
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 23 June 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.918693
This article is part of the Research Topic Advances in Prognosis and Treatment of Endometrial Cancers View all 12 articles
 Hui Zhou1,2*
Hui Zhou1,2* Kai-Fa Lai1,2
Kai-Fa Lai1,2 Qian Xiang1,2
Qian Xiang1,2 Yu Xu3
Yu Xu3 Qian-Wen Zhang3
Qian-Wen Zhang3 Cui Hu4
Cui Hu4 Xi-Guang Mao4
Xi-Guang Mao4 Cheng Chen5
Cheng Chen5 Wu Huang5
Wu Huang5 Gong-Sheng Mi6
Gong-Sheng Mi6 Juan Shen6
Juan Shen6 Yong Tian7
Yong Tian7 Feng-Mei Ke7
Feng-Mei Ke7Objective: To study the oncological safety of diagnostic hysteroscopy for women with apparent early-stage type II endometrial cancer.
Patients and Methods: A total of 429 women with presumed early-stage type II endometrial cancer were included. The 5-year disease-free survival (DFS) and overall survival (OS) were estimated and compared using the Kaplan-Meier method and the log-rank test among patients diagnosed by Dilation & Curettage (D&C) or diagnostic hysteroscopy. The Cox proportional hazards regression model was employed to adjust for potential confounding factors.
Results: 160 cases underwent D&C and 269 cases were diagnosed by diagnostic hysteroscopy. The 5-year DFS rate was 72.17% in the diagnostic hysteroscopy group and 76.16% in the D&C group, diagnostic hysteroscopy was not associated with deteriorated 5-year DFS rate (HR 1.25, 95% CI 0.84-1.86, P=0.281). The 5-year OS rate was 67.23% in the diagnostic hysteroscopy group and 70.71% in the D&C group, diagnostic hysteroscopy did not increase the risk of all-cause death (HR 1.11, 95% CI 0.78-1.57, P=0.573). Multivariable analysis showed that the method of endometrial sampling was not independently associated with DFS (aHR 1.38, 95% CI 0.92-2.07, P=0.122) and OS (aHR 1.23, 95% CI 0.85-1.77, P=0.272).
Conclusion: For apparent early-stage type II endometrial cancer, endometrial sampling by diagnostic hysteroscopy was as safe as D&C.
In developed countries, endometrial cancer ranks first in common gynecological malignancies (1, 2). In 2020, endometrial cancer is diagnosed in about 420,000 women worldwide, and an estimated 98,000 women die from this cancer (3). To make matters worse, the incidence of endometrial cancer and the associated mortality are increasing among women of all backgrounds (2, 3).
In 1983, to reflect the disparate biologic behaviors and to refine the different prognoses, Bokhman classified endometrial cancer to type I cancers and type II cancers (4). Since then, this categorization system of endometrial cancer was universally adopted (2). Unlike type I endometrial cancer, type II endometrial cancer usually develops in nonobese women and is not related to hyperestrogenemia, endometrial hyperplasia, or metabolic syndrome (2, 5). Histologically, type II endometrial cancer is poorly differentiated or undifferentiated, including uterine serous carcinoma (USC), uterine clear cell carcinoma (UCCC), and uterine carcinosarcoma (2, 6, 7). Generally, type II endometrial cancer is clinically aggressive, usually presenting at advanced stages, having high rates of extrauterine involvement, and having a high risk of recurrence (2, 5, 6).
For women with endometrial cancer, the most common manifestation is abnormal uterine bleeding (2, 8). In women with abnormal uterine bleeding, to rule out malignant diseases, ultrasound and endometrial sampling are often required (8). Dilation & Curettage (D&C) and diagnostic hysteroscopy are the two most common methods for endometrial evaluation (8). Compared with D&C, by providing physicians with a visualization of the uterine cavity and facilitating the directed biopsy of suspicious lesions, diagnostic hysteroscopy is considered more accurate (9, 10). However, some researchers present their concerns. They think that in the process of diagnostic hysteroscopy, the elevated pressure in the uterine cavity may increase the risk of dissemination of cancer cells (11, 12). To date, however, many published studies have agreed that diagnostic hysteroscopy, although it can increase the spread of tumor cells into the peritoneal cavity, does not deteriorate the prognosis of endometrial cancer (13–16). But, it is worth noting that in these studies, almost all the included cases were low-risk endometrial cancer (14–16). Due to its rarity, the oncological safety of diagnostic hysteroscopy for type II endometrial cancers is always under-researched. Given the large biological and clinical heterogeneity between type I endometrial cancer and type II endometrial cancer, it is unknown whether diagnostic hysteroscopy is oncological safe for type II endometrial cancer.
Taken together, to explore the oncological safety of diagnostic hysteroscopy for apparent early-stage type II endometrial cancer, we conducted this multicenter retrospective cohort study.
This was a multicenter retrospective cohort study, which was based on six Chinese teaching hospitals. This study was approved by the Institutional Review Board (IRB) of each participating institution. In consideration of the retrospective nature of the study design and this study did not report any identifiable private data, the written informed consent to participate was exempted by the IRBs of the participating centers.
In this study, women with apparent early-stage type II endometrial cancer who had received a diagnosis during the 2011-2016 period and had been managed with surgical staging were included.
Patients would be eligible for this study if they met the following criteria: were between 18 and 80 years old, diagnosed with USC and UCCC by pathological examination, had no signs of a suspicious advanced disease, managed with surgical staging (at least including total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy) within one month after the definite diagnosis, and were consecutively followed up at the participating institutions.
In this study, the signs of suspected advanced diseases were defined as follows: suspicious involvement of the vagina, suspicious metastases of fallopian tubes and/or ovaries, enlarged regional lymph nodes (pelvic and/or para-aortic), or suspicious extrauterine metastases identified by pelvic examination or/and preoperative imaging (including ultrasound, computed tomography, and magnetic resonance imaging). All included cases were staged postoperatively based on the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system for endometrial cancer.
We excluded patients from this study for whom the method of endometrial sampling was unknown, those who lost to follow-up after initial management, those who were managed nonsurgically, those who had undergone neoadjuvant therapy, those who had a history of other malignancies, and those whose postoperative stage of disease was unknown. In this study, patients with a preoperative American Society of Anesthesiologists (ASA) physical status score of IV or larger were also considered not qualified for inclusion.
Demographic, clinical, and pathological data of the included cases were extracted from the medical record management systems of the participating institutions. The data of interest were as follows: year of diagnosis, age at diagnosis, marital status at diagnosis, body mass index (BMI) at diagnosis, the preoperative ASA physical status score, the histological type of the tumor, the grade of tumor differentiation, tumor size, the FIGO stage of disease, the status of lymphovascular space invasion (LVSI), the result of peritoneal cytology, the approach of surgical staging, the scope of lymphadenectomy, the method of endometrial sampling, and the protocol of postoperative adjuvant therapy. Given the retrospective nature of this study, we accepted the clinical heterogeneity in the method of performing diagnostic hysteroscopy among the participating institutions, such as the pressure value of the solution jet, the number of biopsies, and the place of diagnostic hysteroscopy (an office setting or operative room setting), etc.
In this study, disease-free survival (DFS) and overall survival (OS) were the primary outcomes of interest. DFS was defined as the time from diagnosis to disease recurrence or death from endometrial cancer. OS was defined as the time from diagnosis to death from any cause.
All included patients were followed up to death or until January 1, 2022. Data regarding patients with no evidence of recurrence or death were censored at the date of the last follow-up. Data on survival outcomes were collected as follows: vital status, time of disease recurrence, site of disease recurrence, time of death, and cause of death.
Based on the method of endometrial sampling, the included patients were divided into the D&C group and the diagnostic hysteroscopy group. The baseline characteristics were compared between the two groups. When assumptions of normal distribution were confirmed, comparisons of continuous variables would be performed by parametric methods. While the comparisons of non-normally distributed variables and categorical data were performed using nonparametric tests.
The Kaplan–Meier method was employed to generate the survival curves. The comparisons of the survival outcomes between the D&C group and the diagnostic hysteroscopy group were carried out by using the Log-rank test. To adjust the unbalanced confounding factors between the two groups, the Cox proportional hazards regression model was employed to estimate the adjusted hazard ratios (aHR) and 95% confidence intervals (95% CI) for the effect of diagnosis methods on DFS and OS in women with apparent early-stage type II endometrial cancer. To ensure parsimony of the final model, the following variables would be included in the Cox proportional hazards regression model: that was considered clinically relevant to prognosis or that showed a univariate relationship (P-value < 0.2) with outcomes of interest.
Statistical analyses were performed using STATA software, version 17 (StataCorp). Unless otherwise stated, all analyses were carried out with a two-sided significance level of 0.05.
Between January 2011 and January 2016, a total of 11,759 women with endometrial cancer were managed at these participating institutions. After excluding 11,330 patients who were not qualified for the current study, a total of 429 women with apparent early-stage type II endometrial cancer were included in this study. The process of case selection is presented in Figure 1. Among the included patients, 160 patients (37.3%) got diagnosed by diagnostic hysteroscopy, the remaining patients were diagnosed by D&C.
According to the methods of endometrial sampling, the included patients were divided into the D&C group (N=269) and the diagnostic hysteroscopy group (N=160). The comparisons of patient demographics, clinicopathologic characteristics, and treatment variables between the D&C group and the diagnostic hysteroscopy group are summarized in Table 1.
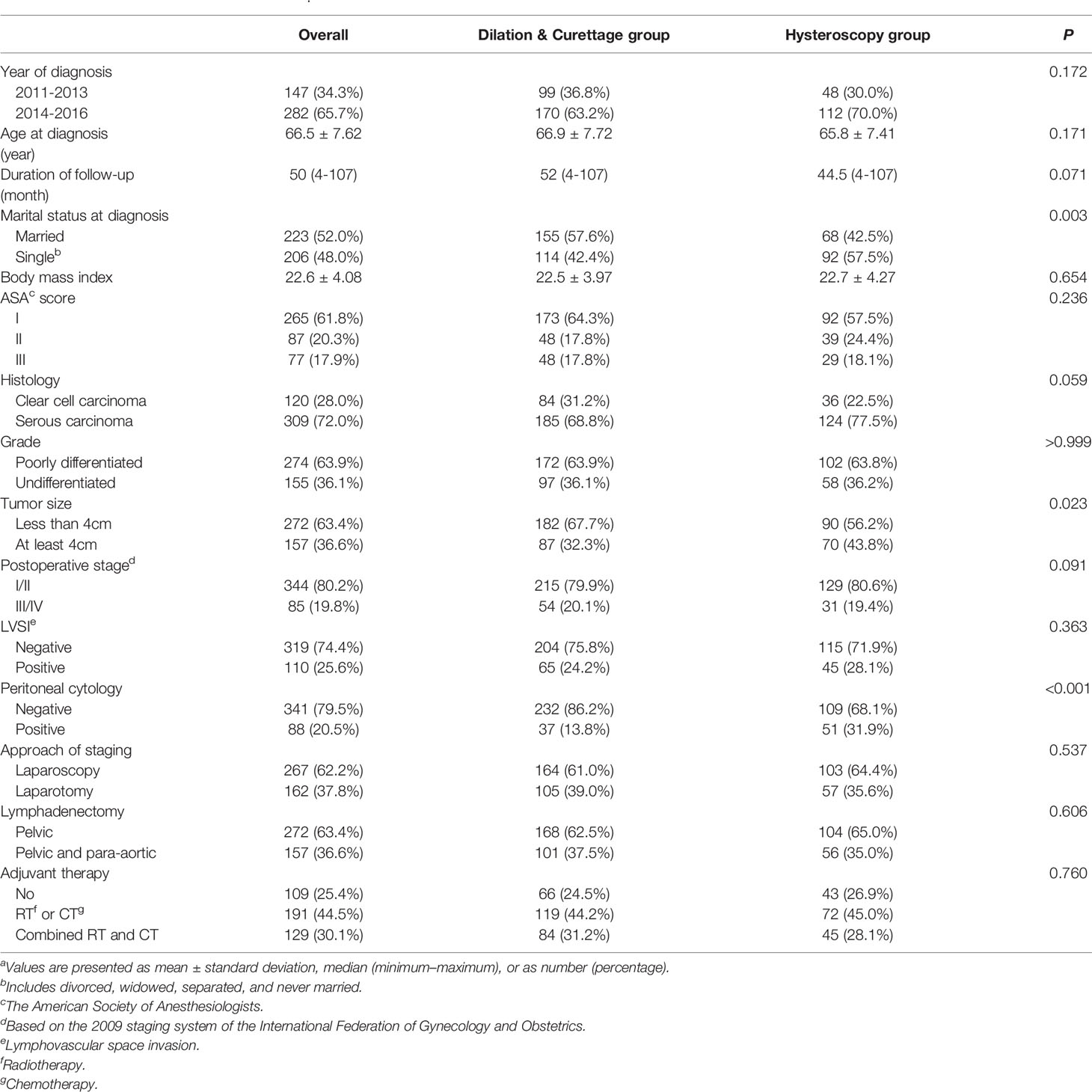
Table 1 Characteristics of the study cohorta.
For the entire cohort, the mean age was 66.5 years (standard deviation: 7.62), and the median duration of follow-up was 50 months (range: 4 months to 107 months). In terms of the age at diagnosis and the duration of follow-up, there was no statistical difference between the two groups (P=0.171 and P=0.071, respectively). There was also no statistical difference in the mean BMI between the two groups, 22.5 kg/m2 and 22.7 kg/m2, respectively. At diagnosis, the proportion of patients being single (including divorced, widowed, separated, and never married) in the hysteroscopy group was significantly higher than that in the D&C group (P=0.003).
In terms of the clinicopathological features of the tumors, 72% of cases were serous carcinomas, about 64% of tumors were poorly differentiated and less than 4 cm, about 20% of cases were found to be advanced (FIGO stage III or IV), 20.5% of patients were identified with positive peritoneal cytology, and 25.6% of the included patients had LVSI. Generally, the histologic type, the grade of tumor differentiation, the size of the tumor, the stage of the disease, and the incidence of LVSI were statistically similar between the D&C group and the diagnostic hysteroscopy group. However, the proportion of patients with positive peritoneal cytology in the diagnostic hysteroscopy group was significantly higher than that in the D&C group, at 31.9% and 13.8%, respectively. The difference in the incidence of positive peritoneal cytology between the two groups was statistically significant (P< 0.001).
Of the included patients, 62.2% got surgical staged by laparoscopy, 36.6% underwent complete regional lymph node removal (combined pelvic and para-aortic lymphadenectomy), and about 75% had postoperative adjuvant therapy (chemotherapy or/and radiotherapy). The protocol of management (surgical approach of staging, extent of lymphadenectomy, and postoperative adjuvant therapy) between the D&C group and the hysteroscopy group was not statistically different.
A total of 55 patients experienced disease recurrence, 18 from the diagnostic hysteroscopy group and the rest from the D&C group, rates of disease recurrence were not statistically different between the two groups (P=0.551). In terms of the pattern of disease recurrence in the two groups, the three most common sites of recurrence are the abdomen (3.0%), lungs (2.6%), and pelvis (1.9%). There was no statistical difference in the pattern of disease recurrence between the two groups (P>0.999). Table 2 shows the pattern and rate of disease recurrence by diagnostic hysteroscopy vs. D&C.
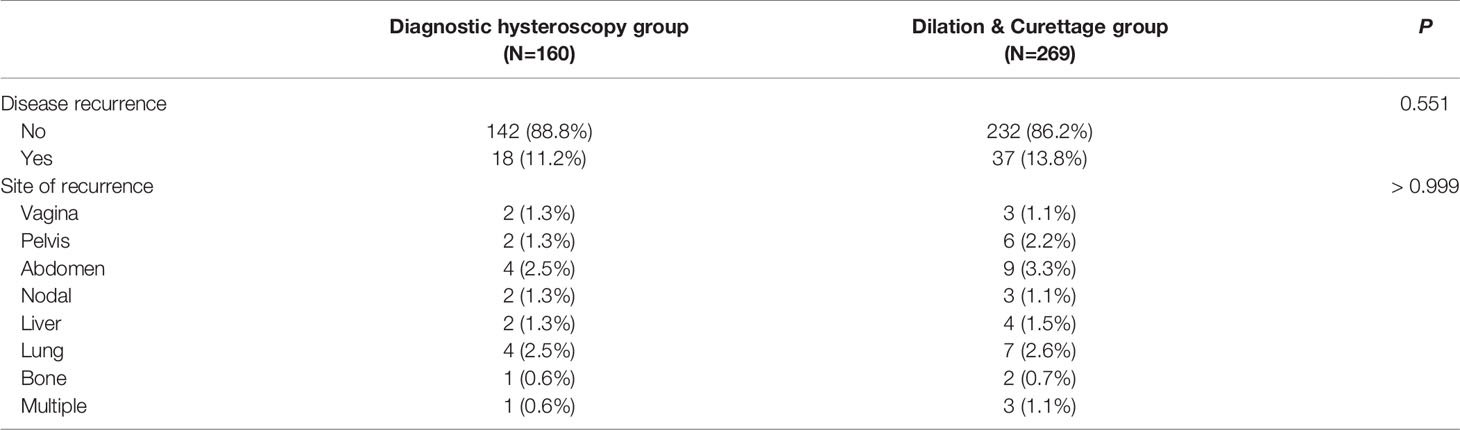
Table 2 Patterns and rates of disease recurrence by diagnostic hysteroscopy vs. Dilation & Curettage.
With a median follow-up of 50 months, a total of 106 cases of recurrence or/and death from endometrial cancer were identified. Supplementary Material 1A shows the DFS curve of the entire cohort. Among them, 63 cases were from the D&C group, and the remaining 43 cases were in the diagnostic hysteroscopy group. The 5-year DFS rate by the Kaplan-Meier method was 72.17% (95% CI 63.68%–79.00%) in the diagnostic hysteroscopy group and 76.16% (95% CI 69.91%–81.29%) in the D&C group. The Log-rank test indicated that for patients with apparent early-stage type II endometrial cancer, diagnostic hysteroscopy was not associated with deteriorated 5-year DFS (HR 1.25, 95% CI 0.84-1.86, P=0.281). Figure 2A shows the Kaplan-Maier curve of DFS (diagnostic hysteroscopy VS. D&C).
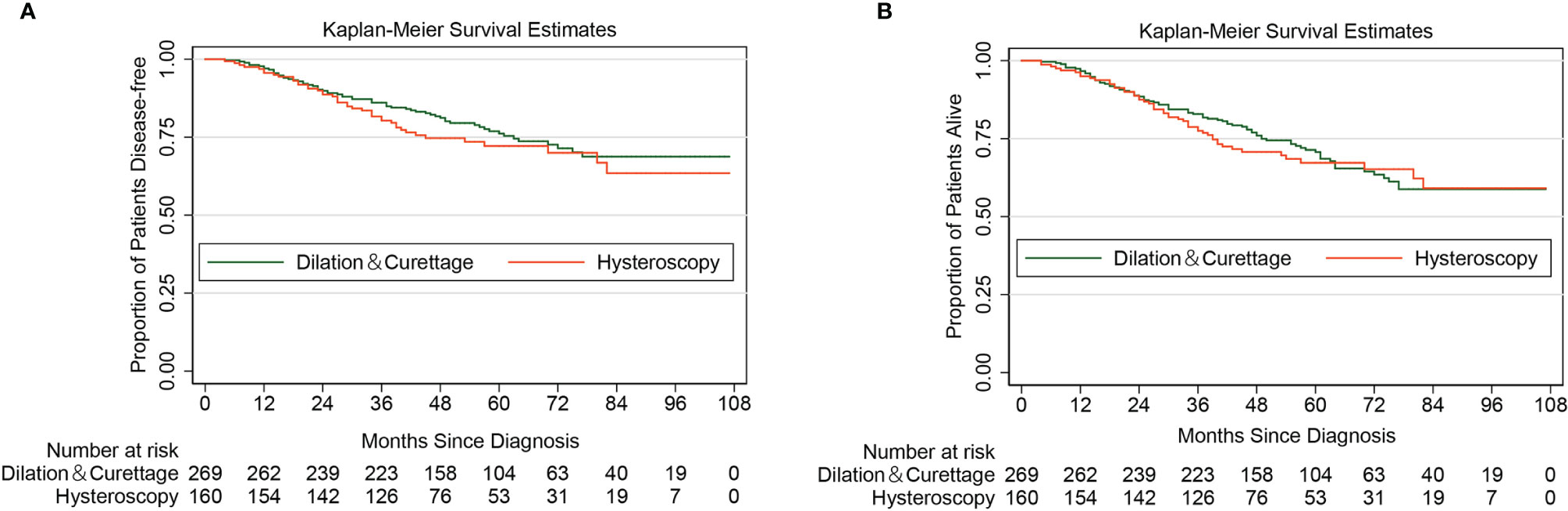
Figure 2 Kaplan-Meier curves of disease-free survival and overall survival for patients with apparent early-stage type II endometrial cancer, by the methods of endometrial sampling. (A for disease-free survival; B for overall survival).
As of January 1, 2022, a total of 135 all-cause deaths have been confirmed. Supplementary Material 1B shows the OS curve of the entire cohort. Among them, 84 cases were from the D&C group, and the remaining 51 cases were in the diagnostic hysteroscopy group. The 5-year OS rate by the Kaplan-Meier method was 67.23% (95% CI 58.60%–74.45%) in the diagnostic hysteroscopy group and 70.71% (95% CI 64.30%–76.18%) in the D&C group. For women with apparent early-stage type II endometrial cancer, diagnostic hysteroscopy did not increase the risk of all-cause death (HR 1.11, 95% CI 0.78-1.57, P=0.573). Figure 2B shows the Kaplan-Maier curve of OS (diagnostic hysteroscopy VS. D&C).
Theoretically, diagnostic hysteroscopy can increase the risk of tumor cells spreading into the peritoneal cavity, this was consistent with the finding of our study (Table 1). However, the Kaplan-Meier method and the Log-rank test showed that for women with apparent early-stage type II endometrial cancer, the positive peritoneal cytology was not associated with the deterioration of DFS (HR 1.03, 95% CI 0.65-1.64, P=0.901) and OS (HR 1.06, 95% CI 0.70-1.60, P=0.797). Figure 3 shows the Kaplan-Maier curves of DFS and OS (positive peritoneal cytology VS. negative peritoneal cytology).
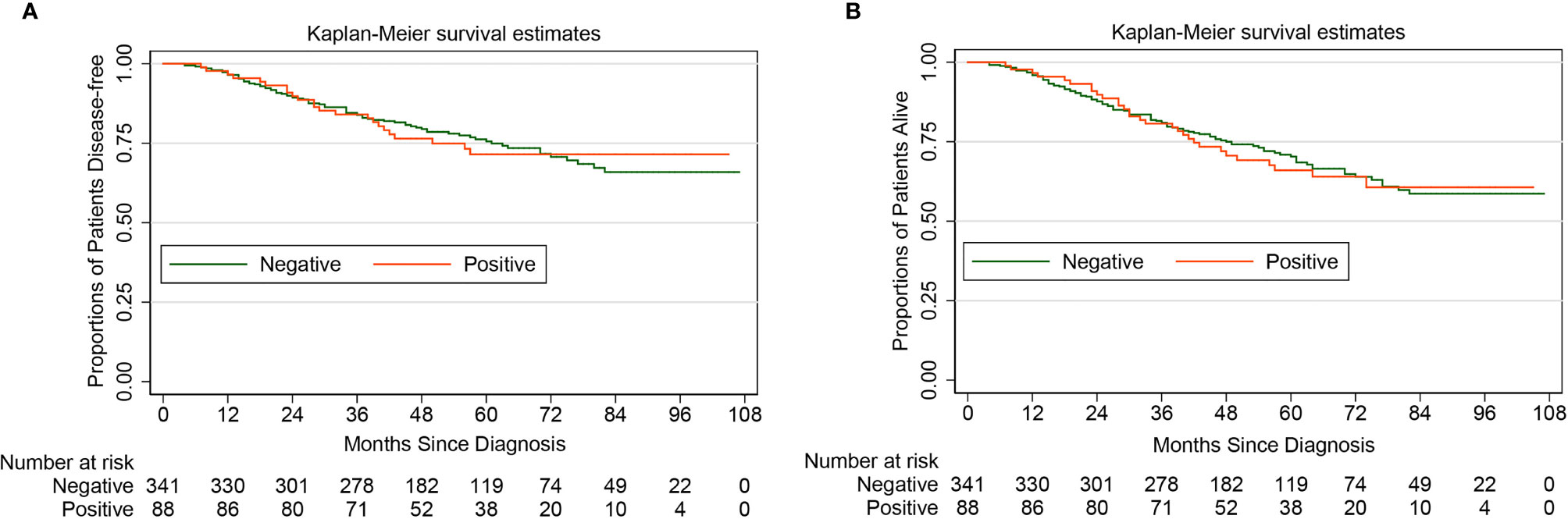
Figure 3 Kaplan-Meier curves of disease-free survival and overall survival for patients with apparent early-stage type II endometrial cancer, by peritoneal cytology. (A for disease-free survival; B for overall survival).
Based on the results of univariate analysis (Supplementary Material 2) and considering the clinical relevance of the candidate variables, the following variables were included in the Cox proportional hazards regression model: age at diagnosis, BMI at diagnosis, the preoperative ASA physical status score, tumor size, the postoperative FIGO stage of the disease, the status of LVSI, adjuvant therapy, and the method of endometrial sampling. The results of the Cox proportional hazards regression analysis demonstrated that for women with apparent early-stage type II endometrial cancer, the methods of preoperative endometrial sampling did not affect the oncological survival (for DFS: diagnostic hysteroscopy VS. D&C, aHR 1.38, 95% CI 0.92-2.07, P=0.122; for OS: diagnostic hysteroscopy VS. D&C, aHR 1.23, 95% CI 0.85-1.77, P=0.272).
The Cox proportional hazards regression model also indicated that for apparent early-stage type II endometrial cancer, having a preoperative ASA physical status score of III (III VS. I: aHR 2.11, 95% CI 1.01-4.43, P=0.048), having an advanced disease (III/IV VS. I/II: aHR 2.68, 95% CI 1.68-4.28, P=0.000), and having LVSI (Yes VS. No: aHR 2.71, 95% CI 1.49-4.95, P=0.001) could worsen the DFS of patients; while postoperative adjuvant therapy was beneficial to the DFS of patients (radiotherapy or chemotherapy VS. without adjuvant therapy: aHR 0.54, 95% CI 0.34-0.87, P=0.011; combined radiotherapy and chemotherapy VS. without adjuvant therapy: aHR 0.39, 95% CI 0.23-0.67, P=0.001). In terms of the risk of all-cause death in patients with apparent early-stage type II endometrial cancer, age at diagnosis (P=0.039), the preoperative ASA physical status score (P=0.029), the stage of disease (P=0.000), the status of LVSI (P=0.000), and postoperative adjuvant therapy (P=0.000) were all independent predictors. Table 3 shows the Cox proportional hazards regression model for survival in patients with apparent early-stage type II endometrial cancer.
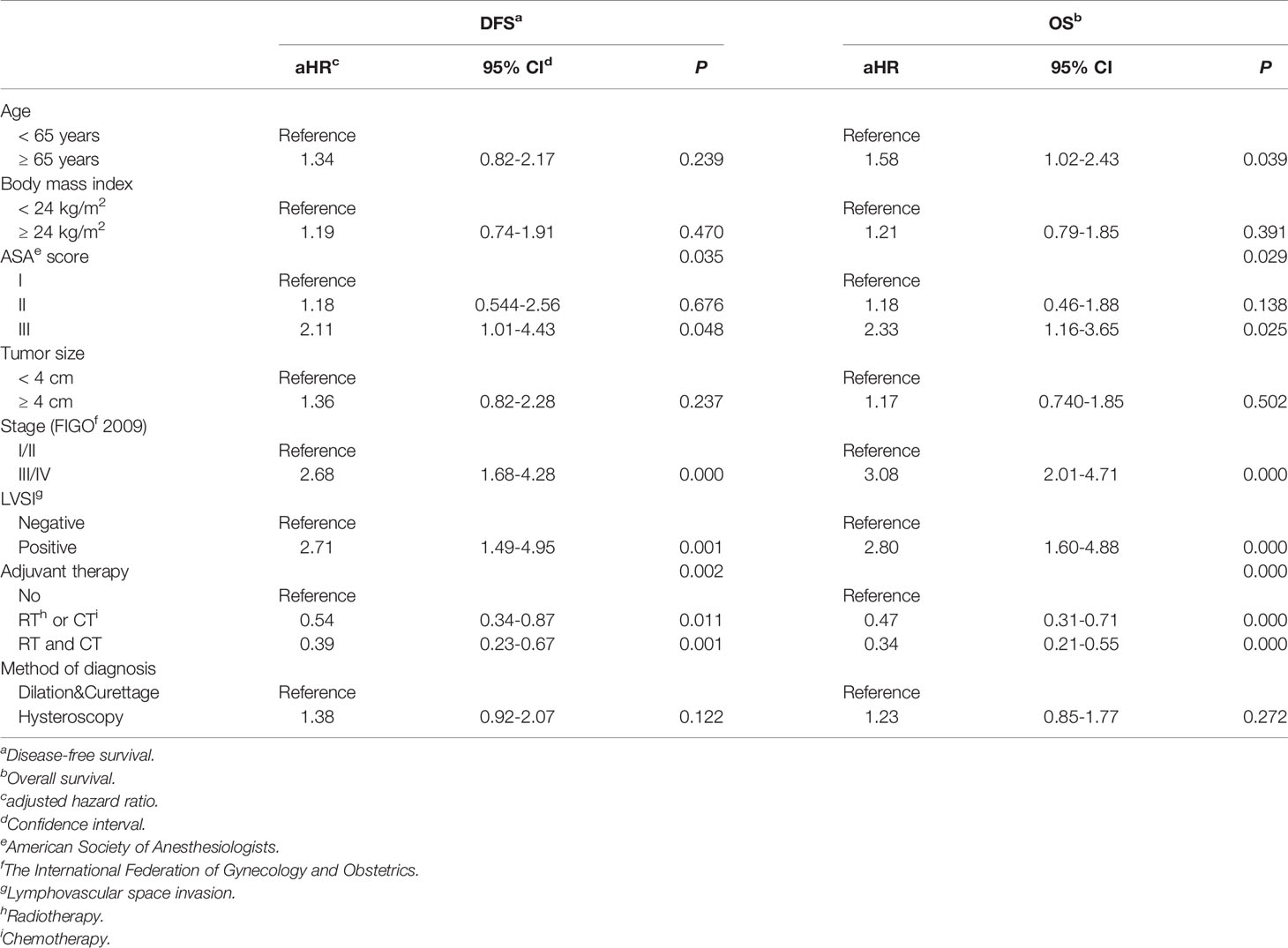
Table 3 Multivariate analysis of prognosis for women with apparent early-stage type II endometrial cancer.
Based on six Chinese tertiary hospitals, this multicenter retrospective cohort study finds that for women with apparent early-stage type II endometrial cancer, diagnostic hysteroscopy was as safe as traditional D&C.
Postmenopausal bleeding, unscheduled bleeding, and menorrhagia are very common gynecologic complaints (17, 18). The main purpose of the management for these women is to rule out malignant lesions or diseases with malignant potentials, such as cancer of the endometrium and endometrial hyperplasia (19). For the elderly with abnormal uterine bleeding, all kinds of evaluations are justified by the common acceptance that postmenopausal bleeding is “cancer until proven otherwise” (20). Thus, for women with abnormal uterine bleeding, the necessity of endometrial sampling is mainly based on the risk of endometrial cancer (20, 21).
The sensitivity of endometrial sampling is high for the identification of endometrial lesions (endometrial cancer included), and D&C has been the standard procedure for diagnosing cancer of the endometrium for years (22). However, with the advances in instrumentation, hysteroscopy plays an increasingly important role in the diagnosis of endometrial cancer, even in an ambulatory setting (23, 24). With endoscopic visualization of the endometrial cavity and the directed biopsy, diagnostic hysteroscopy is considered more accurate and reliable than traditional D&C in diagnosing endometrial lesions (9, 25, 26). A meta-analysis conducted by Bourdel et al. found that for patients with atypical endometrial hyperplasia, compared with D&C, diagnostic hysteroscopy results in a lower underestimation of endometrial cancer (27). However, the high pressure of the uterine cavity during the process of hysteroscopy may facilitate the spreading of tumor cells into the abdominal cavity. Having 1015 women with endometrial cancer included, the study by Polyzos et al. reported that compared with patients who did not undergo diagnostic hysteroscopy, those who underwent diagnostic hysteroscopy had a significantly higher rate of malignant peritoneal cytology (odds ratio 1.78, 95% CI 1.13-2.79, P=0.013) (28). This finding was consistent with that of many other studies (11, 29, 30). In our study, the rate of positive peritoneal cytology in the diagnostic hysteroscopy group was also significantly higher than that in the D&C group, 31.9% and 13.8%, respectively.
But, the negative effects of tumor cells disseminated into the peritoneal cavity during diagnostic hysteroscopy on the prognosis of women with endometrial cancer are not well established. Although the result of peritoneal cytology is no longer a factor to consider in the 2009 FIGO staging system for endometrial cancer, numerous studies still find that malignant peritoneal cytology is strongly associated with the deterioration of long-term prognosis in patients with endometrial cancer (31–34). However, some facts deserve our attention. Almost all of the included cases in the mentioned studies were endometrioid adenocarcinoma of the endometrium (31–34). Few studies have reported the prognostic significance of malignant peritoneal cytology in type II endometrial cancer. What is more, all the malignant peritoneal cytology in the mentioned studies was not associated with diagnostic hysteroscopy (31–34). Whether the malignant cells disseminated into the peritoneal cavity during diagnostic hysteroscopy can survive, colonize, invade the normal tissue, and worsen the prognosis of patients is unknown. A systematic review and meta-analysis by Du et al. showed that for endometrial cancer, although can increase the risk of spreading of malignant cells, diagnostic hysteroscopy did not worsen the prognosis (13). With 127 type II endometrial cancer cases included, the study conducted by Ribeiro et al. also reported that compared with traditional D&C, diagnostic hysteroscopy did not increase the risk of recurrence and all-cause death (35). This result is consistent with ours. But, large and adequately powered prospective studies with long-term follow-up are still needed to testify the safety of diagnostic hysteroscopy for type II endometrial cancer. Until such studies become available, we still need to be careful about the employment of diagnostic hysteroscopy in type II endometrial cancer.
Based on six centers, our study has a sample size of 429 patients. Considering the rarity of type II endometrial cancer, the sample size of the current study is relatively large. Also, the entire cohort underwent a long-term follow-up. However, there are some limitations to our study. First, due to the limited resources, the pathological diagnoses of UCCC and USC were not reviewed again by experts in pathology. We extracted postoperative pathological diagnoses from patients’ electronic medical records. Second, the pressure of the uterine cavity during diagnostic hysteroscopy was not reported in patients’ electronic medical records. Therefore, we could not explore the effect of intrauterine pressure during diagnostic hysteroscopy on the long-term survival of type II endometrial cancer patients who underwent diagnostic hysteroscopy. Third, there was possible confounding by indications of diagnostic hysteroscopy due to our study design. In clinical practice, there is currently no widely accepted indication for diagnostic hysteroscopy in the diagnosis of endometrial cancer. Gynecologists of the participating centers of this study chose the method of endometrial sampling mainly based on their preference and judgment. The last, considering the retrospective nature of the current study, there were some inevitable biases, such as recall bias, selection bias, etc. To reduce these biases as much as possible, we screened cases strictly according to established inclusion and exclusion criteria and excluded those with incomplete data.
For apparent early-stage type II endometrial cancer, endometrial sampling by diagnostic hysteroscopy is as safe as traditional D&C. This finding needs further large and adequately powered prospective studies to verify.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HZ: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, and Supervision. YX: Methodology, Investigation, Data analysis, Writing – original draft, Writing – review & editing, and Project administration. K-FL, QX, Q-WZ, CH, X-GM, CC, WH, G-SM, JS, YT, and F-MK: Methodology, Investigation, Data analysis, and Writing – review & editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.918693/full#supplementary-material
Supplementary Material 1 | Kaplan-Meier curves of disease-free survival and overall survival for patients with apparent early-stage type II endometrial cancer. (A for disease-free survival; B for overall survival).
Supplementary Material 2 | Univariate analysis of prognosis for women with apparent early-stage type II endometrial cancer.
1. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann Oncol (2016) 27(1):16–41. doi: 10.1097/IGC.0000000000000609
2. Lu KH, Broaddus RR. Endometrial Cancer. N Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Bokhman JV. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
5. Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T, et al. Uterine Serous Carcinoma. Gynecol Oncol (2021) 162(1):226–34. doi: 10.1016/j.ygyno.2021.04.029
6. Faber MT, Sperling CD, Bennetsen AKK, Aalborg GL, Kjaer SK. A Danish Nationwide Study of Risk Factors Associated With Type I and Type II Endometrial Cancer. Gynecol Oncol (2021) 161(2):553–8. doi: 10.1016/j.ygyno.2021.02.010
7. Murali R, Soslow RA, Weigelt B. Classification of Endometrial Carcinoma: More Than Two Types. Lancet Oncol (2014) 15(7):e268–278. doi: 10.1016/S1470-2045(13)70591-6
8. Clarke MA, Long BJ, Sherman ME, Lemens MA, Podratz KC, Hopkins MR, et al. Risk Assessment of Endometrial Cancer and Endometrial Intraepithelial Neoplasia in Women With Abnormal Bleeding and Implications for Clinical Management Algorithms. Am J Obstet Gynecol (2020) 223(4):549.e541–549.e513. doi: 10.1016/j.ajog.2020.03.032
9. van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic Hysteroscopy in Abnormal Uterine Bleeding: A Systematic Review and Meta-Analysis. BJOG (2007) 114(6):664–75. doi: 10.1111/j.1471-0528.2007.01326.x
10. Gkrozou F, Dimakopoulos G, Vrekoussis T, Lavasidis L, Koutlas A, Navrozoglou I, et al. Hysteroscopy in Women With Abnormal Uterine Bleeding: A Meta-Analysis on Four Major Endometrial Pathologies. Arch Gynecol Obstet (2015) 291(6):1347–54. doi: 10.1007/s00404-014-3585-x
11. Dong H, Wang Y, Zhang M, Sun M, Yue Y. Whether Preoperative Hysteroscopy Increases the Dissemination of Endometrial Cancer Cells: A Systematic Review and Meta-Analysis. J Obstet Gynaecol Res (2021) 47(9):2969–77. doi: 10.1111/jog.14897
12. Obermair A, Geramou M, Gucer F, Denison U, Graf AH, Kapshammer E, et al. Does Hysteroscopy Facilitate Tumor Cell Dissemination? Incidence of Peritoneal Cytology From Patients With Early Stage Endometrial Carcinoma Following Dilatation and Curettage (D & C) Versus Hysteroscopy and D & C. Cancer (2000) 88(1):139–43. doi: 10.1002/(SICI)1097-0142(20000101)88:1<139::AID-CNCR19>3.0.CO;2-U
13. Du Y, Xu Y, Qin Z, Sun L, Chen Y, Han L, et al. The Oncology Safety of Diagnostic Hysteroscopy in Early-Stage Endometrial Cancer: A Systematic Review and Meta-Analysis. Front Oncol (2021) 11:742761. doi: 10.3389/fonc.2021.742761
14. Namazov A, Gemer O, Helpman L, Hag-Yahia N, Eitan R, Raban O, et al. The Oncological Safety of Hysteroscopy in the Diagnosis of Early-Stage Endometrial Cancer: An Israel Gynecologic Oncology Group Study. Eur J Obstet Gynecol Reprod Biol (2019) 243:120–4. doi: 10.1016/j.ejogrb.2019.10.042
15. Cicinelli E, Tinelli R, Colafiglio G, Fortunato F, Fusco A, Mastrolia S, et al. Risk of Long-Term Pelvic Recurrences After Fluid Minihysteroscopy in Women With Endometrial Carcinoma: A Controlled Randomized Study. Menopause (2010) 17(3):511–5. doi: 10.1097/gme.0b013e3181c8534d
16. de la Cuesta RS, Espinosa JA, Crespo E, Granizo JJ, Rivas F. Does Fluid Hysteroscopy Increase the Stage or Worsen the Prognosis in Patients With Endometrial Cancer? A Randomized Controlled Trial. Eur J Obstet Gynecol Reprod Biol (2004) 115(2):211–5. doi: 10.1016/j.ejogrb.2004.01.029
17. Goldstein SR, Lumsden MA. Abnormal Uterine Bleeding in Perimenopause. Climacteric (2017) 20(5):414–20. doi: 10.1080/13697137.2017.1358921
18. Kaunitz AM. Abnormal Uterine Bleeding in Reproductive-Age Women. Jama (2019) 321(21):2126–7. doi: 10.1001/jama.2019.5248
19. Pennant ME, Mehta R, Moody P, Hackett G, Prentice A, Sharp SJ, et al. Premenopausal Abnormal Uterine Bleeding and Risk of Endometrial Cancer. BJOG (2017) 124(3):404–11. doi: 10.1111/1471-0528.14385
20. Izetbegovic S, Stojkanovic G, Ribic N, Mehmedbasic E. Features of Postmenopausal Uterine Haemorrhage. Med Arch (2013) 67(6):431–4. doi: 10.5455/medarh.2013.67.431-434
21. Papakonstantinou E, Adonakis G. Management of Pre-, Peri-, and Post-Menopausal Abnormal Uterine Bleeding: When to Perform Endometrial Sampling? Int J Gynaecol Obstet (2021). doi: 10.1002/ijgo.13988
22. Ben-Yehuda OM, Kim YB, Leuchter RS. Does Hysteroscopy Improve Upon the Sensitivity of Dilatation and Curettage in the Diagnosis of Endometrial Hyperplasia or Carcinoma? Gynecol Oncol (1998) 68(1):4–7. doi: 10.1006/gyno.1997.4891
23. Giampaolino P, Della Corte L, Di Filippo C, Mercorio A, Vitale SG, Bifulco G. Office Hysteroscopy in the Management of Women With Postmenopausal Bleeding. Climacteric (2020) 23(4):369–75. doi: 10.1080/13697137.2020.1754389
24. Valle RF. Office Hysteroscopy. Clin Obstet Gynecol (1999) 42(2):276–89. doi: 10.1097/00003081-199906000-00011
25. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of Hysteroscopy in the Diagnosis of Endometrial Cancer and Hyperplasia: A Systematic Quantitative Review. Jama (2002) 288(13):1610–21. doi: 10.1001/jama.288.13.1610
26. van Hanegem N, Prins MM, Bongers MY, Opmeer BC, Sahota DS, Mol BW, et al. The Accuracy of Endometrial Sampling in Women With Postmenopausal Bleeding: A Systematic Review and Meta-Analysis. Eur J Obstet Gynecol Reprod Biol (2016) 197:147–55. doi: 10.1016/j.ejogrb.2015.12.008
27. Bourdel N, Chauvet P, Tognazza E, Pereira B, Botchorishvili R, Canis M. Sampling in Atypical Endometrial Hyperplasia: Which Method Results in the Lowest Underestimation of Endometrial Cancer? A Systematic Review and Meta-Analysis. J Minimally Invasive Gynecol (2016) 23(5):692–701. doi: 10.1016/j.jmig.2016.03.017
28. Polyzos NP, Mauri D, Tsioras S, Messini CI, Valachis A, Messinis IE. Intraperitoneal Dissemination of Endometrial Cancer Cells After Hysteroscopy: A Systematic Review and Meta-Analysis. Int J Gynecol Cancer (2010) 20(2):261–7. doi: 10.1111/IGC.0b013e3181ca2290
29. Chang YN, Zhang Y, Wang YJ, Wang LP, Duan H. Effect of Hysteroscopy on the Peritoneal Dissemination of Endometrial Cancer Cells: A Meta-Analysis. Fertil Steril (2011) 96(4):957–61. doi: 10.1016/j.fertnstert.2011.07.1146
30. Revel A, Tsafrir A, Anteby SO, Shushan A. Does Hysteroscopy Produce Intraperitoneal Spread of Endometrial Cancer Cells? Obstet Gynecol Survey (2004) 59(4):280–4. doi: 10.1097/01.OGX.0000120173.09136.4A
31. Shiozaki T, Tabata T, Yamada T, Yamamoto Y, Yamawaki T, Ikeda T. Does Positive Peritoneal Cytology Not Affect the Prognosis for Stage I Uterine Endometrial Cancer?: The Remaining Controversy and Review of the Literature. Int J Gynecol Cancer (2014) 24(3):549–55. doi: 10.1097/IGC.0000000000000072
32. Matsuo K, Yabuno A, Hom MS, Shida M, Kakuda M, Adachi S, et al. Significance of Abnormal Peritoneal Cytology on Survival of Women With Stage I-II Endometrioid Endometrial Cancer. Gynecol Oncol (2018) 149(2):301–9. doi: 10.1016/j.ygyno.2018.02.012
33. Matsuo K, Matsuzaki S, Nusbaum DJ, Machida H, Nagase Y, Grubbs BH, et al. Malignant Peritoneal Cytology and Decreased Survival of Women With Stage I Endometrioid Endometrial Cancer. Eur J Cancer (2020) 133:33–46. doi: 10.1016/j.ejca.2020.03.031
34. Seagle BL, Alexander AL, Lantsman T, Shahabi S. Prognosis and Treatment of Positive Peritoneal Cytology in Early Endometrial Cancer: Matched Cohort Analyses From the National Cancer Database. Am J Obstet Gynecol (2018) 218(3):329.e321–329.e315. doi: 10.1016/j.ajog.2017.11.601
Keywords: uterine serous carcinoma, uterine clear cell carcinoma, diagnostic hysteroscopy, overall survival, disease-free survival
Citation: Zhou H, Lai K-F, Xiang Q, Xu Y, Zhang Q-W, Hu C, Mao X-G, Chen C, Huang W, Mi G-S, Shen J, Tian Y and Ke F-M (2022) Oncological Safety of Diagnostic Hysteroscopy for Apparent Early-Stage Type II Endometrial Cancer: A Multicenter Retrospective Cohort Study. Front. Oncol. 12:918693. doi: 10.3389/fonc.2022.918693
Received: 12 April 2022; Accepted: 16 May 2022;
Published: 23 June 2022.
Edited by:
Marilyn Huang, University of Miami Health System, United StatesReviewed by:
Angelo Finelli, ULSS2 Marca Trevigiana, ItalyCopyright © 2022 Zhou, Lai, Xiang, Xu, Zhang, Hu, Mao, Chen, Huang, Mi, Shen, Tian and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhou, aHVpel9zd211QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.