be statistically significant and P <0.10 as an indicator of trends.
Subgroup analysis stratified by types of hepatectomy [minor versus major hepatectomy, based on the Second International Consensus Conference on Laparoscopic Liver Resections (19)] and age groups (≥65, ≥70, or ≥75) was performed to investigate the potential source of heterogeneity. Finally, a sensitivity analysis was conducted to explore the effect of an individual study by the consecutive exclusion of each study at one time.
Results
Study identification and characteristics
The initial search identified 608 articles (114 from PubMed, 174 from Embase, 274 from Scopus, and 46 from Cochrane Library). Among them, 376 were duplicated articles, and 147 studies were excluded by screening the abstracts. During the evaluation of the full text, 73 studies were further removed for various reasons. Eventually, a total of 12 trials (20–31) involving 1,861 patients (LLR versus OLR: 907 versus 954) were included in our study (flowchart in Figure 1).
Table 1 presents the characteristics of the included studies. The number of patients in each study ranged from a minimum of 51 to 438. Among the 12 included studies, four were performed in China (22, 23, 29, 30), three in Japan (20, 27, 28), two in Korea (25, 31), one in Singapore (26), one in France (21), and one study in Italy, France, and Spain (24), respectively. Different studies define “elder patients” individually. Three studies (21, 30, 31) had an inclusion criterion of ≥65 years, eight studies (20, 22–27, 29) comprised patients aged ≥70 years, and one study (28) included patients who were 75 years old and above. The LLR and the OLR groups were comparable in terms of age, gender, characteristics of the tumor, and the American Society of Anesthesiologists score. The types of hepatectomy were diverse among each study: eight studies (20, 22, 23, 26–30) performed minor hepatectomy and four studies (21, 24, 25, 31) included minor and major hepatectomy. The postoperative complications were graded according to the Clavien–Dindo classification, and a postoperative complication of Clavien–Dindo grade ≥III was defined as a major complication (32).
In addition, the length of hospital stay, surgical time, and blood loss were expressed as median with range or interquartile range. Thus, we converted the above data into mean and standard deviation by utilizing the methodology that was developed by Wan et al. (33).
Quality assessment
Table 2 presents the quality assessment by the Newcastle–Ottawa Scale. All included studies had high quality with a quality score ≥7. Six studies (20, 22, 24–26, 28) did not adjust for some important confounders (such as age, sex) or the covariates included in the PSM model were not reported, and the duration of follow-up in seven studies (21–23, 26–29) was limited.
Funnel plots and Egger regression test for all short-term outcome measures were used to further test for potential publication bias (Supplementary Material 3). No significant differences were found with respect to the endpoints of postoperative complications (P = 0.92), blood loss (P = 0.4164), length of hospital stay (P = 0.8368), or surgical time (P = 0.5373). Furthermore, since the number of trials in the analysis of long-term outcomes was limited, we could not reliably assess the publication bias.
Short-term outcomes
A total of 11 studies presented the postoperative complications (Monden et al. only reported the major postoperative complications). Overall, the incidence of postoperative complications in the LLR group was lower than that in the OLR group, 31.8% (236/741) versus 45.2% (356/788), respectively. Our meta-analysis demonstrated that LLR was associated with a lower incidence of postoperative complications (OR 0.49, 95% CI 0.39 to 0.62, P < 0.00001, I2 = 0%; Table 3, Supplementary Material 3). In addition to overall postoperative complications, the incidence of pulmonary complications was significantly lower in the LLR group (OR 0.24, 95% CI 0.14 to 0.40, P < 0.00001, I2 = 0%; Table 3, Supplementary Material 3). Moreover, six studies reported the rate of R0 resection, and there was no difference in the rate of R0 resection between the OLR and LLR groups (OR 1.06, 95% CI 0.30 to 3.74, P = 0.92, I2 = 69%; Table 3, Supplementary Material 3).
A total of seven studies reported blood loss during the operation. The meta-analysis demonstrated that LLR was associated with a significant less blood loss than OLR (MD −285.69, 95% CI −481.72 to −89.65, P = 0.004, I2 = 96%; Table 3, Supplementary Material 3). Also, LLR was related to a shorter length of hospital stay (MD −7.88, 95% CI −11.38 to −4.37, P < 0.0001, I2 = 96%; Table 3, Supplementary Material 3). Moreover, there was no significant difference in surgical time (MD 17.33, 95% CI −6.17 to 40.83, P = 0.15, I2 = 92%; Table 3, Supplementary Material 3). However, considering the significant heterogeneity in the pooled results, the results should be interpreted with caution.
Long-term outcomes
Four studies (20, 24, 25, 30) reported the long-term outcomes including the 1-, 3-, and 5-year OS and DFS rates, and the meta-analysis indicated that there was no significant difference in the 1-, 3-, and 5-year OS rates between the LLR and the OLR groups (1-year OS: HR 0.60, 95% CI 0.36 to 1.00, P = 0.05, I2 = 7%; 3-year OS: HR 0.82, 95% CI 0.59 to 1.14, P = 0.24, I2 = 0%; 5-year OS: HR 0.77, 95% CI 0.55 to 1.09, P = 0.15, I2 = 20%; Supplementary Material 3). Similarly, the pooled results showed no significant difference in the DFS rates at 1, 3, and 5 years between the LLR and the OLR groups (1-year DFS: HR 0.65, 95% CI 0.43 to 1.00, P = 0.05, I2 = 43%; 3-year DFS: HR 0.82, 95% CI 0.64to 1.04, P = 0.10, I2 = 28%; 5-year DFS: HR 0.79, 95% CI 0.54 to 1.16, P = 0.24, I2 = 60%; Supplementary Material 3).
Subgroup and sensitivity analyses
Prespecified subgroup analyses stratified by types of hepatectomy were performed to investigate the potential discrepant treatment effect and potential sources of heterogeneity (Table 3, Supplementary Material 3). A total of eight studies (20, 22, 23, 26–30) reported patients with minor hepatectomy, and the remaining three studies (21, 24, 25) included both minor and major hepatectomy defined as the combined hepatectomy group.
The pooled ORs for postoperative complications in the two subgroups were 0.44 (95% CI 0.31 to 0.61, P < 0.00001, I2 = 0%) for minor hepatectomy and 0.55 (95% CI 0.40 to 0.75, P = 0.0002, I2 = 0%) for combined hepatectomy. The results indicated that LLR was associated with a lower incidence of postoperative complications for patients with minor or major hepatectomy. Moreover, for patients with minor hepatectomy, LLR was associated with less blood loss (MD −402.09, 95% CI −616.68 to −187.50, P = 0.0002, I2 = 96%), shorter length of hospital stay (MD −8.17, 95% CI −12.24 to −4.10, P < 0.0001, I2 = 95%), and comparable surgical time (MD 10.56, 95% CI −19.79 to 40.90, P = 0.50, I2 = 94%). However, for patients with combined hepatectomy, there was no significant difference in blood loss (MD 22.66, 95% CI −502.36 to 547.68, P = 0.93, I2 = 91%) and length of hospital stay (MD −7.12, 95% CI −14.75 to 0.52, P = 0.07, I2 = 97%), but a longer surgical time (MD 39.26, 95% CI 18.97 to 59.54, P = 0.0001, I2 = 35%) was observed. However, the significant heterogeneity and limited number of studies in this subgroup weakened the credibility of this conclusion.
Furthermore, since there were three different definitions of elderly patients (at least 60, 70, or 75 years old), we performed a subgroup analysis based on the age groups. The subgroup analysis showed that the incidence of postoperative complications was similar in three different subgroups (≥65: OR 0.36, 95% CI 0.21 to 0.61, P = 0.0001, I2 = 0%; ≥70: OR 0.59, 95% CI 0.45 to 0.79, P = 0.0003, I2 = 0%; ≥75: OR 0.36, 95% CI 0.21 to 0.61, P = 0.0002), and the subgroup of ≥70 years old showed similar results with the overall analysis. However, the subgroup of ≥65 years old showed no difference in the length of hospital stay (MD −2.26, 95% CI −4.56 to 0.03, P = 0.05, I2 = 69%) but had longer surgical time (MD 40.82, 95% CI 15.29 to 66.36, P = 0.002, I2 = 65%).
In addition, based on the Clavien–Dindo classification (grades I to II as minor complications, grades III to V as major complications), we divided the data of postoperative complications into minor and major complications. The results indicated that both major and minor postoperative complications were in favor of LLR (major: OR 0.50, 95% CI 0.36 to 0.69, P < 0.0001, I2 = 0%; minor: OR 0.63, 95% CI 0.49 to 0.81, P = 0.0004, I2 = 0%; Table 2, Supplementary Material 3).
Furthermore, the sensitivity analysis by excluding each study showed no significant difference in the short-term outcomes (Supplementary Material 3).
Discussion
Considering the increase in overall life expectancy and the rising incidence of HCC, more elderly patients are considered for liver resection. Despite the advancement of laparoscopic techniques, only a few studies have focused on the potential benefits of LLR in the elderly population. In view of the scarcity of high-quality evidence, we performed this meta-analysis of PSM studies to compare the short- and long-term outcomes of LLR versus OLR for elderly patients with HCC. The results demonstrated that LLR significantly reduces postoperative complications, blood loss, and length of hospital stay, whereas the operation time was insignificantly different. Additionally, in terms of long-term survival rate, there were no significant differences between the LLR and the OLR groups. However, it should be noted that these benefits might only apply to a selected group of patients, undergoing less technically demanding minor laparoscopic hepatectomies.
Generally, the elderly are considered a vulnerable group because of the aging process, with numerous comorbidities and lower reserve capacity (34). In general, elderly patients with underlying functional status can influence the surgeons’ decision-making on surgical procedure selection. OLR for the treatment of HCC is a major abdominal surgery with high risks and difficulties, especially for elderly patients (35, 36). When choosing the clinical outcomes of our study, we compared LLR with OLR on different levels in terms of safety (postoperative complications), difficulty (operative time, blood loss), efficiency (length of hospital stays), and long-term results (OS and DFS rates). The results of our meta-analysis were broadly consistent with previous meta-analyses (35–37), indicating that LLR is a favorable approach for elderly patients that delivers improved short-term outcomes in terms of postoperative complications, blood loss, and length of hospital stay. Moreover, we further analyzed the pulmonary complications and survival rates between LLR and OLR. Our meta-analysis revealed that LLR was associated with an obviously lower incidence of pulmonary complications and no significant difference in OS or DFS rates between the LLR and the OLR groups, thereby dispelling the concerns that the laparoscopic approach may be inferior to the standard open approach in oncological efficiency.
Significantly lower rates of postoperative complications for the LLR group including a lower risk for both minor and major complications were proven in our meta-analysis. Furthermore, pulmonary complications are one of the potentially life-threatening complications after hepatectomy, especially for elderly patients. Our meta-analysis discovered a significantly lower incidence of pulmonary complications in the LLR group, and there might be several reasons for the difference. First, in open hepatectomy, the large abdominal incisions may increase the risk of wound infection and severe pain, which in turn would increase the risk of postoperative pulmonary complications. This might also be associated with delayed postoperative rehabilitation and longer hospital stay. Second, some studies have demonstrated that intraoperative fluid overload is a strong risk factor for pulmonary complications after hepatic surgery (38–40). Therefore, the lower intraoperative blood loss in the LLR group might be helpful in decreasing intraoperative fluid administration.
Another advantage of LLR is less intraoperative blood loss. The decreased blood loss in the LLR group could be attributed to the fact that the length of the incision was relatively small in laparoscopic surgery. Secondly, the hemostatic effect of the artificial pneumoperitoneum and a better view of the surgical field could also diminish blood loss (41, 42). Furthermore, the prevalence of liver cirrhosis differs among studies, but the majority is classified as Child–Pugh A, which might explain the reduced blood loss as well. Nevertheless, considering the significant heterogeneity and potential mistakes in calculating intraoperative blood loss (43), the results need to be interpreted with caution.
Concerning long-term outcomes, we observed that the laparoscopic approach had a potential long-term survival advantage, but it was not statistically significant. Moreover, it is interesting to note that the individual participant data meta-analysis of PSM studies by Syn et al. (44) demonstrated a long-term survival benefit in favor of LLR over OLR for patients with colorectal liver metastases. Although the survival benefit was not definitively confirmed in our meta-analysis, the potential clinical and biological mechanisms underlying the survival benefit associated with LLR should be attracted. First of all, many studies demonstrated that postoperative morbidity was an independent risk factor for long-term survival (45–47). The laparoscopic approach might provide a survival advantage by decreasing postoperative morbidity. Furthermore, by reducing the adverse effects of postoperative morbidity on the timing of postoperative chemotherapy, patients who had LLR have a quicker recovery and earlier resumption of chemotherapy regimens than patients with OLR (48, 49). Secondly, since laparoscopic surgery is a relatively newer surgical technique, it requires skilled surgeons with extensive experience. Thus, surgeons who routinely perform LLR may be more experienced, and the accumulated experience is associated with improved outcomes after hepatectomy for HCC (50). Moreover, the laparoscopic approach could preserve the liver parenchyma and portal pedicles or reduce the rates of dense adhesions, which may also reduce the incidence of postoperative complications and increase the feasibility of salvage surgical resection in the future (51).
However, the current study had several limitations. First and foremost, our study was limited by the retrospective and non-randomized design of the included studies. Although all included studies employed the PSM method to reduce the impact of the measured potential confounders, some unmeasured but important potential confounding factors might be overlooked. Moreover, most of the included studies had a limited sample size. Of those, nine studies were typically defined as small studies (<100 patients per arm), which may lead to a small study effect bias (52).
Secondly, there was a significant between-study heterogeneity in several outcomes, which might be derived from the differences in age ranges, liver function, number and location of lesions, general condition of the individual patient, surgeons’ experience, perioperative care protocols, pre- and postoperative chemotherapy, and other factors. Some studies included patients at wide study intervals, which may introduce biases due to advances in the mastery of surgical skills and improvements in surgical instruments (53). Noteworthy, the covariates for matching were different between the included studies, and some studies did not adjust for some important confounders such as age, sex, and liver function classification. Future research should further dissect the matching covariates to draw more accurate results.
Last but not the least, our meta-analysis only evaluated the overall and pulmonary complications. Some specific and important complications including bile leak, abscesses, and intra-abdominal infection between the two therapies could not be adequately compared, which should be further evaluated in the future.
Conclusion
In conclusion, our meta-analysis of PSM studies suggests that LLR has improved short-term outcomes including a lower incidence of postoperative complications, less blood loss, and shorter length of hospital stay, with comparable long-term outcomes for elderly patients with HCC when compared with the open approach. However, most of the existing data are about the results of minor hepatectomy, and laparoscopic major hepatectomy in elderly patients should be carefully evaluated and preferably performed in expert centers. Furthermore, considering the limited number of included studies with small sample sizes, significant heterogeneity and potential bias were found among the included studies. Well-designed, multicenter RCTs with a large sample size are needed to further evaluate the short- and long-term outcomes of LLR versus OLR for elderly patients with HCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SW and HY conceived the idea, performed the analysis, and wrote the initial draft of this paper. GY, JW and SX contributed to the collection and interpretation of data. QY helped to frame the idea of the study and provided technical support. HY contributed to the revision of this paper and the final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.939877/full#supplementary-material
References
1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (Lond Engl) (2018) 391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
4. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet (2017) 389(10076):1323–35. doi: 10.1016/S0140-6736(16)32381-9
5. Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol (1991) 78(5 Pt 2):956–8.
6. Mirnezami R, Mirnezami AH, Chandrakumaran K, Abu Hilal M, Pearce NW, Primrose JN, et al. Short- and long-term outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB (Oxford) (2011) 13(5):295–308. doi: 10.1111/j.1477-2574.2011.00295.x
7. Witowski J, Rubinkiewicz M, Mizera M, Wysocki M, Gajewska N, Sitkowski M, et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg endoscopy (2019) 33(5):1491–507. doi: 10.1007/s00464-018-6431-6
8. Ciria R, Gomez-Luque I, Ocaña S, Cipriani F, Halls M, Briceño J, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: Updated results from the European guidelines meeting on laparoscopic liver surgery, Southampton, UK. Ann Surg Oncol 2019 (2017) 26(1):252–63. doi: 10.1245/s10434-018-6926-3
9. Pilleron S, Soto-Perez-de-Celis E, Vignat J, Ferlay J, Soerjomataram I, Bray F, et al. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int J Cancer (2021) 148(3):601–8. doi: 10.1002/ijc.33232
10. Gouverneur A, Salvo F, Berdaï D, Moore N, Fourrier-Réglat A, Noize P. Inclusion of elderly or frail patients in randomized controlled trials of targeted therapies for the treatment of metastatic colorectal cancer: A systematic review. J Geriatric Oncol (2018) 9(1):15–23. doi: 10.1016/j.jgo.2017.08.001
11. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med (2014) 33(7):1242–58. doi: 10.1002/sim.5984
12. Dahabreh IJ, Sheldrick RC, Paulus JK, Chung M, Varvarigou V, Jafri H, et al. Do observational studies using propensity score methods agree with randomized trials? A systematic comparison of studies on acute coronary syndromes. Eur Heart J (2012) 33(15):1893–901. doi: 10.1093/eurheartj/ehs114
13. Lonjon G, Boutron I, Trinquart L, Ahmad N, Aim F, Nizard R, et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg (2014) 259(1):18–25. doi: 10.1097/SLA.0000000000000256
14. Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. Jama (2001) 286(7):821–30. doi: 10.1001/jama.286.7.821
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
16. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
19. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann Surg (2015) 261(4):619–29. doi: 10.1097/SLA.0000000000001184
20. Badawy A, Seo S, Toda R, Fuji H, Fukumitsu K, Ishii T, et al. A propensity score-based analysis of laparoscopic liver resection for liver malignancies in elderly patients. J Invest Surg (2019) 32(1):75–82. doi: 10.1080/08941939.2017.1373170
21. Cauchy F, Fuks D, Nomi T, Dokmak S, Scatton O, Schwarz L, et al. Benefits of laparoscopy in elderly patients requiring major liver resection. J Am Coll Surg (2016) 222(2):174–84.e110. doi: 10.1016/j.jamcollsurg.2015.11.006
22. Chan AC, Poon RT, Cheung TT, Chok KS, Dai WC, Chan SC, et al. Laparoscopic versus open liver resection for elderly patients with malignant liver tumors: a single-center experience. J Gastroenterol Hepatol (2014) 29(6):1279–83. doi: 10.1111/jgh.12539
23. Chen Y, Yu L, Quan C. Laparoscopic versus open hepatectomy for elderly patients with hepatocellular carcinoma. J Buon (2020) 25(3):1404–12.
24. Delvecchio A, Conticchio M, Riccelli U, Ferraro V, Ratti F, Gelli M, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A propensity score matching analysis. HPB (Oxford) (2021) 24(6):933–41. doi: 10.1016/j.hpb.2021.10.024
25. Dumronggittigule W, Han HS, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open hepatectomy for hepatocellular carcinoma in elderly patients: A single-institutional propensity score matching comparison. Dig Surg (2020) 37(6):495–504. doi: 10.1159/000510960
26. Goh BKP, Chua D, Syn N, Teo JY, Chan CY, Lee SY, et al. Perioperative outcomes of laparoscopic minor hepatectomy for hepatocellular carcinoma in the elderly. World J Surg (2018) 42(12):4063–9. doi: 10.1007/s00268-018-4741-4
27. Monden K, Sadamori H, Hioki M, Ohno S, Takakura N. Short-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma in older patients: A propensity score matching analysis. BMC Surg (2022) 22(1):63. doi: 10.1186/s12893-022-01518-x
28. Nomi T, Hirokawa F, Kaibori M, Ueno M, Tanaka S, Hokuto D, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: a multi-centre propensity score-based analysis. Surg Endosc (2020) 34(2):658–66. doi: 10.1007/s00464-019-06812-z
29. Wang XT, Wang HG, Duan WD, Wu CY, Chen MY, Li H, et al. Pure laparoscopic versus open liver resection for primary liver carcinoma in elderly patients: A single-center, case-matched study. Med (Baltimore) (2015) 94(43):e1854. doi: 10.1097/MD.0000000000001854
30. Wen N, Liu F, Zhang H, Lu J, Li B, Cheng N. Laparoscopic liver resection for hepatocellular carcinoma presents less respiratory complications compared with open procedure: A propensity score analysis in the elderly. Eur J Surg Oncol (2021) 47(10):2675–81. doi: 10.1016/j.ejso.2021.04.032
31. Kim JM, Kim S, Rhu J, Choi GS, Kwon CHD, Joh JW. Elderly hepatocellular carcinoma patients: Open or laparoscopic approach? Cancers (2020) 12(8):2281. doi: 10.3390/cancers12082281
32. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
33. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
34. Stavropoulou E, Bezirtzoglou E. Human microbiota in aging and infection: A review. Crit Rev Food Sci Nutr (2019) 59(4):537–45. doi: 10.1080/10408398.2017.1379469
35. Notarnicola M, Felli E, Roselli S, Altomare DF, De Fazio M, de'Angelis N, et al. Laparoscopic liver resection in elderly patients: Systematic review and meta-analysis. Surg Endosc (2019) 33(9):2763–73. doi: 10.1007/s00464-019-06840-9
36. Hildebrand N, Verkoulen K, Dewulf M, Heise D, Ulmer F, Coolsen M. Short-term outcomes of laparoscopic versus open hepatectomy in the elderly patient: systematic review and meta-analysis. HPB (Oxford) (2021) 23(7):984–93. doi: 10.1016/j.hpb.2021.01.016
37. Chen K, Pan Y, Maher H, Zhang B, Zheng XY. Laparoscopic hepatectomy for elderly patients: Major findings based on a systematic review and meta-analysis. Med (Baltimore) (2018) 97(30):e11703. doi: 10.1097/MD.0000000000011703
38. Fuks D, Cauchy F, Ftériche S, Nomi T, Schwarz L, Dokmak S, et al. Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: A propensity score analysis. Ann Surg (2016) 263(2):353–61. doi: 10.1097/SLA.0000000000001140
39. Feltracco P, Carollo C, Barbieri S, Pettenuzzo T, Ori C. Early respiratory complications after liver transplantation. World J Gastroenterol (2013) 19(48):9271–81. doi: 10.3748/wjg.v19.i48.9271
40. Pirat A, Ozgur S, Torgay A, Candan S, Zeyneloğlu P, Arslan G. Risk factors for postoperative respiratory complications in adult liver transplant recipients. Transplant Proc (2004) 36(1):218–20. doi: 10.1016/j.transproceed.2003.11.026
41. Skytioti M, Elstad M, Søvik S. Internal carotid artery blood flow response to anesthesia, pneumoperitoneum, and head-up tilt during laparoscopic cholecystectomy. Anesthesiology (2019) 131(3):512–20. doi: 10.1097/ALN.0000000000002838
42. Zhang J, Zhou Z-G, Huang Z-X, Yang K-L, Chen J-C, Chen J-B, et al. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin J Cancer (2016) 35:25–5. doi: 10.1186/s40880-016-0088-0
43. Tomimaru Y, Noguchi K, Morita S, Imamura H, Iwazawa T, Dono K. Is intraoperative blood loss underestimated in patients undergoing laparoscopic hepatectomy? World J Surg (2018) 42(11):3685–91. doi: 10.1007/s00268-018-4655-1
44. Syn NL, Kabir T, Koh YX, Tan HL, Wang LZ, Chin BZ, et al. Survival advantage of laparoscopic versus open resection for colorectal liver metastases: A meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann Surg (2020) 272(2):253–65. doi: 10.1097/SLA.0000000000003672
45. Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg (2010) 251(1):91–100. doi: 10.1097/SLA.0b013e3181bfda3c
46. Ito H, Are C, Gonen M, D'Angelica M, Dematteo RP, Kemeny NE, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg (2008) 247(6):994–1002. doi: 10.1097/SLA.0b013e31816c405f
47. Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol (2008) 15(9):2458–64. doi: 10.1245/s10434-008-9935-9
48. Kawai T, Goumard C, Jeune F, Savier E, Vaillant JC, Scatton O. Laparoscopic liver resection for colorectal liver metastasis patients allows patients to start adjuvant chemotherapy without delay: A propensity score analysis. Surg Endosc (2018) 32(7):3273–81. doi: 10.1007/s00464-018-6046-y
49. Tohme S, Goswami J, Han K, Chidi AP, Geller DA, Reddy S, et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg (2015) 19(12):2199–206. doi: 10.1007/s11605-015-2962-5
50. Navarro JG, Kang I, Rho SY, Choi GH, Han DH, Kim KS, et al. Major laparoscopic versus open resection for hepatocellular carcinoma: A propensity score-matched analysis based on surgeons' learning curve. Ann Surg Oncol (2021) 28(1):447–58. doi: 10.1245/s10434-020-08764-4
51. Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol (2014) 40(5):536–44. doi: 10.1016/j.ejso.2014.01.005
52. Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: A meta-epidemiological study. Crit Care (2013) 17(1):R2. doi: 10.1186/cc11919
Keywords: hepatocellular carcinoma (HCC), laparoscopic liver resection (LLR), open liver resection (OLR), meta-analysis, elderly
Citation: Wang S, Ye G, Wang J, Xu S, Ye Q and Ye H (2022) Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A systematic review and meta-analysis of propensity score-matched studies. Front. Oncol. 12:939877. doi: 10.3389/fonc.2022.939877
Received: 09 May 2022; Accepted: 25 October 2022;
Published: 14 November 2022.
Edited by:
David Geller, University of Pittsburgh, United StatesReviewed by:
Tullio Piardi, Centre Hospitalier Universitaire de Reims, FranceAlessandro Boscarelli, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), Italy
Zenichi Morise, Fujita Health University, Japan
Copyright © 2022 Wang, Ye, Wang, Xu, Ye and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailin Ye, aGFpbGlueWUwMUAxMjYuY29t
 Shi Wang
Shi Wang Hailin Ye
Hailin Ye