
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 July 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.927510
Background: Adding induction chemotherapy to concurrent platinum-based chemoradiotherapy has significantly prolonged the survival time of patients with locoregionally advanced nasopharyngeal carcinoma. In this study, we intend to evaluate the survival outcomes, responses, and incidences of toxicities of induction chemotherapy and the differences between different strategies.
Methods: A comprehensive search was conducted in PubMed, Embase, Web of Science, and Cochrane CENTRAL on August 10, 2021. Single-arm or multi-arm prospective clinical trials on induction chemotherapy without targeted therapies or immune checkpoint inhibitors were included. Primary outcomes included survival outcomes, objective response rate, and disease control rate, and the secondary outcome was the rates of grade 3 or higher treatment-related adverse events.
Results: The 39 studies included in the systematic review and meta-analysis comprised 36 clinical trials and 5389 patients. The estimates for 3-year overall and fail-free survival rates were 87% and 77%. The estimates for 5-year rates of overall and fail-free survival were 81% and 73%. Gemcitabine plus platinum and docetaxel combined with 5-fluorouracil plus platinum strategies were associated with the highest rates of 3-year and 5-year overall survival. The objective response and disease control rates were 85% and 98% after the completion of induction chemotherapy. Neutropenia (27%) and nausea/vomiting (7%) were the most common grade 3 or higher treatment-related hematological and non-hematological adverse events during the induction phase.
Conclusions: Different induction chemotherapeutic strategies appear to have varying effects and risks; a comprehensive summary of the survival outcomes, responses, and toxicities in clinical trials may provide a crucial guide for clinicians.
It is estimated that over 70% of nasopharyngeal carcinoma (NPC) patients presented with locoregional advanced stage (1). For this population, platinum-based concurrent chemoradiotherapy (CCRT) is the backbone of the radical treatment (2, 3). For furtherly elevating the responses and prolonging survival outcomes, induction chemotherapy has been administered before CCRT. For instance, the addition of docetaxel, cisplatin, and 5-fluorouracil reduced 32% and 41% of the 3-year risks of disease progression and death (4); Gemcitabine and cisplatin induction chemotherapy significantly decreased the hazard ratio for 3-year recurrence and death by 49% and 57% in locoregionally advanced NPC patients (5). According to the latest guidelines for nasopharyngeal carcinoma, induction chemotherapy followed by CCRT is recommended as the preferred standard of care for patients with locoregionally advanced NPC (6–8).
Although adding induction chemotherapy to CCRT has been demonstrated to be superior to CCRT alone (9), substantial variations exist in different populations, induction chemotherapeutic regimens, cycles, and CCRT strategies. Ignoring these variations might lead to an inaccurate evaluation of the true efficacy and safety profile associated with induction chemotherapy.
For aiding clinical decision-making, we performed a systematic review and meta-analysis to integrate the benefits and risks of induction chemotherapy in published prospective studies and comprehensively describe the potential differences among a variety of populations, induction chemotherapeutic regimens, cycles, and CCRT strategies.
We conducted this study according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (10). A comprehensive search of English-language prospective clinical trials was performed in PubMed, Embase, Web of Science, and Cochrane CENTRAL with the search terms (nasopharyngeal carcinoma) AND (induction chemotherapy OR neoadjuvant chemotherapy) AND (radiotherapy OR chemoradiotherapy) AND (trial OR clinical trial) on August 10, 2021. The references of relevant published clinical studies and review literatures were also searched for additional eligible trials. Inclusion criteria included: (1) Participants: over 18 years old locoregionally advanced NPC patients; (2) Interventions: induction chemotherapy followed by platinum-based CCRT; (3) Outcomes: data on survival outcomes, responses, and treatment-related adverse events were available. Single-arm and multi-arm studies were eligible. However, patients who received subsequent adjuvant chemotherapy, targeted therapy, or immunotherapy were excluded. Two authors performed the literature search and study selection independently, and any discrepancies were reviewed by a third author and resolved by consensus.
The primary outcome measures comprised the 3- and 5-year survival rates, objective response rate (ORR, defined as the percentage of patients with a response of complete response and partial response), and disease control rate (DCR, defined as the percentage of patients with a response of complete response, partial response, and stable disease) after induction chemotherapy, at the end of CCRT, and at 3 months post CCRT. The secondary outcome was the incidence of grade 3 or higher treatment-related adverse events during induction chemotherapy and CCRT phases. Overall survival (OS) was defined as the time from diagnosis or random assignment to death because of any cause; failure-free survival (FFS) was defined as the time from diagnosis or random assignment to documented disease recurrence; locoregional recurrence-free survival (LRFS) was defined as the time from diagnosis or random assignment to locoregional disease recurrence; distant metastasis-free survival (DMFS) was defined as the time from diagnosis or random assignment to distant metastasis.
Data extraction was conducted by two authors independently and reviewed by a third author. Data regarding the number of patients, study design, region, regimens, dosing schedule, survival rates, responses, and the number of grade 3 or more adverse events were recorded.
The response variable is the number of reported survivals, responses, and grade 3 or higher toxic effects, assumed to follow a binomial distribution. Statistical analyses were performed using R Studio (version 1.4.1717, R Foundation for Statistical Computing). The “meta” package was used to perform the random effects meta-analyses, sensitivity analyses, and tests for heterogeneity (I2 and τ) (11). A random-effects model was selected over a fixed-effects model if I2 > 50% because using random effects is often the preferred technique when conducting a single-arm meta-analysis to guide treatment decisions (12). τ2 = 0 meant that no deviations were found across the studies. Otherwise, deviations existed but did not indicate significant heterogeneity. Pooled proportions were estimated via the metaprop function in the “meta” package, applying a logit transformation and continuity correction of 0.5 and other default settings. The Jadad scoring scale was used to assess the quality of each eligible trial (low quality: a score of ≤ 2; high quality: a score of ≥ 3) (13). Publication bias was evaluated by funnel plots, Egger’s regression tests, and Begg’s test.
Literature search and review of reference lists identified 1434 relevant records. After screening and eligibility assessment, we included in the meta-analysis a total of 36 prospective clinical trials involving 5389 patients (Supplement 1). The trials were published between 2004 and 2021, as displayed in Table 1 (14–52). Patients in 26 trials underwent treatment in China, and patients in the other 10 trials underwent treatments in Italy, Korea, Greece, Australia, Austria, Singapore (Ethnic group: 95.3% of enrolled patients were Chinese), Switzerland, India, and Arabia. Induction chemotherapeutic regimens included (1) taxane plus platinum (TP), (2) platinum plus 5-fluorouracil (PF), (3) taxane plus platinum and 5-fluorouracil (TPF), (4) gemcitabine plus platinum (GP), (5) taxane plus platinum and epirubicin, (6) platinum plus epirubicin, (7) platinum plus capecitabine, (8) gemcitabine plus platinum and taxane, (9) mitomycin C plus epirubicin, platinum, and 5-fluorouracil, and (10) taxane plus ifosfamide and platinum. Two or three cycles of induction chemotherapy were administered. Concurrent chemoradiotherapies comprised weekly and triweekly platinum-based strategies. In addition, T3-4N0 and T3N0-1 NPC patients were excluded in Sun/Li’s and Cao/Yang’s clinical trials, respectively (32, 33, 35, 36).
Supplement 2 shows the quality evaluation for each eligible study, corresponding funnel plots, Egger’s tests (P > 0.1), Begg’s test (P > 0.1), and sensitivity analyses, indicating a moderate-to-high quality for clinical trials enrolled (16 trials were identified as low quality [a score of ≤ 2], while 20 trials as high quality [a score of ≥ 3]) and the sole publication bias in the analysis of 5-year OS (Begg’s test: P = 0.09).
The 3-year OS rate was 87% (95% CI, 84%-90%; I2 = 87%; P < 0.01 for heterogeneity) in 3212 patients across 24 trials, the 3-year FFS rate was 77% (95% CI, 74%-80%; I2 = 68%; P < 0.01) in 3104 patients across 24 trials, the 3-year LRFS rate was 91% (95% CI, 87%-94%; I2 = 85%; P < 0.01) in 2245 patients across 15 trials, and the 3-year DMFS rate was 85% (95% CI, 81%-89%; I2 = 86%; P < 0.01) in 2259 patients across 15 trials (Figure 1).
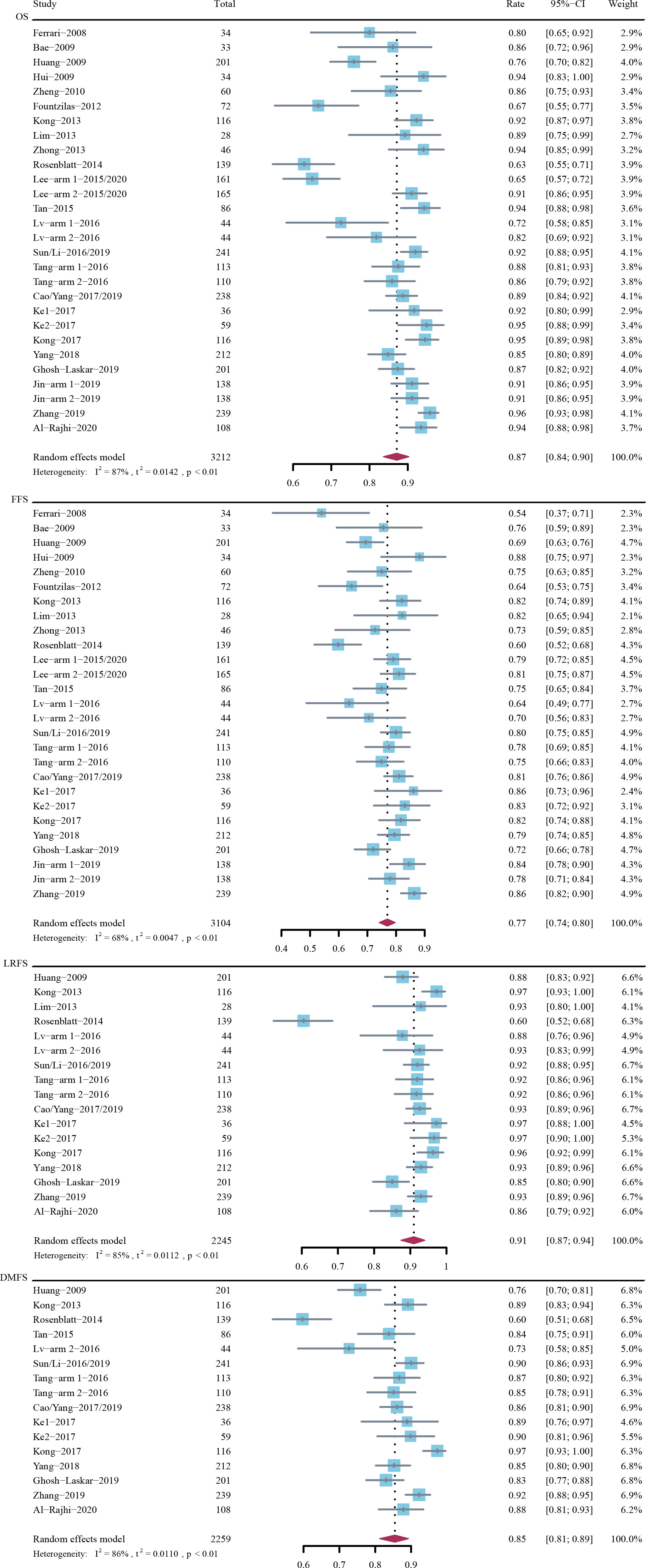
Figure 1 Rates of 3-year overall survival (OS), failure-free survival (FFS), locoregional recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS).
The 5-year OS rate was 81% (95% CI, 76%-85%; I2 = 83%; P < 0.01) in 2009 patients across 9 trials, the 5-year FFS rate was 73% (95% CI, 69%-77%; I2 = 73%; P < 0.01) in 1965 patients across 9 trials, the 5-year LRFS rate was 87% (95% CI, 85%-90%; I2 = 54%; P = 0.03) in 1595 patients across 7 trials, and the 5-year DMFS rate was 83% (95% CI, 78%-88%; I2 = 85%; P < 0.01) in 1595 patients across 7 trials (Figure 2).
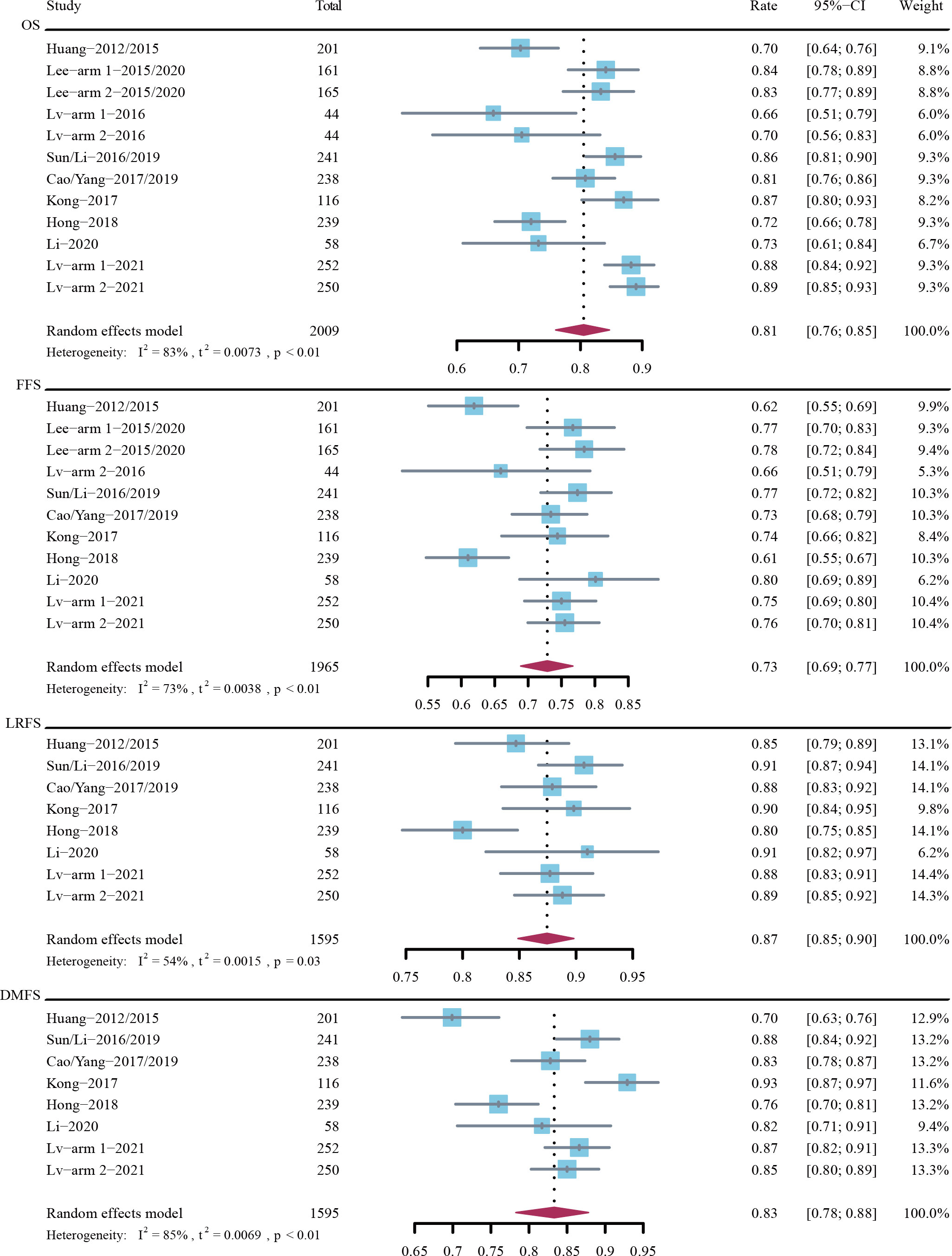
Figure 2 Rates of 5-year overall survival (OS), failure-free survival (FFS), locoregional recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS).
Figure 3 depicts the forest plots for ORR. The estimated ORRs post induction chemotherapy, post CCRT, and post CCRT at 3 months were 85% (95% CI, 80%-90%; I2 = 91%; P < 0.01), 97% (95% CI, 94%-100%; I2 = 80%; P < 0.01), and 98% (95% CI, 96%-99%; I2 = 81%; P < 0.01), respectively.
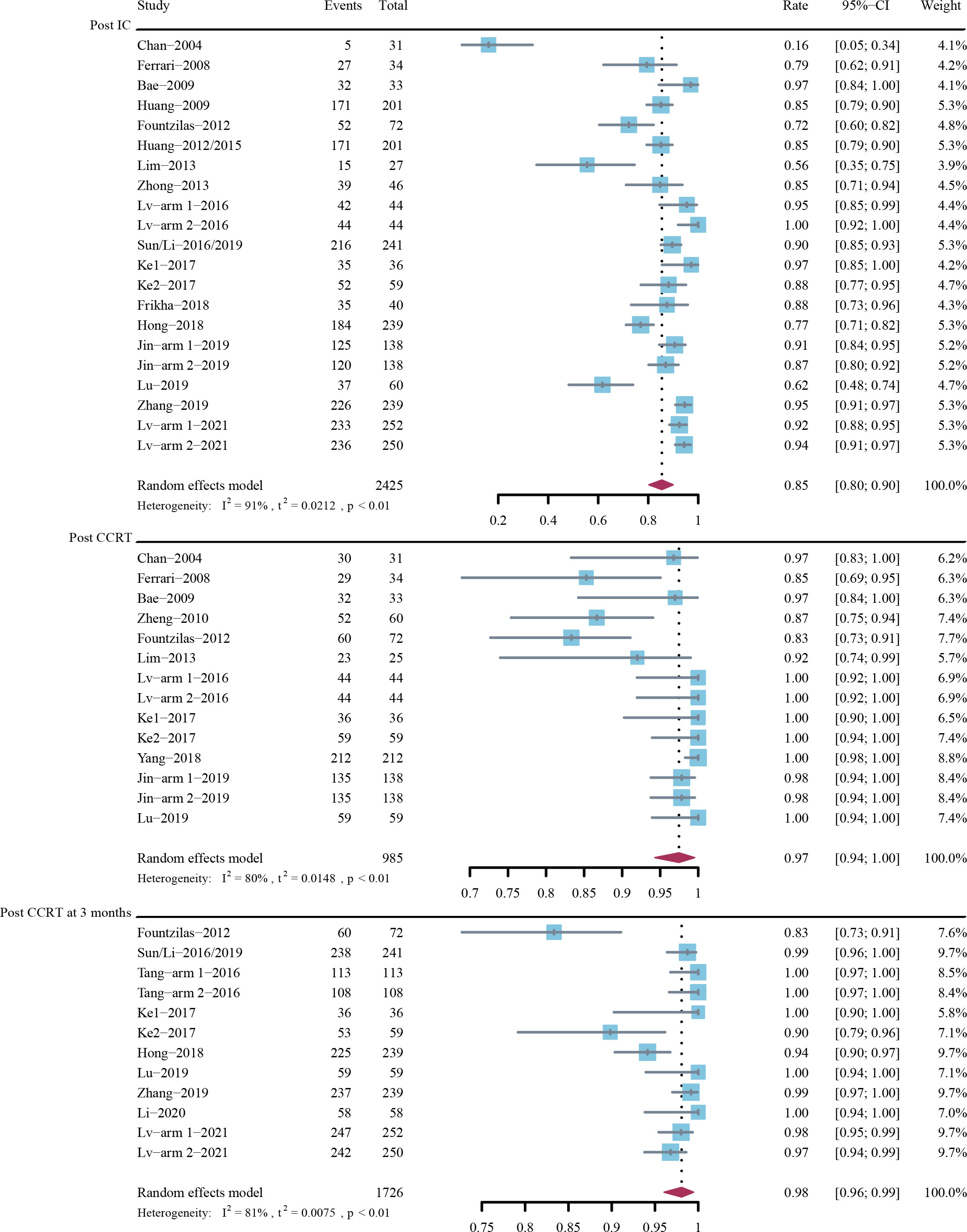
Figure 3 Objective response rate (ORR) post-induction chemotherapy (IC), post-concurrent chemoradiotherapy (CCRT), and post-CCRT at 3 months.
Figure 4 depicts the forest plots for DCR. The estimated DCRs post induction chemotherapy, post CCRT, and post CCRT at 3 months were 98% (95% CI, 97%-100%; I2 = 66%; P < 0.01), 98% (95% CI, 93%-100%; I2 = 71%; P < 0.01), and 96% (95% CI, 87%-100%; I2 = 83%; P < 0.01), respectively.

Figure 4 Disease control rate (DCR) post-induction chemotherapy (IC), post-concurrent chemoradiotherapy (CCRT), and post-CCRT at 3 months.
Figure 5A displays the subgroup analyses regarding population, induction chemotherapeutic regimens, induction chemotherapy cycles, and platinum-based CCRT strategies.
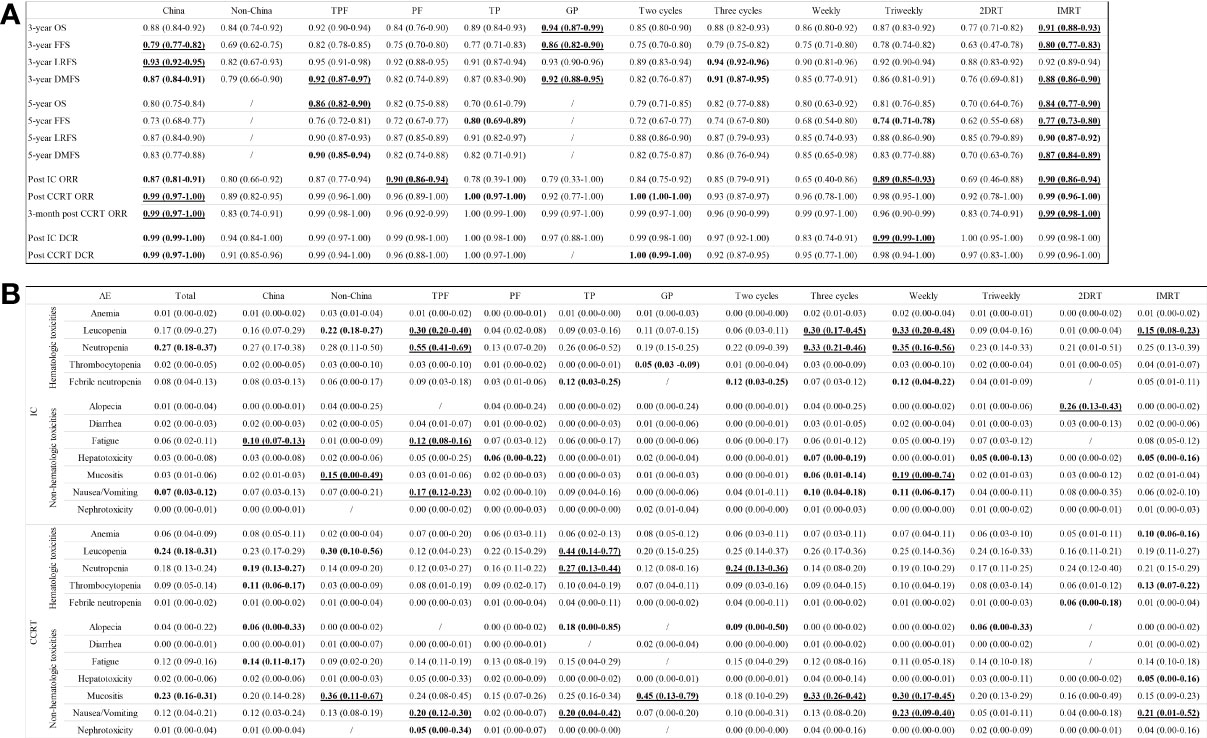
Figure 5 Subgroup analyses of survival outcomes, responses (A), and adverse events (B) in terms of populations, regimens, cycles, and concurrent platinum strategies.
Patients in China achieved higher 3-year FFS (79% [95% CI, 77%-82%] vs. 69% [95% CI, 67%-75]) and LRFS (93% [92%-95%] vs. 82% [95% CI, 67%-93%]) rates, and ORRs (post CCRT: 99% [95% CI, 97%-100%] vs. 89% [95% CI, 82%-95%]; 3-month post CCRT: 99% [95% CI, 97%-100%] vs. 83% [95% CI, 74%-91%]) versus patients outside Chinese region.
GP induction chemotherapy was associated with the highest 3-year OS and FFS rates (OS: 94% [95% CI, 87%-99%]; FFS: 86% [82%-90%]), followed by TPF (92% [95% CI, 90%-94%]; 82% [78%-85%]), TP (89% [95% CI, 84%-93%]; 77% [71%-83%]), and PF (84% [95% CI, 76%-90%]; 75% [70%-80%]). In regard of 5-year OS with an absence of GP data, TPF was associated with the highest rate (86%; 95% CI, 82%-90%), followed by PF (82%; 95% CI, 75%-88%) and TP (70%; 95% CI, 61%-79%). In addition, PF (90%; 95% CI, 86%-94%) had a higher ORR after induction chemotherapy compared to TPF (87%; 95% CI, 77%-94%), GP (79%; 95% CI, 33%-100%), and TP (78%; 95% CI, 39%-100%).
In comparison with two cycles of induction chemotherapy, three cycles of induction chemotherapy might slightly increase the 3-year LRFS (94% [95% CI, 92%-96%] vs. 89% [95% CI, 83%-94%]) and DMFS (91% [95% CI, 87%-95%] vs. 82% [95% CI, 76%-87%]) rates, but fail to improve the ORR (93% [95% CI, 87%-97%] vs. 100% [95% CI, 100%-100%]) and DCR (92% [95% CI, 87%-95%] vs. 100% [95% CI, 99%-100%]) after the completion of CCRT.
Before the administration of platinum-based CCRT, patients who had received induction chemotherapy in the triweekly concurrent platinum therapy group had an 89% (95% CI, 85%-93%) of ORR and a 99% (95% CI, 99%-100%) of DCR that were much higher than the weekly group (65% [95% CI, 40%-86%]; 83% [95% CI, 74%-91%]). In addition, the triweekly group showed an increased 5-year FFS rate versus the weekly group (74% [95% CI, 71%-78%] vs. 68% [95% CI, 54%-80%]). However, patients in both groups achieved comparable rates of 3-year (87% [95% CI, 83%-92%] vs. 86% [95% CI, 80%-92%]) and 5-year (81% [95% CI, 76%-85%] vs. 80% [95% CI, 63%-92%]) OS.
Intensity-modulated radiotherapy (IMRT) has changed outcome of NPC patients significantly. Since three-dimensional radiotherapy (3DRT) data failed to separate from published trials, pooled IMRT and two-dimensional radiotherapy (2DRT) results were sub-analyzed. The IMRT group showed higher rates of post CCRT objective response at 3 months (99% [95% CI, 98%-100%] vs. 83% [95% CI, 74%-91%]), 5-year OS (84% [95% CI, 77%-90%] vs. 70% [95% CI, 64%-76%]), and 5-year PFS (77% [95% CI, 73%-80%] vs. 62% [95% CI, 55%-68%]). Additionally, IMRT could decrease the rate of distant metastasis compared with 2DRT (5-year DMFS: 87% [95% CI, 84%-89%] vs. 70% [95% CI, 63%-76%]).
For the meta-analysis, we focused on the hematological and non-hematological grade 3 or higher adverse events that were recorded during the induction chemotherapy and CCRT phases. A comprehensive list of the incidences of anemia, leucopenia, neutropenia, thrombocytopenia, febrile neutropenia, alopecia, diarrhea, fatigue, hepatotoxicity, mucositis, nausea/vomiting, and nephrotoxicity is provided in Figure 5B.
During the induction chemotherapy phase, the most common hematological grade 3 or higher adverse events were neutropenia (27%; 95% CI, 18%-37%), leucopenia (17%; 95% CI, 9%-27%), and febrile neutropenia (8%; 95% CI, 4%-13%). The most common non-hematological grade 3 or higher adverse events were nausea/vomiting (7%; 95% CI, 3%-12%) and fatigue (6%; 95% CI, 2%-11%). Patients received TPF experienced the highest incidences of grade 3 or higher neutropenia (55%; 95% CI, 41%-69%), leucopenia (30%; 95% CI, 20%-40%), fatigue (12%; 95% CI, 8%-16%), and nausea/vomiting (17%; 95% CI, 12%-23%). Three cycles of induction chemotherapy induced more incidences of grade 3 or higher neutropenia (33% [95% CI, 21%-46%] vs. 22% [95% CI, 9%-39%]) and leucopenia (30% [95% CI, 17%-45%] vs. 6% [95% CI, 3%-11%]) against the two cycles group.
During the CCRT phase, the most common hematological grade 3 or higher adverse events were leucopenia (24%; 95% CI, 18%-31%), neutropenia (18%; 95% CI, 13%-24%), and thrombocytopenia (9%; 95% CI, 5%-14%). The most common non-hematological grade 3 or higher adverse events were mucositis (23%; 95% CI, 16%-31%), fatigue (12%; 95% CI, 9%-16%), and nausea/vomiting (12%; 95% CI, 4%-21%). Patients received TP induction chemotherapy had the highest incidences of grade 3 or higher leucopenia (44%; 95% CI, 14%-77%), neutropenia (27%; 95% CI, 13%-44%), mucositis (20%; 95% CI, 4%-42%), and alopecia (18%; 95% CI, 0%-85%). More cases of grade 3 or higher neutropenia (24% [95% CI, 13%-36%] vs. 14% [95% CI, 8%-20%]) were reported in the two cycles group, while more cases of grade 3 or higher mucositis (33% [95% CI, 26%-42%] vs. 18% [95% CI, 10%-29%]) were reported in the three cycles group. Additionally, patients treated with weekly platinum-based CCRT experienced higher incidences of grade 3 or higher mucositis (30% [95% CI, 17%-45%] vs. 20% [95% CI, 13%-29%]) and nausea/vomiting (23% [95% CI, 9%-40%] vs. 5% [95% CI, 1%-11%]) compared to the patients in the triweekly group. In terms of radiation techniques, patients in the IMRT group showed higher incidences of grade 3 or higher leucopenia (15% [95% CI, 8%-23%] vs. 1% [95% CI, 0%-4%]) and nausea/vomiting (21% [95% CI, 1%-52%] vs. 4% [95% CI, 0%-18%]) against the 2DRT group.
We performed a systematic review of induction chemotherapies and integrated the survival outcomes, responses, and toxic effects in patients with locoregionally advanced NPC who received induction chemotherapy and platinum-based CCRT. To our knowledge, this is the most comprehensive and largest meta-analysis of induction chemotherapy in NPC. Previous meta-analyses mainly demonstrated the benefits of adding induction chemotherapy to CCRT (9). Nevertheless, different populations, induction chemotherapeutic regimens and cycles, and even CCRT strategies may impact the efficacy and tolerability. A comprehensive analysis of the induction chemotherapeutic strategies reported in prospective clinical trials is essential, as the pooled data constitute a critical reference for clinicians. Significant heterogeneity existed among the enrolled studies, however, sensitivity analyses indicated that no substantial changes were found in the pooled survival outcomes and responses.
Although platinum-based induction chemotherapy significantly prolongs survival outcomes, whether adding 5-fluorouracil to TP provides more benefits is hard to judge. Up to now, several studies have compared the efficacy and safety data between TPF and TP. Xiong et al. indicated that TPF failed to improve the OS and PFS in stage III-IV NPC patients compared with TP (53). A Bayesian network meta-analysis of prospective clinical trials involving 1570 patients found that TPF had the highest probability of being the optimal regimen versus TP and PF in terms of OS (50% vs. 47% vs. 2%) (54). In our analysis, we noticed that patients in both TP and TPF subgroups achieved nearly 100% of ORR after completing induction chemotherapy and CCRT. However, TPF had much higher 5-year OS (86% vs. 70%) and DMFS (90% vs. 82%) rates against TP. These results were consistent with the retrospective study published by Tao et al. that patients received TPF had better 5-year OS (85% vs. 79%; p = 0.037), PFS (85% vs. 77%; p = 0.008) and DMFS (90% vs. 82%; p = 0.004) rates than patients received TP (55).
The integrated 3-year survival rates of GP in our analysis showed satisfying effects in treating NPC patients, including 3-year OS, FFS, and DMFS rates. In compared with TPF, GP showed a lower ORR after induction chemotherapy (79% vs. 87%) and comparable 3-year OS (94% vs. 92%), FFS (86% vs. 82%), LRFS (93% vs. 95%), and DMFS (92% vs. 92%) rates. In a comparative retrospective study, GP had a similar 3-year OS (94% vs. 92%), FFS (83% vs. 82%), LRFS (94% vs. 95%), and DMFS (90% vs. 90%) rates versus TPF, and no significant differences were observed (56). Nevertheless, GP induction chemotherapy was demonstrated to be cost-effective compared with TPF for locoregionally advanced NPC patients in real-world practice (57, 58).
On the other hand, published data have demonstrated that TPF achieved significantly better 10-year OS than PF (HR, 0.58; p = 0.005), and the difference between TP and PF was marginally significant (HR, 0.71; p = 0.056) (59). Regarding the 5-year data, TPF regimen significantly improved OS (88% vs. 81; p = 0.042) rate compared with the PF regimen (60). However, according to our analysis, PF had a better 5-year OS rate than TP (82% vs. 70%) and showed the highest ORR after induction chemotherapy (90%), followed by TPF (87%), GP (79%), and TP (78%). It seems hard to deduce that PF is the lowest effective induction chemotherapeutic regimen.
Anthracycline-based induction chemotherapeutic regimens include epirubicin + paclitaxel + cisplatin, epirubicin + cisplatin, and epirubicin + mitomycin C + cisplatin + 5-fluorouracil. These strategies were mainly applied in the non-China population and Taiwan participants (21, 27, 41). In Fountzilas’s study, locoregionally advanced NPC patients treated with epirubicin plus paclitaxel plus cisplatin had a 72% of ORR post-induction chemotherapy and an 83% of ORR post-CCRT (21). In Hong’s report, the ORR after induction chemotherapy was 78% (41). In comparison with our pooled data, the addition of epirubicin to TP may not critically improve the responses in NPC patients. Moreover, since the unreversible cardiotoxicity, epirubicin has a 900 mg/m2 of maximum cumulative dose.
For CCRT strategies, triweekly platinum-based CCRT showed a higher 5-year FFS versus the weekly group (74% vs. 68%) in our analysis, but OS results were similar. A previously pooled analysis of retrospective studies showed no significant differences in 5-year survival outcomes between weekly and triweekly treatments (61). However, the weekly strategy may be associated with improved quality of life than the triweekly regimen (62).
The addition of induction chemotherapy to CCRT has revolutionized the treatment of locoregionally advanced NPC, but the efficacy deserves further elevated. Regardless of complete clinical remission is attained after induction chemotherapy and CCRT, patients may suffer a high risk of locoregional relapse or distant metastasis. Chen et al. reported a phase 3 clinical trial in 2021 and indicated that adding metronomic adjuvant capecitabine after CCRT significantly improved survival outcomes with a manageable safety profile (63). In the subgroup analysis of Chen’s study, we noticed that only patients who received induction chemotherapy could benefit from adjuvant capecitabine treatment (FFS HR 0.49; 95% CI, 0.29-0.83) instead of patients who were treated with CCRT alone (FFS HR 0.51; 95% CI, 0.20-1.30). However, not all locoregionally advanced NPC patients are the suitable population for adjuvant chemotherapy. The changes of plasma EBV DNA across induction chemotherapy and CCRT may provide the necessity of the administration of adjuvant chemotherapy (64, 65). Finding out the suitable populations for induction chemotherapy plus CCRT, CCRT alone, and induction chemotherapy plus CCRT followed by adjuvant chemotherapy is meaningful for developing the treatment of NPC.
In terms of grade 3 or higher treatment-related adverse events, patients who received TPF regimen may suffer more incidences of leucopenia (30%), neutropenia (55%), fatigue (12%), and nausea/vomiting (17%) during the induction chemotherapy phase. In addition, three cycles of induction chemotherapy could induce more grade 3 or higher leucopenia (30%) and neutropenia (33%) versus two cycles. However, these toxicities are manageable. Thus, timely granulocyte colony-stimulating factor treatment could effectively prevent treatment-related severe adverse events or deaths.
The strengths of this analysis included (1) the results are supported by the large sample size from both single-arm and multi-arm prospective clinical trials, and (2) detailed subgroup analyses according to different populations, induction chemotherapeutic regimens, cycles, and CCRT strategies are displayed, because previously published meta-analyses mainly focused on the hazard ratios, odds ratios, or risk ratios in randomized studies comparing induction chemotherapy plus CCRT with CCRT alone or CCRT plus adjuvant chemotherapy. Nevertheless, our study has several limitations. First, heterogeneities existed among the enrolled studies. However, the large heterogeneity could mean that different clinical trials might exhibit inconsistent data of induction chemotherapy in treating locoregionally advanced NPC patients, which was the main point for us to conduct this meta-analysis to analyze the published data of induction chemotherapy comprehensively. In addition, a random-effects model was adopted all through this study to address the heterogeneity. Second, patients were treated with different cycles of induction chemotherapy. The primary reason for the discontinuation of induction chemotherapy was the adverse events, but most of the enrolled patients received two to three treatment cycles. Fortunately, the two-cycle group was not inferior to the three-cycle group. Third, in the CCRT phase, concurrent chemotherapies comprised weekly and triweekly strategies. Although heterogeneities may increase accordingly, our subgroup analysis and previously published pooled analysis had indicated no significant differences between weekly and triweekly strategies.
This meta-analysis has defined survival outcomes, response rates, and the incidences of treatment-related adverse events in locoregionally advanced NPC patients who received induction chemotherapy followed by CCRT. Different population and induction regimens may be associated with different survivals, responses, and adverse events. This global overview of the effects and risks of induction chemotherapies can provide a reference for clinicians and may guide clinical practice for patients with locoregionally advanced NPC.
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
B-CW and G-HL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design, B-CW and G-HL. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript, B-CW, B-HK, and G-HL. Critical revision of the manuscript for important intellectual content, B-CW, X-XL, and QL. Statistical analysis, B-CW and G-HL. Administrative, technical, or material support, QL. Supervision, QL. All authors contributed to the article and approved the submitted version.
This study was supported by the Hubei Provincial Natural Science Foundation (Grant number: 2020CFB397 to B-CW) and the Independent Innovation Foundation of Wuhan Union Hospital (Grant number: 2019-109 to B-CW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.927510/full#supplementary-material
1. Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys (2009) 73:1326–34. doi: 10.1016/j.ijrobp.2008.07.062
2. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
3. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst (2005) 97:536–9. doi: 10.1093/jnci/dji084
4. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
5. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
6. Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, et al. The Chinese society of clinical oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond) (2021) 41:1195–227. doi: 10.1002/cac2.12218
8. Keam B, Machiels JP, Kim HR, Licitra L, Golusinski W, Gregoire V, et al. Pan-Asian adaptation of the EHNS-ESMO-ESTRO clinical practice guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck. ESMO Open (2021) 6:100309. doi: 10.1016/j.esmoop.2021.100309
9. Wang BC, Xiao BY, Lin GH, Wang C, Liu Q. The efficacy and safety of induction chemotherapy combined with concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in nasopharyngeal carcinoma patients: a systematic review and meta-analysis. BMC Cancer (2020) 20:393. doi: 10.1186/s12885-020-06912-3
10. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
11. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with r: a practical tutorial. Evid Based Ment Health (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
12. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making (2005) 25:646–54. doi: 10.1177/0272989X05282643
13. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
14. Chan ATC, Ma BBY, Lo D, Leung SF, Kwan WH, Hui EP, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: Therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol (2004) 22:3053–60. doi: 10.1200/jco.2004.05.178
15. Ferrari D, Chiesa F, Codecà C, Calabrese L, Jereczek-Fossa BA, Alterio D, et al. Locoregionally advanced nasopharyngeal carcinoma: induction chemotherapy with cisplatin and 5-fluorouracil followed by radiotherapy and concurrent cisplatin: a phase II study. Oncology (2008) 74:158–66. doi: 10.1159/000151363
16. Bae WK, Hwang JE, Shim HJ, Cho SH, Lee JK, Lim SC, et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol (2009) 65:589–95. doi: 10.1007/s00280-009-1152-0
17. Huang PY, Mai HQ, Luo DH, Qiu F, Li NW, Xiang YQ, et al. Induction-concurrent chemoradiotherapy versus induction chemotherapy and radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer (2009) 28(10):1033–42. doi: 10.5732/cjc.009.10114
18. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol (2009) 27:242–9. doi: 10.1200/JCO.2008.18.1545
19. Kong L, Zhang YW, Hu CS, Guo Y. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locally advanced nasopharyngeal carcinoma. Chin J Cancer (2010) 29:551–5. doi: 10.5732/cjc.009.10518
20. Zheng JJ, Wang G, Yang GY, Wang DY, Luo XZ, Chen C, et al. Induction chemotherapy with nedaplatin with 5-FU followed by intensity-modulated radiotherapy concurrent with chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Japanese J Clin Oncol (2010) 40:425–31. doi: 10.1093/jjco/hyp183
21. Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic cooperative oncology group (HeCOG) with biomarker evaluation. Ann Oncol (2012) 23:427–35. doi: 10.1093/annonc/mdr116
22. Huang PY, Cao KJ, Guo X, Mo HY, Guo L, Xiang YQ, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol (2012) 48:1038–44. doi: 10.1016/j.oraloncology.2012.04.006
23. Huang PY, Zeng Q, Cao KJ, Guo X, Guo L, Mo HY, et al. Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: A single-institution experience from an endemic area. Eur J Cancer (2015) 51:1760–70. doi: 10.1016/j.ejca.2015.05.025
24. Kong L, Hu C, Niu X, Zhang Y, Guo Y, Tham IWK, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer (2013) 119:4111–8. doi: 10.1002/cncr.28324
25. Lim AM, Corry J, Collins M, Peters L, Hicks RJ, D'Costa I, et al. A phase II study of induction carboplatin and gemcitabine followed by chemoradiotherapy for the treatment of locally advanced nasopharyngeal carcinoma. Oral Oncol (2013) 49:468–74. doi: 10.1016/j.oraloncology.2012.12.012
26. Zhong YH, Dai J, Wang XY, Xie CH, Chen G, Zeng L, et al. Phase II trial of neoadjuvant docetaxel and cisplatin followed by intensity-modulated radiotherapy with concurrent cisplatin in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol (2013) 71:1577–83. doi: 10.1007/s00280-013-2157-2
27. Rosenblatt E, Abdel-Wahab M, El-Gantiry M, Elattar I, Bourque JM, Afiane M, et al. Brachytherapy boost in loco-regionally advanced nasopharyngeal carcinoma: a prospective randomized trial of the international atomic energy agency. Radiat Oncol (2014) 9:67. doi: 10.1186/1748-717x-9-67
28. Lee AWM, Ngan RKC, Tung SY, Cheng A, Kwong DLW, Lu TX, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer (2015) 121:1328–38. doi: 10.1002/cncr.29208
29. Lee AWM, Ngan RKC, Ng WT, Tung SY, Cheng AAC, Kwong DLW, et al. NPC-0501 trial on the value of changing chemoradiotherapy sequence, replacing 5-fluorouracil with capecitabine, and altering fractionation for patients with advanced nasopharyngeal carcinoma. Cancer (2020) 126:3674–88. doi: 10.1002/cncr.32972
30. Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (2015) 91:952–60. doi: 10.1016/j.ijrobp.2015.01.002
31. Lv X, Xia WX, Ke LR, Yang J, Qiu WZ, Yu YH, et al. Comparison of the short-term efficacy between docetaxel plus carboplatin and 5-fluorouracil plus carboplatin in locoregionally advanced nasopharyngeal carcinoma. Oncotar Ther (2016) 9:5123–31. doi: 10.2147/OTT.S103729
32. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
33. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer (2019) 145:295–305. doi: 10.1002/ijc.32099
34. Tang CY, Wu F, Wang RS, Lu HM, Li GS, Liu ML, et al. Comparison between nedaplatin and cisplatin plus docetaxel combined with intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma: a multicenter randomized phase II clinical trial. Am J Cancer Res (2016) 6:2064–75.
35. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer (2017) 75:14–23. doi: 10.1016/j.ejca.2016.12.039
36. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer (Oxf Engl) (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007
37. Ke LR, Xia WX, Qiu WZ, Huang XJ, Yu YH, Liang H, et al. A phase II trial of induction NAB-paclitaxel and cisplatin followed by concurrent chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma. Oral Oncol (2017) 70:7–13. doi: 10.1016/j.oraloncology.2017.04.018
38. Ke LR, Xia WX, Qiu WZ, Huang XJ, Yang J, Yu YH, et al. Safety and efficacy of lobaplatin combined with 5-fluorouracil as first-line induction chemotherapy followed by lobaplatin-radiotherapy in locally advanced nasopharyngeal carcinoma: preliminary results of a prospective phase II trial. BMC Cancer (2017) 17:134. doi: 10.1186/s12885-017-3080-4
39. Kong L, Zhang YW, Hu CS, Guo Y, Lu JDJ. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: Final results of 2 parallel phase 2 clinical trials. Cancer (2017) 123:2258–67. doi: 10.1002/cncr.30566
40. Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol (2018) 29:731–6. doi: 10.1093/annonc/mdx770
41. Hong RL, Hsiao CF, Ting LL, Ko JY, Wang CW, Chang JTC, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan cooperative oncology group (TCOG) 1303 study. Ann Oncol (2018) 29:1972–9. doi: 10.1093/annonc/mdy249
42. Wei JW, Feng HX, Xiao WW, Wang QX, Qiu B, Liu SL, et al. Cycle number of neoadjuvant chemotherapy might influence survival of patients with T1-4N2-3M0 nasopharyngeal carcinoma. Chin J Cancer Res (2018) 30:51. doi: 10.21147/j.issn.1000-9604.2018.01.06
43. Yang H, Chen X, Lin S, Rong J, Yang M, Wen Q, et al. Treatment outcomes after reduction of the target volume of intensity-modulated radiotherapy following induction chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a prospective, multi-center, randomized clinical trial. Radiother Oncol (2018) 126:37–42. doi: 10.1016/j.radonc.2017.07.020
44. Ghosh-Laskar S, Pilar A, Prabhash K, Joshi A, Agarwal JP, Gupta T, et al. Taxane-based induction chemotherapy plus concurrent chemoradiotherapy in nasopharyngeal carcinoma: Prospective results from a non-endemic cohort. Clin Oncol (2019) 31:850–7. doi: 10.1016/j.clon.2019.06.011
45. Jin T, Qin WF, Jiang F, Jin QF, Wei QC, Jia YS, et al. Cisplatin and fluorouracil induction chemotherapy with or without docetaxel in locoregionally advanced nasopharyngeal carcinoma. Trans Oncol (2019) 12:633–9. doi: 10.1016/j.tranon.2019.01.002
46. Lu Y, Chen D, Liang J, Gao J, Luo Z, Wang R, et al. Administration of nimotuzumab combined with cisplatin plus 5-fluorouracil as induction therapy improves treatment response and tolerance in patients with locally advanced nasopharyngeal carcinoma receiving concurrent radiochemotherapy: a multicenter randomized controlled study. BMC Cancer (2019) 19:1262. doi: 10.1186/s12885-019-6459-6
47. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. New Engl J Med (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
48. Zhao C, Miao JJ, Hua YJ, Wang L, Han F, Lu LX, et al. Locoregional control and mild late toxicity after reducing target volumes and radiation doses in patients with locoregionally advanced nasopharyngeal carcinoma treated with induction chemotherapy (IC) followed by concurrent chemoradiotherapy: 10-year results of a phase 2 study. Int J Radiat Oncol Biol Phys (2019) 104:836–44. doi: 10.1016/j.ijrobp.2019.03.043
49. Al-Rajhi NM, Khalil EM, Ahmad S, Soudy H, AlGhazi M, Fatani DM, et al. Low-dose fractionated radiation with induction docetaxel and cisplatin followed by concurrent cisplatin and radiation therapy in locally advanced nasopharyngeal cancer: a randomized phase II–III trial. Hematol/ Oncol Stem Cell Ther (2020) 14(3):199–205. doi: 10.1016/j.hemonc.2020.05.005
50. Li YY, Tian Y, Jin F, Wu WL, Long JH, Ouyang JL, et al. A phase II multicenter randomized controlled trial to compare standard chemoradiation with or without recombinant human endostatin injection (Endostar) therapy for the treatment of locally advanced nasopharyngeal carcinoma: Long-term outcomes update. Curr Problems Cancer (2020) 44(1):100492. doi: 10.1016/j.currproblcancer.2019.06.007
51. Lv X, Cao X, Xia WX, Liu KY, Qiang MY, Guo L, et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III–IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22:716–26. doi: 10.1016/S1470-2045(21)00075-9
52. Yao ZX, Zhang B, Huang JL, Shi L, Cheng B. Radiation-induced acute injury of intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in induction chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a prospective cohort study. Sci Rep (2021) 11(1):7693. doi: 10.1038/s41598-021-87170-6
53. Xiong Y, Shi L, Zhu L, Peng G. Comparison of TPF and TP induction chemotherapy for locally advanced nasopharyngeal carcinoma based on TNM stage and pretreatment systemic immune-inflammation index. Front Oncol (2021) 11:731543. doi: 10.3389/fonc.2021.731543
54. He Y, Guo T, Wang J, Sun Y, Guan H, Wu S, et al. Which induction chemotherapy regimen followed by cisplatin-based concurrent chemoradiotherapy is the best choice among PF, TP and TPF for locoregionally advanced nasopharyngeal carcinoma? Ann Transl Med (2019) 7:104. doi: 10.21037/atm.2019.02.15
55. Tao HY, Zhan ZJ, Qiu WZ, Liao K, Yuan YW, Zheng RH. Docetaxel and cisplatin induction chemotherapy with or without fluorouracil in locoregionally advanced nasopharyngeal carcinoma: A retrospective propensity score matching analysis. Asia Pac J Clin Oncol (2021) 18(2):e111–8. doi: 10.1111/ajco.13565
56. Zhu J, Duan B, Shi H, Li Y, Ai P, Tian J, et al. Comparison of GP and TPF induction chemotherapy for locally advanced nasopharyngeal carcinoma. Oral Oncol (2019) 97:37–43. doi: 10.1016/j.oraloncology.2019.08.001
57. Wu Q, Liao W, Huang J, Zhang P, Zhang N, Li Q. Cost-effectiveness analysis of gemcitabine plus cisplatin versus docetaxel, cisplatin and fluorouracil for induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma. Oral Oncol (2020) 103:104588. doi: 10.1016/j.oraloncology.2020.104588
58. Yang J, Han J, He J, Duan B, Gou Q, Ai P, et al. Real-world cost-effectiveness analysis of gemcitabine and cisplatin compared to docetaxel and cisplatin plus fluorouracil induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Front Oncol (2020) 10:594756. doi: 10.3389/fonc.2020.594756
59. Peng H, Chen B, He S, Tian L, Huang Y. Efficacy and toxicity of three induction chemotherapy regimens in locoregionally advanced nasopharyngeal carcinoma: Outcomes of 10-year follow-up. Front Oncol (2021) 11:765378. doi: 10.3389/fonc.2021.765378
60. Liu GY, Lv X, Wu YS, Mao MJ, Ye YF, Yu YH, et al. Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. Cancer Commun (Lond) (2018) 38:21. doi: 10.1186/s40880-018-0283-2
61. Tang J, Zou GR, Li XW, Su Z, Cao XL, Wang BC. Weekly versus triweekly cisplatin-based concurrent chemoradiotherapy for nasopharyngeal carcinoma: a systematic review and pooled analysis. J Cancer (2021) 12:6209–15. doi: 10.7150/jca.62188
62. Lee JY, Sun JM, Oh DR, Lim SH, Goo J, Lee SH, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: A multicenter randomized phase II trial (KCSG-HN10-02). Radiother Oncol (2016) 118:244–50. doi: 10.1016/j.radonc.2015.11.030
63. Chen YP, Liu X, Zhou Q, Yang KY, Jin F, Zhu XD, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet (2021) 398:303–13. doi: 10.1016/S0140-6736(21)01123-5
64. Hui EP, Li WF, Ma BB, Lam WKJ, Chan KCA, Mo F, et al. Integrating postradiotherapy plasma Epstein-Barr virus DNA and TNM stage for risk stratification of nasopharyngeal carcinoma to adjuvant therapy. Ann Oncol (2020) 31:769–79. doi: 10.1016/j.annonc.2020.03.289
Keywords: induction chemotherapy, nasopharyngeal carcinoma, meta-analysis, concurrent chemoradiotherapy (CCRT), responses, safety
Citation: Wang B-C, Kuang B-H, Liu X-X, Lin G-H and Liu Q (2022) Induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: A systematic review and meta-analysis. Front. Oncol. 12:927510. doi: 10.3389/fonc.2022.927510
Received: 24 April 2022; Accepted: 05 July 2022;
Published: 29 July 2022.
Edited by:
Shao Hui Huang, University Health Network, CanadaReviewed by:
Qiaojuan Guo, Fujian Provincial Cancer Hospital, ChinaCopyright © 2022 Wang, Kuang, Liu, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bi-Cheng Wang, YmNzbm93ZWxsQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.