
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 17 November 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.932212
This article is part of the Research TopicUniversal Health Coverage and Global Health in OncologyView all 13 articles
 Jia Li Low1*
Jia Li Low1* Yiqing Huang1
Yiqing Huang1 Kenneth Sooi1
Kenneth Sooi1 Zhi Yao Chan2
Zhi Yao Chan2 Wei Peng Yong1,3
Wei Peng Yong1,3 Soo Chin Lee1,3,4
Soo Chin Lee1,3,4 Boon Cher Goh1,3,4
Boon Cher Goh1,3,4The rising cost of oncological drugs poses a global challenge to patients, insurers, and policy makers, with the leading drugs worldwide by revenue from immune checkpoint inhibitors (ICIs). Despite its cost, ICI is marked as a paradigm shift, offering the potential of a long-term cure. To reduce cost, an attenuated dose of ICI based on pharmacological principles can be used while maintaining efficacy. This real-world study aims to examine the prescribing patterns, the effect of financial constraints, and the outcomes in non-small cell lung cancer (NSCLC). All patients receiving palliative intent ICI treatment for advanced NSCLC between January 2014 and April 2021 in National University Hospital, Singapore were recruited. Demographics, prescription trends, factors affecting the prescription of attenuated dose ICI (AD ICI) versus standard dose ICI (SD ICI), and the effect of dose on survival outcomes, toxicities, and costs were examined. Two hundred seventy-four received ICI. The majority of them were treated in first-line setting. One hundred sixty-two (59%) of patients received AD ICI, whereas 112 (41%) received SD ICI. Patients who did not have a supplemental private as-charged health insurance plan were more likely to have received AD ICI (OR: 4.53 [2.69–7.61] p < 0.001). There was no difference in progression-free survival (PFS) and overall survival (OS)—adjusted HR 1.07 CI [0.76, 1.50] p = 0.697 and HR 0.95 CI [0.67, 1.34] p = 0.773, respectively, between patients who received AD versus SD ICI. A cost minimization analysis evaluating the degree of cost savings related to drug costs estimated a within study cost saving of USD 7,939,059 over 7 years. Our study provides evidence for AD-ICI as a promising strategy to maximize the number of patients who can be treated with ICI. This has the potential to make significant economic impact and allow more patients to benefit from novel therapies.
Breakthroughs in anti-cancer treatment have altered the treatment paradigm in oncology. However, the costs of treatment pose a global challenge to patients, insurers, and policy makers. Global sales of oncology drugs reached USD 176 billion in 2021. This is more than double that of the next most costly item, vaccines. By 2026, cancer drug sales are expected to almost double to USD 320.6 billion and approach 22% of the pharmaceutical market (1–4). The leading drugs worldwide by revenue currently comes from immune checkpoint inhibitors (ICIs) (5, 6).
Singapore is a high-income economy as defined by World Bank with a gross national income of USD 54,539 per capita. Singapore’s healthcare system is also ranked one of the best in Asia and the world, focusing on quality, efficiency, and cost (7). However, rising national health expenditures is receiving increasing attention. With cancer being the nation’s leading cause of death and rising cost of cancer drugs, the country’s spending on cancer drugs has grown at a compound annual growth rate of 20% between 2017 and 2021. This poses a challenge to the nation’s co-payment healthcare system. Singapore’s healthcare system revolves around a mixed financing system. The country’s public statutory insurance system, MediShield, is a basic insurance plan that covers a portion of hospitalization and outpatient treatment. This is complemented by government subsidies, as well as a compulsory savings account Medisave for each citizen, which pays for inpatient care and selected outpatient services (8–10).
Despite its cost, ICI targeting program death 1 (PD-1) and PD-ligand 1 (PD-L1) are marked as a paradigm shift in cancer treatment and offer the possibility of long-term survival (11–18). However, cost effectiveness and sustainability of these drugs are important issues to be considered in the real world (19, 20). Financial toxicity has not only shown to reduce quality of life, increase symptom burden, and potentially affecting survival of patients (21, 22), but it also threatens the financial sustainability of our healthcare system. The potential impact is the lack of access to drugs and benefits of novel therapies.
With the widespread use of ICI, these escalating healthcare costs are necessitating the practice of value-based oncology. An alternative strategy is the development of lower cost off-label treatment regimens, based on pharmacological rationale. This approach of interventional pharmacoeconomics seeks to decrease costs while maintaining equivalent efficacy (23, 24).
In our study, we looked at the real-world use of ICI in non-small cell lung cancers (NSCLC) in our institution since its approval in 2014 and examined the demographics, factors affecting the prescribing patterns, the effects of financial toxicity, and the survival outcomes of patients treated with ICI.
A retrospective cohort study was carried out for all patients receiving palliative intent ICI treatment for advanced NSCLC between January 2014 and April 2021 in an academic tertiary cancer center (National University Cancer Institute, Singapore; NCIS). NSCLC was selected as ICI has been widely approved for use. All patients were identified retrospectively. Patients receiving ICI and enrolled into clinical trials were excluded from the study. Baseline patient demographics, tumor, and treatment characteristics were extracted from electronic medical records. Local protocols continue treatment until disease progression, unacceptable toxicities, death, patient’s decision to stop treatment, or after 2 years of treatment, although some patients who remained progression free after 2 years continued treatment.
Chest and/or abdominal computed tomography (CT) scans were performed by clinicians every 8–12 weeks, as part of routine clinical care, to evaluate patient’s response and assess for disease progression. Progression-free survival (PFS) was measured from time of initiation of drug to disease progression by RECIST or death due to any cause. Overall survival (OS) was measured from time of initiation of drug to death due to any cause. Safety analysis examined the incidence of ≥ Grade 3 immune-related adverse events (irAEs) and adverse events (AEs) as recorded by clinicians.
Continuous and categorical variables were summarized as median (inter-quartile range) and frequency (percentage), respectively. The differences in baseline characteristics of patients receiving attenuated dose ICI (AD ICI) and standard dose ICI (SD ICI) were evaluated using the multinomial logistic regression model. SD ICI was defined as the FDA-approved dose of pembrolizumab 200 mg every 3 weeks or 400 mg every 6 weeks, nivolumab 240 mg every 2 weeks, or 480 mg 4 weeks, atezolizumab 1200 mg every 3 weeks, and durvalumab 10 mg/kg every 2 weeks. AD ICI was defined as a lower than FDA-approved dose of ICI. In our study, AD ICI was given based on an approximate 2 mg/kg weight-based dose of pembrolizumab and 3 mg/kg weight-based dose of nivolumab. The differences in toxicities of the two doses of ICI were tested using the chi-square or Fisher’s exact test whenever applicable.
We also plotted the Kaplan–Meier curve to find a difference in PFS and OS between the AD ICI and SD ICI. Univariate and multivariable Cox proportional hazard regression model was used to find variables associated with PFS and OS in this population. Quantitative association from Cox regression was expressed as hazard ratio (HR) with its corresponding 95% confidence interval (CI). All the tests used in this study were two sided, and P-values < 0.05 were considered as statistically significant. All these tests were performed using Stata version 17.
Based on an acceptance of non-inferior survival and toxicity outcomes, a limited economic evaluation was carried out using a cost-minimization approach (25). This assessed the monetary savings available from the use of AD ICI instead of SD ICI across the entire study population based on the total cycles received by the study population and price of ICI. A fixed price of ICI was assumed. Sensitivity analysis considered the potential savings within the study population if all patients were to receive AD ICI. The dose of AD ICI for this analysis was calculated at pembrolizumab 100 mg and nivolumab 180 mg based on an approximate weight-based dosing of 2 and 3 mg/kg, respectively, vial size and median weight of 56 kg in our population. Given the identical regimens and observed clinical outcomes, all other costs were assumed to remain constant. This analysis was only performed for patients receiving pembrolizumab and nivolumab, as none of the patients who received durvalumab and atezolizumab were treated at attenuated dose.
Two hundred seventy-four patients received immunotherapy in for advanced NSCLC from 2014 to April 2021 at NCIS. Baseline demographics are shown in Table 1. Median age was 65.1 (range: 28.3–92.2). Majority of the patients were Chinese (214, 78%), Singaporeans (239, 87%), men (202, 73%), had an ECOG status of 0/1 (236, 86%), were current/ex-smokers (177, 65%), married (240, 88%), had children (240, 88%), and worked in the service and sales sector (879, 29%) according to the International Standard Classification of Occupations (ISCO) 8 structure. The average body weight was 56 kg (range: 31–103).
In terms of healthcare services, most patients were government subsidized (214, 78%), had Medisave (234, 85%), had MediShield (244, 89%), and did not have a supplemental private health insurance plan (169, 62%).
Treatment characteristics are summarized in Table 2. The majority of the patients received pembrolizumab (229, 84%), received ICI monotherapy (164, 60%), and were treated in the first-line setting (169, 62%).
Figures 1A, B illustrate the increasing trend of ICI usage in our study population since its approval in 2014 and the shift in the use of ICI in first-line setting, respectively. One hundred sixty-two (59%) of patients received AD-ICI, whereas 112 (41%) received SD-ICI. Using the multinomial logistics regression model, we found that patients who did not have a supplemental private as-charged health insurance plan were more likely to have received LD-ICI (OR: 4.53, 95% CI [2.69, 7.61] p < 0.001) (Table 1).
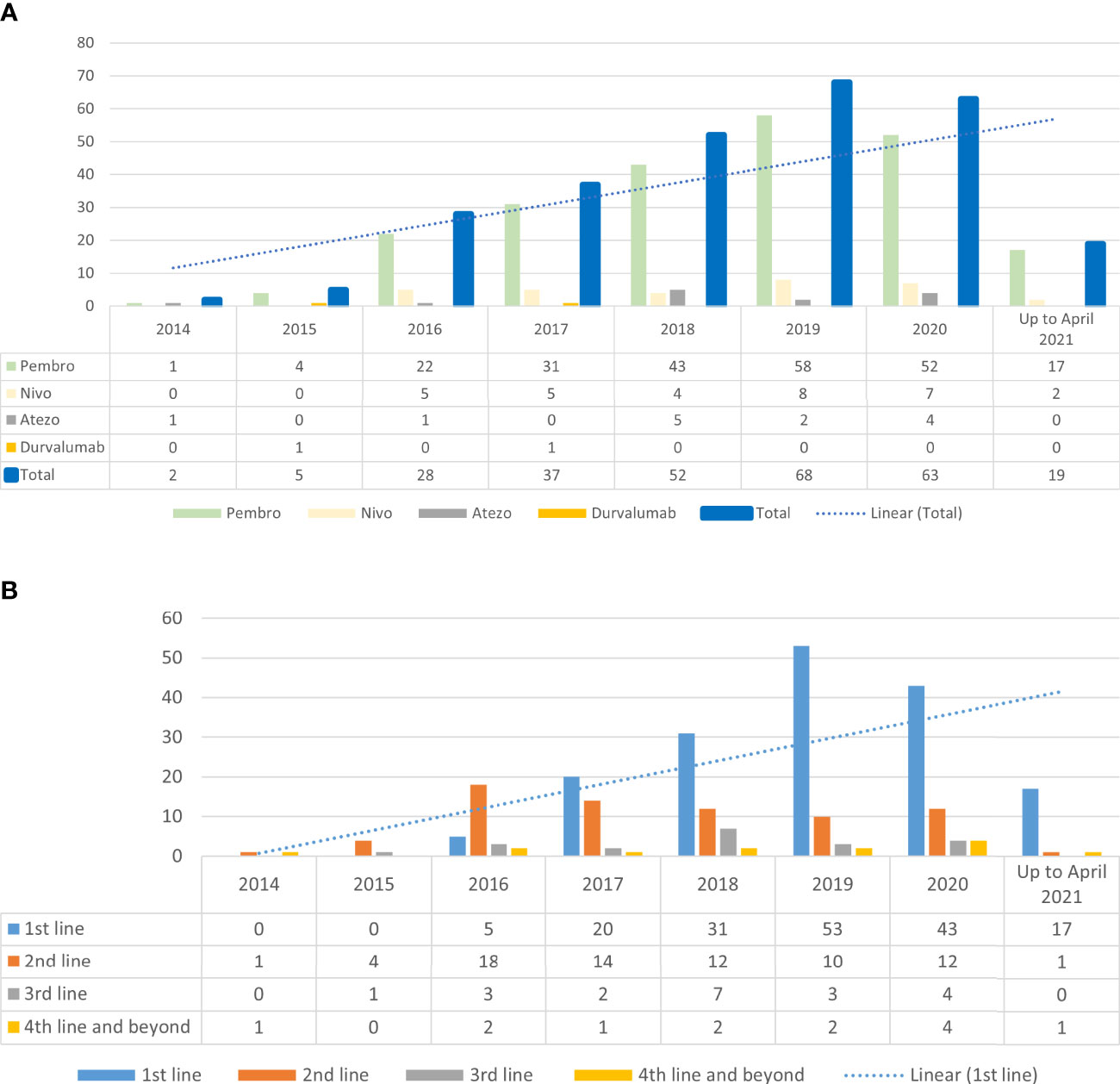
Figure 1 (A) Trend of immune checkpoint inhibitor use since 2014 (B) Immune checkpoint inhibitor and line of treatment.
All patients were included in the survival analysis. Median follow-up duration was 25.1 months.
All variables were analyzed to find independent variables associated with PFS (Table 3) and OS (Table 4). Univariate analysis showed that male gender and a heavier weight were associated with improved PFS, whereas a poorer ECOG status and a later line of treatment were associated with a decreased PFS. For OS, foreigners, heavier weight, and private-paying patients was associated with an improved OS, whereas a poorer ECOG status and a later line of treatment were associated with a decreased OS.
Multivariate logistic regression analysis was conducted to elucidate associations between significant variables found in univariate analysis between PFS and OS. Only a poorer ECOG status and a later line of treatment continued to be associated with both a decreased PFS and OS.
The Kaplan–Meier curves for PFS and OS are demonstrated in Figures 2A, B. The median PFS and OS for AD ICI and SD ICI were 4.6 and 6.1 months and 11.9 and 17.9 months, respectively. The univariate Cox regression model demonstrates no significant difference in PFS (raw HR 1.21, 95% CI [0.91, 1.61], p = 0.183, and OS (raw HR 1.34, 95% CI [0.99, 1.83], p = 0. 0.060). When adjusted for significant variables found in the univariate analysis, the multivariate Cox regression model shows no significant difference in PFS (adjusted HR 1.07, 95% CI [0.76, 1.50], p = 0.843) and OS (adjusted HR 0.95, 95% CI [0.67, 1.34], p = 0.773) between AD ICI and SD ICI.
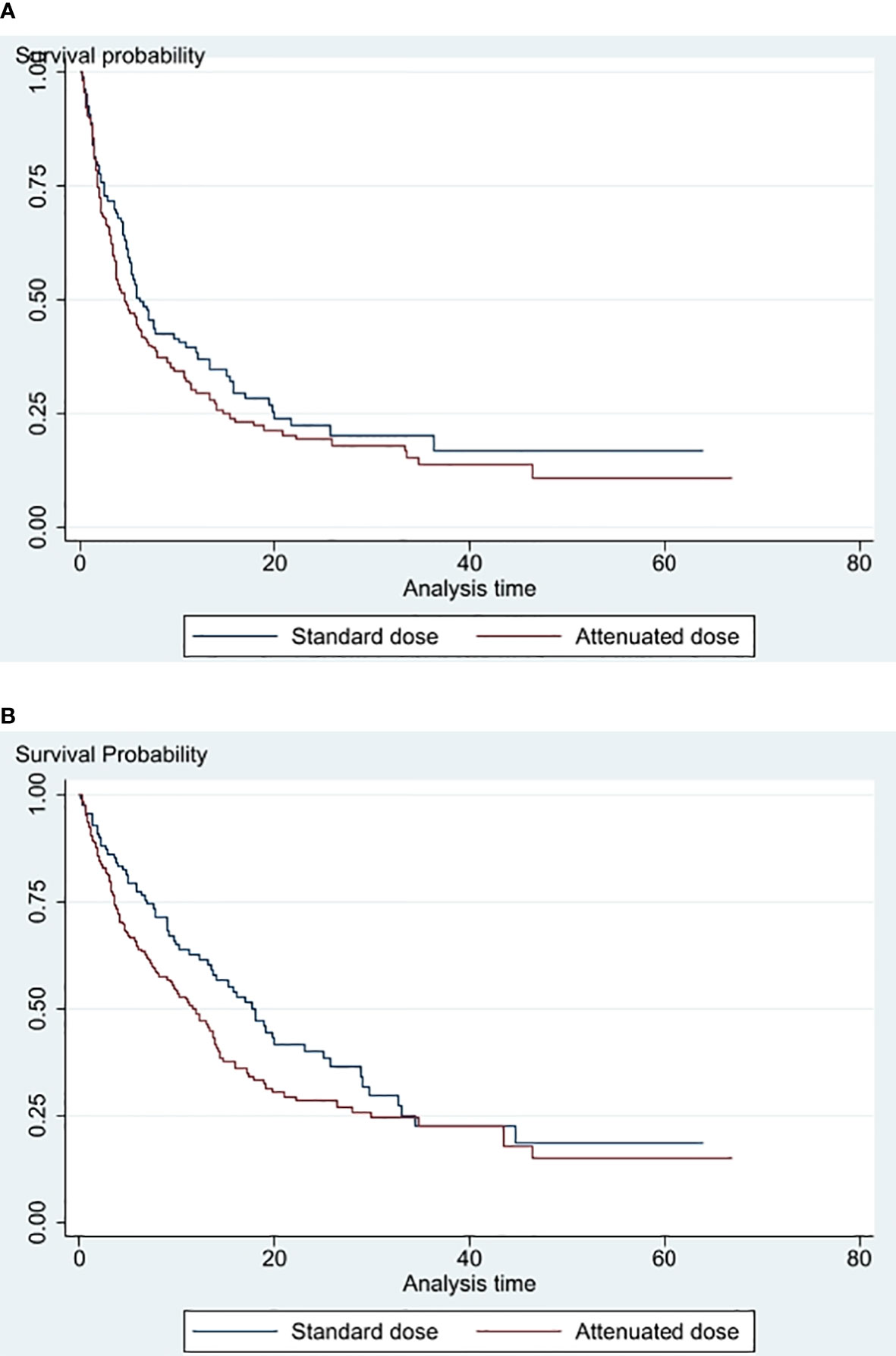
Figure 2 (A) PFS of standard dose vs attenuated dose immune checkpoint inhibitors (B) Overall survival of standard dose versus attenuated dose immune checkpoint inhibitors.
Thirty-seven (12%) of the patients discontinued treatment because of toxicities. There was no dose relationship between ICI and serious irAEs or deaths. The rates of G3 or more AEs and deaths were 10% versus 18% (p = 0.056) and 3% and 4% (p = 0. 0.386) for AD and SD treatments, respectively (Table 5).
In our study, a lower than FDA-approved dose of ICI was routinely delivered based on an approximate 2 mg/kg weight-based dose of pembrolizumab and 3 mg/kg weight-based dose of nivolumab for patients who did not have adequate financial reimbursement plan or based on physician’s preference. None of the patients who received durvalumab or atezolizumab received a lower than approved dose (Table 2).
In our institution, a 100-mg vial of pembrolizumab costs USD 3,778, whereas a 100- and 40-mg vial of nivolumab costs USD 976 and 433, respectively, in Singapore. The total number of cycles of pembrolizumab and nivolumab received in our study was 3,743, median cycles 8 and 7, respectively. Cycles (2,313 vs. 1,430) were delivered in the AD ICI and SD ICI groups. We estimated a total cost savings in our study population of USD 7,939,059 based on the total number of cycles of ICI received in the AD ICI group. This translates to cost savings per cycle for each patient of USD 3,778 and USD 433 for pembrolizumab and nivolumab, respectively.
The cost minimization analysis demonstrates a cost saving of USD 12,863,264 if a weight-based dose of AD ICI was used instead of SD ICI. This would translate to a cost saving of USD 55,692 and USD 5,335 per patient receiving pembrolizumab and nivolumab, respectively (Table 6).
To our knowledge, our study represents the largest cohort to date to evaluate the real-world use of ICI and the efficacy of an attenuated dose of ICI in NSCLC.
The overall use of ICI and the use in the first-line setting have increased over the years in our institution since its approval in 2014 for use in NSCLC, which is reflective of the global trend (26–28). The majority of patients also received ICI upfront in their treatment, in line with FDA’s approval of ICI in NSCLC (29).
However, 162 (59%) of patients in our institute did not receive SD ICI. Only 105 (38%) of the patients had a supplemental as-charged private insurance plan on top of Singapore’s public statutory insurance system, and this was significantly associated with the use of SD ICI with odds ratio of 4.53. Despite financial barriers to prescribing SD ICI, multivariate analysis showed no significant differences in PFS and OS despite the discrepancy in the doses of ICI with an adjusted HR of 1.07 and 0.95, respectively. Only a poorer ECOG status and treatment in later lines were significantly associated with both a poorer PFS and OS, which were within expectations.
Pharmacological principles for dose reduction and weight-based dosing were employed for patients who did not have adequate financial reimbursement. It is known that there are nonlinear relationships between dose of ICI and clinical outcomes. The pharmacokinetic analysis of doses of 200 mg and 2 mg/kg of pembrolizumab has shown similar exposure distributions with no advantage to either dosing approach. Pembrolizumab kinetics has also shown that there is 95% trough target engagement with dosing of 0.8 mg/kg every 3 weeks with saturation of PD-1 receptors at a dose of ≥1 mg/kg. Similarly, for nivolumab, a dose ranging phase 1b study showed that PD-1 receptor occupancy was already saturated at a dose of 0.3 mg/kg (30–38). In our study, the median dose of patients receiving AD ICI was close to 2 and 3 mg/kg for pembrolizumab and nivolumab, respectively. This could explain why we did not see an efficacy difference between the AD ICI and SD ICI.
A weight-based dosing of ICI also appears to be cost efficient. Goldstein et al. demonstrated huge cost savings to the U.S. healthcare system by using a personalized dosing of 2 mg/kg of pembrolizumab (20). In our study population, an estimated in study cost savings was USD 8,154,100. This could increase to USD 13,207,243 if all patients received AD ICI. Other than cost savings, adoption of a weight-based dosing approach will also decrease the dosage drugs needed and may allow more global access to effective yet value-driven therapeutics. While the development of ICI has improved the survival of people with several kinds of cancer, it is not available to most people in low- and middle-income countries (39). In fact, while the importance of immune-oncology drugs was recognized, it is not listed in the World Health Organisation essential medical list (WHO EML) at the 23rd WHO meeting on essential medicines held in September 2021 due to their high cost (40). In a study to evaluate the concordance of medications included in the WHO EML and availability on the frontline of clinical care, striking barriers to accessing high-priority medicines in low- and middle- income countries remain. Core medications such as doxorubicin, cisplatin, and tamoxifen continue to be associated with risks of catastrophic out-of-pocket expenditure (41). The fact that substantial proportion cannot even afford older generic cytotoxic drugs, let alone ICI, highlights a major barrier in access to core medicines. The result of our study reinforces the sustainability and efficacy of use of weight-based dosing approach and may be a step toward addressing the affordability of oncology drugs, allowing more uniform global access to effective yet value-driven therapeutics.
Our study has its limitations. The PFS and OS were numerically better in SD ICI group but the retrospective nature of the study, differing baseline characteristics and limited sample size does not allow for valid efficacy comparison among different dosing strategies. In addition, the relatively small sample size limits the power of the study to demonstrate a statistically significant difference. Given the uncertainty of clinical outcome between the 2-dose groups, a prospective randomized controlled clinical trial is needed to clarify this. The use of SD-ICI was more likely in patients who had a supplemental as-charged private insurance plan on top of Singapore’s public statutory insurance system. This is a potential source of bias due to a positive relationship between health insurance coverage and health-related outcomes (42, 43). Other ICI such as tislelizumab, a China-developed anti-PD1 antibody, has also shown improve PFS in advanced non-squamous NSCLC when combined with chemotherapy (44) and was also reported to be cost effective (45) but is not yet approved or available in Singapore and, hence, not used in this study. Data to support the use of these newer anti-PD1 antibodies to the currently approved ones will also take time to accumulate. Finally, given no differences were identified in the clinical outcomes of the two regimens, a cost minimization analysis was used to examine the cost savings provided by AD ICI. This was not pre-planned and simply provides an indication of cost savings. The costs assessed are only those of the drug and do not include regimen-related costs such as drug administration, pre-medications, clinic visits, subsequent therapy, and management of AEs. While the costs are not anticipated to vary based on the study outcomes, further formal assessment of cost utility of AD ICI should be considered alongside future prospective randomized study.
Despite these limitations, our study reflects the real-world application of ICI where cost is prohibitive, outside the controlled setting of conventional clinical trials (39). It also suggests the efficacy of an attenuated dose of ICI, which can provide considerable cost savings to both patients and the healthcare system.
Increasing cost of drugs contributes to the increasing cost of healthcare. This problem needs to be urgently tackled. Our real-world study demonstrates efficacy of AD ICI, based on a pharmacological rationale, which has the potential to make significant economic impact yet allow our patients to benefit from novel therapies. With the expanding role of ICI in various tumor types, this value driven approach will be highly relevant to patients, oncologists, and policy makers.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by National Health Group Domain Specific Review Board (NHG DSRB) (Reference number: 2017/012654). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JL, YH, KS, and ZC participated in data acquisition and collection. JL analyzed the data. JL, WY, SL, and BG contributed to manuscript writing. All authors contributed to the article and approved the submitted version.
This research is supported by the Singapore Ministry of Health’s National Medical Research Council under its NMRC Centre Grant Programme CGAug16M005.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Demartino PC, Miljković MD, Prasad V. Potential cost implications for all US food and drug administration oncology drug approvals in 2018. JAMA Internal Med (2021) 181(2):162–7. doi: 10.1001/jamainternmed.2020.5921
2. Haslam A, Lythgoe MP, Greenstreet Akman E, Prasad V. Characteristics of cost-effectiveness studies for oncology drugs approved in the united states from 2015-2020. JAMA Netw Open (2021) 4(11):e2135123. doi: 10.1001/jamanetworkopen.2021.35123
3. Turck R. Oncology drug costs-the imaginary crisis? Ann Oncol (2017) 28(2):427–31. doi: 10.1093/annonc/mdw548
4. Feinberg BA, Gajra A, Zettler ME, Phillips TD, Phillips EG, Kish JK. Use of real-world evidence to support FDA approval of oncology drugs. Value Health (2020) 23(10):1358–65. doi: 10.1016/j.jval.2020.06.006
5. Kim H, Liew D, Goodall S. Cost-effectiveness and financial risks associated with immune checkpoint inhibitor therapy. Br J Clin Pharmacol (2020) 86(9):1703–10. doi: 10.1111/bcp.14337
6. Neubauer A. Immunotherapy of cancer with checkpoint inhibitors: Not only in malignant melanoma. Internist (2017) 58(4):409–23. doi: 10.1007/s00108-017-0208-1
7. Ramesh M, Bali AS. The remarkable healthcare performance in Singapore. In: Great policy successes (Oxford: Oxford Academic) (2019). doi: 10.1093/oso/9780198843719.003.0003
8. Yin JDC, He AJ. Health insurance reforms in Singapore and Hong Kong: How the two ageing Asian tigers respond to health financing challenges? Health Policy (New York) (2018) 122(7):693–7. doi: 10.1016/j.healthpol.2018.04.012
9. Tan SC, Low PY, Chia MTXY. Drug access through sharing public and individual responsibilities in the public health care system of Singapore. Value Health (2014) 17(7):PA790. doi: 10.1016/j.jval.2014.08.429
10. Lim J. Sustainable health care financing: The Singapore experience. Glob Policy (2017) 8(S2):103–9. doi: 10.1111/1758-5899.12247
11. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol (2020) 38(14). doi: 10.1200/JCO.19.03136
12. Cheng Y, Zhang L, Hu J, Wang D, Hu CP, Zhou J, et al. Pembrolizumab plus chemotherapy for Chinese patients with metastatic squamous NSCLC in KEYNOTE-407. JTO Clin Res Rep (2021) 2(10):100225. doi: 10.1016/j.jtocrr.2021.100225
13. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37(7). doi: 10.1200/JCO.18.00149
14. Nasser NJ, Gorenberg M, Agbarya A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals (2020) 13(11):10.3390/ph13110373. doi: 10.3390/ph13110373
15. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet (2019) 394:10212. doi: 10.1016/S0140-6736(19)32591-7
16. Low JL, Walsh RJ, Ang Y, Chan G, Soo RA. The evolving immuno-oncology landscape in advanced lung cancer: First-line treatment of non-small cell lung cancer. Ther Adv Med Oncol (2019) 11. doi: 10.1177/1758835919870360
17. Lopes G, Wu YL, Kudaba I, Kowalski D, Cho BC, Castro G, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol (2018) 36(18_suppl). doi: 10.1200/jco.2018.36.18_suppl.lba4
18. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Final analysis of the phase III KEYNOTE-042 study: Pembrolizumab (Pembro) versus platinum-based chemotherapy (Chemo) as first-line therapy for patients (Pts) with PD-L1–positive locally advanced/metastatic NSCLC. Ann Oncol (2019) 30(Supplement 2):I38. doi: 10.1093/annonc/mdz063
19. Low JL, Huang Y, Sooi K, Ang Y, Chan ZY, Spencer K, et al. Low-dose pembrolizumab in the treatment of advanced non-small cell lung cancer. Int J Cancer. (2021) 149(1):169–76. doi: 10.1002/ijc.33534
20. Goldstein DA, Gordon N, Davidescu M, Leshno M, Steuer CE, Patel N, et al. A phamacoeconomic analysis of personalized dosing vs fixed dosing of pembrolizumab in firstline PD-L1-Positive non-small cell lung cancer. J Natl Cancer Inst (2017) 109(11):djx063. doi: 10.1093/jnci/djx063
21. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol (2016) 34(15):1732–40. doi: 10.1200/JCO.2015.63.2232
22. Ramsey SD, Bansal A, Fedorenko CR, Blough DK, Overstreet KA, Shankaran V, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol (2016) 34(9):980–6. doi: 10.1200/JCO.2015.64.6620
23. Goldstein DA, Strohbehn GW, Serritella A v., Hyman DA, Lichter AS, Ratain MJ. Interventional pharmacoeconomics. Cancer J (United States) (2020) 26(4):330–4. doi: 10.1097/PPO.0000000000000461
24. Ratain MJ, Goldstein DA, Lichter AS. Interventional pharmacoeconomics - a new discipline for a cost-constrained environment. JAMA Oncol (2019) 5(8):1097–8. doi: 10.1001/jamaoncol.2019.1341
25. Bonk RJ. Cost-minimization analysis. In: Pharmacoeconomics in perspective (Binghamton, New York: Pharmaceutical Product Press) (2020). doi: 10.1201/9781439814277-8
26. Xu C, Zhang S, Zhang Y, Tang SQ, Fang XL, Zhu GL, et al. Evolving landscape and academic attitudes toward the controversies of global immuno-oncology trials. Int J Cancer (2021) 149(1):108–18. doi: 10.1002/ijc.33503
27. Tang J, Pearce L, O’donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov (2018) 17:783–4. doi: 10.1038/nrd.2018.167
28. Kushi LH, Lasiter L, Belli AJ, Boyd M, Bruinooge SS, Christian J, et al. Trends in immunotherapy use in patients with advanced non-small cell lung cancer (aNSCLC) patients: Analysis of real-world data. J Clin Oncol (2020) 38(15_suppl). doi: 10.1200/jco.2020.38.15_suppl.e19311
29. Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA Approval summary: Pembrolizumab for treatment of metastatic non-small cell lung cancer: First-line therapy and beyond. Oncologist (2017) 22(11):1392–9. doi: 10.1634/theoncologist.2017-0078
30. Li H, Yu J, Liu C, Liu J, Subramaniam S, Zhao H, et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn (2017) 44(5):1392–9. doi: 10.1007/s10928-017-9528-y
31. Lala M, Li TR, de Alwis DP, Sinha V, Mayawala K, Yamamoto N, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer (2020) 131:68–75. doi: 10.1016/j.ejca.2020.02.016
32. Ogungbenro K, Patel A, Duncombe R, Clark J, Lorigan P. A rational approach to dose optimisation of pembrolizumab and nivolumab using cost analysis and pharmacokinetic modelling and simulation. Ann Oncol (2016) 27(Supplement 6):VI370. doi: 10.1093/annonc/mdw378.36
33. Ogungbenro K, Patel A, Duncombe R, Nuttall R, Clark J, Lorigan P. Dose rationalization of pembrolizumab and nivolumab using pharmacokinetic modeling and simulation and cost analysis. Clin Pharmacol Ther (2018) 103(4):582–90. doi: 10.1002/cpt.875
34. Parchment RE, Doroshow JH. Pharmacodynamic endpoints as clinical trial objectives to answer important questions in oncology drug development. Semin Oncol (2016) 43(4):514–25. doi: 10.1053/j.seminoncol.2016.07.002
35. Renner A, Burotto M, Rojas C. Immune checkpoint inhibitor dosing: Can we go lower without compromising clinical efficacy? J Glob Oncol (2019) 2019(5). doi: 10.1200/JGO.19.00142
36. Elassaiss-Schaap J, Rossenu S, Lindauer A, Kang S, de Greef R, Sachs J, et al. Using model-based “learn and confirm” to reveal the pharmacokinetics-pharmacodynamics relationship of pembrolizumab in the KEYNOTE-001 trial. CPT Pharmacometrics Syst Pharmacol (2017) 6(1):21–8. doi: 10.1002/psp4.12132
37. Freshwater T, Kondic A, Ahamadi M, Li CH, de Greef R, de Alwis D, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer (2017) 5(1). doi: 10.1186/s40425-017-0242-5
38. Yoo SH, Keam B, Kim M, Kim SH, Kim YJ, Kim TM, et al. Low-dose nivolumab can be effective in non-small cell lung cancer: Alternative option for financial toxicity. ESMO Open (2018) 3(5):E000332. doi: 10.1136/esmoopen-2018-000332
39. Patel A, Goldstein DA, Tannock IF. Improving access to immunotherapy in low- and middle-income countries. Ann Oncol (2022) 33(4):360–1. doi: 10.1016/j.annonc.2022.01.003
40. Executive summary. In: The selection and use of essential medicines 2021 report of the 23 rd WHO expert committee on the selection and use of essential medicines (Geneva: Who Health Organisation). Available at: http://apps.who.int/bookorders.
41. Fundytus A, Sengar M, Lombe D, Hopman W, Jalink M, Gyawali B, et al. Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey. Lancet Oncol (2021) 22(10):1367–77. doi: 10.1016/S1470-2045(21)00463-0
42. Institute of Medicine (US) Committee on the Consequences of Uninsuranc. Care Without Coverage: Too Little, Too Late. Washington (DC): National Academies Press (US) (2002).
43. Michael McWilliams J. Health consequences of uninsurance among adults in the united states: Recent evidence and implications. Milbank Q (2009) 87(2):1367–77. doi: 10.1111/j.1468-0009.2009.00564.x
44. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol (2021) 16(9):1367–77. doi: 10.1016/j.jtho.2021.05.005
Keywords: PDL1, attenuated, lung cancer, immunotherapy, immune check inhibitor (ICI), dose, non-small cell lung cancer
Citation: Low JL, Huang Y, Sooi K, Chan ZY, Yong WP, Lee SC and Goh BC (2022) Real-world assessment of attenuated dosing anti-PD1 therapy as an alternative dosing strategy in a high-income country (as defined by World Bank). Front. Oncol. 12:932212. doi: 10.3389/fonc.2022.932212
Received: 29 April 2022; Accepted: 17 October 2022;
Published: 17 November 2022.
Edited by:
Marine Hovhannisyan, Yerevan State Medical University, ArmeniaReviewed by:
Carrie Langstraat, Mayo Clinic, United StatesCopyright © 2022 Low, Huang, Sooi, Chan, Yong, Lee and Goh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Li Low, amlhX2xpX2xvd0BudWhzLmVkdS5zZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.