
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Oncol., 24 August 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.935383
This article is part of the Research TopicNew Understandings and Research in Anal Squamous Cell CarcinomaView all 9 articles
Background: Squamous carcinoma of the anal canal (SCAC) is a human papillomavirus (HPV)-driven cancer with poor prognosis in locally advanced or recurrent settings. Carboplatin–paclitaxel is the preferred first-line regimen for unresectable locally advanced or metastatic SCAC, with the reported median progression-free survival (PFS) and overall survival (OS) of 8.1 and 20.0 months, respectively. Immune checkpoint blockade (ICB) demonstrates improved survival in HPV-driven cervical and head and neck cancers. Retifanlimab (INCMGA00012) is an investigational humanized, hinge-stabilized, immunoglobulin G4κ monoclonal antibody targeting programmed cell death-1 (PD-1), with characteristics common to the ICB class. In POD1UM-202, retifanlimab showed substantial clinical activity and an expected safety profile in patients with advanced SCAC who progressed on platinum-based chemotherapy. Based on these encouraging results, POD1UM-303/InterAACT 2 (NCT04472429), a phase III, double-blind, randomized, multiregional study, investigates the addition of retifanlimab to the standard of care (SOC) carboplatin–paclitaxel in patients with inoperable locally recurrent or metastatic SCAC not previously treated with systemic chemotherapy.
Methods and analysis: Patients ≥18 years with inoperable locally recurrent or metastatic SCAC, measurable disease per RECIST v1.1, and no prior systemic chemotherapy or PD-(L)1-directed therapy will be enrolled and stratified by PD-L1 expression, region, and extent of disease. Patients with well-controlled human immunodeficiency virus infection are eligible. Planned enrollment is approximately 300 patients worldwide, with a 1:1 randomization to retifanlimab or placebo. Patients will receive up to six induction cycles (24 weeks) of carboplatin (area-under-the-curve 5 on day 1) and paclitaxel (80 mg/m2 on days 1, 8, and 15) every 28 days per SOC. Concurrently, retifanlimab 500 mg or placebo will be administered intravenously in a blinded fashion on day 1 of each 28-day cycle for up to 13 cycles (1 year) in the absence of unacceptable toxicity, disease progression, withdrawal of consent, loss to follow-up, or premature discontinuation. Crossover to open-label retifanlimab will be allowed for patients assigned to placebo upon verification of progression by blinded independent central radiographic review (BICR). The primary study endpoint is PFS per RECIST v1.1 by BICR. Secondary endpoints are OS, objective response rate, duration of response, disease control rate, safety, and retifanlimab pharmacokinetics. The study is currently recruiting.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT04472429; https://clinicaltrialsregister.eu/ctr-search/search?query=2020-000826-24
Anal cancer is a rare malignancy accounting for 2.6% of approximately 1.9 million global cases of colorectal cancer in 2020, and of the almost 51,000 cases reported, 57% occurred in women (1). Squamous carcinoma of the anal canal (SCAC) is the predominant tumor type (80%) in anal cancer (2, 3). Epidemiological studies have reported an increasing incidence of SCAC in Europe and North America during the period 1989–2007 (4) and in the United States for 2001–2015 (5). The risk factors for SCAC include sexual practices (e.g., anal receptive intercourse, lifetime number of partners), history of sexually transmitted diseases, vulvar or cervical carcinoma/dysplasia, chronic immunosuppression [e.g., related to human immunodeficiency virus (HIV) or subsequent to organ transplantation], and smoking (6–8).
Chemoradiation is the standard of care (SOC) for localized SCAC, with a reported 5-year disease-free survival of 57.8% with cisplatin–fluorouracil plus radiotherapy (RT) and 67.8% with mitomycin–fluorouracil plus RT (9). Carboplatin–paclitaxel is currently the preferred first-line regimen for unresectable locally advanced or metastatic SCAC, with the reported median progression-free survival (PFS) and overall survival (OS) of 8.1 and 20.0 months, respectively (10).
The dominant etiology of SCAC is human papillomavirus (HPV) infection (6, 7, 11), with approximately 90% of tumors found to be HPV-positive (2, 12, 13). Immune checkpoint blockade (ICB) with programmed cell death (ligand)-1 [PD-(L)1] inhibitors is a promising approach to HPV-driven malignancy based on clinical experience in head and neck squamous cell carcinoma (HNSCC) (14) and cervical cancer (15).
Retifanlimab (INCMGA00012) is an investigational humanized, hinge-stabilized, immunoglobulin G4κ monoclonal antibody targeting PD-1, with characteristics common to the ICB class (16–19). Retifanlimab monotherapy demonstrated encouraging clinical efficacy in the treatment of 94 patients with advanced SCAC who progressed on or during a platinum-based regimen; the single-arm phase II POD1UM-202 study achieved an overall median PFS and OS of 2.3 and 10.1 months, respectively (20). Thirteen (13.8%) patients achieved an objective response (one complete response, 12 partial responses) and the median duration of responses was 9.5 months (range, 5.6–not estimable) (20). Thirty-three (35.1%) patients had stable disease, resulting in an overall disease control rate of 48.9%. Importantly, responses in the POD1UM-202 study were observed regardless of PD-L1 expression, liver metastases, or HIV status; retifanlimab was well-tolerated with a low incidence (2.1%) of treatment discontinuation because of immune-related adverse events (20). Retifanlimab response in locally advanced and/or metastatic SCAC results is supported by the results with other single-agent PD-(L)1 inhibitors (21–24), with the reported median PFS and OS of 4.1 and 11.5 months for nivolumab (21), 2.0 and 13.9 months for avelumab (24), and up to 3.0 and 11.9 months for pembrolizumab (22, 23), respectively.
Based on the encouraging results seen with retifanlimab in the platinum-refractory SCAC patient population in POD1UM-202, the phase III POD1UM-303/InterAACT 2 study was designed to examine the addition of retifanlimab to SOC in chemotherapy-naive patients with advanced SCAC.
POD1UM-303/InterAACT 2 (NCT04472429) is a global, multiregional, double-blind, randomized study investigating carboplatin–paclitaxel in combination with retifanlimab or placebo in patients with inoperable locally recurrent or metastatic SCAC not previously treated with systemic chemotherapy. Patients will receive up to six induction 28-day cycles (24 weeks) of carboplatin (area-under-the-curve 5 on day 1) and paclitaxel (80 mg/m2 on days 1, 8, and 15) as per SOC. Concurrently, retifanlimab 500 mg or placebo will be administered intravenously in a blinded fashion on day 1 of each 28-day cycle (every 4 weeks) for up to 13 cycles (six induction cycles plus seven retifanlimab or placebo cycles) in the absence of unacceptable toxicity, disease progression, withdrawal of consent, loss to follow-up, or premature discontinuation (Figure 1).
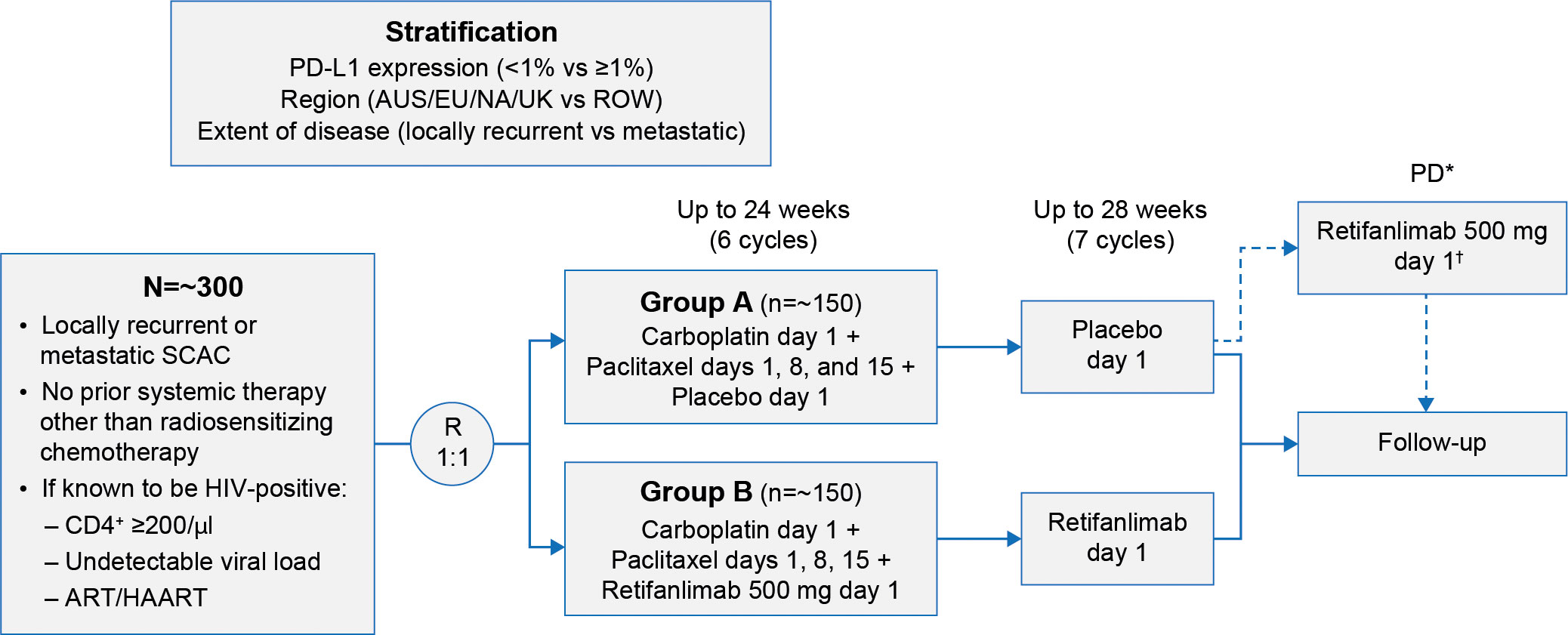
Figure 1 POD1UM-303/InterAACT 2 study design. *Verified by BICR. †Optional crossover period for qualified patients. ART/HAART, antiretroviral therapy/highly active antiretroviral therapy; AUS, Australia; BICR, blinded independent central radiographic review; EU, European Union; HIV, human immunodeficiency virus; NA, North America; PD, progressive disease; PD-L1, programmed cell death ligand 1; R, randomization; ROW, rest of the world; SCAC, squamous carcinoma of the anal canal; UK, United Kingdom.
Crossover to open-label retifanlimab will be allowed for patients assigned to placebo upon verification of disease progression by blinded independent central radiographic review (BICR). Patients will be followed until all study participants have received 13 cycles (1 year) of retifanlimab or placebo, all assigned study drugs have been discontinued, or they experience second disease progression.
The key inclusion and exclusion criteria for the study are presented in Table 1. In brief, patients ≥18 years with inoperable locally recurrent or metastatic SCAC, measurable disease per RECIST v1.1 (25), and no prior systemic therapy received will be enrolled. Prior neoadjuvant or adjuvant therapy if completed ≥6 months before study entry is permitted. Patients who have received prior PD-(L)1-directed therapy, radiotherapy (with or without radiosensitizing chemotherapy) within 28 days of cycle 1 day 1, or palliative radiotherapy (≤30 Gy and not directed to the pelvic region) within 14 days of cycle 1 day 1 will be excluded. Tumor tissue biopsies collected during screening or archival biopsy samples will be evaluated for PD-L1 expression (central laboratory result is required for stratification and randomization), HPV, and microsatellite instability. Patients with well-controlled HIV infection (defined as CD4+ count ≥200/µl, undetectable viral load, and receiving antiretroviral therapy for ≥4 weeks before study enrollment) are eligible to be enrolled in the trial. Patients with impaired cardiac function, clinically significant cardiac disease, or history of organ or allogeneic stem cell transplant will not be eligible for the study.
Enrolled patients will be stratified by PD-L1 expression (<1% versus ≥1%), extent of disease (locally recurrent versus metastatic), and region. Planned enrollment is approximately 300 patients worldwide, with 1:1 randomization to retifanlimab or placebo. The study is being conducted in Australia, Belgium, Denmark, France, Germany, Italy, Japan, Norway, Spain, the United Kingdom, and the United States.
The primary study endpoint is PFS per RECIST v1.1 by BICR. The key secondary endpoint is OS; additional secondary endpoints include overall response rate (ORR), duration of response (DOR), disease control rate (DCR) by BICR, safety, and retifanlimab pharmacokinetics. A summary of the study endpoints including exploratory endpoints is presented in Table 2.
Adverse events, including immune-related adverse events, will be coded by the Medical Dictionary for Regulatory Activities (MedDRA), with treatment-emergent adverse events (TEAEs) tabulated by preferred term and system organ class for all events, treatment-related events, and grade ≥3 events. Clinical safety laboratory analyses will include blood chemistries, hematology assessments, coagulation tests, endocrine function, and urinalysis. Additional laboratory monitoring in patients known to be HIV-positive will include CD4+ cell count and HIV viral load.
Several pharmacokinetic parameters will be assessed including maximum observed plasma concentration, time to maximum plasma concentration, minimum observed plasma concentration over the dose interval, and area under the plasma or serum concentration–time curve from time 0 to the last measurable concentration at time t.
At a one-sided overall 2.5% level of significance and true hazard ratio (HR) of 0.67 (under the alternative hypothesis), a total of 207 PFS events are required to have 83% power to reject the null hypothesis (HR = 1) using a log-rank test. Approximately 300 patients will therefore be randomized to the two treatment arms in a 1:1 ratio.
The full analysis set includes all randomized patients and will be the primary population for all efficacy analysis. The safety population will include all randomized patients who received at least one dose of the study drug. The pharmacokinetic (PK) evaluable population will include all patients who received at least one dose of the study drug and provided at least one postdose sample (one PK measurement).
PFS will be assessed via BICR according to RECIST v1.1, with survival data analyzed by the Kaplan–Meier method and the estimated median with 95% confidence interval (CI) reported. There are no interim analyses for PFS.
OS data will be analyzed by the Kaplan–Meier method, and the estimated medians with 95% CI reported.
ORR and DCR will be assessed via BICR according to RECIST v1.1. ORR and DCR including the respective 95% CIs will be determined. The odds ratio from the Cochran–Mantel–Haenszel test will be calculated for ORR. DOR data will be analyzed by the Kaplan–Meier method, and estimated medians with 95% CIs reported.
TEAEs will be tabulated by the MedDRA preferred term and system organ class for all events, related events, and grade ≥3 events. A data monitoring committee will meet at regular intervals to assess ongoing safety.
The collected PK data will be analyzed by standard population PK methods using appropriate software (e.g., NONMEM) or alternatively pooled with the data from other studies for a population PK analysis.
PFS 2 (PFS on the next line therapy) will be assessed using the same methods as PFS. ORR crossover (percentage of patients in the retifanlimab monotherapy crossover period having investigator-assessed CR or PR) will be determined if data permit. Translational analyses such as blood and/or tumor analytes, immune cell profiles and viral profiles, and assessment of patient-reported outcomes including the Quality of Life Questionnaire for Anal Cancer (QLQ-ANL27) are also planned.
The combination of PD-(L)1 inhibitors with SOC chemotherapy has improved the outcomes for many patients, including other tumor types that are primarily driven by HPV. Specifically, improved survival has been demonstrated with the addition of pembrolizumab to SOC chemotherapy in recurrent or metastatic cervical cancer (15) and in combination with platinum–5-fluorouracil-based chemotherapy for the treatment of recurrent or metastatic HNSCC (26). Improvement in overall survival has also been shown with nivolumab in advanced HNSCC following progression on platinum-based chemotherapy (14) and with cemiplimab in the treatment of platinum-refractory cervical cancer (27).
The current preferred first-line regimen for unresectable locally advanced or metastatic SCAC is carboplatin–paclitaxel, with the reported PFS and OS of 8.1 and 20.0 months, respectively (20). Although new treatment options have been investigated (23, 24), there have been no approved therapies for SCAC in recent years. POD1UM-303 is one of the largest well-controlled, randomized trials of a novel therapeutic in locally inoperable or metastatic SCAC. It is anticipated that adding retifanlimab will increase PFS and OS over what can be achieved with a carboplatin–paclitaxel regimen. As observed with other immunotherapies, the results of this trial may have applicability to other HPV-driven tumor types adding further rationale and importance to this global phase III registration study.
Patient recruitment for POD1UM-303/InterAACT 2 began on 19 October 2020. The study protocol at the time of writing this report is INCMGA0012-303 Amendment 1 (Version 2), December 2021.
Patient and public input were solicited during the design of the study, including a patient advocacy board held to gain insight regarding participation in the trial.
POD1UM-303/InterAACT 2 is conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Practice, the principles of the Declaration of Helsinki, and other applicable local ethical and legal requirements. The protocol and all amendments are reviewed and approved by the Institutional Review Boards or Independent Ethics Committees before the start of the study. Informed consent is obtained before patient participation in any study-related procedures. Study data and findings are planned to be presented at congresses and submitted for publication in peer-reviewed medical journals.
All authors contributed to the study design, drafting and critical review of the manuscript, and provided approval of the final version to be published.
This study is sponsored by Incyte Corporation (Wilmington, DE).
The authors wish to thank the patients and their families, the investigators, and the site personnel participating in this study. The authors would like to thank Mark Cornfeld (Incyte Corporation, Wilmington, DE) for the critical review of this report. Medical writing assistance was provided by Matthew Bidgood, Ph.D., of Envision Pharma Group (Philadelphia, PA).
SR reports advisory role or honoraria from Amgen, Celgene, and Shire and travel grants from Bayer, Celgene, and Incyte Corporation. MJ, JB, and CT report employment and stock ownership for Incyte Corporation. J-PS reports honoraria from AstraZeneca, Biogaran, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Leo Pharma, Mylan, Myriad Genetics, Novartis, Pfizer, and Pierre Fabre; a consulting or advisory role for Merck Sharp & Dohme and Roche; and a grant from MSD Avenir.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer (2004) 101:270–80. doi: 10.1002/cncr.20365
3. Valvo F, Ciurlia E, Avuzzi B, Doci R, Ducreux M, Roelofsen F, et al. Cancer of the anal region. Crit Rev Oncol Hematol (2019) 135:115–27. doi: 10.1016/j.critrevonc.2018.12.007
4. Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol (2017) 46:924–38. doi: 10.1093/ije/dyw276
5. Deshmukh AA, Suk R, Shiels MS, Sonawane K, Nyitray AG, Liu Y, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst (2020) 112:829–38. doi: 10.1093/jnci/djz219
6. Morris V, Eng C. Summary of emerging targets in anal cancer: The case for an immunotherapy based-approach. J Gastrointest Oncol (2016) 7:721–6. doi: 10.21037/jgo.2016.08.03
7. Morton M, Melnitchouk N, Bleday R. Squamous cell carcinoma of the anal canal. Curr Probl Cancer (2018) 42:486–92. doi: 10.1016/j.currproblcancer.2018.11.001
8. Pessia B, Romano L, Giuliani A, Lazzarin G, Carlei F, Schietroma M. Squamous cell anal cancer: Management and therapeutic options. Ann Med Surg (Lond) (2020) 55:36–46. doi: 10.1016/j.amsu.2020.04.016
9. Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB 3rd, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol (2012) 30:4344–51. doi: 10.1200/jco.2012.43.8085
10. Rao S, Sclafani F, Eng C, Adams RA, Guren MG, Sebag-Montefiore D, et al. International rare cancers initiative multicenter randomized phase II trial of cisplatin and fluorouracil versus carboplatin and paclitaxel in advanced anal cancer: InterAAct. J Clin Oncol (2020) 38:2510–8. doi: 10.1200/jco.19.03266
11. Jacome AA, Eng C. Experimental and investigational drugs for the treatment of anal cancer. Expert Opin Investig Drugs (2018) 27:941–50. doi: 10.1080/13543784.2018.1543659
12. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer (2006) 118:3030–44. doi: 10.1002/ijc.21731
13. Morris VK, Rashid A, Rodriguez-Bigas M, Das P, Chang G, Ohinata A, et al. Clinicopathologic features associated with human papillomavirus/p16 in patients with metastatic squamous cell carcinoma of the anal canal. Oncologist (2015) 20:1247–52. doi: 10.1634/theoncologist.2015-0091
14. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
15. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med (2021) 385:1856–67. doi: 10.1056/NEJMoa2112435
16. Lakhani N, Mehnert JM, Rasco D, Gordon M, Lohr J, Sharma S, et al. A phase 1 study of the safety, tolerability, and pharmacokinetics (PK) of MGA012 (anti-PD-1 antibody) in patients with advanced solid tumors. J Immunother Cancer (2017) 5:87. P249. doi: 10.1186/s40425-017-0288-4
17. Mehnert JM, Joshua A, Lakhani N, Banerji U, Rasco D, Lugowska I, et al. First-in-human phase 1 study of INCMGA00012 in patients with advanced solid tumors: interim results of the cohort expansion phase. J Immunother Cancer (2018) 6:115. P669. doi: 10.1186/s40425-018-0423-x
18. Condamine T, Owens S, Feldman P, Motte-Mohs RL, Muth P, Sumrow B, et al. Pharmacodynamic correlates in a phase I study of INCMGA00012, a PD-1 antagonistic monoclonal antibody. Cancer Res (2019) 79:Abstract CT085. doi: 10.1158/1538-7445.Am2019-ct085
19. Chen X, Wang P, Kaul S, Sumrow B, Yeleswaram S. Assessment of flat dosing strategy for INCMGA00012 in patients with advanced tumors. Cancer Res (2019) 79:Abstract LB–268. doi: 10.1158/1538-7445.Am2019-lb-268
20. Rao S, Capdevila J, Gilbert D, Kim S, Dahan L, Kayyal T, et al. LBA42 POD1UM-202: phase II study of retifanlimab in patients (pts) with squamous carcinoma of the anal canal (SCAC) who progressed following platinum-based chemotherapy. Ann Oncol (2020) 31:S1170–S1. doi: 10.1016/j.annonc.2020.08.2272
21. Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol (2017) 18:446–53. doi: 10.1016/s1470-2045(17)30104-3
22. Ott PA, Piha-Paul SA, Munster P, Pishvaian MJ, van Brummelen EMJ, Cohen RB, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol (2017) 28:1036–41. doi: 10.1093/annonc/mdx029
23. Marabelle A, Cassier PA, Fakih M, Kao S, Nielsen D, Italiano A, et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: Results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol Hepatol (2022) 7:446–54. doi: 10.1016/s2468-1253(21)00382-4
24. Lonardi S, Prete AA, Morano F, Messina M, Formica V, Corsi DC, et al. Randomized phase II trial of avelumab alone or in combination with cetuximab for patients with previously treated, locally advanced, or metastatic squamous cell anal carcinoma: The CARACAS study. J Immunother Cancer (2021) 9:e002996. doi: 10.1136/jitc-2021-002996
25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
26. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet (2019) 394:1915–28. doi: 10.1016/s0140-6736(19)32591-7
Keywords: anal cancer, carboplatin, paclitaxel, retifanlimab, squamous carcinoma
Citation: Rao S, Jones M, Bowman J, Tian C and Spano J-P (2022) POD1UM-303/InterAACT 2: A phase III, global, randomized, double-blind study of retifanlimab or placebo plus carboplatin–paclitaxel in patients with locally advanced or metastatic squamous cell anal carcinoma. Front. Oncol. 12:935383. doi: 10.3389/fonc.2022.935383
Received: 03 May 2022; Accepted: 25 July 2022;
Published: 24 August 2022.
Edited by:
Stefano Kim, Centre Hospitalier Universitaire de Besançon, FranceReviewed by:
Robert Glynne-Jones, Mount Vernon Cancer Centre, United KingdomCopyright © 2022 Rao, Jones, Bowman, Tian and Spano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheela Rao, U2hlZWxhLlJhb0BybWgubmhzLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.