- 1Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
- 2Sir Peter MacCallum Department of Oncology, The University of Melbourne, Parkville, VIC, Australia
- 3Personalised Oncology, Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC, Australia
- 4Department of Medical Oncology, St Vincent’s Hospital Melbourne, Melbourne, VIC, Australia
- 5Department of Surgery, Royal Melbourne Hospital, University of Melbourne, Melbourne, VIC, Australia
- 6Department of Cancer Surgery, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
- 7Department of Medical Oncology, Chris O’Brien Lifehouse, Camperdown, NSW, Australia
- 8Faculty of Medicine and Health, University of Sydney, Camperdown, NSW, Australia
- 9Department of Urology, Chris O’Brien Lifehouse, Camperdown, NSW, Australia
- 10ONTrac at Peter Mac, Victorian Adolescence and Young Adult Cancer Service, Melbourne, VIC, Australia
Purpose: Post-chemotherapy retroperitoneal lymph node dissection (pcRPLND) for residual nodal masses is a critical component of care in metastatic testicular germ cell tumour (GCT). However, the procedure is not of therapeutic value in up to 50% of individuals in whom histopathology demonstrates post-treatment necrosis or fibrosis alone. Improved diagnostic tools and clinicopathologic features are needed to separate individuals who benefit from pcRPLND and avoid surgery in those who do not.
Methods: A prospectively registered meta-analysis of studies reporting clinicopathologic features associated with teratoma, GCT and/or necrosis/fibrosis at pcRPLND for metastatic non-seminoma GCT (NSGCT) was undertaken. We examined the effect of various clinicopathologic factors on the finding of necrosis/fibrosis at pcRPLND. The log odds ratios (ORs) of each association were pooled using random-effects models.
Results: Using the initial search strategy, 4,178 potentially eligible abstracts were identified. We included studies providing OR relating to clinicopathologic factors predicting pcRPLND histopathology, or where individual patient-level data were available to permit the calculation of OR. A total of 31 studies evaluating pcRPLND histopathology in 3,390 patients were eligible for inclusion, including two identified through hand-searching the reference lists of eligible studies. The following were associated with the presence of necrosis/fibrosis at pcRPLND: absence of teratomatous elements in orchidectomy (OR 3.45, 95% confidence interval [CI] 2.94-4.17); presence of seminomatous elements at orchidectomy (OR 2.71, 95% CI 1.37-5.37); normal pre-chemotherapy serum bHCG (OR 1.96, 95% CI 1.62-2.36); normal AFP (OR 3.22, 95% CI 2.49–4.15); elevated LDH (OR 1.72, 95% CI 1.37-2.17); >50% change in mass during chemotherapy (OR 4.84, 95% CI 3.94-5.94); and smaller residual mass size (<2 cm versus >2 cm: OR 3.93, 95% CI 3.23-4.77; <5 cm versus >5 cm: OR 4.13, 95% CI 3.26-5.23).
Conclusions: In this meta-analysis, clinicopathologic features helped predict the presence of pcRPLND necrosis/fibrosis. Collaboration between centres that provide individual patient-level data is required to develop and validate clinical models and inform routine care to direct pcRPLND to individuals most likely to derive benefits.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021279699
Introduction
Improvements in survival for individuals diagnosed with testicular germ cell tumour (GCT) have been heralded as one of the most significant advances within oncology (1, 2). GCT most commonly affects younger people, and with an ever-growing survivorship cohort, increasing attention is being placed on reducing the treatment-related morbidity given the potential wide-ranging consequences that can occur many years after cure (3, 4).
The resection of residual, post-chemotherapy masses >1 cm in marker-negative, advanced non-seminomatous GCT (NSGCT) continues to form an important part of the treatment paradigm. Owing to the risks associated with residual teratoma or viable GCT harboured within residual masses after chemotherapy, the aggressive resection of residual disease is the recommended approach in international consensus guidelines (5–8). However, post-chemotherapy retroperitoneal lymph node dissection (pcRPLND) is associated with a range of specific risks including pain, intraoperative vascular or lymphatic injury and ejaculatory dysfunction (9–12). Whilst pcRPLND is critically important in the 10%–15% and 40%–45% of individuals whose specimens contain residual viable GCT or teratoma, respectively (13, 14), improved diagnostic tools and algorithms are required to predict which individuals require pcRPLND and spare the remaining individuals with fibrosis/necrosis from unnecessary treatment from which they derive no therapeutic benefit.
Predictive models to guide decision-making regarding the appropriate selection for pcRPLND have been available for at least two decades (15–17). Clinicopathologic variables such as orchidectomy histology, particularly the presence of teratoma, pre-chemotherapy serum tumour marker levels and size of residual mass have all been reported to help predict histopathology at pcRPLND (5, 18). Accordingly, clinical models that provide weighting to each covariate have been developed (15–17). However, a lack of prospective cohorts evaluating these models has limited their use and a meta-analysis of contemporary datasets has not been performed. Thus, we performed a meta-analysis of existing literature to validate which clinicopathologic variables accurately predict pcRPLND histopathology and aid patient selection for surgery.
Materials and methods
Search strategy and study eligibility criteria
We searched PubMed/Medline, Embase and the Cochrane Central Register of Controlled Trials to identify studies that investigated pcRPLND histopathology in NSGCT. The search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021279699) (19). Any studies with the keywords retroperitoneal (OR) lymphadenectomy (OR) node dissection (OR) RPLND, (AND) testicular (OR) germ cell (OR) non-semin*, (AND) post-chemo* (AND) histo* (OR) viable (OR) terato* (OR) patho* were retrieved. The last search update was performed on 17 May 2022.
All studies reporting histopathological outcomes at pcRPLND for metastatic NSGCT, where odds ratios (ORs) relating to clinicopathologic variables predicting necrosis or fibrosis were either reported or able to be calculated from individual patient-level data were included in the meta-analysis.
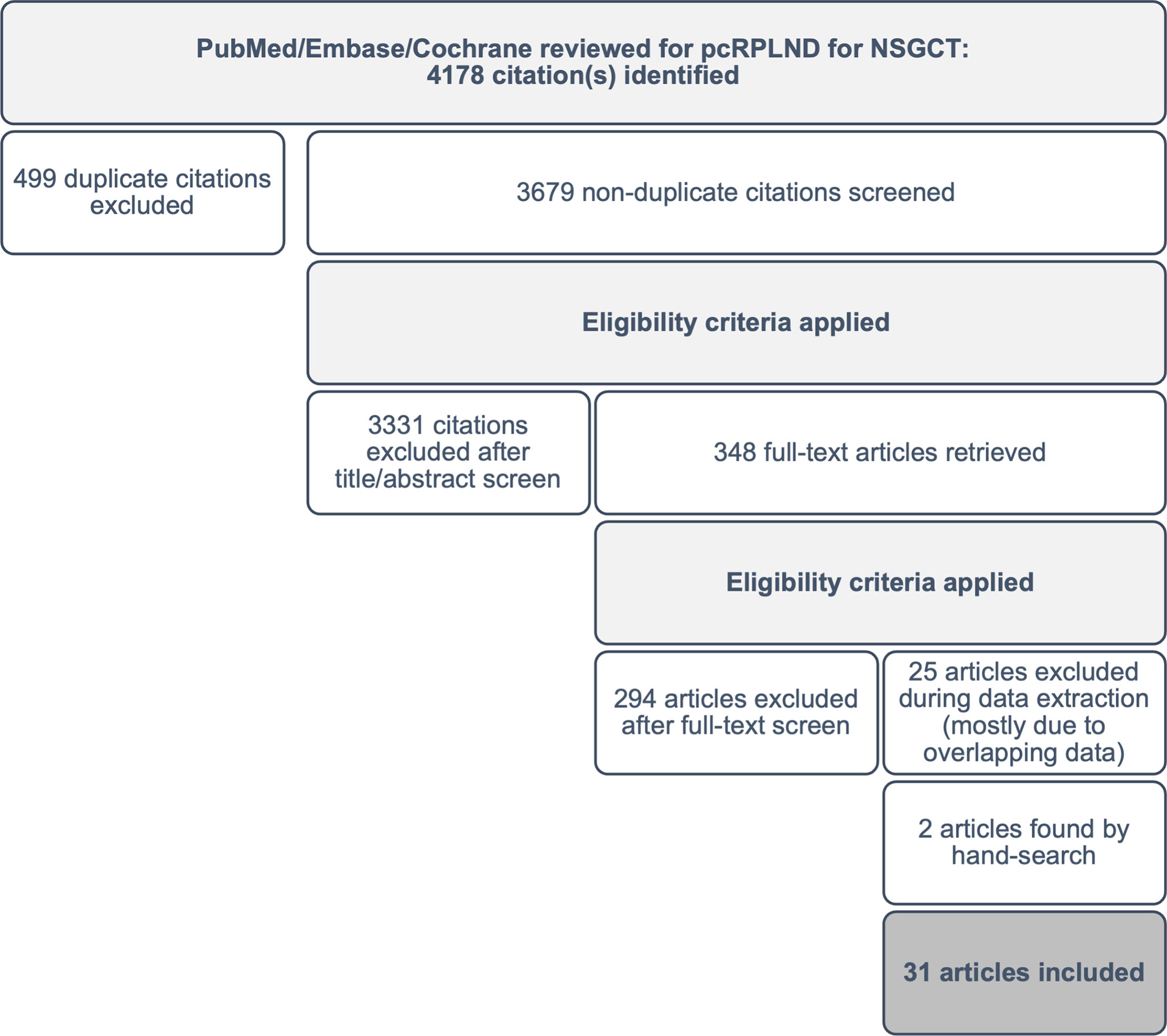
After the removal of duplicates, two investigators screened the abstracts from each database to identify potentially eligible studies for a full-text review. Pre-defined exclusion criteria including reviews, expert opinions, case reports, irrelevant topics (i.e. pure seminoma or non-germ cell tumour, primary RPLND) and non-English language were applied during screening. The initial search yielded 4,178 articles, and after the application of exclusion criteria, 348 potentially eligible studies remained available for a full-text review.
Articles that were considered potentially eligible were retrieved in full text and reviewed to confirm eligibility. Any discrepancies were discussed, and consensus reached. Reference lists from all eligible studies were surveyed to identify other potentially eligible studies that may have been missed during screening (n=2). Following a review of the full text of the 348 studies, 293 citations (84%) were excluded from the analysis. The most common reason for exclusion was an incorrect population or outcome of interest or inadequate patient-level data to permit OR calculation. An additional 25 (7%) studies were excluded during data extraction due to overlapping datasets. A total of 31 studies, including 2 sourced from the reference lists of eligible papers, were included in the meta-analysis (see Figure 1).
Data extraction
For each eligible trial, we recorded in an electronic spreadsheet the first author’s name, journal, year of publication, sample size, pcRPLND histopathology and clinicopathologic variables including orchidectomy histology, disease stage, International Germ Cell Cancer Collaborative Group (IGCCCG) prognostic groups, serum tumour marker elevation [including beta-human chorionic gonadotropin (bHCG), alpha-fetoprotein (AFP) and lactate dehydrogenase (LDH)], residual mass size and change in the mass size during chemotherapy, where applicable. Where reported, we also collected pre-calculated ORs relating to predictive factors for necrosis or fibrosis at pcRPLND (with no associated teratoma or viable GCT). 95% confidence interval (CI) were reported. The risk of bias was evaluated using the Quality in Prognosis Studies tool (20) for all eligible articles across domains including participation, attrition, outcome measurement, confounding and statistical reporting.
The primary analysis comprised a pooled analysis of studies reporting or permitting calculation of ORs for clinicopathologic variables predicting necrosis/fibrosis at pcRPLND.
Statistical analysis
We performed an aggregate patient data meta-analysis. The log OR effect estimates of each clinicopathologic factor on necrosis/fibrosis was pooled using the DerSimonian and Laird random effects model, with confidence intervals computed using the Cornfield method. Heterogeneity was estimated by comparing the result of each study with a Mantel–Haenszel fixed-effect meta-analysis result and assessed using the using the I² statistic. Publication bias was assessed using funnel plots and Egger’s test to look for small study effects. Analyses were conducted using Stata version 15.1 with the ‘metan’ and ‘metafunnel’ packages.
Results
Eligible studies
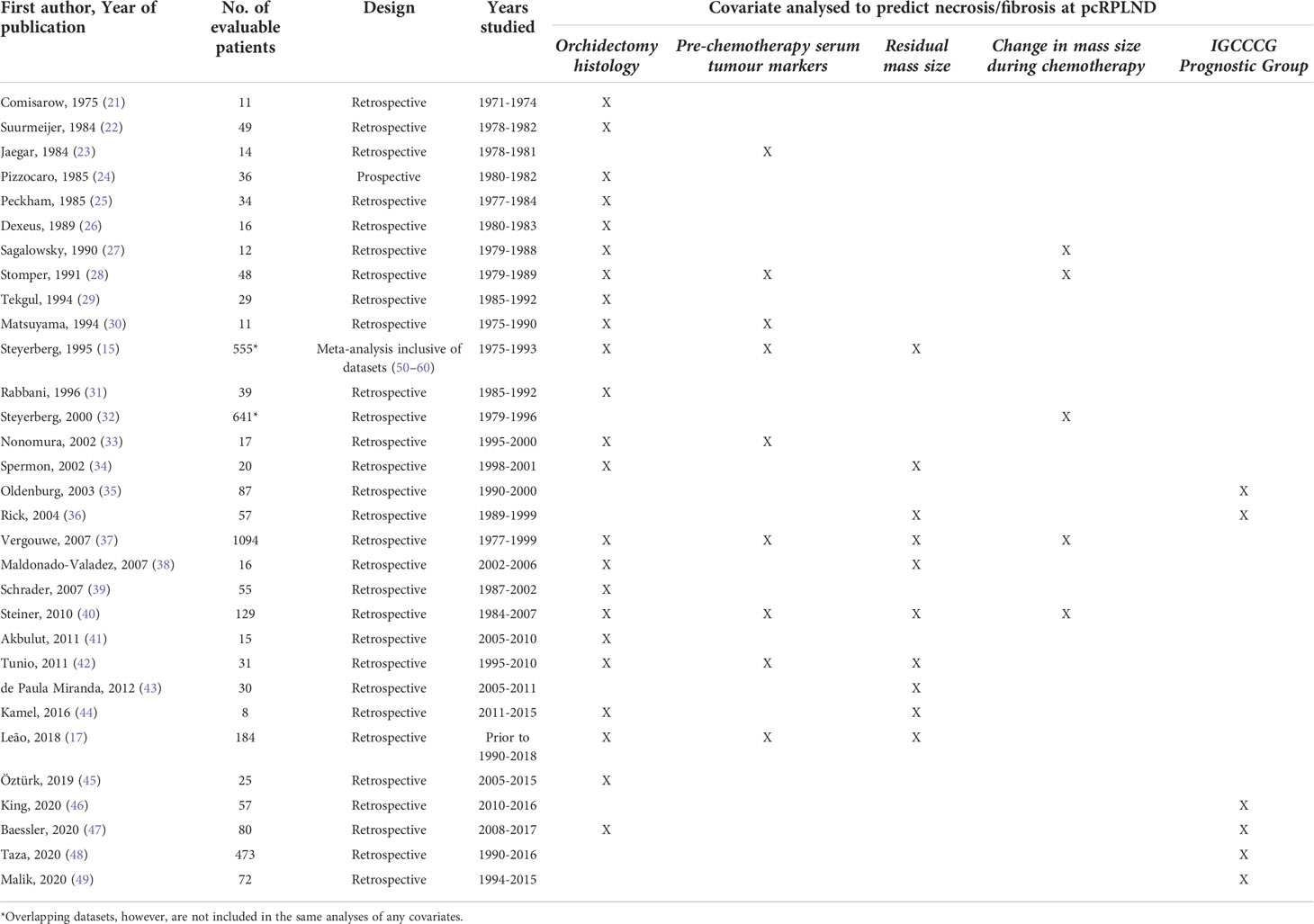
A total of 31 studies, including 3,390 individual patients were considered eligible for the analysis (15, 17, 21–49) (see Table 1). Patients were considered eligible for inclusion in the meta-analysis if they had NSGCT and underwent pcRPLND [± additional surgical resection(s)] for a residual mass, and clinicopathologic data were available. The meta-analysis of clinicopathologic variables predicting necrosis/fibrosis at pcRPLND, including orchidectomy histology, IGCCCG prognostic groups, pre-chemotherapy serum tumour marker elevation, residual mass size and other clinicopathologic features was conducted where there were two or more studies with the required information were eligible. Notably, two studies that evaluated different clinicopathologic variables in overlapping patients were included amongst these 31 papers (15, 32); however, both papers were not included in the same analysis of any covariate, and the larger dataset (32) formed the analysis of patient characteristics.
Patient characteristics
Clinicopathologic factors were reported variably between studies. Of the 3,390 eligible patients, the reported age range at pcRPLND was 6–71 years old. The IGCCCG prognostic group was recorded in 1,033 (30%) patients, and 605 (59%), 211 (20%) and 217 (21%) participants had IGCCCG good-, intermediate- and poor-risk disease, respectively. Staging at enrolment was documented in a minority of participants only (10%). Of those where serum tumour markers were reported, AFP and bHCG were elevated prior to chemotherapy in at least 68% and 63% of patients, respectively.
Histopathology at orchidectomy and pcRPLND was variably documented, with some studies reporting the presence or absence of histologic subtypes within mixed tumours and others reporting predominant histologic subtypes only or grouping histologic subtypes, for example, NSGCT, not otherwise specified (NOS), non-teratoma NSGCT or simply not necrosis/fibrosis. Overall, orchidectomy histology was described in 3,290 (97%) patients, with teratoma being the most reported histologic subtype (n=1,507, 46%), followed by NSGCT, NOS (n=1,110, 34%). The most common histopathology reported at pcRPLND was necrosis/fibrosis (n=1,352, 40%), with a relative minority of patients having teratoma (n=1,107, 33%) or viable tumour reported (n=238, 7%) within the specimen.
All participants received chemotherapy prior to RPLND; however, the line of treatment, number of cycles, and choice of chemotherapy was variably documented between studies. Of those where the line of treatment was documented, 94% of participants received first-line chemotherapy prior to surgery (n=965). Where chemotherapy was documented, bleomycin, etoposide, cisplatin (BEP) was the most common regimen (n=321, 56%).
Orchidectomy histology and post-chemotherapy retroperitoneal lymph node dissection histopathology
Twenty-three studies (15, 17, 21, 22, 24–31, 33, 34, 37–42, 44, 45, 47) evaluating the impact of orchidectomy histology on pcRPLND histopathology were included in the analysis, including a meta-analysis of relevant studies (50–60). The presence of teratomatous elements within the orchidectomy sample was analysed in 20 studies (15, 17, 21, 22, 24–29, 31, 33, 34, 37, 39–41, 44, 45, 47), and 2,438 patients did not predict necrosis/fibrosis at pcRPLND (OR 0.29, 95% CI 0.24-0.34) over residual teratoma or viable GCT at pcRPLND (see Figure 2A). The presence of seminoma at orchidectomy (as a component of mixed GCT) was evaluated in nine studies (21, 22, 27, 28, 30, 33, 34, 41, 45) and 208 patients and was also shown to be predictive of necrosis/fibrosis at pcRPLND (OR 2.71, 95% CI 1.37-5.37) (see Figure 2B).
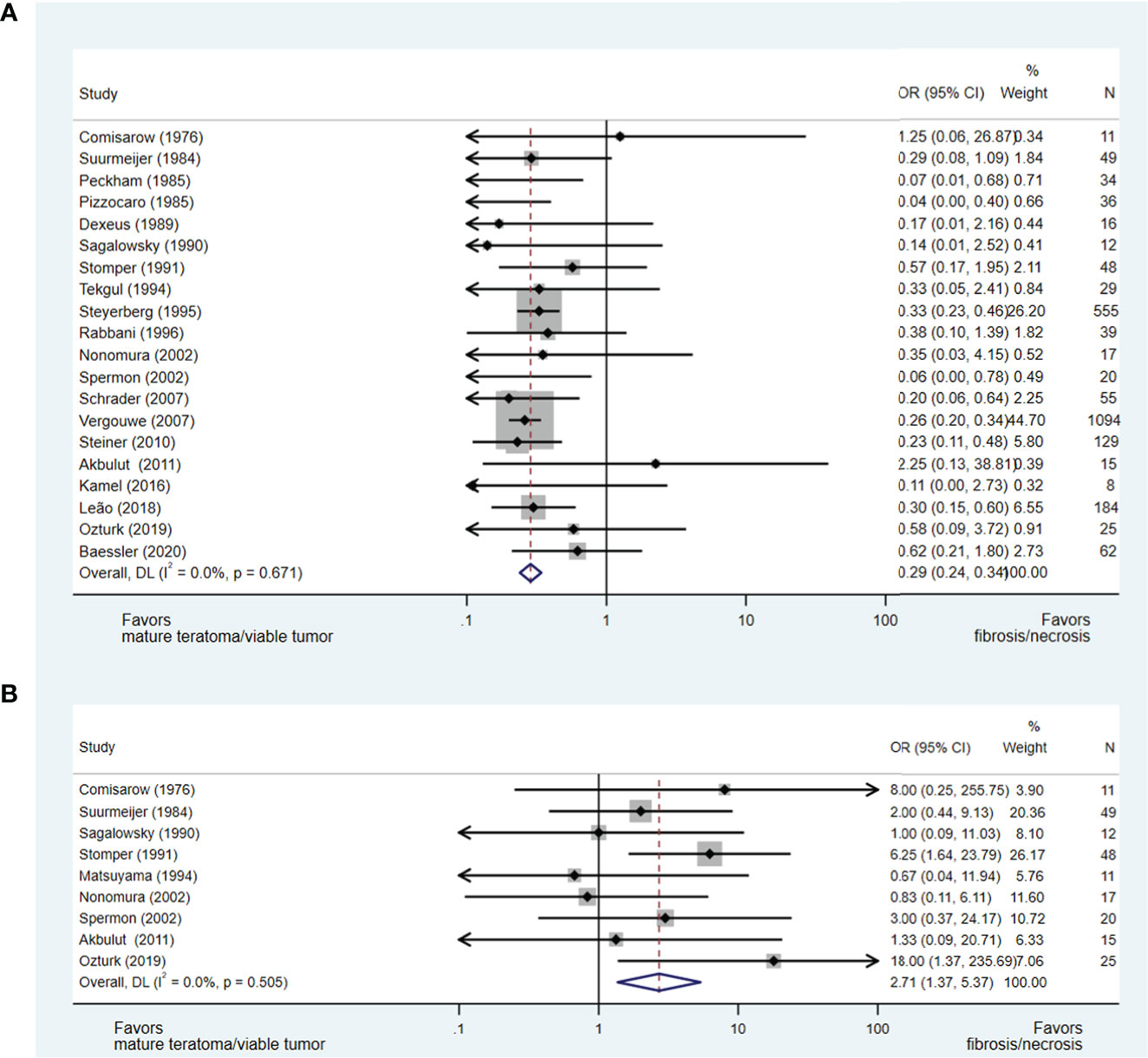
Figure 2 Forest plot of eligible studies evaluating relationship between presence of teratomatous elements (A) and seminoma (B) within orchidectomy and necrosis/fibrosis at pcRPLND.
In contrast, necrosis/fibrosis at pcRPLND could not be reliably predicted over residual teratoma or viable GCT by the presence of other histopathologies within the orchidectomy sample, including embryonal carcinoma (OR 0.87, 95% CI 0.46-1.66) (17, 28–30, 33, 34, 42, 45) or yolk sac tumour (OR 1.25, 95% CI 0.62-2.50) (22, 28, 30, 33, 34, 38, 41, 42, 45), where there was no significant association between these variables and necrosis/fibrosis at pcRPLND (see Supplements 1A- B).
Pre-chemotherapy serum tumour marker level and pcRPLND histopathology
A total of nine studies including 2,053 patients addressed the issue of the predictive value of pre-chemotherapy serum tumour marker levels on necrosis/fibrosis at pcRPLND (15, 17, 23, 28, 30, 33, 37, 40, 42), including the aforementioned meta-analysis (15). Necrosis/fibrosis was significantly more likely to be identified in post-chemotherapy residual masses when compared with teratoma or viable GCT when AFP (OR 3.22, 95% CI 2.49-4.15) (15, 17, 28, 30, 37, 40, 42) or bHCG (OR 1.96, 95% CI 1.62-2.36) (15, 17, 28, 33, 37, 40, 42) were normal at the commencement of chemotherapy. In contrast, normal LDH prior to chemotherapy was negatively associated with this necrosis/fibrosis at pcRPLND (OR 0.58, 95% CI 0.46-0.73) (15, 17, 23, 33, 37, 40) (see Figures 3A–C).
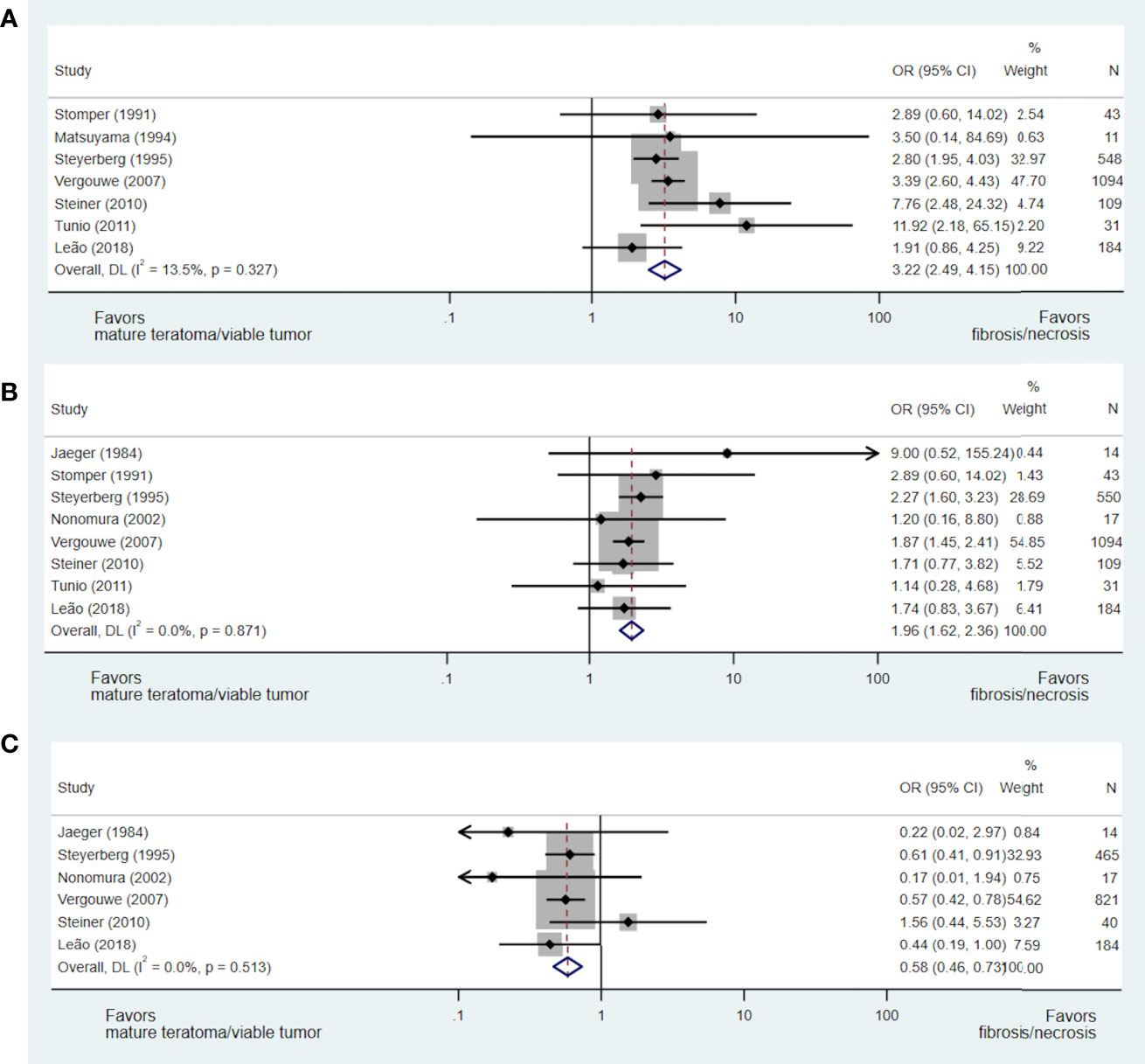
Figure 3 Forest plot of eligible studies evaluating relationship between normal AFP (A), bHCG (B) and LDH (C) and necrosis/fibrosis at pcRPLND.
Change in mass size during chemotherapy and histopathology
A reduction in mass size during chemotherapy was evaluated consistently in five studies as a predictor of residual mass histopathology (27, 28, 32, 37, 40), with each study permitting the development of an OR of less than or greater than 50%, 70% and/or 90% reduction in mass size during chemotherapy.
In an analysis of four studies (28, 32, 37, 40), which addressed the predictive value of less than or greater than 50% reduction in mass size during chemotherapy and included 1,912 patients, a greater than 50% reduction in mass size was strongly predictive of necrosis/fibrosis over residual teratoma or viable GCT when compared to those with less than 50% reduction in mass size (OR 4.84, 95% CI 3.94-5.94) (see Figure 4A). Similarly, in the analyses of studies evaluating less than or greater than 70% (27, 28, 32, 37, 40) and 90% (27, 28, 40) reduction in mass size, masses undergoing greater change were more likely to represent necrosis/fibrosis than residual teratoma or viable GCT when compared to masses undergoing lesser change (≥70%: OR 4.36, 95% CI 3.49-5.44; ≥90%: OR 2.19, 95% CI 1.16-4.11) (see Figures 4B, C).
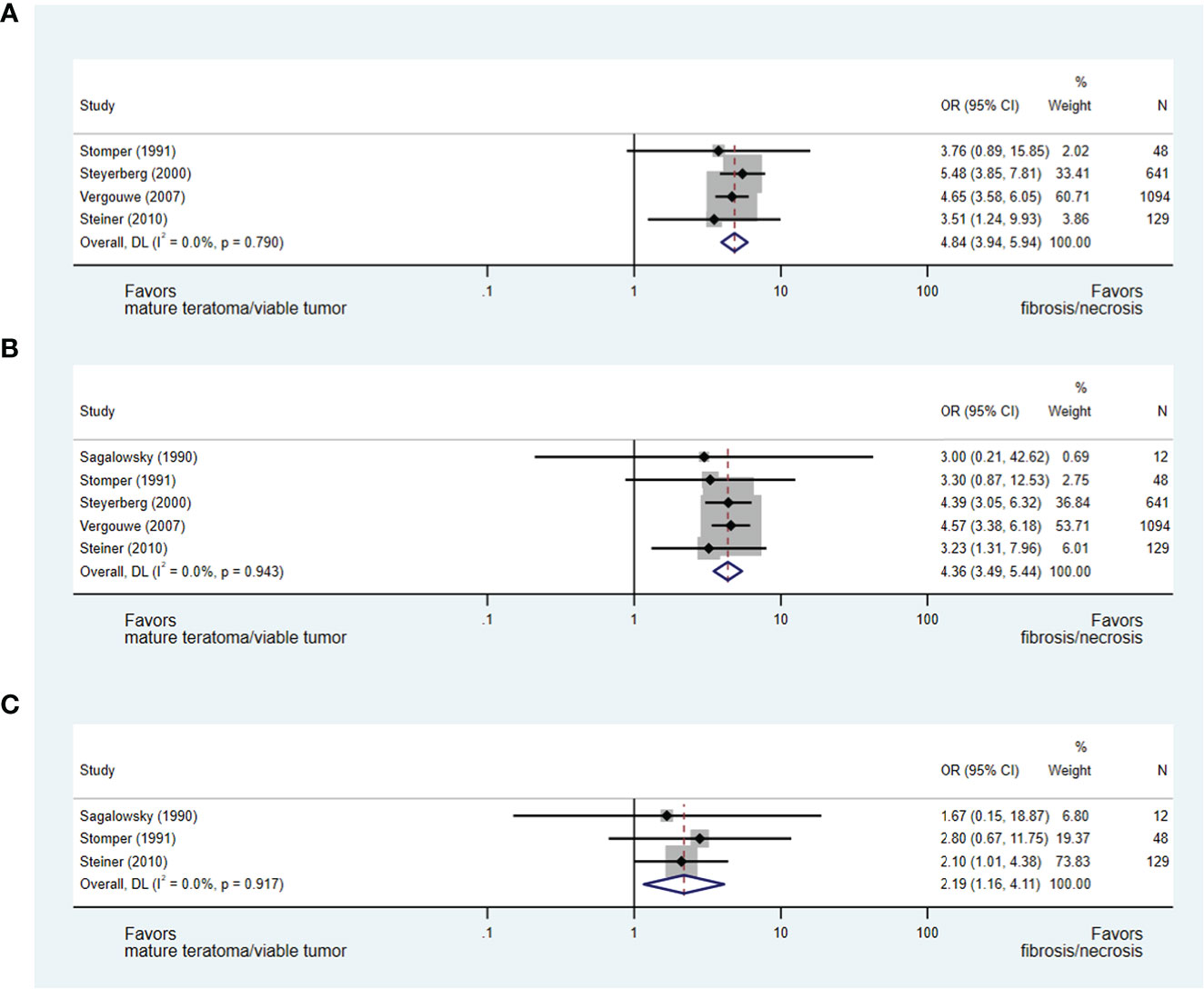
Figure 4 Forest plot of eligible studies evaluating relationship between ≥50% (A), ≥70% (B) and ≥90% (C) change in mass size during chemotherapy and necrosis/fibrosis at pcRPLND.
Post-chemotherapy residual mass size and histopathology
The size of residual mass was evaluated in several eligible papers; however, only 10 studies utilising consistent parameters for the measurement of the residual mass were evaluable (15, 17, 34, 36–38, 40, 42–44). Each evaluable paper described the measurement of the longest transverse (axial) dimension to assign patients to groups defined as: less than or greater than 2 cm and less than or greater than 5 cm. Notably, Vergouwe et al. (37) included 136 (12%) patients proceeding to pcRPLND with residual masses <1 cm.
Ultimately, in an analysis of seven studies (15, 17, 36–38, 40, 42), which addressed the predictive value of a residual mass size of less than or greater than 2 cm for necrosis/fibrosis and included 2,126 patients (including 6% with post-chemotherapy residual masses <1 cm), a residual mass size of <2 cm was significantly associated with necrosis/fibrosis over residual teratoma or viable GCT when compared with residual masses measuring >2 cm (OR 3.93, 95% CI 3.23-4.77). When a residual mass size of less than or greater than 5 cm was analysed in eight studies and 2,132 patients (15, 17, 34, 37, 40, 42–44), a residual mass of <5 cm was also predictive of necrosis/fibrosis at pcRPLND (OR 4.13, 95% CI 3.26-5.23) (see Figures 5A, B).
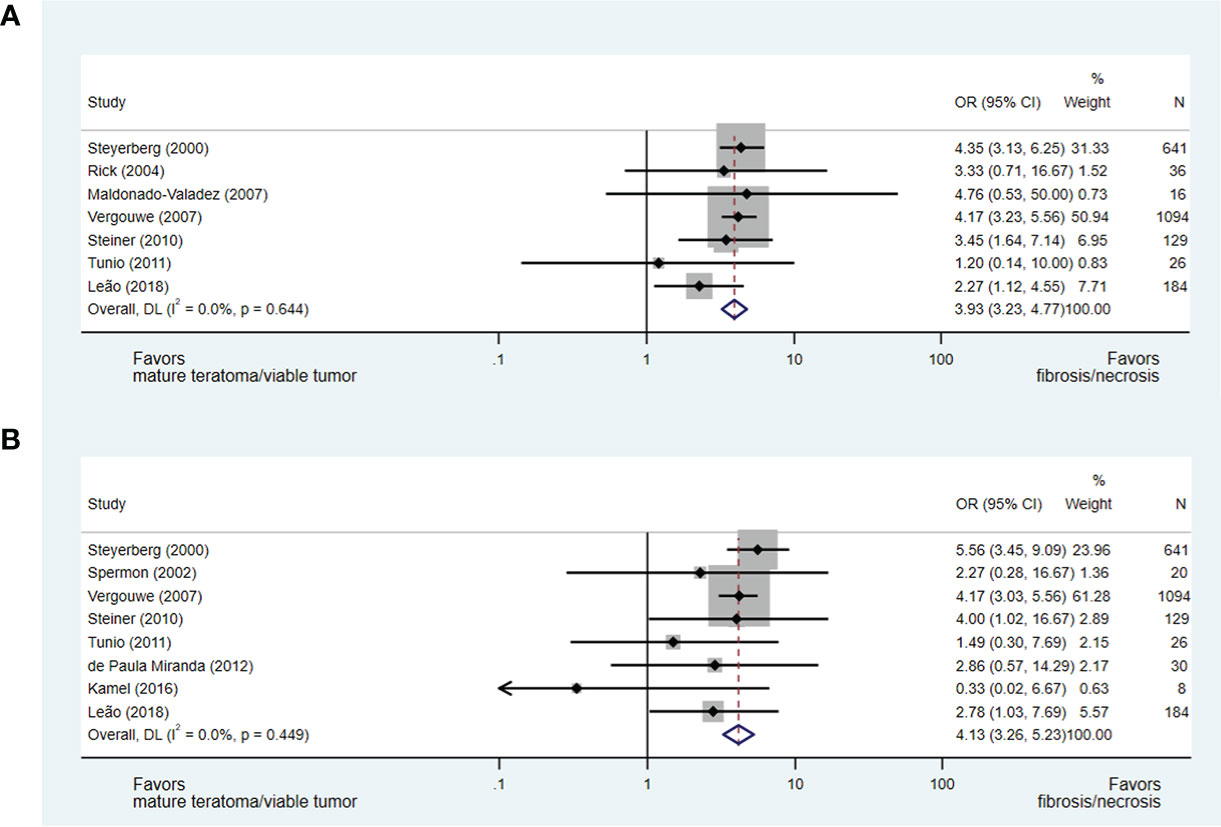
Figure 5 Forest plot of eligible studies evaluating relationship between residual mass size <2cm (A), <5cm (B) and necrosis/fibrosis at pcRPLND.
International Germ Cell Cancer Collaborative Group prognostic group and post-chemotherapy retroperitoneal lymph node dissection histology
The predictive value of IGCCCG prognostic groups for pcRPLND necrosis/fibrosis was evaluable in six studies of 794 patients (35, 36, 46–49) (see Supplement 2). There was no significant association of necrosis/fibrosis at pcRPLND between IGCCCG good-risk NSGCT compared to IGCCCG intermediate-risk (OR 0.97, 95% CI 0.48-1.97), poor-risk (OR 0.72, 95% CI 0.40-1.31), and intermediate- or poor-risk disease (OR 1.12, 95% CI 0.66-1.93).
Studies of heterogeneity and publication bias
Apart from the analyses of the relationship between pcRPLND histopathology and the IGCCCG prognostic group, which revealed moderate heterogeneity (I2 = 35%–52%), there was minimal heterogeneity amongst the eligible studies in other analyses (I2 = 0%–13.5%). Small-study effects were not detected for any pooled analyses (see Supplement 3).
Discussion
The aggressive resection of residual masses in advanced NSGCT represents an integral component of treatment for many patients (5). pcRPLND shields them from risks associated with growing teratoma syndrome, late relapse, or progressive malignancy; however, pcRPLND exposes up to half of patients with post-chemotherapy residual masses to the short- and long-term hazards of surgery with no therapeutic value (13, 14). While clinicopathologic factors predicting pcRPLND histopathology have been published (5, 18), this is the first meta-analysis to comprehensively summarise contemporary literature of the value of clinicopathologic variables in predicting necrosis/fibrosis.
Principal findings
We confirmed several key clinical findings reported in earlier studies (5, 18). Clinicopathologic factors associated with necrosis/fibrosis rather than teratoma or viable tumour within the residual mass at pcRPLND included: the absence of teratomatous elements in orchidectomy; a greater change in mass size during chemotherapy and a smaller size of post-chemotherapy residual mass; seminoma as a component of a mixed GCT resected at orchidectomy; and normal serum bHCG and AFP at the commencement of chemotherapy. The single clinicopathologic variable most predictive of necrosis/fibrosis was a >50% change in mass size during chemotherapy, which was 4.8× more likely to contain necrosis/fibrosis than teratoma/viable tumour at pcRPLND, when compared to masses undergoing <50% change during chemotherapy. Notably, the IGCCCG prognostic group did not significantly interact with pcRPLND histopathology. This has been identified previously in the abstract form where IGCCCG prognostic groups had little effect in predicting the pcRPLND pathology in a retrospective German series of 392 patients (61). Whilst some studies reported the presence of lymphovascular invasion (17, 62) in the orchidectomy sample as an additional predictor of pcRPLND histopathology, we were unable to include it in our meta-analysis as insufficient patient-level data were available to permit the calculation of OR for these covariates. The incorporation of craniocaudal lymph node length (63) as a predictor of pcRPLND histopathology was also not possible based on eligible studies; however, it would be important in the design of prospective studies of this issue.
Strengths and limitations of the study
Our study has several strengths. Firstly, the meta-analysis was conducted using a prospectively registered protocol and in accordance with PRISMA guidelines. Secondly, it incorporated citations from the three most comprehensive databases within the field and it is unlikely that citations have been inadvertently excluded from consideration. Finally, the analysis represents data from 3,390 eligible patients and is the largest analysis of clinicopathologic variables predicting pcRPLND histopathology in NSGCT, providing a platform to propel ongoing research in this area.
However, we acknowledge a number of limitations. Firstly, as the eligible studies were almost exclusively retrospective in their design, confounding variables, such as the choice of chemotherapy regimen and number of cycles was not routinely captured. Additionally, many studies evaluated alternative primary endpoints whilst reporting pcRPLND histopathology and as such, there were significant amounts of missing data. Furthermore, due to changes in staging systems and treatment patterns during the lifetime of the eligible studies, the impact of the disease stage and chemotherapy type on pcRPLND histopathology was unable to be evaluated. This speaks to the potential issue of heterogeneity within datasets, which has plagued the existing clinical models of this issue (5). However, heterogeneity was generally considered to be low in our analysis, except for the IGCCCG prognostic group. Additionally, the inclusion of a small number of participants with residual masses less than 1 cm (15, 37) and stage 1S disease [n=1 (46)], which lies outside current treatment recommendations, introduces possible bias. Additionally, the large dataset by Vergouwe at al (37). contributed a significant amount of weight (up to 55%) to some OR calculations exposing them to potential issues; however, funnel plots reassuringly demonstrated minimal publication bias. Studies only permitting the calculation of the predictive value of clinicopathologic variables for teratoma and not necrosis/fibrosis were also excluded, given the limited clinical relevance where the treatment of alternate histopathology is strictly divergent.
Implications for clinicians
Whilst our study evaluated almost 3,400 patients and was able to identify several clinicopathologic factors that predict necrosis/fibrosis, insufficient patient-level data were a recurrent barrier to study inclusion and will be required to aggregate clinically relevant models in the future. In rare tumours like testicular cancer, collaborative networks are needed to transform care. Existing clinical models have evaluated upwards of 1,500 patients cumulatively (15, 17, 37) and offer high specificity (>96%) and concordance (C-index >0.7) when predicting benign histology (necrosis/fibrosis) at pcRPLND. Unfortunately, each model has problems with diagnostic accuracy with false negatives, which may relate to the inclusion of heterogeneous populations recruited and analysed over several decades while the patterns of care change (5, 64). Additionally, these models lack prospective validation and clinical guidelines largely have not included them and continue to recommend the resection of all post-chemotherapy residual masses >1 cm in NSGCT provided that serum tumour markers are normal or plateauing (6–8). Where patients receiving treatment for testicular cancer will, on average, gain three decades of life following curative treatment (1), the risk of misclassifying an individual’s risk of residual teratoma or viable GCT is significant. Collaboration between leading centres treating testicular cancer will be required to move this forward in the coming years.
Meanwhile, pcRPLND continues to be a cornerstone of management for patients with NSGCT with residual masses (65, 66) erring on the side of over-treatment and preservation of cure. Historically, an open pcRPLND ensuring complete resection within a short time frame from the end of chemotherapy has been the preferred approach; however, robotic strategies are increasingly utilised in some centres to limit specific post-operative complications, such as the length of stay and post-operative pain and ileus (12, 67), but require an ongoing prospective evaluation to ensure equivalent oncologic outcomes. Regardless of the surgical strategy, referral to a high-volume centre specialising in pcRPLND is strongly recommended (8) to aid in clinical decision-making, limit potential complications, and improve the chances of a cure.
Newer techniques are required to allow a safe de-escalation of therapies in testicular cancer with a view to directing treatment towards those most likely to yield benefits and protecting the quality of life in survivors (2). One potential approach in the evaluation of post-chemotherapy residual masses in advanced NSGCT (68) is the use of microribonucleic acids (miRNAs). miR-371a-3p, -373-3p and -367-3p have been evaluated and demonstrate relatively higher serum levels when residual viable tumour was identified at pcRPLND. Unfortunately, in the same analysis, miR-371a-3p was unable to differentiate between necrosis/fibrosis and teratoma, which classically expresses lower levels of miR-371 compared to other testicular cancer subtypes, leaving an area of clinical need (69), and the studies of miR-375 have also yielded variable results (70, 71). No studies of miRNA were eligible for our analysis, and research into other candidate biomarkers is also underway (72–75).
In summary, this meta-analysis of clinicopathologic variables predicting necrosis/fibrosis at pcRPLND in NSGCT has confirmed the presence of important clinicopathologic factors, which may assist in directing personalised treatment to patients, whilst other technologies are awaited. A collaboration between key international research groups to contribute individual patient-level data, as well as the inclusion of novel molecular techniques, is required to develop and prospectively validate a clinical model for use in the clinic.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors made substantial contributions to the conceptionand design of the work. CC, WH and FM made substantial contributions to the acquisition, analysis and interpretation ofdata. CC was responsible for the drafting the manuscript. JL provided critical supervision and guidance to conception,acquisition, interpretation and drafting. All authors provided critical revision for important intellectual content and approval of the final version to be submitted.
Conflict of interest
BTr reports grants and personal fees from Amgen, grants and personal fees from Astra Zeneca, grants from Astellas, grants and personal fees from BMS, grants and personal fees from Janssen, grants and personal fees from Pfizer, grants and personal fees from MSD, grants and personal fees from Ipsen, personal fees from IQVIA, personal fees from Sanofi, personal fees from Tolmar, personal fees from Novartis, grants and personal fees from Bayer and personal fees from Roche, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.931509/full#supplementary-material
Supplement 1 | Forest plot of eligible studies evaluating relationship between presence of embryonal carcinoma (A) and yolk sac tumour (B) within orchidectomy and necrosis/fibrosis at pcRPLND.
Supplement 2 | Forest plot of eligible studies evaluating relationship between IGCCCG (A) good- versus intermediate-risk, (B) good- versus poor-risk and (C) good- versus intermediate- or poor-risk and necrosis/fibrosis at pcRPLND.
Supplement 3 | Funnel plots of publication bias in analyses of (A) primary teratoma, (B) primary seminoma, (C), normal pre-chemotherapy AFP, (D) normal pre-chemotherapy bHCG, (E) normal pre-chemotherapy LDH, (F) greater than or equal to 50% change in mass during chemotherapy, (G) greater than or equal to 70% change in mass during chemotherapy, (H) greater than or equal to 90% change in mass during chemotherapy, (I) residual mass size less than or greater than 2cm, and (J) residual mass size of less than or greater than 5cm for necrosis/fibrosis at pcRPLND.
References
1. Hanna NH, Einhorn LH. Testicular cancer–discoveries and updates. N Engl J Med (2014) 371(21):2005–16. doi: 10.1056/NEJMra1407550
2. Gillessen S, Sauvé N, Collette L, Daugaard G, de Wit R, Albany C, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): Results from the IGCCCG update consortium. J Clin Oncol (2021) 39(14):1563–74. doi: 10.1200/JCO.20.03296
3. Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J Clin Oncol (2010) 28(30):4649–57. doi: 10.1200/JCO.2010.29.9362
4. Kerns SL, Fung C, Monahan PO, Ardeshir-Rouhani-Fard S, Abu Zaid MI, Williams AM, et al. Cumulative burden of morbidity among testicular cancer survivors after standard cisplatin-based chemotherapy: A multi-institutional study. J Clin Oncol (2018) 36(15):1505–12. doi: 10.1200/JCO.2017.77.0735
5. Heidenreich A, Pfister D. Retroperitoneal lymphadenectomy and resection for testicular cancer: An update on best practice. Ther Adv Urol (2012) (4):187–205. doi: 10.1177/1756287212443170
6. Heidenreich A, Haidl F, Paffenholz P, Pape C, Neumann U, Pfister D. Surgical management of complex residual masses following systemic chemotherapy for metastatic testicular germ cell tumours. Ann Oncol (2017) 28(2):362–7. doi: 10.1093/annonc/mdw605
7. Oldenburg J, Berney DM, Bokemeyer C, Climent M, Daugaard G, Gietema JA, et al. Testicular seminoma and non-seminoma: ESMO-EURACAN clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33:362–75. doi: 10.1016/j.annonc.2022.01.002
8. National Comprehensive Cancer Network. Testicular cancer, version 2.2022. In: NCCN clinical practice guidelines in oncology (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf.
9. Donohue JP, Thornhill JA, Foster RS, Bihrle R. Vascular considerations in postchemotherapy. Retroperitoneal lymph-node dissection: Part II. World J Urol. (1994) 12(4):187–9. doi: 10.1007/BF00185669
10. McMahon CG, Abdo C, Incrocci L, Perelman M, Rowland D, Waldinger M, et al. Disorders of orgasm and ejaculation in men. J Sex Med (2004) 1(1):58–65. doi: 10.1111/j.1743-6109.2004.10109.x
11. Pearce SM, Golan S, Gorin MA, Luckenbaugh AN, Williams SB, Ward JF, et al. Safety and early oncologic effectiveness of primary robotic retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer. Eur Urol. (2017) 71(3):476–82. doi: 10.1016/j.eururo.2016.05.017
12. Gerdtsson A, Håkansson U, Törnblom M, Jancke G, Negaard HFS, Glimelius I, et al. Surgical complications in postchemotherapy retroperitoneal lymph node dissection for nonseminoma germ cell tumour: A population-based study from the Swedish Norwegian testicular cancer group. Eur Urol Oncol (2020) 3(3):383–9. doi: 10.1016/j.euo.2019.08.002
13. Carver BS, Serio AM, Bajorin D, Motzer RJ, Stasi J, Bosl GJ, et al. Improved clinical outcome in recent years for men with metastatic nonseminomatous germ cell tumors. J Clin Oncol (2007) 25(35):5603–8. doi: 10.1200/JCO.2007.13.6283
14. Heidenreich A, Pfister D, Witthuhn R, Thüer D, Albers P. Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol. (2009) 55(1):217–24. doi: 10.1016/j.eururo.2008.09.027
15. Steyerberg EW, Keizer HJ, Fosså SD, Sleijfer DT, Toner GC, Schraffordt Koops H, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: Multivariate analysis of individual patient data from six study groups. J Clin Oncol (1995) 13(5):1177–87. doi: 10.1200/JCO.1995.13.5.1177
16. Vergouwe Y, Steyerberg EW, de Wit R, Roberts JT, Keizer HJ, Collette L, et al. External validity of a prediction rule for residual mass histology in testicular cancer: An evaluation for good prognosis patients. Br J Cancer. (2003) 88(6):843–7. doi: 10.1038/sj.bjc.6600759
17. Leão R, Nayan M, Punjani N, Jewett MAS, Fadaak K, Garisto J, et al. A new model to predict benign histology in residual retroperitoneal masses after chemotherapy in nonseminoma. Eur Urol Focus. (2018) 4(6):995–1001. doi: 10.1016/j.euf.2018.01.015
18. Vergouwe Y, Steyerberg EW, Foster RS, Habbema JD, Donohue JP. Validation of a prediction model and its predictors for the histology of residual masses in nonseminomatous testicular cancer. J Urol. (2001) 165(1):84–8. doi: 10.1097/00005392-200101000-00021
19. Conduit C, Hong W. A review of clinicopathologic features that predict residual invasive germ cell tumour or teratoma at post-chemotherapy retroperitoneal lymph node dissection in individuals receiving treatment for non-seminoma. In: PROSPERO (2021). Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021279699.
20. Moons C, Hooft L, Hayden J eds. Systematic reviews of prognosis studies II: Assessing bias in studies of prognostic factors using the QUIPS tool. In: Abstracts of the global evidence summit. Cape Town, South Africa: Cochrane Database of Systematic Reviews.
21. Comisarow RH, Grabstald H. Re-exploration for retroperitoneal lymph node metastases from testis tumors. J Urol. (1976) 115(5):569–71. doi: 10.1016/S0022-5347(17)59286-1
22. Suurmeijer AJ, Oosterhuis JW, Sleijfer DT, Koops HS, Fleuren GJ. Non-seminomatous germ cell tumors of the testis: Morphology of retroperitoneal lymph node metastases after chemotherapy. Eur J Cancer Clin Oncol (1984) 20(6):727–34. doi: 10.1016/0277-5379(84)90208-6
23. Jaeger N, Weissbach L, Vahlensieck W. Second-look lymphadenectomy in the treatment of germ cell testis tumors. Eur Urol. (1984) 10(1):10–6. doi: 10.1159/000463503
24. Pizzocaro G, Salvioni R, Pasi M, Zanoni F, Milani A, Pilotti S, et al. Early resection of residual tumor during cisplatin, vinblastine, bleomycin combination chemotherapy in stage III and bulky stage II nonseminomatous testicular cancer. Cancer (1985) 56(2):249–55. doi: 10.1002/1097-0142(19850715)56:2<249::AID-CNCR2820560207>3.0.CO;2-8
25. Peckham MJ, Hendry WF. Clinical stage II non-seminomatous germ cell testicular tumours. results of management by primary chemotherapy. Br J Urol. (1985) 57(6):763–8. doi: 10.1111/j.1464-410x.1985.tb07050.x.
26. Dexeus FH, Shirkhoda A, Logothetis CJ, Chong C, Sella A, Ogden S, et al. Clinical and radiological correlation of retroperitoneal metastasis from nonseminomatous testicular cancer treated with chemotherapy. Eur J Cancer Clin Oncol (1989) 25(1):35–43. doi: 10.1016/0277-5379(89)90048-5
27. Sagalowsky AI, Ewalt DH, Molberg K, Peters PC. Predictors of residual mass histology after chemotherapy for advanced testis cancer. Urology (1990) 35(6):537–42. doi: 10.1016/0090-4295(90)80112-Z
28. Stomper PC, Kalish LA, Garnick MB, Richie JP, Kantoff PW. CT and pathologic predictive features of residual mass histologic findings after chemotherapy for nonseminomatous germ cell tumors: can residual malignancy or teratoma be excluded? Radiology (1991) 180(3):711–4. doi: 10.1148/radiology.180.3.1651526
29. Tekgul S, Ozen HA, Celebi I, Ozgu I, Ergen A, Demircin M, et al. Postchemotherapeutic surgery for metastatic testicular germ cell tumors: Results of extended primary chemotherapy and limited surgery. Urology (1994) 43(3):349–54. doi: 10.1016/0090-4295(94)90078-7
30. Matsuyama H, Yamamoto N, Sakatoku J, Suga A, Hayashida S, Kamiryo Y, et al. Predictive factors for the histologic nature of residual tumor mass after chemotherapy in patients with advanced testicular cancer. Urology (1994) 44(3):392–8. doi: 10.1016/S0090-4295(94)80099-5
31. Rabbani F, Gleave ME, Coppin CM, Murray N, Sullivan LD. Teratoma in primary testis tumor reduces complete response rates in the retroperitoneum after primary chemotherapy. The case for primary retroperitoneal lymph node dissection of stage IIb germ cell tumors with teratomatous elements. Cancer (1996) 78(3):480–6. doi: 10.1002/(SICI)1097-0142(19960801)78:3<480::AID-CNCR15>3.0.CO;2-V
32. Steyerberg EW, Keizer HJ, Sleijfer DT, Fossâ SD, Bajorin DF, Gerl A, et al. Retroperitoneal metastases in testicular cancer: Role of CT measurements of residual masses in decision making for resection after chemotherapy. Radiology (2000) 215(2):437–44. doi: 10.1148/radiology.215.2.r00ma02437
33. Nonomura N, Nishimura K, Takaha N, Inoue H, Nomoto T, Mizutani Y, et al. Nerve-sparing retroperitoneal lymph node dissection for advanced testicular cancer after chemotherapy. Int J Urol. (2002) 9(10):539–44. doi: 10.1046/j.1442-2042.2002.00520.x
34. Spermon JR, De Geus-Oei LF, Kiemeney LA, Witjes JA, Oyen WJ. The role of (18)fluoro-2-deoxyglucose positron emission tomography in initial staging and re-staging after chemotherapy for testicular germ cell tumours. BJU Int (2002) 89(6):549–56. doi: 10.1046/j.1464-410X.2002.02641.x
35. Oldenburg J, Alfsen GC, Lien HH, Aass N, Waehre H, Fossa SD. Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol (2003) 21(17):3310–7. doi: 10.1200/JCO.2003.03.184
36. Rick O, Bokemeyer C, Weinknecht S, Schirren J, Pottek T, Hartmann JT, et al. Residual tumor resection after high-dose chemotherapy in patients with relapsed or refractory germ cell cancer. J Clin Oncol (2004) 22(18):3713–9. doi: 10.1200/JCO.2004.07.124
37. Vergouwe Y, Steyerberg EW, Foster RS, Sleijfer DT, Fosså SD, Gerl A, et al. Predicting retroperitoneal histology in postchemotherapy testicular germ cell cancer: a model update and multicentre validation with more than 1000 patients. Eur Urol. (2007) 51(2):424–32. doi: 10.1016/j.eururo.2006.06.047
38. Maldonado-Valadez R, Schilling D, Anastasiadis AG, Sturm W, Stenzl A, Corvin S. Post-chemotherapy laparoscopic retroperitoneal lymph-node dissection in testis cancer patients. J Endourol. (2007) 21(12):1501–4. doi: 10.1089/end.2006.0441
39. Schrader AJ, Seger M, Konrad L, Olbert P, Hegele A, Hofmann R, et al. Clinical impact of MDR1-expression in testicular germ cell cancer. Exp Oncol (2007) 29(3):212–6.
40. Steiner H, Berg B, Stöhr B, Fritzer A, Ramoner R, Aigner F, et al. Prediction of retroperitoneal histology in metastatic nonseminomatous testicular cancer patients after chemotherapy based on clinical and radiological parameters. Curr Urol. (2010) 4:142–51. doi: 10.1159/000253441
41. Akbulut Z, Canda AE, Atmaca AF, Caglayan A, Asil E, Balbay MD. Is positron emission tomography reliable to predict post-chemotherapy retroperitoneal lymph node involvement in advanced germ cell tumors of the testis? Urol J (2011) 8(2):120–6.
42. Tunio M, Hashmi A, Naimutallah N, Mohsin R, Maqbool A. Fifteen years of experience in the management of non-seminomatous testicular germ cell tumors at a tertiary care center in Pakistan. Pak J Med Sci (2011) 27(4):806.
43. de Paula Miranda E, Abe DK, Nesrallah AJ, dos Reis ST, Crippa A, Srougi M, et al. Predicting necrosis in residual mass analysis after retroperitoneal lymph node dissection: a retrospective study. World J Surg Oncol (2012) 10:203. doi: 10.1186/1477-7819-10-203
44. Kamel MH, Littlejohn N, Cox M, Eltahawy EA, Davis R. Post-chemotherapy robotic retroperitoneal lymph node dissection: Institutional experience. J Endourol. (2016) 30(5):510–9. doi: 10.1089/end.2015.0673
45. Öztürk Ç, Been LB, van Ginkel RJ, Gietema JA, Hoekstra HJ. Laparoscopic resection of residual retroperitoneal tumor mass in advanced nonseminomatous testicular germ cell tumors; a feasible and safe oncological procedure. Sci Rep (2019) 9(1):15837. doi: 10.1038/s41598-019-52109-5
46. King J, Kawakami J, Heng D, Gan CL. Post-chemotherapy retroperitoneal lymph node dissection for non-seminomatous germ cell tumors: A single-surgeon, Canadian experience. Can Urol Assoc J (2020) 14(9):E407–e11. doi: 10.5489/cuaj.6219
47. Baessler B, Nestler T, Pinto Dos Santos D, Paffenholz P, Zeuch V, Pfister D, et al. Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur Radiol (2020) 30(4):2334–45. doi: 10.1007/s00330-019-06495-z
48. Taza F, Chovanec M, Snavely A, Hanna NH, Cary C, Masterson TA, et al. Prognostic value of teratoma in primary tumor and postchemotherapy retroperitoneal lymph node dissection specimens in patients with metastatic germ cell tumor. J Clin Oncol (2020) 38(12):1338–45. doi: 10.1200/JCO.19.02569
49. Malik K, Raja A, Radhakrishnan V, Kathiresan N. A retrospective analysis of patients undergoing postchemotherapy retroperitoneal lymph node dissection and metastasectomy in advanced nonseminomatous germ cell tumors. Indian J Urol. (2020) 36(2):112–6. doi: 10.4103/iju.IJU_301_19
50. Gelderman WA, Koops HS, Sleijfer DT, Oosterhuis JW, Oldhoff J. Treatment of retroperitoneal residual tumor after PVB chemotherapy of nonseminomatous testicular tumors. Cancer (1986) 58(7):1418–21. doi: 10.1002/1097-0142(19861001)58:7<1418::AID-CNCR2820580706>3.0.CO;2-T
51. Nijman JM, Schraffordt Koops H, Kremer J, Sleijfer DT. Gonadal function after surgery and chemotherapy in men with stage II and III nonseminomatous testicular tumors. J Clin Oncol (1987) 5(4):651–6. doi: 10.1200/JCO.1987.5.4.651
52. Donohue JP, Rowland RG, Kopecky K, Steidle CP, Geier G, Ney KG, et al. Correlation of computerized tomographic changes and histological findings in 80 patients having radical retroperitoneal lymph node dissection after chemotherapy for testis cancer. J Urol. (1987) 137(6):1176–9. doi: 10.1016/S0022-5347(17)44439-9
53. Gelderman WA, Schraffordt Koops H, Sleijfer DT, Oosterhuis JW, van der Heide JN, Mulder NH, et al. Results of adjuvant surgery in patients with stage III and IV nonseminomatous testicular tumors after cisplatin-vinblastine-bleomycin chemotherapy. J Surg Oncol (1988) 38(4):227–32. doi: 10.1002/jso.2930380405
54. Fosså SD, Aass N, Ous S, Høie J, Stenwig AE, Lien HH, et al. Histology of tumor residuals following chemotherapy in patients with advanced nonseminomatous testicular cancer. J Urol. (1989) 142(5):1239–42. doi: 10.1016/S0022-5347(17)39044-4
55. Fosså SD, Ous S, Lien HH, Stenwig AE. Post-chemotherapy lymph node histology in radiologically normal patients with metastatic nonseminomatous testicular cancer. J Urol. (1989) 141(3):557–9. doi: 10.1016/S0022-5347(17)40892-5
56. Toner GC, Panicek DM, Heelan RT, Geller NL, Lin SY, Bajorin D, et al. Adjunctive surgery after chemotherapy for nonseminomatous germ cell tumors: recommendations for patient selection. J Clin Oncol (1990) 8(10):1683–94. doi: 10.1200/JCO.1990.8.10.1683
57. Mulders PF, Oosterhof GO, Boetes C, de Mulder PH, Theeuwes AG, Debruyne FM. The importance of prognostic factors in the individual treatment of patients with disseminated germ cell tumours. Br J Urol. (1990) 66(4):425–9. doi: 10.1111/j.1464-410X.1990.tb14967.x
58. de Graaff WE, Oosterhuis JW, van der Linden PJ, van der Heide JN, Koops HS, Sleijfer D. Residual mature teratoma after chemotherapy for nonseminomatous germ cell tumors of the testis occurs significantly less often in lung than in retroperitoneal lymph node metastases. J Urogen Pathol (1991) 1:75–81.
59. Fosså SD, Qvist H, Stenwig AE, Lien HH, Ous S, Giercksky KE. Is postchemotherapy retroperitoneal surgery necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses? J Clin Oncol (1992) 10(4):569–73. doi: 10.1200/JCO.1992.10.4.569
60. Steyerberg EW, Keizer HJ, Zwartendijk J, Van Rijk GL, Van Groeningen CJ, Habbema JDF, et al. Prognosis after resection of residual masses following chemotherapy for metastatic nonseminomatous testicular cancer: a multivariate analysis. Br J Cancer. (1993) 68:195–200. doi: 10.1038/bjc.1993.313
61. Pfister D, Busch J, Winter A, Albers P, Schrader M, Dieckmann KP. Pathohistological findings in patients with nonseminomatous germ cell tumours who undergo postchemotherapy retroperitoneal lymph node dissection for small tumours. J Urol (2011) 29(7_suppl):224. doi: 10.1016/j.juro.2011.02.650
62. Dusaud M, Malavaud B, Bayoud Y, Sebe P, Hoepffner JL, Salomon L, et al. Post-chemotherapy retroperitoneal teratoma in nonseminomatous germ cell tumors: Do predictive factors exist? results from a national multicenter study. J Surg Oncol (2016) 114(8):992–6. doi: 10.1002/jso.24464
63. Howard SA, Gray KP, O'Donnell EK, Fennessy FM, Beard CJ, Sweeney CJ. Craniocaudal retroperitoneal node length as a risk factor for relapse from clinical stage I testicular germ cell tumor. Am J Roentgenol (2014) 203(4):W415–20. doi: 10.2214/AJR.13.11615
64. Paffenholz P, Nestler T, Hoier S, Pfister D, Hellmich M, Heidenreich A. External validation of 2 models to predict necrosis/fibrosis in postchemotherapy residual retroperitoneal masses of patients with advanced testicular cancer. Urol Oncol (2019) 37(11):809.e9–.e18. doi: 10.1016/j.urolonc.2019.07.021
65. Albers P, Weissbach L, Krege S, Kliesch S, Hartmann M, Heidenreich A, et al. Prediction of necrosis after chemotherapy of advanced germ cell tumors: results of a prospective multicenter trial of the German testicular cancer study group. J Urol. (2004) 171(5):1835–8. doi: 10.1097/01.ju.0000119121.36427.09
66. Daneshmand S, Albers P, Fosså SD, Heidenreich A, Kollmannsberger C, Krege S, et al. Contemporary management of postchemotherapy testis cancer. Eur Urol. (2012) 62(5):867–76. doi: 10.1016/j.eururo.2012.08.014
67. Rodrigues GJ, Guglielmetti GB, Orvieto M, Seetharam Bhat KR, Patel VR, Coelho RF. Robot-assisted retroperitoneal lymphadenectomy: The state of art. Asian J Urol. (2021) 8(1):27–37. doi: 10.1016/j.ajur.2020.09.002
68. Leão R, van Agthoven T, Figueiredo A, Jewett MAS, Fadaak K, Sweet J, et al. Serum miRNA predicts viable disease after chemotherapy in patients with testicular nonseminoma germ cell tumor. J Urol. (2018) 200(1):126–35. doi: 10.1016/j.juro.2018.02.068
69. Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. (2016) 13(12):715–25. doi: 10.1038/nrurol.2016.170
70. Lafin J, Kenigsberg A, Meng X, Abe D, Savelyeva A, Singla N, et al. Serum small RNA sequencing and miR-375 assay do not identify the presence of pure teratoma at postchemotherapy retroperitoneal lymph node dissection. Eur Urol Open Science. (2021) 26:83–7. doi: 10.1016/j.euros.2021.02.003
71. Nappi L, Thi M, Adra N, Hamilton RJ, Leao R, Lavoie JM, et al. Integrated expression of circulating miR375 and miR371 to identify teratoma and active germ cell malignancy components in malignant germ cell tumors. Eur Urol. (2021) 79(1):16–9. doi: 10.1016/j.eururo.2020.10.024
72. Fichtner A, Richter A, Filmar S, Gaisa NT, Schweyer S, Reis H, et al. The detection of isochromosome i(12p) in malignant germ cell tumours and tumours with somatic malignant transformation by the use of quantitative real-time polymerase chain reaction. Histopathology (2021) 78(4):593–606. doi: 10.1111/his.14258
73. Freitag CE, Sukov WR, Bryce AH, Berg JV, Vanderbilt CM, Shen W, et al. Assessment of isochromosome 12p and 12p abnormalities in germ cell tumors using fluorescence in situ hybridization, single-nucleotide polymorphism arrays, and next-generation sequencing/mate-pair sequencing. Hum Pathol (2021) 112:20–34. doi: 10.1016/j.humpath.2021.03.008
74. Nestler T, Kremer L, Wagener-Ryczek S, Wittersheim M, von Brandenstein M, Paffenholz P, et al. Differentially expressed mRNA/proteins can distinguish viable germ cell tumors and teratomas from necrosis in retroperitoneal lymph node resections after chemotherapy (pcRPLND). J Clin Oncol (2022) 40(suppl 6; abstr 408):408. doi: 10.1200/JCO.2022.40.6_suppl.408
75. Pfister D, Seger M, Schrader AJ, Ohlmann CH, Heidenreich A. 588: Prediction of residual retroperitoneal mass histology following postchemotherapy retroperitoneal surgery for metastatic nonseminomatous germ cell tumors: Role of MDR-1 and mismatch repair genes. J Urol (2007) 177:197. doi: 10.1016/S0022-5347(18)30828-0
Keywords: testicular neoplasms, germinoma, teratoma, pathology, meta-analysis
Citation: Conduit C, Hong W, Martin F, Thomas B, Lawrentschuk N, Goad J, Grimison P, Ahmadi N, Tran B and Lewin J (2022) A meta-analysis of clinicopathologic features that predict necrosis or fibrosis at post-chemotherapy retroperitoneal lymph node dissection in individuals receiving treatment for non-seminoma germ cell tumours. Front. Oncol. 12:931509. doi: 10.3389/fonc.2022.931509
Received: 29 April 2022; Accepted: 18 July 2022;
Published: 17 August 2022.
Edited by:
Ricardo Leao, University of Coimbra, PortugalReviewed by:
Ardalanejaz Ahmad, University Health Network (UHN), CanadaDiogo Alpuim Costa, CUF Oncologia, Portugal
Copyright © 2022 Conduit, Hong, Martin, Thomas, Lawrentschuk, Goad, Grimison, Ahmadi, Tran and Lewin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy Lewin, amVyZW15Lmxld2luQHBldGVybWFjLm9yZw==
 Ciara Conduit
Ciara Conduit Wei Hong
Wei Hong Felicity Martin1
Felicity Martin1 Benjamin Thomas
Benjamin Thomas Nathan Lawrentschuk
Nathan Lawrentschuk Peter Grimison
Peter Grimison Nariman Ahmadi
Nariman Ahmadi Jeremy Lewin
Jeremy Lewin