- 1School of Public Health, Weifang Medical University, Weifang, China
- 2Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 3Cheeloo College of Medicine, Shandong University, Jinan, China
- 4School of Public Health, Shandong University, Jinan, China
- 5School of Public Health, Sun Yat-Sen University, Guangzhou, China
Background: The low uptake rate of upper gastrointestinal cancer (UGC) screening substantially reduces the benefits of endoscopic screening. This study aimed to obtain residents’ UGC screening preferences to optimize screening strategies and increase the participation rate.
Methods: A discrete choice experiment (DCE) was conducted to assess UGC screening preferences of 1,000 rural residents aged 40 to 70 years from three countries (Linqu, Feicheng, and Dongchangfu) of Shandong province in China. The DCE questionnaire was developed from five attributes: out-of-pocket costs, screening interval, regular follow-up for precancerous lesions, mortality reduction, and screening technique. The data from the DCE were analyzed within the framework of random utility theory using a mixed logit model.
Results: In total, 926 of 959 residents who responded were analyzed. The mean (SD) age was 57.32 (7.22) years. The five attributes all significantly affected residents’ preferences, and the painless endoscopy had the most important impact (β=2.927, P<0.01), followed by screening interval of every year (β = 1.184, P<0.01). Policy analyses indicated that switching the screening technique to painless endoscopy would increase the participation rate up to 89.84% (95%CI: 87.04%-92.63%). Residents aged 40–49, with a history of cancer, with a family income of more than ¥30,000 were more likely to participate in a screening.
Conclusions: UGC screening implementation should consider residents’ preferences to maximize the screening participation rate. Resources permitting, we can carry out the optimal screening program with shorter screening intervals, lower out-of-pocket costs, less pain, follow-up, and higher UGC mortality reduction.
Introduction
Upper gastrointestinal cancer (UGC), including esophageal and gastric cancer, is one of the most commonly diagnosed cancers worldwide. An estimated 16.04 million new UGC cases (8.31% of total cancers) and 13.13 million UGC deaths (13.13% of total cancer deaths) occurred in 2020 throughout the world, with China alone accounting for above 50% of cases and deaths, respectively (1). The survival rate of UGC patients largely depends on the disease stage at diagnosis. The overall 5-year survival rate of UGC patients is only about 30% when diagnosed at an advanced stage, but it can reach 90% and above if detected and treated earlier (2, 3).
Endoscopy followed by biopsy is the gold standard with high sensitivity and specificity for the diagnosis of UGC (4), which has been widely adopted in many East Asian countries, such as China, Japan, and Korea. Among these countries, Japan and Korea have implemented a national endoscopic screening for gastric cancer and achieved good results (5, 6). In China, since the implementation of population-based UGC screening in 2005, endoscopic screening for esophageal cancer and gastric cancer has been performed in more than 194 high-risk areas throughout the country, and 32,000 patients have been found, with a detection rate of 1.69% (2, 4). The current evidence from population studies has confirmed that endoscopic screening is an effective intervention method to reduce the morbidity and mortality of esophageal cancer and gastric cancer (7, 8). In addition, several economic evaluation studies (9–11) from South Korea, USA, and China showed that endoscopic screening for esophageal and gastric cancer is cost-effective compared with no screening.
However, the participation rate of the target population largely impacts the effectiveness of cancer screening (12). The endoscopic screening compliance is found to be only 48.62% in China (7). In some developed countries where population-based endoscopic screening has been going on for a long time, such as Japan and Korea, the participation rate is still lower than 50% (13, 14). This low participation rate is a huge challenge that needs to be addressed to maximize the benefits of endoscopic screening. Individuals’ screening preferences (willingness) have been shown to largely determine the UGC screening participation rate (9). Introducing preference factors into the decision-making process will improve residents’ experience and satisfaction and increase the screening compliance (15).
In recent years, discrete choice experiments (DCEs) have increasingly been used to quantify individuals’ preferences in cancer screening areas, especially in colorectal cancer, breast cancer, and cervical cancer (16–19). These data provide emphasis on the importance of DCE to obtain individuals’ preference trade-off in different screening strategies to optimize screening strategies and improve the participation rate. However, there are few DCE studies on individuals’ preferences for UGC screening, and no related study has been done in China.
This study, therefore, aims to determine individuals’ preferences and willingness to pay (WTP) on UGC screening in rural China by using a DCE and predict the participation rate of various UGC screening options to help policymakers design effective population-based screening programs.
Materials and methods
Discrete choice experiment
A discrete choice experiment (DCE) is a stated preference (SP) survey that explicates how people make decision by balancing product factors (e.g., characteristics of screening programs) and has been widely used in healthcare research (20, 21). In a DCE, respondents are asked to choose the most effective option among several alternative programs composed of different attribute levels (22). DCEs can figure out which characteristics (attribute levels) influence whether people choose to take a UGC screening program. In this study, we adhered to the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guideline for DCE (21).
Attributes and levels definition
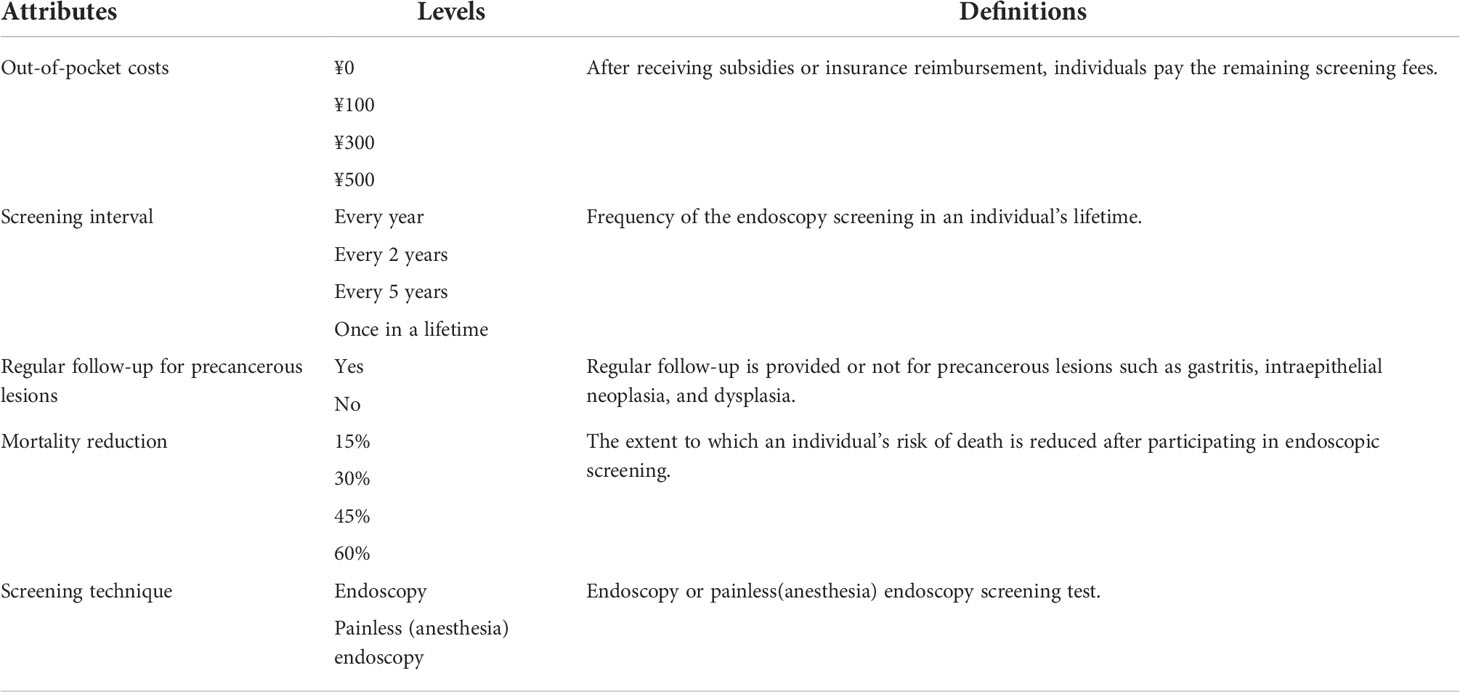
The attributes and levels were selected in a stepwise manner including literature review, expert interview, and focus groups with the target population (23). Firstly, we conducted a literature review to identify attributes and levels. Attributes on screening technique, interval, cost, sensitivity, specificity, mortality reduction, follow-up, pain, waiting time for screening reports, and location of the test had a great influence on residents’ preferences of UGC screening. Since endoscopy is the gold standard for the diagnosis of UGC in China, we did not retain the attributes of sensitivity and specificity (4, 12, 14, 17, 24–30). Secondly, interviews with six experts (three clinicians specialized in UGC from Shandong Cancer Hospital, an expert who majored in public health from Shandong University, and two experts specialized in endoscopic screening from Feicheng People’s Hospital) were conducted to confirm these attributes, resulting in the following six attributes: out-of-pocket costs, time waiting for screening results, screening interval, screening technique, mortality reduction, and regular follow-up for precancerous lesions. Then, two focus-group interviews with 10 residents aged 40 to 70 from the Endoscopic Screening Department of Feicheng People’s Hospital were conducted. In the two focus groups (n = 5 × 2), they were asked to indicate which attribute of UGC screening tests they would expect to be important and to rank them in order of importance in their decision to participate in a screening program. Finally, the survey results included five attributes: out-of-pocket costs, screening interval, screening technique, mortality reduction, and regular follow-up for precancerous lesions. In addition, we identified the extreme ranges of the attribute levels from the literature review of existing UGC screenings. The selected attributes and their levels are summarized in Table 1.
Questionnaire design
A total of 256 (43 × 22) possible scenarios and 32,640 ((256 × 255)/2) unique choice sets were generated by the full factorial design (31), which were not feasible to ask residents to complete. To reduce the response burden, 16 choice sets were generated and divided into two blocks using D-efficiency design with SAS9.4 software. One fixed choice set (27) (option A is definitely better than option B) was included in each block to check the residents’ understanding of the questionnaire (32). Therefore, this study had a total of two different questionnaire versions containing nine DCE choice sets. In each choice set, two hypothetical UGC screening options (option A and option B) and an opt-out option were included (see Supplementary Table S1). Then a pilot study was conducted, and we found that the length of the questionnaire and residents’ understanding were in line with expectations, without any significant change.
Study population and data collection
The methods for determining the sample size varies at present (33). The most common method, which is proposed by Johnson and Orem (34, 35), is the rule of thumb: n > 500*c/(t*a), where 500 is a fixed variable, c denotes the largest number of levels for a certain attribute, a indicates the number of DCE choice sets per block of questionnaire, and t means the number of alternatives per DCE choice set (not including “Opt-out”). Accordingly, the sample size required for this study should be more than 112 respondents (500 × 4/(2 × 9) = 112). Considering the possibility of conducting further subgroup analyses, we increased the sample size to n = 1,000.
Then, we used a face-to-face interview to collect data. Based on different economic development levels, we selected 1,000 Chinese rural residents aged 40–70 years from three countries (i.e., Linqu, Feicheng, and Dongchangfu) of Shandong province in China. In the first step, residents were asked to choose the screening program that generates the highest utility to them from two hypothetical programs, and in the second step, they were asked to answer whether to undergo the screening program in real life.
Data analysis
The out-of-pocket costs attribute was set as a continuous variable to calculate the WTP, and all other attributes were set as classification variables coded by dummy variables (36). All statistical analyses were conducted using Stata 16.0 software.
The data from the DCE were analyzed within the framework of random utility theory using a mixed logit (MIXL) model. Based on the random utility framework, the utility function can be expressed as:
where Unij refers to the utility obtained by respondent n choosing alternatives i in choice scenario j. Vnij is a systematic component specified as the observable total utility, and ϵnij is the error term. X refers to the five attributes and their levels, and β reflects the values of each attribute and the horizontal regression coefficient (β0 represents a fixed constant term).
Importance scores for each attribute showed the contribution of each attribute relative to other attributes in decision making. Scores were calculated by dividing the maximum utility of an attribute by the total utility of all attributes.
The WTP can be estimated as the ratio of the value of the coefficient of other attribute levels to the negative of the cost attribute (). In this context, the WTP showed the relative monetary value that respondents place on different screening characteristics, which would facilitate our understanding of the relative importance of non-monetary attributes in a DCE.
A useful output was how the probability of choosing a given screening test changes as levels of attributes are changed. To assess the expected uptake of a screening program, we applied the model as shown in the following form (37).
where x is a vector of attribute level coefficients, and β reflects the values of each attribute level and the horizontal regression coefficient. The attitudes of respondents to participate in a screening test are calculated by entering the constant term ASC (β0) into the model. The size of the coefficient indicates how important the attribute level is to the respondent’s choice. A positive sign implies that the attribute has a positive impact on the take-up of a given screening test; a negative coefficient is the opposite (37). In the results of a DCE, the mean coefficients reflect the relative preference weights, and the standard deviation reflects the extent of preference heterogeneity (38).
All respondents provided written informed consent. This study was approved by the Institutional Ethical Review Board of Shandong Cancer Hospital and Institute (Reference No. SDTHEC201909001).
Results
Respondents
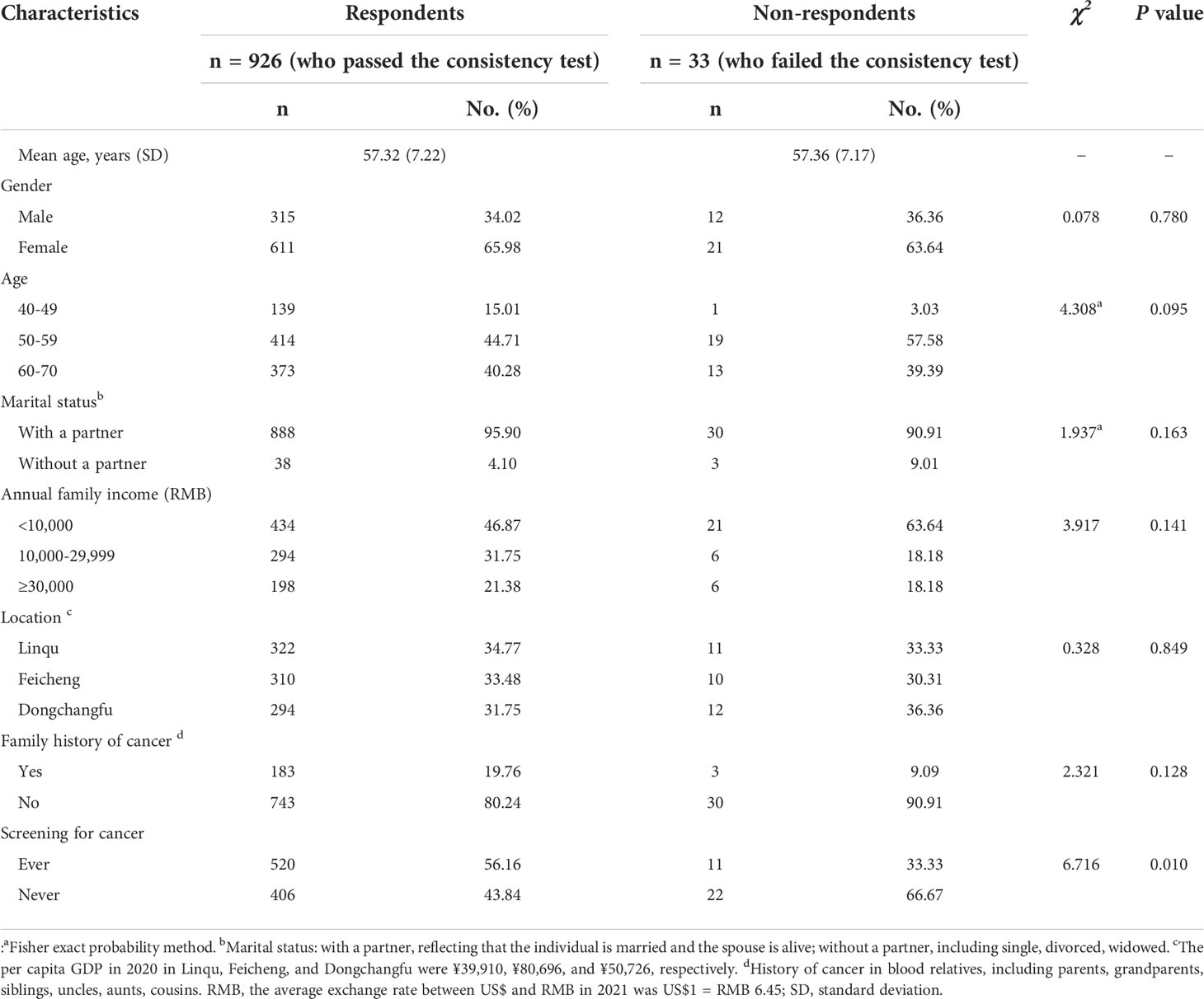
Of the 1,000 invited residents, 959 (including 33 respondents who did not pass the consistency test) completed the questionnaires (response rate: 95.9%). The sensitivity analyses indicated that removing respondents who failed the rationality test did not considerably change the outcome (see Supplementary Table S2). Considering the accuracy of the results, 926 respondents who passed the consistency test were finally included for preference estimation by the mixed logit model. Table 2 summarizes the demographic of the 926 respondents. The mean age (SD) was 57.32 (7.22 years), and 66% of the respondents were women. A family history of cancers was reported by 19.76% of the respondents, and 434 respondents’ annual family income was lower than ¥10,000. No statistical differences between respondents and non-respondents were found.
Preferences estimates
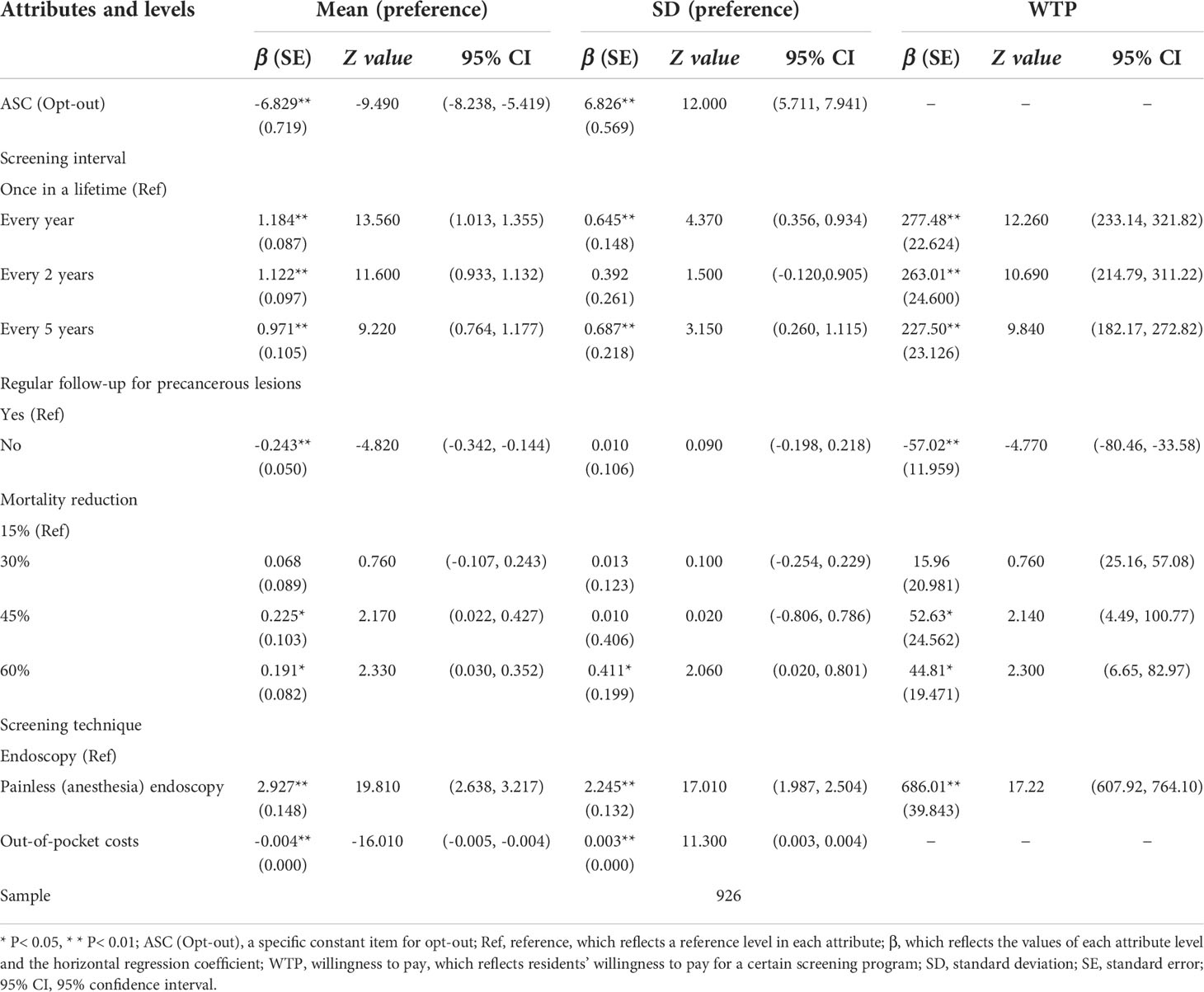
Table 3 shows the results of the final preferences model. The participants preferred the UGC screening over no screening (β = -6.829; (95%CI, -8.238 to -5.419)). All five attributes had statistical effects on residents, and the direction was consistent with our expectations. The screening technique had the most important impact on respondents, followed by out-of-pocket costs and screening interval (see Supplementary Figure S1). In all attribute levels, most of the residents preferred painless endoscopy (β = 2.927; (95%CI, 2.638 to 3.217)). Overall, the respondents preferred a screening program that has a shorter screening interval, causes less pain, has follow-up, pays lower costs, and results in a higher decrease in UGC-related mortality.
Willingness to pay
The results of WTP are shown in Table 3. When converting endoscopy to painless endoscopy, the respondents were willing to pay ¥686.01 (95% CI, 607.92 to 764.10). Compared with screening once in a lifetime, they were willing to pay about ¥250 for every year, every 2 years, and every 5 years. Theoretically, if regular follow-up was not provided, the respondents should be compensated ¥57.02 (95% CI, -80.46 to -33.58).
Subgroup analyses
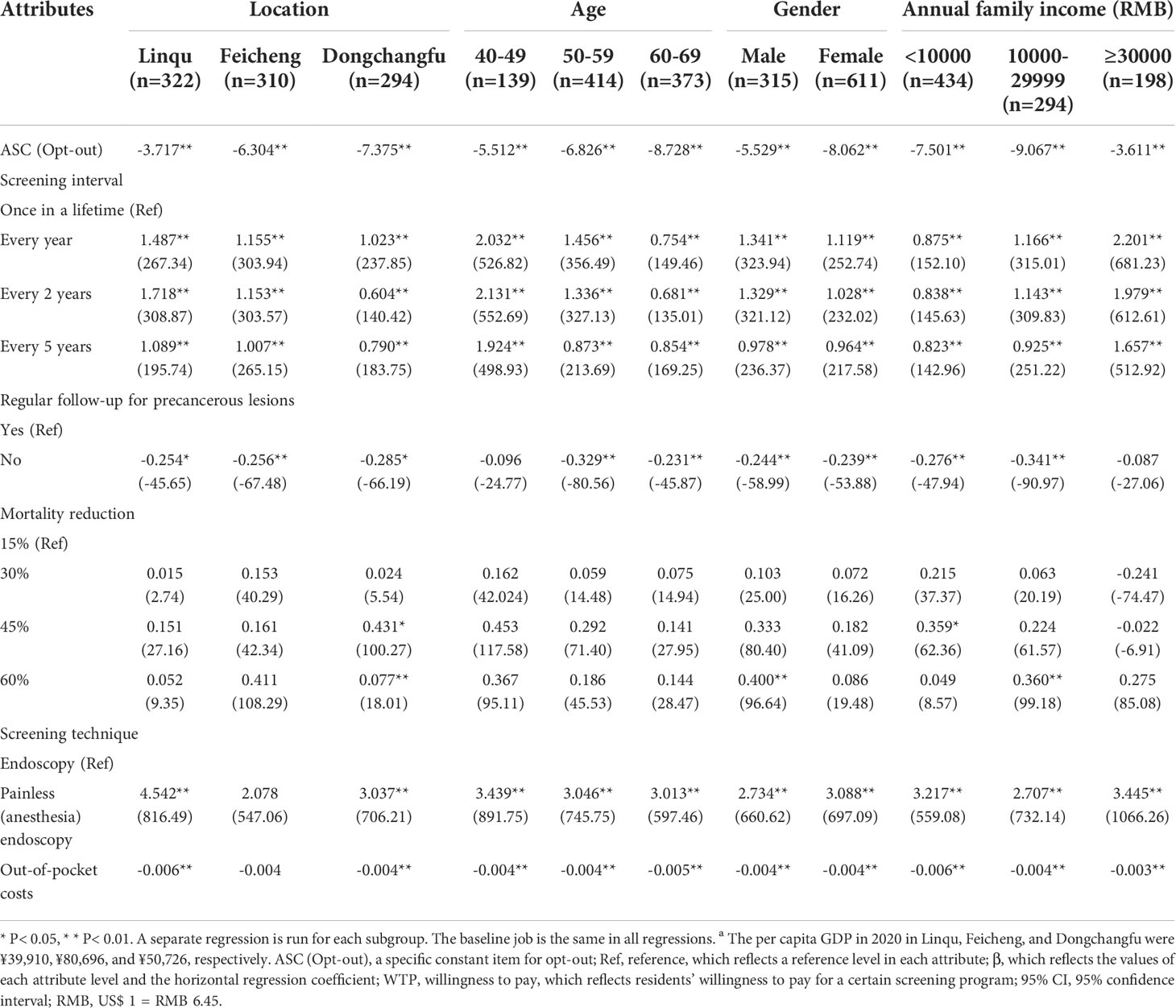
The results of MIXL and WTP among different subgroups are shown in Table 4 (see Supplementary Table S3-6, which shows more details). Apart from the screening technique and screening interval attributes, the preferences for other attributes between different subgroups were relatively similar. Residents who came from Linqu placed more value on the screening interval attribute, and they were willing to pay ¥308.87 (95% CI, 215.64 to 402.10) to shorten the intervals from once in a lifetime to every 2 years. Compared with residents whose family income was less than ¥10,000, residents with a family income of more than ¥30,000 were willing to pay ¥681.23 (95% CI, 457.06 to 905.40) for the screening interval of every year. In comparison to other age groups, residents aged 40 to 49 favored a painless endoscopy approach. In addition, women preferred to participate in a screening test with painless endoscopy than men (β: 3.088 vs. 2.734, P< 0.01).
Expected UGC screening uptake
Figure 1 depicts the probability of respondents as attributes and levels were changed at the baseline level (baseline: ¥0, once in a lifetime, with follow-up, a 15% mortality reduction, and endoscopy). Increased out-of-pocket costs from ¥0 to ¥500 resulted in a 78.8% drop in participation, but changing the screening technique from endoscopy to painless endoscopy would raise participation to 89.84%. Under the optimal screening scenario (i.e., free, 1-yearly, with follow-up, 45% mortality reduction, and painless endoscopy), the participation rate of endoscopic screening would increase to 97.42%.
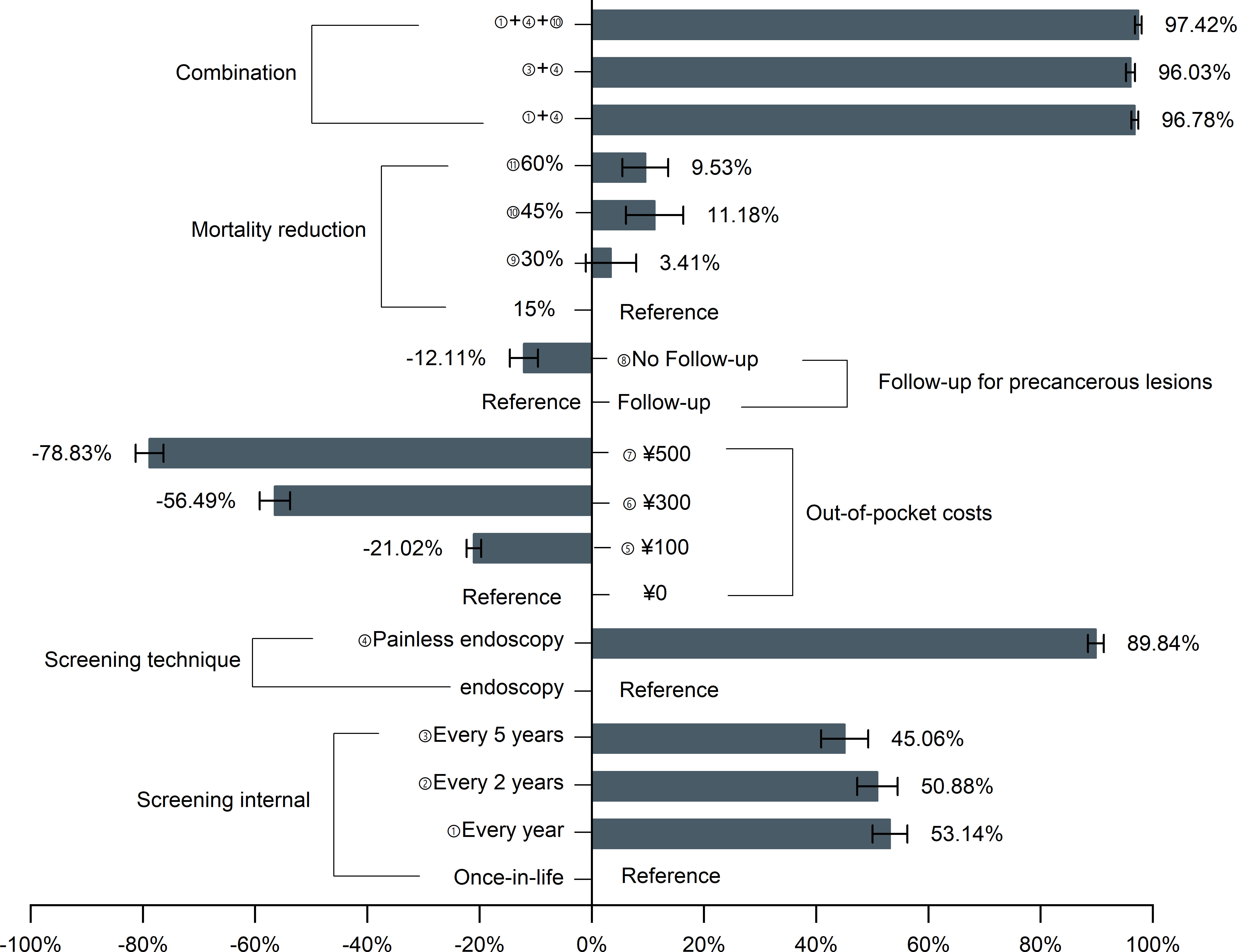
Figure 1 Effects of changing the screening program characteristics on the probability of participation in upper gastrointestinal cancer screening. UGC, upper gastrointestinal cancer.
Furthermore, the participation rates at different out-of-pocket cost levels were as shown in Figure 2. The initial participation rate of out-of-pocket cost of ¥0 is 47.46%, whereas the participation rate of ¥500 was only 6.42%. However, with the exception of ¥500, the participation rate of other cost levels achieved above 90% based on the ideal screening scenario combination.
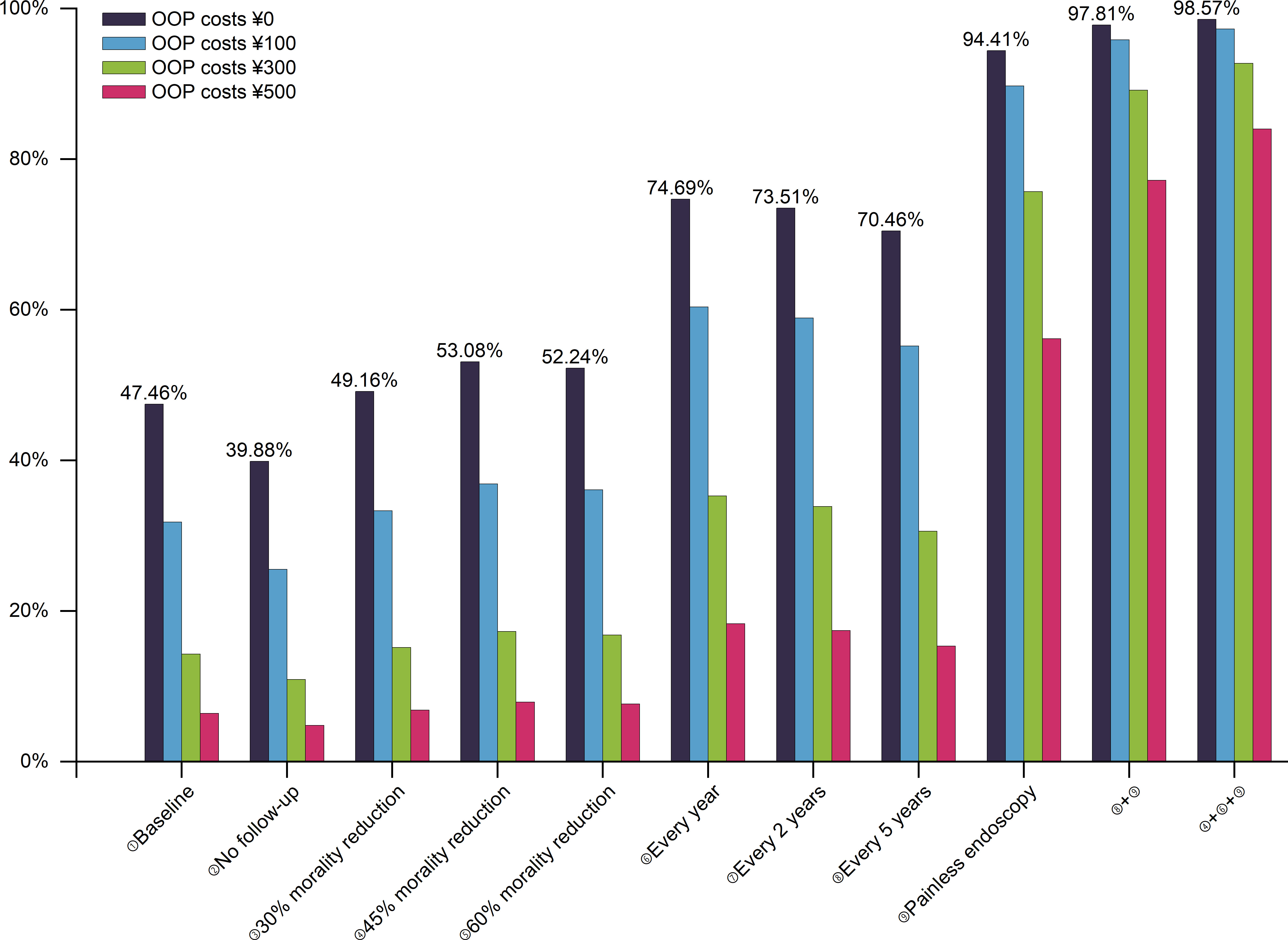
Figure 2 The participation rates for UGC screening at different levels of out-of-pocket costs. Baseline: once in a lifetime, with follow-up, a 15% mortality reduction, and endoscopy; UGC, upper gastrointestinal cancer; OOP, out-of-pocket.
Discussion
To our knowledge, this was the first DCE study to explore the UGC screening preferences of rural residents in China. The results were consistent with our assumptions based on the current screening status in China. All five attributes considered in our study were found to be statistically significant. Rural residents preferred screening programs with a higher decreased UGC-related mortality, shorter intervals, follow-up, less pain, and lower costs.
In our study, respondents were mostly driven by painless endoscopy (WTP = ¥686.01). The respondents’ strong preference for painless endoscopy largely reflected their pursuit of comfort in the screening process. In the Netherlands, two DCEs for esophageal cancer screening showed that the participants equally preferred a screening program with less pain and discomfort (12, 39). Other studies for colorectal cancer screening concluded that the respondents preferred a no-pain or mild-pain screening program (17, 22, 40–42). The target population may ignore the improvement of other attributes in pursuit of a more comfortable screening experience. Thus, it was an effective means to improve the participation rate of UGC screening by advocating the painless endoscopy.
A previous DCE study on Barrett’s esophagus concluded that surveillance every 5 years would lead to a 26% reduction, while surveillance every 3 to 3.5 years would result in a 7% increase in participation (30). The subjects preferred endoscopic screening every 1–2 years compared to a longer screening interval (43). Similar to the above studies, the subjects in our study preferred a shorter screening interval, in which the screening interval of every year is the most popular. However, it may be unrealistic to establish an annual screening interval in some countries with limited resources. We further found that raising the screening interval from once-lifetime to every 5 years can increase the probability by 45.06%, but increasing it from every 5 years to 2 years increases the probability by only 5.82%. Clearly, when the screening interval was shortened to every 5 years, the increase in participation rate slowed down apparently. Existing studies have also shown that every 5 years or a longer screening interval were more popular among the population (17, 18).
Our study also revealed the heterogeneity of population preferences in different subgroups. Consistent with the findings of Peter et al. (12), women preferred a screening program with less pain, and men preferred a lower risk of death. The subjects of different ages had significant differences in preferences for cancer screening and treatment (24, 44). Residents aged 40–49 years with a higher family income had a higher WTP for screening, and they seemed to have a higher demand and better compliance for UGC screening.
A useful output when using DCEs was how the probability of choosing a given option changes with attribute levels (37). We found that the 47.46% participation rate of current screening strategies was very close to the actual uptake rates of 48.62% and 49% (7, 10), indicating that the predicted results in this study were accurate and dependable. We also found that the UGC screening strategies with follow-up, shorter interval, less pain, higher mortality reduction, and lower costs had a higher participation rate. However, as UGC screening strategies, in addition to the participation rate, the cost of health economics also needs to be considered. Xia et al. (10) noted that an endoscopic screening for EC and GC would be more cost-effective than no screening regardless of the initial screening age or screening interval. This finding is consistent with the conclusion reported in our previous research, and the selected attributes and levels in this study draw on the dominance strategies screened from our previous work. It can be considered that the screening strategies consisting of different attributes and levels in this study are cost-effective and feasible under the level of per capita GDP to screen high-risk groups aged 40–70 in China. Nonetheless, further studies are still needed to validate the economics of the UGC screening strategies proposed in this study.
One study pointed out that the cost was always the most important attribute in colorectal cancer screening, and respondents preferred a lower cost (22). Another study on esophageal cancer screening also indicated that the participation rate would decrease by 48% if subjects were required to pay $500 (30). In line with their findings, we discovered that as out-of-pocket costs rise, participation rates decline dramatically. Nonetheless, by optimizing combinations of attributes and levels, the high-cost screening program can be made more appealing. Further analysis showed that the participation rate of out-of-pocket costs of ¥100 and ¥0 was similar when converting an endoscopy to a painless endoscopy. Even if residents have to pay ¥500 in the optimal screening scenario, their participation rate still reached over 80%. However, there were few studies on cancer screening financing mechanisms and user out-of-pocket cost levels in China. Moreover, how to ensure the participation rate of users when paying needs further empirical research.
Contrary to other studies (17, 40, 42), the mortality reduction in this study showed an insignificant effect, and the 30% reduction of death risk was not statistically significant. There were probably two reasons. First, all respondents in this study were rural residents aged 40–70, and they were far less concerned about reducing the risk of cancer deaths than younger generations. Second, the respondents did not understand the concept of death risk at all. Nevertheless, only a minority (3.44%) of respondents failed the test of rationality, suggesting that most respondents understand the DCE questions. In addition, previous studies on colorectal cancer screening preference concluded that characteristics associated with accuracy (sensitivity and specificity) seemed to be more important than those related to screening procedures (45–47). However, the accuracy attribute was not used in this study since endoscopy as the gold standard of UGC screening (an accuracy rate of more than 95%) had been used widely in China.
This study is characterized by several strengths. First, our sample size of 1,000 was substantially larger than the recommended at least 20 respondents per version by the DCE User’s Guide (37), which makes our results more representative and sufficient to provide references for other countries. In addition, an opt-out option was included, since it better reflects actual screening participation and prevents overestimation of screening uptake. Finally, this is the first DCE study on UGC endoscopic screening in a developing country. In a global context, these results are important for optimizing future endoscopic screening strategies in light of the impact of the high morbidity and mortality of UGC, especially in developing nations.
Several limitations need to be discussed. First, only five attributes were included in the DCE to simplify the choice tasks. Thus, not all aspects of the UGC screening test were captured in this DCE. In the design stage, we conducted a literature review, expert interviews, and two focus groups to identify the five most important attributes that can explain the target population’s preference to the greatest extent. Second, this study is a stated preference, which may be different from revealed preferences (48). The revealed preferences should be examined after implementing the UGC screening programs. Third, our results showed that residents preferred a 45% reduction in death risk than 60%. In the context of this study, it is difficult for us to determine exactly whether the phenomenon is due to the rural residents’ lack of understanding or they do not care about morality reduction, and further research is needed to explore.
In conclusion, residents were positive for UGC screening, and their participation rate was greatly affected by the implementation of painless endoscopy. For maximizing the population uptake rate, an optimal UGC endoscopic screening with shorter screening intervals, lower out-of-pocket costs, painlessness, follow-up, and higher UGC mortality reduction should be implemented according to resources’ availability. Our data provided some insights for clinicians and policymakers to develop screening programs with a higher population uptake.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Institutional Ethical Review Board of Shandong Cancer Hospital and Institute (Reference No. SDTHEC201909001). The patients/participants provided their written informed consent to participate in this study.
Author contributions
RL, study concept and design, data collection, data entry, data interpretation, and statistical analysis, drafting of manuscript, and critical revision of the manuscript. YHL, critical revision of the manuscript. YFL and WW, data collection, data entry, and suggestion with revision of the manuscript. CS, QZ, XW, and JW, suggestion with revision of the manuscript. NZ, study concept and design, residents’ recruitment, data interpretation, and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 71904109.
Acknowledgments
The authors wish to thank all of the residents, collaborators, and graduate students who participated in the field survey and discrete choice experiment study, as well as the experts and work staff in sample regions who assisted with participant recruitment and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.917622/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Wang GQ, Wei WQ. The new transformation of upper gastrointestinal cancer screening and early diagnosis and treatment programs: opportunistic screening. China J Prev Med (2019) 11:1084–1085-1086-1087.
3. Zheng X, Mao X, Xu K, Lü L, Peng X, Wang M, et al. Massive endoscopic screening for esophageal and gastric cancers in a high-risk area of China. PLoS One (2015) 10:e0145097. doi: 10.1371/journal
4. Wang GQ, Wei WQ. The technical scheme for screening, early diagnosis and early treatment of upper gastrointestinal cancer (trial version in 2020). Beijing: People's Health Publishing House (2020). 151p.
5. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the national cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev (2011) 12:725–30.
6. Hamashima C. Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol (2018) 48:673–83. doi: 10.1093/jjco/hyy077
7. Wei WQ, Chen ZF, He YT, Feng H, Hou J, Lin DM, et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol (2015) 33:1951–7. doi: 10.1200/JCO.2014.58.0423
8. Chen R, Liu Y, Song G, Li B, Zhao D, Hua Z, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut (2021) 70:251–60. doi: 10.1136/gutjnl-2019-320200
9. Saumoy M, Schneider Y, Shen N, Kahaleh M, Sharaiha RZ, Shah SC. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology (2018) 155:648–60. doi: 10.1053/j.gastro.2018.05.026
10. Xia R, Zeng H, Liu W, Xie L, Shen M, Li P, et al. Estimated cost-effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high-risk areas in China. JAMA Netw Open (2021) 4:e2121403. doi: 10.1001/jamanetworkopen
11. Chang HS, Park EC, Chung W, Nam CM, Choi KS, Cho E, et al. Comparing endoscopy and upper gastrointestinal X-ray for gastric cancer screening in south Korea: a cost-utility analysis. Asian Pac J Cancer Prev (2012) 13:2721–8. doi: 10.7314/apjcp.2012.13.6.2721
12. Peters Y, van Grinsven E, van de Haterd M, van Lankveld D, Verbakel J, Siersema PD. Individuals' preferences for esophageal cancer screening: A discrete choice experiment. Value Health (2020) 23:1087–95. doi: 10.1016/j.jval.2020.03.013
13. Suh M, Song S, Cho HN, Park B, Jun JK, Choi E, et al. Trends in participation rates for the national cancer screening program in Korea, 2002-2012. Cancer Res Treat (2017) 49:798–806. doi: 10.4143/crt.2016.186
14. Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol (2014) 20:13767–74. doi: 10.3748/wjg.v20.i38
15. Zhang QY, Zheng Y, Zhang JY. Application of discrete choice experiment and analytic hierarchy process in decision-making of colorectal cancer screening program. Chin Oncol (2020) 29:177–84.
16. de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ (2012) 21:145–72. doi: 10.1007/s40273-014-0170-x
17. Phisalprapa P, Ngorsuraches S, Wanishayakorn T, Kositamongkol C, Supakankunti S, Chaiyakunapruk N. Estimating the preferences and willingness-to-pay for colorectal cancer screening: an opportunity to incorporate the perspective of population at risk into policy development in Thailand. J Med Econ (2021) 24:226–33. doi: 10.1080/13696998.2021.1877145
18. Pignone MP, Crutchfield TM, Brown PM, Hawley ST, Laping JL, Lewis CL, et al. Using a discrete choice experiment to inform the design of programs to promote colon cancer screening for vulnerable populations in north Carolina. BMC Health Serv Res (2014) 14:611. doi: 10.1186/s12913-014-0611-4
19. Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol (2016) 2:322–9. doi: 10.1007/s10549-021-06131-9
20. Mandeville KL, Lagarde M, Hanson K. The use of discrete choice experiments to inform health workforce policy: a systematic review. BMC Health Serv Res (2014) 14:367. doi: 10.1186/1472-6963-14-367
21. Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health (2011) 14:403–13. doi: 10.1016/j.jval.2010.11.013
22. Cheng J, Pullenayegum E, Marshall DA, Marshall JK, Thabane L. An empirical comparison of methods for analyzing correlated data from a discrete choice survey to elicit patient preference for colorectal cancer screening. BMC Med Res Methodol (2012) 12:15. doi: 10.1186/1471-2288-12-15
23. Coast J, Horrocks S. Developing attributes and levels for discrete choice experiments using qualitative methods. J Health Serv Res Policy (2007) 12:25–30. doi: 10.1258/135581907779497602
24. Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsmann M. Patients' preferences for outcome, process and cost attributes in cancer treatment: A systematic review of discrete choice experiments. Patient (2017) 10:553–65. doi: 10.1007/s40271-017-0235-y
25. Vallejo-Torres L, Melnychuk M, Vindrola-Padros C, Aitchison M, Clarke CS, Fulop NJ, et al. Discrete-choice experiment to analyse preferences for centralizing specialist cancer surgery services. Br J Surg (2018) 105:587–96. doi: 10.1002/bjs.10761
26. Saengow U, Birch S, Geater A, Chongsuwiwatvong V. Willingness to pay for colorectal cancer screening and effect of copayment in southern Thailand. Asian Pac J Cancer Prev (2018) 19:1727–34. doi: 10.22034/APJCP.2018.19.6.1727
27. Johnson FR, Yang JC, Reed SD. The internal validity of discrete choice experiment data: A testing tool for quantitative assessments. Value Health (2019) 22:157–60. doi: 10.1016/j.jval.2018.07.876
28. Li HQ, Xue H, Yuan H, Wan GY, Zhang XY. Preferences of first-degree relatives of gastric cancer patients for gastric cancer screening: a discrete choice experiment. BMC Cancer (2021) 21:959. doi: 10.1186/s12885-021-08677-9
29. Guo L, Zhang S, Liu S, Zheng L, Chen Q, Cao X, et al. Determinants of participation and detection rate of upper gastrointestinal cancer from population-based screening program in China. Cancer Med (2019) 8:7098–107. doi: 10.1002/cam4.2578
30. Bulamu NB, Chen G, Bright T, Ratcliffe J, Chung A, Fraser RJL, et al. Preferences for surveillance of barrett's oesophagus: a discrete choice experiment. J Gastrointest Surg (2019) 23:1309–17. doi: 10.1007/s11605-018-4049-6
31. Cheung GW, Rensvold RB. Testing factorial invariance across groups: A reconceptualization and proposed new method. J Management (2016) 25:1–27. doi: 10.1177/014920639902500101
32. Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Mar Res (1996) 33:307–17. doi: 10.1177/002224379603300305
33. de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient (2015) 8:373–84. doi: 10.1007/s40271-015-0118-z
34. Orme B. Sample size issues for conjoint analysis studies Sequim: Sawtooth software technical paper. Sequim,WA: Sawtooth Software (1998) p.6–9
35. Johnson R, Orme B. Getting the most from CBC. Sequim sawtooth software research paper series. . Sequim,WA: Sawtooth Software (2003). 7 p.
36. Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR conjoint analysis good research practices task force. Value Health (2016) 19:300–15. doi: 10.1016/j.jval.2016.04.004
37. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics (2008) 26:661–77. doi: 10.2165/00019053-200826080-00004
38. Train K. Mixed logit with bounded distributions of correlated partworths. Dordrecht: Springer (2005) p. 5–8.
39. Peters Y, Siersema PD. Public preferences and predicted uptake for esophageal cancer screening strategies: A labeled discrete choice experiment. Clin Transl Gastroenterol (2020) 11:e00260. doi: 10.14309/ctg.0000000000000260
40. Hendry G, North D, Zewotir T, Naidoo RN. The application of subset correspondence analysis to address the problem of missing data in a study on asthma severity in childhood. Stat Med (2014) 33:3882–93. doi: 10.1002/sim.6189
41. Marshall DA, Johnson FR, Kulin NA, Ozdemir S, Walsh JM, Marshall JK, et al. How do physician assessments of patient preferences for colorectal cancer screening tests differ from actual preferences? a comparison in Canada and the united states using a stated-choice survey. Health Econ (2009) 18:1420–39. doi: 10.1002/hec.1437
42. Nayaradou M, Berchi C, Dejardin O, Launoy G. Eliciting population preferences for mass colorectal cancer screening organization. Med Decis Making (2010) 30:224–33. doi: 10.1177/0272989X09342747
43. Kruijshaar ME, Essink-Bot ML, Donkers B, Looman CW, Siersema PD, Steyerberg EW. A labelled discrete choice experiment adds realism to the choices presented: preferences for surveillance tests for Barrett esophagus. BMC Med Res Methodol (2009) 9:31. doi: 10.1186/1471-2288-9-31
44. González JM, Ogale S, Morlock R, Posner J, Hauber B, Sommer N, et al. Patient and physician preferences for anticancer drugs for the treatment of metastatic colorectal cancer: a discrete-choice experiment. Cancer Manag Res (2017) 9:149–58. doi: 10.2147/CMAR.S125245
45. Bilger M, Özdemir S, Finkelstein EA. Demand for cancer screening services: Results from randomized controlled discrete choice experiments. Value Health (2020) 23:1246–55. doi: 10.1016/j.jval.2020.06.004
46. Howard K, Salkeld G.. Does attribute framing in discrete choice experiments influence willingness to pay? Results from a discrete choice experiment in screening for colorectal cancer. Value Health (2009) 12(2):354–63. doi: 10.1111/j.1524-4733.2008.00417.x
47. Wortley S, Wong G, Kieu A, Howard K. Assessing stated preferences for colorectal cancer screening: a critical systematic review of discrete choice experiments. Patient (2014) 7:271–82. doi: 10.1007/s40271-014-0054-3
Keywords: upper gastrointestinal cancer, endoscopic screening, discrete choice experiment, preference, rural residents
Citation: Liu R, Lu Y, Li Y, Wei W, Sun C, Zhang Q, Wang X, Wang J and Zhang N (2022) Preference for endoscopic screening of upper gastrointestinal cancer among Chinese rural residents: a discrete choice experiment. Front. Oncol. 12:917622. doi: 10.3389/fonc.2022.917622
Received: 11 April 2022; Accepted: 01 July 2022;
Published: 27 July 2022.
Edited by:
Alessandra Buja, University of Padova, ItalyReviewed by:
Lanwei Guo, Henan Provincial Cancer Hospital, ChinaXin Qi, Xi’an Jiaotong University, China
Copyright © 2022 Liu, Lu, Li, Wei, Sun, Zhang, Wang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Zhang, bmFuemhhbmdAdmlwLjEyNi5jb20=
 Ruyue Liu
Ruyue Liu Youhua Lu2
Youhua Lu2 Qianqian Zhang
Qianqian Zhang Xin Wang
Xin Wang Nan Zhang
Nan Zhang