
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 17 August 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.934978
This article is part of the Research TopicMethods in Surgical OncologyView all 34 articles
 Liu Ouyang1†
Liu Ouyang1† Hao Hu1†
Hao Hu1† Gang Nie1†
Gang Nie1† Li-xue Yang2†
Li-xue Yang2† Zhi-ping Huang3†
Zhi-ping Huang3† Chen-ming Ni1
Chen-ming Ni1 Zhuo Shao1
Zhuo Shao1 Kai-lian Zheng1
Kai-lian Zheng1 Wei Jing1
Wei Jing1 Bin Song1
Bin Song1 Gang Li1*
Gang Li1* Xian-gui Hu1*
Xian-gui Hu1* Gang Jin1*
Gang Jin1*Objectives: This study aimed to examine the incidence of bifid pancreatic duct (BPD) in pancreaticoduodenectomy (PD) and clarify its impact on clinically relevant postoperative pancreatic fistula (CR-POPF).
Background: Until now, all the literature about BPD during PD are published as case reports, and the incidence of BPD in PD and its impact on CR-POPF remain unknown.
Results: A total of 438 consecutive PDs were divided into two groups: the former year group and the latter year group. The former year group included 215 consecutive PDs, while the latter year group included 223. In the latter year group, we found 16 BPDs during PD (O-BPD); the incidence of O-BPD is 7.17%. Of them, there were eight patients who had BPD in the preoperative imaging (I-BPD). All the I-BPDs are O-BPDs; which means that 50% of O-BPDs were a single pancreatic duct in the preoperative imaging (I-SPD). There were 17 I-BPDs in the 438 consecutive PDs; the incidence of I-BPD is 3.88%. In the former year group, the rate of severe complications of I-BPD and I-SPD is 77.78% and 27.18%, respectively (p = 0.003); the rate of CR-POPF of I-BPD is higher than I-SPD, 55.56% vs. 27.18%, but there were no statistically significant differences. In the latter year group, the rate of severe complications of O-BPD and O-SPD is 50% and 18.36%, and the rate of CR-POPF of O-BPD and O-SPD is 37.5% and 22.22%, respectively; both of them have statistically significant differences, and the p-value is 0.003 and 0.006, respectively. In the subgroup analysis, both the rate of severe complications and the rate of CR-POPF of I-BPD were higher than O-BPD, 77.78% vs. 50%, and 55.56% vs. 37.5%, but there were no statistically significant differences in both of them; the p-value is 0.174 and 0.434, respectively. Univariate and multivariate analyses showed that BPD was an independent risk factor of CR-POPF.
Conclusions: The incidence of O-BPD in PD is 7.17%, 50% of O-BPDs were I-SPD, and the incidence of I-BPD is 3.88%. BPD is an independent risk factor of CR-POPF. The suture closure method may be a simple, safe, and effective method in dealing with BPD in PD.
Pancreaticoduodenectomy (PD) represents the standard surgical procedure for neoplasms of the pancreatic head and periampullary region. It involves the removal of the pancreatic head, duodenum, gallbladder, and common bile duct, with or without the removal of the gastric antrum; after resection, pancreaticojejunostomy (PJ), cholangiojejunostomy, and gastrojejunostomy must be performed. The most common complications after PD are delayed gastric emptying (DGE), pancreatic fistulae, hemorrhage, chyle leaks, endocrine and exocrine pancreatic insufficiency, and surgical site infections (1). The mortality rate of PD is about 2%–5%; the two most frequent causes of death were a leak from an anastomosis with sepsis/multiple organ system failure and bleeding. Clinically relevant postoperative pancreatic fistula (CR-POPF) occurs in up to 20% of patients and is typically associated with an increased hospital stay, cost, and reintervention rates. It is one of the most important initiating factors of severe complications and even death after PD (2).
The validated Fistula Risk Score (FRS) by Callery et al. (3) is the most cited and best used POPF prediction model. The FRS consists of gland texture, pancreatic duct diameter, intraoperative blood loss, and definitive pathology. The alternative Fistula Risk Score for PD (a-FRS) by Mungroop et al. (4) was based on pancreatic texture, duct diameter, and body mass index (BMI), without blood loss and pathology, and was successfully validated for both the 2005 and 2016 POPF definition. Kantor et al. (5) derived a modified Fistula Risk Score (mFRS) for preoperative risk stratification in patients undergoing PD, which included five predictors: sex, BMI, preoperative total bilirubin, pancreatic ductal diameter, and gland texture.
Except for the above risk factors, Shukla et al. (6) believed that the anatomy of the main pancreatic duct plays an important role in determining the outcomes of pancreatic anastomoses, and an investigation to identify its correlation is necessary. Bifid pancreatic duct (BPD) represents a relatively rare anatomical variation of the pancreatic ductal system, presenting a major bifurcation in the main pancreatic duct along its length. Halpert et al. (7) first reported a patient with bifid pancreas in 1990, diagnosed by ERCP. Steger et al. (8) investigated the anatomy of the pancreatic duct in 25 human cadaveric pancreas with a focus on the corpus area, and they found that in addition to the main and accessory pancreatic duct in the head, an additional BPD was observed within the pancreas corpus in 16% of the cases. Since then, there were some case reports about the BPD during PD (9–13). On 20 March 2015, we found two pancreatic duct orifices in the remnant of the pancreas during PD for the first time, and the BPD anatomy was confirmed via intraoperative probing, direct visualization of the ductal orifices, and dissecting the resected specimen postoperatively. As mentioned above, all the literature about the BPD during PD are case reports now; we do not know the incidence of BPD in PD, and the relationship between BPD and CR-POPF. This study aims to address these points.
Between 20 March 2015 and 19 March 2016, 223 consecutive PDs were performed by a single surgeon in the Department of Hepatobiliary and Pancreatic Surgery (HBP Surgery) of Changhai Hospital Affiliated with the Naval Military Medical University. After transecting the pancreas, we inspected the cut surface of the residual dorsal pancreas carefully; if there are two pancreatic duct orifices in the remnant of the pancreatic body, and both of the two pancreatic ducts are more than 2 mm in diameter, we confirmed the BPD via intraoperative probing, direct visualization of the ductal orifices intraoperatively, and dissecting the resected specimen postoperatively (Figure 1).
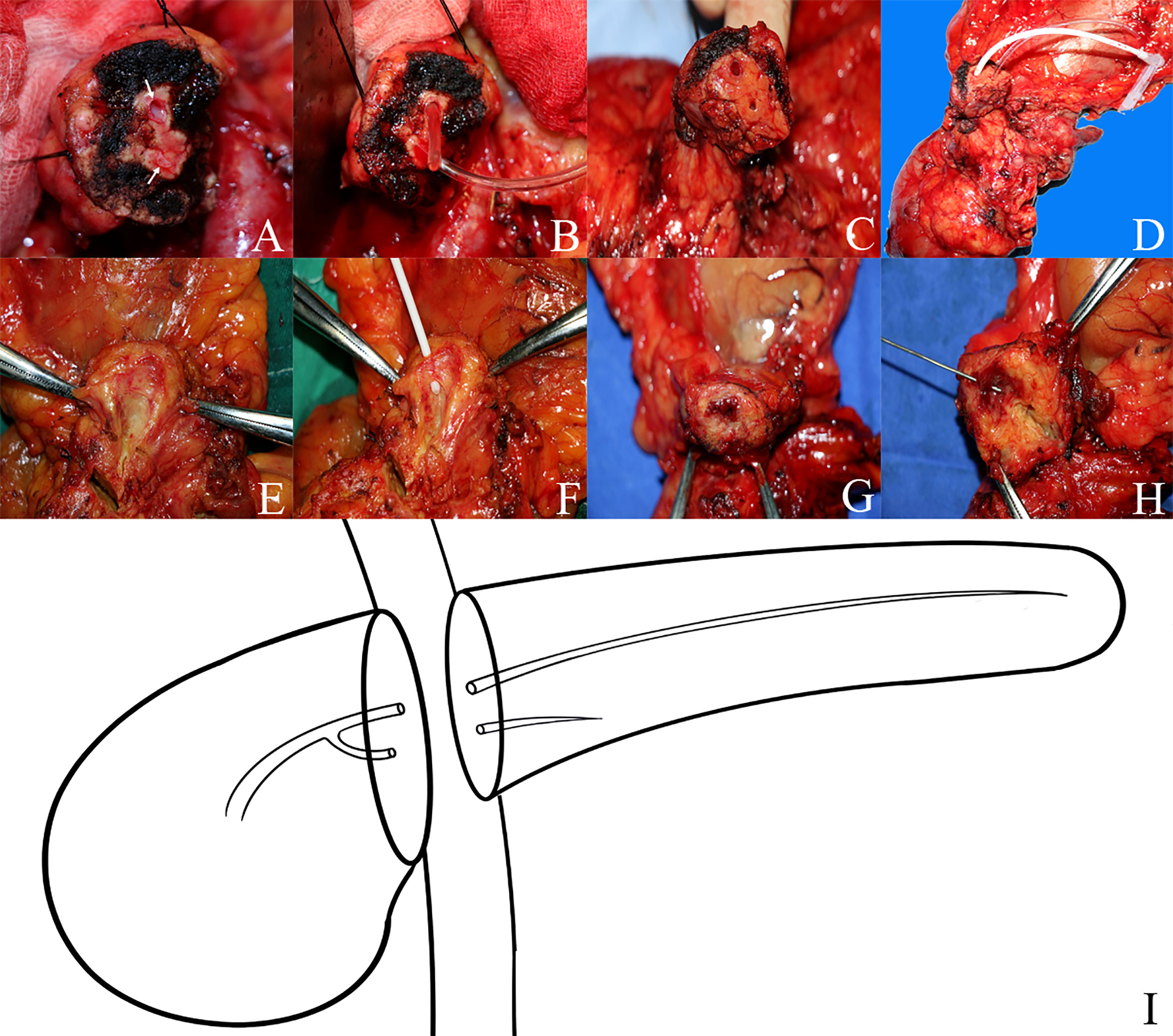
Figure 1 The anatomy of bifid pancreatic duct (A–D) Two pancreatic duct orifices in the remnant pancreas body during pancreaticoduodenectomy and the bifid pancreatic duct anatomy were confirmed via intraoperative probing and direct visualization of the ductal orifices. (E–H) The bifid pancreatic duct anatomy was confirmed by dissecting the resected specimen postoperatively; the bifid pancreatic duct in the body of the pancreas joins at the pancreatic head and drains through the major papilla. (I) Diagram of the anatomy of bifid pancreatic duct.
In the former year (between 20 March 2014 and 19 March 2015), we did not pay attention to BPD, and all the 215 consecutive PDs performed by the same surgeon were treated as a single pancreatic duct (SPD). The preoperative imaging data of the two groups of patients were reviewed retrospectively, and all the patients were divided into four subgroups: imaging single pancreatic duct (I-SPD), imaging bifid pancreatic duct (I-BPD), operative single pancreatic duct (O-SPD), and operative bifid pancreatic duct (O-BPD). In I-BPD, the bifurcation and the joints of the two pancreatic ducts must be seen in the preoperative imaging.
The PD operation consists of an en bloc removal of the pancreatic head, the duodenum, the common bile duct, the gall bladder, and the distal portion of the stomach together with the adjacent lymph nodes. Pylorus-preserving pancreaticoduodenectomy (PPPD) leaves the functional pylorus at the gastric outlet. Because the neck of the pancreas is a vascular watershed between celiac and superior mesenteric arterial systems, the transection plane was 1.5–2.0 cm to the left of the neck of the pancreas (14, 15) in this study. In the O-BPD group, we sutured and ligated the small pancreatic duct with silk thread (suture closure method), and the large pancreatic duct was anastomosed with the jejunum by the double-layer continuous suturing PJ method (16) with 5-0 and 3-0 Prolene, respectively.
Demographic, histopathologic, and perioperative data of all the patients in the 2 years were collected comprehensively from the electronic medical record. The staging was based on the American Joint Committee on Cancer (AJCC) TNM Staging of Pancreatic Cancer (8th ed., 2017). The diagnosis of postoperative pancreatic fistulas (POPFs), postpancreatectomy hemorrhage (PPH), and delayed gastric emptying (DGE) was according to the International Study Group of Pancreatic Surgery (ISGPS) definition, POPF-ISGPS (2016) (17), PPH-ISGPS (2007) (18), and DGE-ISGPS (2007) (19), respectively. The assessment of postoperative complications was according to the Clavien–Dindo classification (2004) (20).
Continuous variables were reported as median (interquartile range), and were compared using Mann–Whitney U test. Categorical variables were presented as whole numbers, and proportions and were compared by the χ2 test or Fisher’s exact test when appropriate. The cutoff value of certain parameters was determined using the receiver operating characteristic curve. Logistic regression analyses were applied in univariate and multivariate risk factor analysis, then a nomogram was established for predicting CR-POPF according to the results of multivariate risk factor analysis. The nomogram was validated via concordance index analysis, receiver operating characteristic curve, and calibration plot. Statistical analyses were conducted using GraphPad Prism 9, SPSS 24.0 software, and R software version 4.1.2. All the statistical significance levels were two-sided, with p-values less than 0.05.
In this study, there were 438 consecutive PDs performed by the same surgeon, and they were divided into two groups: the former year group (between 20 March 2014 and 19 March 2015) and the latter year group (between 20 March 2015 and 19 March 2016). Of them, the former year group included 215 consecutive PDs, while the latter year group included 223. The main clinicopathological characteristics and postoperative complications of the two groups are shown in Table 1. There were no statistically significant differences in baseline characteristics among the two groups except that the postoperative complication is fewer in the latter year group compared with the former year group using the Clavien–Dindo classification (p = 0.036).
In the latter year group, we found 16 BPDs during PD (O-BPD); thus, the incidence of O-BPD is 7.18% (16/223). Of them, there were eight patients who had BPD in the preoperative imaging (I-BPD). All the I-BPDs are O-BPDs; it means that 50% of O-BPDs were SPD in the preoperative imaging (I-SPD). There were 17 BPDs in the 438 consecutive PDs in the preoperative imaging (I-BPD); thus, the incidence of I-BPD is 3.88% (17/438) in this study (Figure 2).
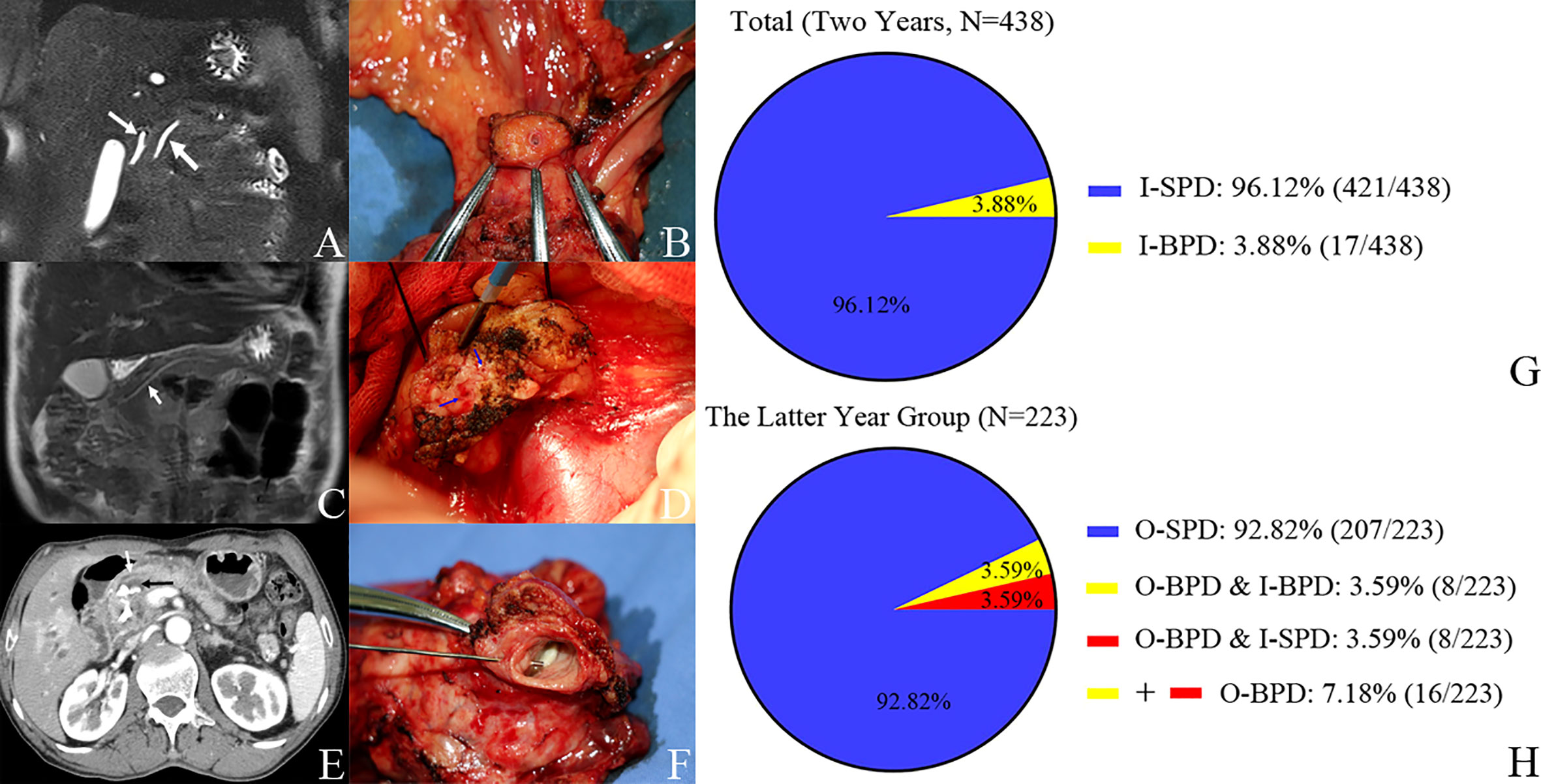
Figure 2 Pancreatic duct in preoperative imaging and operation and the incidence of bifid pancreatic duct. Case 1. (A) I-SPD vs. (B) O-SPD. (A) I-SPD: common bile duct (left arrow) and main pancreatic duct (right arrow) in preoperative imaging. (B) O-SPD: one pancreatic duct orifice in the remnant of pancreas. Case 2. (C) I-SPD vs. (D) O-BPD. (C) I-SPD: main pancreatic duct (arrow) in preoperative imaging. (D) O-BPD: bifid pancreatic duct (two green arrows) in pancreaticoduodenectomy. Case 3. (E) I-BPD vs. (F) O-BPD. (E) I-BPD: bifid pancreatic duct (white arrow and black arrow) in preoperative imaging. (F) O-BPD: bifid pancreatic duct in resected specimen; the bifid pancreatic duct in the body of the pancreas joins at the pancreatic head (probe in the small pancreatic duct). (G, H) Incidence of bifid pancreatic duct in preoperative imaging and operation. I-SPD, imaging single pancreatic duct; O-SPD, operative single pancreatic duct; I-BPD, imaging bifid pancreatic duct; O-BPD, operative bifid pancreatic duct.
There were no statistically significant differences in preoperative baseline characteristics among the two groups, including the rate of CR-POPF (p = 0.227). In the former year group, the rate of severe complications (Clavien–Dindo classification ≥ IIIa) of I-BPD and I-SPD is 77.78% (7/9) and 27.18% (56/206), respectively (p = 0.003); the rate of CR-POPF of I-BPD is higher than I-SPD, 55.56% (5/9) vs. 27.18% (56/206), but there were no statistically significant differences (p = 0.122). In the latter year group, the rate of severe complications of O-BPD and O-SPD is 50% (8/16) and 18.36% (38/207), and the rate of CR-POPF of O-BPD and O-SPD is 37.5% (6/16) and 22.22% (46/207), respectively; both of them have statistically significant differences, and the p-value is 0.003 and 0.006, respectively. In the subgroup analysis, both the rate of severe complications and the rate of CR-POPF of I-BPD were higher than O-BPD, 77.78% (7/9) vs. 50% (8/16), and 55.56% (5/9) vs. 37.5% (6/16), but there were no statistically significant differences in both of them; the p-value is 0.174 and 0.434, respectively. The effect of BPD on postoperative complications in PD is shown in Table 2.
The perioperatively obtained variables, include age, gender, hypertension, cardiovascular disease, smoking history, history of alcoholism, history of pancreatitis, history of abdominal surgery, BMI, TNM stage, pathology, blood type, NYHA score, ASA score, NNIS score, APACHE score, intraoperative bleeding, intraoperative blood transfusion, and indexes of blood or serum tests, were subjected to univariate and multivariate analyses. Our results showed that BPD (hazard ratio [HR] 2.396, 95% confidence interval [CI] 1.054–5.433), pancreatic duct diameter <0.2 cm (3.515, 2.041–6.054), tumor diameter ≤2 cm (3.31, 2.021–5.423), ASA score (1.914, 1.186–3.089), pathology except for pancreatic cancer and pancreatitis (4.371, 2.691–7.101), and BMI ≥ 23 (2.808, 1.809–4.359) were independent risk factors of CR-POPF (Table 3).
As listed in Table 3, BPD, pancreatic duct diameter <0.2 cm, tumor diameter ≤2 cm, ASA score, pathology except for pancreatic cancer and pancreatitis, and BMI ≥ 23 were selected in the construction of nomogram predicting CR-POPF (Figure 3). The concordance index was 0.795, and the area under the curve (AUC) was 0.790 according to the receiver operating characteristic (ROC) curve (Figure 4). The concordance index is a measure of the predictive accuracy of the model being tested, which ranges from 0.5 (completely random prediction) to 1 (perfect prediction). The apparent incidence of CR-POPF, the ideal incidence, and the bias-corrected incidence were shown as different lines in a calibration plot (Figure 5). The bias-corrected (also known as overfitting-corrected or optimism-corrected) line is produced using a bootstrap approach to estimate predicted and observed values based on a nonparametric smoother applied to a sequence of predicted values. These three lines were closely aligned, demonstrating good calibration.
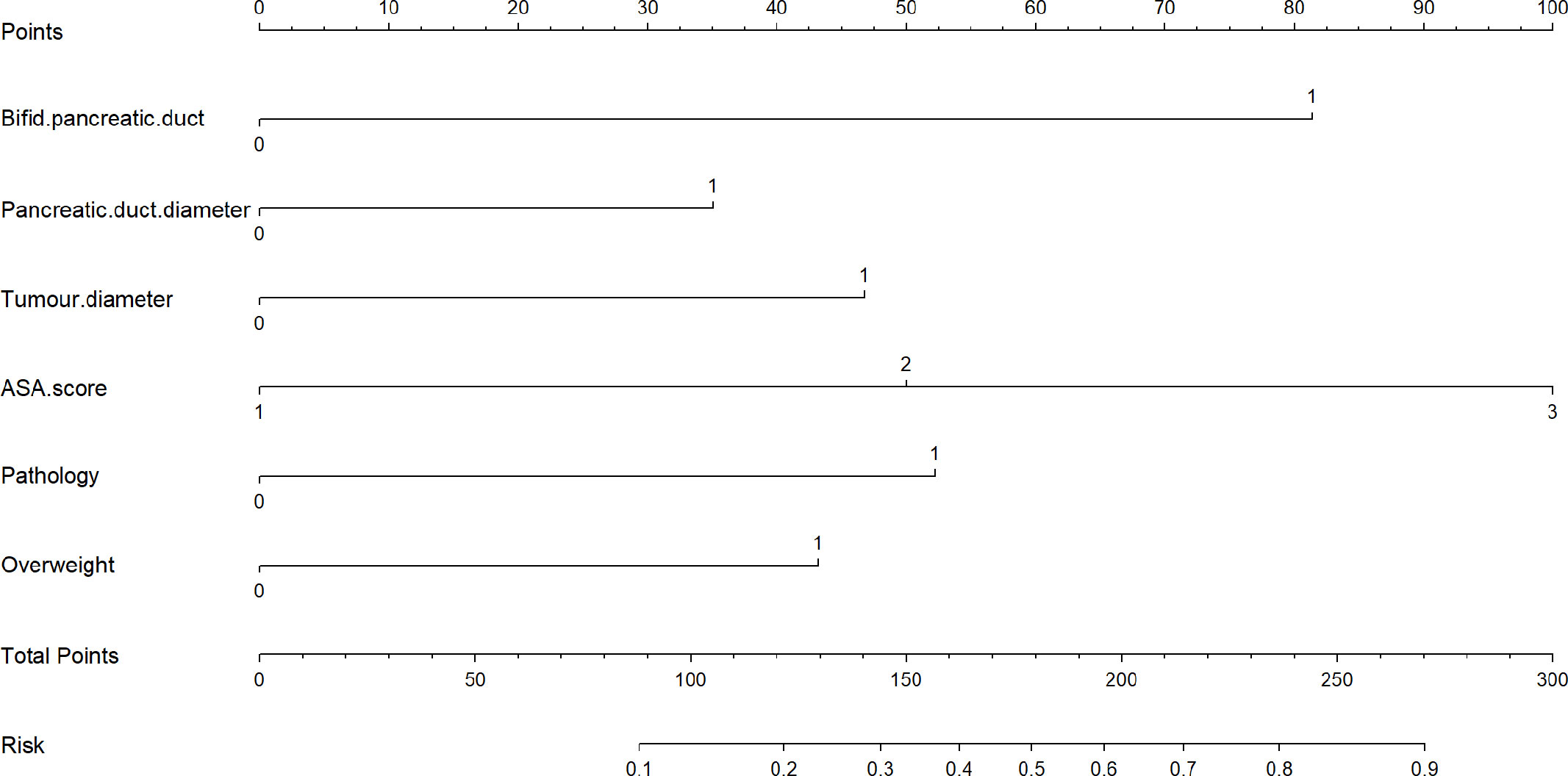
Figure 3 A novel nomogram for predicting CR-POPF of patients who underwent pancreaticoduodenectomy. The nomogram is used by adding up the points identified on the points scale for each variable. According to the sum of these points projected on the bottom scales, the nomogram can provide the incidence of CR-POPF for an individual patient. CR-POPF, clinically relevant postoperative pancreatic fistula.
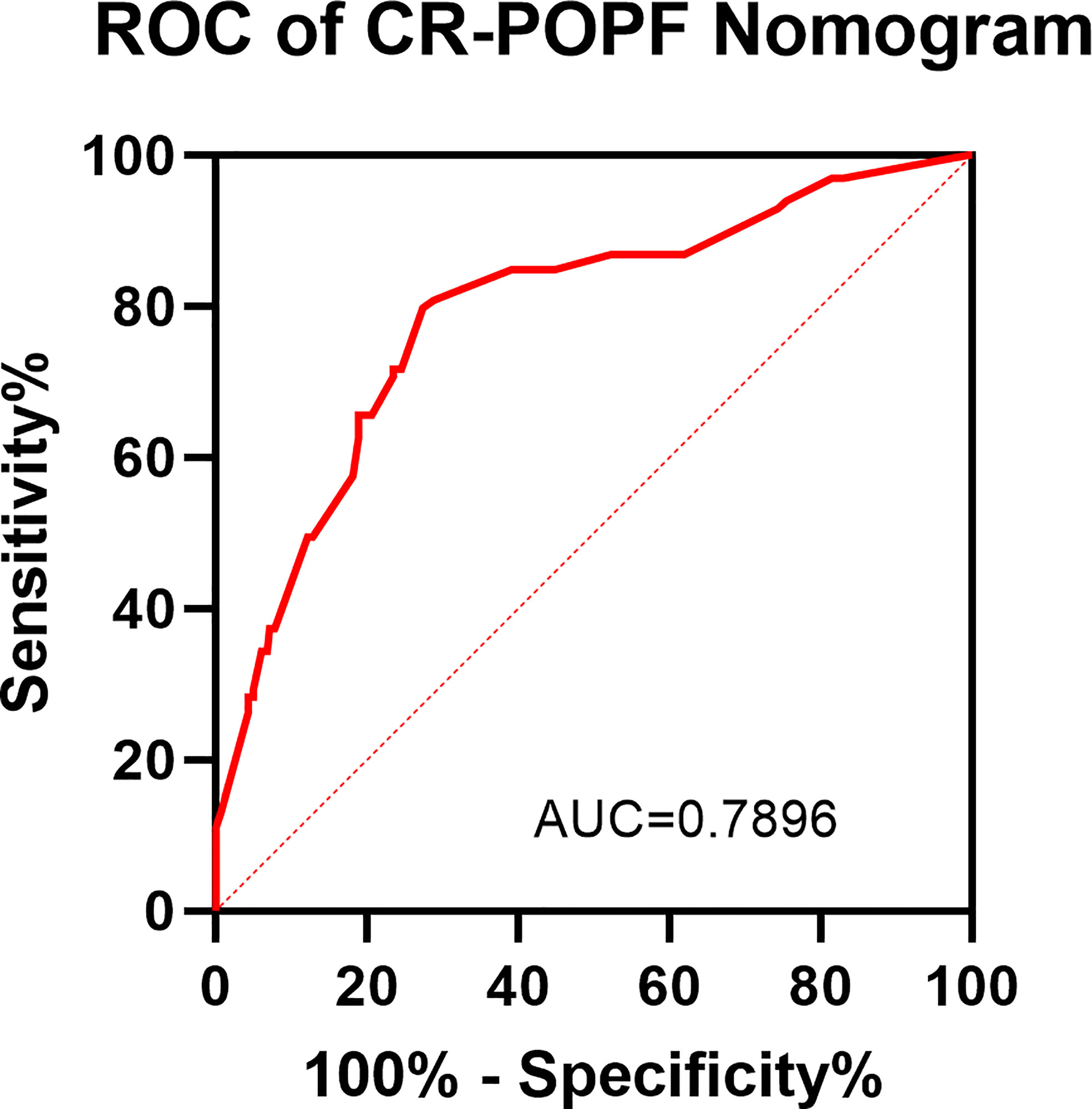
Figure 4 ROC of the CR-POPF nomogram. A receiver operating characteristic (ROC) curve was conducted for assessing the model. The area under the ROC curve is 0.7896.
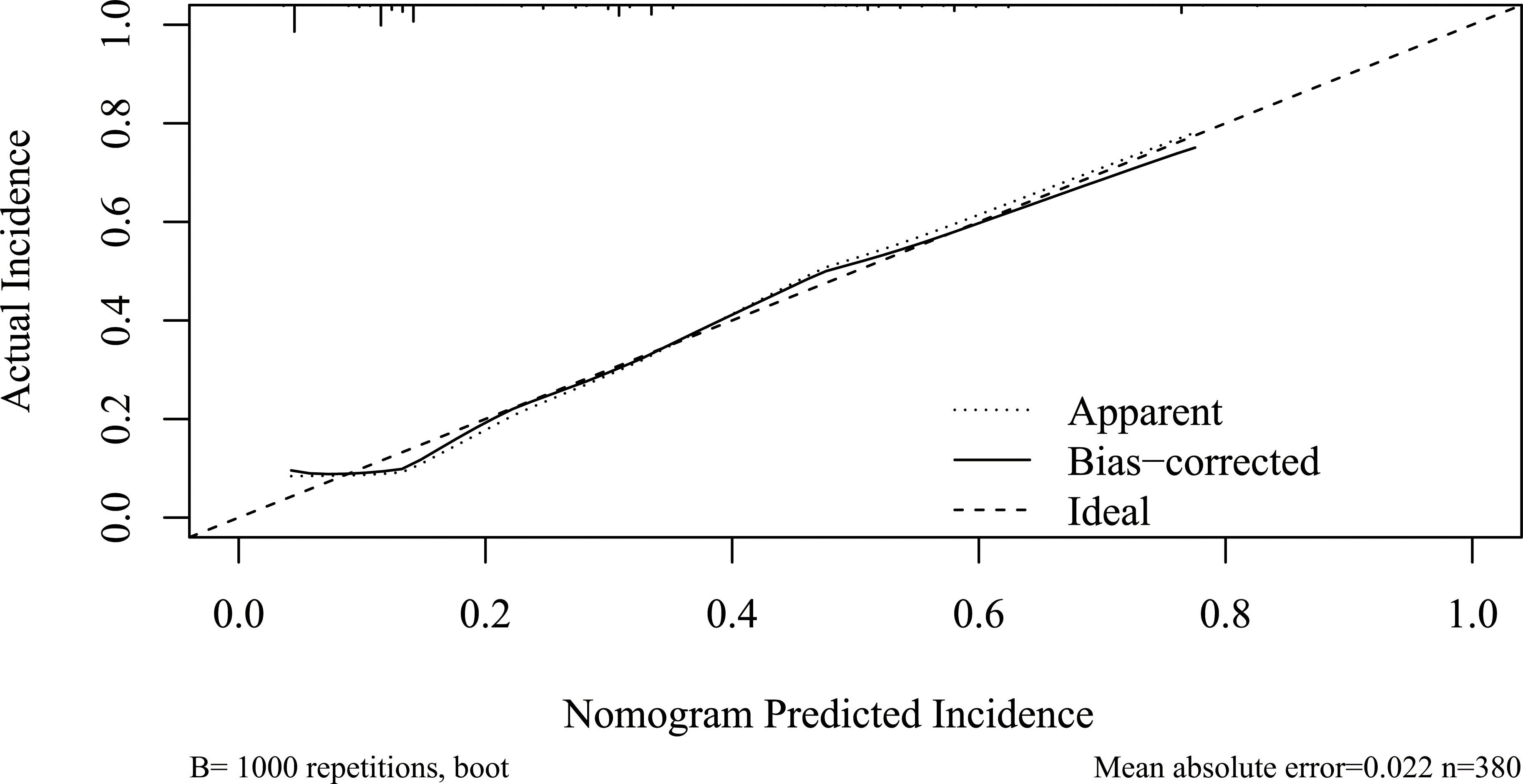
Figure 5 Calibration plot of the nomogram. Calibration plot of the nomogram predicting CR-POPF. The x-axis represents the nomogram-predicted survival, and the actual survival is plotted on the y-axis. The apparent incidence of CR-POPF, the ideal incidence, and the bias-corrected incidence were shown as different lines. CR-POPF, clinically relevant postoperative pancreatic fistula.
On 20 March 2015, we found BPD in the remnant pancreatic body during PD for the first time, and the BPD anatomy was confirmed via intraoperative probing, direct visualization of the ductal orifices, and dissecting of the resected specimen postoperatively. BPD represents a relatively rare anatomical variation of the pancreatic ductal system, presenting a major bifurcation in the main pancreatic duct along its length; it is different from the main pancreatic duct (Wirsung duct) and accessory pancreatic duct (Santorini duct) in the head of the pancreas (Figure 1). Steger et al. (8) investigated the anatomy of the pancreatic duct in 25 human cadaveric pancreas with a focus on the corpus area, and they found BPD within the pancreas corpus in 16% of the cases (Figure 6). There are also some case reports about the BPD during PD (9–13), and since all the literature about BPD are published as case reports, the incidence of BPD in PD remains unclear. In this study, we defined BPD as the diameter of both pancreatic ducts larger than 2 mm.
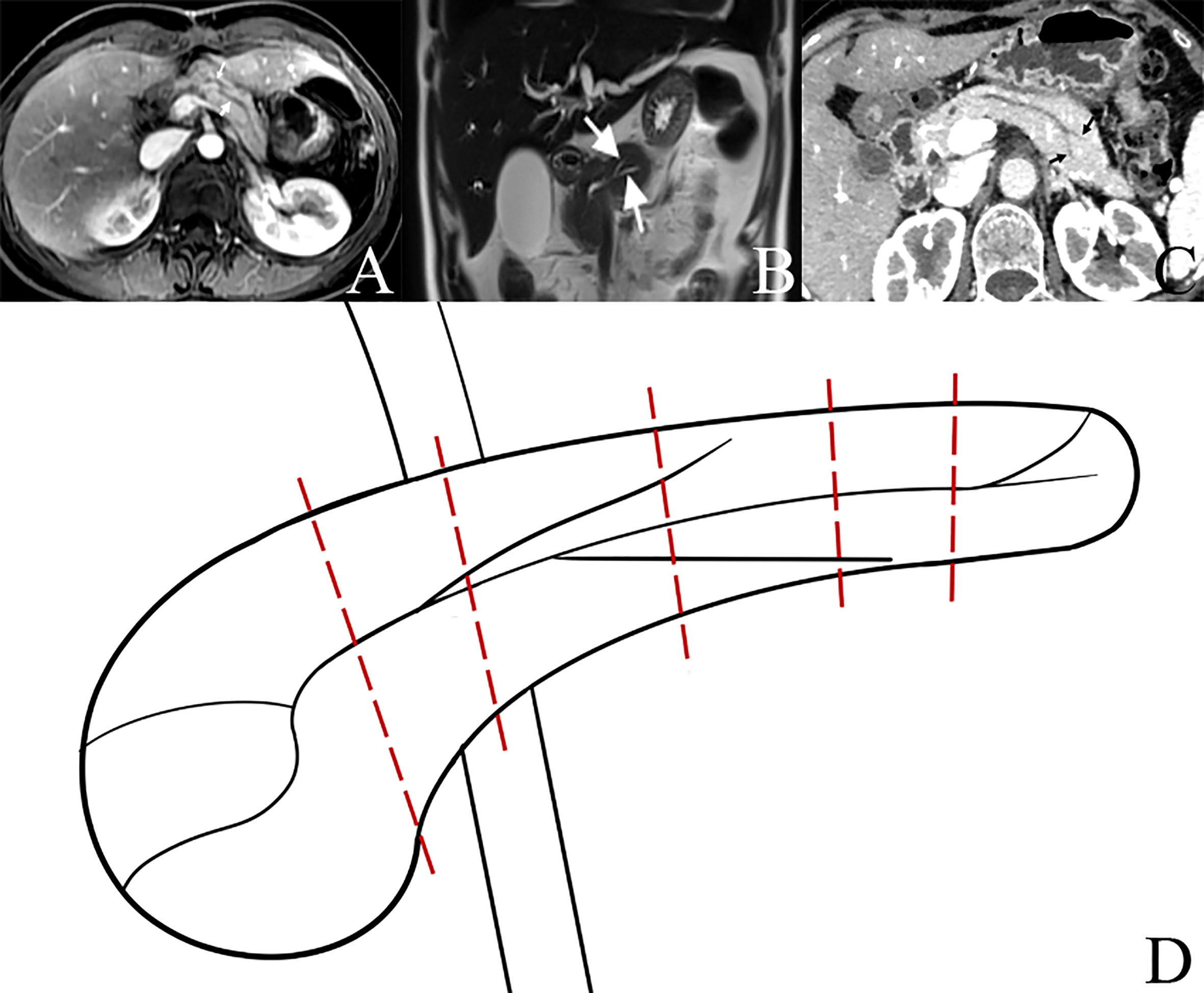
Figure 6 Bifid pancreatic duct at the neck, body, and tail of pancreas. (A) Bifid pancreatic duct (two white arrows) at the neck of pancreas. (B) Bifid pancreatic duct (two white arrows) at the body of pancreas. (C) Bifid pancreatic duct (two black arrows) at the tail of pancreas. (D) Diagram of bifid pancreatic duct at different locations of pancreas; different pancreatic transections result in different numbers of pancreatic duct orifices in the remnant of pancreas during operation.
In this study, during PD, the incidence of O-BPD is 7.17% (16/223), and 50% of O-BPDs were SPD in the preoperative imaging (I-SPD); thus, during PD, a careful intraoperative inspection of the cut surface of the residual dorsal pancreas is needed to identify the presence of BPD even in I-SPD patients. There were 17 BPDs in the 438 consecutive PDs in the preoperative imaging (I-BPD); thus, the incidence of I-BPD is 3.88% (17/438). Obstruction of the pancreatic duct and transecting the pancreas at the left of the pancreatic neck may increase the incidence of BPD in PD (8).
There are two pancreatic duct orifices in the remnant pancreatic body in patients with BPD during PD; in this study, we found that both the rate of severe complications and the rate of CR-POPF of BPD were higher than SPD, and both the rate of severe complications and the rate of CR-POPF of I-BPD in the former year (untreated) were higher than O-BPD (treated with the suture closure method), suggesting that we must deal with the BPD in PD. Yoshida et al. (9) and Vasiliadis et al. (10) reported one case of double duct-to-mucosa PJ for BPD following PD. On the other hand, Ball et al. (11) and Shim et al. (12) sutured and ligated the small BPD, and the large pancreatic duct was anastomosed with the jejunum using a standard duct-to-mucosa PJ. Recently, Ishida et al. (13) presented a novel technique named the “two-in-one” method in a case of PD for BPD; they anastomosed one jejunal hole to a double pancreatic duct. In this study, we sutured and ligated the small pancreatic duct with silk thread (suture closure method) in O-BPD patients, and the large pancreatic duct was anastomosed with the jejunum by double-layer continuous suturing PJ (16) with 5-0 and 3-0 Prolene, respectively (Figure 7). By using the suture closure method, both the rate of severe complications and the rate of CR-POPF decreased, suggesting that the suture closure method may be a simple, safe, and effective method in dealing with BPD in PD. Different from duct-to-mucosa PJ, invagination PJ is performed by invagination of 1–2 cm of the proximal end of the pancreatic stump into the jejunum (21); thus, invagination PJ may be superior to duct-to-mucosa PJ in patients with BPD.
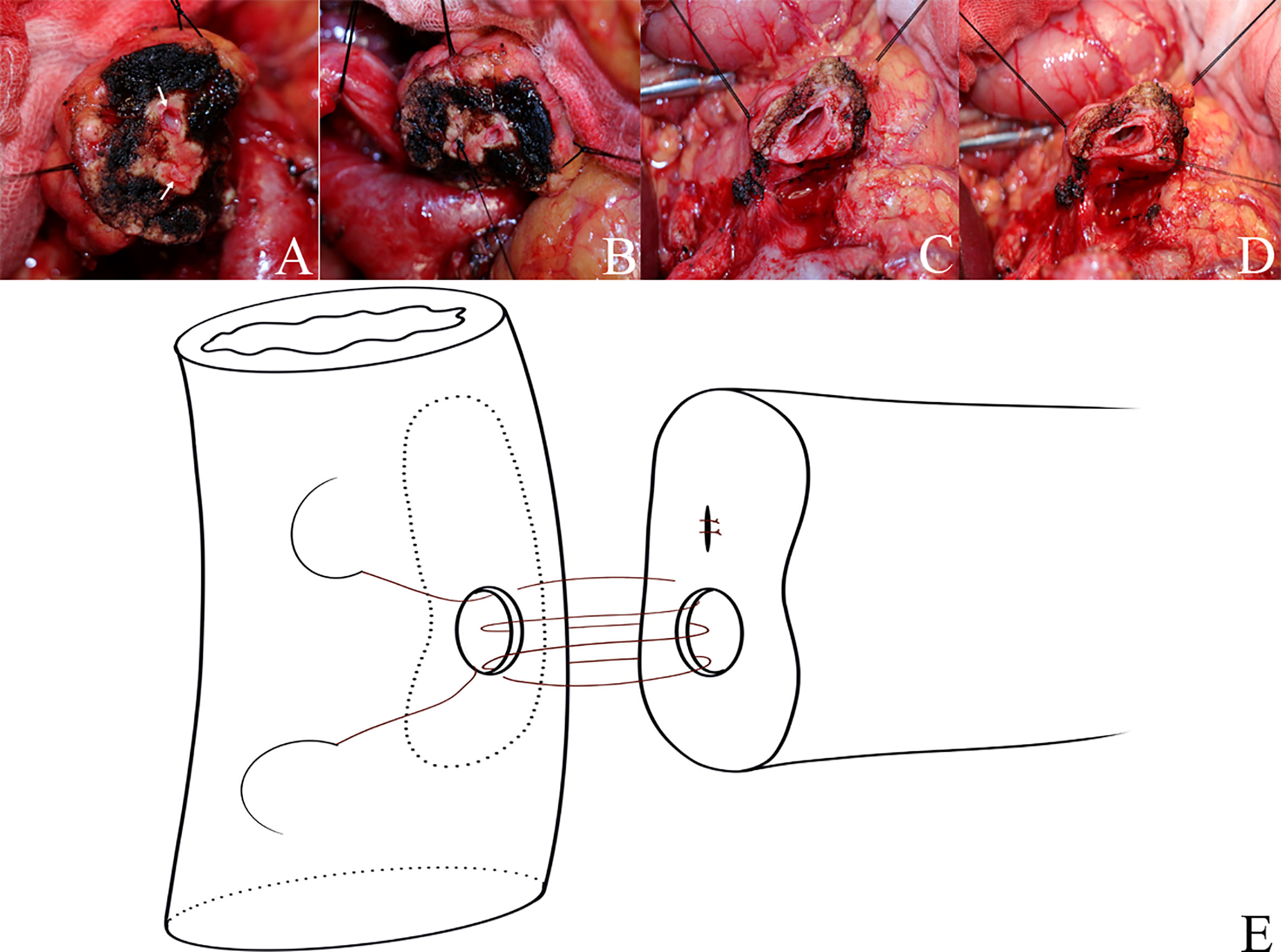
Figure 7 Suture closure method and double-layer continuous suturing pancreaticojejunostomy. (A–D) Suture closure method (suture and ligate the small pancreatic duct with silk thread). (E) The large pancreatic duct was anastomosed with the small intestine by double-layer continuous suturing pancreaticojejunostomy with 5-0 and 3-0 Prolene, respectively.
In the study of the anatomy of the pancreatic duct in the human cadaveric pancreas, fewer transversely cut side branches were observed within the plane of the portal vein as compared to the resection planes 2–4 cm beneath (8), suggesting that it is appropriate to transect the pancreas within the plane of the portal vein when it does not affect the radical PD.
When the transection plane of the pancreas is obscured by burning or bleeding during PD, intraoperative ultrasonography (IOUS) was useful in identifying the exact location of the BPD. Ohkubo et al. (22) used the IOUS and IOUS-guided pancreatography to clarify the exact location of BPD during PD; the transection line was set on the proximal side of the pancreatic duct bifurcation, which helped prevent the inadvertent suture of the second pancreatic duct or leave the second duct without anastomosis, which may result in CR-POPF and severe complications. By using IOUS to confirm the exact location of the pancreatic duct bifurcation as well as the tumor extension, Tajima et al. (23) performed a distal pancreatectomy, instead of a middle pancreatectomy, with a cutting line at the downstream pancreas to the duct bifurcation point, which resulted in a favorable outcome without any postoperative complications. It suggests that IOUS-guided pancreatography should be recommended to confirm the relationship between the transection line of the pancreas and the duct bifurcation point when performing PD if BPD is suspected.
Postoperative pancreatic fistula (POPF) is one of the most threatening complications after PD. POPF occurs in up to 20% of patients and is typically associated with an increased hospital stay, cost, reintervention rates, and mortality. Different factors may predict POPF, including gland texture, pancreatic duct diameter, intraoperative blood loss, definitive pathology, BMI, sex, and preoperative total bilirubin (3–5). Except for the above risk factors, the anatomy of the main pancreatic duct plays an important role in determining the outcomes of pancreatic anastomoses (6). BPD represents a relatively rare anatomical variation of the pancreatic ductal system, presenting a major bifurcation in the main pancreatic duct along its length. Our results showed that BPD, pancreatic duct diameter <0.2 cm, tumor diameter ≤2 cm, ASA score, pathology except for pancreatic cancer and pancreatitis, and BMI ≥ 23 were independent risk factors of CR-POPF.
Nonetheless, our study has some limitations. First, this study is a single-center retrospective study; a selection bias may be suggested by the retrospective nature. Second, BPD in the former year group was not intraoperatively investigated and estimation of I-BPD is difficult even after reviewing MR imaging, and as the incidence of BPD is low, and the sample size of this study was not large enough, we put the I-BPD cases from the former year group together with the O-BPD from the latter year group in the univariate and multivariate analyses. Third, as mentioned above, because the incidence of BPD is low, and the sample size of this study was not large enough, we have not performed a propensity matching score (PSM) study. Fourth, BPD should be treated during PJ; in this article, because we only used the suture closure method, we do not know the result of other methods [such as double duct-to-mucosa PJ (9, 10) and the two-in-one method (13)]; hence, a large-sample-size, multicenter, and randomized controlled trial needs to be performed in the future.
In conclusion, in this study, the incidence of O-BPD is 7.18% during PD, 50% of O-BPDs were SPD in the preoperative imaging (I-SPD), and the incidence of I-BPD is 3.88%. BPD is an independent risk factor of CR-POPF after PD, and the suture closure method may be a simple and safe method in dealing with BPD, with a potential reduction of CR-POPF rate, although the effectiveness still needs to be proven in further clinical research.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
This study was reviewed and approved by the Ethics Committee of Changhai hospital. The patients/participants provided their written informed consent to participate in this study.
Contributions: (I)†contribute equally; (II)Conception and design: GJ, X-GH, and GL. (III) Administrative support: GJ, X-GH, and GL. (IV) Provision of study patients: GJ, LO, ZS, K-LZ, WJ. (V) Collection and assembly of data: LO, HH, GN. (VI) Data analysis and interpretation: All authors; (VII) Manuscript writing: LO. All authors contributed to the article and approved the submitted version.
This study was supported by the Shanghai ShenKang hospital development center (No. SHDC2020CR2001A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Simon R. Complications after pancreaticoduodenectomy. Surg Clin North Am (2021) 101(5):865–74. doi: 10.1016/j.suc.2021.06.011
2. Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg (2015) 220(4):530–6. doi: 10.1016/j.jamcollsurg.2014.12.031
3. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg (2013) 216(1):1–14. doi: 10.1016/j.jamcollsurg.2012.09.002
4. Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): Design and international external validation. Ann Surg (2019) 269(5):937–43. doi: 10.1097/SLA.0000000000002620
5. Kantor O, Talamonti MS, Pitt HA, Vollmer CM, Riall TS, Hall BL, et al. Using the NSQIP pancreatic demonstration project to derive a modified fistula risk score for preoperative risk stratification in patients undergoing pancreaticoduodenectomy. J Am Coll Surg (2017) 224(5):816–25. doi: 10.1016/j.jamcollsurg.2017.01.054
6. Shukla PJ, Sakpal SV, Maharaj R. Does pancreatic ductal anatomy play a role in determining outcomes of pancreatic anastomoses? Med Hypotheses (2011) 76(2):230–3. doi: 10.1016/j.mehy.2010.10.003
7. Halpert RD, Shabot JM, Heare BR, Rogers RE. The bifid pancreas: a rare anatomical variation. Gastrointest Endosc (1990) 36(1):60–1. doi: 10.1016/S0016-5107(90)70926-1
8. Steger U, Range P, Mayer F, Waschke J, Timm S, Schlegel N. Pancreatic duct anatomy in the corpus area: Implications for closure and anastomotic technique in pancreas surgery. Langenbecks Arch Surg (2010) 395(3):201–6. doi: 10.1007/s00423-009-0526-4
9. Yoshida T, Ninomiya S, Morii Y, Matsumata T, Arimura E, Hidaka H. Double duct-to-mucosa pancreaticojejunostomy for bifid pancreatic duct. Hepatogastroenterology. (2004) 51(58):1196–7. doi: 10.1002/hed.20037
10. Vasiliadis K, Papavasiliou C, Lamprou N, Delivorias P, Papaioannou S, Karagiannidis A, et al. Double duct to mucosa pancreaticojejunostomy for bifid pancreatic duct following pylorus preserving pancreaticoduodenectomy: A case report. Case Rep Med (2012) 2012:657071. doi: 10.1155/2012/657071
11. Ball CG, Schmidt CM, Lillemoe KD. A bifid neck of the pancreas. J Gastrointest Surg (2010) 14(9):1462–3. doi: 10.1007/s11605-010-1217-8
12. Shim JR, Park SJ, Park HM, Lee EC, Han SS. Are you prepared for pancreas bifidum? A case report. Ann Surg Treat Res (2018) 94(1):49–51. doi: 10.4174/astr.2018.94.1.49
13. Ishida J, Toyama H, Tsugawa D, Kido M, Fukumoto T. Two-in-one method: Novel pancreaticojejunostomy technique for the bifid pancreas. Ann Gastroenterol Surg (2019) 4(2):175–9. doi: 10.1002/ags3.12302
14. Strasberg SM, McNevin MS. Results of a technique of pancreaticojejunostomy that optimizes blood supply to the pancreas. J Am Coll Surg (1998) 187(6):591–6. doi: 10.1016/S1072-7515(98)00243-9
15. Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, Ehlers JP, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: Effect on anastomotic failure in the Whipple procedure. J Am Coll Surg (2002) 194(6):746–58; discussion 759-60. doi: 10.1016/S1072-7515(02)01202-4
16. Ji W, Shao Z, Zheng K, Wang J, Song B, Ma H, et al. Pancreaticojejunostomy with double-layer continuous suturing is associated with a lower risk of pancreatic fistula after pancreaticoduodenectomy: a comparative study. Int J Surg (2015) 13:84–9. doi: 10.1016/j.ijsu.2014.11.034
17. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery (2017) 161(3):584–91. doi: 10.1016/j.surg.2016.11.014
18. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery (2007) 142(1):20–5. doi: 10.1016/j.surg.2007.02.001
19. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surgery (2007) 142(5):761–8. doi: 10.1016/j.surg.2007.05.005
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
21. Senda Y, Shimizu Y, Natsume S, Ito S, Komori K, Abe T, et al. Randomized clinical trial of duct-to-mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br J Surg (2018) 105(1):48–57. doi: 10.1002/bjs.10727
22. Ohkubo T, Imamura H, Sugawara Y, Takayama T, Makuuchi M. Successful pancreatic duct-to-jejunum anastomosis in a patient with a double pancreatic duct: usefulness of intraoperative ultrasonography (IOUS) and IOUS-guided pancreatography. Hepatogastroenterology. (2002) 49(48):1724–5. doi: 10.1002/hed.10155
Keywords: bifid pancreatic duct, morbidity, pancreaticoduodenectomy, CR-POPF, nomogram
Citation: Ouyang L, Hu H, Nie G, Yang L-x, Huang Z-p, Ni C-m, Shao Z, Zheng K-l, Jing W, Song B, Li G, Hu X-g and Jin G (2022) Incidence of bifid pancreatic duct in pancreaticoduodenectomy and its impact on clinically relevant postoperative pancreatic fistula. Front. Oncol. 12:934978. doi: 10.3389/fonc.2022.934978
Received: 03 May 2022; Accepted: 25 July 2022;
Published: 17 August 2022.
Edited by:
Michele Ammendola, University Magna Graecia of Catanzaro, ItalyReviewed by:
Cosimo Sperti, University of Padua, ItalyCopyright © 2022 Ouyang, Hu, Nie, Yang, Huang, Ni, Shao, Zheng, Jing, Song, Li, Hu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Jin, amluZ2FuZ0BzbW11LmVkdS5jbg==; Xian-gui Hu, aHhpYW5ndWlAMTI2LmNvbQ==; Gang Li, bGlfZ2FuZzE2OUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.