- 1General Surgery Department, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 2Clinical Center for Liver Cancer, Capital Medical University, Beijing, China
- 3Medical Affairs Department, Acornmed Biotechnology Co. Ltd., Beijing, China
The identification of ERBB2 (HER2) alteration in some solid tumors has become critically important due to the actionable events predictive of response to anti-HER2 therapy. However, the efficacy of ERBB2 mutated hilar cholangiocarcinoma (hCCA) against ERBB2 is rarely reported. Here we report a 76-year-old female diagnosed with hCCA complicated by liver metastases after radical resection. The next-generation sequencing assay showed that the tumor had an ERBB2 mutation. Then, the patient was treated with trastuzumab plus capecitabine. After 2 months of treatment, she had a partial response. Until now, the patient is still alive. This study has shown the potential of trastuzumab combined with capecitabine as an effective treatment for hilar cholangiocarcinoma complicated by liver metastases harboring ERBB2 alterations.
Introduction
Cholangiocarcinoma (CCA) is a heterogeneous malignancy that can occur at every point in the biliary tree, from the canals of Hering to the main bile duct (1){Banales, 2016 #3}{Banales, 2016 #3}. CCAs are classified into intrahepatic (iCCA) and extrahepatic (eCCA) categories on the basis of anatomical origin (2). The eCCA can be further separated into two subtypes: the proximal or hilar (HCCA) accounts for 60–70% and the distal for 20–30% of CCA (3).
hCCA is a common malignant tumor with a relatively poor prognosis. The overall 5-year survival rate is approximately 13–40% (4). The gold standard of treatment for patients with unresectable hilar cholangiocarcinoma is palliative chemotherapy. However, there was a poor outcome with radiation and systemic chemotherapy, such as with gemcitabine and cisplatin (5). Thus, a novel therapeutic approach is warranted.
Targeted therapies are rapidly transforming the therapeutic paradigm for biliary tract cancer, particularly for iCCA, where targeting FGFR2 fusions, IDH 1/2 mutations, and BRAF V600E mutations are becoming a reality (6, 7). Unlike iCCA, targeted therapies for hCCA are rare.
Herein we report a rare case of a female patient with ERBB2-mutant hCCA who responds well to the treatment of trastuzumab in combination with capecitabine.
Case Presentation
A 76-year-old woman was admitted to a local hospital after she experienced itchy skin plus dark yellow urine for several days. The patient was diagnosed with Bismuth type IV hCCA after imageological diagnosis and received percutaneous transhepatic cholangiodrainage (PTCD) on February 5, 2021. The total bilirubin concentration was 400 µmol/L before PTCD, and the daily bile drainage was approximately 300-400 ml. The preoperative total bilirubin level was reduced to the normal level of 19.6 µmol/L. Then, she visited Beijing Youan Hospital, Capital Medical University, for further operative treatment. Enhanced CT of the upper abdomen and magnetic resonance cholangiopancreatography indicated high biliary obstruction and a high possibility of hilar cholangiocarcinoma, involving the upper to the middle common bile duct, and lesions after PTCD. On March 17, 2021, the patient underwent hilar radical resection, and the resection segments were reconstructed by “basin-shape” hepaticojejunostomy. The clinical data were collected and analyzed. A postoperative pathological examination revealed adenocarcinoma with lymph node metastasis. The patient fell accidentally after discharge from the hospital and experienced a fracture of the left femoral neck. Therefore, she did not undergo postoperative treatment until she came to the hospital for review in September 2021. Abdominal contrast-enhanced CT revealed a low-density mass in the left medial hepatic lobe of the liver. After receiving multidisciplinary treatment, the patient was considered unsuitable for surgical treatment, ablation therapy, and radiotherapy.
With the patient’s consent, the tissue sample obtained during surgery was subjected to next-generation sequencing (NGS) using an 808-gene panel in a College of American Pathologists (CAP)-certified lab. Somatic gene mutations have been detected in the tissue, including ERBB2 R678Q, PTCH1 c.747-2A>T, TP53 E286Q, ARID1A Q2176X, SMAD4 D493Y, and SMAD4 R361H (Table 1). The NGS results indicated low-level microsatellite instability and low tumor mutational burden (5.58 mutants/Mb).
With the patient’s informed consent, we considered trastuzumab combined with capecitabine as the first-line treatment strategy. The initial loading dose of trastuzumab was 420 mg, followed by 380 mg at 3 weeks later, concomitant with capecitabine 2,000 mg/m2 for 14 days in 21-day cycles. Tumor response is “objectified” according to the response evaluation criteria in solid tumors, whereby the longest diameter of the target lesion that can be best visualized is monitored over time—depending on the clinical situation, e.g., every 2 months. After 2 months, the MR scan showed that the volume of some lesions was reduced by 51.9%, from 27 to 13 mm, indicating that the patient had a partial response (PR). We then continued to treat the patient with trastuzumab combined with capecitabine. After another 2 months, the lesion diameter was still 13 mm, which was considered a stable disease (SD), and no metastatic lesions in other organs were found. In addition, the levels of serum tumor markers were significantly reduced during the treatment (Figure 1). At the time of this report, the patient was alive.
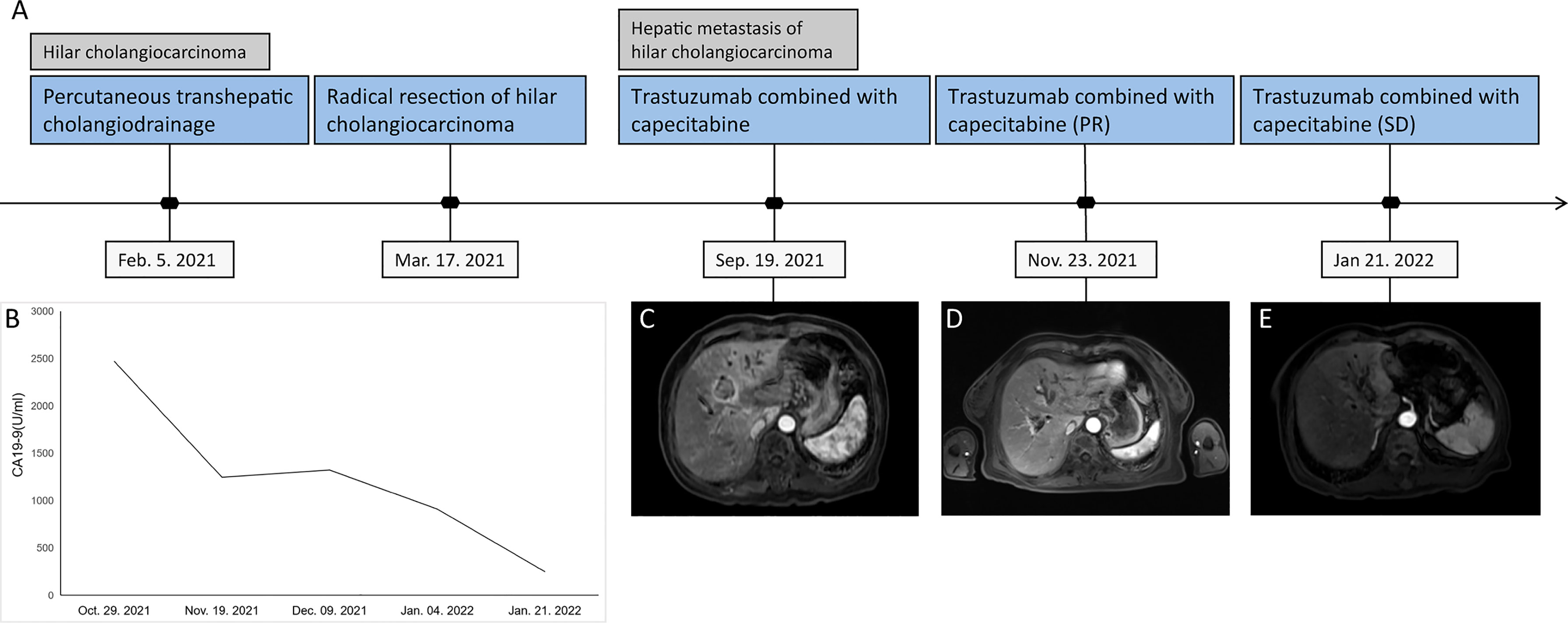
Figure 1 Tumor progression of the patient with postoperative liver metastases from hilar cholangiocarcinoma. (A) The timeline of therapies and tumor progression are indicated. (B) Line chart showing changes in the levels of the tumor marker CA 19-9 during the course of treatment. (C–E) MR scan of the lesion in the bile duct before and after 2 and 4 months of capecitabine plus trastuzumab treatment. MR, magnetic resonance; CA 19-9, cancer antigen 19-9.
Discussion
Advances in genomic research have promoted the development of personalized medicine for the treatment of cancer patients. Here we reported an hCCA patient harboring the ERBB2 R678Q mutation who acquired a response to trastuzumab and capecitabine treatment. To our knowledge, this is the first report to claim that ERBB2 mutations can be used as trastuzumab targets in hCCA.
ERBB2-targeted therapy has achieved remarkable success in the treatment of metastatic breast cancer and gastric cancer. Similarly, recent studies have identified that patients with ERBB2 overexpression or amplification could potentially benefit from ERBB2-targeted therapy in biliary tract cancer (trastuzumab, not lapatinib, has therapeutic effects on Chinese patients with HER2-positive cholangiocarcinoma) (8){Law, 2012 #21;Law, 2012 #21}.
Jeong reported that a biliary tract cancer patient with HER2 overexpression received trastuzumab-pkrb, gemcitabine, and cisplatin and showed an overall response rate of 50.0% (including SD and PR) (9). Yarlagadda et al. demonstrated that a CCA patient with ERBB2 amplification was treated with dual HER2-directed therapy (trastuzumab/pertuzumab) and responded very well with regression of tumor on imaging (10). Consistent with this result, our study showed that an hCCA patient with ERBB2 mutated can benefit from capecitabine combined with trastuzumab treatment. This may implicate that ERBB2-targeted therapy could be a favorable option in hCCA with ERBB2 variation.
ERBB receptors contain an extracellular domain (ECD), a transmembrane domain (TMD), an intracellular region that consists of a juxtamembrane domain (JMD), a kinase domain, and a carboxy terminal tail domain (11). Several studies reported that TMD variation and ECD of ERBB2 may stabilize ERBB2 heterodimerization with other EGFR family members and favor a kinase-active conformation (12). TMD variations are located within the glycine zipper motif at the N‐terminal portion of TMD, which is critically important to the dimerization of ERBB2 to other EGFR family members (12).
Trastuzumab is a monoclonal antibody specifically designed to target the ERBB2. The mechanisms of action of trastuzumab include antibody-dependent cellular cytotoxicity, antibody-mediated ERBB2 internalization followed by receptor degradation, and inhibition of ERBB2 dimerization (13). ERBB2 amplification with corresponding HER2 protein overexpression is associated with sensitivity to therapies targeting HER2, including lapatinib, pertuzumab, and trastuzumab, which were approved by the US Food and Drug Administration. Recent evidence has demonstrated that the presence of ERBB2 mutation can predict clinical responses to anti-HER2-targeted therapies (14).
The recent results from the SUMMIT study have shown that the clinical benefit from ERBB2-targeted therapies (neratinib) may be dependent on the type of ERBB2 alteration and type of tumor (15). Mou et al. reported that a CCA patient with ERBB2 gene S310F in the ECD received trastuzumab combined with chemotherapy and showed therapeutic effects, decreased tumor burden, and improved quality of life (16). Herein we found an hCCA patient, with Pahuja et al. indicating that the ERBB2 R678Q mutation located at the JMD might have a significant effect on TMD geometry and dimerization (17). Therefore, we speculated that the JMD mutant R678Q might respond to trastuzumab.
Concluding Remarks
In conclusion, we were able to identify ERBB2 JMD mutations on NGS in a patient with hCCA and found anti-HER2 therapy as an effective treatment strategy. This will have implications for other patients with hCCA in terms of the identification of this target and considerations toward anti-HER2 therapy on or off trial.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XZ and LD participated in collecting data. LL and YS drafted the manuscript. HL and YZ revised and commented on the draft. DZ and GL contributed to the scientific review and final approval of this manuscript. All authors read and approved the final manuscript.
Conflict of Interest
Author LL, YS, YZ, and HL were employed by the company Acornmed Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert Consensus Document: Cholangiocarcinoma: Current Knowledge and Future Perspectives Consensus Statement From the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol (2016) 13(5):261–80. doi: 10.1038/nrgastro.2016.51
2. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global Trends in Mortality From Intrahepatic and Extrahepatic Cholangiocarcinoma. J Hepatol (2019) 71(1):104–14. doi: 10.1016/j.jhep.2019.03.013
3. Sapisochin G, Ivanics T, Subramanian V, Doyle M, Heimbach JK, Hong JC. Multidisciplinary Treatment for Hilar and Intrahepatic Cholangiocarcinoma: A Review of the General Principles. Int J Surg (2020) 82s:77–81. doi: 10.1016/j.ijsu.2020.04.067
4. Ma Y, Chen Z, Zhu W, Yu J, Ji H, Tang X. Chemotherapy Plus Concurrent Irreversible Electroporation Improved Local Tumor Control in Unresectable Hilar Cholangiocarcinoma Compared With Chemotherapy Alone. Int J hyperthermia: Off J Eur Soc Hyperthermic Oncol North Am Hyperthermia Group (2021) 38(1):1512–8. doi: 10.1080/02656736.2021.1991008
5. Yamashita-Kashima Y, Yoshimura Y, Fujimura T, Shu S, Yanagisawa M, Yorozu K, et al. Molecular Targeting of HER2-Overexpressing Biliary Tract Cancer Cells With Trastuzumab Emtansine, an Antibody-Cytotoxic Drug Conjugate. Cancer chemother Pharmacol (2019) 83(4):659–71. doi: 10.1007/s00280-019-03768-8
6. Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular Targeted Therapies: Ready for “Prime Time” in Biliary Tract Cancer. J Hepatol (2020) 73(1):170–85. doi: 10.1016/j.jhep.2020.03.007
7. Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib Plus Trametinib in Patients With BRAF(V600E)-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol (2020) 21(9):1234–43. doi: 10.1016/S1470-2045(20)30321-1
8. Law LY. Dramatic Response to Trastuzumab and Paclitaxel in a Patient With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Cholangiocarcinoma. J Clin Oncol (2012) 30(27):e271–3. doi: 10.1200/JCO.2012.42.3061
9. Jeong H, Jeong JH, Kim KP, Lee SS, Oh DW, Park DH, et al. Feasibility of HER2-Targeted Therapy in Advanced Biliary Tract Cancer: A Prospective Pilot Study of Trastuzumab Biosimilar in Combination With Gemcitabine Plus Cisplatin. Cancers (Basel) (2021) 13(2):161. doi: 10.3390/cancers13020161
10. Yarlagadda B, Kamatham V, Ritter A, Shahjehan F, Kasi PM. Trastuzumab and Pertuzumab in Circulating Tumor DNA ERBB2-Amplified HER2-Positive Refractory Cholangiocarcinoma. NPJ Precis Oncol (2019) 3:19. doi: 10.1038/s41698-019-0091-4
11. Kovacs E, Zorn JA, Huang J, Barros T, Kuriyan J. A Structural Perspective on the Regulation of the Epidermal Growth Factor Receptor. Annu Rev Biochem (2015) 84:739–64. doi: 10.1146/annurev-biochem-060614-034402
12. Fan Y, Qiu J, Yu R, Cao R, Chen X, Ou Q, et al. Clinical and Molecular Characteristics of Chinese non-Small Cell Lung Cancer Patients With ERBB2 Transmembrane Domain Mutations. Mol Oncol (2020) 14(8):1731–9. doi: 10.1002/1878-0261.12733
13. Zhao J, Mohan N, Nussinov R, Ma B, Wu WJ. Trastuzumab Blocks the Receiver Function of HER2 Leading to the Population Shifts of HER2-Containing Homodimers and Heterodimers. Antibodies (Basel) (2021) 10(1):7. doi: 10.3390/antib10010007
14. Ross JS, Gay LM, Wang K, Ali SM, Chumsri S, Elvin JA, et al. Nonamplification ERBB2 Genomic Alterations in 5605 Cases of Recurrent and Metastatic Breast Cancer: An Emerging Opportunity for Anti-HER2 Targeted Therapies. Cancer (2016) 122(17):2654–62. doi: 10.1002/cncr.30102
15. Ross JS, Gay LM, Wang K, Ali SM, Chumsri S, Elvin JA, et al. HER Kinase Inhibition in Patients With HER2- and HER3-Mutant Cancers. Nature (2018) 554(7691):189–94. doi: 10.1038/nature25475
16. Mou HB, Li WD, Shen YJ, Shi JP, Guo XD, Yao M, et al. Trastuzumab, Not Lapatinib, Has Therapeutic Effects on Chinese Patients With HER2-Positive Cholangiocarcinoma. Hepatobiliary Pancreat Dis Int (2018) 17(5):477–9. doi: 10.1016/j.hbpd.2018.09.011
Keywords: cholangiocarcinoma, ERBB2 mutation, treatment, trastuzumab, case report
Citation: Zeng D, Zhao X, Di L, Lou L, Song Y, Zhang Y, Liu H and Li G (2022) Effectiveness of Trastuzumab Combined With Capecitabine Treatment in a Patient With Hilar Cholangiocarcinoma Complicated by Liver Metastases With an ERBB2-Activating Mutation: A Case Report. Front. Oncol. 12:918297. doi: 10.3389/fonc.2022.918297
Received: 12 April 2022; Accepted: 13 June 2022;
Published: 07 July 2022.
Edited by:
Zongli Zhang, Qilu Hospital of Shandong University, ChinaReviewed by:
Jiansheng Guo, First Hospital of Shanxi Medical University, ChinaShanglei Ning, Shandong University, China
Copyright © 2022 Zeng, Zhao, Di, Lou, Song, Zhang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangming Li, bGlndWFuZ21pbmc5MTdAMTYzLmNvbQ==
 Daobing Zeng1,2
Daobing Zeng1,2 Luyan Lou
Luyan Lou Yanfang Song
Yanfang Song Huanhuan Liu
Huanhuan Liu