- Department of Hepatobiliary Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
In the past few decades, tumor diagnosis and treatment theory have developed in a variety of directions. The number of people dying from pancreatic cancer increases while the mortality rate of other common tumors decreases. Traditional imaging methods show the boundaries of pancreatic tumor, but they are not sufficient to judge early micrometastasis. Although carcinoembryonic antigen (CEA) and carbohydrate antigen19-9 (CA19-9) have the obvious advantages of simplicity and minimal invasiveness, these biomarkers obviously lack sensitivity and specificity. Circulating tumor cells (CTCs) have attracted attention as a non-invasive, dynamic, and real-time liquid biopsy technique for analyzing tumor characteristics. With the continuous development of new CTCs enrichment technologies, substantial progress has been made in the basic research of CTCs clinical application prospects. In many metastatic cancers, CTCs have been studied as an independent prognostic factor. This article reviews the research progress of CTCs in the treatment and prognosis of pancreatic cancer.
Introduction
As one of the most malignant abdominal tumors, pancreatic adenocarcinoma (PDAC) is the fourth leading cause of tumor-related death in Western countries (1). A predictive study of the burden of cancer in 28 European countries using a time-linear Poisson regression model showed that pancreatic cancer will surpass breast cancer as the third leading cause of cancer-related deaths by 2025 (2, 3). By 2030, it will be the second leading cause of cancer-related deaths in the United States (3). In the past few decades, despite the great progress made in adjuvant therapy, neoadjuvant therapy, and chemotherapy, the prognosis of pancreatic cancer is still very poor. Although multidisciplinary precision treatment has been applied, and fine perioperative management has been implemented, the median survival time is less than 3–6 months, and less than 9% of diagnosed patients survive beyond 5 years (4, 5). The main reason for these dismal results is the difficulty in early diagnosis. The highly malignant nature of PDAC manifested as highly invasive early metastasis and unremarkable symptoms before the tumors evolve from the early stage to the late stage, which means that surgical treatment is not feasible. Still, we have no approach to stratify the risks for tumor metastasis, which are key indicators to guide neoadjuvant and adjuvant therapies, despite detecting the tumor in the early stage. The pathogenesis and etiology of PDAC are complicated and multifactorial, consisting of genetic and environmental factors. According to the United States Preventive Services Task Force (USPSTF) epidemiological study of pancreatic cancer, type 2 diabetes, obesity, advanced age, history of smoking, and chronic pancreatitis are predominant risk factors of PDAC (6).
Computed tomography (CT) is still the most effective means for clinical diagnosis and staging of PDAC and is a common method to monitor treatment response. CT and magnetic resonance imaging (MRI) display the anatomical boundaries of the tumor lesion, but they are inefficient to accurately assess the presence of tumor metastasis especially very small metastases in the early stage. Positron emission tomography (PET) is also incapable to detect micrometastases of PDAC because of the resolution ratio limitation that ranged from 4 to 10 mm (7). The ability to detect stage T1 pancreatic cancer with a tumor diameter of less than 2 cm can be used as a measure of various imaging techniques to diagnose early-stage pancreatic cancer (Table 1). Based on the comparison, it is clear that PET-CT is the imaging method with the highest detection rate for early-stage pancreatic cancer. PET-CT is useful as a whole-body examination in assessing the M-stage of tumors and has unique advantages in detecting distant metastases. Although PET-CT is more sensitive in detecting early pancreatic cancer, it is less specific and may give false-negative results for active chronic pancreatitis, plasmacytic cystadenoma, and enlarged lymph nodes in the head of the pancreas. Also, PET-CT is imprecise in its anatomical localization. Additionally, such imaging requires specialized and expensive equipment. Histological and cytological analysis is still the primary method to get an accurate diagnosis. Ultrasound-guided fine needle aspiration (FNA) is the most commonly used tissue acquisition method. Although the sensitivity of EUS seems to be the highest, this comes at the cost of invasive pathology (EUS-FNA). Unfortunately, the methods require superb expertise and there is a corresponding risk of complications. If the puncture extracts the matrix of the tumor tissue instead of cancer cells, it means a failure of diagnosis, and multiple fine needle aspirations may promote tumor proliferation.
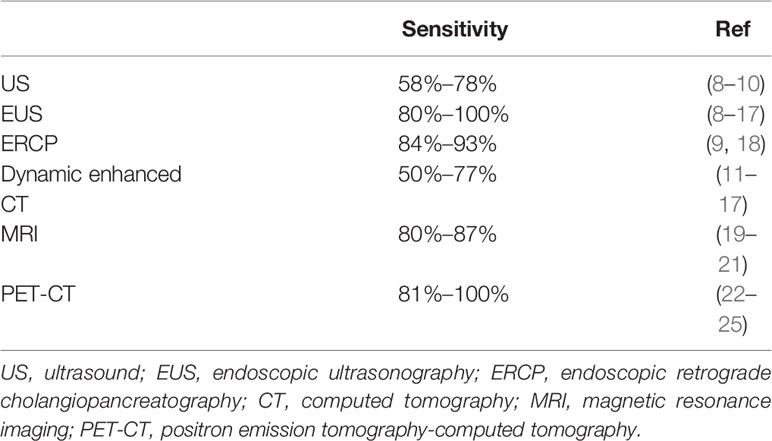
Table 1 The ability of various imaging techniques to detect early-stage pancreatic carcinoma with a tumor diameter of less than 2 cm.
The measurements of CA19-9 and CEA involve a few available blood-derived biomarkers. In particular, CA19-9 is the only serum biomarker approved by the United States Food and Drug Administration. Although blood-based tumor biomarkers have significant advantages (they are cheap, simple, and minimally invasive), these common tumor biomarkers have apparent limitations (they lack sensitivity and specificity); thus, they are not recommended for use in the early screening of pancreatic cancer. To date, the best marker, CA19-9, a sialylated Lewis antigen derived from the mucin 1 (MUC1) protein, has an overall sensitivity of 80% and a specificity of 82%. However, 5%–10% of the Caucasian population are incapable of synthesizing the CA19-9 epitope because of their Lewis a- and b‐genotype blood type (26). In addition, when we detect the blood of patients with benign diseases such as cholangitis, obstructive jaundice, and cirrhosis, CA19-9 is also elevated, thus increasing the difficulty of discrimination (27). On the other hand, CA19-9 does not increase in 35% of the patients diagnosed with resectable pancreatic cancer (28). However, for pancreatic cancers less than 1 cm in diameter and confined to the ductal epithelium, the 5-year survival rate after surgical resection can reach 100% (29). Therefore, we urgently need a reliable and all-purpose method to synthesize early detection of PDAC, real-time monitoring of radiotherapy and chemotherapy response, and prediction of the risk of tumor recurrence or metastasis.
Circulating tumor cells (CTCs) are tumor cells that have the capability to enter the blood circulating system. This cellular population circulates through peripancreatic vessels and their capillaries, along with tumor-induced neovessels, ultimately leading to metastasis progression in multiple organs (30). Research has shown that CTCs can enter the blood circulation in two ways: released from the tumor surface through shedding passively or by an active epithelial-to-mesenchymal transition (EMT) mechanism. Many tumor cells shed at the early stage of tumor formation through the first mechanism (31, 32). In the course of tumor development, some epithelial cells experience cellular changes in EMT. This change enhances their capacity of leaving the site of the primary tumor by increasing its mobility (33). CTCs analysis is beneficial in that its immunophenotypic and molecular genetic features may accurately represent the primary tumor serving as a “liquid biopsy” for metastatic tumors. At present, the two main assay methods widely used for CTCs detection are an immunomagnetic technique led by the CellSearch system and real-time quantitative PCR (qRT-PCR). A large number of derivative measurement technologies based on them are gradually being developed.
In recent years, with the continuous development of new CTCs enrichment technologies, substantial progress has been made in basic research on the prospects of CTCs clinical applications. For example, in many metastatic cancers, CTCs have been studied as an independent prognostic factor to predict patients’ progression-free survival (PFS) and overall survival (OS) (34–38). We found two studies that directly compared the diagnostic efficacy of CTCs and CA19-9. A prospective study in 2022 included blood samples from 82 patients with PDAC. This study aimed to compare the diagnostic efficacy of CTCs and CA19-9 in PDAC (39). In view of the false-negative results of CA19-9 in some Lewis antigen-negative populations, the researchers divided patients into a low-expression group (CA19-9 < 37 U/ml) and a high-expression group (CA19-9 ≥ 37 U/ml). In the low-expression group, 18 PDAC patients were misdiagnosed as negative by CA19-9, while CTCs correctly diagnosed 14 of the 18 misdiagnosed patients while maintaining high specificity. Zhang et al. obtained the patient’s portal vein blood to detect CTCs to evaluate their diagnostic and prognostic value for pancreatic cancer. The AUC demonstrated the diagnostic efficacy of different indicators: POV CTCs+CA19-9 (AUROC = 0.987), POV CTCs (AUROC = 0.942), and CA19-9 (AUROC = 0.806) (40). Their findings also suggested that the combination of CTCs and CA19-9 has a higher diagnostic power than individual indicators. In the neoadjuvant setting, the detection of CTCs is also an independent prognostic risk factor (41). In particular, strong evidence from epidemiological studies for CTCs as independent and stable prognostic factors has been published for breast carcinoma (42). During the past few years, attention has been paid to CTCs as a noninvasive, dynamic, and real-time liquid biopsy to analyze the characteristics of the tumor (43). In this review, we mainly incorporate relevant literature from the last 10 years, with a special focus on the clinical application of CTCs in pancreatic cancer in the current clinical context, where the prognostic value of CTCs in the treatment of pancreatic cancer is the main focus. We also focused on the comparison of the advantages and disadvantages of some imaging techniques and the comparison between CTCs and traditional markers. We briefly outline the classical mechanisms of CTCs generation.
Epithelial–Mesenchymal Transformation and Circulating Tumor Cells
EMT is the process in which epithelial cells change their polarity, restructure their intercellular junctional complexes, and obtain the phenotypes of interstitial cells under the influence of physiological or pathological stimulus factors. The physiological EMT process begins at the embryonic stage and plays a significant role in the differentiation of organs and tissues during embryonic development. Pathological EMT can adversely cause organ fibrosis and promote carcinoma progression through a variety of mechanisms. The EMT process causes the loss of cell adhesion and changes in the cytoskeleton, which enable tumor cells and the external environment to develop new interactions. The EMT program is highly conservative because the execution program during the embryonic development and carcinogenesis process has high similarity. EMT endows cells with migration and invasion properties, induces stem cell properties, prevents cell apoptosis and senescence, and promotes immune suppression. Through this, it obtains interstitial cell characteristics including infiltration ability and migration ability (44). In various tumor cell lines, scholars have studied multiple signaling pathways that induce EMT, such as the TGF-β signaling pathway, the Notch pathway, and the Wnt signaling pathway. The best-studied signal pathway is TGF-β signaling; research on the combined in vivo/in vitro carcinogenic model indicates that in the progression of advanced tumors, the activation of the TGF-β pathway can effectively promote the EMT process of cancer cells (45). The activation of these signal transduction pathways will induce the expression of zinc finger transcription factors with high affinity to E-box at the gene transcription level, such as snail, slug, and zeb (46). These transcription factors inhibit the transcription of E-cadherin, causing the loss of adhesion junctions mediated by it. Functioning as a key gatekeeper for cells to maintain an epithelial state, its inhibition is widely involved in various stages of tumor metastasis and progression (46).
Changing the cytoskeleton is another important strategy of EMT to promote tumor cell metastasis. According to the cellular tensegrity model theory, cellular morphology is subtly maintained by the tension force of microtubule stents and the counter tension force of actin mesh, which achieve a stable balance to stabilize the shape of cells (47). EMT causes the rearrangement and alteration of the actin cytoskeleton, which is responsible for the dissemination of CTCs (48). In the process of EMT, the stability of microtubules is a key step to improve the efficiency of CTCs transfer. In the post-translational modification of α-tubulin, a constitutive protein of microtubules, glutamic acid residues replaced tyrosine at the carboxyl end. Such alteration is extremely common in human cancers and sarcomas, especially during tumor progression. Tyr-microtubules can only last for 3–5 min, while glu-microtubules last for hours in vivo (49, 50). The orientation of glutamate microtubules is the same as that of cell migration, implying that it may be related to the aggressiveness of the tumors (51) (Figure 1). Another mechanism that stabilizes microtubules is binding microtubule-associated proteins. Microtubule-associated proteins are a large family with multiple functions. MAP1 and MAP2/tau protein families are the most widely studied in human malignancies. As a soluble microtubule-binding protein, the function of tau is to promote microtubule bundling and stabilization by enhancing the structural strength of microtubules. Compared with bare microtubules, tau-modified microtubules can withstand a greater deformation force in the stress resistance to actin (52). The binding of tau to microtubules and the depolymerization of actin can significantly promote the formation of microtentacles (McTN) (53). This long and dynamic microtubule-rich micro antenna is formed by the extension of the cell membrane of CTCs cells (54). This tau-induced McTN not only increases the reattachment of suspended cells in capillaries to endothelial cells, but also increases the probability of CTCs retention in blood vessels (53). McTN promotes the accumulation of tumor cells of the same type and is of great significance for their survival outside the primary lesion, because effective aggregation can protect CTCs from mechanical damage caused by shear and collision forces and damage from immune monitoring (55).
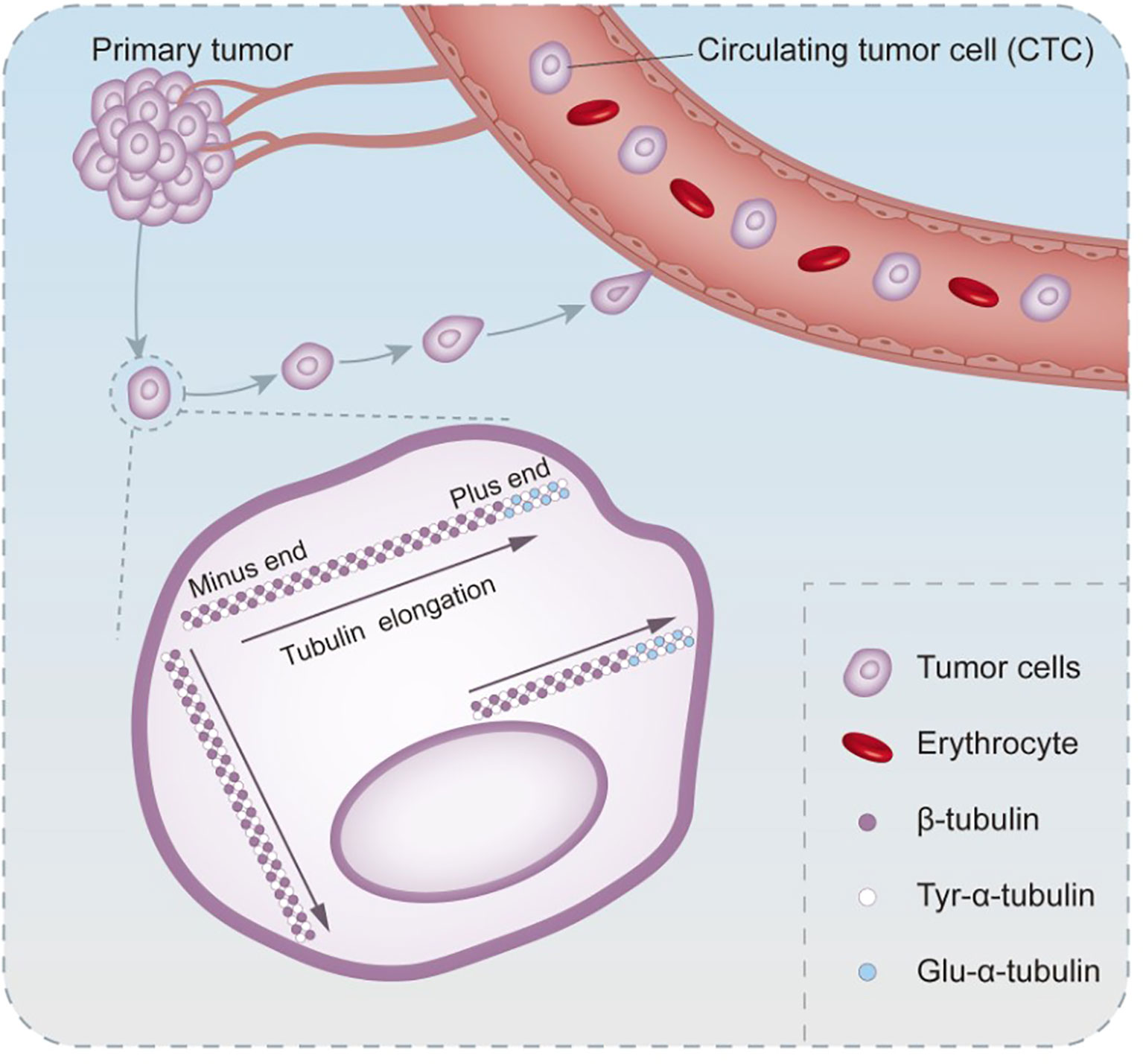
Figure 1 The process of altered microtubule stability in circulating tumor cells undergoing EMT. Post-translational modification of the microtubule constituent protein α-microtubulin occurs when the tyrosine at the carboxyl terminus is replaced by a glutamate residue. Glutamate microtubules are more stable than tyrosine microtubules, and glutamate microtubules extend in the same direction as CTCs migrate.
CTC Assay for Treatment Management of Pancreatic Carcinoma
It has become a consensus that malignancy is a systemic disease. The therapy of surgically resectable pancreatic cancer is always a combination of local excision and subsequent systemic treatment, and it is therefore helpful in the context of surgical treatment to divide our tumors into localized lesions and systemic lesions. Achieving R0 resection of localized lesions through pancreatic surgery is key to the treatment of localized lesions. For antitumor battles occurring systemically, the detection and characterization of CTCs obtained in the blood can help inform decisions about treatment. For example, patients with a low preoperative CTCs load benefit less from neoadjuvant therapy than those with a high load, and may be better served by surgical treatment alone.
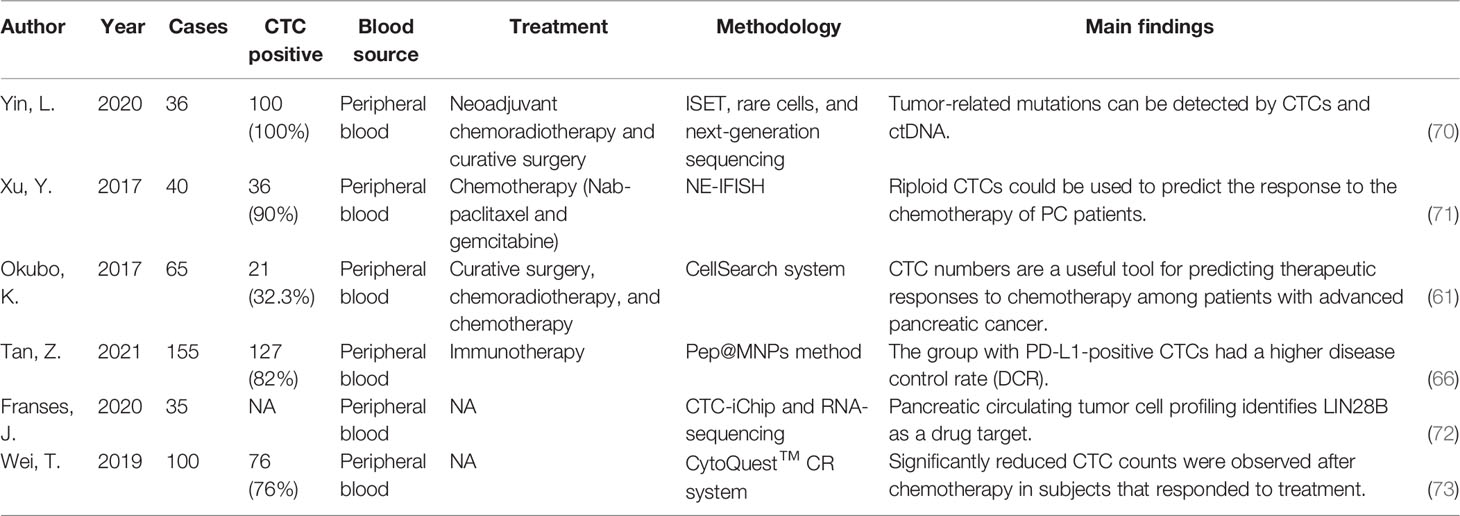
A great advantage of CTCs identification in the treatment process is that it can be dynamically sampled multiple times during the clinical process, so as to distinguish different CTCs subgroups and find specific molecules that drive tumor metastasis and promote chemotherapy resistance. In a study of somatic mutations in complete pathological remission after neoadjuvant therapy (56), the researchers tried to use the genomic analysis of the excised samples combined with liquid biopsy data to reveal the molecular characteristics of the complete pathological remission samples of patients with excised PDAC. Their results showed that after complete pathological remission, even if there are no remaining cancer cells, there are still somatic mutations in the body. This mutation can be monitored after surgery with CTCs and ctDNA. In the tumor self-seeding theory, CTCs colonize the tumor site where it originated through the self-seeding process (57). In animal models, the self-seeding of multiple tumors has been confirmed to be mediated by aggressive CTCs (58). The research of Comen et al. showed that, on the whole, “self-seeding” will change the tumor microenvironment to make it more supportive of tumor growth, including accelerating tumor growth, inducing angiogenesis, and activating matrix recruitment (59). If this microenvironmental change is intervened during the treatment process, theoretically, it should lead to a decrease in CTCs returning to the original site of the tumor. In fact, in locally advanced pancreatic cancer, randomized clinical trials have shown that, compared with chemotherapy alone, adding local radiotherapy to chemotherapy can improve the survival rate of patients (60). Okubo et al. evaluated the change in the number of CTCs before and after treatment in 40 patients with advanced unresectable pancreatic carcinoma. The rate of positive CTCs was significantly higher in patients with progressive disease (45.4%) than with progressive disease or partial response (24.1%), suggesting that the CTCs changes were associated with the tumor response to treatment (61). Bobek et al. (62). proposed a method to isolate CTCs based on cell size and included 24 patients with PDAC. They detected positive CTCs in 66.7% of the patients, and the positive rate of CTCs in patients with T3 PDAC could reach 80%; CTCs can be detected in 77% of patients who have lost the chance of surgery, which provides a hint that patients can be pre-screened before surgery to assess operability. This suggests that the development of new CTCs isolation methods may contribute to more sensitive isolation of CTCs from patient blood. CTCs act as a “bridge” from the primary tumor to the metastasis, and help to find the genotype or phenotype difference between the primary tumor and the metastasis. This difference may be caused by the clonal proliferation process of the primary tumor heterogeneity in metastases and subpopulations in the proliferation of genes or phenotype differentiation (63). If this differentiation results in a difference in sensitivity to chemotherapy drugs, alternative treatment options need to be considered. The current decision-making on the treatment of metastatic tumors is based on the analysis of the primary tumor. In order to reduce the invasive examination of metastatic lesions, “liquid biopsy” of CTCs, which are representative of metastatic lesions, may be a good choice for “experimental biopsy”. If mutations can be found in CTCs using deep sequencing techniques, resistance to therapy can be assessed in real time, which has already been attempted in colorectal and prostate cancers (64, 65). CTCs-assisted personalized therapy has been used in breast and lung cancers. However, susceptible targets have not been successfully identified in pancreatic cancer. We hope that once an effective drug for pancreatic cancer is discovered, based on the information provided by CTCs obtained before and after treatment (such as a patient’s susceptibility genes), it is possible to subclassify patients and select the best treatment for individual cases, such as gene therapy and immunotherapy. In a retrospective study in 2021, Tan et al. studied 155 patients with advanced malignancies receiving anti-PD-1 immunotherapy, including six cases of pancreatic cancer, who were tested for PD1/PDL1 by CTCs obtained from peripheral blood. The results showed that the disease control rate was higher in the PDL1-positive CTCs group (71.56%), and in the remaining patients, the disease control rate was only 39.29%. The reduction in counts and rates of PD-L1-positive CTCs and PD-L1-high CTCs reflected a favorable response to PD-1/PD-L1 inhibitors (66). Haber et al. found T790M resistance mutations in patients with clinical tumor progression who received tyrosine kinase inhibitor treatment by analyzing CTCs (67). In vitro models using CTCs help to identify potential therapeutic targets for tumors. For instance, Dimitrov-Markov et al. established a xenograft mouse model using CTCs derived from patients with highly metastatic PDAC. They sequenced RNA from CTCs isolated from patients and identified survivin, a potential therapeutic target for pancreatic cancer. They actually used the CTCs to create a reproducible system to mimic the metastatic process of cancer cells (68). In the future, it is expected that the changes in genotype and phenotype will be continuously monitored during the treatment of pancreatic cancer to ensure timely detection of the emergence of drug resistance, so as to guide changes in treatment strategies. Min Yu et al. used a microfluidic device to efficiently isolate CTCs from endogenous genetically engineered mouse pancreatic cancer models, and through single-molecule RNA sequencing, they found that CTCs are rich in Wnt2 genes (69). The expression of Wnt2 gene in pancreatic cancer cells inhibits cell anoikis, and the non-canonical WNT signaling pathway may contribute to the metastasis of pancreatic cancer. In pancreatic cancer patients, 5 out of 11 pancreatic CTCs (45%) showed abundant WNT signals. The effectiveness of TAK1 in inhibiting this effect establishes a new potential drug target for inhibiting metastasis. Therefore, molecular analysis of CTCs may identify candidate therapeutic targets for preventing the distant spread of cancer. We present some related studies in Table 2. In conclusion, the use of CTCs in the clinical management of pancreatic cancer is expected to offer an alternative to “real-time biopsies,” allowing assessment of tumor biological activity. Therefore, detection and characterization of CTCs in pancreatic cancer may be used in preoperative differential diagnosis, prediction of risk of recurrence after surgery, examination of pharmacodynamic biomarkers, detection of treatment resistance profiles, assistance in the designation of treatment strategies, assessment of response to treatment, and assessment of prognostically important. This role is significant, especially in distinguishing pancreatic cancer from neuroendocrine tumors preoperatively and when primary and metastatic tumors are too small to be detected on CT scans.
The Role of CTCs in Prognosis
The basis for the use of CTCs for early detection of pancreatic tumors is hematogenous dissemination of pancreatic cancer is an early event. As demonstrated in an interesting mouse model of the pancreas, the researchers found that pancreatic cells with characteristics of CTCs circulate in the blood and reach and seed the liver before the primary tumor is found in the pancreas (74). The characterization of CTCs in pancreatic carcinoma not only is an important part of the diagnostic process but also can reveal the risk of tumor recurrence. In addition, mainly due to the subjectivity of tumor plasticity and evaluation criteria, traditional tumor prognostic factors such as tumor stage, pathological grade, lymph node metastasis, vascular and nerve invasion, and tumor resection margin status can better predict the prognosis of patients with pancreatic cancer. However, it can only be evaluated after surgery. CA19-9 can predict the prognosis of pancreatic cancer, but it is susceptible to biliary obstruction, infection, and the patient’s age and gender (75, 76). Conventional prognostic indicators for predicting the prognosis of patients are usually not impeccable. Therefore, there is an urgent need to establish a new and sensitive prognostic method to identify patients with poor prognosis or patients with rapid progress.
Several studies have identified CTCs as a potential minimally invasive mechanism that can be used to analyze patients with primary carcinoma and their risk of subsequent metastasis (77–79). In several different kinds of tumors, CTCs have been identified as a new prognostic biomarker, including lung cancer, locally advanced breast cancer, and liver cancer (80–82). In a large prospective study (n = 472 patients), Van Dalum et al. reported that the detection rate is similar before and after surgery (24% to 20%); only the detection of CTCs before surgery is a prognostic factor, while the detection of CTCs after surgery is not associated with a poor prognosis (83). After adjuvant chemotherapy is completed for high-risk colorectal cancer patients, patients with high CTCs have a higher recurrence rate (83, 84). The detection technology used in most studies is FDA-approved CellSearch® technology. A phase 2 study (n = 80) in patients with non-metastatic gastric cancer showed that using a technique that claims to have an abnormally high detection rate of CTCs (>90% of patients), CTCs reduction during neoadjuvant chemotherapy is an independent survival prognostic factor (85).
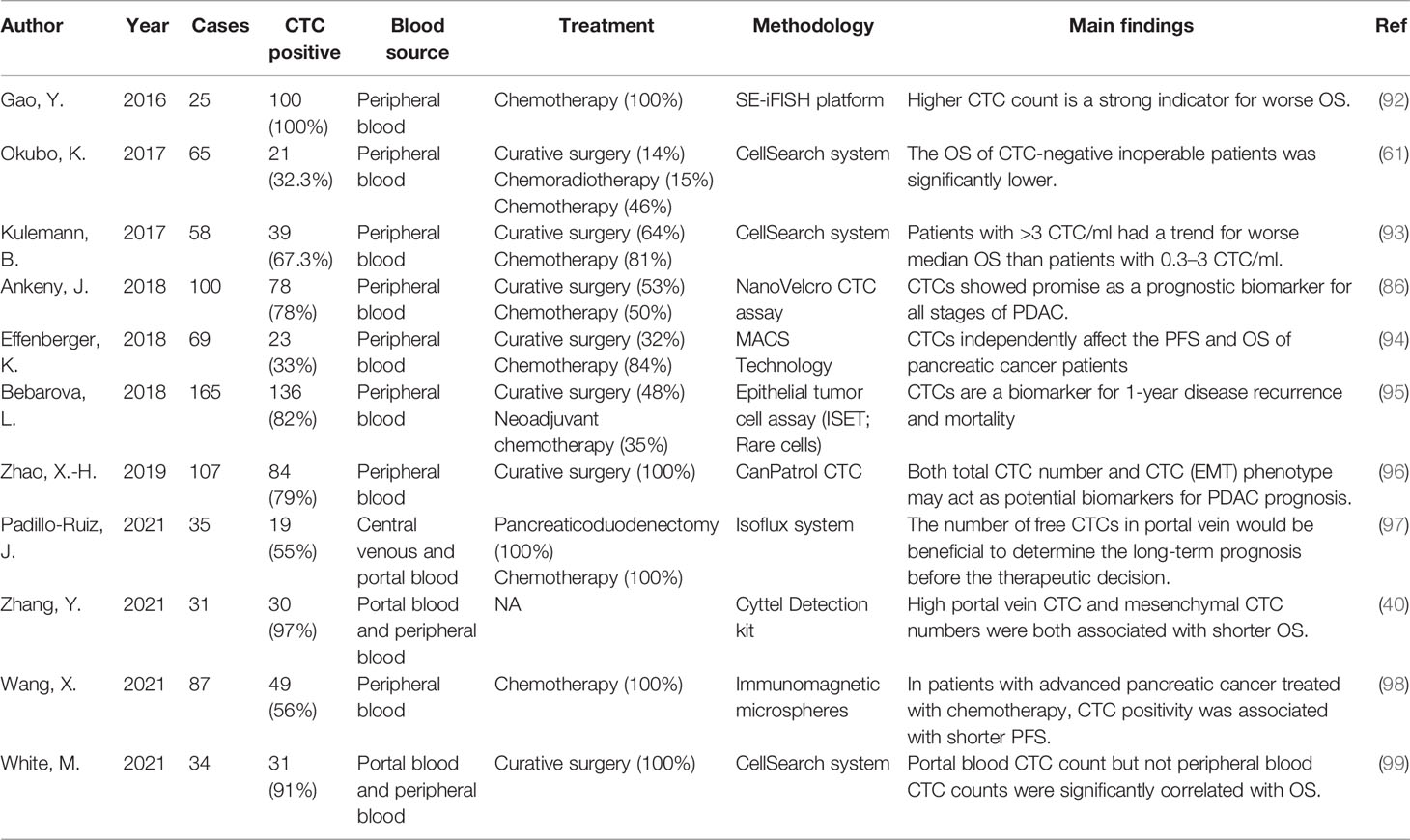
Research on the prognostic role of CTCs in pancreatic cancer is mainly focused on PDAC (86). CTCs have been identified in the blood of patients at all stages of PDAC. Previous studies have shown that there is an association between the presence of CTCs and a poor prognosis (87–91). We list several studies on the prognostic relevance of CTCs and PDAC in the last 5 years (Table 3). In the report of Okubo et al., they proved that CTCs are associated with liver metastasis in patients with pancreatic cancer (p = 0.0002). They also proved that the presence or absence of CTCs detected by intraoperative molecular RT-PCR detection is associated with a high risk of blood-borne metastasis in patients with pancreatic cancer. Their conclusions indicate that the detection of CTCs can compensate for the insufficient sensitivity and specificity of enhanced CT in detecting disease recurrence (61). However, other studies have shown that the correlation between CTCs and the prognosis of patients with pancreatic cancer is very poor (88, 100). In view of the conflict results of previous studies, Lu Han et al. conducted a meta-analysis of all eligible studies to clarify the prognostic role of CTCs in patients with pancreatic cancer. They included nine cohorts of 268 CTCs-positive patients, and CTCs were significantly associated with poorer PFS (p < 0.001) and OS (p < 0.001) in patients. Similar results were obtained for subgroup analyses by ethnicity, test method, and treatment method (101). A meta-analysis by Pang et al. included more than 50 patients in nine studies, six of which found a significant relationship between CTCs and OS or PFS (102). A meta-analysis in 2019 included the largest number of studies and patients, including 19 studies with 1,320 confirmed individuals. Their results also support the idea that CTCs-positive patients have worse OS and PFS than CTCs-negative patients, while they suggest that CTCs may serve as a predictive biomarker for pancreatic cancer patients prior to treatment (103). Although there is no consensus on the best detection method and CTCs cutoff value for predicting the clinical outcome of pancreatic cancer, their analysis indicated that CTCs-positive pancreatic cancer patients may have more severe PFS and OS than CTCs-negative patients and the detection of CTCs in peripheral blood may be a promising biomarker for pancreatic cancer detection and prognosis. The differences in the results of previous studies may be due to the small sample size of patients in these studies and the differences in testing and treatment methods.
However, the prognostic role of CTCs in pancreatic cancer is not without its limitations. In a previous study, in 2,183 blood samples from 964 metastatic carcinoma patients, 36% (781 of 2,183) of the specimens had only 2 CTCs (104). The rarity of carbon tetrachloride in the peripheral blood of patients with non-metastatic cancer greatly limits its use as a predictor of metastasis. The reason may be that the diameter of CTCs is about 25 mm, which is too large for them to pass through capillaries (about 8 mm in diameter) (104). Studies in animal models have shown that most of the radiolabeled tumor cells injected into blood vessels are trapped in the capillary bed of the first organ that cancer cells reach (similar to the liver’s first-pass elimination effect), which is difficult to detect in peripheral blood (105). However, this problem is not insoluble. Rahbari et al. studied the difference in the interval distribution of CTCs in patients with colorectal cancer, and the results show that the detection rate of CTCs is higher in tumor drainage (mesenteric) vessels compared with peripheral venous blood. Theoretically, the concentration of CTCs detected in the portal blood of patients with pancreatic cancer is higher than that of peripheral blood. Therefore, in patients with pancreatic cancer, the count of CTCs in portal blood is expected to be an early indicator of liver micrometastasis. In a cohort study(n = 29) (106), the researchers used ultrasound-guided transhepatic puncture to analyze the CTCs in the portal blood of patients with advanced pancreatic cancer. CTCs were detected in 29 cases of portal vein blood, and the absolute number of pancreatic cancer cells circulating in the portal vein was significantly higher than that in peripheral blood. The study found that the portal vein CTCs count is highly correlated with intrahepatic metastasis. The OS of patients with portal vein CTCs exceeding 150/7.5 ml is significantly shortened, suggesting that patients with advanced pancreatic cancer have a poor prognosis. The results show that the analysis of portal vein CTCs is a powerful method for the prognosis of patients with advanced pancreatic cancer. It is worth noting that due to the sensitivity difference between different technologies, the CTCs cutoff shown by one CTCs detection technique is a prognostic factor, and another technique cannot be inferred. The timing of CTCs detection is also critical. Positive CTCs before, during, or after surgery may have different biological and clinical significance. In many studies, portal blood was obtained from patients undergoing radical pancreatic surgery for CTCs, and it was clear that the detection rate was higher in portal blood than in peripheral veins; however, it is difficult to determine whether this difference is related to the compression of the cancerous tissue caused by the surgery or to the elimination effect of the liver. If future studies can obtain portal blood, central venous blood, and peripheral blood simultaneously and compare the levels of CTCs in the three types of blood, and correlate them with tumor recurrence and time of liver metastasis, this mystery will be revealed.
The ultimate fate of CTCs depends on their resistance to denervation, immune killing, and apoptosis. Some studies have explored the relationship between the apoptosis level of CTCs and the prognosis of breast cancer. They found that the higher the expression level of the apoptotic factor BCL-2 in CTCs, the better the prognosis of patients. This may be the result of a multifactorial effect, in addition to the possible activation of immune killing by more CTCs and the association of less deformable CTCs with vascular anatomy, and tumor metastasis should also be considered (107).
Summary
In the era of multidisciplinary collaborative diagnosis and treatment, pancreatic cancer is still one of the most malignant tumors. As a promising direction of tumor research, CTCs have actively explored the treatment of pancreatic cancer and its prognosis. With the continuous development of various CTCs enrichment and detection equipment, it can be speculated that there will be more surprising discoveries in the future to challenge the existing practice of diagnosis and treatment of pancreatic malignancies. As new assay technologies continue to emerge and the heterogeneity of CTCs becomes better understood, we need in-depth genetic and gene expression pattern studies of different types of CTCs, and we may need CTCs assays that are specific to different types of tumors, rather than one assay technology that covers all cancer types. In fact, a tumor cell-specific assay should precisely match the characteristics of that tumor, in line with the concept of precision medicine, and one of the disadvantages of CA199 compared to CTCs is that it cannot be specific to each tumor. In the future, we believe that “whole process monitoring” of CTCs will provide more information for decision-making in the treatment of tumors, such as preoperative differential diagnosis, prognostic assessment, real-time monitoring of drug resistance mutations during treatment, assessment of treatment response, and timely detection of micrometastases. All of these aspects are of most interest to clinicians battling pancreatic cancer. Although we have repeatedly discussed the increased risk of metastasis and spread of pancreatic cancer with invasive testing compared to “liquid biopsy”, more prospective studies are needed to prove this point in the future.
Similarly, do diagnostic and therapeutic interventions (e.g., surgery and chemotherapy), and access to CTCs in portal blood or even in pancreatic ducts, result in more CTCs being released into the bloodstream and, if so, does this release alter the prognosis of pancreatic cancer? It is conceivable, given the right conditions, whether test specimens obtained from different routes would alter the results; which test specimens obtained from different routes would be most appropriate for surgical and non-surgical patients, respectively; and whether portal blood or even pancreatic fluid would be preferable to central venous blood and peripheral blood when considering convenience and economic cost. New and surprising findings may challenge our current paradigm and medical practice. Admittedly, with only one assay, the CellSearch system, now in the transitional phase of FDA approval to clinical application, new assays must be validated in clinical trials to prove their efficacy. In any case, it is always helpful to explore all aspects of the utility of CTCs as one of the few new markers now in development. It is hoped that this review will provide some insight into the clinical application of CTCs and the clinical validation of CTCs as new biomarkers.
Author Contributions
KL did the bibliographic search. KL, XW, and XZ wrote the initial manuscript. ZL, SH, and RL critically revised the manuscript and actively contributed to it. All authors contributed to the article and approved the submitted version.
Funding
RL is supported by the 2022 Henan Province Key R&D and Promotion Special Support Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vincent A, Herman J, Schulick R, Hruban R H, Goggins M. Pancreatic Cancer. Lancet (2011) 378:607–20. doi: 10.1016/S0140-6736(10)62307-0
2. Ferlay J, Partensky C, Bray F. More Deaths From Pancreatic Cancer Than Breast Cancer in the EU by 2017. Acta Oncol (Stockholm Sweden) (2016) 55:1158–60. doi: 10.1080/0284186X.2016.1197419
3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
4. Siegel R, Miller K, Fuchs H, Jemal A. Cancer Statistics. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
5. Feng RM, Zong YN, Cao SM, Xu RH. Current Cancer Situation in China: Good or Bad News From the 2018 Global Cancer Statistics? Cancer Commun (2019;) 39:1–12. doi: 10.1186/s40880-019-0368-6
6. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. Jama (2019;) 322:438–44. doi: 10.1001/jama.2019.10232
7. Tien YW, Kuo H-C, Ho B-I, Chang M-C, Chang Y-T, Cheng M-F, et al. A High Circulating Tumor Cell Count in Portal Vein Predicts Liver Metastasis From Periampullary or Pancreatic Cancer: A High Portal Venous CTC Count Predicts Liver Metastases. Medicine (2016) 95(16):e3407. doi: 10.1097/MD.0000000000003407
8. Ariyama J, Suyama M, Satoh K, Sai J. Imaging of Small Pancreatic Ductal Adenocarcinoma. Pancreas (1998) 16:396–401. doi: 10.1097/00006676-199804000-00030
9. Furukawa H, Okada S, Saisho H, Ariyama J, Karasawa E, Nakaizumi A, et al. Clinicopathologic Features of Small Pancreatic Adenocarconoma: A Collective Study. Cancer: Interdiscip Int J Am Cancer Soc (1996) 78:986–90. doi: 10.1002/(SICI)1097-0142(19960901)78:5<986::AID-CNCR7>3.0.CO;2-A
10. Ishikawa O, Ohigashi H, Imaoka S, Nakaizumi A, Uehara H, Kitamura T, et al. Minute Carcinoma of the Pancreas Measuring 1 Cm or Less in Diameter–Collective Review of Japanese Case Reports. Hepato-gastroenterology (1999) 46:8–15. doi: 10.1016/S0016-5107(99)70472-4
11. D’Onofrio M, Crosara S, Signorini M, De Robertis R, Canestrini S, Principe F, et al. Comparison Between CT and CEUS in the Diagnosis of Pancreatic Adenocarcinoma. Ultraschall der Medizin-European J Ultrasound (2013) 34:377–81. doi: 10.1055/s-0032-1325324
12. Sugiyama M, Hagi H, Atomi Y, Saito M. Diagnosis of Portal Venous Invasion by Pancreatobiliary Carcinoma: Value of Endoscopic Ultrasonography. Abdominal Imaging (1997) 22:434–8. doi: 10.1007/s002619900227
13. Legmann P, Vignaux O, Dousset B, Baraza A-J, Palazzo L, Dumontier I, et al. Pancreatic Tumors: Comparison of Dual-Phase Helical CT and Endoscopic Sonography AJR. Am J roentgenology (1998) 170:1315–22. doi: 10.2214/ajr.170.5.9574609
14. Shoup M, Hodul P, Aranha GV, Choe D, Olson M, Leya J, et al. Defining a Role for Endoscopic Ultrasound in Staging Periampullary Tumors. Am J Surg (2000) 179:453–6. doi: 10.1016/S0002-9610(00)00379-2
15. Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, et al. Detection of Small Pancreatic Tumors With Multiphasic Helical CT. Am J Roentgenology (2004) 182:619–23. doi: 10.2214/ajr.182.3.1820619
16. Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, et al. Dynamic Imaging of Pancreatic Diseases by Contrast Enhanced Coded Phase Inversion Harmonic Ultrasonography. Gut (2004) 53:854–9. doi: 10.1136/gut.2003.029934
17. Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of Contrast-Enhanced Endoscopic Ultrasonography for Diagnosis of Small Pancreatic Carcinomas. Ultrasound Med Biol (2008) 34:525–32. doi: 10.1016/j.ultrasmedbio.2007.09.018
18. Riker A, Libutti SK, Bartlett DL. Advances in the Early Detection, Diagnosis, and Staging of Pancreatic Cancer. Surg Oncol (1997) 6:157–69. doi: 10.1016/S0960-7404(97)00025-X
19. Kikuyama M, Kamisawa T, Kuruma S, Chiba K, Kawaguchi S, Terada S, et al. Early Diagnosis to Improve the Poor Prognosis of Pancreatic Cancer. Cancers (2018) 10:48. doi: 10.3390/cancers10020048
20. Grenacher L, Klauss M, Dukic L, Delorme S, Knaebel H, Düx M, et al. Diagnosis and Staging of Pancreatic Carcinoma: MRI Versus Multislice-CT–a Prospective Study. RoFo: Fortschr auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin (2004) 176:1624–33. doi: 10.1055/s-2004-813642
21. Harrington KA, Shukla-Dave A, Paudyal R, Do RK. MRI of the Pancreas. J Magnetic Resonance Imaging (2021) 53:347–59. doi: 10.1002/jmri.27148
22. Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-Fluorodeoxyglucose Positron Emission Tomography in Differentiating Malignant From Benign Pancreatic Cysts: A Prospective Study. J gastrointestinal Surg (2005) 9:22–9. doi: 10.1016/j.gassur.2004.10.002
23. Seo S, Machimoto T, Kami K, Masui T, Hatano E, Ogawa K, et al. Contribution of 18F-Fluorodeoxyglucose Positron Emission Tomography to the Diagnosis of Early Pancreatic Carcinoma. J Hepato-Biliary-Pancreatic Surg (2008) 15:634–9. doi: 10.1007/s00534-007-1339-x
24. Okano K, Kakinoki K, Akamoto S, Hagiike M, Usuki H, Yamamoto Y, et al. 18F-Fluorodeoxyglucose Positron Emission Tomography in the Diagnosis of Small Pancreatic Cancer. World J gastroenterology: WJG (2011) 17:231. doi: 10.3748/wjg.v17.i2.231
25. Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, et al. Evaluation of Delayed Additional FDG PET Imaging in Patients With Pancreatic Tumour. Nucl Med Commun (2005) 26:895–901. doi: 10.1097/00006231-200510000-00008
26. Steinberg W. The Clinical Utility of the CA 19-9 Tumor-Associated Antigen. Am J Gastroenterol (Springer Nature) (1990) 85(4):350.
27. Duffy M, Sturgeon C, Lamerz R, Haglund C, Holubec V, Klapdor R, et al. Tumor Markers in Pancreatic Cancer: A European Group on Tumor Markers (EGTM) Status Report. Ann Oncol (2010) 21:441–7. doi: 10.1093/annonc/mdp332
28. Goggins M. Molecular Markers of Early Pancreatic Cancer. J Clin Oncol (2005) 23:4524–31. doi: 10.1200/JCO.2005.19.711
29. Ariyama J. Detection and Prognosis of Small Pancreatic Ductal Adenocarcinoma. Nihon Geka Gakkai Zasshi (1997) 98:592–6. doi: 10.1097/00006676-200105000-00015
30. Robinson K, McGrath R, McGrew E. Circulating Cancer Cells in Patients With Lung Tumors. Surgery (1963) 53:630–6.
31. Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic Spread is an Early Step in Breast Cancer. Cancer Cell (2008) 13:58–68. doi: 10.1016/j.ccr.2007.12.003
32. Pantel K, Brakenhoff RH. Dissecting the Metastatic Cascade. Nat Rev Cancer (2004) 4:448–56. doi: 10.1038/nrc1370
33. Thiery JP. Epithelial–mesenchymal Transitions in Tumour Progression. Nat Rev Cancer (2002) 2:442–54. doi: 10.1038/nrc822
34. Bidard F-C, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. Clinical Validity of Circulating Tumour Cells in Patients With Metastatic Breast Cancer: A Pooled Analysis of Individual Patient Data. Lancet Oncol (2014) 15:406–14. doi: 10.1016/S1470-2045(14)70069-5
35. Huang X, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. Meta-Analysis of the Prognostic Value of Circulating Tumor Cells Detected With the CellSearch System in Colorectal Cancer. BMC Cancer (2015) 15:202. doi: 10.1186/s12885-015-1218-9
36. Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia X, et al. Circulating Tumor Cell Biomarker Panel as an Individual-Level Surrogate for Survival in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol (2015) 33:1348. doi: 10.1200/JCO.2014.55.3487
37. Krebs MG. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients with Non-Small-Cell Lung Cancer. J Clin Oncol (2011) 29:1556. doi: 10.1200/JCO.2010.28.7045
38. Hou J-M, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients With Small-Cell Lung Cancer. J Clin Oncol (2012) 30:525–32. doi: 10.1200/JCO.2010.33.3716
39. Chen J, Wang H, Zhou L, Liu Z, Tan X. A Combination of Circulating Tumor Cells and CA199 Improves the Diagnosis of Pancreatic Cancer. J Clin Lab Anal (2022) 36:e24341. doi: 10.1002/jcla.24341
40. Zhang Y, Su H, Wang H, Xu C, Zhou S, Zhao J, et al. Endoscopic Ultrasound-Guided Acquisition of Portal Venous Circulating Tumor Cells as a Potential Diagnostic and Prognostic Tool for Pancreatic Cancer. Cancer Manage Res (2021) 13:7649. doi: 10.2147/CMAR.S330473
41. Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga J-Y, et al. Circulating Tumor Cells: Clinical Validity and Utility. Int J Clin Oncol (2017) 22:421–30. doi: 10.1007/s10147-017-1105-2
42. Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-Analysis of the Prognostic Value of Circulating Tumor Cells in Breast Cancer. Clin Cancer Res (2012) 18:5701–10. doi: 10.1158/1078-0432.CCR-12-1587
43. Pantel K, Alix-Panabières C. Real-Time Liquid Biopsy in Cancer Patients: Fact or Fiction? Cancer Res (2013) 73:6384–8. doi: 10.1158/0008-5472.CAN-13-2030
44. Kalluri R, Weinberg RA. The Basics of Epithelial-Mesenchymal Transition. J Clin Invest (2009) 119:1420–8. doi: 10.1172/JCI39104
45. Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, et al. Ras and Tgfβ Cooperatively Regulate Epithelial Cell Plasticity and Metastasis: Dissection of Ras Signaling Pathways. J Cell Biol (2002) 156:299–314. doi: 10.1083/jcb.200109037
46. Yang J, Weinberg RA. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev Cell (2008) 14:818–29. doi: 10.1016/j.devcel.2008.05.009
47. Ingber DE, Tensegrity I. Cell Structure and Hierarchical Systems Biology. J Cell Sci (2003) 116:1157–73. doi: 10.1242/jcs.00359
48. Korb T, Schlüter K, Enns A, Spiegel H-U, Senninger N, Nicolson GL, et al. Integrity of Actin Fibers and Microtubules Influences Metastatic Tumor Cell Adhesion. Exp Cell Res (2004) 299:236–47. doi: 10.1016/j.yexcr.2004.06.001
49. Kreitzer G, Liao G, Gundersen GG. Detyrosination of Tubulin Regulates the Interaction of Intermediate Filaments With Microtubules In Vivo via a Kinesin-Dependent Mechanism. Mol Biol Cell (1999) 10:1105–18. doi: 10.1091/mbc.10.4.1105
50. Mialhe A, Lafanechèere L, Treilleux I, Peloux N, Dumontet C, Brémond A, et al. Tubulin Detyrosination is a Frequent Occurrence in Breast Cancers of Poor Prognosis. Cancer Res (2001) 61:5024–7. doi: 10.1046/j.1523-5394.2001.94010.x
51. Gundersen GG, Bulinski JC. Selective Stabilization of Microtubules Oriented Toward the Direction of Cell Migration. Proc Natl Acad Sci (1988) 85:5946–50. doi: 10.1073/pnas.85.16.5946
52. Schaap IA, Hoffmann B, Carrasco C, Merkel R, Schmidt CF. Tau Protein Binding Forms a 1 Nm Thick Layer Along Protofilaments Without Affecting the Radial Elasticity of Microtubules. J Struct Biol (2007) 158:282–92. doi: 10.1016/j.jsb.2006.11.010
53. Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, et al. Metastatic Breast Tumors Express Increased Tau, Which Promotes Microtentacle Formation and the Reattachment of Detached Breast Tumor Cells. Oncogene (2010) 29:3217–27. doi: 10.1038/onc.2010.68
54. Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin Filaments Support Extension of Tubulin-Based Microtentacles in Detached Breast Tumor Cells. Cancer Res (2008) 68:5678–88. doi: 10.1158/0008-5472.CAN-07-6589
55. Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, et al. Intravascular Metastatic Cancer Cell Homotypic Aggregation at the Sites of Primary Attachment to the Endothelium. Cancer Res (2003) 63:3805–11. doi: 10.1016/j.urolonc.2003.12.009
56. Yin L, Pu N, Thompson ED, Miao Y, Wolfgang CL, Yu J. Improved Assessment of Response Status in Patients With Pancreatic Cancer Treated With Neoadjuvant Therapy Using Somatic Mutations and Liquid Biopsy Analysis. Clin Cancer Res (2020) 27(3):740–8. doi: 10.1158/1078-0432.CCR-20-1746
57. Norton L, Massagué J. Is Cancer a Disease of Self-Seeding? Nat Med (2006) 12:875–8. doi: 10.1038/nm0806-875
58. Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L, et al. Tumor Self-Seeding by Circulating Cancer Cells. Cell (2009) 139:1315–26. doi: 10.1016/j.cell.2009.11.025
59. Comen E, Norton L, Massague J. Clinical Implications of Cancer Self-Seeding. Nat Rev Clin Oncol (2011) 8:369–77. doi: 10.1038/nrclinonc.2011.64
60. . Loehrer >SrPJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine Alone Versus Gemcitabine Plus Radiotherapy in Patients With Locally Advanced Pancreatic Cancer: An Eastern Cooperative Oncology Group Trial. J Clin Oncol (2011) 29:4105. doi: 10.1200/JCO.2011.34.8904
61. Okubo K, Uenosono Y, Arigami T, Mataki Y, Matsushita D, Yanagita S, et al. Clinical Impact of Circulating Tumor Cells and Therapy Response in Pancreatic Cancer. Eur J Surg Oncol (EJSO) (2017) 43:1050–5. doi: 10.1016/j.ejso.2017.01.241
62. Bobek V, Gurlich R, Eliasova P, Kolostova K. Circulating Tumor Cells in Pancreatic Cancer Patients: Enrichment and Cultivation. World J gastroenterology: WJG (2014) 20:17163. doi: 10.3748/wjg.v20.i45.17163
63. Cen P, Ni X, Yang J, Graham DY, Li M. Circulating Tumor Cells in the Diagnosis and Management of Pancreatic Cancer. Biochim Biophys Acta (BBA)-Reviews Cancer (2012) 1826:350–6. doi: 10.1016/j.bbcan.2012.05.007
64. Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, et al. Complex Tumor Genomes Inferred From Single Circulating Tumor Cells by Array-CGH and Next-Generation Sequencing. Cancer Res (2013) 73:2965–75. doi: 10.1158/0008-5472.CAN-12-4140
65. Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, Cruz-Gordillo P, et al. Whole-Exome Sequencing of Circulating Tumor Cells Provides a Window Into Metastatic Prostate Cancer. Nat Biotechnol (2014) 32:479–84. doi: 10.1038/nbt.2892
66. Tan Z, Yue C, Ji S, Zhao C, Jia R, Zhang Y, et al. Assessment of PD-L1 Expression on Circulating Tumor Cells for Predicting Clinical Outcomes in Patients With Cancer Receiving PD-1/PD-L1 Blockade Therapies. Oncologist (2021) 26:e2227–38. doi: 10.1002/onco.13981
67. Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of Mutations in EGFR in Circulating Lung-Cancer Cells. New Engl J Med (2008) 359:366–77. doi: 10.1056/NEJMoa0800668
68. Dimitrov-Markov S, Perales-Patón J, Bockorny B, Dopazo A, Muñoz M, Baños N, et al. Discovery of New Targets to Control Metastasis in Pancreatic Cancer by Single-Cell Transcriptomics Analysis of Circulating Tumor Cells. Mol Cancer Ther (2020) 19:1751–60. doi: 10.1158/1535-7163.MCT-19-1166
69. Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, et al. RNA Sequencing of Pancreatic Circulating Tumour Cells Implicates WNT Signalling in Metastasis. Nature (2012) 487:510–3. doi: 10.1038/nature11217
70. Yin L, Pu N, Thompson E, Miao Y, Wolfgang C, Yu J. Improved Assessment of Response Status in Patients With Pancreatic Cancer Treated With Neoadjuvant Therapy Using Somatic Mutations and Liquid Biopsy Analysis. Clin Cancer Res (2021) 27:740–8. doi: 10.1158/1078-0432.CCR-20-1746
71. Xu Y, Qin T, Li J, Wang X, Gao C, Xu C, et al. Detection of Circulating Tumor Cells Using Negative Enrichment Immunofluorescence and an in Situ Hybridization System in Pancreatic Cancer. . Int J Mol Sci (2017) 18:622. doi: 10.3390/ijms18040622
72. Franses JW, Philipp J, Missios P, Bhan I, Liu A, Yashaswini C, et al. Pancreatic Circulating Tumor Cell Profiling Identifies LIN28B as a Metastasis Driver and Drug Target. Nat Commun (2020) 11:1–12. doi: 10.1038/s41467-020-17150-3
73. Wei T, Zhang X, Zhang Q, Yang J, Chen Q, Wang J, et al. Vimentin-Positive Circulating Tumor Cells as a Biomarker for Diagnosis and Treatment Monitoring in Patients With Pancreatic Cancer. Cancer Lett (2019) 452:237–43. doi: 10.1016/j.canlet.2019.03.009
74. Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell (2012) 148:349–61. doi: 10.1016/j.cell.2011.11.025
75. Kondo N, Murakami Y, Uemura K, Nakagawa N, Takahashi S, Ohge H, et al. Comparison of the Prognostic Impact of Pre-and Post-Operative CA19-9, SPan-1, and DUPAN-II Levels in Patients With Pancreatic Carcinoma. Pancreatology (2017) 17:95–102. doi: 10.1016/j.pan.2016.10.004
76. Junior PLU, Callegaro-Filho D, Bugano DD, Moura F, Maluf FC. Predictive Value of Serum Carbohydrate Antigen 19-9 (CA19-9) for Early Mortality in Advanced Pancreatic Cancer. J gastrointestinal Cancer (2018) 49:481–6. doi: 10.1007/s12029-017-0007-x
77. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. New Engl J Med (2004) 351:781–91. doi: 10.1056/NEJMoa040766
78. Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology. Nature (2007) 450:1235–9. doi: 10.1038/nature06385
79. Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, et al. Circulating Tumor Cell Analysis in Patients With Progressive Castration-Resistant Prostate Cancer. Clin Cancer Res (2007) 13:2023–9. doi: 10.1158/1078-0432.CCR-06-2701
80. Vona G, Estepa L, Béroud C, Damotte D, Capron F, Nalpas B, et al. Impact of Cytomorphological Detection of Circulating Tumor Cells in Patients With Liver Cancer. Hepatology (2004) 39:792–7. doi: 10.1002/hep.20091
81. Pierga J-Y, Bidard F-C, Mathiot C, Brain E, Delaloge S, Giachetti S, et al. Circulating Tumor Cell Detection Predicts Early Metastatic Relapse After Neoadjuvant Chemotherapy in Large Operable and Locally Advanced Breast Cancer in a Phase II Randomized Trial. Clin Cancer Res (2008) 14:7004–10. doi: 10.1158/1078-0432.CCR-08-0030
82. Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Fléjou JF, et al. Preoperative Circulating Tumor Cell Detection Using the Isolation by Size of Epithelial Tumor Cell Method for Patients With Lung Cancer Is a New Prognostic Biomarker. Clin Cancer Res (2011) 17:827–35. doi: 10.1158/1078-0432.CCR-10-0445
83. Sotelo M, Sastre J, Maestro M, Veganzones S, Vieitez J, Alonso V, et al. Role of Circulating Tumor Cells as Prognostic Marker in Resected Stage III Colorectal Cancer. Ann Oncol (2015) 26:535–41. doi: 10.1093/annonc/mdu568
84. Lu C, Tsai H, Uen Y, Hu H, Chen C, Cheng T, et al. Circulating Tumor Cells as a Surrogate Marker for Determining Clinical Outcome to mFOLFOX Chemotherapy in Patients With Stage III Colon Cancer. Br J Cancer (2013) 108:791–7. doi: 10.1038/bjc.2012.595
85. Ma J, Yao S, Li X-S, Kang H-R, Yao F-F, Du N. Neoadjuvant Therapy of DOF Regimen Plus Bevacizumab can Increase Surgical Resection Ratein Locally Advanced Gastric Cancer: A Randomized, Controlled Study. Medicine (2015) 94(42):e1489. doi: 10.1097/MD.0000000000001489
86. Ankeny JS, Sho S, Winograd P, Hou S, Song M, Wainberg ZA, et al. Circulating Tumor Cells Predict Occult Metastatic Disease and Prognosis in Pancreatic Cancer. Ann Surg Oncol (2018) 25:1000–8. doi: 10.1245/s10434-017-6290-8
87. De Albuquerque A, Kubisch I, Breier G, Stamminger G, Fersis N, Eichler A, et al. Multimarker Gene Analysis of Circulating Tumor Cells in Pancreatic Cancer Patients: A Feasibility Study. Oncology (2012) 82:3–10. doi: 10.1159/000335479
88. Soeth E, Grigoleit U, Moellmann B, Röder C, Schniewind B, Kremer B, et al. Detection of Tumor Cell Dissemination in Pancreatic Ductal Carcinoma Patients by CK 20 RT-PCR Indicates Poor Survival. J Cancer Res Clin Oncol (2005) 131:669–76. doi: 10.1007/s00432-005-0008-1
89. Z’graggen K, Centeno BA, Fernandez-del Castillo C, Jimenez RE, Werner J, Warshaw AL. Biological Implications of Tumor Cells in Blood and Bone Marrow of Pancreatic Cancer Patients. Surgery (2001) 129:537–46. doi: 10.1067/msy.2001.113819
90. Tjensvoll K, Nordgård O, Smaaland R. Circulating Tumor Cells in Pancreatic Cancer Patients: Methods of Detection and Clinical Implications. Int J Cancer (2014) 134:1–8. doi: 10.1002/ijc.28134
91. Kurihara T, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Tsuji S, et al. Detection of Circulating Tumor Cells in Patients With Pancreatic Cancer: A Preliminary Result. J hepato-biliary-pancreatic Surg (2008) 15:189–95. doi: 10.1007/s00534-007-1250-5
92. Gao Y, Zhu Y, Zhang Z, Zhang C, Huang X, Yuan Z. Clinical Significance of Pancreatic Circulating Tumor Cells Using Combined Negative Enrichment and Immunostaining-Fluorescence In Situ Hybridization. J Exp Clin Cancer Res (2016) 35:1–8. doi: 10.1186/s13046-016-0340-0
93. Kulemann B, Rösch S, Seifert S, Timme S, Bronsert P, Seifert G, et al. Pancreatic Cancer: Circulating Tumor Cells and Primary Tumors Show Heterogeneous KRAS Mutations. Sci Rep (2017) 7:1–11. doi: 10.1038/s41598-017-04601-z
94. Effenberger KE, Schroeder C, Hanssen A, Wolter S, Eulenburg C, Tachezy M, et al. Improved Risk Stratification by Circulating Tumor Cell Counts in Pancreatic Cancer. Clin Cancer Res (2018) 24:2844–50. doi: 10.1158/1078-0432.CCR-18-0120
95. Bebarova L, Skalický P, Srovnal J, Prokopová A, Zapletalová J, Hajdúch M, et al. The Effect of Circulating Tumor Cells on the Survival of Patients With Pancreatic Cancer 5-Year Results. Rozhledy v Chirurgii: Mesicnik Ceskoslovenske Chirurgicke Spolecnosti (2018) 97:94–8.
96. Zhao X-H, Wang Z-R, Chen C-L, Di L, Bi Z-F, Li Z-H, et al. Molecular Detection of Epithelial-Mesenchymal Transition Markers in Circulating Tumor Cells From Pancreatic Cancer Patients: Potential Role in Clinical Practice. World J Gastroenterol (2019) 25:138. doi: 10.3748/wjg.v25.i1.138
97. Padillo-Ruiz J, Suarez G, Pereira S, Calero-Castro FJ, Tinoco J, Marin L, et al. Circulating Tumor Cells Enumeration From the Portal Vein for Risk Stratification in Early Pancreatic Cancer Patients. Cancers (2021) 13:6153. doi: 10.3390/cancers13246153
98. Wang X, Hu L, Yang X, Chen F, Xu H, Yu H, et al. Clinical Prognostic Value of Circulating Tumor Cells in the Treatment of Pancreatic Cancer With Gemcitabine Chemotherapy. Exp Ther Med (2021) 22:1–11. doi: 10.3892/etm.2021.10574
99. White MG, Lee A, Vicente D, Hall C, Kim MP, Katz MH, et al. Measurement of Portal Vein Blood Circulating Tumor Cells is Safe and may Correlate With Outcomes in Resected Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol (2021) 28:4615–22. doi: 10.1245/s10434-020-09518-y
100. Uchikura K, Takao S, Nakajo A, Miyazono F, Nakashima S, Tokuda K, et al. Intraoperative Molecular Detection of Circulating Tumor Cells by Reverse Transcription-Polymerase Chain Reaction in Patients With Biliary-Pancreatic Cancer is Associated With Hematogenous Metastasis. Ann Surg Oncol (2002) 9:364–70. doi: 10.1007/BF02573871
101. Han L, Chen W, Zhao Q. Prognostic Value of Circulating Tumor Cells in Patients With Pancreatic Cancer: A Meta-Analysis. Tumor Biol (2014) 35:2473–80. doi: 10.1007/s13277-013-1327-5
102. Pang TC, Po JW, Becker TM, Goldstein D, Pirola RC, Wilson JS, et al. Circulating Tumour Cells in Pancreatic Cancer: A Systematic Review and Meta-Analysis of Clinicopathological Implications. Pancreatology (2021) 21:103–14. doi: 10.1016/j.pan.2020.11.022
103. Wang Y, Yu X, Hartmann D, Zhou J. Circulating Tumor Cells in Peripheral Blood of Pancreatic Cancer Patients and Their Prognostic Role: A Systematic Review and Meta-Analysis. HPB (2020) 22:660–9. doi: 10.1016/j.hpb.2019.11.003
104. Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas But Not in Healthy Subjects or Patients With Nonmalignant Diseases. Clin Cancer Res (2004) 10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378
105. Fidler IJ. Metastasis: Quantitative Analysis of Distribution and Fate of Tumor Emboli Labeled With 125I-5-Iodo-2′-Deoxyuridine. J Natl Cancer Institute (1970) 45:773–82. doi: 10.1093/jnci/45.4.773
106. Liu X, Li C, Li J, Yu T, Zhou G, Cheng J, et al. Detection of CTCs in Portal Vein was Associated With Intrahepatic Metastases and Prognosis in Patients With Advanced Pancreatic Cancer. J Cancer (2018) 9:2038. doi: 10.7150/jca.23989
Keywords: circulating tumor cell, pancreatic carcinoma, EMT, prognosis, circulating tumor cell (CTCs)
Citation: Luo K, Wang X, Zhang X, Liu Z, Huang S and Li R (2022) The Value of Circulating Tumor Cells in the Prognosis and Treatment of Pancreatic Cancer. Front. Oncol. 12:933645. doi: 10.3389/fonc.2022.933645
Received: 01 May 2022; Accepted: 31 May 2022;
Published: 04 July 2022.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Vuong Trieu, University of Valencia, SpainMujib Ullah, Stanford University, United States
Copyright © 2022 Luo, Wang, Zhang, Liu, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renfeng Li, ejE3MjYzODgzOTgyQDE2My5jb20=
†These authors have contributed equally to this work
 Kai Luo
Kai Luo Xiangkun Wang
Xiangkun Wang Xudong Zhang
Xudong Zhang Zhongyuan Liu†
Zhongyuan Liu†