
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 July 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.926840
This article is part of the Research TopicLymph Node Assessment in Cervical CancerView all 9 articles
The aims of this study were to investigate the short-term and long-term efficacies and chronic radiotoxicity of concurrent chemoradiotherapy (CCRT) combined with image-guided adaptive brachytherapy (IGABT) in patients with locally advanced cervical cancer (LACC) and identify prognostic factors in this patient population. The clinical data of 204 patients with cervical cancer who completed CCRT and subsequent brachytherapy in our hospital between February 2015 and March 2017 were retrospectively analyzed. Short-term and long-term outcomes, chronic radiotoxicity, and prognostic factors were assessed. The median follow-up was 61.1 months. The short-term objective response (OR) rate was 85%. Lymph node metastasis before treatment was an independent predictor of OR (HR = 6.290, 95% CI: 2.211-17.897, p = 0.001). Fifty-two patients developed recurrence, with a median recurrence-free survival of 9.9 months (range, 2.4-52.2 months) and a post-recurrence survival of 12.1 months (range, 2.9-58.1 months). At 3 years, the cumulative incidence of overall recurrence was 26% (95% CI: 17-36). Multivariate analysis showed that Stage IIIB (HR = 2.332, 95% CI: 1.195-4.551, p = 0.013; reference, Stage IIB) and lymph node metastasis (HR = 4.462, 95% CI: 2.365-8.419, p < 0.001) were significant independent predictors of recurrence. Fifty-three patients developed chronic radiation proctitis (CRP). The incidence of severe CRP was approximately 5%, and the average rectal accumulation in patients with severe CRP was 73.4 Gy which is 3.9 Gy higher than that in patients without CRP (p = 0.013). At 4 years, the overall survival (OS) and disease-free survival rates were 65% and 62%, respectively, and lymph node metastasis before treatment was an independent prognostic risk factor for OS. The short-term and long-term efficacies of CCRT combined with IGABT for the treatment of LACC patients were relatively satisfactory. However, the short-term and long-term efficacies of patients with lymph node metastasis before treatment were poor. For patients with lymph node metastasis before treatment, more active individualized treatment strategies should be adopted. When designing a radiotherapy plan, it is necessary to strictly limit the rectal accumulation to prevent serious CRP.
According to the World Cancer Report 2020 released by the International Agency for Research on Cancer, cervical cancer is the most common malignant tumor of the female reproductive system worldwide, and it accounts for 3.1% and 3.4% of cancer-related morbidities and mortalities, respectively (1). According to the 2009 staging guidelines of the International Federation of Gynecology and Obstetrics (FIGO), locally advanced cervical cancer (LACC) includes Stages IB2 and IIA2-IVA cervical cancers, and the standard of care is brachytherapy after concurrent chemoradiotherapy (CCRT) (2, 3).
There have been great advancements in the treatment of cervical cancer in recent years, especially for LACC. For LACC, image-guided intensity modulated radiation therapy (IG-IMRT) and image-guided adaptive brachytherapy (IGABT) can more accurately provide a high dose to the gross tumor volume and reduce the dose to peripheral organs at risk (OARs) compared with 3-dimensional conformal radiation therapy and traditional 2-dimensional brachytherapy (4–8). In view of the significant improvement in local control and treatment-related toxicity, IG-IMRT plus IGABT combined with platinum-based concurrent chemotherapy has become the latest expert consensus for the treatment of LACCy (9–11). Many studies have reported the short-term and long-term efficacies of CCRT in LACC or its advantages over other treatment schemes, as well as prognostic factors (12–15). However, the specific radiotherapy techniques and chemotherapy schemes of these studies rarely reflect the latest expert consensus. Here, we retrospectively analyzed the clinical data of 278 cervical cancer patients who were treated with CCRT combined with subsequent brachytherapy by Professor Ye Weijun’s group in the Department of Radiotherapy, Sun Yat-sen University Cancer Center between February 2015 and March 2017. All patients underwent radiotherapy with IG-IMRT+IGABT and concurrent chemotherapy with cisplatin. The short-term and long-term efficacies and chronic radiotoxicity were assessed, and risk factors of relapse, metastasis, and survival were analyzed and summarized to provide a reference for individualized treatment of LACC.
The clinical data of 278 patients with cervical cancer who received CCRT and subsequent brachytherapy from Professor Ye Weijun’s group at the Department of Radiotherapy, Sun Yat-sen University Cancer Center between February 2015 and March 2017 were retrospectively analyzed. The inclusion criteria were as follows: (1) no previous major surgery and no history of pelvic radiotherapy; (2) treatment with radical CCRT and subsequent brachytherapy; (3) Stage IB2 or IIA2-IVA LACC according to the 2009 FIGO staging guidelines 2; (4) histological diagnosis of squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma confirmed by pathological examination; and (5) clinical examination and specialized examination using MRI, PET-CT, and/or CT to evaluate the local and metastatic spread of the tumor. In detail, to obtain data regarding tumor size, vaginal involvement, and parametrial invasion, each patient in the lithotomy position was examined by two experienced gynecologic oncologists with vaginal inspection and bimanual vaginal and rectal palpation. At the same time, MRI was used to assist in the assessment of parametrial involvement. And MRI, PET-CT and/or CT were performed to assess regional lymph nodes. The exclusion criteria were as follows: (1) other synchronous malignant tumors; (2) serious untreated complications; and (3) loss to follow-up or incomplete records of relevant research data. There were 204 patients with cervical cancer who met the criteria, and the effective rate was 73.4%. Table 1 provides details on the general clinical data.
All patients received CCRT followed by brachytherapy. Pelvic external irradiation was performed using image-guided volume-modulated arc therapy (VMAT) at a total dose of 45-50 Gy in 180-200 cGy/fraction. The primary tumor and metastatic pelvic lymph nodes were simultaneously administered 60 Gy in 240 cGy/fraction. The target volume was delineated according to the RTOG guidelines (16). In the late course of external irradiation, an Elekta high-dose-rate 192Ir afterloading unit was used to conduct CT image-guided IGABT, 7 Gy/fraction, 2 times/week, for a total of 2 weeks. The target volume was delineated according to the GEC-ESTRO guidelines (17). IGABT was administered using manual interstitial brachytherapy combined with intracavitary brachytherapy. Concurrent chemotherapy of 40 mg/m2 intravenous platinum was administered once a week for 5-7 weeks.
Using the quadratic linear model (α/β = 10 was adopted for gross tumor volume, and α/β = 3 was adopted for OARs), the physical dose of IGABT was converted into the bioequivalent dose of 2 Gy, and the dose received by 90% of the clinical target volume (HRCTV) of the primary tumor (D90%) and the irradiation dose received by 2 cm3 () of the high-dose area to the OARs were calculated (18, 19). Then, the doses of IGABT and pelvic external irradiation were added to obtain the cumulative D90% to the HRCTV and the cumulative to the OARs.
Imaging and gynecological examination were used to assess the efficacy of treatment 3 months after treatment according to the Response Evaluation Criteria In Solid Tumors (20). Complete remission (CR) was defined as complete disappearance of the lesions and normal tumor marker levels for more than 4 weeks. Partial response (PR) was defined as a ≥30% decrease in the lesion diameter for more than 4 weeks. Stable disease (SD) was defined as a <30% decrease or a <20% increase in the lesion diameter. Progressive disease (PD) was defined as a ≥20% increase in the lesion diameter and an increase in the absolute value by ≥5 cm or the appearance of new lesions. The objective response (OR) rate was calculated as the percentage of patients achieving CR or PR. Late toxicities were judged as Grades 1-4 according to the EORTC/RTOG late effects of normal tissues guidelines (21–23). Chronic toxicity was judged and graded by three senior doctors in combination with clinical symptoms and endoscopic and imaging examination results. This study only reported the results of chronic radiation proctitis (CRP) because of its incidence and degree of impact.
Follow-up was conducted by hospital reexaminations and telephone calls. The cutoff date for follow-up was March 6, 2022. All patients were followed up regularly for more than 5 years after treatment or until death. The median follow-up time was 61.1 months (range, 3.3–80.7 months). Reexaminations were performed every 1-3 months in the 1st year, every 3 months in the 2nd to 3rd years, every 6 months in the 3rd to 5th years, and annually thereafter. Overall survival (OS) was defined as the time from the beginning of treatment to death for any reason or the last follow-up, and disease-free survival (DFS) was defined as the time from the beginning of treatment to recurrence, metastasis, or death for any reason or the last follow-up. Distant metastasis (DM)-free survival was defined as the time from the beginning of treatment to the first distant metastasis or death for any reason, and locoregional recurrence (LR)-free survival was defined as the time from the beginning of treatment to the first locoregional recurrence
SPSS V.26.0 (IBM, New York, USA) and GraphPad Prism V.9.0 (GraphPad Software Company, Beijing, China) software were used for statistical analysis. For the univariate analysis of short-term efficacy, the Mann-Whitney U test was used for categorical data and χ2 test was used for continuous data; binary logistic regression analysis was used for multivariate analysis. The relationship between CRP and rectal was assessed by ANOVA, and LSD analysis was used for multiple comparisons. The Kaplan-Meier method was used for the analysis of recurrence and survival, and the Cox risk regression model was used for univariate and multivariate analysis. P < 0.05 indicated statistical significance.
Three months after treatment by CCRT combined with IGABT, 120 patients (59%) had achieved CR, 53 (26%) had achieved PR, 16 (8%) had SD, and 15 (7%) had PD (Figure 1A). The OR rate was 85% (173/204).
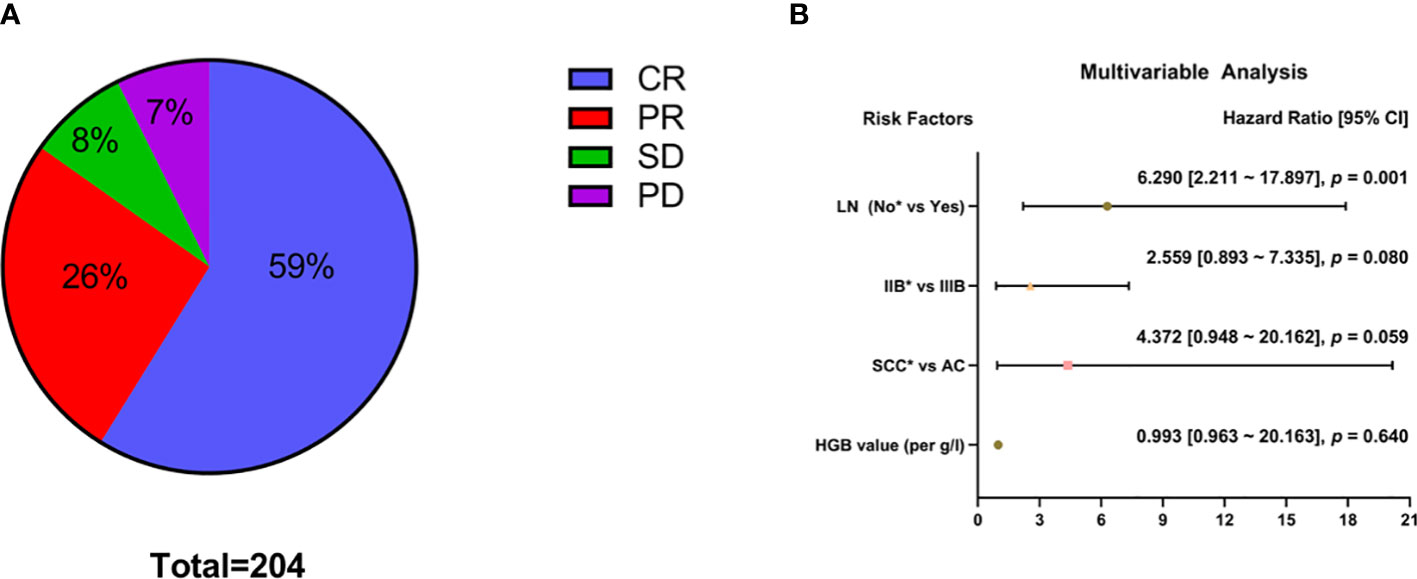
Figure 1 Analysis of short-term efficacy (N = 204). Specific patterns of (A) short-term efficacy. (B) Multivariable analysis; *Reference item.
Univariate analysis showed that histological type (squamous cell carcinoma vs adenocarcinoma: χ² = 4.183, p = 0.041) and lymph node metastasis before treatment (χ² = 22.532, p < 0.001) had significant effects on short-term efficacy (Table 2). Factors with p < 0.2 in the univariate analysis (HGB level before treatment, histological type, FIGO stage, and lymph node metastasis before treatment) were included in the multivariate analysis. The results showed that CCRT combined with IGABT had poor short-term efficacy in LACC patients with lymph node metastasis before treatment (HR = 6.290, 95% CI: 2.211-17.897, p = 0.001), whereas HGB levels (OR = 0.993, 95% CI: 0.963-1.024, p = 0.640), histology (adenocarcinoma vs. squamous cell carcinoma: OR = 4.372, 95% CI: 0.948-20.162, p = 0.059), and tumor stage (Stage IIIB vs Stage IIB: OR = 2.559, 95% CI: 0.893-7.335, p = 0.080) were not significantly associated with short-term efficacy (Figure 1B).
During the follow-up period, 52 patients experienced recurrence (including LR and DM), with a median recurrence-free survival of 9.9 months (range, 2.4-52.2 months). At 3 years, the cumulative incidence of overall recurrence (CIR) was 26% (95% CI: 17-36) (Figure 2A), and the LR and DM rates were 15% (95% CI: 6-28) and 15% (95% CI: 6-27), respectively (Figure 2B). Fifty patients with recurrence died; the median post-recurrence survival was 12.1 months (range, 2.9-58.1 months), and more than 90% of patients with recurrence died within 3 years after recurrence (Figure 2C). Twenty-one (40%), 24 (46%), and 7 (14%) patients had LR only, DM only, and locoregional and distant recurrence (LDR), respectively (Figure 2D). Of the 28 patients with LR, 12 had central recurrence (cervical/parauterine/vaginal), 4 had pelvic lymph node metastasis (N1), 9 had paraaortic/inguinal lymph node metastasis (N2), and 3 had central type combined with N2 (Figure 2E). There were 31 cases of DM, and patients often had metastases at multiple sites. The most commonly affected parenchymal organs were the lungs and liver; the lymph nodes and bone were also common metastatic sites (Figure 2F).
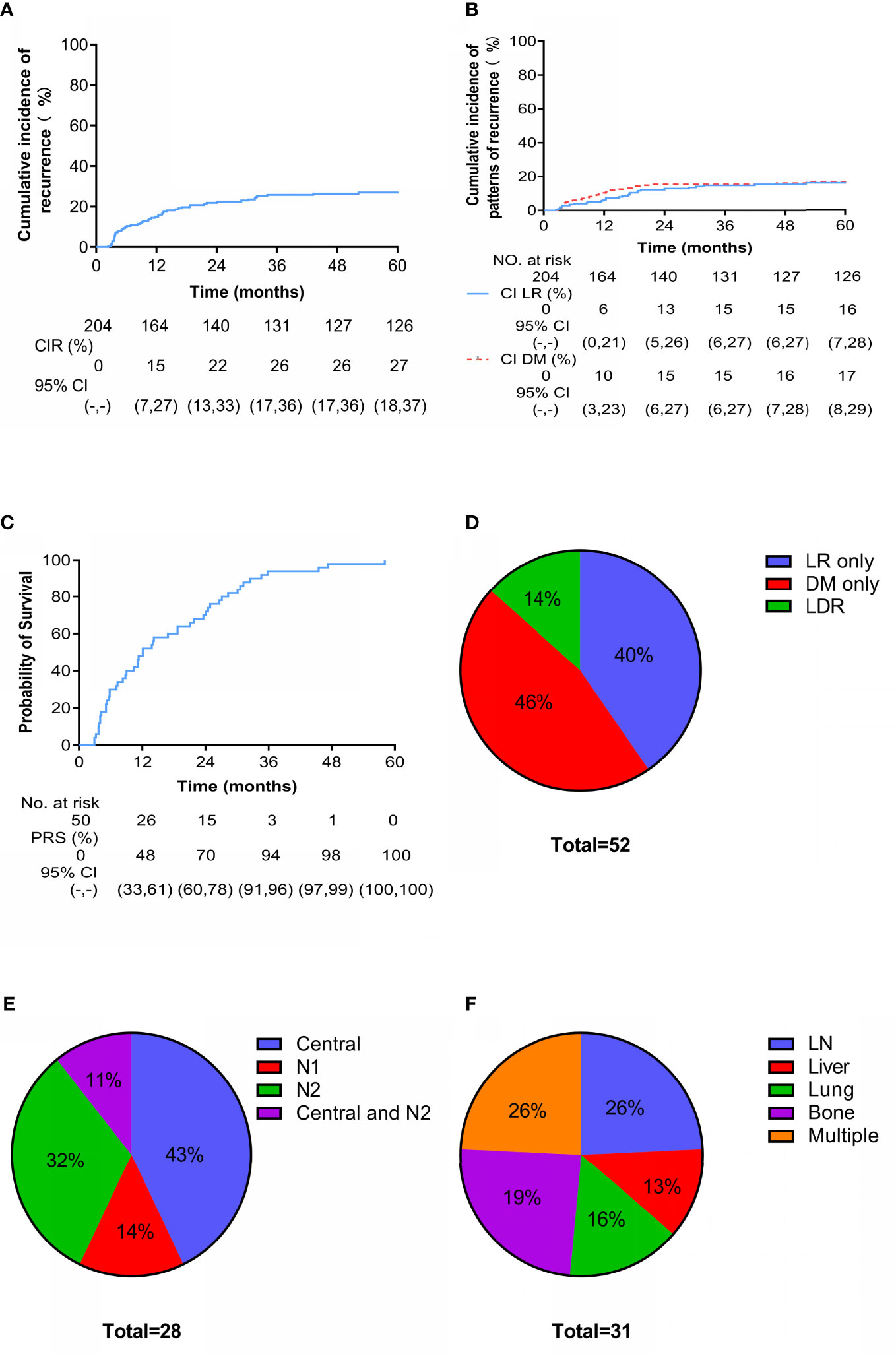
Figure 2 Cumulative incidence and patterns of recurrence. Cumulative incidence of (A) overall recurrence and (B) locoregional (LR) versus distant metastasis (DM) since treatment. Cumulative incidence of (C) death after recurrence. Site-specific patterns of (D) recurrence, (E) LR (N1 are pelvic lymph nodes and N2 are para-aortic lymph nodes or/and inguinal lymph nodes) and (F) DM.
Univariate analysis showed that stage was significantly associated with recurrence (Stage IIIB vs Stage IIB: HR = 2.562, 95% CI: 1.340-4.899, p = 0.013), and lymph node metastasis before treatment was a high-risk factor for recurrence (HR = 3.523, 95% CI: 2.024-6.131, p < 0.001). Age, tumor diameter, SCC level before treatment, HGB level before treatment, HRCTV D90%, histology, and tumor growth morphology (exophytic or endophytic) were not significantly associated with recurrence (Figure 3A). Factors with p < 0.2 in the univariate analysis (tumor diameter, FIGO stage, and lymph node metastasis before treatment) were included in the multivariate analysis. Tumor stage (Stage IIIB vs. Stage IIB: HR = 2.332, 95% CI: 1.195-4.551, p = 0.013) and lymph node metastasis before treatment (HR = 4.462, 95% CI: 2.365-8.419, p < 0.001) were both significant independent predictors of recurrence (Figure 3B).
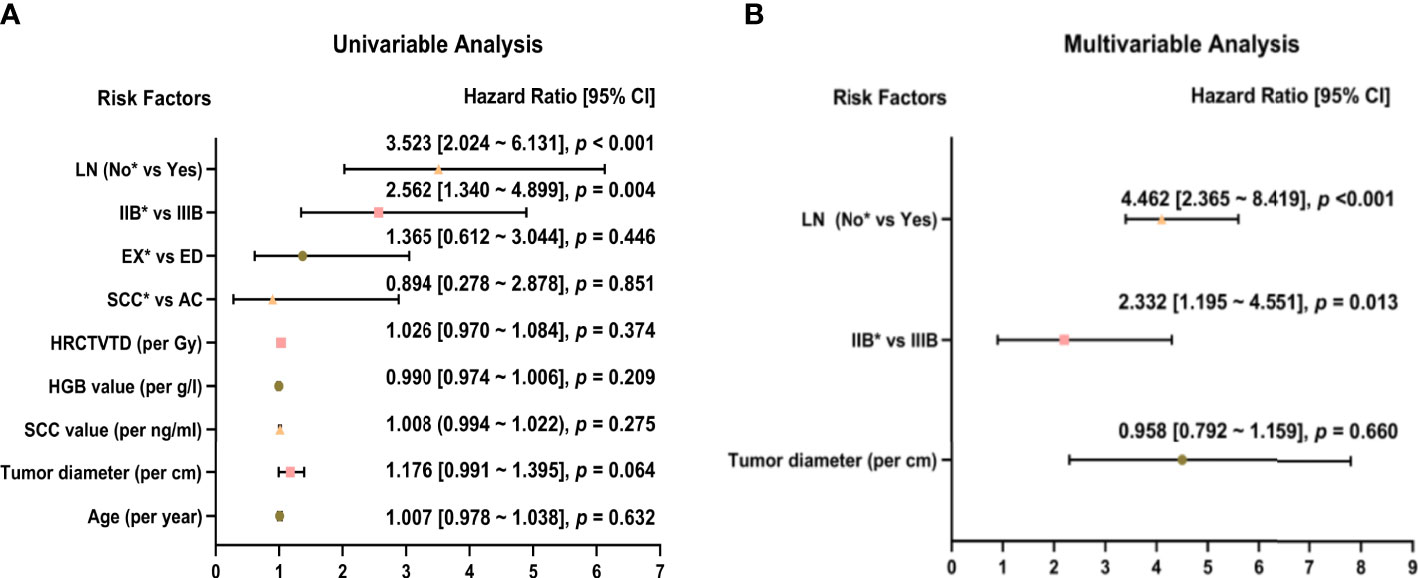
Figure 3 Univariate (A) and multivariate (B) analysis of 52 patients with recurrence. *Reference item.
Fifty-three patients (26%) developed CRP, including 25, 17, 4, and 7 with Grades 1, 2, 3, and 4 CRP, respectively (Figure 4A). Grades 1 and 2 CRP were classified as mild (42 cases), and Grades 3 and 4 CRP were classified as severe (11 cases). The incidence of severe CRP was approximately 5%. The average cumulative rectal of patients without CRP, those with mild CRP, and those with severe CRP were 69.5 Gy ( ± 5.1 Gy, range, 57.3-91.2 Gy), 73.1 Gy ( ± 4.0 Gy, range, 65.9-84.6 Gy), and 73.4 Gy ( ± 6.2 Gy, range, 59.2-84.3 Gy), respectively. The cumulative rectal of patients without CRP was 3.6 Gy lower than that of patients with mild CRP (p < 0.001) and 3.9 Gy lower than that of patients with severe CRP (p = 0.013). There was no statistically significant difference in the cumulative rectal between patients with mild and severe CRP (p = 0.853) (Figure 4B and Table 3).
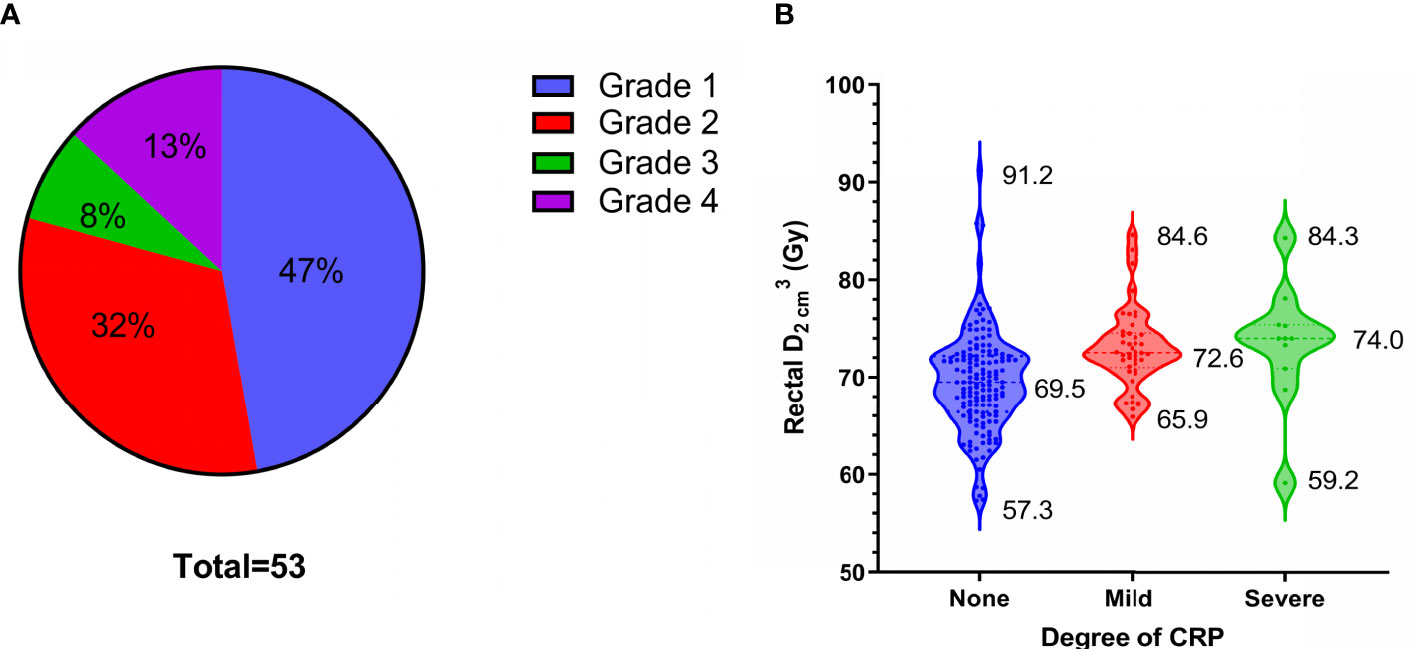
Figure 4 53 patients of chronic radiation proctitis (CRP). (A) The proportion of each grade of CRP. (B) Rectal grouped by degree of CRP.
A total of 77 patients died during the follow-up: 1 patient died of acute leukemia, 27 (35%) died of tumor progression, 20 (26%) died of LR, 23 (29%) died of DM, and 7 (9%) died of LDR (Figure 5A). At 4 years, the cumulative OS and DFS rates were 65% and 62%, respectively (Figures 5B, C).
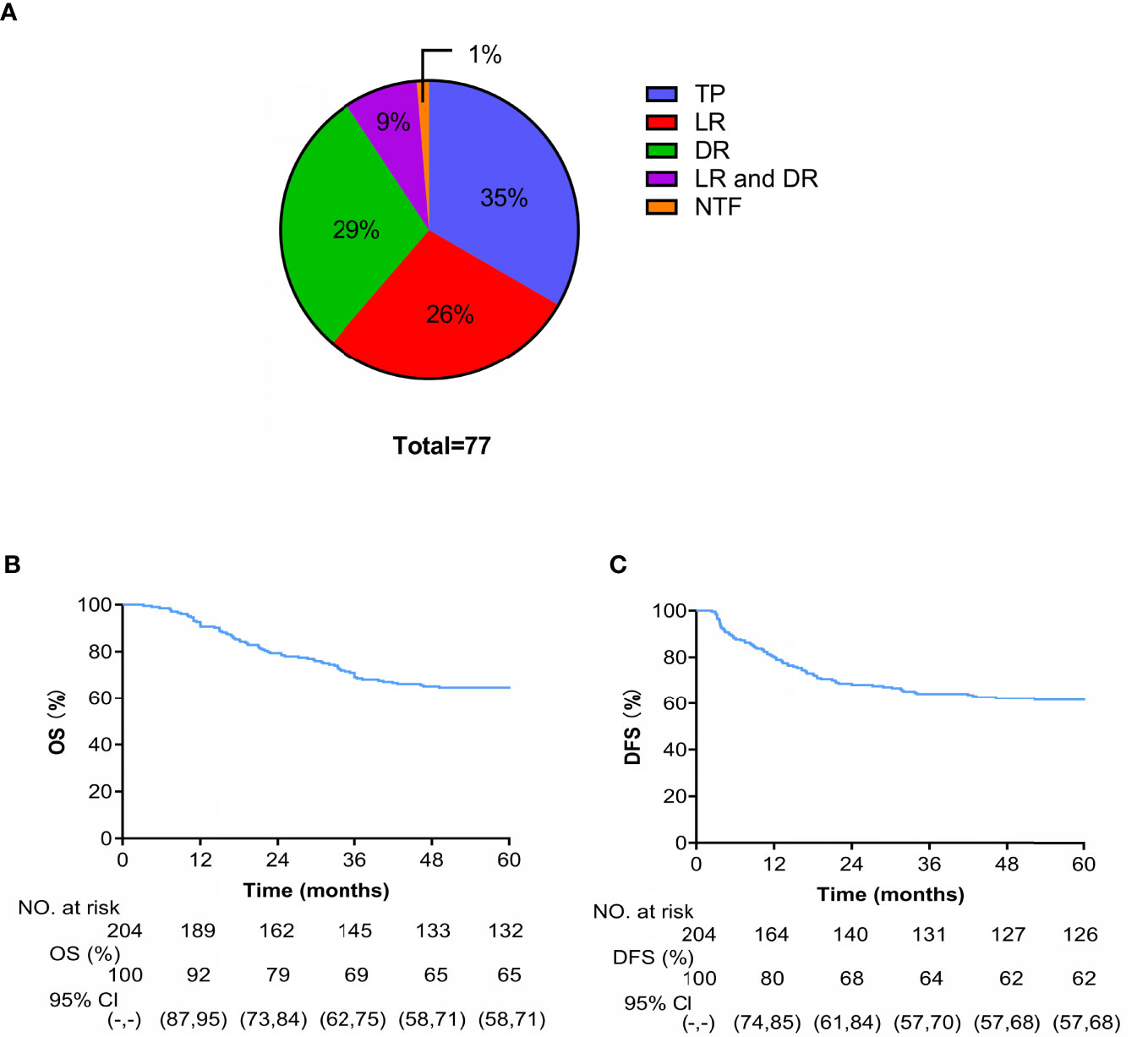
Figure 5 (A) Cause-specific of death (TP is tumor progression and NTF is non-tumor factors). Kaplan-Meier curves of OS (B) and (C) DFS.
Univariate analysis showed that tumor diameter, HGB level before treatment, FIGO stage, and lymph node metastasis before treatment were significantly associated with OS. Factors with p < 0.2 in the univariate analysis (tumor diameter, SCC and HGB levels before treatment, FIGO stage, and lymph node metastasis before treatment) were included in the multivariate analysis. Lymph node metastasis before treatment was the only independent prognostic risk factor for OS (HR = 6.765, 95% CI: 3.826-11.964, p < 0.001) (Table 4).
In this study, patients with LACC received concurrent VMAT external irradiation and cisplatin-based chemotherapy combined with IGABT, and the OR and OS rates were 85% and 65%, respectively. Previous studies reported that, under the standard treatment scheme, the local control and long-term OS rates of LACC were approximately 80% and 60%, respectively (24–26). Our results were similar to those in these studies, indicating that our efficacy was satisfactory. The 5-year CIR was 27%, among which LR only, DR only, and LDR accounted for 40%, 46%, and 14% of recurrence patterns, respectively. In similar studies, the recurrence rate of LACC patients was 20%-30% (27, 28). The most common recurrence pattern in these studies was DR with lymph node metastasis, which is similar to our results.
CRP is a common complication of radiotherapy for cervical cancer (29, 30). Patients who develop CRP may have permanent changes in defecation habits, and symptoms such as gastrointestinal bleeding may also occur, which seriously affects patient quality of life. In this study, the incidences of mild CRP (Grades 1-2) and severe CRP (Grades 3-4) were approximately 21% and 5%, respectively. Dosimetry analysis showed that the average rectal cumulative of patients with severe CRP was 73.4 Gy, which was 3.9 Gy higher than that of patients without CRP. Therefore, rectal should be fully considered and limited when planning radiotherapy.
We analyzed factors influencing short-term and long-term outcomes, including clinical features and radiation doses. There were many single factors affecting prognosis, but local lymph node metastasis before treatment was the main independent risk factor of short-term and long-term outcomes. Previous studies have reported that pelvic or abdominal paraaortic lymph node metastasis before treatment is an independent prognostic risk factor for LACC patients (31–33). In addition, the size and number of metastatic lymph nodes are independent prognostic factors for LACC (34, 35). We did not find any influence of the HRCTV on short-term or long-term outcomes, which might be because the HRCTV was over 83 Gy in all patients, over 85.0 Gy in 97% of patients, and over 90 Gy in 91% of patients. Bandyopadhyay et al. (36) reported a significant difference in the HRCTV between patients who did and did not develop recurrence (83.97 Gy and 77.96 Gy, respectively) among patients with LACC treated with CCRT combined with IGABT. Therefore, we suggest that the cumulative HRCTV of radiotherapy for LACC patients should be above 85 Gy.
The limitation of this study is its retrospective design. Further, although the number of cases in this study was relatively large, we included mainly patients with FIGO Stage IIB and IIIB disease, and there were no patients with Stage IVA disease. Additionally, most patients had squamous cell carcinoma, and the specific locations and numbers of pelvic metastatic lymph nodes were not statistically analyzed before treatment. Many studies have reported that patients with cervical squamous cell carcinoma have better short-term efficacy, OS, and DFS than those with adenocarcinoma (37–39), which is speculated to be related to the low radiosensitivity of adenocarcinoma. Tumor diameter has been also reported as an independent prognostic factor for LACC (40), which is considered to be related to the proportion of hypoxic tumor cells, as hypoxic cells are less sensitive to radiation. Furthermore, we assessed pre-treatment metastatic lymph nodes by MRI, PET-CT, and/or CT, which is sometimes difficult and may lead to some errors. A systematic review covering two hundred and twenty studies concluded that minimally invasive laparoscopic staging in patients for LACC is feasible, especially in patients with microscopic metastasis. What’s more, overtreatment could be spared by the strategy (41). Objectively, the minimally invasive laparoscopic staging indeed has some advantages over imaging examination, but superior skills and constant training are required staging procedure to avoid operative complications. In addition, we would take further aggressive treatment such as surgery, re-radiotherapy and re-chemotherapy for some recurrent patients. Nevertheless, in order to ensure the consistency of treatment scheme of all patients enrolled in the study, we excluded patients who accepted aggressive treatment after recurrence. Some studies have reported the use of expanded lateral pelvic resection and therapeutic lymphatic dissection for gynecological malignancies (42, 43). This strategy may be a good option for recurrent or chemoradiotherapy-insensitive LACC.
In summary, we examined the short-term and long-term outcomes after concurrent VMAT external radiotherapy and platinum-based chemotherapy combined with IGABT in patients with LACC. The OR rate was 85%, the cumulative OS and DFS rates at 5 years were 65% and 62%, respectively, and the short-term and long-term efficacies were satisfactory. Lymph node metastasis before treatment was an independent risk factor of short-term and long-term outcomes. The incidence of severe CRP was 5%, and the average cumulative rectal in patients with severe CRP was 73.4 Gy, which was 3.9 Gy higher than that in patients without CRP. For patients with lymph node metastasis before treatment, more active individualized treatment measures should be taken. When designing a radiotherapy plan, it is necessary to strictly limit the rectal accumulation to prevent serious CRP.
The datasets of this research are also backed up on the Research Data Deposit (RDD, https://www.researchdata.org.cn, approval number: RDDA2022129802) and are available on reasonable request.
The studies involving human participants were reviewed and approved by Sun Yat-sen University Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Q-hP is responsible for project design and article writing, KC and J-yL are responsible for patient diagnosis and treatment, LC guides the project, and W-jY is in charge of clinical guidance and article revision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (12075329)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Pecorelli S, Zigliani L, Odicino F. Revised FIGO Staging for Carcinoma of the Cervix. Int J Gynaecol Obstet (2009) 105:107–8. doi: 10.1016/j.ijgo.2009.02.009
4. Liang YQ, Feng SQ, Xie WJ, Jiang QZ, Yang YF, Luo R, et al. Comparison of Survival, Acute Toxicities,and Dose-Volume Parameters Between Intensity-Modulated Radiotherapy With or Without Internal Target Volume Delineation Method and Three-Dimensional Conformal Radiotherapy in Cervical Cancer Patients: A Retrospective and Propensity Score-Matched Analysis. Cancer Med (2022) 11:151–65. doi: 10.1002/cam4.4439
5. Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, et al. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J Clin Oncol (2018) 36:2538–44. doi: 10.1200/JCO.2017.77.4273
6. Tiwari R, Narayanan GS, Narayanan S, Suresh KP. Long-Term Effectiveness and Safety of Image-Based,Transperineal Combined Intracavitary and Interstitial Brachytherapy in Treatment of Locally Advanced Cervical Cancer. Brachytherapy (2020) 19:73–80. doi: 10.1016/j.brachy.2019.10.003
7. Potter R, Tanderup K, Schmid MP, Jürgenliemk-Schulz I, Haie-Meder C, Fokdal LU, et al. MRI-Guided Adaptive Brachytherapy in Locally Advanced Cervical Cancer (EMBRACE-I): A Multicentre Prospective Cohort Study. Lancet Oncol (2021) 22:538–47. doi: 10.1016/S1470-2045(20)30753-1
8. Sturdza A, Potter R, Fokdal LU, Haie-Meder C, Tan LT, Mazeron R, et al. Image Guided Brachytherapy in Locally Advanced Cervical Cancer: Improved Pelvic Control and Survival in RetroEMBRACE, a Multicenter Cohort Study. Radiother Oncol (2016) 120:428–33. doi: 10.1016/j.radonc.2016.03.011
9. Faye MD, Alfieri J. Advances in Radiation Oncology for the Treatment of Cervical Cancer. Curr Oncol (2022) 29:928–44. doi: 10.3390/curroncol29020079
10. Cho O, Chun M. Management for Locally Advanced Cervical Cancer: New Trends and Controversial Issues. Radiat Oncol J (2018) 36:254–64. doi: 10.3857/roj.2018.00500
11. Lee J, Lin JB, Sun FJ, Chen YJ, Chang CL, Jan YT, et al. Safety and Efficacy of Semiextended Field Intensity-Modulated Radiation Therapy and Concurrent Cisplatin in Locally Advanced Cervical Cancer Pa Tients: An Observational Study of 10-Year Experience. Med (Baltimore) (2017) 96:e6158. doi: 10.1097/MD.0000000000006158
12. Kim TE, Park BJ, Kwack HS, Kwon JY, Kim JH, Yoon SC. Outcomes and Prognostic Factors of Cervical Cancer After Concurrent Chemoradiation. J Obstet Gynaecol Res (2012) 38:1315–20. doi: 10.1111/j.1447-0756.2012.01871.x
13. Bermudez A, Bhatla N, Leung E. Cancer of the Cervix Uteri. Int J Gynaecol Obstet (2015) 131 Suppl 2:S88–95. doi: 10.1016/j.ijgo.2015.06.004
14. Tangjitgamol S, Tharavichitkul E, Tovanabutra C, Rongsriyam K, Asakij T, Paengchit KTangjitgamol S, et al. A Randomized Controlled Trial Comparing Concurrent Chemoradiation Versus Concurrent Chemoradiation Followed by Adjuvant Chemotherapy in Locally Advanced Cervical Cancer Patients: ACTLACC Trial. J Gynecol Oncol (2019) 30:e82. doi: 10.3802/jgo.2019.30.e82
15. Tovanabutra C, Asakij T, Rongsriyam K, Tangjitgamol S, Tharavichitkul E, Sukhaboon J, et al. Long-Term Outcomes and Sites of Failure in Locally Advanced Cervical Cancer Patients Treated by Concurrent Chemoradiation With or Without Adjuvant Chemotherapy: ACTLACC Trial. Asian Pac J Cancer Prev (2021) 22:2977–85. doi: 10.31557/APJCP.2021.22.9.2977
16. Lim K, Small WJ, Portelance L, Creutzberg C, Jürgenliemk-Schulz IM, Mundt A, et al. Consensus Guidelines for Delineation of Clinical Target Volume for Intensity-Modulated Pelvic Radiotherapy for the Definitive Treatment of Cervix Cancer. Int J Radiat Oncol Biol Phys (2011) 79:348–55. doi: 10.1016/j.ijrobp.2009.10.075
17. Haie-Meder C, Potter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations From Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and Terms in 3D Image Based 3D Treatment Planning in Cervix Cancer Brachytherapy With Emphasis on MRI Assessment of GTV and CTV. Radiother Oncol (2005) 74:235–45. doi: 10.1016/j.radonc.2004.12.015
18. Anonymous. Prescribing, Recording, and Reporting Brachytherapy for Cancer of the Cervix. J ICRU (2013) 13(1-2):NP. doi: 10.1093/jicru/ndw027
19. Fowler JF. 21 Years of Biologically Effective Dose. Br J Of Radiol (2010) 83:554–68. doi: 10.1259/bjr/31372149
20. Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. New Response Evaluation Criteria in Solid Tumours-Revised RECIST Guideline (Version 1.1). Gan To Kagaku Ryoho (2009) 36:2495–501.
21. Rubin P, Constine LR, Fajardo LF, Phillips TL, Wasserman TH, EORTC Late Effects Working Group. Overview of Late Effects Normal Tissues (LENT) Scoring System. Radiother Oncol (1995) 35:9–10. doi: 10.1016/0167-8140(95)97447-L
22. Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, et al. EORTC Late Effects Working Group. Late Effects Toxicity Scoring: The SOMA Scale. Int J Radiat Oncol Biol Phys (1995) 31:1043–7. doi: 10.1016/0360-3016(95)00059-8
23. Cox JD, Stetz J, Pajak TF. Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys (1995) 31:1341–6. doi: 10.1016/0360-3016(95)00060-C
24. Todo Y, Watari H. Concurrent Chemoradiotherapy for Cervical Cancer: Background Including Evidence-Based Data, Pitfalls of the Data, Limitation of Treatment in Certain Groups. Chin J Cancer Res (2016) 28:221–7. doi: 10.21147/j.issn.1000-9604.2016.02.10
25. Datta NR, Stutz E, Liu M, Rogers S, Klingbiel D, Siebenhüner A, et al. Concurrent Chemoradiotherapy vs. Radiotherapy Alone in Locally Advanced Cervix Cancer: A Systematic Review and Meta-Analysis. Gynecol Oncol (2017) 145:374–85. doi: 10.1016/j.ygyno.2017.01.033
26. Schefter T, Winter K, Kwon JS, Stuhr K, Balaraj K, Yaremko BP, et al. RTOG 0417: Efficacy of Bevacizumab in Combination With Definitive Radiation Therapy and Cisplatin Chemotherapy in Untreated Patients With Local Ly Advanced Cervical Carcinoma. Int J Radiat Oncol Biol Phys (2014) 88:101–5. doi: 10.1016/j.ijrobp.2013.10.022
27. De Foucher T, Bendifallah S, Ouldamer L, Bricou A, Lavoue V, Varinot J, et al. Patterns of Recurrence and Prognosis in Locally Advanced FIGO Stage IB2 to IIB Cervical Cancer: Retrospective Multicentre Study From the FRANCOGYN Group. Eur J Surg Oncol (2019) 45:659–65. doi: 10.1016/j.ejso.2018.11.014
28. Kilic C, Kimyon CG, Cakir C, Yuksel D, Codal B, Kilic F, et al. Recurrence Pattern and Prognostic Factors for Survival in Cervical Cancer With Lymph Node Metastasis. J Obstet Gynaecol Res (2021) 47:2175–84. doi: 10.1111/jog.14762
29. Tagkalidis PP, Tjandra JJ. Chronic Radiation Proctitis. ANZ J Surg (2001) 71:230–7. doi: 10.1046/j.1440-1622.2001.02081.x
30. Tabaja L, Sidani SM. Management of Radiation Proctitis. Dig Dis Sci (2018) 63:2180–8. doi: 10.1007/s10620-018-5163-8
31. Endo D, Todo Y, Okamoto K, Minobe S, Kato H, Nishiyama N. Prognostic Factors for Patients With Cervical Cancer Treated With Concurrent Chemoradiotherapy: A Retrospective Analysis in a Japanese Cohort. J Gynecol Oncol (2015) 26:12–8. doi: 10.3802/jgo.2015.26.1.12
32. Chen CC, Wang L, Lin JC, Jan JS. The Prognostic Factors for Locally Advanced Cervical Cancer Patients Treated by Intensity-Modulated Radiation Therapy With Concurrent Chemotherapy. J Formos Med Assoc (2015) 114:231–7. doi: 10.1016/j.jfma.2012.10.021
33. Queiroz A, Fabri V, Mantoan H, Sanches SM, Guimarães APG, Ribeiro ARG, et al. Risk Factors for Pelvic and Distant Recurrence in Locally Advanced Cervical Cancer. Eur J Obstet Gynecol Reprod Biol (2019) 235:6–12. doi: 10.1016/j.ejogrb.2019.01.028
34. Pinto P, Chen MJ, Santos NE, Faloppa CC, De Brot L, Guimaraes APG, et al. Prognostic Factors in Locally Advanced Cervical Cancer With Pelvic Lymph Node Metastasis. Int J Gynecol Cancer (2022) 32:239–45. doi: 10.1136/ijgc-2021-003140
35. Wang SC, Lin LC, Kuo YT, Lin YW. Radiographic Number of Positive Pelvic Lymph Nodes as a Prognostic Factor in Cervical Cancer Treated With Definitive Concurrent Chemoradiother Apy or Intensity-Modulated Radiotherapy. Front Oncol (2018) 8:546. doi: 10.3389/fonc.2018.00546
36. Bandyopadhyay A, Ghosh AK, Chhatui B, Das D. Dosimetric and Clinical Outcomes of CT Based HR-CTV Delineation for HDR Intracavitary Brachytherapy in Carcinoma Cervix - a Retrospective Study. Rep Pract Oncol Radiother (2021) 26:170–8. doi: 10.5603/RPOR.a2021.0023
37. Hu K, Wang W, Liu X, Meng Q, Zhang F. Comparison of Treatment Outcomes Between Squamous Cell Carcinoma and Adenocarcinoma of Cervix After Definitive Radiotherapy or Concurrent Chemoradiotherapy. Radiat Oncol (2018) 13:249. doi: 10.1186/s13014-018-1197-5
38. Intaraphet S, Kasatpibal N, Sogaard M, Khunamornpong S, Patumanond J, Chandacham A, et al. Histological Type-Specific Prognostic Factors of Cervical Small Cell Neuroendocrine Carcinoma, Adenocarcinoma, and Squamous Cell Carcinoma. Onco Targets Ther (2014) 7:1205–14. doi: 10.2147/OTT.S64714
39. Kang JH, Cho WK, Yeo HJ, Jeong SY, Noh JJ, Shim JI, et al. Prognostic Significance of Tumor Regression Rate During Concurrent Chemoradiotherapy in Locally Advanced Cervix Cancer: Analysis by Radiation Phase and Histologic Type. J Clin Med (2020) 9:3471. doi: 10.3390/jcm9113471
40. Chen W, Xiu S, Xie X, Guo H, Xu Y, Bai P, et al. Prognostic Value of Tumor Measurement Parameters and SCC-Ag Changes in Patients With Locally-Advanced Cervical Cancer. Radiat Oncol (2022) 17:6. doi: 10.1186/s13014-021-01978-0
41. Capozzi VA, Sozzi G, Monfardini L, Di Donna MC, Giallombardo V, Lo Balbo G, et al. Transperitoneal Versus Extraperitoneal Laparoscopic Aortic Lymph Nodal Staging for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2021) 47(9):2256–64. doi: 10.1016/j.ejso.2021.04.036
42. Höckel M, Wolf B, Hentschel B, Horn LC. Surgical Treatment and Histopathological Assessment of Advanced Cervicovaginal Carcinoma: A Prospective Study and Retrospective Analysis. Eur J Can (2017) 70:99–110. doi: 10.1016/j.ejca.2016.10.016
Keywords: locally advanced cervical cancer, concurrent chemoradiotherapy, short-term and long-term efficacies, chronic radiation proctics, prognostic factor
Citation: Peng Q-h, Chen K, Li J-y, Chen L and Ye W-j (2022) Analysis of Treatment Outcomes and Prognosis After Concurrent Chemoradiotherapy for Locally Advanced Cervical Cancer. Front. Oncol. 12:926840. doi: 10.3389/fonc.2022.926840
Received: 23 April 2022; Accepted: 09 June 2022;
Published: 06 July 2022.
Edited by:
Benedetta Guani, Fribourg Cantonal Hospital, SwitzerlandReviewed by:
Cristiana Sessa, Oncology Institute of Southern Switzerland (IOSI), SwitzerlandCopyright © 2022 Peng, Chen, Li, Chen and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chen, Y2hlbmxpQHN5c3VjYy5vcmcuY24=; Wei-jun Ye, eWV3akBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.