- 1Department of Neurosurgery, Huashan Hospital, Shanghai Medical College, Fudan University, National Center for Neurological Disorders, National Key Lab. for Medical Neurobiology, Institutes of Brain Science, Shanghai Key Lab. of Brain Function and Regeneration, Institute of Neurosurgery, MOE Frontiers Center for Brain Science, Shanghai, China
- 2Department of Orthopaedics Surgery, Fudan University Huashan Hospital, Shanghai, China
Objective: To our knowledge, the impact of area-level socioeconomic status (SES) has not yet been described in primary central nervous system lymphomas (PCNSLs). Current study sought to explore the association of socioeconomic deprivation, measured using the Area Deprivation Index (ADI), with PCNSL outcomes.
Methods: The Surveillance, Epidemiology, and End Results (SEER) database was used to identify PCNSL patients diagnosed between 2006 and 2015 for our analyses. The impact of ADI on overall survival (OS) and cancer-specific survival (CSS) were investigated. Survival analyses were conducted using Kaplan-Meier method with log-rank tests. The Inverse Probability Weighting (IPW) analysis and multivariate cox proportional hazards regression analysis were employed to make covariate adjustments. Multiple mediation analysis (MMA) was performed to estimate the mediating effects.
Results: A total of 3159 PCNSL patients classified into low and high ADI subgroups according to the median ADI score were studied. The Kaplan-Meier analyses showed that low ADI was significantly associated with higher OS rates (HR 1.15, 95%CI 1.06-1.26, P<0.01) and CSS rates (HR 1.15, 95%CI 1.05-1.27, P<0.01). Similar results were observed in analyses adjusted via IPW and multivariate cox methods. Subgroup analyses revealed that ADI could remain a prognostic indictor among different subsets. MMA revealed that several factors including chemotherapy and HIV status making up about 40% of the overall effect, mediated PCNSL survival disparities related to the ADI. Finally, multivariable logistic regression analysis showed that ADI as well as several other factors were independently related to receipt of chemotherapy.
Conclusions: Our study highlights the role of area-level SES in prognosis of PCNSLs. And several factors including chemotherapy and HIV status of PCNSL patents contributed to the CSS disparities between ADI subgroups were uncovered by MMA. Such relationships would highlight the importance of policies development to enhance healthcare delivery and promote awareness of HIV prevention and treatment in low-resource neighborhoods.
Introduction
Primary central nervous system lymphoma (PCNSL) is a type of highly aggressive extranodal non-Hodgkin lymphoma that confined to the central nervous system (CNS) including leptomeninges, brain, eyes, or spinal cord without evidence of systemic disease (1–4). Immunosuppressive patients have a higher risk of developing this disease (5, 6). Historically, PCNSL has carried a poor prognosis with a 5-year survival of only 15-30% (1, 7). Regarding therapeutic measures, high-dose methotrexate-based systemic chemotherapy is identified as the standard first-line treatment (3, 4). Over the past decades, major progresses have been achieved in the treatment for this disease, however, significant disparities in PCNSL outcomes persist.
Among most tumors, black patients usually tend to have worse outcomes than white patients. And increasing research have shown many important clinical differences between cancer patients with different races and ethnicities (8–10). Also, socioeconomic status (SES), as measured by the state of income, wealth, education, occupation, and living conditions, has been found to be associated with cancer survival (11, 12). In PCNSL, few studies have examined the association of SES with disease outcomes. A previous study of PCNSL found that treatment selection in elderly patients was significantly influenced by sex, facility type, degree of urbanization, and type of insurance (13). Another observational study reported that lack of private insurance and residence in poorer areas were significantly associated with the worse outcomes in PCNSL patients (14). Many population-based studies using large database also have demonstrated that area-based SES was an important risk factor for worse prognosis across a variety of tumors (15, 16). However, these researches have tended to employ single-domain SES measures (income, education, poverty, etc.) or create overly simplistic composite neighborhood SES measures (16–19), which had been questioned as underexamined (20), thereby making inaccurate assessment. As a metrics of socioeconomic deprivation, the area deprivation index (ADI) integrates 17 measures of education, employment, housing quality, and poverty based on the long-form US Census data (21, 22); and it can be used to better assess PCNSL prognosis in the context of neighborhood socioeconomic disadvantage.
Therefore, using the Surveillance, Epidemiology, and End Results (SEER) database, we investigated the association of ADI with PCNSL outcomes to gain better insight into the impact of socioeconomic inequality. In-depth understanding the relation between socioeconomic deprivation and cancer prognosis may support policies for ongoing investments in lower-resource neighborhoods, thereby reducing health disparities.
Methods
Study population
We used the SEER database to extract research data. Patients diagnosed with PCNSL from 2006 to 2015 were obtained for our analyses. PCNSL patients were identified according to the International Classification of Diseases for Oncology Third Edition (ICD-O-3) histology codes (9590–9599, 9670–9699, 9700–9719, 9720–9729) with the location limited to the central nervous system, as demonstrated in our previous article (23). PCNSLs included in our analysis were restricted to primary cancers and patients diagnosed without histological confirmation or diagnosed by autopsy were excluded.
Area deprivation index and covariates
As a comprehensive composite measure of neighborhood SES, ADI can be used for county-level SES estimation. Based on the American Community Survey (ACS) 5-year estimates (2006 to 2010 and 2011 to 2015), we employed “sociome” package to calculate the ADI of each patient according to the patient’s five-digit geographic identifiers (24). And all patients were assigned into low- and high-group based on the median ADI score for further research. Data regarding patient demographics (age at diagnosis, sex, race, marital status, insurance status), tumor characteristics (tumor location and tumor histology types), treatment information (surgery type, radiotherapy and chemotherapy) and follow-up time were also extracted from SEER database. The primary endpoints of this study were overall survival (OS) and cancer-specific survival (CSS).
Statistical analysis
The χ2 tests were employed to compare distributions of categorical covariates stratified by ADI level among PCNSL patients. Survival analyses were conducted using Kaplan-Meier method with log-rank tests. Multivariate Cox proportional hazards regression analyses were employed to make covariate adjustments. For further results enhancement, the Inverse Probability Weighting (IPW) analysis was performed via a propensity model to adjust for imbalances by ADI (25). The absolute Standardized Mean Differences (SMDs) were calculated to verify the covariate balances after the IPW adjustment; and a difference of SMD equal to zero indicates ideal balance.
Subgroup analyses were conducted to examine the robustness of ADI effect. Furthermore, we used multiple mediation analysis (MMA) which proposed by Yu et al. to explore how much effect from multiple mediators/confounders involving in the ADI disparity on cancer-specific survival (26). We proposed a mediation model to identify the presence and relative contributions of factors that are influenced by the independent variable (ADI) and that may exert indirect effects on the PCNSL survival. Direct effect and indirect effect were estimated via MMA using “mma” package. Indirect effect is the different cancer-specific survival between low and high ADI groups that can be accounted for by selected mediators, while the direct effect is opposite. The purpose of this approach is to explore the existence and feasibility of identifying processes that mediate known ADI disparity. We hypothesize that the relationships between low-SES neighborhood and poor PCNSL outcomes are partially mediated/moderated by treatment or other factors. And by revealing such internal relationships, it would benefit the development of targeted policies for low-SES neighborhood. Finally, we performed multivariable logistic regression analysis to further evaluate the association between clinical and sociodemographic factors and chemotherapy use.
The R software version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses and a two-sided P value of <0.05 indicated statistically significant.
Results
Population characteristics
A total of 3159 PCNSL patients met the study’s eligibility criteria, with 1580 cases identified as low ADI patients and 1579 identified as high ADI patients, were further studied. The sociodemographic, clinical characteristics, as well as treatment information of the included patients are summarized in Table 1. Overall, most patients (61.3%) were 60 years old or older, about half of patients (53.2%) were male, and 78.6% were white. 42.2% of the tumors were supratentorial and DLBCL (78.7%) was the most common subtype. In terms of treatment measures, majority of patients (69.9%) received chemotherapy, whereas only 23.3% underwent surgical excision and 30.1% received radiotherapy. Additionally, the vast majority of patients were HIV negative. Comparison of patient characteristics between ADI subgroups showed that high ADI was significantly associated with black race, negative HIV status and no chemotherapy implement.
Survival analysis
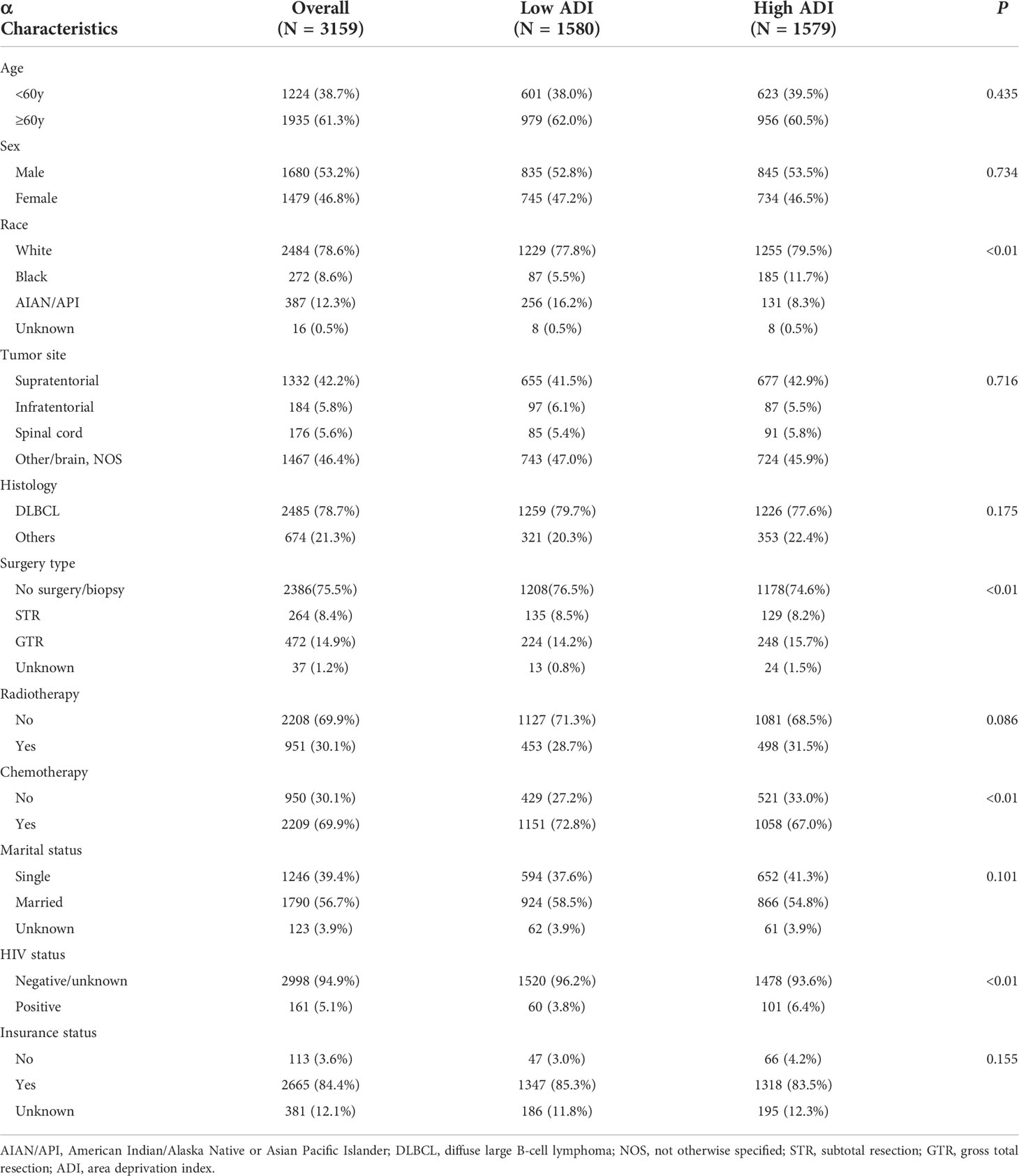
We then conducted Kaplan-Meier survival analysis of OS and CSS for low and high ADI patients. As shown Figure 1, in crude KM analysis, low ADI was significantly associated with higher OS rates (HR 1.15, 95%CI 1.06-1.26, P<0.01). The 1- and 3-year OS were 56.8% (95%CI, 54.4%-59.3%) and 44.3% (95%CI, 41.9%-47.0%) in low ADI cohort, whereas the corresponding OS were 52.7% (95%CI, 50.0%-55.2%) and 38.8% (95%CI, 36.4%-41.4%) in high ADI patients. In terms of endpoint of CSS, similar result with higher CSS rates in low ADI patients was also observed (HR 1.15, 95%CI 1.05-1.27, P<0.01). And the 1-year and 3-year CSS were 60.7% (95%CI, 58.3%-63.2%) and 49.4% (95%CI, 46.9%-52.1%) in low ADI group, whereas the corresponding CSS were 56.8% (95%CI, 54.4%-59.4%) and 44.1% (95%CI, 41.5%-46.8%) in high ADI group. To further intensify our findings, we additionally performed IPW analysis to adjust the potential confounding. Excellent balances between the two ADI groups were achieved regarding all covariates (Figure 2). And IPW-adjusted survival analysis showed that low ADI still demonstrated better OS and CSS [OS: IPTW-adjusted HR 1.10, 95%CI 1.01-1.20, P<0.01; CSS: IPTW-adjusted HR 1.10, 95%CI 1.00-1.21, P<0.01; Figure 1]. Multivariable Cox proportional hazards regression further revealed increased adjusted overall mortality (HR 1.10, 95%CI 1.01-1.20) and cancer-specific mortality (HR1.10, 95%CI 1.00-1.21) for high ADI patients.
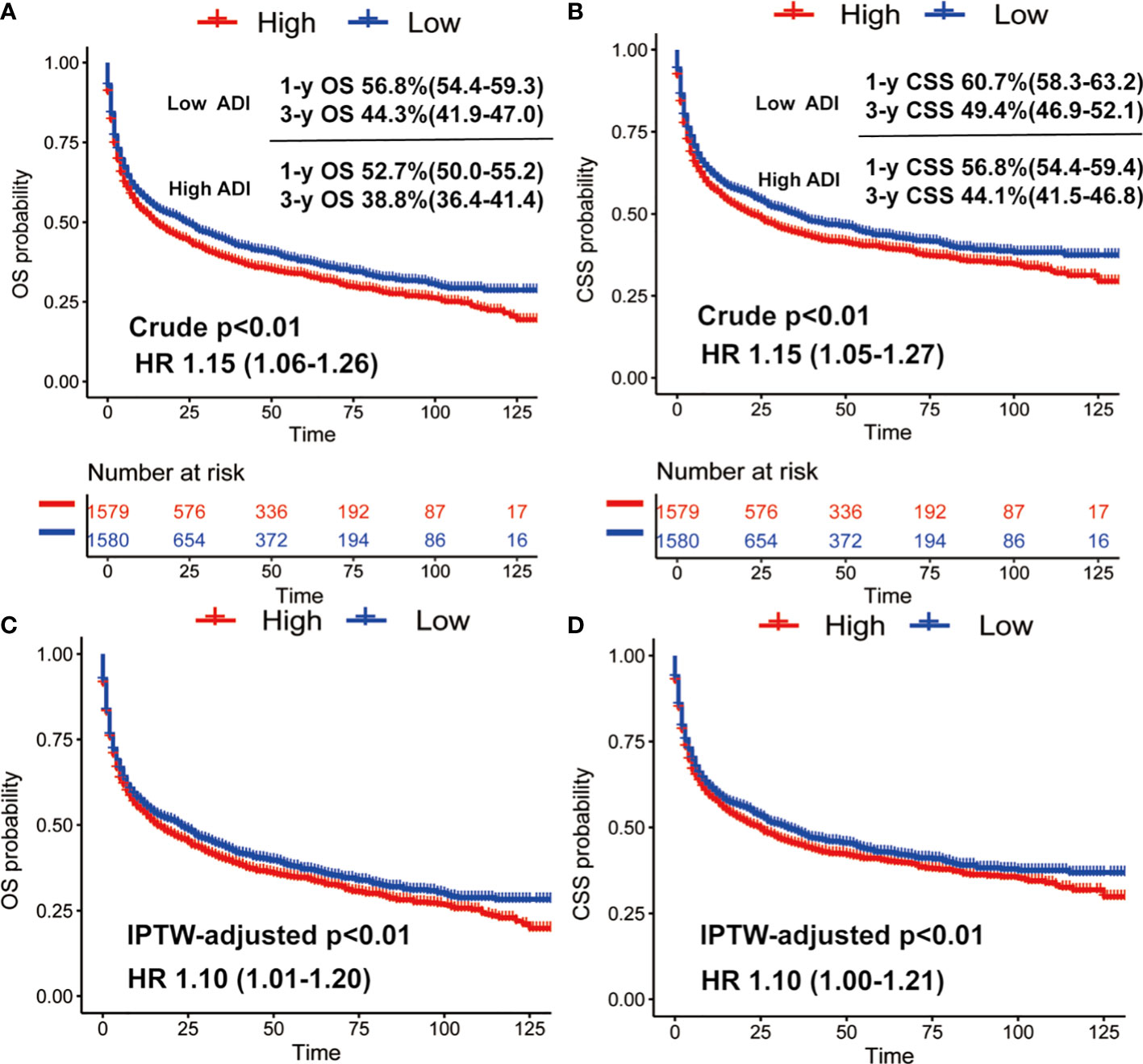
Figure 1 Crude Kaplan-Meier survival curves stratified by ADI for overall survival (A) and cancer-specific survival (B); IPW-adjusted Kaplan-Meier survival curves stratified by ADI for overall survival (C) and cancer-specific (D).
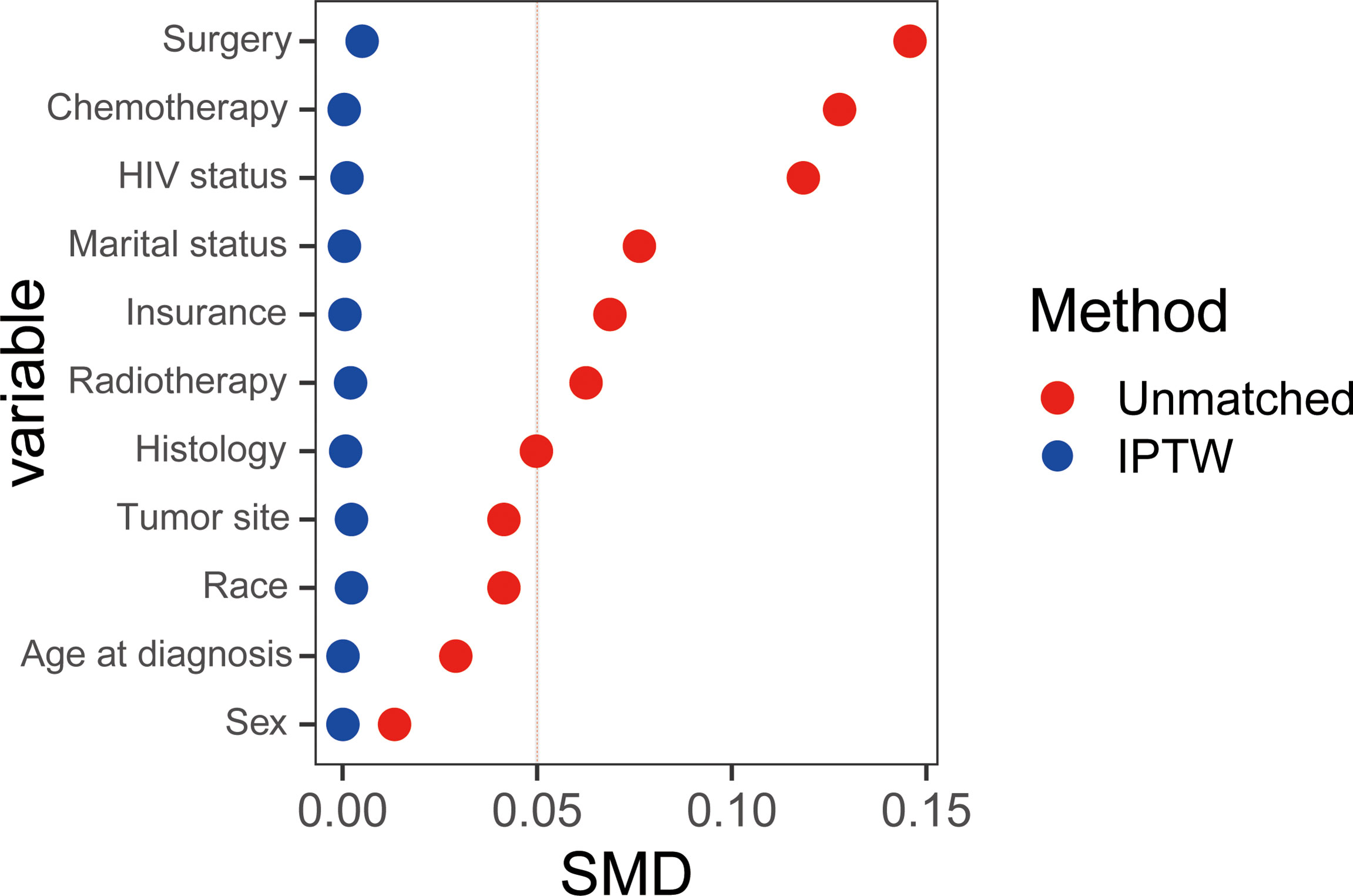
Figure 2 The absolute Standardized Mean Differences (SMDs) were calculated to verify the covariate balances after the IPW adjustment and a difference of SMD equal to zero indicates ideal balance.
Subgroup analysis
To explore the relation between ADI and CSS in different patient subsets, subgroup analyses were conducted and results are summarized in Figure 3. We found ADI could remain a prognostic indictor among subgroups. And ADI demonstrated more relatively robust in patients who were less than 60 years old, male, AIAN/API, with infratentorial tumors, with other PCNSL subtypes, single, with negative HIV status and insured.
Figure 3 Results for the subgroup analyses for cancer-specific survival are summarized in a forest plot. AIAN/API, American Indian/Alaska Native or Asian Pacific Islander; DLBCL, diffuse large B-cell lymphoma; NOS, not otherwise specified.
Multiple mediation analysis
Subsequently, we further performed MMA to investigate the contribution of estimated direct and indirect effects to the CSS disparities between two ADI subgroups. As illustrated in Figure 4, we found estimated direct effect that mediating factors failed to account for was 57.8% (95%CI, 54.2%-62.2%), whereas indirect effect was 42.2% (95%CI, 38.9%-45.8%) where the chemotherapy (27.9%; 95%CI, 21.3%-34.0%) made a greatest contribution, followed by HIV status (17.2%, 95%CI, 11.5%-23.0%).

Figure 4 The estimation of direct and indirect effects contributing to the disparities related to the ADI on cancer-specific survival in PCNSL patients.
Association of ADI with chemotherapy application
Chemotherapy is the main treatment for PCNSL patients, and almost 70% patients initiated some form of chemotherapy in our cohort. Finally, we examined the extent to which area-based socioeconomic deprivation as well as other sociodemographic variables predicted receipt of chemotherapy. In multivariable logistic regression, ADI was found to be significantly related to the odds of initiating chemotherapy (odds ratio of 0.82 for the high ADI compared with the low ADI; 95% CI 0.70-0.97, P=0.018). Moreover, older age, black race, other PCNLS subtypes, single status, positive HIV status and no insured were observed to be significantly associated with less use of chemotherapy (all P<0.05, Table 2).

Table 2 Multivariable logistic regression to predict odds of receiving chemotherapy for PCNSL patients.
Discussion
The results of our national, population-based study showed that neighborhood SES, as measured by ADI, was independently associated with both OS and CSS in PCNSL. And the survival disparities remained stable after adjusting for multiple factors via IPW analysis and multivariable Cox proportional hazards regression analysis. Furthermore, MMA revealed that several factors including chemotherapy and HIV status making up about 40% of the overall effect, mediated PCNSL survival disparities related to the ADI. These findings add to the growing literatures uncovering the impact of disadvantaged neighborhood SES on cancer outcomes.
To our knowledge, this is the first study to focus on the impact of the neighborhood SES, measured by ADI, on PCNSL survival. Instead of applying single-metric or one-off composite neighborhood SES features, our study complemented prior works via employing a comprehensive composite measure to evaluate the complexity of community environment. Socioeconomic deprivation has been observed to be in connection with increased cancer incidence, lack of treatments and inferior outcomes across multiple tumors (15, 20, 27). Yu et al. reported that ADI level partially accounted for the tumor characteristics at presentation and survival disparities in colorectal cancers, and they further analyzed mediating factors to gain deeper understand on neighborhood SES (28). Also, our study broadens the type of tumors affected by the neighborhood SES, and neighborhood disadvantage was found to play an important role in survival of these highly malignant tumors.
Through MMA, the application of chemotherapy was observed to mediate the greatest share of ADI disparity on PCNSL survival. Approximately one-fourth of the ADI disparity on PCNSL survival was attributed to differences in the receipt of chemotherapy. High-dose methotrexate-based chemotherapy has demonstrated high efficacy and it has greatly improved PCNSL outcomes. And omission of chemotherapy was found to be closely related to indicators of poor socioeconomic status (14). Consistently, our study also confirmed that ADI was significantly associated with the odds of initiating chemotherapy. These may partially explain MMA results. Meanwhile, multivariable logistic regression showed other factors including age, race, marital status, insurance status and HIV status were independently related to receipt of chemotherapy. The disparities in application of chemotherapy for PCNSL highlights the need to improve delivery of systemic treatment in the community setting. And In-depth researches are needed to explore the internal connection.
Furthermore, HIV status was observed to be associated with 17.2% of the effect of ADI. Many studies have demonstrated that HIV diagnosis rates are higher for individuals from low-SES communities compared with those from high-SES communities (29, 30). Also, once infected with HIV, persons with acquired immune deficiency syndrome (AIDS) had a markedly increased risk of malignancies including PCNSLs (31). Given the comorbidity and poor performance status of HIV-related PCNSL patients, as well as the association between positive HIV status and the nonreceipt of chemotherapy which was also found in our analysis, they usually have a worse prognosis relative to counterparts with normal immune function, indicating the importance of HIV prevention and treatment. Therefore, through a mediation model, we demonstrated that policies development to enhance health delivery at the community level is a vital step to improve equity on PCNSL prognosis. And health policy makers and medical institutions should also take multilevel initiatives to strengthen HIV prevention and treatment.
Moreover, multiple factors have been found to account for cancer survival inequality in deprived neighborhoods including limited access to healthcare resources, lack of socioeconomic support, and barriers to travel for initial and follow-up care (20, 32, 33). Although SES information at the individual level may be more accurate and indicative, county-based socioeconomic deprivation has been reported to be associated with nonoptimal treatment and inferior survival independently of individual SES (20); and it can offer insight into cultural and group-level phenomena, may exerting more guiding effect on the implementation of macro medical policy. Besides, Unger et al. reported that association of high area-level socioeconomic deprivation with worse cancer outcomes persisted in clinical trials where cancer patients get access to protocol-directed care (34), suggesting that neighborhood SES should be cautiously considered for researchers to design and interpret clinical trials. The findings of our analysis may provide some meaningful implications for PCNSL-related clinical trial design and healthcare policy. Future studies are also needed to explore the role of ADI in other type of tumors to support health policy interventions at the community level.
There are some several limitations should be acknowledged. Although ADI has excellent quantitative ability for neighborhood socioeconomic disadvantage which had been validated in some articles (20, 28), it cannot reflect every aspect of neighborhood SES. Due to inherent limitations of the SEER database, some prognostic factors such as comorbidities, Karnofsky performance status, and specific therapeutical program, as well as individual SES were failed to be adjusted. Moreover, the findings of this study are based on the SEER database, and whether they can be generalized to other groups needs further verification. In spite of these shortcomings mentioned above, the methods applied in our analyses were rigorously designed and the results still offer certain referential value.
Conclusions
The current study found that ADI was significantly associated with receipt of treatment and cancer prognosis in PCNSL patients. And several factors including chemotherapy and HIV status of PCNSL patents contributed to the CSS disparities between ADI subgroups were uncovered by MMA. Such relationships would highlight the importance of policies development to enhance healthcare delivery and promote awareness of HIV prevention and treatment in low-resource neighborhoods. And this study also supports policies for ongoing investments in low-SES communities. Policymakers and payers should take socioeconomic deprivation into consideration to maximize the efficiency and potency of healthcare strategies. Furthermore, individual-level SES is underexamined in our study given the inaccessibility of data of individual-level SES in SEER database, future studies need#146;to investigate contribution of individual patient-level SES to PCNSL survival, and to better assess cancer outcomes in the context of both neighborhood and individual socioeconomic disadvantage.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data-software/.
Author contributions
XD designed the study; XD, XY, and CY contributed to data analysis. XD wrote the initial draft of the manuscript; XD, XY, CY, KC, JuZ, QZ, TL, QT, and JiZ reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by grants (2018YFA0107900, 92168103, 32171417, 2019CXJQ01), from Ministry of Science and Technology of China, National Nature Science Foundation and Shanghai Municipal Government, Peak Disciplines (Type IV) of Institutions of Higher Leaning in Shanghai.
Acknowledgments
The authors would like to thank the SEER database for the availability of the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Han C, Batchelor T. Diagnosis and management of primary central nervous system lymphoma. Cancer (2017) 123(22):4314–24. doi: 10.1002/cncr.30965
2. Grommes C, DeAngelis L. Primary CNS lymphoma. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(21):2410–8. doi: 10.1200/jco.2017.72.7602
3. Schaff L, Grommes C. Primary central nervous system lymphoma. Blood (2021). doi: 10.1182/blood.2020008377
4. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger A, Preusser M, Rudà R, et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European association for neuro-oncology. Lancet Oncol (2015) 16(7):e322–32. doi: 10.1016/s1470-2045(15)00076-5
5. Franca R, Travaglino A, Varricchio S, Russo D, Picardi M, Pane F, et al. HIV Prevalence in primary central nervous system lymphoma: A systematic review and meta-analysis. Pathol Res Pract (2020) 216(11):153192. doi: 10.1016/j.prp.2020.153192
6. Kaulen L, Galluzzo D, Hui P, Barbiero F, Karschnia P, Huttner A, et al. Prognostic markers for immunodeficiency-associated primary central nervous system lymphoma. J Neuro-oncol (2019) 144(1):107–15. doi: 10.1007/s11060-019-03208-w
7. Kasenda B, Ferreri A, Marturano E, Forst D, Bromberg J, Ghesquieres H, et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)–a systematic review and individual patient data meta-analysis. Ann Oncol Off J Eur Soc Med Oncol (2015) 26(7):1305–13. doi: 10.1093/annonc/mdv076
8. Ko N, Hong S, Winn R, Calip G. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol (2020) 6(3):385–92. doi: 10.1001/jamaoncol.2019.5672
9. Malinowski C, Lei X, Zhao H, Giordano S, Chavez-MacGregor M. Association of Medicaid expansion with mortality disparity by race and ethnicity among patients with de novo stage IV breast cancer. JAMA Oncol (2022). doi: 10.1001/jamaoncol.2022.0159
10. Ostrom Q, Cote D, Ascha M, Kruchko C, Barnholtz-Sloan J. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol (2018) 4(9):1254–62. doi: 10.1001/jamaoncol.2018.1789
11. Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc (2007) 99(9):1013–23.
12. Singh GK, Jemal A. Socioeconomic and Racial/Ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: Over six decades of changing patterns and widening inequalities. J Environ Public Health (2017) 2017:2819372. doi: 10.1155/2017/2819372
13. Samhouri Y, Mustafa Ali M, Khan C, Wegner R, Lee S, Lister J. The trend of combined modality treatment and its outcomes in elderly patients with primary CNS lymphoma: A 12-year population-based analysis using propensity score. Anticancer Res (2022) 42(4):1867–77. doi: 10.21873/anticanres.15663
14. Fallah J, Qunaj L, Olszewski A. Therapy and outcomes of primary central nervous system lymphoma in the United States: Analysis of the national cancer database. Blood Adv (2016) 1(2):112–21. doi: 10.1182/bloodadvances.2016000927
15. Cote DJ, Ostrom QT, Gittleman H, Duncan KR, CreveCoeur TS, Kruchko C, et al. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer (2019) 125(19):3390–400. doi: 10.1002/cncr.32328
16. Lin D, Gold H, Schreiber D, Leichman L, Sherman S, Becker D. Impact of socioeconomic status on survival for patients with anal cancer. Cancer (2018) 124(8):1791–7. doi: 10.1002/cncr.31186
17. Shariff-Marco S, Yang J, John E, Sangaramoorthy M, Hertz A, Koo J, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: The neighborhood and breast cancer study. Cancer Epidemiol Biomarkers Prev (2014) 23(5):793–811. doi: 10.1158/1055-9965.Epi-13-0924
18. Wiese D, Stroup A, Maiti A, Harris G, Lynch S, Vucetic S, et al. Socioeconomic disparities in colon cancer survival: Revisiting neighborhood poverty using residential histories. Epidemiology (2020) 31(5):728–35. doi: 10.1097/ede.0000000000001216
19. Zheng Y, Zhang X, Lu J, Liu S, Qian Y. Association between socioeconomic status and survival in patients with hepatocellular carcinoma. Cancer Med (2021) 10(20):7347–59. doi: 10.1002/cam4.4223
20. Cheng E, Soulos PR, Irwin ML, Cespedes Feliciano EM, Presley CJ, Fuchs CS, et al. Neighborhood and individual socioeconomic disadvantage and survival among patients with nonmetastatic common cancers. JAMA Netw Open (2021) 4(12):e2139593. doi: 10.1001/jamanetworkopen.2021.39593
21. Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med (2018) 378(26):2456–8. doi: 10.1056/NEJMp1802313
22. Kind A, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann Internal Med (2014) 161(11):765–74. doi: 10.7326/m13-2946
23. Deng X, Xu X, Lin D, Zhang X, Yu L, Sheng H, et al. Real-world impact of surgical excision on overall survival in primary central nervous system lymphoma. Front Oncol (2020) 10:131. doi: 10.3389/fonc.2020.00131
24. Singh GK. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health (2003) 93(7):1137–43. doi: 10.2105/ajph.93.7.1137
25. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med (2015) 34(28):3661–79. doi: 10.1002/sim.6607
26. Yu Q, Wu X, Li B, Scribner RA. Multiple mediation analysis with survival outcomes: With an application to explore racial disparity in breast cancer survival. Stat Med (2019) 38(3):398–412. doi: 10.1002/sim.7977
27. Fairfield K, Black A, Ziller E, Murray K, Lucas F, Waterston L, et al. Area deprivation index and rurality in relation to lung cancer prevalence and mortality in a rural state. JNCI Cancer Spectr (2020) 4(4):pkaa011. doi: 10.1093/jncics/pkaa011
28. Yu KX, Yuan WJ, Huang CH, Xiao L, Xiao RS, Zeng PW, et al. Socioeconomic deprivation and survival outcomes in patients with colorectal cancer. Am J Cancer Res (2022) 12(2):829–38.
29. Denning PH, DiNenno EA, Ryan E Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence — 24 cities, United States, 2006-2007. MMWR Morbidity Mortal Wkly Rep (2011) 60(31):1045–9.
30. Santelli J, Chen I, Nabukalu D, Lutalo T, Spindler E, Chang L, et al. HIV Combination prevention and declining orphanhood among adolescents, rakai, Uganda, 2001-18: An observational community cohort study. Lancet HIV (2022) 9(1):e32–41. doi: 10.1016/s2352-3018(21)00275-7
31. Brandsma D, Bromberg J. Primary CNS lymphoma in HIV infection. Handb Clin Neurol (2018) 152:177–86. doi: 10.1016/b978-0-444-63849-6.00014-1
32. Roche A, Fedewa S, Shi L, Chen A. Treatment and survival vary by race/ethnicity in patients with anaplastic thyroid cancer. Cancer (2018) 124(8):1780–90. doi: 10.1002/cncr.31252
33. Kirkwood M, Bruinooge S, Goldstein M, Bajorin D, Kosty M. Enhancing the American society of clinical oncology workforce information system with geographic distribution of oncologists and comparison of data sources for the number of practicing oncologists. J Oncol Pract (2014) 10(1):32–8. doi: 10.1200/jop.2013.001311
Keywords: socioeconomic deprivation, primary central nervous system lymphoma, SEER, overall survival (OS), cancer-specific survival
Citation: Deng X, Yang X, Yang C, Chen K, Ren J, Zeng J, Zhang Q, Li T, Tang Q and Zhu J (2022) Socioeconomic deprivation and survival outcomes in primary central nervous system lymphomas. Front. Oncol. 12:929585. doi: 10.3389/fonc.2022.929585
Received: 27 April 2022; Accepted: 05 August 2022;
Published: 26 August 2022.
Edited by:
Shengwen Calvin Li, Children’s Hospital of Orange County, United StatesReviewed by:
Gwenn Menvielle, Institut National de la Santéet de la Recherche Médicale (INSERM), FranceYasuo Iwadate, Chiba University, Japan
Copyright © 2022 Deng, Yang, Yang, Chen, Ren, Zeng, Zhang, Li, Tang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhong Zhu, anpodUBmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work
 Xiangyang Deng1†
Xiangyang Deng1† Chunlei Yang
Chunlei Yang Tianwen Li
Tianwen Li Jianhong Zhu
Jianhong Zhu