- 1Department of Oncologic and Urologic Surgery, The 903rd People's Liberation Army (PLA) Hospital, Wenzhou Medical University, Hangzhou, China
- 2Center for Radiation Oncology, Affiliated Hangzhou Cancer Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Neurology, The 903rd People's Liberation Army (PLA) Hospital, Wenzhou Medical University, Hangzhou, China
- 4Department of Urology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Enze Hospital, Taizhou Enze Medical Center (Group), Taizhou, China
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of renal cancer. The top four mutant genes affecting the occurrence and progression of ccRCC are VHL, PBRM1, BAP1, and SETD2, respectively. Tyrosine kinase/mammalian target of rapamycin inhibitors (TKI/mTORis) with or without immunotherapy are the standard and effective therapy to metastatic ccRCC. Once TKI/mTORis fail to ccRCC, there is still a lack of other effective therapies. In this study, we reported a case in which a metastatic ccRCC patient (T2aN1M1) presented resistance after a 28-month treatment by sorafenib–axitinib–everolimus (TKI-TKI-mTORi). Subsequently, a frame shift pathogenic mutation, c.799_800del (p.Q267fs) in the exon10 of BAP1 in ccRCC, was revealed by targeted sequencing. Oral administration of nilapanib (PARP inhibitor) was further given, which may provide a new therapy for TKI/mTORi-resistance metastatic ccRCC. Fortunately, a partial response has been achieved and lasted for 5 months. Since the frequency of BAP1 mutations in ccRCC patients was approximately 10%–20%, as reported previously, we also tried to explore the potential mechanisms benefitting from the nilapanib. Moreover, the literature concerning BAP1 mutation and associated cancers including ccRCC is reviewed.
Introduction
Clear cell renal cell carcinoma (ccRCC) is the most common pathological subtype of renal cell cancer, with a proportion of more than 75%. CcRCC is characterized by a high frequency, more than 90%, of von Hippel–Lindau (VHL) gene inactivation, which plays a key role in regulating angiogenesis through a hypoxia-driven pathway and affects the expression of multiple genes, such as the vascular endothelial growth factor (VEGF) and its receptor (VEGFR) (1–3). Due to the lack of effective chemotherapy, interferon, and cytokines, the tyrosine kinase inhibitor (TKI), which target those pathways including VEGF and VEGFR genes, played an important role in the treatment of advanced ccRCC in the past 20 years (4). With the development of immunotherapy, especially since 2019, TKI alone is instead by the TKI combined with immunotherapy for the metastatic ccRCC as the first-line therapy (5, 6). Alhough TKI with or without immunotherapy did prolong metastatic ccRCC patients’ progression-free survival (PFS) time. The second line therapy is limited to change one TKI to another (TKI to TKI) or the mammalian target of rapamycin inhibitor (mTORi), and in the absence of precision medicine based on gene sequencing.
Actually, VHL is not the only inactivation gene that appeared in ccRCC. Secondarily mutated genes, including PBRM1, BAP1, and SETD2, are also involved in the formation of ccRCC, with a frequency up to 30%–41%, 10%–20%, and 10%–20%, respectively (7–9). Here, we present a metastatic ccRCC patient with a somatic mutation BAP1 detected by targeted genes and next-generation sequencing (targeted sequencing; range 808 genes®). After a 28-month treatment of TKI to TKI to mTORi, the patient presented resistance and tumor progression based on RECIST v1.1 (10). Subsequently, nilapanib [poly ADP-ribose polymerase inhibitor (PARPi)] was further given, and partial response was achieved, which has not been reported before. This may provide a new insight and drug therapy for advanced-resistance ccRCC. Moreover, we also systematically reviewed the previous literature, and assessed the BAP1 alteration frequency and possible mechanism in cancers, particularly in ccRCC.
Case Description
A 49-year-old man without urologic symptoms and renal cancer family history received a conventional physical examination in a local hospital in May 2018. A huge hypoechoic mass in the left renal was occasionally showed by urologic Doppler ultrasound (US) examination. Computed tomography (CT) revealed an enhancing mass measuring 9.3 × 8.2 × 7.7 cm in the left renal (Figure 1A), multiple enlarged lymph nodes in the retroperitoneal, and a quasi-circular mass (maximum size 3.0 cm) in the liver but normal alpha-fetoprotein levels (1.54 ng/ml; normal <5.0 ng/ml). Afterwards, a US-guided fine-needle aspiration (FNA) was performed on the left renal mass. The histological Hematoxylin-Eosin (H&E) examination of biopsy specimens showed features positive for malignancy, and immunohistochemistry revealed that the tumor cells were positive expressions of PAX-8, CAIX, CD10, and negative for CK7, suggesting ccRCC [T2aN1M1 (11)]. Meanwhile blood routine testing showed the patient with a lower serum hemoglobin of 97 g/L, an elevated platelet of 393 × 109/L, and Ca2+ 3.05 mmol/L, respectively. Based on these blood markers and less than 1 year from diagnosis to treatment, the patient was classified as a poor-risk group according to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria (11). Sorafenib (400 mg, bid) was subsequently taken as the first-line therapy based on the European Association of Urology (EAU) Guidelines–2018 (11). In July 2019, after the patient achieved a partial response confirmed by magnetic resonance imaging (MRI) after sorafenib-targeted therapy for 14 months (Figure 1B) (10), cytoreductive nephrectomy (CN) was further performed. Histopathological examination revealed ccRCC with lymph node metastases. Two weeks later, a radiofrequency ablation of liver metastases was also conducted. After 2 months of the CN operation, sorafenib was replaced by axitinib (5 mg, bid) due to an adverse event of grade 3 maculopapular erythroderma, and the disease was stable continuously. In May 2020, the patient began to lose weight and have a headache. Chest CT identified the areas of new abnormal metabolism in both lungs and brain MRI showed a mass in the right optic canal. Everolimus (10 mg per day; mTORi) was subsequently given as the third line but showed ineffective treatment.
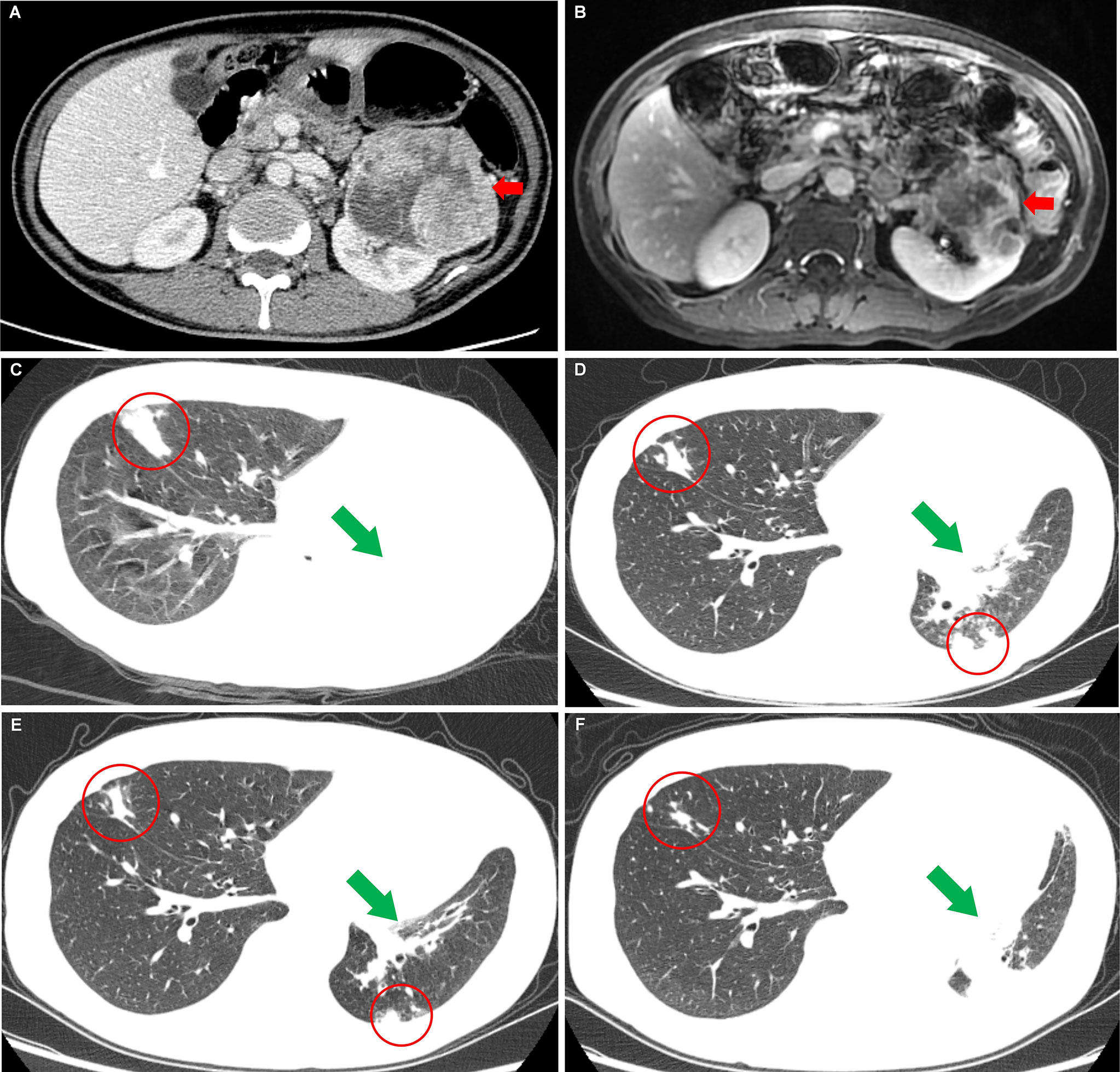
Figure 1 Images of the left renal carcinoma and the changes of metastases when the patient took nilapanib. (A) Computed tomography image examination showed an enhancing mass measuring 9.3 cm × 8.2 cm × 7.7 cm (red arrow) in the left renal. (B) Magnetic resonance image examination revealed a significantly reduced mass [7.3 cm × 6.8 cm × 7.0 cm (red arrow)] in the left renal after sorafenib-targeted therapy for 14 months. (C) Before nilapanib; (D) After nilapanib for 2 months; (E) After nilapanib for 4 months; (F) After nilapanib for 5 months. Chest CT showed metastases in the lungs (red circle) and atelectasis in the left lung (green arrows) due to the tumor invasion of the left bronchus, received continuous remission in the first 4 months after nilapanib (C–E). (F) showed that new metastases appeared in the hilus of the left lung, resulting in atelectasis.
In September 2020, the patient came to our hospital with a severe headache and dyspnea. The admission assessment showed a 4-grade Eastern Cooperative Oncology Group (12) and an 8-grade Visual Analogue Scale (VAS) Pain (13). Chest CT showed atelectasis in the left lung due to the tumor invasion of the left bronchus and metastases in the right lung (Figure 1C). Brain MRI showed a mass in the right optic canal. Targeted genetic testing was further performed on a ccRCC specimen from nephrectomy [formalin-fixed paraffin-embedded (FFPE)] by utilizing the Illumina HiSeq 4000 platform. A frame shift pathogenic mutation c.799_800del (p.Q267fs) in the exon 10 of BAP1 (https://www.ncbi.nlm.nih.gov/clinvar/variation/1070749/) was found. Owing to the patient’s worse performance status and high cost of drugs, immune checkpoint inhibitors (PD-1/PD-L1/CTLA-4/LAG-3/CD47) were not a priority treatment option and should be refused. On the contrary, based on BAP1 mutation detected in the tumor in the patient, PARPi should be recommended. In October 2020, niraparib (200 mg per day) was taken after obtaining the patient and his family’s full informed consent, followed by CyberKnife radiosurgery (25 Gy; 5 cycles) for the treatment of intracranial metastatic lesion and supplemented nutritional support therapy. To our excited, the patient displayed a partial response in both lungs after niraparib for 2 months (Figure 1D). The intracranial lesion also shrunk due to radiotherapy and the headache were totally released. The Lactate Dehydrogenase (LDH) and Carcino-Embryonic Antigen (CEA), which elevated to abnormal when the patient admitted to our hospital, returned to normal again (Figure 2). In the following 2 months, the patient received continuous remission (Figure 1E). However, in March 2021, 5 months after taking niraparib, new metastases appeared in the brain and the hilus of the left lung, resulting in atelectasis (Figure 1F). Then, the patient began to suffer from breathing difficulties and refused any further treatment, except palliative care. Eventually, the patient died in June 2021 according to his family’s message.
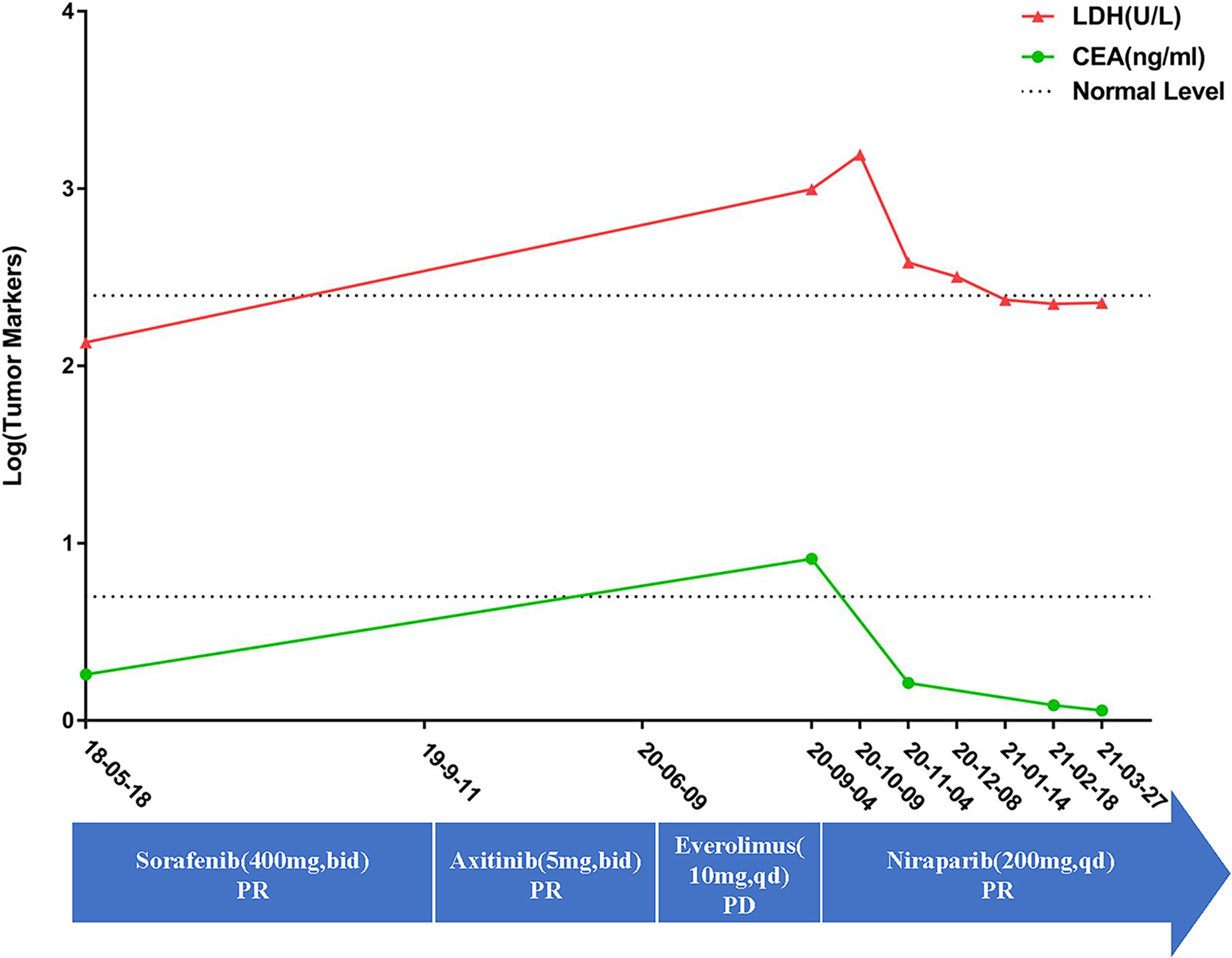
Figure 2 The patient’s detailed treatment process and the tumor marker changes. The curve showed that the tumor markers is still in the normal level upon first diagnosis. However, they were already higher than normal when the patient was admitted in our hospital. However, after taking nilapanib, the tumor markers returned to normal again. The blue arrow showed the detailed medication information along the treatment. On the other hand, there is still the lack of specific tumor markers for ccRCC. The changes of LDH and CEA may be inconsistent with changes in the tumors.
Moreover, we clustered data that were identified from other patients with cancer and BAP1 alteration by the “cBioPortal” (http://www.cbioportal.org). This identified a total of 32 large-scale TCGA PanCancer Atlas Studies containing 10,967 samples. The data from these publications are clustered for analysis as shown in Figure 3A.
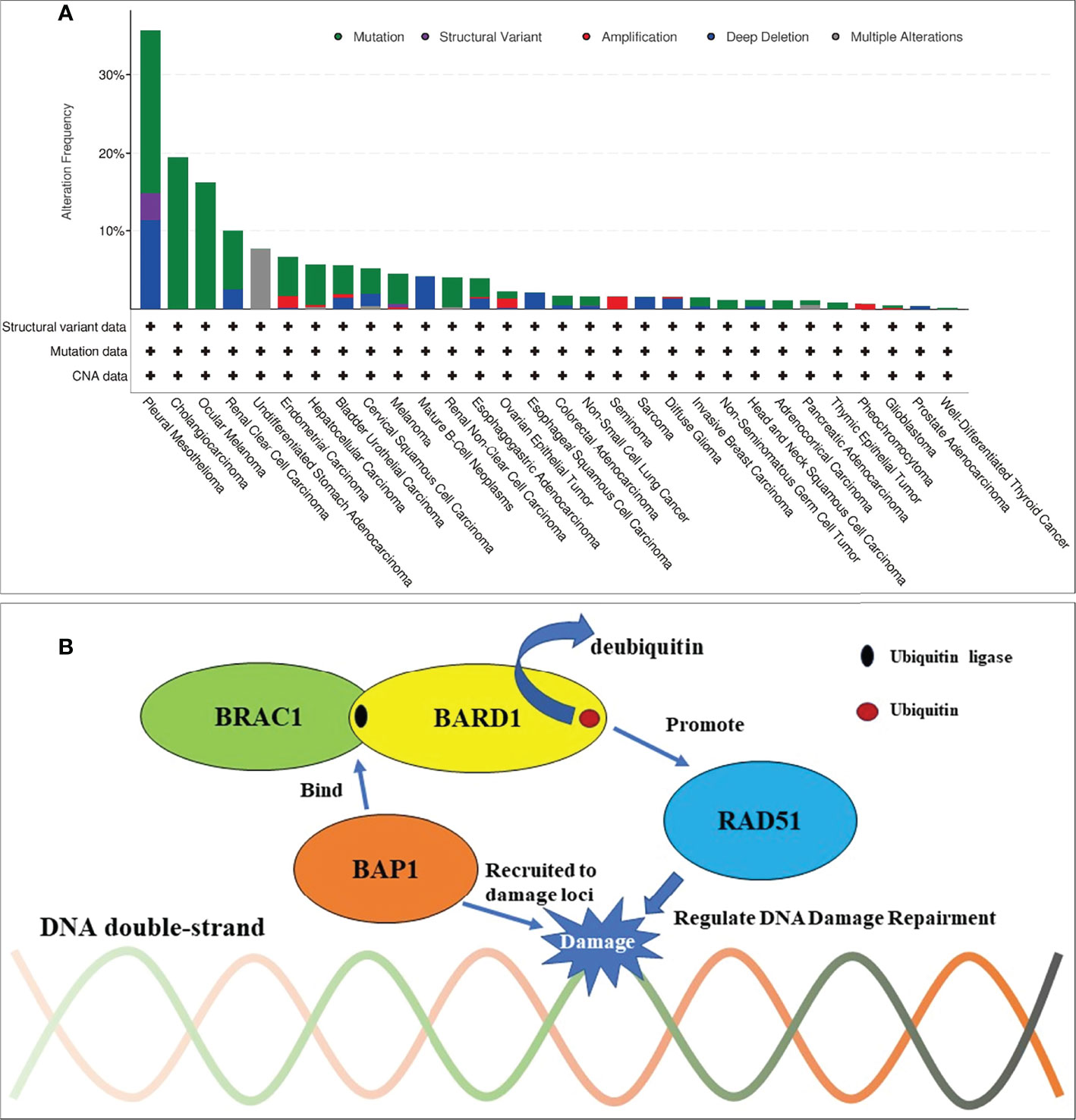
Figure 3 Frequencies of BAP1 alteration across cancer types and mechanism of BAP1 in DNA damage repairment. (A) BAP mutations most commonly occurred in pleural mesotheliomas, cholangiocarcinoma, ocular melanoma, and renal clear cell carcinoma from a total of 32 large-scale TCGA PanCancer Atlas Studies that contained 10,967 samples. (B) Mechanism of BAP1 in DNA damage repairment. BRCA1/BARD1 complex is an E3 ubiquitin ligase and interacts with the recombinase RAD51 to regulate the homologous recombination repairment. BAP1 can be recruited to DNA damage loci and bind to the BRCA1/BARD1 complex. By this, BAP1 can inhibit the E3 ligase activity of BRCA1/BARD1 to protect against additional ubiquitination and deubiquitinate preexisting ubiquitin chains (bent arrow).
Discussion
BAP1, which is located on the chromosome 3p21.1, is a ubiquitin carboxy-terminal hydrolase (14). It was recognized as a tumor suppressor, and a mass of processes including chromatin modification, programmed cell death, cell cycle control, DNA damage repair, and the immune response were regulated by its deubiquitinating activity (14, 15). BAP1 can undergo germline or somatic alterations, and tumors that are relevant to germline are semblable to those with somatic BAP1 alterations (15). The inactivation of the BAP1 gene (either germline or somatic form) often led to the development of a number of cancers, such as melanoma, malignant mesothelioma, and ccRCC (14, 16). This may indicate the common mechanisms of tumorigenesis and the potential target therapies to BAP1 in these highly relevant tumors (15). CcRCC with a BAP1 mutation often showed clinicopathologic features as a high pathological stage, high renal vein involvement rate, and high metastasis rate and always had a worse prognosis, even in patients with low-risk tumors (17–19). In this study, the presence of the patient by physical examination showed that the occasional ccRCC had advanced and distant (bone and liver) metastasis (T2aN1M1), implying a worse prognosis. Moreover, the traditional targeted therapy for ccRCC with BAP1 mutation, for example, antiangiogenic therapy-TKI, might also show a poor outcome (20). In contrast, this patient received a 24-month PFS time in total by the two TKI (sorafenib to axitinib) therapies, showing a certain degree of efficacy. On the contrary, mTORi (everolimus) was used but not effective, lasting 3 months continuously. The treatment options for ccRCC patients regardless with or without the BAP1 alterations are limited to traditional therapies. TKI with or without immunotherapy was still the main treatment method. Once TKI failed, the optimal further treatment is scanty. Immunotherapy was an option for a subsequent treatment; however, patients’ poor performance status and the high cost of immune checkpoint inhibitors always prevented its application, such as this patient. It is interesting that several therapies targeting the BAP1 alterations showed a positive potential in cancers, according to the recent studies (21, 22). The enhancer of zeste homolog 2 inhibitor (EZH2i) and histone deacetylase inhibitors (HDACis), which target the mechanism of BAP1 in transcriptional regulation and chromatin modification, respectively, are potential targeted therapies (21, 22). An EZH2i study had positive results obtained in a phase 2 trial conducted in BAP1-altered malignant pleural mesothelioma in vivo (22), as well as HDACi warrants further exploration whether BAP1 aberrations modulate response in VANTAGE 014 study (21), although BAP1 downregulation increases the sensitivity to HDACi in vitro (23). Unfortunately, EZH2i and HDACi were not available in China, potentially, PARPi should be chosen as the further treatment for this TKI/mTORi-resistance advanced ccRCC.
BAP1 is first known as a protein interacted with BRCA1, which is the famous homologous recombination repair gene (24, 25). Nowadays, research has also reported that BAP1 regulates the DNA damage repair in many ways, for instance, binding the BRCA1/BARD1 complex. The BRAC1/BARD1 complex, which functions as an E3 ubiquitin ligase, is known to play a significant role in the DNA damage response through recruiting RAD51 to the damaged loci and so on (26, 27). Jensen et al. showed that BAP1 can interact with BRCA1 and augment the tumor suppressor activity of BRCA1 (28). Nishikawa et al. showed that BARD1 is the major binding partner of BAP1 (29). By binding to the BRCA1/BARD1 complex, BAP1 can inhibit the E3 ligase activity of BRCA1/BARD1 to protect against additional ubiquitination and deubiquitinate preexisting ubiquitin chains (29). The dual role of BAP1 toward BRCA1/BARD1 could be important to ensure the inhibition of ubiquitination in cellular pathways, though the interacting mechanism of these molecules between BAP1 and BRCA1/BARD1 needs further study (Figure 3B) (24–30). Thus, PARPi would have a synthetic lethal effect on BAP1-mutated tumors, theoretically. Given the known experiment of BAP1-mutated cancers, PARPi was effective in the treatment of malignant neoplasm—pleural mesotheliomas, osteosarcomas in vitro (31–34) and mesotheliomas, intrahepatic cholangiocarcinoma in vivo (35–38), and so on (Table 1). The ongoing phase II clinical studies of niraparib and olaparib also react to the safety and feasibility of PARPi in treating patients with BAP1 alterations (NCT03207347, NCT03531840, NCT03786796). As long as the mutated genes are identical, the same targeted drugs are feasible for different diseases (39). To control tumor progression, PARPi may have the potential to target BAP1-altered ccRCC. Based on these findings, niraparib was taken after obtaining the patient’s full informed consent. The exciting part is that the tumors in the lungs achieved a partial response for 5 months, suggesting that niraparib is a relatively effective treatment for TKI-refractory metastatic ccRCC with BAP1 alteration. Although owing the lack of specific tumor markers for ccRCC, the effect of the treatment cannot always be reflected by tumor markers. The reduction of LDH and CEA, which elevated to abnormal when the patient was admitted to hospital, may indicate that niraparib worked to some extent. Moreover, for ccRCC patients with a BAP1 alteration, immunotherapy and/or immunocombination therapy may improve efficacy. Recent reports revealed that BAP1 alterations in cancer confer distinct immunogenic phenotypes that may be particularly susceptible to novel cancer immunotherapies (40). BAP1 mutations in ccRCC correlate with increased CCR5 expression and immunosuppression (41). These studies speculated that both PARPi and immunotherapy seemed to show enormous potential in treating ccRCC with BAP1 alteration. Unfortunately, the patient never received the immunotherapy from the beginning to the end.
Conclusion
This study demonstrates that PARPi may be another potential therapy for TKI/mTORi-resistance ccRCC with a BAP1 mutation, and, additionally, immunotherapy and/or immunocombination may also have effect on ccRCC with a BAP1 mutation, although this warrants further validation in clinical studies. An individualized comprehensive approach for advanced ccRCC with BAP1 mutation is beneficial.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The 903rd Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conception/design: X-PQ and B-JL; Provision of study material or patients: KZ, W-YC, FL, and X-DF; Data collection and analysis: B-JL, KZ, W-YC, X-DF, and ZD; Manuscript writing: B-JL and X-PQ; All authors have read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81472861), the Key Project of Zhejiang Province Science and Technology Plan, China (2014C03048-1), and Hangzhou Municipal Commission of Health and Family Planning Science and Technology Program (B20210355, OO20190253).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the patient and family members who agreed to publish the case in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.927250/full#supplementary-material
References
1. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol (2016) 70(1):93–105. doi: 10.1016/j.eururo.2016.02.029
2. Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL Tumour Suppressor Gene in Renal Carcinoma. Nat Genet (1994) 7(1):85–90. doi: 10.1038/ng0594-85
3. Rini BI, Campbell SC, Escudier B. Renal Cell Carcinoma. Lancet (2009) 373(9669):1119–32. doi: 10.1016/S0140-6736(09)60229-4
4. Motzer RJ, Molina AM. Targeting Renal Cell Carcinoma. J Clin Oncol (2009) 27(20):3274–6. doi: 10.1200/JCO.2009.21.8461
5. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
6. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab Plus Ipilimumab Versus Sunitinib for First-Line Treatment of Advanced Renal Cell Carcinoma: Extended 4-Year Follow-Up of the Phase III CheckMate 214 Trial. ESMO Open (2020) 5(6):e001079. doi: 10.1136/esmoopen-2020-001079
7. Jonasch E, Walker CL, Rathmell WK. Clear Cell Renal Cell Carcinoma Ontogeny and Mechanisms of Lethality. Nat Rev Nephrol (2021) 17(4):245–61. doi: 10.1038/s41581-020-00359-2
8. Guo G, Gui Y, Gao S, Tang A, Hu X, Huang Y, et al. Frequent Mutations of Genes Encoding Ubiquitin-Mediated Proteolysis Pathway Components in Clear Cell Renal Cell Carcinoma. Nat Genet (2011) 44(1):17–9. doi: 10.1038/ng.1014
9. Creighton CJ, Morgan M, Gunaratne PH, Wheeler DA, Gibbs RA, Robertson AG, et al. Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Clear Cell Renal Cell Carcinoma. Nature (2013) 499(7456):43–9. doi: 10.1038/nature12222
10. Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. New Response Evaluation Criteria in Solid Tumours-Revised RECIST Guideline (Version 1.1). Gan To Kagaku Ryoho (2009) 36(13):2495–501.
11. Ljungberg B, Albiges L, Bensalah K, Bex A, Giles RH, Hora M, et al. EAU Guidelines on Rencal Cell Carcinoma 2018. Arnhem, Netherlands: EAU Annual Congress Copenhagen (2018).
12. Oken MM, Creech RH, Tormey DC. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol (1982) 5(6):649–55. doi: 10.1097/00000421-198212000-00014
13. McCormack HM, Horne DJ, Sheather S. Clinical Applications of Visual Analogue Scales: A Critical Review. Psychol Med (1988) 18(4):1007–19. doi: 10.1017/S0033291700009934
14. Wang A, Papneja A, Hyrcza M, Al-Habeeb A, Ghazarian D. Gene of the Month: BAP1. J Clin Pathol (2016) 69(9):750–3. doi: 10.1136/jclinpath-2016-203866
15. Louie BH, Kurzrock R. BAP1: Not Just a BRCA1-Associated Protein. Cancer Treat Rev (2020) 90:102091. doi: 10.1016/j.ctrv.2020.102091
16. Laitman Y, Newberg J, Molho RB, Jin DX, Friedman E. The Spectrum of Tumors Harboring BAP1 Gene Alterations. Cancer Genet (2021) 256-257:31–5. doi: 10.1016/j.cancergen.2021.03.007
17. Joseph RW, Kapur P, Serie DJ, Eckel-Passow JE, Parasramka M, Ho T, et al. Loss of BAP1 Protein Expression is an Independent Marker of Poor Prognosis in Patients With Low-Risk Clear Cell Renal Cell Carcinoma. Cancer (2014) 120(7):1059–67. doi: 10.1002/cncr.28521
18. Tan G, Xuan Z, Li Z, Huang S, Chen G, Wu Y, et al. The Critical Role of BAP1 Mutation in the Prognosis and Treatment Selection of Kidney Renal Clear Cell Carcinoma. Transl Androl Urol (2020) 9(4):1725–34. doi: 10.21037/tau-20-1079
19. Gallan AJ, Parilla M, Segal J, Ritterhouse L, Antic T. BAP1-Mutated Clear Cell Renal Cell Carcinoma. Am J Clin Pathol (2021) 155(5):718–28. doi: 10.1093/ajcp/aqaa176
20. Hsieh JJ, Chen D, Wang PI, Marker M, Redzematovic A, Chen YB, et al. Genomic Biomarkers of a Randomized Trial Comparing First-Line Everolimus and Sunitinib in Patients With Metastatic Renal Cell Carcinoma. Eur Urol (2017) 71(3):405–14. doi: 10.1016/j.eururo.2016.10.007
21. Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, et al. Vorinostat in Patients With Advanced Malignant Pleural Mesothelioma Who Have Progressed on Previous Chemotherapy (VANTAGE-014): A Phase 3, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Oncol (2015) 16(4):447–56. doi: 10.1016/S1470-2045(15)70056-2
22. Zauderer MG, Szlosarek P, Moulec SL, Popat S, Fennell DA. Phase 2, Multicenter Study of the EZH2 Inhibitor Tazemetostat as Monotherapy in Adults With Relapsed or Refractory (R/R) Malignant Mesothelioma (MM) With BAP1 Inactivation. J Clin Oncol (2018) 36(15_suppl):8515–5. doi: 10.1200/JCO.2018.36.15_suppl.8515
23. Sacco JJ, Kenyani J, Butt Z, Carter R, Chew HY, Cheeseman LP, et al. Loss of the Deubiquitylase BAP1 Alters Class I Histone Deacetylase Expression and Sensitivity of Mesothelioma Cells to HDAC Inhibitors. Oncotarget (2015) 6(15):13757–71. doi: 10.18632/oncotarget.3765
24. Ismail IH, Davidson R, Gagne JP, Xu ZZ, Poirier GG, Hendzel MJ. Germline Mutations in BAP1 Impair its Function in DNA Double-Strand Break Repair. Cancer Res (2014) 74(16):4282–94. doi: 10.1158/0008-5472.CAN-13-3109
25. Wu J, Lu LY, Yu X. The Role of BRCA1 in DNA Damage Response. Protein Cell (2010) 1(2):117–23. doi: 10.1007/s13238-010-0010-5
26. Zhao W, Steinfeld JB, Liang F, Chen X, Maranon DG, Jian Ma C, et al. BRCA1-BARD1 Promotes RAD51-Mediated Homologous DNA Pairing. Nature (2017) 550(7676):360–5. doi: 10.1038/nature24060
27. Tarsounas M, Sung P. The Antitumorigenic Roles of BRCA1-BARD1 in DNA Repair and Replication. Nat Rev Mol Cell Biol (2020) 21(5):284–99. doi: 10.1038/s41580-020-0218-z
28. Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: A Novel Ubiquitin Hydrolase Which Binds to the BRCA1 RING Finger and Enhances BRCA1-Mediated Cell Growth Suppression. Oncogene (1998) 16(9):1097–112. doi: 10.1038/sj.onc.1201861
29. Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, et al. BRCA1-Associated Protein 1 Interferes With BRCA1/BARD1 RING Heterodimer Activity. Cancer Res (2009) 69(1):111–9. doi: 10.1158/0008-5472.CAN-08-3355
30. Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor Suppressor and Deubiquitinase BAP1 Promotes DNA Double-Strand Break Repair. Proc Natl Acad Sci USA (2014) 111(1):285–90. doi: 10.1073/pnas.1309085110
31. Srinivasan G, Sidhu GS, Williamson EA, Jaiswal AS, Najmunnisa N, Wilcoxen K, et al. Synthetic Lethality in Malignant Pleural Mesothelioma With PARP1 Inhibition. Cancer Chemother Pharmacol (2017) 80(4):861–7. doi: 10.1007/s00280-017-3401-y
32. Engert F, Kovac M, Baumhoer D, Nathrath M, Fulda S. Osteosarcoma Cells With Genetic Signatures of BRCAness are Susceptible to the PARP Inhibitor Talazoparib Alone or in Combination With Chemotherapeutics. Oncotarget (2017) 8(30):48794–806. doi: 10.18632/oncotarget.10720
33. Borchert S, Wessolly M, Schmeller J, Mairinger E, Kollmeier J, Hager T, et al. Gene Expression Profiling of Homologous Recombination Repair Pathway Indicates Susceptibility for Olaparib Treatment in Malignant Pleural Mesothelioma In Vitro. BMC Cancer (2019) 19(1):108. doi: 10.1186/s12885-019-5314-0
34. Parrotta R, Okonska A, Ronner M, Weder W, Stahel R, Penengo L, et al. A Novel BRCA1-Associated Protein-1 Isoform Affects Response of Mesothelioma Cells to Drugs Impairing BRCA1-Mediated DNA Repair. J Thorac Oncol (2017) 12(8):1309–19. doi: 10.1016/j.jtho.2017.03.023
35. Ghafoor A, Mian I, Wagner C, Mallory Y, Agra MG, Morrow B, et al. Phase 2 Study of Olaparib in Malignant Mesothelioma and Correlation of Efficacy With Germline or Somatic Mutations in BAP1 Gene. JTO Clin Res Rep (2021) 2(10):100231. doi: 10.1016/j.jtocrr.2021.100231
36. Dudnik E, Bar J, Moore A, Gottfried T, Moskovitz M, Dudnik J, et al. BAP1-Altered Malignant Pleural Mesothelioma: Outcomes With Chemotherapy, Immune Check-Point Inhibitors and Poly(ADP-Ribose) Polymerase Inhibitors. Front Oncol (2021) 11:603223. doi: 10.3389/fonc.2021.603223
37. Fennell DA, King A, Mohammed S, Branson A, Brookes C, Darlison L, et al. Rucaparib in Patients With BAP1-Deficient or BRCA1-Deficient Mesothelioma (MiST1): An Open-Label, Single-Arm, Phase 2a Clinical Trial. Lancet Respir Med (2021) 9(6):593–600. doi: 10.1016/S2213-2600(20)30390-8
38. Sabbatino F, Liguori L, Malapelle U, Schiavi F, Tortora V, Conti V, et al. Case Report: BAP1 Mutation and RAD21 Amplification as Predictive Biomarkers to PARP Inhibitor in Metastatic Intrahepatic Cholangiocarcinoma. Front Oncol (2020) 10:567289. doi: 10.3389/fonc.2020.567289
39. Lian B, Zhang W, Wang T, Yang Q, Jia Z, Chen H, et al. Clinical Benefit of Sorafenib Combined With Paclitaxel and Carboplatin to a Patient With Metastatic Chemotherapy-Refractory Testicular Tumors. Oncologist (2019) 24(12):e1437–42. doi: 10.1634/theoncologist.2019-0295
40. Shrestha R, Nabavi N, Lin YY, Mo F, Anderson S, Volik S, et al. BAP1 Haploinsufficiency Predicts a Distinct Immunogenic Class of Malignant Peritoneal Mesothelioma. Genome Med (2019) 11(1):8. doi: 10.1186/s13073-019-0620-3
Keywords: clear cell renal cell carcinoma, resistance, BAP1 mutation, niraparib, case report
Citation: Lian B-J, Zhang K, Fang X-D, Li F, Dai Z, Chen W-Y and Qi X-P (2022) Clinical Benefit of Niraparib to TKI/mTORi-Resistance Metastatic ccRCC With BAP1-Frame Shift Mutation: Case Report and Literature Review. Front. Oncol. 12:927250. doi: 10.3389/fonc.2022.927250
Received: 24 April 2022; Accepted: 06 June 2022;
Published: 06 July 2022.
Edited by:
Katy Beckermann, Vanderbilt University, United StatesReviewed by:
Ruhee Dere, Baylor College of Medicine, United StatesScott Haake, Vanderbilt University Medical Center, United States
Copyright © 2022 Lian, Zhang, Fang, Li, Dai, Chen and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Ping Qi, cXhwbG1kQDE2My5jb20=; Wei-Ying Chen, Y2hlbnd5ODMzNUBlbnplbWVkLmNvbQ==
†These authors have contributed equally to this work
 Bi-Jun Lian
Bi-Jun Lian Ke Zhang2†
Ke Zhang2† Xu-Dong Fang
Xu-Dong Fang Zhao Dai
Zhao Dai Xiao-Ping Qi
Xiao-Ping Qi