
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 15 November 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.917366
This article is part of the Research TopicReproducibility and Rigour in Cancer EpidemiologyView all 4 articles
Objective: The overexpression of polo-like kinase 1 (PLK-1) has been found in a broad spectrum of human tumors, making it an attractive prognostic tumor biomarker. Nowadays, PLK-1 is considered a cancer therapeutic target with clinical therapeutic value. The aim of the present study was to systematically review the prognostic and therapeutic value of PLK-1 in different malignant neoplasms.
Methods: A systematic literature search of the Cochrane Library, PubMed, Web of Science, and China National Knowledge Internet (CNKI) databases was conducted between December 2018 and September 2022. In total, 41 published studies were screened, comprising 5,301 patients. We calculated the pooled odds ratios (ORs) and corresponding 95%CIs for the clinical parameters of patients included in these studies, as well as the pooled hazard ratios (HRs) and corresponding 95% CIs for 5-year overall survival (OS).
Results: Our analysis included 41 eligible studies, representing a total of 5,301 patients. The results showed that overexpression of PLK-1 was significantly associated with poor OS (HR, 1.57; 95% CI, 1.18–2.08) and inferior 5-year disease-free survival/relapse-free survival ((HR, 1.89; 95% CI, 1.47–2.44). The pooled analysis showed that PLK-1 overexpression was significantly associated with lymph node metastasis, histological grade, clinical stages (p < 0.001 respectively), and tumor grade (p < 0.001). In digestive system neoplasms, PLK-1 overexpression was significantly associated with histopathological classification, primary tumor grade, histological grade, and clinical stages (p = 0.002, p = 0.001, p < 0.0001, respectively). In breast cancer, PLK-1 was significantly associated with 5-year overall survival, histological grade, and lymph node metastasis (p < 0.001, p = 0.003, p < 0.001, respectively). In the female reproductive system, PLK-1 was significantly associated with clinical stage (p = 0.011). In the respiratory system, PLK-1 was significantly associated with clinical stage (p = 0.021).
Conclusion: Our analysis indicates that high PLK-1 expression is associated with aggressiveness and poor prognosis in malignant neoplasms. Therefore, PLK-1 may be a clinically valuable target for cancer treatment.
Cancer remains one of the leading causes of death worldwide, largely because of tumor cells’ unlimited replication potential and ability to resist apoptosis and escape immune destruction (1). The International Agency for Research on Cancer estimated an incidence of about 19.3 million new cancer cases and 10.0 million cancer deaths in 2020 alone (2). One of the most serious problems associated with cancer treatment is multidrug resistance (MDR), is a common cause of chemotherapy failure and cancer recurrence with a very low survival rate (3). Improvements in cancer diagnosis and treatment therefore represent one of the greatest challenges facing researchers in the coming decades.
Polo-like kinase (PLK) is a serine/threonine protein kinase that is widely expressed in eukaryotic cells. It includes five family members (PLK-1, PLK-2, PLK-3, PLK-4, and PLK-5), of which PLK-1 has been the most extensively studied. PLK-1 is key for cell division, mitotic progression, and DNA damage repair (4, 5). In addition, recent research has found that PLK-1 is also related to epithelial–mesenchymal transition, cell death, and the immune system (6). This protein kinase is differentially expressed in a variety of human cancers, and an increasing number of studies have shown that PLK-1 overexpression is associated with tumor progression and patient prognosis (7). To date, several inhibitors of PLK-1, such as BI2536 (8), volasertib (9), onvansertib (10), and rigosertib (11), have been widely used in various tumor studies, and two of them (BI2536 and volasertib) have entered phase II clinical trials (8, 9). PLK-1 inhibition, in combination with other targeted drugs such as cisplatin and paclitaxel, has thus become a new strategy for the treatment of malignant tumors. (6) This phenomenon indicates that PLK-1 is widely present in various types of tumors, suggesting that it will be a clinically valuable therapeutic target.
PLK-1 has been postulated as a potential oncogene. Therefore, the aim of this study was to perform a systematic review of the literature and a meta-analysis to evaluate the prognostic value of PLK-1 expression in different cancer types.
A systematic literature search of the Cochrane Library, PubMed, Web of Science, and China National Knowledge Internet (CNKI) databases was conducted between December 2018 and September 2022. The search terms used were as follows: (“PLK1,” “polo like kinase 1,” “plk1,” “PLK-1,” or “plk-1”) and (“cancer” or “tumor” or “neoplasm” or “carcinoma”) with no subheading or language restrictions. The reference lists of related articles were also reviewed.
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (12). The inclusion criteria for each study were as follows (1): detected PLK-1 expression in human tumor tissues, (2) evaluated the correlation between PLK-1 expression and prognosis in any type of human malignant neoplasm, and (3) provided sufficient data to calculate the odds ratios (ORs) and 95% CIs of clinicopathological information or the hazard ratios (HRs) and 95% CIs related to 5-year overall survival. Studies were excluded for the following reasons: (1) lacked the necessary data to obtain information on clinical parameters and 5-year overall survival; (2) contained older and incomplete data from two articles reporting results from the same study; and (3) case reports, review articles, and experiments on animals or cell lines alone.
Data extracted from each study included the first author; publication year; country of origin; cancer type; cutoff value for PLK-1 expression; patients’ age, gender, and population characteristics; tumor size and status of distant metastasis; and method of sample analysis. Clinicopathological information included lymph node metastasis, primary tumor grade, histological grade, and clinical stages. Prognostic information included 5-year overall survival, which was extracted from studies providing univariate and multivariate data analyses. Alternatively, where these data were not provided directly in the study, HRs were extracted from Kaplan-Meier curves using Engauge Digitizer software, version 5.1, as described by Tierney et al. (13) STATA software, version 15.1 (StataCorp, LP, College Station, TX, USA) was used for the pooled HRs or ORs with each corresponding 95% CI. Study quality was evaluated by the Newcastle-Ottawa Quality Assessment Scale (NOS), with score items classified into three major categories: selection, assessment of outcome, and comparability. Scores of 7 and above were considered to correspond to high study quality.
In this study, the prognostic values of high PLK-1 expression for multiple human cancer prognoses were estimated by HRs and corresponding 95% CIs. An observed HR > 1 implied that patients with high PLK-1 expression had a worse clinical outcome. Pooled ORs and 95% CIs were used to evaluate the association between PLK-1 expression and clinicopathological features. The heterogeneity of the studies was estimated by chi-squared and I2 tests. Where the heterogeneity was not significant (I2 < 50% or p > 0.1), we used the fixed-effect model. In all other cases, random-effects models were applied. Publication bias was estimated by Begg’s and Egger’s tests (14). A p-value of less than 0.05 was considered statistically significant. In each system, no further analysis was performed if the clinicopathological or 5-year overall survival information contained fewer than three references.
Initially, 428 relevant studies were identified according to the search strategy. From there, 387 articles were excluded for not meeting the inclusion criteria (Figure 1). Ultimately, 41 studies were included in this meta-analysis, comprising 29 studies from China, 5 from Germany, 3 from Japan, and 1 each from Spain, England, Australia, and Belgium. In total, 5,301 patients were included in the analysis, representing 3,604 cases from China, 586 from Germany, 445 from Japan, and 666 from the other four countries. The cancer types included hepatocellular carcinoma (HCC), breast cancer (BC), colorectal cancer (CRC), gastric cancer (GC), lung cancer, osteosarcoma, laryngeal neoplasms, ovarian carcinoma, endometrial carcinoma, gallbladder cancer, B-cell lymphoma, malignant glioma, thyroid cancer, pancreatic cancer, and synovial sarcoma (SS). For the detection of PLK-1 expression, quantitative real-time (qRT)-PCR was used in four studies and immunohistochemistry was used in the other 37 studies. The characteristics of all included studies are listed in Table 1.
Analysis of PLK-1 and 5-year overall survival in 18 studies (comprising 2,630 cases) (15–32) suggested that high PLK-1 expression (HR, 1.64; 95% CI, 1.25–2.14; p < 0.0001; Figure 2A) was significantly associated with poor 5-year overall survival in cancer patients. Because the heterogeneity was significant (I2 > 50%, p < 0.1), we grouped the included literature into “Asia” and “non-Asia” categories to find the source of heterogeneity by subgroup analysis. The results showed that the heterogeneity derived mainly from the Asian literature (Figure 2B).
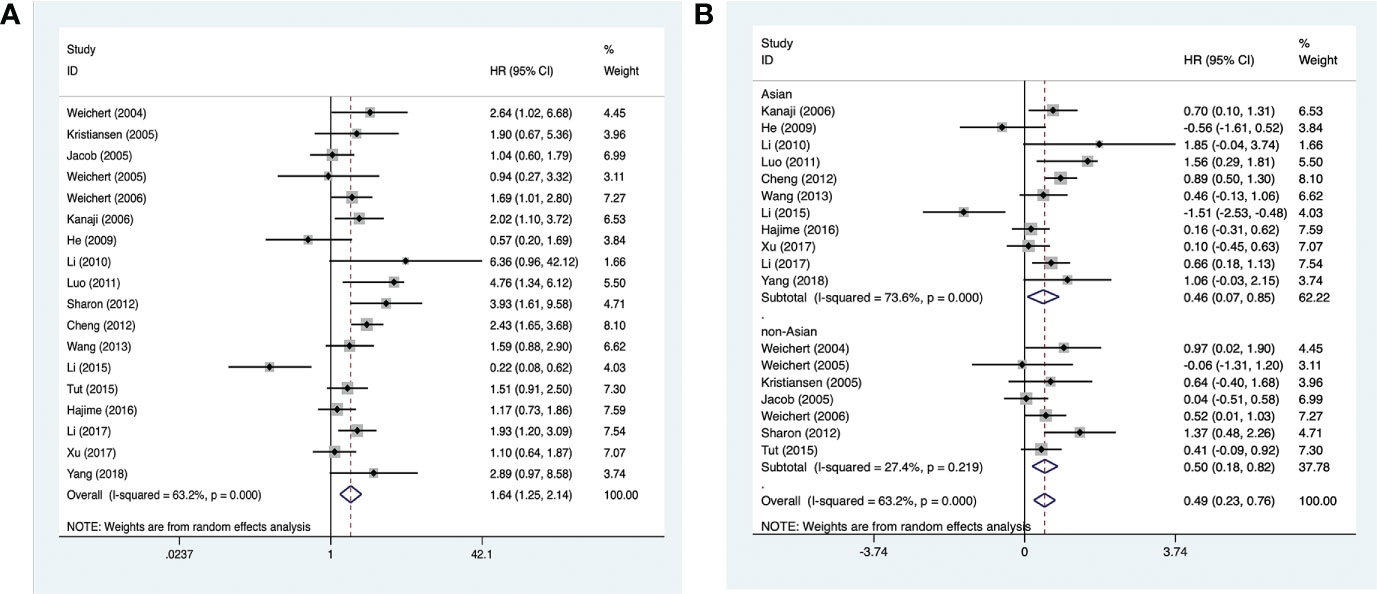
Figure 2 Forest plots of studies evaluating (A) the association between PLK-1 expression and 5-year overall survival and (B) the 5-year overall survival analysis of PLK-1 expression in Asian and non-Asian patients.
Clinicopathological information was extracted from the included 41 studies. Among them, 28 studies (17–19, 21–23, 25–31, 33–47) provided information on the histological grade of the tumor; 19 studies (16, 17, 20, 23, 28–31, 33–35, 39–41, 44–48) provided the primary tumor grade; 28 studies (16, 18, 20, 22, 23, 25, 27–30, 33–42, 44–51) provided information on lymph node metastasis; and 26 studies (15–17, 19, 20, 24, 26, 30–40, 44–48, 51–53) provided information on clinical staging. The results indicated that high expression of PLK-1 was significantly associated with histological grade (OR, 1.94; 95% CI, 1.66–2.27; p < 0.001; Figure 3A), primary tumor grade (OR, 1.96; 95% CI, 1.37–2.81; p < 0.001; Figure 3B), lymph node metastasis (OR, 1.61; 95% CI, 1.20–2.16; p = 0.001; Figure 3C), and clinical stage (OR, 2.53; 95% CI, 1.87–3.42; p < 0.001, Figure 3D). The details of these results are shown in Table 2.
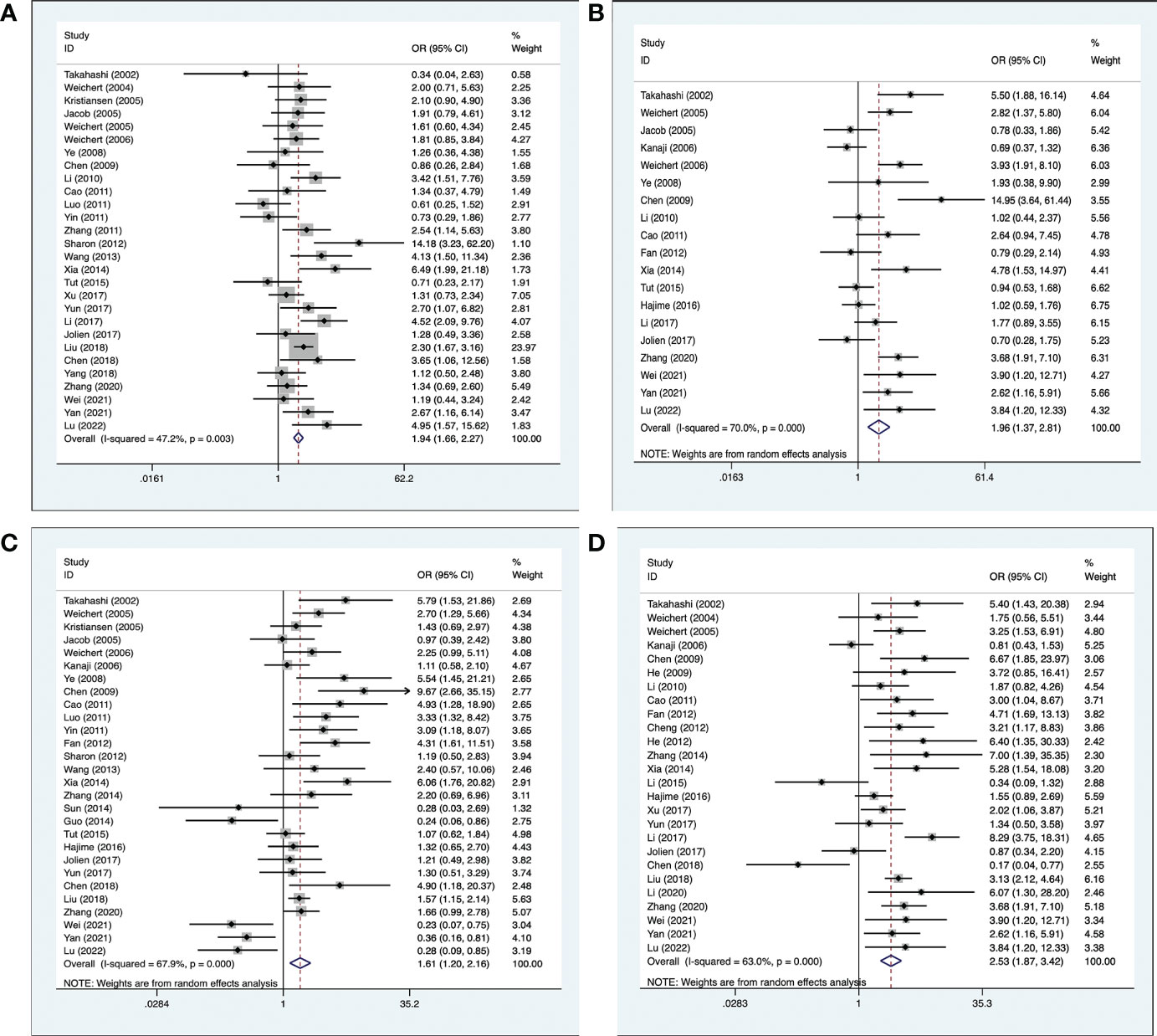
Figure 3 Meta-analysis for the association between PLK-1 expression levels with clinicopathological parameters (A) histological grade; (B) primary tumor grade; (C) lymph node metastasis; (D) clinical stage.

Table 2 Main results for the meta-analysis between PLK-1 and clinicopathological features in multiple cancers.
However, we found no statistically significant relationship between high PLK-1 expression and age (OR, 1.06; 95% CI, 0.76–1.47; p = 0.722; Figure S1A), gender (OR, 1.16; 95% CI, 0.97–1.39; p = 0.102; Figure S1B), tumor size (OR, 1.41; 95% CI, 0.80–2.49; p = 0.232; Figure S1C), or distant metastasis (OR, 1.28; 95% CI, 0.53–3.09; p = 0.577; Figure S1D).
Information on digestive system neoplasms was extracted from 22 studies, comprising 2,517 cases (16–18, 20, 21, 23–25, 28–30, 32, 34, 35, 39, 42, 45–48, 50, 54). Of these, 11 studies (16, 17, 20, 21, 23–25, 28–30, 32) provided information on 5-year overall survival, 14 studies (17, 21, 23, 25, 28–30, 34, 35, 39, 42, 45–47) provided information on the histological grade, 14 studies (16, 17, 20, 23, 28–30, 34, 35, 39, 45–48) provided the primary tumor grade, 16 studies (16, 20, 23, 25, 28–30, 34, 35, 39, 42, 45–48, 55) provided information on lymph node metastasis, and 13 studies (16, 17, 20, 30, 32, 34, 35, 39, 45–48, 56) provided information on clinical staging.
The analysis showed no significant association between high PLK-1 levels and 5-year overall survival (HR, 1.31; 95% CI, 0.97–1.76; p = 0.079; Figure 4A) or lymph node metastasis ((OR, 1.52; 95% CI, 0.94–2.46; p = 0.089; Figure 4B), but the other results indicated that high PLK-1 expression was significantly associated with histological grade (OR, 2.08; 95% CI, 1.35–3.19; p = 0.002; Figure 4C), primary tumor grade (OR, 2.08; 95% CI, 1.35–3.19; p = 0.001; Figure 4D), and clinical stage (OR, 2.90; 95% CI, 1.78–4.72; p < 0.001; Figure 4E). The details of these results are provided in Table 3.

Figure 4 Meta-analysis for the association between PLK-1 expression levels and clinicopathological parameters in cancers of the digestive system. (A) 5-year survival; (B) lymph node metastasis; (C) histological grade; (D) primary tumor grade; (E) clinical stages.
Clinicopathological information on breast cancer was extracted from six studies, comprising 1,656 cases (18, 22, 27, 36–38, 44). An analysis of three of the studies (18, 22, 27) suggested that high PLK-1 expression (HR, 3.60; 95% CI, 2.17–5.97; p < 0.001; Figure 5A) was significantly associated with poor 5-year overall survival in breast cancer patients. These seven studies also provided information on the histological grade and lymph node metastasis, and an analysis of these factors indicated that high PLK-1 expression was significantly associated with both histological grade (OR, 2.12; 95% CI, 1.29–3.50; p = 0.003; Figure 5B) and lymph node metastasis (OR, 1.64; 95% CI, 1.31–2.05; p < 0.001; Figure 5C).
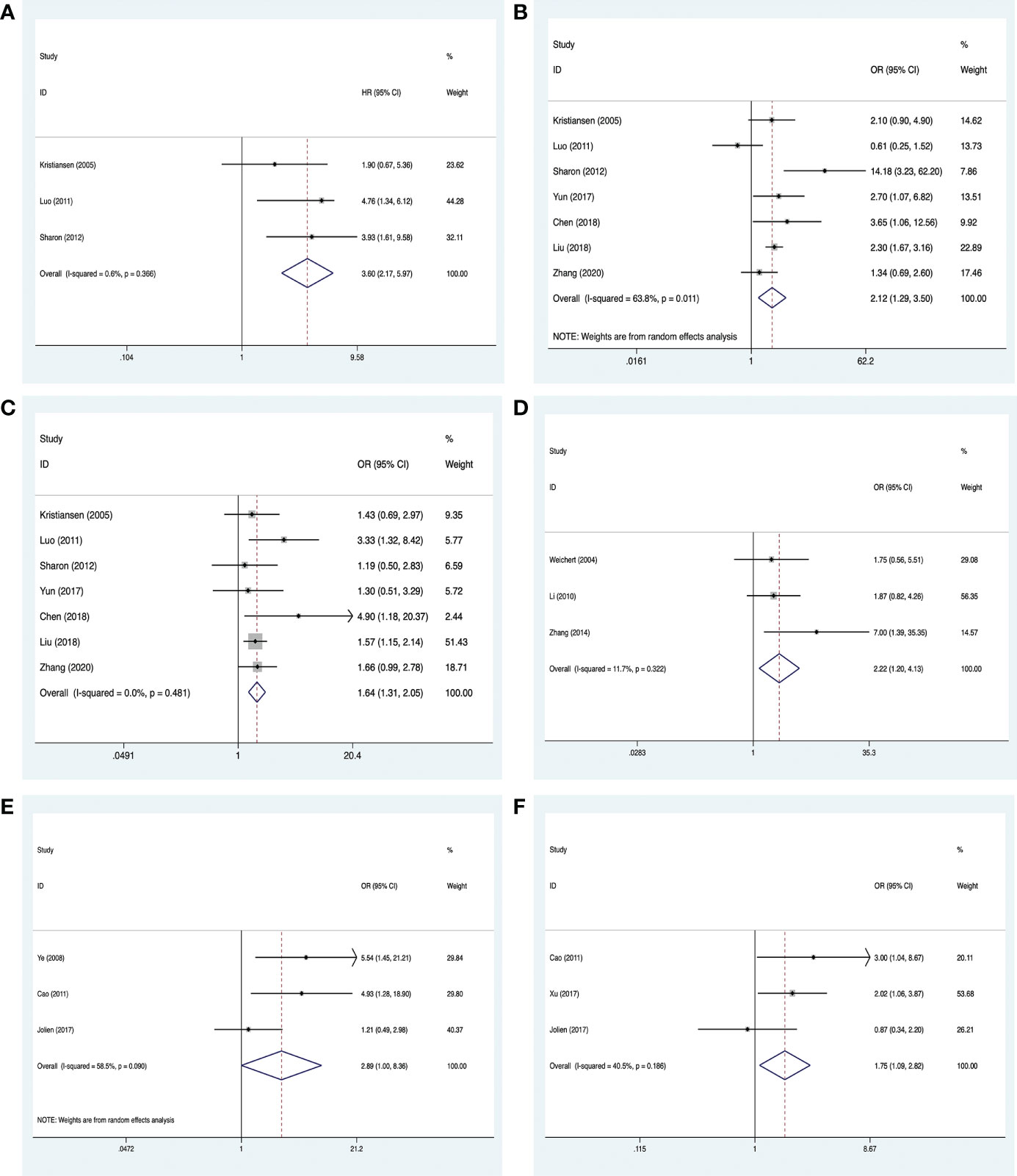
Figure 5 Meta-analysis for the association between PLK-1 expression levels, breast cancer, and cancers of the respiratory system and female reproductive system. (A) 5-year overall survival for breast cancer; (B) histological grades in breast cancer; (C) lymph node metastasis in breast cancer; (D) clinical stages in cancers of the female reproductive system; (E) lymph node metastasis in cancers of the respiratory system; (F) clinical stages in cancers of the respiratory system.
Clinicopathological information on cancers of the female reproductive system was extracted from three studies, comprising 296 cases (26, 31, 51). All three studies provided information on the clinical stage, and our analysis found that high PLK-1 expression was significantly associated with clinical stage (OR, 2.22; 95% CI, 1.20–4.13; p = 0.011; Figure 5D).
Clinicopathological information on respiratory system neoplasms was extracted from four studies, comprising 494 cases (19, 33, 40, 41). Three of these studies (33, 40, 41) provided information on lymph node metastasis, and three (19, 33, 40) provided information on the clinical stage. The results of our analysis indicated that high PLK-1 expression was not associated with lymph node metastasis (OR, 2.89; 95% CI, 1.00–8.36; p = 0.05; Figure 5E), but was associated with clinical stage (OR, 1.75; 95% CI, 1.09–2.82; p = 0.021; Figure 5F). The lack of a significant association with lymph node metastasis may be due to the relatively small number of included studies. The details of these results are shown in Table 3.
A sensitivity analysis of the 5-year overall survival studies was conducted by skipping one article per round to evaluate the influence of each data set on high PLK-1 expression and the pooled HRs. The results indicated that there no single study had a disproportionate effect on the combined HRs (Figure 6). Additionally, there was no obvious publication bias among all the analyses of PLK-1 expression and 5-year overall survival. The results of Begg’s test for each analysis are presented in Table 2.
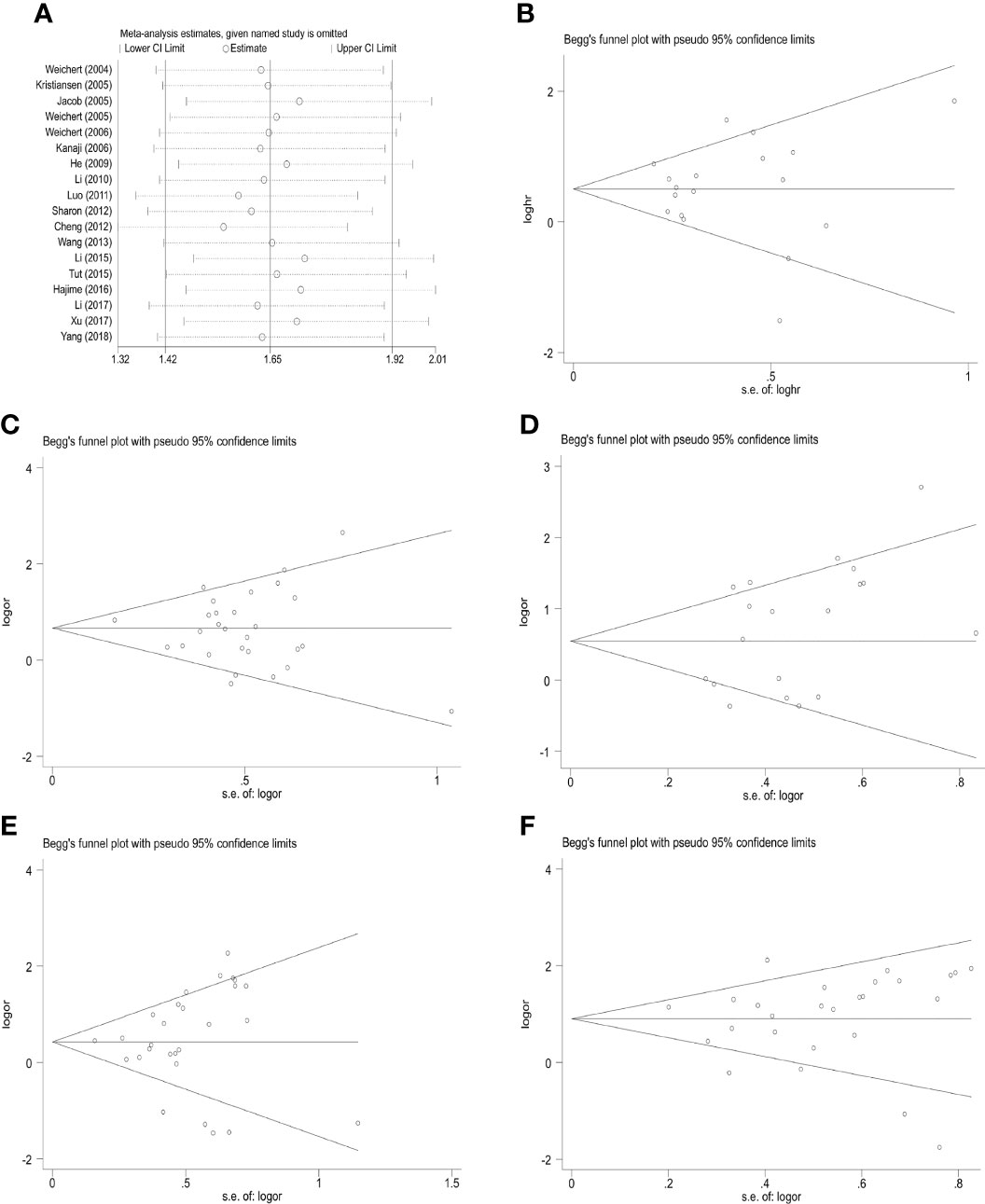
Figure 6 Sensitivity analysis and funnel plot for publication bias testing of the PLK-1 analyses and the clinicopathological parameters of cancer patients. (A) sensitivity analysis for 5-year overall survival; (B) publication bias plots for analyses of 5-year overall survival; (C) histological grade; (D) primary tumor grade; (E) lymph node metastasis; (F) clinical stages.
A sensitivity analysis and an analysis of possible publication bias confirmed the stability of our results. The random-effects model was also used to reduce the impact of heterogeneity on our results.
Prompt, precise, personalized treatment of malignant tumors is critical for improving survival rates and clinical management. PLK-1 has been identified as a treatment target due to its overexpression in various cancers, such as lung, gastric, breast, and colorectal cancer, as well as in other malignancies (52), and its carcinogenic oncogene functions have been confirmed. Some studies have shown that PLK-1 is highly expressed in synovial sarcoma and can promote its occurrence and development (53). On the basis of this extensive literature, PLK-1 has been regarded as a promising prognostic factor in multiple cancers.
We found that there was no statistical significance between PLK-1 expression and 5-year overall survival in digestive system cancers, which was inconsistent with the conclusions of the study by Lin et al. (57), which found that increased expression of PLK-1 was significantly correlated with the survival rate of gastric cancer patients. As a potential prognostic marker of gastric cancer, PLK-1 functions through the MEK-ERK pathway (58).
We also reviewed and compared the results of the meta-analyses of PLK-1 in breast cancer and found that the high PLK-1 expression was significantly different from the tumor size (59). Nevertheless, PLK-1 may become a therapeutic target in the study of triple-negative breast carcinoma (60). The reason for this inconsistent conclusion may be due to the expansion of the research scope, from one cancer to the whole system, leading to differences in the final results. However, in the published meta-analyses on PLK-1, prognoses and pathological stages are affected by the high expression of PLK-1, which is consistent with the results of this study (57, 59).
Several recent investigations have confirmed that PLK-1 plays an important role in cell cycle events, DNA damage repair, epithelial–mesenchymal transition (EMT), and autophagy. During the cell cycle, it is a regulator of mitotic entry and cytokinesis in tumor cells. The significance of PLK-1 in tumor cells is reflected in that its expression often increases when mitosis enters the S phase, reaches its peak in the M phase, and decreases rapidly after mitosis (61). In addition, PLK-1 regulates the initiation and cessation of mitosis by regulating the activities of the cyclinB1/CDK1 complex and APC/C (62, 63). By inhibiting PLK-1 expression, tumor cell mitosis can be arrested in the G2/M phase. Inhibiting PLK-1 in this way also promotes the activation of apoptotic proteins Bax and Bak, causes the inactivation of the anti-apoptotic Bcl-2 protein family, and finally activates caspase-3 and caspase-9 to promote tumor cell apoptosis (64). Recent studies have demonstrated that PLK-1 inhibits DNA damage repair through the P53 signaling pathway and also affects transcriptional processes and apoptotic activity (65). Our investigations have also found that PLK-1 phosphorylates cRAF, which induces the MEK/ERK cascade, eventually activating the ZEB1 and ZEB2 transcription factors, leading to the expression of EMT genes (66). Interestingly, the inhibition of PLK-1 led to autophagy induction through mTORC1 dephosphorylation (67). Due to the effect of PLK-1 on the cell cycle and apoptosis, PLK-1 is highly expressed in tumors and affects the development of tumors and the 5-year overall survival of patients.
The deregulation of the PI3K/Akt pathway, which plays a crucial role in human cancers, has been confirmed. Interestingly, PLK-1 is a downstream gene activated by the PI3K/Akt signal pathway. Mao et al. (68) found that combination therapy, especially therapy targeting the PLK-1/PI3K/AKT pathway, may be a feasible approach for the treatment of pancreatic cancer. Meanwhile, Tan et al. (69) confirmed that PLK-1 is a key member of the pdk1-PLK-1-myc pathway and jointly maintains the growth and differentiation of tumor cells (68). Moreover, PLK-1 can also pass the IKKS of the NF-κB signaling pathway, which is involved in the regulation of normal and tumor cell proliferation. (70) These results indicate that PLK-1 is an important regulator of tumor cell growth and proliferation and can also be used as a target for the treatment of malignant tumors. For this reason, targeting PLK-1 through the development of small molecule inhibitors as anticancer drugs has become an area of intense study.
For example, BI2536, an ATP-competitive inhibitor of PLK-1, has been evaluated for patients in the preclinical setting, with promising results (71). Jeong et al. (72) found that the proliferation and migration ability of breast cancer cells was significantly reduced through the use of BI2536 and by activating the cRaf/ERK signaling pathway, which significantly reduced the cells’ EMT capabilities. Meanwhile, inhibition of PLK-1 expression reduced the forming ability of breast cancer cells and the expression level of tumor stem cell marker proteins (c-myc, Sox2, Oct4, b-catenin, etc.).
By using another PLK-1 inhibitor, BI6727, Dang et al. (73) inhibited PLK-1 expression and caused obvious arrest of the gastric cancer cell cycle in the G2 phase. Moreover, the proliferation and migration of the gastric cancer cells were significantly decreased. BI6727 has therefore been shown to be highly efficacious in inducing tumor regression.
Poloxin and thymoquinone are selective PLK-1 inhibitors targeting the polo-box domain of PLK-1. They can block the correct orientation of PLK-1, thereby preventing cancer cell mitosis (74). Zhao et al. (71) silenced PLK-1 expression by using siRNA, which significantly inhibited the proliferation of esophageal squamous cell carcinoma cells and promoted apoptosis. In addition, clinical trials have shown that using the PLK-1 inhibitors, including BI2536 and volasertib, in combination with decitabine has made some progress against leukemia type 1B (6).
Although this meta-analysis included 41 studies and enrolled 5,301 cancer patients overall, several inherent limitations still exist. First, variability in the detection of PLK-1 expression and subsequent cutoff value selection introduces a potential source of bias. In practice, the lack of a standardized threshold contributes to potential heterogeneity. Second, several studies detected PLK-1 by qRT-PCR, whereas other studies used immunohistochemistry, leading to methodological differences. Third, as some studies did not provide accurate overall survival data, some of the survival data were indirectly extracted from Kaplan-Meier curves via software. Accordingly, the corresponding HR and 95% CI may lack credibility.
To summarize, PLK-1 provides significant prognostic value in a number of human malignancies. Overexpression of PLK-1 suggests poor prognosis and aggressiveness. Given the complicated regulatory mechanism between PLK-1 and its target genes, further investigation and additional relevant studies are needed to establish the clinical significance of PLK-1 as aprognostic biomarker and potential therapeutic target.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
M-WW, LZ, and CLH contributed the research concept and design. M-WW, L-JP, and YQ were responsible for developing the methodology and for the writing, review, and revision of the paper. NW, JD, and J-MH provided data acquisition, analysis, and interpretation, as well as statistical analysis. YQ provided technical and material support. All authors read and approved the final paper.
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81860471), the international cooperation projects of Shihezi University (grant no. GJHZ201710), the Zhanjiang Science and Technology Development Special Fund Competitive Allocation Project—key projects of disease prevention and control (2021A05145), and the Provincial Science and Technology Special Fund (“College items + task list”) project—the special topic for basic and applied research (2021A05236).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.917366/full#supplementary-material
Supplementary Figure 1 | PLK-1 expression with other information: (A) age; (B) gender; (C) tumor size; (D) distant metastases.
1. Iqbal J, Abbasi B, Ahmad R, Mahmood T, Kanwal S, Ali B, et al. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2018) 108:752–6. doi: 10.1016/j.biopha.2018.09.096
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2020) 70(4):313. doi: 10.3322/caac.21609
3. Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
4. van Vugt M, Medema R. Checkpoint adaptation and recovery: back with polo after the break. Cell Cycle (Georgetown Tex) (2004) 3(11):1383–6. doi: 10.4161/cc.3.11.1248
5. Zitouni S, Nabais C, Jana S. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol (2014) 15(7):433–52. doi: 10.1038/nrm3819
6. Chiappa M, Petrella S, Damia G, Broggini M, Guffanti F, Ricci F. Present and future perspective on PLK1 inhibition in cancer treatment. Front Oncol (2022) 12:903016. doi: 10.3389/fonc.2022.903016
7. Lu L, Yu X. The balance of polo-like kinase 1 in tumorigenesis. Cell division (2009) 4:4. doi: 10.1186/1747-1028-4-4
8. Awad M, Chu Q, Gandhi L, Stephenson J, Govindan R, Bradford D, et al. An open-label, phase II study of the polo-like kinase-1 (Plk-1) inhibitor, BI 2536, in patients with relapsed small cell lung cancer (SCLC). Lung Cancer (Amsterdam Netherlands) (2017) 104:126–30. doi: 10.1016/j.lungcan.2016.12.019
9. Ottmann OG, Müller-Tidow C, Krämer A, Schlenk RF, Lübbert M, Bug G, et al. Phase I dose-escalation trial investigating volasertib as monotherapy or in combination with cytarabine in patients with relapsed/refractory acute myeloid leukaemia. Br J haematology (2019) 184(6):1018–21. doi: 10.1111/bjh.15204
10. Weiss GJ, Jameson G, Hoff DDV, Valsasina B, Davite C, Giulio CD, et al. Phase I dose escalation study of NMS-1286937, an orally available polo-like kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Investigational New Drugs (2018) 36(1):85–95. doi: 10.1007/s10637-017-0491-7
11. Navada SC, Fruchtman SM, Odchimar-Reissig R, Demakos EP, Petrone ME, Zbyszewski PS, et al. A phase 1/2 study of rigosertib in patients with myelodysplastic syndromes (MDS) and MDS progressed to acute myeloid leukemia. Leukemia Res (2018) 64:10–6. doi: 10.1016/j.leukres.2017.11.006
12. Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London England) (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
13. Tierney J, Stewart L, Ghersi D. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
14. Egger M, Davey Smith G, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed) (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
15. Cheng M, Wang B, Weng Z. Clinicopathological significance of polo-like kinase 1 (PLK1) expression in human malignant glioma. Acta histochemica (2012) 114(5):503–9. doi: 10.1016/j.acthis.2011.09.004
16. Kanaji S, Saito H, Tsujitani S. Expression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinoma. Oncology (2006) 70(2):126–33. doi: 10.1159/000093003
17. Li H, Wang H, Sun Z, Guo Q, Shi H, Jia Y. The clinical and prognostic value of polo-like kinase 1 in lung squamous cell carcinoma patients: immunohistochemical analysis. Bioscience Rep (2017) 37(4):e28–35. doi: 10.1042/bsr20170852
18. Zhi-Feng LI, Luo MX, Wang BC, Geng HC. Expression of polo-like kinase 1 mRNA and its prognostic value in breast cancer. Prog Modern Biomedicine (2011) 2011(18):3442–5+513. doi: 10.13241/j.cnki.pmb.2011.18.032
19. Xu Y, Shen X, Liu B. The abnormal expression and the clinicopathologic significance of polo-like kinase 1 ( PLK1 ) in adenocarcinoma of the lung. J Southeast University(Medical Sci Edition) (2017) 05):804–10. doi: 10.3969/j.issn.1671-6264.2017.05.025
20. Otsu H, Iimori M, Ando K, Saeki H, Aishima S, Oda Y, et al. Gastric cancer patients with high PLK1 expression and DNA aneuploidy correlate with poor prognosis. Oncology (2016) 91(1):31–40. doi: 10.1159/000445952
21. Yang Y, Wang J, Han Y, Shuang S. Expression of polo - like kinase 1 in liver cancer and its clinical significance. Chin J Cancer Prev Treat (2018) S1):2. doi: CNKI:SUN:QLZL.0.2018-S1-005
22. King S, Purdie C, Bray S. Immunohistochemical detection of polo-like kinase-1 (PLK1) in primary breast cancer is associated with TP53 mutation and poor clinical outcom. Breast Cancer Res BCR (2012) 14(2):R40. doi: 10.1186/bcr3136
23. Tut T, Lim S, Dissanayake I. Upregulated polo-like kinase 1 expression correlates with inferior survival outcomes in rectal cancer. PloS One (2015) 10(6):e0129313. doi: 10.1371/journal.pone.0129313
24. Li W, Liu K, Lin D, Xin X, Wang M. Low expression of polo-like kinase 1 is associated with poor prognosis in liver cancer. Cancer Trans Med (2015) 1(6):196. doi: 10.4103/2395-3977.172858
25. Wang R, Song Y, Xu X. The expression of Nek7, FoxM1, and Plk1 in gallbladder cancer and their relationships to clinicopathologic features and survival. Clin Trans Oncol Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico (2013) 15(8):626–32. doi: 10.1007/s12094-012-0978-9
26. Weichert W, Denkert C, Schmidt M. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer (2004) 90(4):815–21. doi: 10.1038/sj.bjc.6601610
27. Weichert W, Kristiansen G, Winzer K. Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Archiv an Int J Pathol (2005) 446(4):442–50. doi: 10.1007/s00428-005-1212-8
28. Weichert W, Schmidt M, Jacob J. Overexpression of polo-like kinase 1 is a common and early event in pancreatic cancer. Pancreatology Off J Int Assoc Pancreatology (IAP) [et al] (2005) 5:259–65. doi: 10.1159/000085280
29. Weichert W, Ullrich A, Schmidt M. Expression patterns of polo-like kinase 1 in human gastric cancer. Cancer Sci (2006) 97(4):271–6. doi: 10.1111/j.1349-7006.2006.00170.x
30. Weichert W, Kristiansen G, Schmidt M. Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol (2005) 11(36):5644–50. doi: 10.3748/wjg.v11.i36.5644
31. Li S, Shi S, Liu F, Zhang W, Zhao X. The correlation of PLK1 expression with biologic behaver and prognosis in endometrial carcinoma. Oncol (2010) 8(03):295–8+4. doi: 10.3969/j.issn.1672-1535.2010.03.022
32. He ZL, Zhong DW, Zheng H, Miao XY, Hu JX, Wen Y. Expression of gene Plk1 and its relationship with prognosis of hepatocellular carcinoma. World Chin J Digestology (2009) 17(02):146–50. doi: 10.3969/j.issn.1009-3079.2009.02.007
33. Cao H, Shi G. Expression of PLK1 in laryngeal squamous cell carcinoma. Chin Arch Otolaryngology-Head Neck Surg (2011) 18(01):9–11. doi: 10.16066/j.1672-7002.2011.01.005.
34. Chen Z, Dai Q, Lan B, Zhang H, Chen S, Gao A. Expression and significance of PLK1, RAF-1 and ki-67 in gastric cancer. J Fujian Med Univ (2009) 43(02):121–4. doi: 10.7666/d.y950115
35. Xia D. Expression and significance of Nek2, Plk1 and Cdk1 in gastric cancer. Chin J Clin Exp Pathol (2014) 30(11):1266–9. doi: 10.13315/j.cnki.cjcep.2014.11.016
36. Chen X, Jin H. Expression of MEL-18, notch-1 and PLK-1 in invasive breast cancer. Health Res (2018) 38(02):172–4. doi: 10.3969/j.issn.1674-6449.2018.02.015
37. Liu J, Zhou B, Ma Q, Che H, Zhang J. Expression and clinical significance of FOXM1, PLK1, HIF-1α and EZH2 in breast cancer. Med J Chin People's Armed Police Forces (2018) 29(04):353–7. doi: 10.14010/j.cnki.wjyx.2018.04.009
38. Tian Y, Wang YK, Yuan XT, Meng NL, Pathology DO. Expression of Mel-18,Notch-1 and PLK-1 in different breast tissues and their significance. Chin J Diagn Pathol (2017) 24(1):30–33. doi: 10.3969/j.issn.1007-8096.2017.01.008
39. Takahashi T, Sano B, Nagata T. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci (2003) 94(2):148–52. doi: 10.1111/j.1349-7006.2003.tb01411.x
40. VdB J, Christophe D, OdB K. Towards prognostic profiling of non-small cell lung cancer: New perspectives on the relevance of polo-like kinase 1 expression, the TP53 mutation status and hypoxia. J Cancer (2017) 8(8):1441–52. doi: 10.7150/jca.18455
41. Ye M, Zhou T, Lu H. Expression and significance of polo-like kinase 1 in 63 cases of non-small cell lung cancer. Chin J Oncol (2008) 02):46–8. doi: 10.3969/j.issn.1004-0242.2008.02.019
42. Yin L, Chen D, Hu Q. Expression and clinical significance of polo-like kinase 1 and p53 in esophageal squamous cell carcinoma. J Jiangsu University(Medicine Edition) (2011) 21(01):58–61. doi: 10.13312/j.issn.1671-7783.2011.01.016
43. Zhang Z, Zhang G, Kong C. High expression of polo-like kinase 1 is associated with the metastasis and recurrence in urothelial carcinoma of bladder. Urologic Oncol (2013) 31(7):1222–30. doi: 10.1016/j.urolonc.2011.11.028
44. Zhang Y, Li J. Expression and clinical significance of FOXM1, PLK1, HIF-1α and EZH2 in breast invasive ductal carcinoma. Modern Cancer Med (2020) 28(12):2067–71. doi: 10.3969/j.issn.1672-4992.2020.12.016
45. Wei W, Zong L, Cui P, Hu W. Expression of polo-like kinase 1 and murine double microgene-2 in gastric cancer and its clinical significance. Chin J Exp Surg (2021) 38(11):2236–8. doi: 10.3760/cma.j.cn421213-20201224-01466
46. Yan P, Liu P, Shi L, Xu K, Sun X. Expression and clinical significance of metastasis-associated gene 1 and polo-like kinase 1 in colorectal cancer. Chin J Exp Surg (2021) 38(12):2502–4. doi: 10.3760/cma.j.cn421213-20210108-01010
47. Lu W, Zheng L, Ma T, Chen X, Zhu J, Zhong X. Expression of polo-like kinase 1 and cyclin 20 and their relationship with biological behavior of esophageal squamous cell carcinoma. Chin J Exp Surg (2022) 39(04):726–8. doi: 10.3760/cma.j.cn421213-20210907-01260
48. Fan P, Zheng W, Gao B. Expression of PLK1 in esophageal squamous cell carcinoma and its effect on invasion and migration of esophageal cancer cells. Shandong Med J (2012) 52(34):5–7. doi: 10.3969/j.issn.1005-9202.2013.02.027
49. Guo M. Plk1 and p27 expression in thyroid papillary carcinoma and prognostic value. J Modern Oncol (2014).
50. Sun W, Su Q, Cao X, Shang B, Chen A, Yin H, et al. High expression of polo-like kinase 1 is associated with early development of hepatocellular carcinoma. Int J Genomics (2014) 2014:312130. doi: 10.1155/2014/312130
51. Zhang R, Shi H, Fang R, Zhang M, Xia L, Gynecology DO, et al. Expression and significance of PLK1 and P53 in epithelial ovarian cancer. Chin J Clinicians(Electronic Edition) (2014) (8):1432–6. doi: 10.3969/cma.j.issn.1674-0785.2014.08.008
52. He R, Xie L, Zhao Q. Expression and clinical significance of E2F1, BIRC5 and PLK1 in b-cell lymphoma. Chin J Clin Exp Pathol (2012) 28(03):298–301. doi: 10.13315/j.cnki.cjcep.2012.03.007
53. Li Z, Liang W, Zhang Z, Wang N, Meier T, Zou H, et al. Expression of PLK1 in synovial sarcoma and its clinical significance and prognosis. J Shihezi Univ (Natural Sci Edition) (2020) 38(04):496–501. doi: 10.13880/j.cnki.65-1174/n.2020.22.010
54. Cebrián A, Pulgar T, Fernández-Aceñero MJ. Decreased PLK1 expression denotes therapy resistance and unfavourable disease-free survival in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Pathol - Res Pract (2016), 212(12) :1133–7. doi: 10.1016/j.prp.2016.09.012
55. Yang Y, Wang J, Han Y. Expression and clinical significance of polo-like kinase 1 in hepatocellular carcinoma. Chin J Cancer Prev Treat (2018) S1):8–9. doi: 10.16073/j.cnki.cjcpt.2018.s1.005
56. Li W, Peng Y, Lin Q. Expression of PLK1 in primary hepatocellular carcinoma. Lingnan Modern Clinics Surg (2011) 11(03):183–5. doi: 10.3969/j.issn.1009-976X.2011.03.008
57. Lin P, Xiong D, Dang Y. The anticipating value of PLK1 for diagnosis, progress and prognosis and its prospective mechanism in gastric cancer: a comprehensive investigation based on high-throughput data and immunohistochemical validation. Oncotarget (2017) 8(54):92497–521. doi: 10.18632/oncotarget.21438
58. Dang S-C, Fan Y-Y, Cui L, Chen J-X, Qu J-G, Gu M. PLK1 as a potential prognostic marker of gastric cancer through MEK-ERK pathway on PDTX models. Onco Targets Ther (2018) 11:6239–47. doi: 10.2147/ott.S169880
59. Zhang Y, Wu Z, Liu D, Wang M, Xiao G, Wang P, et al. Augmented expression of polo-like kinase 1 indicates poor clinical outcome for breast patients: a systematic review and meta-analysis. Oncotarget (2017) 8(34):57723–32. doi: 10.18632/oncotarget.17301
60. Salama M, Khairy D. Polo-like kinase 1(PLK1) immunohistochemical expression in triple negative breast carcinoma: A probable therapeutic target. Asian Pacific J Cancer Prev APJCP (2021) 22(12):3921–5. doi: 10.31557/apjcp.2021.22.12.3921
61. Petronczki M, Lénárt P, Peters J. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell (2008) 14(5):646–59. doi: 10.1016/j.devcel.2008.04.014
62. Barr A, Gergely F. Aurora-a: the maker and breaker of spindle poles. J Cell Sci (2007) 120:2987–96. doi: 10.1242/jcs.013136
63. Sandison H, Usher S, Karimiani E. PLK1 and YY1 interaction in follicular lymphoma is associated with unfavourable outcome. J Clin Pathol (2013) 66(9):764–7. doi: 10.1136/jclinpath-2013-201461
64. Weiß L, Hugle M, Romero S. Synergistic induction of apoptosis by a polo-like kinase 1 inhibitor and microtubule-interfering drugs in Ewing sarcoma cells. Int J Cancer (2016) 138(2):497–506. doi: 10.1002/ijc.29725
65. Korns J, Liu X, Takiar V. A review of plks: Thinking outside the (polo) box. Mol carcinogenesis (2022) 61(2):254–63. doi: 10.1002/mc.23388
66. Wu J, Ivanov AI, Fisher PB, Fu Z. Polo-like kinase 1 induces epithelial-to-mesenchymal transition and promotes epithelial cell motility by activating CRAF/ERK signaling. eLife (2016) 5:e10734. doi: 10.7554/eLife.10734
67. Tao Y-F, Li Z-H, Du W-W, Xu L-X, Ren J-L, Li X-L, et al. Inhibiting PLK1 induces autophagy of acute myeloid leukemia cells via mammalian target of rapamycin pathway dephosphorylation. Oncol Rep (2017) 37(3):1419–29. doi: 10.3892/or.2017.5417
68. Mao Y, Xi L, Li Q. Regulation of cell apoptosis and proliferation in pancreatic cancer through PI3K/Akt pathway via polo-like kinase 1. Oncol Rep (2016) 36(1):49–56. doi: 10.3892/or.2016.4820
69. Tan J, Li Z, Lee P. PDK1 signaling toward PLK1-MYC activation confers oncogenic transformation, tumor-initiating cell activation, and resistance to mTOR-targeted therapy. Cancer Discovery (2013) 3(10):1156–71. doi: 10.1158/2159-8290.Cd-12-0595
70. Higashimoto T, Chan N, Lee Y-K, Zandi E. Regulation of IκB kinase complex by phosphorylation of γ-binding domain of IκB kinase β by polo-like kinase 1. The Journal of biological chemistry (2008) 283(51):35354–67. doi: 10.1074/jbc.M806258200
71. Frost A, Mross K, Steinbild S, Hedbom S, Unger C, Kaiser R, et al. Phase i study of the Plk1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours. Curr Oncol (Toronto Ont) (2012) 19(1):e28–35. doi: 10.3747/co.19.866
72. Jeong SB, Im JH, Yoon J-H. Essential role of polo-like kinase 1 (Plk1) oncogene in tumor growth and metastasis of tamoxifen-resistant breast cancer. Mol Cancer Ther (2018) 17(4):825–37. doi: 10.1158/1535-7163
73. Dang S, Fan Y, Cui L. PLK1 as a potential prognostic marker of gastric cancer through MEK-ERK pathway on PDTX models. OncoTargets Ther (2018) 11:6239–47. doi: 10.2147/ott.S169880
Keywords: PLK-1, malignant neoplasm, prognosis, survival, meta-analysis
Citation: Wang M-W, Li Z, Chen L-H, Wang N, Hu J-M, Du J, Pang L-J and Qi Y (2022) Polo-like kinase 1 as a potential therapeutic target and prognostic factor for various human malignancies: A systematic review and meta-analysis. Front. Oncol. 12:917366. doi: 10.3389/fonc.2022.917366
Received: 11 April 2022; Accepted: 06 October 2022;
Published: 15 November 2022.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Gagan Chhabra, University of Wisconsin-Madison, United StatesCopyright © 2022 Wang, Li, Chen, Wang, Hu, Du, Pang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Qi, cWl5YW55YW4tMTk5OEAxNjMuY29t; Li-Juan Pang, b2NlYW4xMjM0NTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.