- 1Department of Critical Medicine, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Gynaecology and Obstetrics, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Objective: Several studies have found that MMP-9, one of the extracellular matrix-degrading proteinases, was involved in EC’s (endometrial cancer) clinical progression and prognosis. However, the results involving the associations of MMP-9 expression with risk, clinical features and prognosis of EC were conflicting. Therefore, we performed a systematic review and meta-analysis to clarify the correlation of MMP-9 expression with EC.
Methods: Relative studies involving the associations between MMP-9 expression and EC were retrieved from PubMed, Embase, Web of Science and CNKI (China National Knowledge Infrastructure) electronic databases. OR (odds ratio) with 95% CI (confidence interval) was applied to evaluate the associations of MMP-9 expression with risk and clinical features of EC. Furthermore, we evaluated the role of MMP-9 expression in prognosis of EC using HR and 95% CI. The funnel plots and Begg test were used to assess the publication bias.
Results: A total of 28 eligible studies were acquired from Pubmed, Embase, Web of science and CNKI databases. We found MMP-9 overexpression was significantly associated with the risk of EC (OR = 11.02, 95% CI = 7.51-16.16, P < 0.05). In the meantime, MMP-9 overexpression was significantly associated with the tumor grade, FIGO stage, lymph node metastasis and myometrial invasion (Tumor grade: OR = 1.68, 95% CI = 1.09-2.58, P < 0.05; FIGO stage: OR = 3.25, 95% CI = 1.73-6.08, P < 0.05; Lymph node metastasis: OR = 2.98, 95% CI = 1.27-7.03, P < 0.05; Myometrial invasion: OR = 2.42, 95% CI = 1.42-4.12, P < 0.05) in Asians. In addition, the overall results showed that MMP-9 overexpression predicted a worse prognosis of EC (OR = 1.82, 95% CI = 1.01-2.62, P < 0.05).
Conclusions: MMP-9 overexpression might be a potential predictor of poor clinical progression and prognosis of EC.
Introduction
EC is the most common gynecologic malignancy in developed countries, while its incidence and mortality are rising (1). Most of the patients were diagnosed at 70 years or older (2). In the past several years, estrogen therapy, tamoxifen therapy, and surgical treatment significantly improved the survival rates of EC patients. However, 42,000 women still died of EC (3). Early menstruating, late menopause, infertility, polycystic ovary syndrome, increased age, hypertension and diabetes increased the risk of EC. It has been reported that obesity and conditions associated with metabolic syndrome were significantly linked with the development of EC. Obesity rates continued to rise in developed countries, which might aggravate the occurrence of EC (4). In addition, racial disparity in death rates of EC patients was found in genetic studies (4). These studies suggested that many risk factors increased EC mortality. Although the 5-year survival of EC patients with early stage was estimated to be 90%, those patients with advanced stage had a worse prognosis of EC (5). Therefore, identification of novel and reliable markers for the diagnosis, prediction for clinical progression and prognosis of EC were urgently needed.
Endometrial carcinoma could invade the basement membrane and myometrium through gelatinase, penetrating the lymphatic vascular lumen and spreading (6). MMP-9 gene was located at chromosome 20q13.12 which encoded Gelatinase B. Gelatinase B could degrade gelatin, collagen and elastin through proteolytic cleavage to regulate extracellular matrix (ECM) remodeling (7). Furthermore, Gelatinase B could directly cleave polypeptides after MMP-9 was secreted into the extracellular space (8). Therefore, MMP-9 was involved in many biological processes such as proteolytic degradation of ECM, cleavage of cell surface proteins and alteration of cell-cell or cell-ECM interactions (9). Published studies showed that MMP-9 significantly affected tumor invasion, metastasis, angiogenesis and tumor microenvironment (9–37). Therefore, MMP-9 might be a potential biological target for prediction and treatment of EC. However, the expression of MMP-9 in EC patients at different stages was still controversial in published studies (10–37). Therefore, the meta-analysis carried out a quantitative analysis to explore whether the high MMP-9 expression predicted EC’s risk, clinical progression and prognosis.
Methods
Search strategy
The meta-analysis was conducted according to the PRISMA 2015 statement (38). The search strategy of “(“Matrix Metalloproteinase 9”[Mesh]) AND “Endometrial Neoplasms”[Mesh]” were used to searched all studies involving the associations of MMP-9 expression with risk, clinical outcome and prognosis of EC from PubMed, Embase, Web of Science, and CNKI databases until April 2022. The following search terms were also used: “MMP-9”, “matrix metallopeptidase 9”, “prognosis”, “survival”, “neoplasms”, “EC”, “endometrial carcinoma” and “carcinoma of endometrium”. In addition, references in the eligible literature were reviewed to obtain the relevant articles.
Study selection criteria
The literature’s inclusion and exclusion criteria were established to select and eliminate retrieved literature. All included articles should meet the inclusion criteria: 1) Studies evaluating the role of MMP-9 in the risk, clinical progression, and prognosis of EC; 2) Articles providing enough data to calculate the ORs and 95% CI; 3) Literature containing HRs with 95% CI or survival curve about the prognosis of EC. 4) Studies that the detection method of MMP-9 protein expression was IHC. Studies were excluded if they met the following exclusion criteria: 1) studies with insufficient data for calculating the OR, HR and 95% CI; 2) publications with duplicate data; 3) studies carried out in cells or animals. Two authors independently identified the eligible studies according to the inclusion and exclusion criteria.
Data extraction
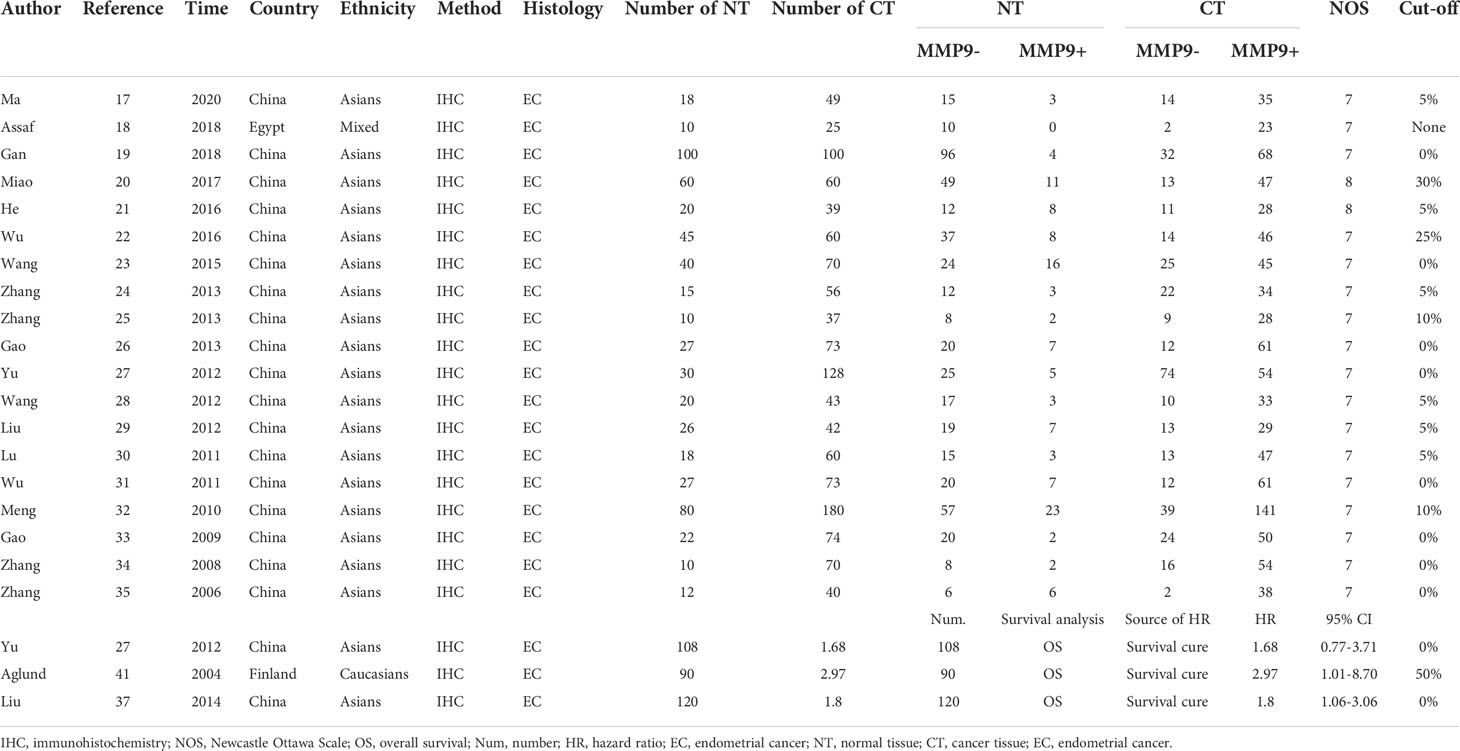
Two reviewers independently extracted relevant data from the included studies. The following information was extracted: first author’s name, year of publication, country, ethnicity, disease type, time of follow-up, the detection method of MMP-9 protein expression, cut-off values of MMP-9 protein expression and HRs with 95% CI about overall survival time of EC. If studies only provided a survival curve about the overall survival time of EC patients, we used Engauge Digitizer 4.1 software (http://digitizer.sourceforge.net/) to extract the HRs and 95% CI (39). The quality of included studies was assessed with the Newcastle Ottawa Scale (NOS) table (40). The scores of eligible studies were from 0 to 9, while 7 to 9 scores were considered as high-quality.
Statistical analysis
Chi-squared test and I2 statistic were applied to assess the heterogeneity among studies, and I2 > 50 or P-value < 0.05 presented significant heterogeneity (41, 42). The random-effects model was adopted if significant heterogeneity existed, and the fixed-effects model was used when heterogeneity was not found (43). We drew the funnel plots by conducting the Begg’s test to evaluate the publication bias (44). Sensitivity analysis was performed to test the stability of the pooled results. In addition, subgroup analysis was used to examine the source of heterogeneity among the included studies. All statistical analysis of the present study were performed with STATA 12.0 software (Stata Corp., College Station, TX, USA). All statistical tests were two-sided, and a P-value < 0.05 was considered statistical significance.
Results
Study inclusion and characteristics
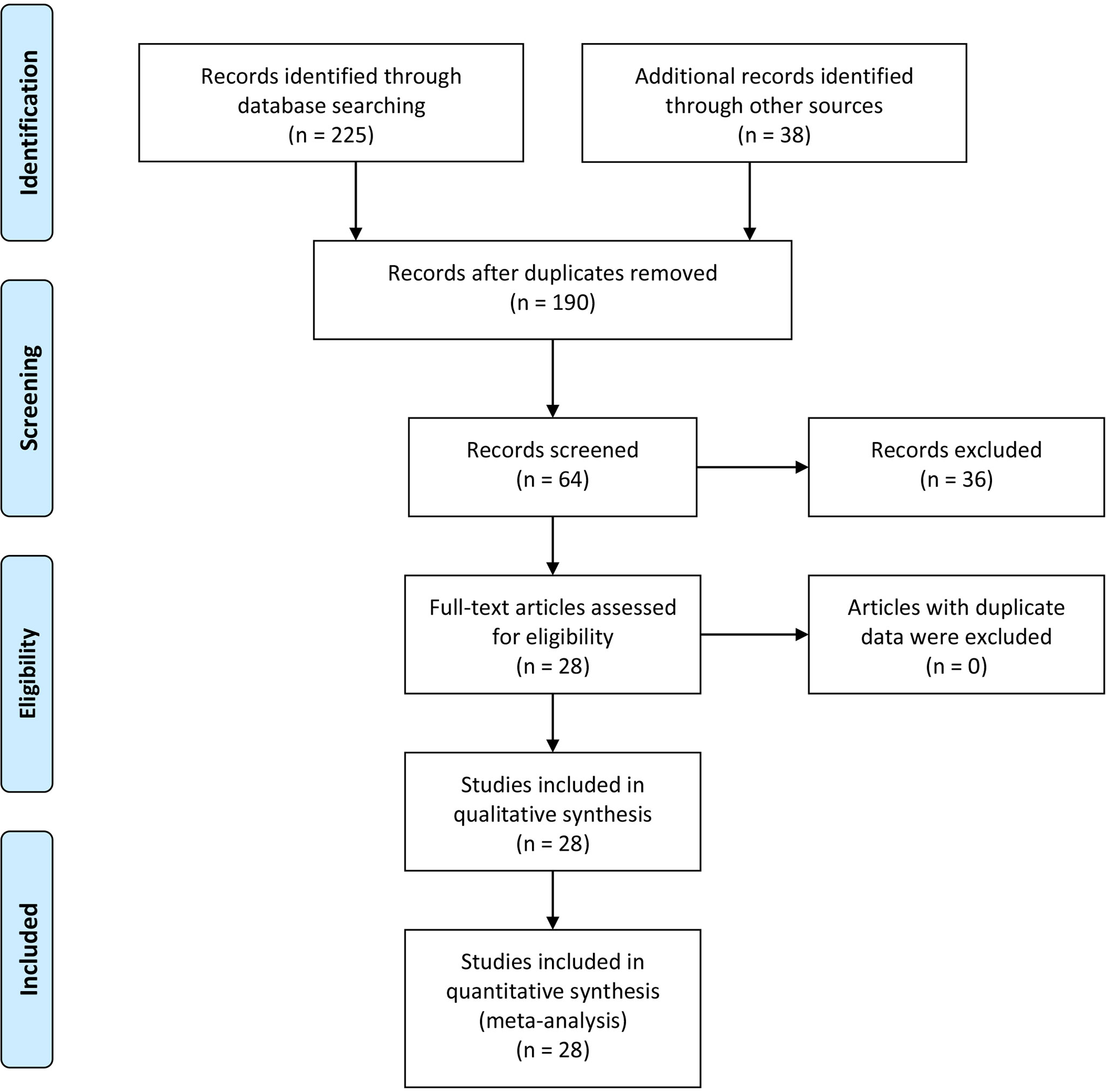
The initial search based on the inclusion and exclusion criteria identified 190 articles from electronic databases. Among them, 70 reports were duplicated and therefore were removed. Moreover, three articles were review types, so they were excluded. After titles and abstracts were read, 23 studies were excluded since they were unrelated to the associations of MMP-9 expression and risk, clinical features or prognosis of EC. In addition, 13 articles were excluded because of insufficient data. Finally, 28 eligible studies were included in the meta-analysis (10–37) (Figure 1 and Table 1). In the included studies, 8 reports were carried out in Caucasians and 20 articles were performed in Asians. In addition, the information of included studies for the analysis of associations between MMP-9 expression and EC clinical features was presented in Supplementary Table 1.
Meta-analysis results
The pooled results revealed that there was a significant association between MMP-9 overexpression and risk of EC (OR = 11.02, 95% CI = 7.51 – 16.16, P < 0.05). Small heterogeneity was observed (I2 = 50.5, P = 0.006), and a random-effects model was applied. Moreover, subgroup analysis based on ethnicity or cut-off values was performed. The results showed that MMP-9 expression was significantly correlated with risk of EC in Asians (OR = 10.55, 95% CI = 7.27 – 15.30, P < 0.05). In the meantime, the subgroup analysis based on cut-off values indicated that high expression of MMP-9 was still an increased risk for EC (cut-off value: 0%, OR = 11.62, 95% CI = 5.28 – 25.60, P < 0.05; 5%, OR = 8.32, 95% CI = 4.91 – 14.08, P < 0.05; 10%, OR = 9.28, 95% CI = 5.27 – 16.36, P < 0.05). Moreover, heterogeneity among the included studies significantly decreased in the subgroup analysis. Thus, ethnicity and cut-off values of MMP-9 expression might contribute to the heterogeneity (Figure 2 and Table 2).

Figure 2 The forest plot and funnel plots for the correlations of MMP-9 overexpression with risk and prognosis of EC. (A) Forest plot for the risk of EC. (B) Forest plot for the overall survival of EC. (C) Funnel plot for the risk of EC. (D) Funnel plot for the overall survival of EC. OR, odds ratio; HR, hazard ratio; EC, endometrial cancer.
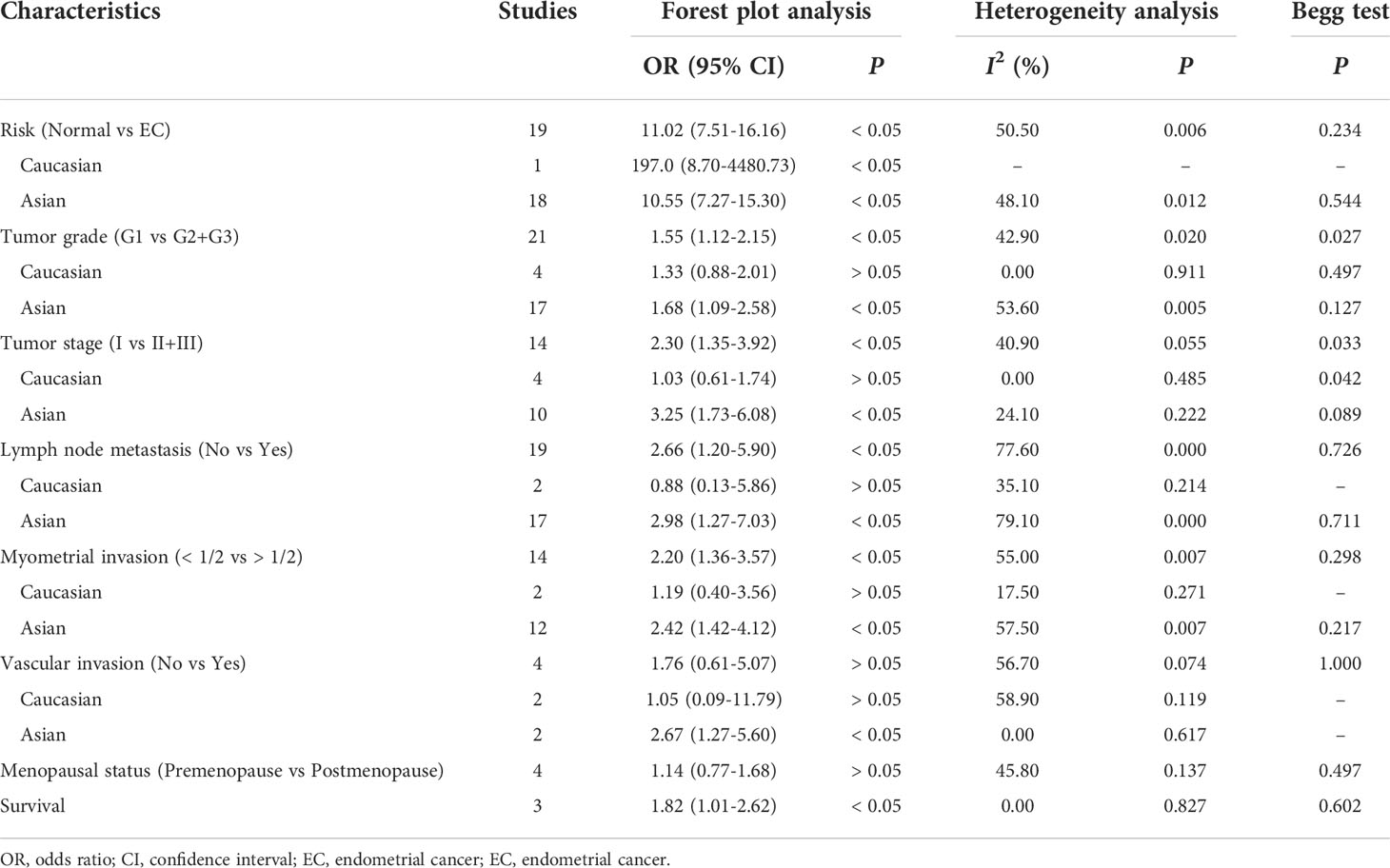
Table 2 Meta-analysis of the correlations of MMP-9 expression with risk, clinical features and prognosis of EC.
Then we performed a meta-analysis to explore the role of MMP-9 overexpression in the clinical characteristics of EC. The results indicated that patients with high G2-G3 grade had higher MMP-9 expression than that with G1 grade in Asians (OR = 1.68, 95% CI = 1.09 – 2.58, P < 0.05). And, high MMP-9 expression might represent the III-IV FIGO stage in Asians (OR = 3.25, 95% CI = 1.73 – 6.08, P < 0.05). In addition, high MMP-9 expression was significantly associated with lymph node metastasis (OR = 2.98, 95% CI = 1.27 – 7.03, P < 0.05), myometrial invasion (OR = 2.42, 95% CI = 1.42 – 4.12, P < 0.05) and vascular invasion (OR = 2.67, 95% CI = 1.27 – 5.60, P < 0.05) of EC in Asians. Some heterogeneities were found in the analysis of the associations of MMP-9 expression with lymph node metastasis, myometrial invasion and vascular invasion of EC, so the random-effects model was used. Moreover, the subgroup analysis based on the cut-off values significantly reduced the heterogeneity among studies and significant associations were still found (Figures 3, 4 and Tables 2, 3). Therefore, the pooled results were convincing. According to the results of the present meta-analysis, some studies have suggested that MMP-9 expression was associated with the development of EC, while others have obtained opposite results which can be seen in Figure 3.

Figure 3 Forest plots for the associations of MMP-9 overexpression with clinical features of EC in Asians and Caucasians. (A) Forest plot for FIGO stage of EC. (B) Forest plot for endometrial tumor grade. (C) Forest plot for lymph node metastasis of EC. (D) Forest plot for myometrial invasion of EC. OR, odds ratio; EC, endometrial cancer.
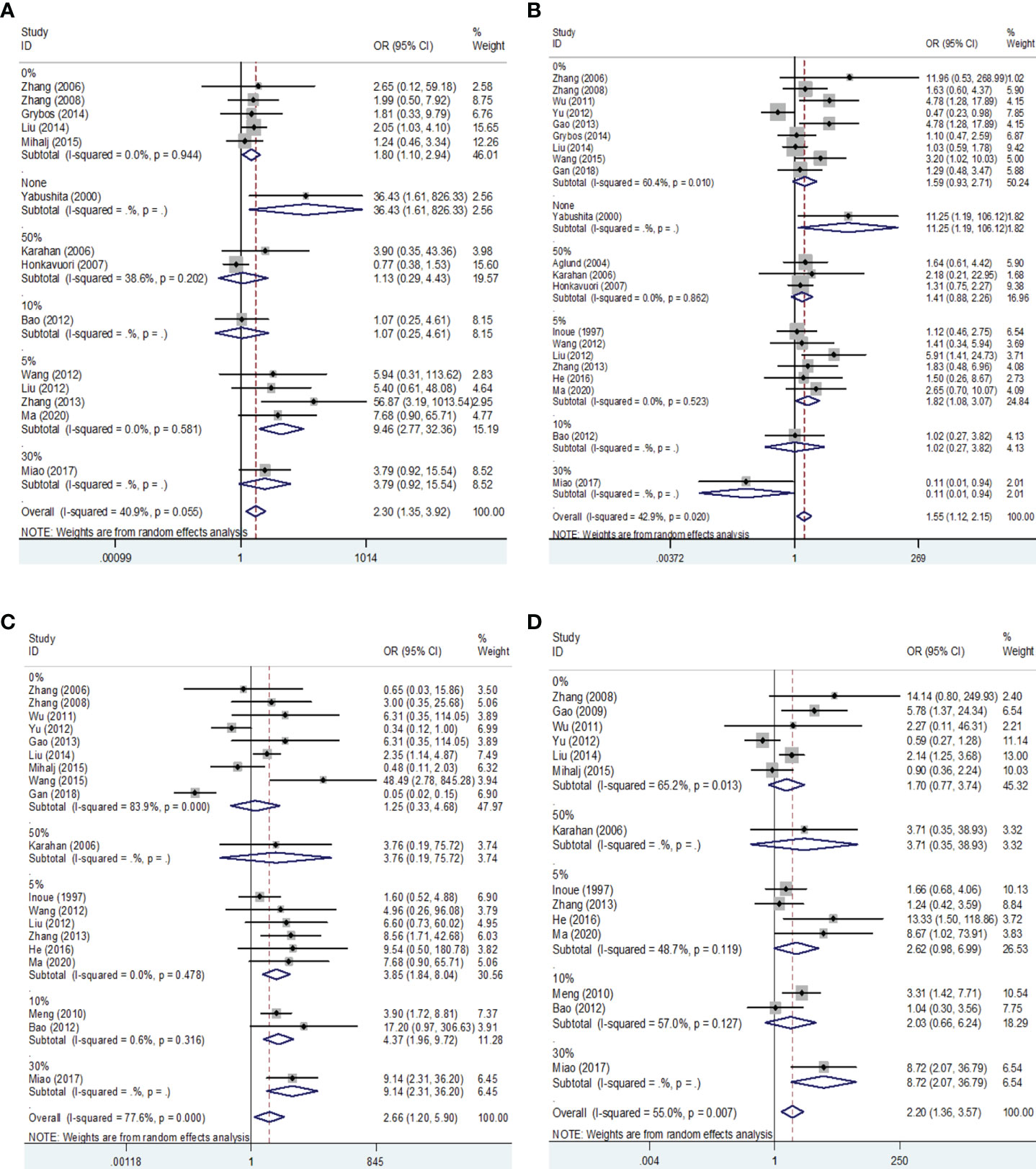
Figure 4 Forest plots for the associations of MMP-9 overexpression with clinical features of EC in different cut-off values of IHC. (A) Forest plot for FIGO stage of EC. (B) Forest plot for endometrial tumor grade. (C) Forest plot for lymph node metastasis of EC. (D) Forest plot for myometrial invasion of EC. IHC, immunocytochemistry. OR, odds ratio; EC, endometrial cancer.
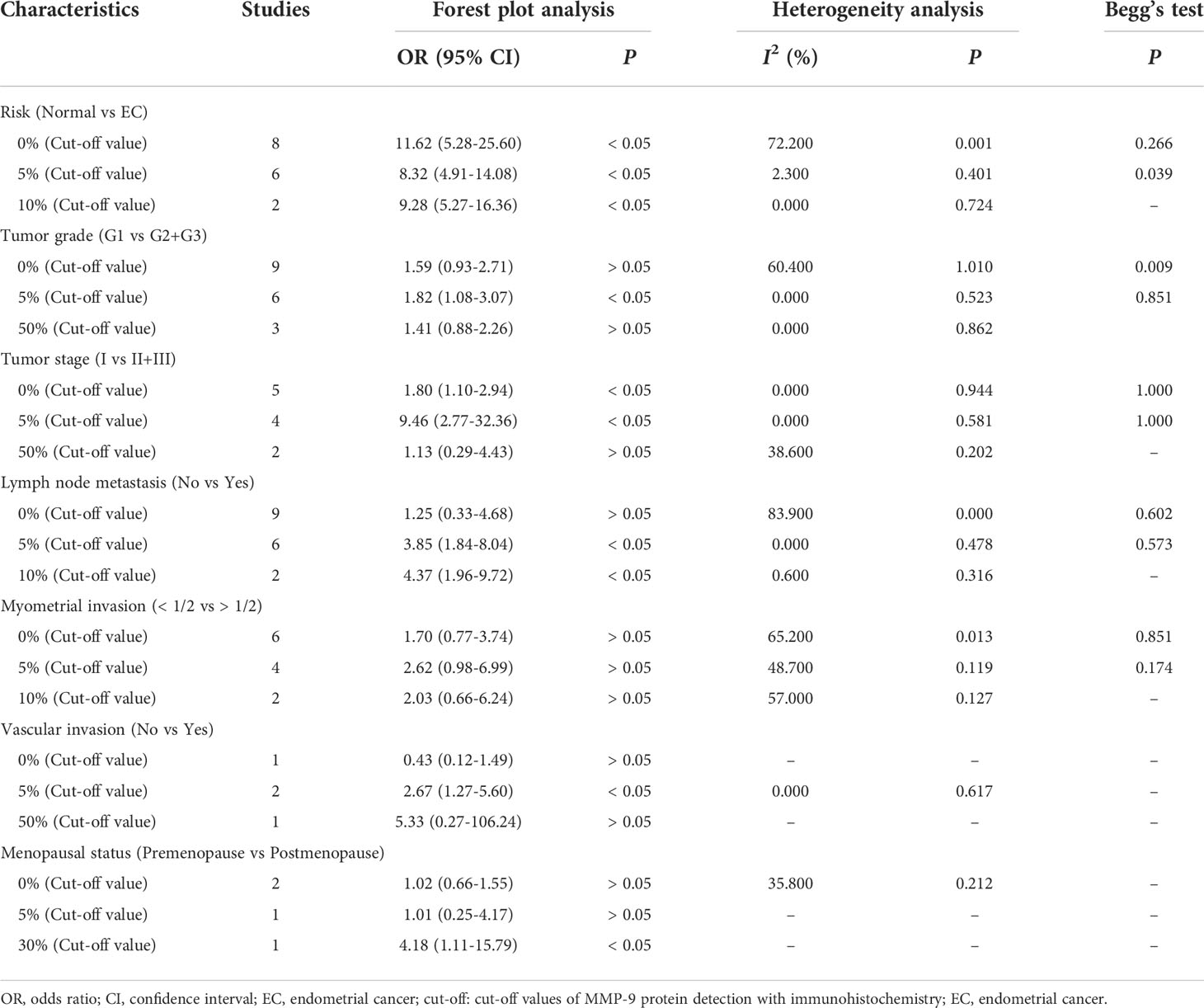
Table 3 The subgroup analysis based on cut-off values of MMP-9 expression for the risk and clinical features of EC.
The HR and 95% CI values were extracted from included studies and combined to evaluate the correlation between MMP-9 expression and overall survival of EC. The outcome showed that higher MMP-9 expression represented a worse overall survival of EC (OR = 1.82, 95% CI = 1.01 – 2.62, P < 0.05). No significant heterogeneity was found in the meta-analysis for the overall survival of EC (I2 = 0, P = 0.827). In the three included studies for the overall survival of EC, two studies believed MMP-9 expression affected the survival of EC, while negative result obtained in the study of Yu et al. (Figure 2 and Table 2).
Publication bias and sensitivity analysis
No significant publication bias was found in the meta-analysis of the present study. In the meantime, the results of sensitivity analysis suggested that the overall results were stable (Figure 2 and Tables 2, 3).
Discussion
It has been documented that MMP-9 protein plays a pivotal part in various diseases. For instance, MMP-9 could degrade components of the extracellular matrix and numerous nonmatrix proteins in fibrosis disease. Surprisingly, although MMP-9 levels were increased in the alveolar lavage fluid of idiopathic pulmonary fibrosis patients, MMP-9 promoted abnormal epithelial cell migration and lung tissue repair (45). In the lung fibrosis model of MMP-9-/- mice, deficiency of MMP-9 protected mice from alveolar bronchiolization (46). On the contrary, some studies have shown that MMP-9 overexpression in liver tissue was a risk factor for advanced T category, tumor stage and poor outcome (47, 48). Therefore, MMP-9 might have different roles in diverse diseases. Previous studies have reported overexpression of MMP-9 was associated with the clinical progression of EC (10, 13, 29, 32, 37, 49). However, other studies found no significant associations between MMP-9 overexpression and the clinical stage of EC (50, 51). Furthermore, many inconsistent results involving the associations between MMP-9 expression and clinical features of EC were reported (10–37). Therefore, the role of MMP-9 overexpression in EC needs to be studied urgently. In fact, functional studies have showed that MMP-9 was expressed in proliferative phase endometrium, hyperplastic endometrium and EC (52, 53). In the peritoneal endometriotic lesions, positive cells (59.1%) were more than colorectal endometriosis (44.4%). Nevertheless, EC patients had the highest levels of MMP-9 expression (54). Therefore, the expression level of MMP-9 might increase with the development of endometrial disease.
This was the first meta-analysis assessing the associations of MMP-9 overexpression with EC risk, clinicopathological features and prognosis. The results indicated the significant associations between MMP-9 expression and EC risk. However, only one included study was performed on Caucasians for the analysis of risk. So, the overall results for the risk of EC might be more applicable to Asian population. Furthermore, stratified analysis based on the cut-off values of MMP-9 expression significantly decreased the heterogeneity among studies and a significant correlation for risk was still found in the stratified analysis, which showed that the results of the meta-analysis were convinced. It’s worth noting that MMP-9 expressed highly in EC patients with G2-G3 grade, III-IV FIGO stage, lymph node metastasis, myometrial invasion, vascular invasion or postmenopausal, indicating that high MMP-9 expression promoted clinical progress of EC. According to our results, ethnicity might be an important influencing factor because MMP-9 overexpression mainly promoted the clinical progression of EC in Asians. One meta-analysis has also found MMP-9 expression of bladder cancer tissue presented significant race diversity (55). Therefore, MMP-9 expression might have a potential association with ethnicity. In the meantime, stratified analysis based on the cut-off values of MMP-9 expression also significantly reduced the heterogeneity among studies for clinical features of EC, which hinted different cut-off values of MMP-9 expression were used in the included studies of the meta-analysis. Besides, the overexpression of MMP-9 was significantly associated with a worse prognosis for EC, which demonstrated MMP-9 overexpression reduced the survival time of EC patients. Therefore, the expression level of MMP-9 should be reduced to improve the prognosis in the clinical treatment of EC patients. Moreover, no significant heterogeneity among included studies for the association of EC survival with MMP-9 overexpression was found, which indicated that the pooled result was convincing. Sensitivity analysis revealed that no individual study significantly affected the overall results which showed that the pooled results were stable.
Several limitations of the present study should be pointed out. First, the number of included studies conducted on Caucasians was too small for the meta-analysis of EC risk. Second, cut-off values of MMP-9 expression were evaluated dependent on included studies for the associations of EC clinical features with MMP-9 overexpression, which significantly affected the heterogeneity among the eligible studies. Third, other factors might be involved in the heterogeneity among the included studies. However, these factors were not quantified or provided in the eligible studies. Thus, more multi-center studies with more clinical information were warranted to verify the role of MMP-9 overexpression in EC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.925424/full#supplementary-material
References
1. Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Journal of the National Cancer Institute (2020) 126(10):2225–49. doi: 10.1002/cncr.32802
2. Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. (2016) 93(6):468–74.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
4. Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
5. Bradford LS, Rauh-Hain JA, Schorge J, Birrer MJ, Dizon DS. Advances in the management of recurrent endometrial cancer. Am J Clin Oncol (2015) 38(2):206–12. doi: 10.1097/COC.0b013e31829a2974
6. Di Nezza LA, Misajon A, Zhang J, Jobling T, Quinn MA, Ostör AG, et al. Presence of active gelatinases in endometrial carcinoma and correlation of matrix metalloproteinase expression with increasing tumor grade and invasion. Cancer (2002) 94(5):1466–75. doi: 10.1002/cncr.10355
7. Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase b or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol (2002) 37(6):375–536. doi: 10.1080/10409230290771546
8. Cauwe B, Opdenakker G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol (2010) 45(5):351–423. doi: 10.3109/10409238.2010.501783
9. Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors (Basel). (2018) 18(10):3249. doi: 10.3390/s18103249
10. Ma XH, Wang HC, Liu SS. Expression and significance of VEGF-d and MMP-9 in endometrial carcinoma. China Acad J (2020) 42(4):267–9.
11. Assaf MI, Abd El-Aal W, Mohamed SS, Yassen NN, Mohamed EA. Role of morphometry and matrix metalloproteinase-9 expression in differentiating between atypical endometrial hyperplasia and low grade endometrial adenocarcinoma. Asian Pac J Cancer Prev (2018) 19(8):2291–7. doi: 10.22034/APJCP.2018.19.8.2291
12. Gan L, Chen Y, Ju WH. Expression and clinical significance of VEGF and MMP-9 in endometrial carcinoma. Hebei Med J (2018) 40(8):1168–71.
13. Miao JT, Wang SY, Yan Y, Yin TZ. Positive expression rates of HMGB1 and MMP−9 in endometrial carcinoma and correlation analysis. Maternal Child Health Care China. (2017) 32(13):3036–9.
14. He YJ, Jiang X, Han SQ, Cao J, Cai YY. Expressions and clinical significance of ezrin and MMP−9 in endometrial cancer. Maternal Child Health Care China. (2016) 31(20):4287–9.
15. Wu FL. Expression of RECK in endometrial carcinoma and its correlation with MMP-9. Med Res Education. (2016) 33(5):6–9. doi: 10.1016/S0002-9440(10)65293-5
16. Wang ZY, Xiao L, Yang YY, Liu XB, Yue LL. Expression and significance of AP-2α, Eif4E and MMP-9 in endometrial cancer. J Med Forum. (2015) 36(4):70–2.
17. Zhang J, Lv XP and Feng S. Expression, clinical significance of ki-67 and MMP-9 in endometrial carcinoma. Acta Acad Med Weifang. (2013) 35(1):49–52.
18. Zhang YJ, Wu ZH, Liu L, M XG. Expression of adhesion molecule β1 integrin and MMP-9 in endometrial carcinoma. Maternal Child Health Care China. (2013) 28:3930–2.
19. Gao P, Li XY, Chen XL, Wang XN, Yang AJ, Ren YX. Expression and significance of MMP-9 and CD44v6 in endometrial carcinoma. China J Pharm Economics. (2013) 6:300–1.
20. Yu F, Jiang QP, Zhou Y, Yang Z, Yu XL, Wang H, et al. Abnormal expression of matrix metalloproteinase-9 (MMP9) correlates with clinical course in Chinese patients with endometrial cancer. Dis Markers. (2012) 32(5):321–7. doi: 10.1155/2012/269279
21. Wang F, Wang YC, Zhou J. Expression of MMP-9 and ezrin in endometrial carcinoma and its correlation with ER and PR. J Pract Oncol (2012) 27(3):240–4.
22. Liu JJ, Gu ZP, Yao LJ, Li P, Liu ZH. Expression of RECK and MMP -9 in endometrial carcinoma and its clinical significance. J Int Obstet Gynecol. (2012) 39(1):95–106. doi: 10.1016/j.ijgo.2011.11.002
23. Lu BY, Wang YP. Expression of matrix metalloproteinase MMP-9 and its inhibitor TIMP-1 in endometrial adenocarcinoma and its clinical significance. Hei Long Jiang Med Pharmacy. (2011) 34(6):49–50.
24. Wu XQ, Chen XL, Bao Y, Zheng FY. Expression and clinical significance of matrix metalloproteinases, tissue inhibitors of metalloproteinases and CD44v6 in the endometrial carcinoma. J Wenzhou Med College. (2011) 41(1):48–52.
25. Meng Q. Expressions of MMP-9, COX-2 in endometrial carcinoma and its correlation with angiogenesis. China Modern Doctor. (2010) 48(12):1–5.
26. Gao SM, Li XZ, Zhang JM, Zhang YX, Zhang N. Relationship between microvessel density and MMP-9 expression in endometrioid adenocarcinoma AND its significance. Acta Acad Med Weifang. (2009) 31(1):63–5.
27. Zhang Y, Tong SC, Wei L, Wang YL. Expression of galectin-3 and MMP-9 in endometrial adenocarcinoma. Shanxi Med J (2008) 37(11):969–71.
28. Zhang QH, Li SL, Peng ZP, Zhou ZP, Li J, Hu BY, et al. Expression of PTEN and MMP-9 in endometrial carcinoma and their correlation and clinical significance. Tumor (2006) 26(5):476–9.
29. Grybos A, Bar J. The relationships between the immunoexpression of KAI1, MMP-2, MMP-9 and steroid receptors expression in endometrial cancer. Folia Histochemicaet Cytobiologica. (2014) 52(3):187–94. doi: 10.5603/FHC.2014.0022
30. Liu TB, Gao HY, Yang M, Zhao TT, Liu YD, Lou G. Correlation of TNFAIP8 overexpression with the proliferation, metastasis, and disease-free survival in endometrial cancer. Tumor Biol (2014) 35(6):5805–14. doi: 10.1007/s13277-014-1770-y
31. Bao MY, Zhang HQ, Ling HP. Expression and clinicopathological significance of CD44v6, MMP-9 in endometrial carcinoma. China Modern Doctor. (2012) 50(16):41–2.
32. Honkavuori M, Talvensaari-Mattila A, Soini Y, Turpeenniemi-Hujanen T, Santala M. MMP-2 expression associates with CA 125 and clinical course in endometrial carcinoma. Gynecol Oncol (2007) 104(1):217–21. doi: 10.1016/j.ygyno.2006.08.006
33. Karahan N, Güney M, Baspinar S, Oral B, Kapucuoglu N, Mungan T. Expression of gelatinase (MMP-2 and MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J Gynaecol Oncol (2007) 28(3):184–8.
34. Aglund K, Rauvala M, Puistola U, Angström T, Turpeenniemi-Hujanen T, Zackrisson B, et al. (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to the grade and the stage. Gynecol Oncol (2004) 94(3):699–704. doi: 10.1016/j.ygyno.2004.06.028
35. Yabushita H, Narumiya H, Hiratake K, Yamada H, Shimazu M, Sawaguchi K, et al. The association of transforming growth factor-beta 1 with myometrial invasion of endometrial carcinomas through effects on matrix metalloproteinase. J Obstet Gynaecol Res (2000) 26(3):163–70. doi: 10.1111/j.1447-0756.2000.tb01305.x
36. Inoue Y, Abe K, Obata K, Yoshioka T, Ohmura G, Doh K, et al. Immunohistochemical studies on matrix metalloproteinase-9 (MMP-9) and type-IV collagen in endometrial carcinoma. J Obstet Gynaecol Res (1997) 23(2):139–45. doi: 10.1111/j.1447-0756.1997.tb00822.x
37. Srdelić Mihalj S, Kuzmić-Prusac I, Zekić-Tomaš S, Šamija-Projić I and Čapkun V. Lipocalin-2 and matrix metalloproteinase-9 expression in high-grade endometrial cancer and their prognostic value. Histopathology (2015) 67(2):206–15. doi: 10.1111/his.12633
38. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015: elaboration and explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
39. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med (1998) 17(24):2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
40. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
41. DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med (1996) 15(12):1237–48. doi: 10.1002/(SICI)1097-0258(19960630)15:12<1237::AID-SIM301>3.0.CO;2-N
42. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
43. Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More than numbers: the power of graphs in meta-analysis. Am J Epidemiol. (2009) 169(2):249–55. doi: 10.1093/aje/kwn340
44. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
45. Henry MT, McMahon K, Mackarel AJ, Prikk K, Sorsa T, Maisi P, et al. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J (2002) 20(5):1220–7. doi: 10.1183/09031936.02.00022302
46. Betsuyaku T, Fukuda Y, Parks WC, Shipley JM, Senior RM. Gelatinase b is required for alveolar bronchiolization after intratracheal bleomycin. Am J Pathol (2000) 157(2):525–35. doi: 10.1016/S0002-9440(10)64563-4
47. Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis c: Comparison with PIIINP and hyaluronic acid. Am J Gastroenterol (2004) 99(2):271–9. doi: 10.1111/j.1572-0241.2004.04055.x
48. Gong L, Wu D, Zou J, Chen J, Chen L, Chen Y, et al. Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget (2016) 7(14):18458–68. doi: 10.18632/oncotarget.7607
49. Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E, Sompolska-Rzechuła A, Chudecka K, Bulsa M, et al. Clinical relevance of NGAL/MMP-9 pathway in patients with endometrial cancer. Dis Markers. (2017) 2017:6589262. doi: 10.1155/2017/6589262
50. Gershtein ES, Mushtenko SV, Ermilova VD, Levchenko NE, Kushlinskii NE. Matrix metalloproteinases and their tissue inhibitors in blood serum of patients with endometrial cancer: Clinical and morphological correlations. Bull Exp Biol Med (2018) 165(1):75–9. doi: 10.1007/s10517-018-4103-0
51. Shaco-Levy R, Sharabi S, Benharroch D, Piura B, Sion-Vardy N. Matrix metalloproteinases 2 and 9, e-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol (2008) 139(2):226–32. doi: 10.1016/j.ejogrb.2008.01.004
52. Graesslin O, Cortez A, Fauvet R, Lorenzato M, Birembaut P, Metalloproteinase-2 EDaraïVerifyta. -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation study. Ann Oncol (2006) 17(4):637–45. doi: 10.1093/annonc/mdj129
53. Zhang X, Qi C, Lin J. Enhanced expressions of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factors (VEGF) and increased microvascular density in the endometrial hyperplasia of women with anovulatory dysfunctional uterine bleeding. Fertil Steril. (2010) 93(7):2362–7. doi: 10.1016/j.fertnstert.2008.12.142
54. Ria R, Loverro G, Vacca A, Ribatti D, Cormio G, Roccaro AM, et al. Angiogenesis extent and expression of matrix metalloproteinase-2 and-9 agree with progression of ovarian endometriomas. Eur J Clin Invest. (2002) 32(3):199–206. doi: 10.1046/j.1365-2362.2002.00960.x
Keywords: MMP-9, endometrial cancer, clinical progression, prognosis, meta-analysis
Citation: Li X, Zha L, Li B, Sun R, Liu J and Zeng H (2022) Clinical significance of MMP-9 overexpression in endometrial cancer: A PRISMA-compliant meta-analysis. Front. Oncol. 12:925424. doi: 10.3389/fonc.2022.925424
Received: 21 April 2022; Accepted: 06 October 2022;
Published: 26 October 2022.
Edited by:
Shmuel Jaffe Cohen, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Irina-Draga Caruntu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaWeimin Kong, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, China
Copyright © 2022 Li, Zha, Li, Sun, Liu and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Zeng, aGd3emVuZ0AxMjYuY29t
 Xia Li1
Xia Li1 Hongwei Zeng
Hongwei Zeng