- 1Department of Hematology, Huashan Hospital, Fudan University, Shanghai, China
- 2Department of Hematology, Huashan Hospital North, Fudan University, Shanghai, China
- 3Division of Hematology, Department of Internal Medicine, Mayo Clinic, Rochester, MN, United States
The prognosis of relapsed/refractory (R/R) primary central nervous system lymphoma (PCNSL) is dismal, and there are limited treatment options for these patients. This was a prospective single-arm phase II study of combined pemetrexed and lenalidomide for salvage treatment of R/R PCNSL. Patients with R/R PCNSL (n = 38) who had undergone two or more different therapeutic regimens and experienced disease progression or recurrence were enrolled. The primary endpoint was overall response rate (ORR). Secondary endpoints were progression-free survival (PFS) and overall survival (OS). Patients were followed up for a median of 18 (range, 1–36) months. ORR was 68.4%, with median PFS and OS of 6 and 18 months, respectively. Adverse events (AEs) included myelosuppression, fatigue, nausea, fever, infection, cardiac disease, and thrombogenesis. Commonly observed grade ≥ 3 AEs included neutropenia (5.3%), leukopenia (2.6%), thrombocytopenia (7.9%), and infection (2.6%). Elevated lactate dehydrogenase (LDH) levels (χ2 = 13.25; P = 0.0003) and bulky disease (P = 0.032; χ2 = 4.580) were associated with short PFS. Elevated serum LDH level (P = 0.011; χ2 = 6.560), abnormal lymphoma cells in the cerebrospinal fluid (CSF) [P = 0.011; χ2 = 6.445], and multiple lesions (P = 0.036; χ2 = 4.404) were significantly associated with poorer OS. Abnormal lymphoma cells in the CSF were an independent predictor of poor prognosis on multivariate analysis (P = 0.034; hazard ratio (HR) = 2.836; 95% confidence interval, 1.082–7.434). Our results indicate that pemetrexed plus lenalidomide is effective for heavily treated R/R PCNSL, with moderate toxicity. Trial registration: #ChiCTR1900028070.
Introduction
Primary central nervous system lymphoma (PCNSL) is an invasive extranodal lymphoma localized in the brain parenchyma, spinal cord, cerebrospinal fluid (CSF), or vitreoretinal space, with no evidence of systemic lymphoma at initial diagnosis (1). The incidence of PCNSL is 0.4–0.5 per 100,000 individuals annually, accounting for 4% of intracranial malignant tumors and 4%–6% of extranodal lymphomas (2). Regarding pathological type, more than 90% of patients with PCNSL have diffuse large B-cell lymphoma (DLBCL), and the majority originating from non-germinal center B-cells (non-GCBs) (3). Due to the rarity of this disease in the relapsed/refractory setting, there are no large-scale randomized clinical trials, and limited treatment options exist. At present, chemotherapy with high-dose methotrexate (HD-MTX) is generally recommended as the first-line treatment for PCNSL, with an overall response rate (ORR) of 70%–87% and a 2-year overall survival (OS) rate of 58%–67% (4, 5); however, approximately 10%–30% of patients have primarily refractory disease and 50% of patients relapse after remission (6, 7). Although various treatment options, including chemotherapy, radiotherapy, and autologous hematopoietic stem cell transplantation (ASCT), have been evaluated for use in patients with relapsed/refractory (R/R) PCNSL (8–14), a standard treatment strategy has yet to be established and patient prognosis remains extremely poor. These studies treatments revealed heterogeneous survivals (range, 2-59) months.
Pemetrexed, similar to methotrexate, is an antifolate agent that contains a pyrrolidine core that exerts anti-tumor effects by disrupting the normal intracellular floate dependent metabolic process. Lenalidomide is a novel immunomodulator with immunoregulatory and antitumor functions that shows preferential anti-lymphoma activity against non-GCB subtypes of systemic DLBCL (15). Previous studies have reported that pemetrexed (13, 16, 17) or lenalidomide (12, 18, 19) alone, or in combination with other agents, are active drugs in patients with R/R PCNSL. Both drugs can penetrate the blood-brain barrier. Pemetrexed has been reported to be involved in the activation of NF-κB signaling, which may induce pemetrexed resistance, and lenalidomide could block NF-κB signaling. We hypothesize that lenalidomide can overcome pemetrexed resistance and the combination of these two drugs may have a synergistic effect. Therefore, we conducted this prospective phase II study to determine the efficacy and safety of pemetrexed combined with lenalidomide for treating patients with R/R PCNSL.
Methods
Patients
Thirty-eight patients admitted to our institution with a confirmed diagnosis of R/R PCNSL from January 2018 to June 2020 were enrolled in this study.
Inclusion and Exclusion Criteria
Inclusion Criteria
The inclusion criteria were: 1) age of 18–75 years; 2) confirmed CNS or vitreoretinal involvement with lymphoma; 3) histologically confirmed DLBCL diagnosis confirmed through histopathology and immunohistochemical staining; 4) no evidence of systemic involvement based on imaging; 5) disease progression or recurrence after having undergone at least two different therapeutic regimens before recruited in the trial, including HD-MTX (3.5–8 g/m2) based chemotherapy as the primary regimen, with or without whole-brain radiotherapy (WBRT) and/or ASCT.
Exclusion Criteria
Exclusion criteria were: 1) Current or prior systemic evidence of lymphoma; 2) severe cardiac insufficiency (New York Heart Association class III or IV); 3) severe liver insufficiency (alanine or aspartate transferase to levels 2-fold higher than the upper normal limit); 4) renal insufficiency (serum creatinine levels higher than the upper normal limit); 5) allergies or contraindications pemetrexed or lenalidomide; 6) other malignant tumors or serious uncontrolled concurrent disease; 7) active hepatitis A, B, or C, or tuberculosis infection, or other causes of immunosuppression; 8) active pregnancy or lactation; 9) participation in other clinical trials within one month.
R/R PCNSL Definition
In this study, relapsed PCNSL was defined as biopsy-proven PCNSL initially with complete response (CR) but presence of new lesions. Refractory PCNSL was defined as the presence of stable disease (SD) or progressive disease (PD) after receiving two or more treatment regimens. With regard to the imaging examinations performed, patients with relapsed PCNSL were evaluated with contrast-enhanced magnetic resonance imaging (MRI) or whole-body positron emission tomography-computed tomography.
Study Design and Regimen
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the Ethics Committee of our hospital. This was registered study on the Chinese Clinical Trial Registry website (#ChiCTR1900028070). All patients provided written informed consent. Pemetrexed [900 mg/m2, intravenous infusion, day (d) 1] and lenalidomide (25 mg/day, orally, d1–21) were administered in a 28 day cycle. Additionally, folic acid (5 mg, orally, three times daily), vitamin B12 (1 mg supplementation, intramuscular injection, on the day before pemetrexed), and dexamethasone (15 mg, intravenous infusion, d1–3) were administered to reduce the toxicity of pemetrexed, and aspirin (100 mg/day, orally, d1–21) was given to prevent lenalidomide-induced thrombosis.
Primary and Secondary Endpoints and Evaluation of Response Efficacy and Adverse Effects
Our trial treatment continued until lymphoma progression or intolerable toxicity developed. Treatment continued to a maximum of eight cycles. The primary endpoint was ORR (CR + CRu + PR), and patients were required to complete at least one cycle of the study regimen to be evaluable. According to International PCNSL Collaborative Group criteria, ORR includes CR, unconfirmed CR (CRu), and partial response (PR) (20). Secondary endpoints included progression-free survival (PFS) and OS. Patient response to PCNSL treatment was assessed after every cycle. Assessments included contrast-enhanced MRI of the brain parenchyma and spinal cord, lumbar puncture of CSF, and detailed ophthalmic examination of eyes. After each cycle, patient’s condition was observed, and blood tests were performed routinely. In accordance with the National Cancer Institute Standard for Common Terminology Criteria for Adverse Events version 4.0, adverse events (AEs) were assessed as grade 1–5, with each grade indicating mild, moderate, severe, life-threatening, or death, respectively.
Statistical Analysis
We used the PASS 15 statistical software for single-stage phase II clinical trials to calculate the sample size for this study. Assuming α = 0.05, β = 0.1, power = 0.9, P0 = 0.25 (where P0 is the maximum response proportion to a poor treatment), and P1 = 0.5 (where P1 is the minimum response proportion to a good treatment), the minimum sample size was estimated to be 33. Estimating a dropout rate of 10%, the final enrollment was calculated to 38. Medians, ranges or proportions were used to describe patient clinical baseline characteristics. PFS and OS were calculated using Kaplan-Meier curves. Univariate and multivariate prognostic analyses were performed using the log-rank test and a Cox regression model, respectively. Statistical significance was determined from two-tailed P-values and 95% confidence intervals (CI), where P-values < 0.05 indicated statistically significant differences. SPSS 26.0 (IBM Company, USA) and GraphPad Prism 7 software (Insightful Science Company, San Diego, California, USA) were used for data analyses.
Results
Patient Baseline Characteristics
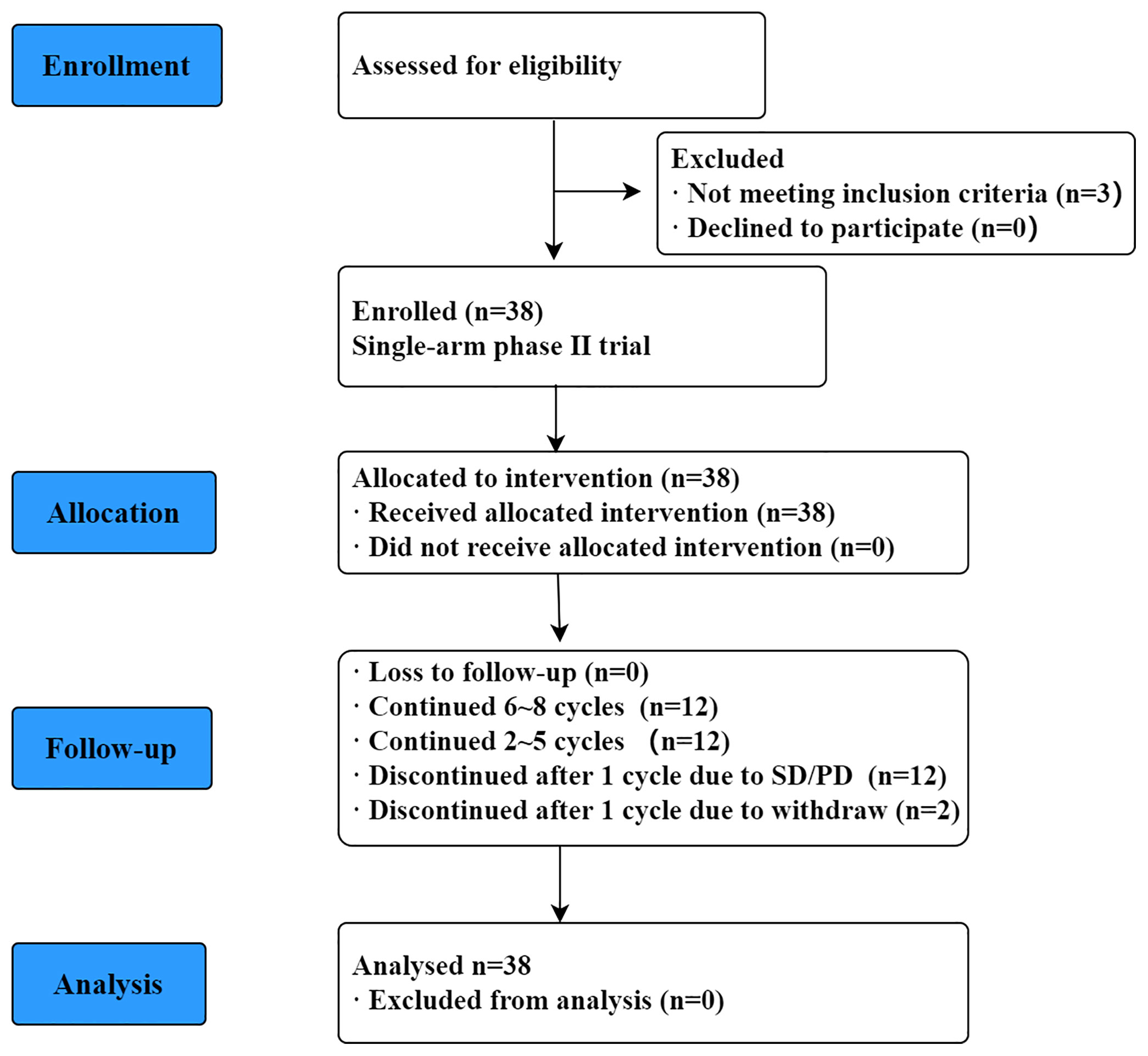
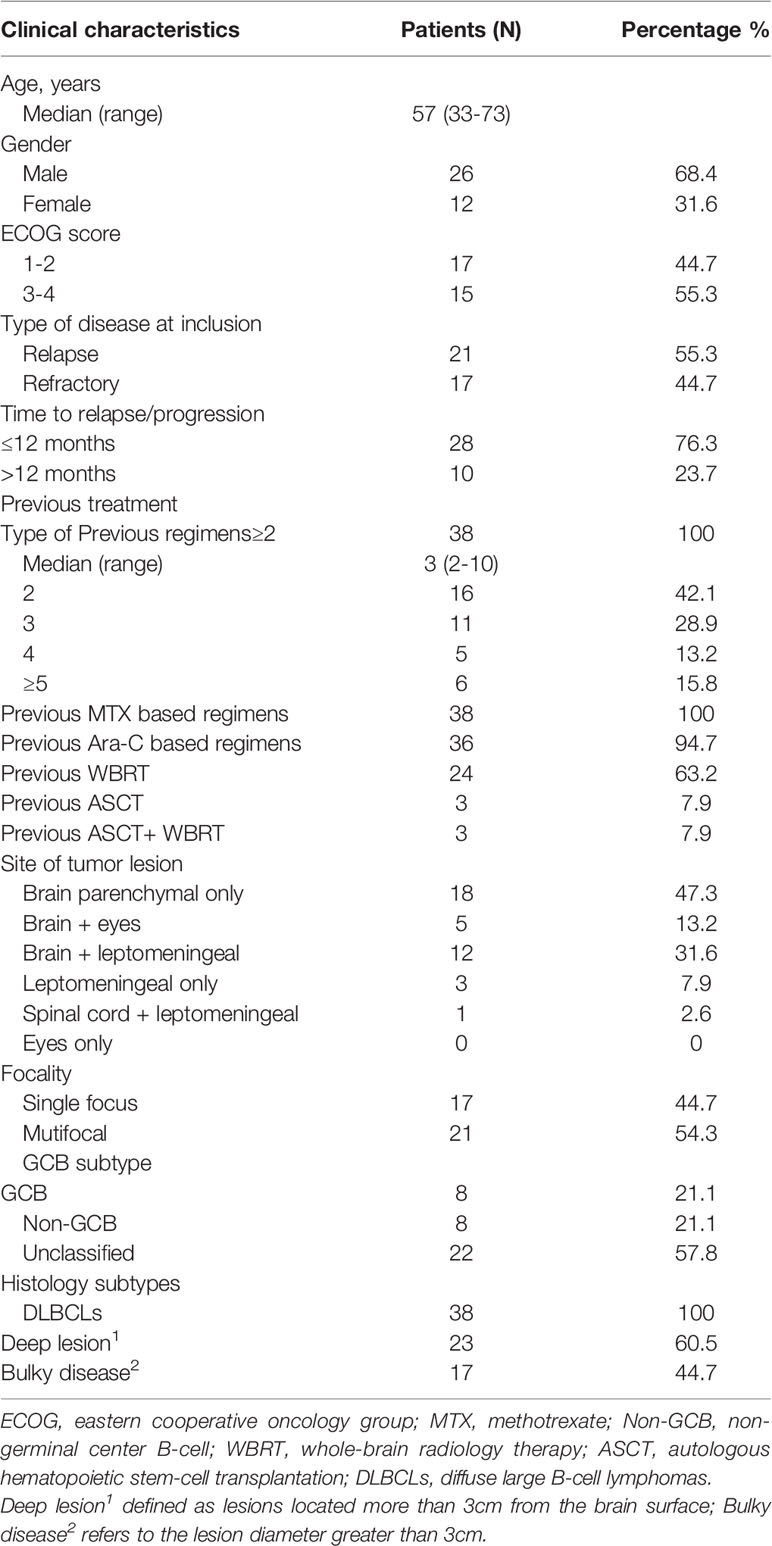
Figure 1 is a CONSORT flowchart illustrating the process of patient inclusion in our study. A total of 38 patients with R/R PCNSL were enrolled in our study from January 2018 to June 2020, and their median age was 57 (range, 33–73) years. The clinical baseline characteristics of the patients are shown in Table 1. Eastern Cooperative Oncology Group (ECOG) score was 1–2 for 17 patients (44.7%) and 3–4 for 21 patients (55.3%). At the time of inclusion, 21 (55.3%) and 17 (44.7%) patients were diagnosed as having relapsed and refractory PCNSL, respectively. Of all patients, 73.7% experienced recurrence or progression within 12 months of initial diagnosis. Patients underwent a median of 3 (range, 2–10) prior treatment regimens to enrollment. Twenty-four patients (63.2%) had previously undergone WBRT, and three patients received ASCT in addition to WBRT. Eighteen patients had only recurrence/progression involving brain parenchyma, 12 patients had brain parenchymal involvement and leptomeningeal abnormalities, and 5 patients had brain parenchymal and vitreoretinal involvement. A further 3 patients presented with only leptomeningeal abnormalities, and one patient presented with only spinal cord lesions. Other characteristics, such as focality, histology pathological, GCB or non-GCB subtype, deep lesions, and bulky disease were shown in Table 2.
Treatment Response and Survival
All 38 patients received the trail regimen, of which 12 patients completed 6-8 cycles 12 patients continued 2-5 cycles to SD/PD, 12 patients discontinued after one cycle due to SD/PD, and 2 patients discontinued after one cycle due to withdrew. Median number of cycles was 3 (range, 1-8).
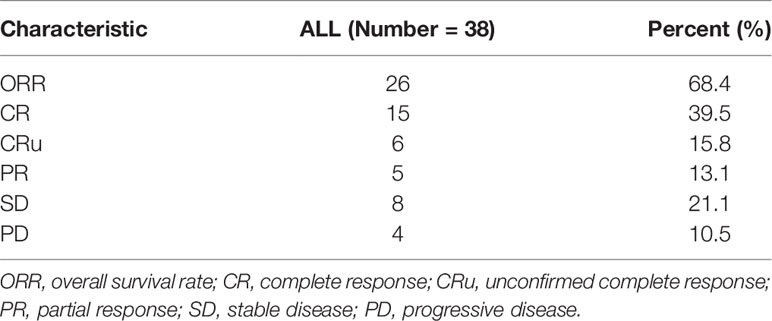
All patients were evaluated. As shown in Table 2, the best responses were observed in 15 patients (39.5%) with CR, while 6 (15.8%) achieved CRu, 5 (13.1%) with PR, 8 (21.1%) with SD, and 4 (10.5%) with PD, resulting in an ORR of 68.4% and disease control rate (CR + PR + SD) of 89.5% (Table 2).
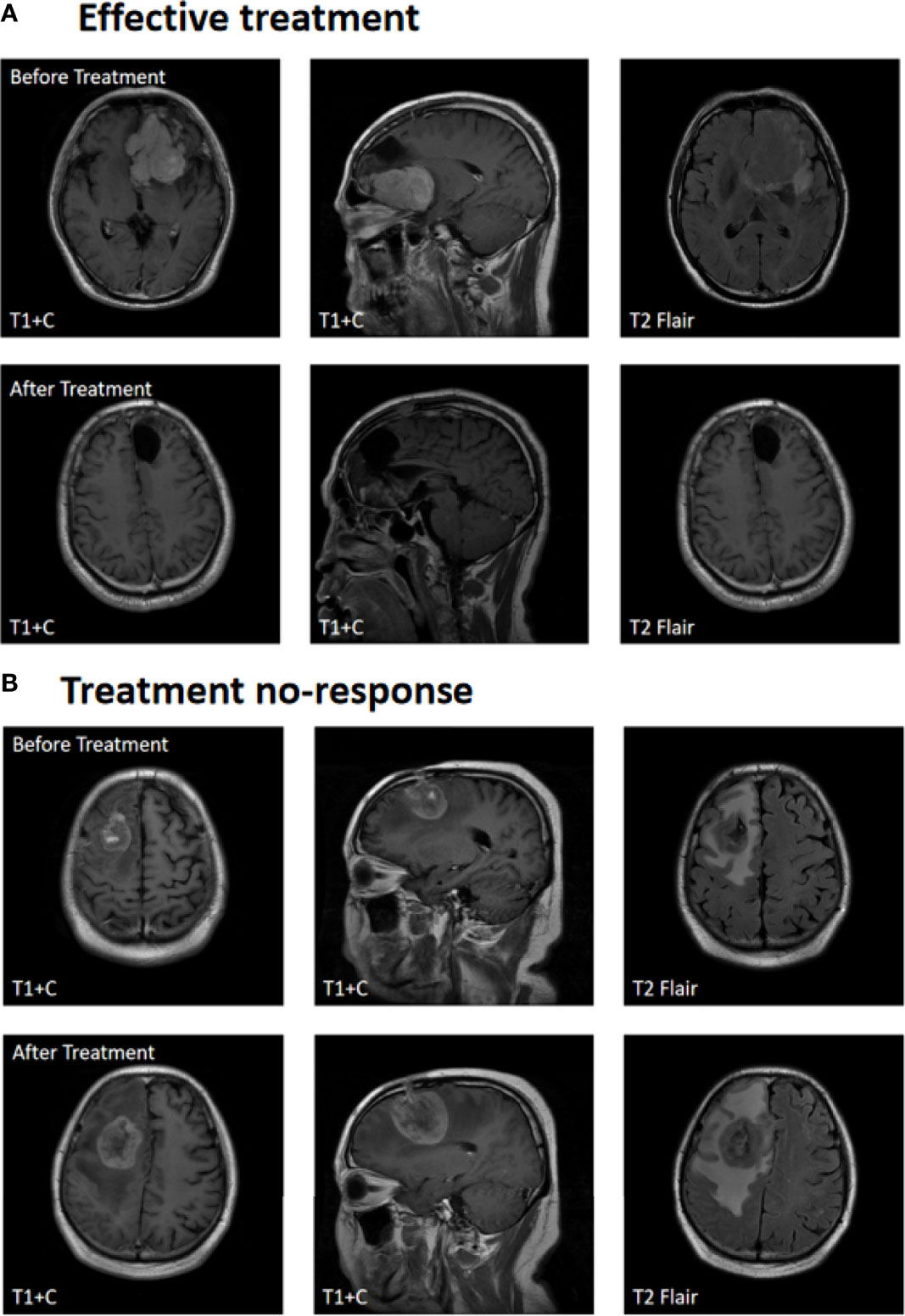
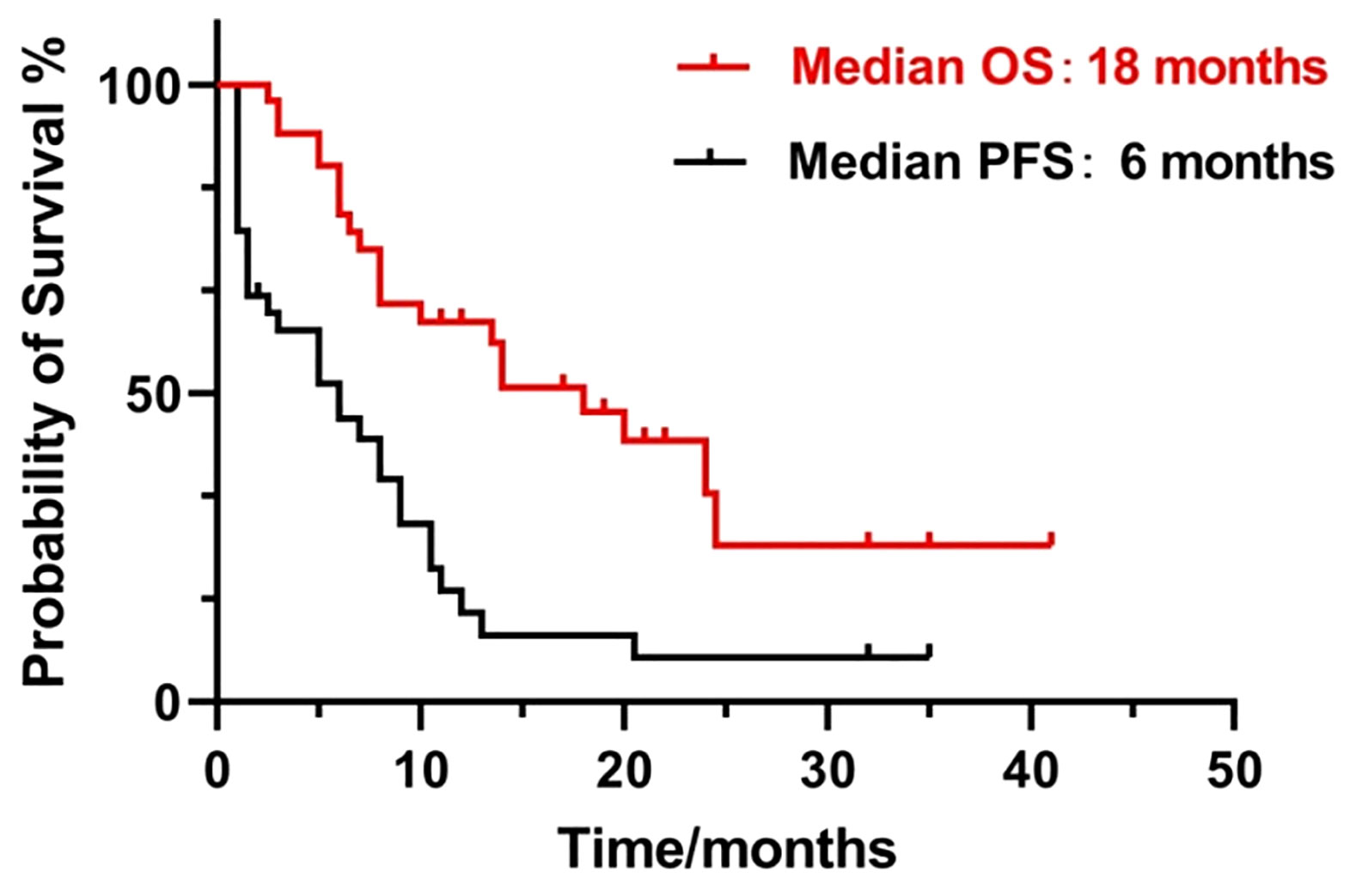
Figure 2A shows contrast-enhanced MRI findings (before and after treatment) of a sample patient who had an effective treatment response and Figure 2B shows the contrast-enhanced MRI findings of a patient with no response. After treatment, the median PFS time was 6 months, with 6-month PFS rates, and the 12-month PFS rates were 50% and 10.5%, respectively. The median OS time was 18 months, and the 1-year and 2-year overall survival rates were 52.6% and 13.2%, respectively (Figure 3).
Safety
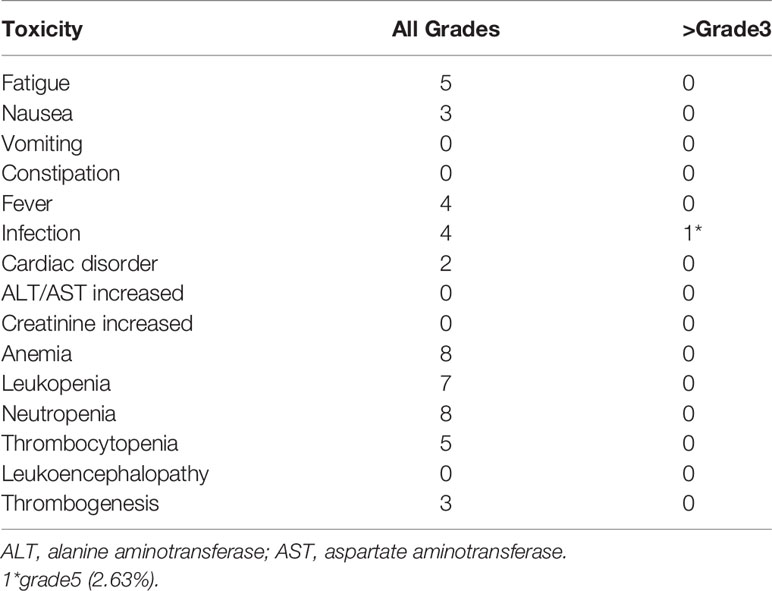
Common AEs included fatigue, nausea, fever, infection, and myelosuppression. Five patients developed fatigue (grade 1, 4 cases and grade 2, 1 case). Three patients developed nausea (grade 1, 2 cases and grade 2, 1 case). Four patients developed fever (grade 1, 3 cases and grade 2, 1 case). Four patients developed infection (grade 1, 3 cases and grade 5, 1 case), one of which grade 5 died of pneumonia. Myelosuppression mainly included leukopenia (grade 1, 6 cases and grade 3, 1 case), neutropenia (grade 1, 6 cases and grade 3, 2 cases), anemia (grade 1, 8 cases), and thrombocytopenia (grade 2, 1 case and grade 3, 3 cases). Other AEs included cardiac disorder (grade 1, 2 cases) and thrombogenesis (grade 2, 3 cases). No incidence of vomiting, abnormal liver and kidney functions, constipation, or leukoencephalopathy was noted (Table 3).
Analysis of Prognostic Factors
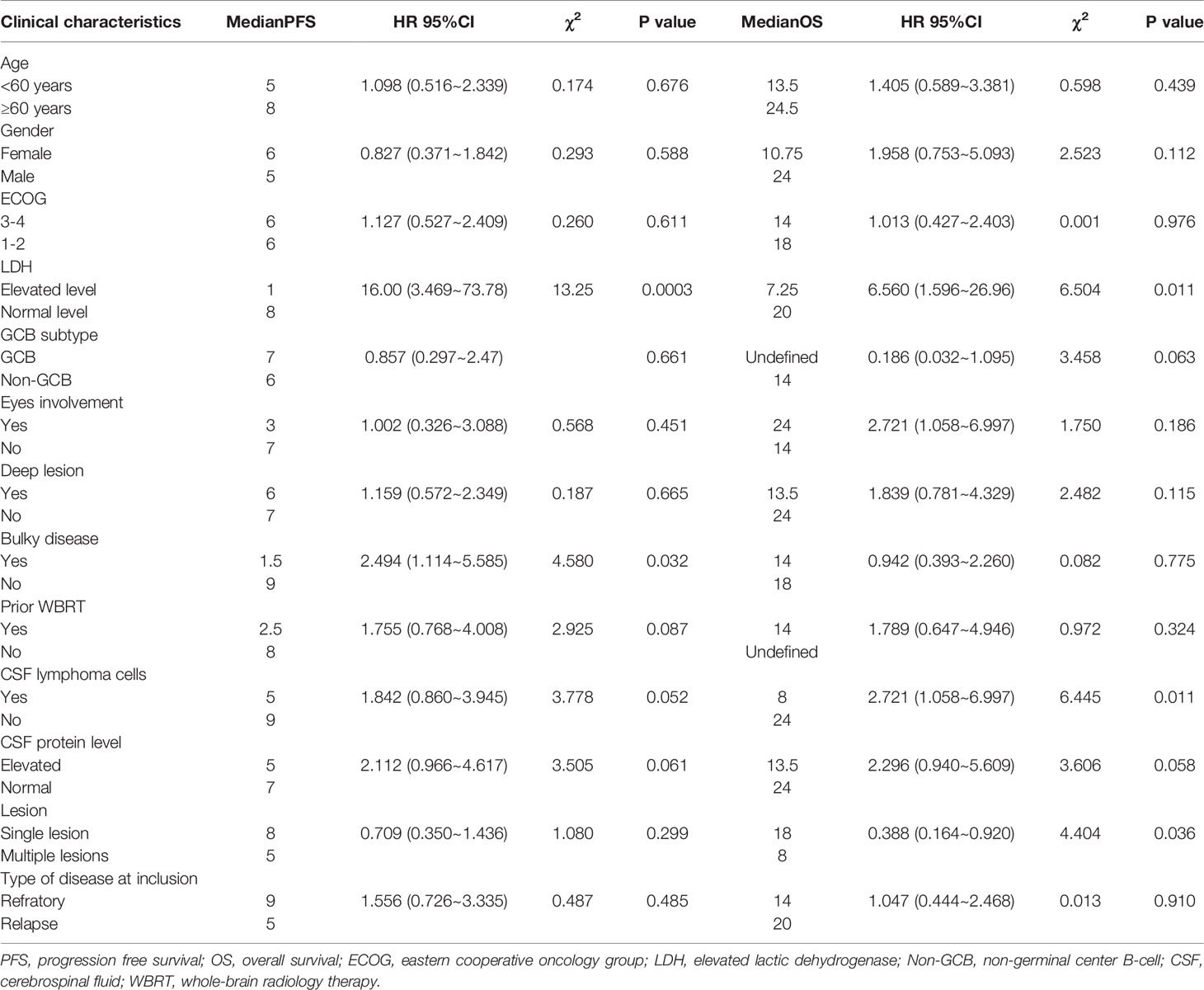
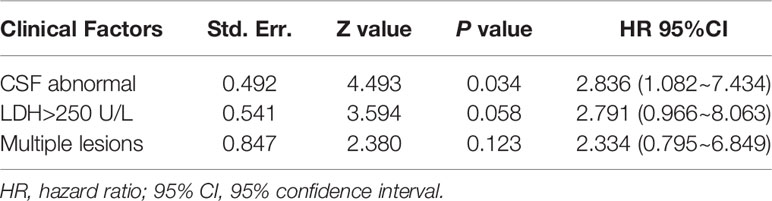
Univariate analysis of the PFS and OS of patients with R/R PCNSL was performed using the log-rank test. As shown in Table 4, elevated serum lactate dehydrogenase (LDH) levels (P = 0.0003; χ2 = 13.25) and bulky disease (refers to the lesion diameter greater than 3cm) (P = 0.032; χ2 = 4.580) were significantly related to shorter PFS (Figures 4A, B). Elevated serum LDH levels (P = 0.011; χ2 = 6.560), abnormal lymphoma cells in the CSF (P = 0.011; χ2 = 6.445), and multiple lesions (P = 0.036; χ2 = 4.404) were significantly associated with poor OS (Figures 4C–E). Multivariate Cox regression analysis for OS was performed to select prognostic factors with statistically significant differences as per univariate analysis (P < 0.05), and the results showed that abnormal lymphoma cells in the CSF was an independent poor prognostic factor [P = 0.034; HR = 2.836; 95% CI, 1.082–7.434] (Table 5).

Figure 4 Kaplan-Meier survival curve and prognosis analysis. (A) Univariate analysis of LDH level for PFS. (B) Univariate analysis of Bulky disease for PFS. (C) Univariate analysis of LDH level for OS. (D) Univariate analysis of CSF lymphoma cells for OS. (E) Univariate analysis of Multiple lesions for OS.
Discussion
Although various salvage treatments, including chemotherapy, radiotherapy, and ASCT are available for patients with R/R PCNSL, a standard effective treatment has not been established, and the prognosis remains unsatisfactory. Due to the rarity of the disease, no data from randomized phase III trials on R/R PCNSL have been reported; however, without effective treatment, the OS of patients with R/R PCNSL is ≤ 2 months (21). Previous studies have reported that pemetrexed (alone or in combination with other agents) (13, 16, 17) or lenalidomide (alone or in combination with other agents) (12, 18, 19) are active drugs in patients with R/R PCNSL; however, long-term survival is observed only in a few patients. Pemetrexed has been reported to be involved in the activation of NF-κB signaling, which may induce pemetrexed resistance, and lenalidomide could block NF-κB signaling. Both drugs can penetrate the blood-brain barrier. We hypothesize that lenalidomide can overcome pemetrexed resistance and the combination of these two drugs may have a synergistic effect. Therefore, we conducted this prospective phase II study to determine the efficacy and safety of pemetrexed combined with lenalidomide for treating patients with R/R PCNSL.
The primary analysis included all enrolled eligible patients who had received at least one cycle of our study regimen. According to our results, the ORR was 68.4% and the disease control rate was 89.5%. The best response rates were as follows: CR + CRu, 55.3%; PR, 13.1%; SD, 21.1%; and PD, 10.5% (Table 2). Notably, some patients who had relapsed or progressed after received four or more regimens, still demonstrated good response in our study regimen. After treatment, the median PFS and OS were 6 and 18 months, respectively (Figure 3). Patients who did not respond or progress would be rechallenged with HD-MTX based regimens (when MTX interval >1year), or other regimens, including BTKi, Pomalidomide, PD-1, or WBRT. These treatments may have prolonged the survival. Our results are comparable or superior to those of other chemotherapeutic agents used for the treatment of R/R PCNSL. Several studies evaluated pemetrexed monotherapy or in combination with other drugs in the treatment of R/R PCNSL, with an OS rate of 55-62.9%, a median PFS time of 4.2-7.8 months, and a median OS time of 7.8-19.5 months (16, 17, 22, 23). Caroline et al. (19) first reported lenalidomide as salvage treatment for relapsed PCNSL, 2 of 6 patients achieved a confirmed CR and one patient achieved PR. A prospective phase II study (18) reported a combination of lenalidomide and rituximab treatment for R/R PCNSL with an ORR of 64.7%, the median PFS and OS were 7.8 months and 17.7 months. Our study regimen shows an advantage response rate over pemetrexed or lenalidomide monotherapy, and was comparable to pemetrexed or lenalidomide in combination with other chemotherapeutic agents used for the treatment of R/R PCNSL.
Prospective studies using other agents, such as topotecan, temozolomide, and rituximab, have shown ORR of 31%–55% and PFS of 1.6–5.7 months (24–26). Previous studies have also reported that intensive chemotherapy followed by ASCT can be effective against R/R PCNSL. Few studies reported the effect of ASCT for R/R PCNSL, with a 2-year OS rate of 45-68%, 2-year-PFS rate of 43-54%, and a median OS of 18.3-86months (27–29). In recent years, several studies with novel targeted drugs that show efficacy in R/R PCNSL have been conducted, including the use of Bruton’s tyrosine kinase inhibitors [ibrutinib (30, 31) and tirabrutinib (10)], mTOR inhibitors [temsirolimus (32)], immunomodulatory drugs [pomalidomide (33)], and immune checkpoint inhibitors [anti-PD1 monoclonal antibodies (34)]. An increasing number of these drugs are being evaluated for use in patients with R/R PCNSL, some of which have been incorporated into the National Comprehensive Cancer Network guidelines.
Moderate toxicity was observed in patients enrolled in this clinical study. The most common AEs included fatigue, nausea, fever, infection, and myelosuppression. Other rare AEs included cardiac dysfunction and thrombosis. One patient died of pneumonia (grade 5). There were no other AEs, such as abnormalities of liver and kidney function, constipation, or leukoencephalopathy (Table 3). Treatment with granulocyte colony stimulating factor and thrombopoietin corrected myelosuppression from treatment. Patients with thrombosis, which manifested as deep vein thrombosis, responded to anticoagulant therapy. The low number of AEs was likely related to effective early prevention and dynamic monitoring. We also compared our safety data with other trials using pemetrexed or lenalidomide and found that the two-drug combination did not increase unexpected toxicity.
Currently, there are two widely used prognostic models for PCNSL: Memorial Sloan Kettering Cancer Center (MSKCC) (35) and International Extranodal Lymphoma Study Group (IELSG) (36). The MSKCC prognostic score includes age and Karnofsky performance status and divides patients into three different prognostic classes. The IELSG prognostic score system includes five parameters: age, ECOG score, serum LDH level, protein level in the CSF, and deep brain lesions. We also performed univariate and multivariate analysis for the 38 patients utilizing these and other factors. Univariate analysis showed that elevated serum LDH level and bulky disease were poor prognostic factors for PFS. Elevated serum LDH level, abnormal lymphoma cells in the CSF, and multiple lesions were significantly related to poor OS. Multivariate survival analysis for OS showed that abnormal lymphoma cells in the CSF was an independent prognostic factor [P = 0.034; HR = 2.836; 95% CI, 1.082–7.434].
This study is limited by being a single-center study that included a small sample size. In addition, a non-randomized research design was used. Therefore, multicenter, randomized clinical trials are needed to confirm the results. Nevertheless, the trial regimen was well-tolerated and resulted in good PFS indicating that this drug combination may be another option for patients with R/R PCNSL.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Huashan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JM, ZL, TD, YM, and BC prepared and conceived the study. JM, ZL, and TD conducted and analyzed the data. JM, YM, and BC interpreted the results. JM wrote the manuscript. All authors discussed the results and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This prospective phase II study is the part of a research project (SHDC12020112) that was funded by Shanghai Shenkang Clinical Innovation Project. This study was also funded by the Clinical Research Plan (SHDC2020CR6005-002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schaff LR, Grommes C. Updates on Primary Central Nervous System Lymphoma. Curr Oncol Rep (2018) 20(2):11. doi: 10.1007/s11912-018-0666-1
2. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, Gender, and Racial Differences in Incidence and Survival in Primary CNS Lymphoma. Brit J Cancer (2011) 105(9):1414–8. doi: 10.1038/bjc.2011.357
3. Camilleri-Broët S, Martin A, Moreau A, Angonin R, Hénin D, Gontier MF, et al. Primary Central Nervous System Lymphomas in 72 Immunocompetent Patients: Pathologic Findings and Clinical Correlations. Am J Clin Pathol (1998) 110(5):607–12. doi: 10.1093/ajcp/110.5.607
4. Batchelor T. Treatment of Primary CNS Lymphoma With Methotrexate and Deferred Radiotherapy: A Report of NABTT 96-07. J Clin Oncol (2003) 21(6):1044–9. doi: 10.1200/JCO.2003.03.036
5. Ferreri AJM. Therapy of Primary CNS Lymphoma: Role of Intensity, Radiation, and Novel Agents. Hematology (2017) 2017(1):565–77. doi: 10.1182/asheducation-2017.1.565
6. Kim JE, Yoon DH, Kim S, Lee DH, Kim JH, Yoon YH, et al. Relapse Pattern and Prognostic Factors for Patients With Primary Central Nervous System Lymphoma. Korean J Hematol (2012) 47(1):60–6. doi: 10.5045/kjh.2012.47.1.60
7. Langner-Lemercier S, Houillier C, Soussain C, Ghesquières H, Chinot O, Taillandier L, et al. Primary CNS Lymphoma at First Relapse/Progression: Characteristics, Management, and Outcome of 256 Patients From the French LOC Network. Neuro-Oncology (2016) 18(9):1297–303. doi: 10.1093/neuonc/now033
8. Holdhoff M, Wagner-Johnston N, Roschewski M. Systemic Approach to Recurrent Primary CNS Lymphoma: Perspective on Current and Emerging Treatment Strategies. Onco Targets Ther (2020) 13:8323–35. doi: 10.2147/OTT.S192379
9. Kasenda B, Ihorst G, Schroers R, Korfel A, Schmidt-Wolf I, Egerer G, et al. High-Dose Chemotherapy With Autologous Haematopoietic Stem Cell Support for Relapsed or Refractory Primary CNS Lymphoma: A Prospective Multicentre Trial by the German Cooperative PCNSL Study Group. Leukemia (2017) 31(12):2623–9. doi: 10.1038/leu.2017.170
10. Narita Y, Nagane M, Mishima K, Terui Y, Arakawa Y, Yonezawa H, et al. Phase 1/2 Study of Tirabrutinib, a Second-Generation Bruton’s Tyrosine Kinase Inhibitor, in Relapsed/Refractory Primary Central Nervous System Lymphoma. Neuro-Oncol (2020) 23(1):122–33. doi: 10.1093/neuonc/noaa145
11. Choi MK, Kang ES, Kim DW, Ko YH, Seok H, Park JH, et al. Treatment Outcome of Relapsed/Refractory Primary Central Nervous System Diffuse Large B-Cell Lymphoma: A Single-Center Experience of Autologous Stem Cell Transplantation. Int J Hematol (2013) 98(3):346–54. doi: 10.1007/s12185-013-1403-z
12. Rubenstein JL, Geng H, Fraser EJ, Formaker P, Chen L, Sharma J, et al. Phase 1 Investigation of Lenalidomide/Rituximab Plus Outcomes of Lenalidomide Maintenance in Relapsed CNS Lymphoma. Blood Adv (2018) 2(13):1595–607. doi: 10.1182/bloodadvances.2017014845
13. Dietrich J, Versmee L, Drappatz J, Eichler AF, Nayak L, Norden A, et al. Pemetrexed in Recurrent or Progressive Central Nervous System Lymphoma: A Phase I Multicenter Clinical Trial. Oncologist (2020) 25(9):747–e1273. doi: 10.1634/theoncologist.2020-0489
14. Plotkin SR, Betensky RA, Hochberg FH, Grossman SA, Lesser GJ, Nabors LB, et al. Treatment of Relapsed Central Nervous System Lymphoma With High-Dose Methotrexate. Clin Cancer Res (2004) 10(17):5643–6. doi: 10.1158/1078-0432.CCR-04-0159
15. Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR, et al. Higher Response to Lenalidomide in Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Nongerminal Center B-Cell-Like Than in Germinal Center B-Cell-Like Phenotype. Cancer (2011) 117(22):5058–66. doi: 10.1002/cncr.26135
16. Raizer JJ, Rademaker A, Evens AM, Rice L, Schwartz M, Chandler JP, et al. Pemetrexed in the Treatment of Relapsed/Refractory Primary Central Nervous System Lymphoma. Cancer (2012) 118(15):3743–8. doi: 10.1002/cncr.26709
17. Zhao HT, Chen J, Shi SB, Tian J, Tao RJ. Pemetrexed Plus Rituximab as Second-Line Treatment for Primary Central Nervous System Lymphoma. Med Oncol (2015) 32(1):351. doi: 10.1007/s12032-014-0351-7
18. Ghesquieres H, Chevrier M, Laadhari M, Chinot O, Choquet S, Moluçon-Chabrot C, et al. Lenalidomide in Combination With Intravenous Rituximab (REVRI) in Relapsed/Refractory Primary CNS Lymphoma or Primary Intraocular Lymphoma: A Multicenter Prospective 'Proof of Concept' Phase II Study of the French Oculo-Cerebral Lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann Oncol (2019) 30(4):621–8. doi: 10.1093/annonc/mdz032
19. Houillier C, Choquet S, Touitou V, Martin-Duverneuil N, Navarro S, Mokhtari K, et al. Lenalidomide Monotherapy as Salvage Treatment for Recurrent Primary CNS Lymphoma. Neurology (2015) 84(3):325–6. doi: 10.1212/WNL.0000000000001158
20. Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an International Workshop to Standardize Baseline Evaluation and Response Criteria for Primary CNS Lymphoma. J Clin Oncol (2005) 23(22):5034–43. doi: 10.1200/JCO.2005.13.524
21. Reni M, Ferreri AJ, Villa E. Second-Line Treatment for Primary Central Nervous System Lymphoma. Brit J Cancer (1999) 79(3-4):530–4. doi: 10.1038/sj.bjc.6690083
22. Sun Y, Wang Y, Han S, Xing B, Li H, Zhu Y, et al. Efficacy and Safety of Pemetrexed on Recurrent Primary Central Nervous System Lymphomas in China: A Prospective Study. Onco Target Ther (2017) 10:2595–600. doi: 10.2147/OTT.S134684
23. Zhang JP, Lee EQ, Nayak L, Doherty L, Kesari S, Muzikansky A, et al. Retrospective Study of Pemetrexed as Salvage Therapy for Central Nervous System Lymphoma. J Neuro Oncol (2013) 115(1):71–7. doi: 10.1007/s11060-013-1196-1
24. Fischer L, Thiel E, Klasen HA, Birkmann J, Jahnke K, Martus P, et al. Prospective Trial on Topotecan Salvage Therapy in Primary CNS Lymphoma. Ann Oncol (2006) 17(7):1141–5. doi: 10.1093/annonc/mdl070
25. Reni M, Zaja F, Mason W, Perry J, Mazza E, Spina M, et al. Temozolomide as Salvage Treatment in Primary Brain Lymphomas. Brit J Cancer (2007) 96(6):864–7. doi: 10.1038/sj.bjc.6603660
26. Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab Monotherapy for Patients With Recurrent Primary CNS Lymphoma. Neurology (2011) 76(10):929–30. doi: 10.1212/WNL.0b013e31820f2d94
27. Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive Chemotherapy Followed by Hematopoietic Stem-Cell Rescue for Refractory and Recurrent Primary CNS and Intraocular Lymphoma: Société Française De Greffe De Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol (2008) 26(15):2512–8. doi: 10.1200/JCO.2007.13.5533
28. Alimohamed N, Daly A, Owen C, Duggan P, Stewart DA. Upfront Thiotepa, Busulfan, Cyclophosphamide, and Autologous Stem Cell Transplantation for Primary CNS Lymphoma: A Single Centre Experience. Leuk Lymphoma (2012) 53(5):862–7. doi: 10.3109/10428194.2011.633250
29. Seidel S, Nilius-Eliliwi V, Kowalski T, Vangala DB, Schlegel U, Schroers R. High-Dose Chemotherapy With Autologous Hematopoietic Stem Cell Transplantation in Relapsed or Refractory Primary CNS Lymphoma: A Retrospective Monocentric Analysis of Long-Term Outcome, Prognostic Factors, and Toxicity. Cancers(Basel) (2022) 14(9):2100. doi: 10.3390/cancers14092100
30. Chamoun K, Choquet S, Boyle E, Houillier C, Larrieu-Ciron D, Al Jijakli A, et al. Ibrutinib Monotherapy in Relapsed/Refractory CNS Lymphoma: A Retrospective Case Series. Neurology (2017) 88(1):101–2. doi: 10.1212/WNL.0000000000003420
31. Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, et al. Ibrutinib Monotherapy for Relapse or Refractory Primary CNS Lymphoma and Primary Vitreoretinal Lymphoma: Final Analysis of the Phase II 'Proof-of-Concept' iLOC Study by the Lymphoma Study Association (LYSA) and the French Oculo-Cerebral Lymphoma (LOC) Network. Eur J Cancer (2019) 117:121–30. doi: 10.1016/j.ejca.2019.05.024
32. Korfel A, Schlegel U, Herrlinger U, Dreyling M, Schmidt C, von Baumgarten L, et al. Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol (2016) 34(15):1757–63. doi: 10.1200/JCO.2015.64.9897
33. Tun HW, Johnston PB, DeAngelis LM, Atherton PJ, Pederson LD, Koenig PA, et al. Phase 1 Study of Pomalidomide and Dexamethasone for Relapsed/Refractory Primary CNS or Vitreoretinal Lymphoma. Blood (2018) 132(21):2240–8. doi: 10.1182/blood-2018-02-835496
34. Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B, et al. PD-1 Blockade With Nivolumab in Relapsed/Refractory Primary Central Nervous System and Testicular Lymphoma. Blood (2017) 129(23):3071–3. doi: 10.1182/blood-2017-01-764209
35. Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. J Clin Oncol (2006) 24(36):5711–5. doi: 10.1200/JCO.2006.08.2941
Keywords: primary central nervous system lymphoma, relapsed/refractory, pemetrexed, lenalidomide, efficacy and safety
Citation: Ma J, Lin Z, Ding T, Li Q, Zhang M, Kang H, Johnston PB, Ma Y and Chen B (2022) Pemetrexed Plus Lenalidomide for Relapsed/Refractory Primary Central Nervous System Lymphoma: A Prospective Single-Arm Phase II Study. Front. Oncol. 12:938421. doi: 10.3389/fonc.2022.938421
Received: 07 May 2022; Accepted: 20 June 2022;
Published: 11 July 2022.
Edited by:
Sylvain Choquet, Hôpitaux Universitaires Pitié Salpêtrière, FranceReviewed by:
Zimu Gong, Houston Methodist Hospital, United StatesEmmanuel Gyan, Centre Hospitalier Universitaire de Tours, France
Copyright © 2022 Ma, Lin, Ding, Li, Zhang, Kang, Johnston, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bobin Chen, bbchen@fudan.edu.cn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Jingjing Ma
Jingjing Ma Zhiguang Lin1,2†
Zhiguang Lin1,2† Qing Li
Qing Li Yan Ma
Yan Ma Bobin Chen
Bobin Chen