
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 09 August 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.916840
This article is part of the Research TopicPersonalized Radiation Therapy: Guided with Imaging TechnologiesView all 20 articles
Aim: To investigate the value of pretreatment blood biomarkers combined with magnetic resonance imaging (MRI) in predicting the efficacy of neoadjuvant chemoradiotherapy (NCRT) in patients with locally advanced rectal cancer (LARC).
Methods: This study involved patients with LARC who received NCRT and subsequently underwent total mesenteric excision from June 2015 to June 2021 at the First Affiliated Hospital of Soochow University. Patients with incomplete courses of neoadjuvant therapy, comorbidities with other malignancies or diseases that affect the study outcome, and those who underwent unplanned surgery were ultimately excluded. Laboratory data such as albumin, CEA, various blood cell levels, and MRI related data such as tumor regression grade assessed by magnetic resonance imaging (mrTRG) were collected from the included patients one week prior to NCRT. MrTRG is a common clinical imaging metric used to assess the degree of tumor regression in rectal cancer, primarily based on morphological assessment of residual tumor. Furthermore, pretreatment blood biomarkers such as neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), albumin to fibrinogen ratio (AFR), and prealbumin to fibrinogen ratio (PFR) were assessed. The independent variables for pathologic complete response (pCR) to NCRT were determined by univariate and multivariate logistic regression analyses. Receiver operating characteristic (ROC) curve analysis was used to examine the performance of MRI with or without pretreatment blood biomarkers in predicting pCR using DeLong’s method. A nomogram was created and confirmed internally.
Results: Fifty-nine individuals with LARC satisfied the inclusion criteria, among which 23 showed pCR after NCRT. Logistic regression analysis demonstrated that pretreatment CEA (≤ 3 µg/L, OR = 0.151, P = 0.039), NLR (OR = 4.205, P = 0.027), LMR (OR = 0.447, P = 0.034), and PFR (OR = 0.940, P = 0.013) were independent predictors of pCR to NCRT. The AUCs of mrTRG alone and mrTRG plus the above four pretreatment blood biomarkers were 0.721 (P =0.0003) and 0.913 (P <0.0001), respectively. The constructed nomogram showed a C-index of 0.914.
Conclusion: Pretreatment blood biomarkers combined with MRI can help clinical efforts by better predicting the efficacy of NCRT in patients with locally advanced rectal cancer.
According to the global cancer statistics in 2020, colorectal cancer (CRC) is the second deadliest malignant tumor worldwide (1). Rectal cancer accounts for approximately 30% of colorectal cancer cases, and the proportion is increasing annually. In addition, most patients with rectal cancer are already locally advanced at the time of diagnosis and have a poor prognosis.
Since studies have reported the superiority of preoperative chemoradiotherapy over postoperative chemoradiotherapy, the conventional treatment modality of locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (NCRT) followed by total mesenteric excision (TME) (2, 3). In recent years, the total neoadjuvant therapy (TNT) modality and the watch-and-wait (W&W) strategy have received increasing attention (4–7).
However, a significant variation in individual responses to NCRT has been noted during clinical treatment. Approximately 50%-60% of rectal cancer patients show staged shrinkage after NCRT, whereas about 10%-30% show pathologic complete response (pCR) (5). However, approximately one-third of patients show poor sensitivity to chemoradiotherapy, and NCRT efficacy is strongly linked to the prognosis of these patients (8). Therefore, the early prediction of NCRT efficacy is particularly important in the diagnosis and treatment of locally advanced rectal cancer.
Based on the principles of pathological tumor regression grading (pTRG), Patel et al. proposed magnetic resonance imaging for assessing tumor regression grade (mrTRG) in 2011 (9). However, this traditional morphological qualitative assessment based on T2-weighted imaging may fail to predict treatment response when assessing residual tumors (10). Since conventional MRI provides only morphological information, it is difficult to distinguish treatment-induced fibrosis, necrosis, and tumor residuals (11). In contrast, functional MRI such as diffusion-weighted imaging (DWI) can provide information at the molecular level of the tumor (12). DWI indirectly reflects the biology of human tissues by assessing the diffusive motion of water molecules and providing a quantitative index of the apparent diffusion coefficient (ADC). Recent studies have used DWI techniques to assess the efficacy of neoadjuvant therapy in patients with rectal cancer (13, 14). The efficacy of ADC values in predicting the efficacy of neoadjuvant therapy for rectal cancer remains controversial. The reasons for this may be related to factors such as the use of different methods to outline the region of interest (ROI) and different b-values. Therefore, until a uniform standard is reached in clinical as well as scientific research, assessment based on a single imaging image is inevitably a bit subjective.
Several economically feasible blood markers have been explored in recent clinical studies to predict tumor regression response after NCRT, such as neutrophil to lymphocyte ratio (NLR), prognostic nutritional index (PNI), and carcinoembryonic antigen (CEA) (15–23). Some foreign scholars explored whether the combined use of magnetic resonance imaging (MRI) parameters with CEA levels could better predict the efficacy of NCRT than MRI parameters alone. It was found that the combination of mrTRG and CEA improved the AUC value from 0.680 to 0.728 compared to mrTRG alone (24). However, although the performance of MRI parameters in combination with CEA for predicting pTRG improved, it was still unsatisfactory. Therefore, it was natural to question whether more satisfactory results could be obtained using additional and more valuable blood biomarkers in combination with MRI.
Thus, in this study, we aimed to investigate whether combining multiple blood biomarkers with T2WI-based mrTRG could significantly improve the power of MRI in predicting the efficacy of neoadjuvant chemoradiotherapy in patients with LARC. We also established a new model of MRI parameters and multiple blood markers. We have reason to believe that this is the first study to combine multiple blood biomarkers with MRI to predict the efficacy of neoadjuvant chemoradiotherapy in rectal cancer. This study will provide new ideas and methods for the selection of treatment strategies for neoadjuvant chemoradiotherapy in patients with rectal cancer.
This retrospective study initially screened LARC patients who underwent NCRT and subsequent surgery at the First Affiliated Hospital of Soochow University from June 2015 to June 2021. The follow-up period was from the clinical diagnosis of rectal cancer to 2 weeks after TME surgery, encompassing the entire neoadjuvant treatment. The inclusion criteria were as follows: (1) rectal cancer with positive clinical stage T3-T4 or positive lymph nodes as determined by preoperative MRI, without distant metastases; (2) adenocarcinoma of the rectum less than 10 cm from the anal verge as confirmed by pathology of the colonoscopic biopsy specimen; (3) no previous chemotherapy or pelvic radiotherapy experience; (4) complete clinical process information, including laboratory test results within 7 days before the start of NCRT and tumor pathological characteristics; (5) complete imaging information, including rectal MRI images 4 weeks before NCRT and 6-8 weeks after NCRT; and (6) complete resection without positive tumor margins. The standards for exclusion were as follows: (1) incomplete completion of preoperative chemoradiotherapy treatment; (2) evidence of acute and chronic infections, autoimmune diseases, and hematological disorders; (3) palliative surgery or partial resection or emergency surgery; and (4) synchronous malignancies or medical history of other malignancies. This study was approved by the ethics committee of the First Affiliated Hospital of Soochow University.
In this study, all patients received neoadjuvant chemoradiotherapy. Patients received preoperative radiation in the pelvic region in 25 fractions at a dose of 45 Gy, and the original tumor was irradiated with an additional 5.4 Gy in three doses, making the maximum dosage 50.4 Gy (25). Capecitabine was administered at a dose of 825 mg/m2 twice daily from Monday to Friday throughout the radiotherapy period. In the interval after radiotherapy and before surgery, patients received 2 to 3 cycles of neoadjuvant chemotherapy in one of two regimens, the CapeOX (43 cases, 72.9%) and the FOLFOX (16 cases, 27.1%). All patients underwent surgery according to the principle of TME at 4 to 8 weeks after NCRT. Patients were considered for adjuvant chemotherapy 3–4 weeks following surgery.
Pathological response to NCRT was evaluated by two independent pathologists according to the four-tier American Joint Committee on Cancer (AJCC) seventh edition tumor regression grade (TRG) classification. The pathological TRGs (pTRGs) system was defined as follows: pTRG0, no remaining viable cancer cells; pTRG1, single cells or rare residual cancer cells; pTRG2, residual cancer with a desmoplastic response; and pTRG3, minimal evidence of tumor response (26). The pCR was defined as pTRG 0 and the other grades were defined as non-pCR.
All patients underwent rectal MRI 4 weeks before and 6-8 weeks after NCRT. The assessment of rectal cancer MRI parameters was performed by two radiologists with more than 3 years of experience in rectal cancer MRI staging. T-stage, N-stage, the distance from the anal verge to the lower edge of the tumor, and the status of the circumferential resection margin (mrCRM) were assessed by rectal MRI 4 weeks prior to NCRT. If the distance between the tumor and the mesorectal fascia on MRI was greater than or equal to 1 mm, the case was considered definitive mrCRM (9). The assessment of mrTRG was based on rectal MRI 6-8 weeks after NCRT: grade 1, mucosal or mucosal inferior 1 to 2 mm scar or marked normalization of the rectal wall; grade 2, dense fibrosis with no obvious residual tumor; grade 3, more than 50% of fibrosis or mucus and visible residual tumor signal; grade 4, minimal fibrosis/mucinous degeneration, mostly tumor; and grade 5, same as a primary tumor or tumor progression (10). Like pTRGs, mrTRGs were classified into good response and poor response, with mrTRG 1 or 2 and mrTRG 3, 4, or 5 indicating good and poor response, respectively.
All patients underwent routine blood tests, liver and kidney function tests, coagulation tests, and serum CEA tests. All blood specimens were tested in our laboratory one week before the start of NCRT. The pretreatment blood biomarkers were calculated as follows:
In previous studies different cut-off values have been used for these biomarkers. For example, for NLR, Braun LH et al. adopted a cut-off value of 4.06, and neoadjuvant therapy tended to work well in patients with rectal cancer with pre-treatment NLR below 4.06 (15). However, some studies have also used 2.0 and 3.05 as cut-off values for NLR (22, 23). And there are also some scholars who did not convert these biomarkers into dichotomous variables (20, 21). Therefore, our study used continuous variables for all biomarkers.
The Statistical Package for the Social Sciences, version 20.0, was used to conduct statistical analyses (IBM SPSS Inc., Chicago, USA). Continuous variables were analyzed using the Student’s t-test for normally distributed variables or the Mann-Whitney U test for skewed distributed variables. Categorical variables were assessed using the Chi-square test or Fisher’s exact test (if the expected frequencies were <5). A univariate and multivariate logistic regression model was utilized to determine predictive factors for pCR to NCRT. DeLong’s technique was used to compare the areas under the curves (AUC) based on receiver operating characteristic (ROC) curves analysis of mrTRG alone versus the combination of mrTRG and pretreatment blood biomarkers for the prediction of pCR. A predictive nomogram was developed using R version 4.1.3 (R-Project, Institute of Statistics and Mathematics, Vienna, Austria) based on the findings of multivariate logistic regression analysis. The nomogram’s performance was evaluated using internal validation and AUC. Furthermore, the Harrell’s concordance index (C-index) was calculated to evaluate the discriminating capability of the nomogram. A two-sided P < 0.05 was considered statistically significant.
From June 2015 to June 2021, we initially enrolled 100 LARC patients to receive neoadjuvant therapy, with 59 patients ultimately completing the study (see Figure 1). Patients who have not completed their course of chemoradiotherapy (n = 13), those who received concomitant targeted agents during NCRT (n = 9), those with incomplete laboratory records or imaging data (n = 9), and those with metastases to other organs (n = 10), were excluded from the study. Ultimately, 59 patients who satisfied all criteria were included in the study. All patients included underwent rectal MRI for clinical staging and assessment of treatment outcome before and after neoadjuvant chemoradiotherapy. For all 59 patients, the pTRGs according to each mrTRG are displayed in Table 1. Patient characteristics are summarized in Table 2. Among the 59 patients, pCR (pTRG 0) was observed in 23 (29.0%) patients, pTRG 1 in 16 (27.1%), pTRG 2 in 8 (13.6%) and pTRG 3 in 12 (20.3%). The median pretreatment biomarkers levels of serum albumin, prealbumin, hemoglobin, NLR, PLR, LMR, SII, PNI, AFR, and PFR were 41.1 g/L (range,33.1-49.2), 222.6 mg/L (range,149.9-345.5), 136 g/L (range,73-147), 2.65 (range,1.46-4.55), 133.85 (range,50.23-562.50), 3.59 (range,2.03-9.00), 552.3 (range,241.66-1644.78), 41.11 (range,33.11-49.21), 18.19 (range,14.13-22.17), and 95.95 (range, 60.69-157.05), respectively. The number of patients with CEA >3µg/L was 41(69.5%).
The relationships between patient demographics, tumor features, pretreatment biomarkers and MRI parameters, and pCR are shown in Table 2. Clinical biomarkers such as gender, age, BMI, the distance from the anal verge to the lower edge of the tumor, T stage, N stage, and mrCRM, and pretreatment blood biomarkers such as serum albumin, serum prealbumin, hemoglobin, PLR, SII, and PNI were not associated with pCR to NCRT (all P > 0.05).
According to the univariate analysis, mrTRG (1-2 vs. < 3-5, OR = 0.129, 95% CI 0.038-0.432, P = 0.001), pretreatment CEA level (≤ 3.0 vs. > 3.0, OR = 0.183, 95% CI 0.055-0.608, P = 0.006), pretreatment NLR (OR = 2.648, 95% CI 1.202-5.834, P = 0.016), pretreatment LMR (OR = 0.581, 95% CI 0.396-0.851, P < 0.001), pretreatment AFR (OR = 0.674, 95% CI 0.456-0.997, P = 0.048), and pretreatment PFR (OR = 0.969, 95% CI 0.941-0.998, P = 0.036) were significantly associated with pCR to NCRT (Table 3). Multivariate Logistic regression analysis demonstrated that mrTRG (1-2 vs. < 3-5, OR = 0.074, 95% CI 0.011-0.499, P = 0.007), pretreatment CEA level (≤ 3.0 vs. > 3.0, OR = 0.151, 95% CI 0.025-0.913, P = 0.039), pretreatment NLR (OR = 4.205, 95% CI 1.175-15.052, P = 0.027), pretreatment LMR (OR = 0.447, 95% CI 0.212-0.939, P < 0.034), and pretreatment PFR (OR = 0.940, 95% CI 0.896-0.987, P = 0.013) were independent predictors of pCR to NCRT (Table 3).
Overall, the pCR group had higher LMR and PFR, but lower NLR and CEA levels.
Figure 2 shows the ROCs for mrTRG alone (Figure 2A) and mrTRG plus pretreatment blood biomarkers for predicting pCR (Figures 2B-D). The AUCs for mrTRG plus biomarkers for predicting pCR were significantly larger than that for mrTRG alone (Table 4).
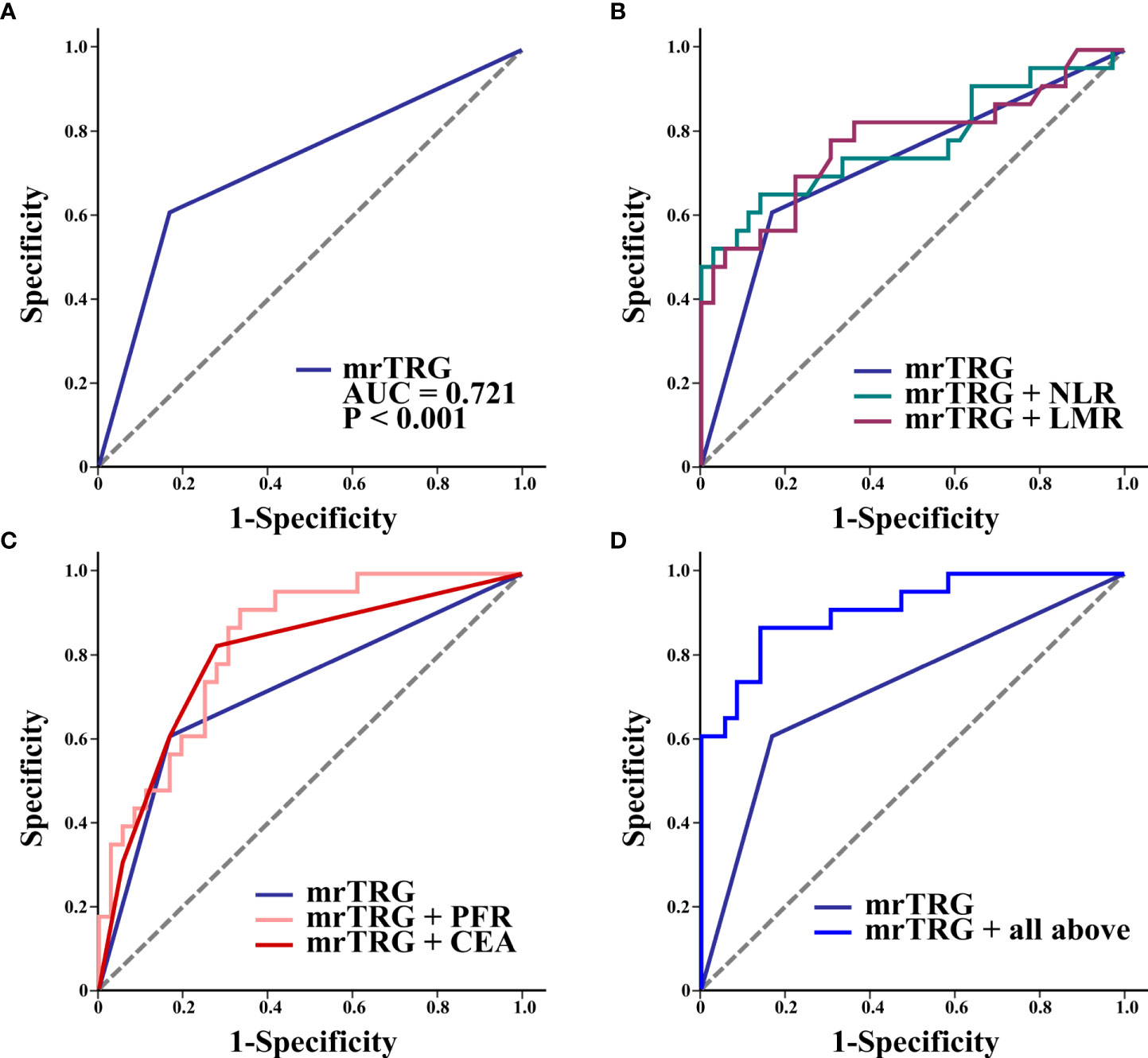
Figure 2 ROC curves of mrTRG (1-2 vs. 3-5) alone (A) and mrTRG plus pretreatment biomarkers (NLR, LMR, PFR, CEA and all above four biomarkers) (B–D) for the prediction of pCR.
Based on the significant predictors in the logistic regression analysis, a nomogram for the prediction of pCR to NCRT in LARC patients was developed, as shown in Figure 3A. The predicted probability of pCR for NCRT could be easily obtained by adding up the scores of each variable and then drawing a straight line. The patients with higher total scores tended to achieve a higher probability of pCR to NCRT. The internally validated calibration curves revealed good agreement between the predicted and actual probability of pCR to NCRT (Figure 3B). The nomogram performance was verified internally, and it exhibited a C-index of 0.914 (95% CI 0.838-0.988) and AUC of 0.913, as illustrated in Figure 3C.
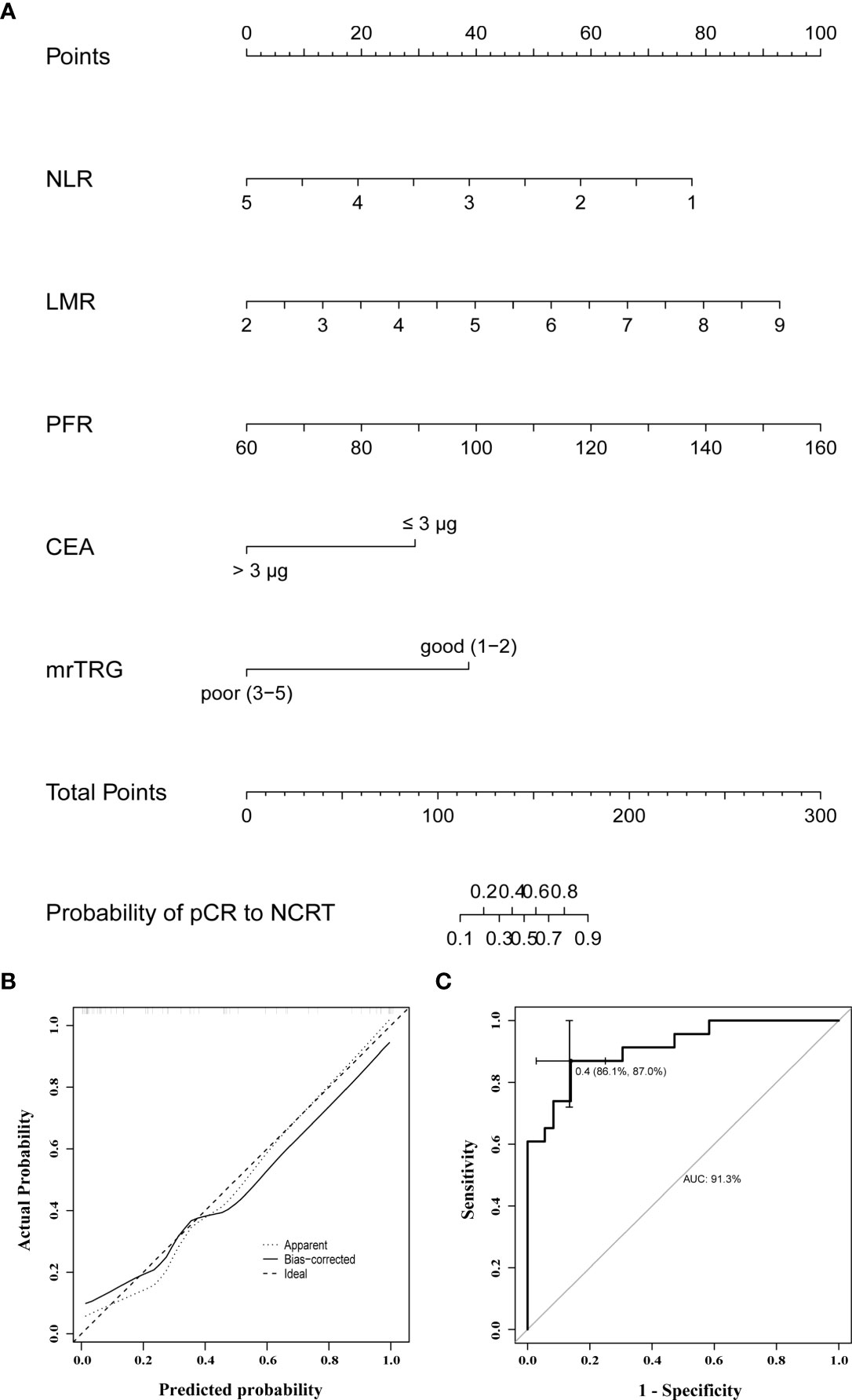
Figure 3 (A) A nomogram for predicting the probability of pCR to NCRT in LARC patients; (B) curves with internal validation for the nomogram; (C) ROC analysis of the nomogram. NCRT, neoadjuvant chemoradiotherapy; pCR, pathologic complete response; mrTRG, tumor regression grade assessed by magnetic resonance imaging; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PFR, prealbumin to fibrinogen ratio; CEA, carcinoembryonic antigen.
The results of this study revealed that 23 (39.0%) of 59 LARC patients who received NCRT achieved pCR. CEA, NLR, LMR, and PFR were significant predictors of pCR, superior to markers such as PLR, SII, and PNI. High values of pretreatment LMR and PFR and low values of NLR and CEA were positively correlated with pCR. High pre-NCRT AFR was positively correlated with pCR in univariate logistic regression analysis, but not in multivariate logistic regression analysis. This discrepancy may be due to the high correlation between PFR and AFR in multivariate regression analysis. In the ROC curves analysis, the AUCs of individual biomarkers (NLR, LMR, PFR, and CEA) in combination with mrTRG were 0.774, 0.778, 0.831, and 0.798, respectively, which were all higher than that of mrTRG alone (AUC of 0.721). Furthermore, expectedly, the AUC of mrTRG in combination with all four biomarkers (NLR, LMR, PFR, and CEA) was the highest (0.913).
Scholars are increasingly recognizing a possible cross-link between systemic inflammatory responses and nutritional risk, as well as tumor-associated immune responses. Various inflammatory cells and inflammatory mediators are important components of the tumor microenvironment. For example, lymphocytes can induce cytotoxicity leading to tumor cell death and inhibit tumor cell proliferation and migration (27, 28). Sustained local and systemic inflammatory responses can be involved in the development, progression and prognosis of many malignancies through various mechanisms such as inhibition of DNA damage and apoptosis by inflammatory cytokines (29, 30). Malignancies can in turn lead to severe nutritional imbalances and even cachexia, directly activating proteolysis and lipolysis in target organs through a variety of pathways, such as pro-inflammatory factors with catabolic effects that can act as mediators of cachexia (31). This catabolism occurs mainly in skeletal muscle, adipose tissue, and myocardium, and its consequences include increased chemotherapy toxicity, complication rates of surgery, and increased mortality (32). In contrast, the cytotoxic effect of chemoradiotherapy causes necrosis of tumor cells and alters the local and systemic inflammatory response, thus increasing the recognition of tumor antigens by the body’s immune system (33). Therefore, early assessment of the sensitivity of patients with malignant tumors to radiotherapy is essential.
In imaging, conventional rectal MRI is a classic tool for clinical assessment of rectal cancer staging and the effectiveness of neoadjuvant therapy. The application of apparent diffusion coefficient (ADC) values from diffusion-weighted imaging (DWI) to predict the tumor regression response after NCRT in rectal cancer has been studied (34–36). Some studies have investigated the performance of maximum standardized uptake values (SUVs) of 18F-FDG PET or its dynamics before and after NCRT in predicting pCR in rectal cancer patients (37, 38). However, many tools and parameters are currently not up to a uniform standard.
In recent years, some inflammatory biomarkers and nutritional biomarkers can directly or indirectly respond to the inflammatory response and nutritional status of the body and have been found to be independent prognostic factors in patients with rectal cancer treated with NCRT (39–43). Most of the previous studies have focused on one or a few biomarkers, exploring their relationship with the efficacy of neoadjuvant chemotherapy in rectal cancer (44–48). However, it is clear that no single biomarker is currently powerful enough to achieve accurate prediction independently, and new models combining blood biomarkers and imaging parameters can achieve better outcomes. In this study, valuable blood biomarkers were filtered and combined with mrTRG to take advantage of the unique advantages of the different parameters as much as possible. A common feature of previous studies is the conversion of the obtained biomarkers from continuous variables to dichotomous variables, thus grouping patients in a simple way. However, it generates the problem of using different cut-off values in different studies. We did not perform simple dichotomization of the raw data in this study. It not only avoids the problem of various cut-off values due to sample differences, but also retains the advantage of continuous variables.
Our study has a few limitations. First, since this is a retrospective study with limited sample size, the possibility of selection bias during data collection cannot be excluded. Second, the blood biomarkers analyzed in this study are non-specific and may be influenced by various physiological or pathological factors. Hence, their values can vary over time. Nevertheless, our study focused only on the predictive role of these blood biomarkers prior to NCRT. Moreover, to analyze the efficacy of NCRT in treating rectal cancer, the final results should be tracked to determine long-term patient outcomes. In this study, the biomarkers’ long-term prognostic ability was not investigated. Thus, further large sample-sized studies are needed to determine these effects.
This study extensively screened a variety of valuable pre-neoadjuvant blood biomarkers, such as CEA, NLR, LMR, and PFR, that could serve as predictors of pathologic complete regression and help improve the performance of MRI in predicting the efficacy of neoadjuvant therapy in patients with locally advanced rectal cancer. Combining pretreatment blood biomarkers with MRI metrics to create a clinical prediction model can effectively predict the efficacy of neoadjuvant therapy and thus help determine the optimal individual treatment regimen for LARC patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The present retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Suzhou, China; approval no. 2022099), with a waiver of informed consent. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SH: design and guidance, study supervision, and critical revision of the manuscript. XS: statistical analysis of the data, interpretation of the significance of the outcome, and manuscript drafting. MZ: software application, and production of partial charts. BS, GC, HY, JC, DW and WG: data acquisition. All authors have made contributions to this article and agree with the final version of the article.
This work was supported by National Science Foundation (NSF) of Jiangsu Province of China grants (BK20191172), Project of Gusu Medical Key Talent of Suzhou City of China (GSWS2020005), and Project of New Pharmaceutics and Medical Apparatuses of Suzhou City of China (SLJ2021007).
The author's thank all staff working in the department of general surgery in the First Affiliated Hospital of Soochow University, Soochow, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med (2004) 351(17):1731–40. doi: 10.1056/NEJMoa040694
3. Song JH, Jeong JU, Lee JH, Kim SH, Cho HM, Um JW, et al. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: A meta-analysis of randomized controlled trials. Radiat Oncol J (2017) 35(3):198–207. doi: 10.3857/roj.2017.00059
4. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol (2019) 37(34):3212–22. doi: 10.1200/JCO.19.00308
5. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (IWWD): An international multicentre registry study. Lancet (2018) 391(10139):2537–45. doi: 10.1016/S0140-6736(18)31078-X
6. Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol (2016) 17(2):174–83. doi: 10.1016/S1470-2045(15)00467-2
7. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2017) 2(7):501–13. doi: 10.1016/S2468-1253(17)30074-2
8. Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol (2012) 30(15):1770–6. doi: 10.1200/JCO.2011.39.7901
9. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol (2011) 29(28):3753–60. doi: 10.1200/JCO.2011.34.9068
10. van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: A systematic review and meta-analysis. Radiology (2013) 269(1):101–12. doi: 10.1148/radiol.13122833
11. Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer (2005) 92(9):1599–610. doi: 10.1038/sj.bjc.6602550
12. Pham TT, Liney GP, Wong K, Barton MB. Functional MRI for quantitative treatment response prediction in locally advanced rectal cancer. Br J Radiol (2017) 90(1072):20151078. doi: 10.1259/bjr.20151078
13. Tang Z, Zhang XY, Liu Z, Li XT, Shi YJ, Wang S, et al. Quantitative analysis of diffusion weighted imaging to predict pathological good response to neoadjuvant chemoradiation for locally advanced rectal cancer. Radiother Oncol (2019) 132:100–8. doi: 10.1016/j.radonc.2018.11.007
14. Delli Pizzi A, Caposiena D, Mastrodicasa D, Trebeschi S, Lambregts D, Rosa C, et al. Tumor detectability and conspicuity comparison of standard b1000 and ultrahigh b2000 diffusion-weighted imaging in rectal cancer. Abdom Radiol (NY) (2019) 44(11):3595–605. doi: 10.1007/s00261-019-02177-y
15. Braun LH, Baumann D, Zwirner K, Eipper E, Hauth F, Peter A, et al. Neutrophil-to-Lymphocyte ratio in rectal cancer-novel biomarker of tumor immunogenicity during radiotherapy or confounding variable? Int J Mol Sci (2019) 20(10):2448. doi: 10.3390/ijms20102448
16. Zhang Y, Liu X, Xu M, Chen K, Li S, Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep (2020) 10(1):8017. doi: 10.1038/s41598-020-64684-z
17. Deng YX, Lin JZ, Peng JH, Zhao YJ, Sui QQ, Wu XJ, et al. Lymphocyte-to-monocyte ratio before chemoradiotherapy represents a prognostic predictor for locally advanced rectal cancer. Onco Targets Ther (2017) 10:5575–83. doi: 10.2147/OTT.S146697
18. Dolan RD, Alwahid M, McSorley ST, Park JH, Stevenson RP, Roxburgh CS, et al. A comparison of the prognostic value of composite ratios and cumulative scores in patients with operable rectal cancer. Sci Rep (2020) 10(1):17965. doi: 10.1038/s41598-020-73909-0
19. Wang YY, Liu ZZ, Xu D, Liu M, Wang K, Xing BC. Fibrinogen-albumin ratio index (FARI): A more promising inflammation-based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol (2019) 26(11):3682–92. doi: 10.1245/s10434-019-07586-3
20. Wang Y, Chen L, Zhang B, Song W, Zhou G, Xie L, et al. Pretreatment inflammatory-nutritional biomarkers predict responses to neoadjuvant chemoradiotherapy and survival in locally advanced rectal cancer. Front Oncol (2021) 11:639909. doi: 10.3389/fonc.2021.639909
21. Li A, He K, Guo D, Liu C, Wang D, Mu X, et al. Pretreatment blood biomarkers predict pathologic responses to neo-CRT in patients with locally advanced rectal cancer. Future Oncol (2019) 15(28):3233–42. doi: 10.2217/fon-2019-0389
22. Sun Y, Huang Z, Chi P. An inflammation index-based prediction of treatment response to neoadjuvant chemoradiotherapy for rectal mucinous adenocarcinoma. Int J Clin Oncol (2020) 25(7):1299–307. doi: 10.1007/s10147-020-01670-5
23. Kim TG, Park W, Kim H, Choi DH, Park HC, Kim SH, et al. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy. Tumori (2019) 105(5):434–40. doi: 10.1177/0300891618792476
24. Yoo GS, Park HC, Yu JI, Choi DH, Cho WK, Park YS, et al. Carcinoembryonic antigen improves the performance of magnetic resonance imaging in the prediction of pathologic response after neoadjuvant chemoradiation for patients with rectal cancer. Cancer Res Treat (2020) 52(2):446–54. doi: 10.4143/crt.2019.261
25. Roels S, Duthoy W, Haustermans K, Penninckx F, Vandecaveye V, Boterberg T, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys (2006) 65(4):1129–42. doi: 10.1016/j.ijrobp.2006.02.050
26. Schrag D, Weiser MR, Goodman KA, Gonen M, Cercek A, Reidy DL, et al. Neoadjuvant FOLFOX-bev, without radiation, for locally advanced rectal cancer. J Clin Oncol (2010) 28(Suppl):15S. doi: 10.1200/jco.2010.28.15_suppl.3511
27. Kim HJ, Choi GS, Park JS, Park S, Kawai K, Watanabe T. Clinical significance of thrombocytosis before preoperative chemoradiotherapy in rectal cancer: Predicting pathologic tumor response and oncologic outcome. Ann Surg Oncol (2015) 22(2):513–9. doi: 10.1245/s10434-014-3988-8
28. Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol (2010) 28(26):4045–51. doi: 10.1200/JCO.2010.27.9992
29. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
30. Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell (2012) 22(5):645–55. doi: 10.1016/j.ccr.2012.09.009
31. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers (2018) 4:17105. doi: 10.1038/nrdp.2017.105
32. Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol (2016) 54:2–10. doi: 10.1016/j.semcdb.2015.09.001
33. Ko EC, Formenti SC. Radiotherapy and checkpoint inhibitors: A winning new combination? Ther Adv Med Oncol (2018) 10:1758835918768240. doi: 10.1177/1758835918768240
34. Bulens P, Couwenberg A, Haustermans K, Debucquoy A, Vandecaveye V, Philippens M, et al. Development and validation of an MRI-based model to predict response to chemoradiotherapy for rectal cancer. Radiother Oncol (2018) 126(3):437–42. doi: 10.1016/j.radonc.2018.01.008
35. Barbaro B, Vitale R, Valentini V, Illuminati S, Vecchio FM, Rizzo G, et al. Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys (2012) 83(2):594–9. doi: 10.1016/j.ijrobp.2011.07.017
36. Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E, et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: Preliminary results. Int J Radiat Oncol Biol Phys (2012) 82(2):863–70. doi: 10.1016/j.ijrobp.2010.12.063
37. Murcia Duréndez MJ, Frutos Esteban L, Luján J, Frutos MD, Valero G, Navarro Fernández JL, et al. The value of 18F-FDG PET/CT for assessing the response to neoadjuvant therapy in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging (2013) 40(1):91–7. doi: 10.1007/s00259-012-2257-y
38. Leccisotti L, Gambacorta MA, de Waure C, Stefanelli A, Barbaro B, Vecchio FM, et al. The predictive value of 18F-FDG PET/CT for assessing pathological response and survival in locally advanced rectal cancer after neoadjuvant radiochemotherapy. Eur J Nucl Med Mol Imaging (2015) 42(5):657–66. doi: 10.1007/s00259-014-2820-9
39. Yang J, Xu H, Guo X, Zhang J, Ye X, Yang Y, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep (2018) 8(1):3044. doi: 10.1038/s41598-018-21093-7
40. Jung SW, Park IJ, Oh SH, Yeom SS, Lee JL, Yoon YS, et al. Association of immunologic markers from complete blood counts with the response to preoperative chemoradiotherapy and prognosis in locally advanced rectal cancer. Oncotarget (2017) 8(35):59757–65. doi: 10.18632/oncotarget.15760
41. An L, Yin W-T, Sun D-W. Albumin-to-alkaline phosphatase ratio as a promising indicator of prognosis in human cancers: Is it possible? BMC Cancer (2021) 21(1):247. doi: 10.1186/s12885-021-07921-6
42. Ishikawa D, Nishi M, Takasu C, Kashihara H, Tokunaga T, Higashijima J, et al. The role of neutrophil-to-lymphocyte ratio on the effect of CRT for patients with rectal cancer. In Vivo (2020) 34(2):863–8. doi: 10.21873/in vivo.11850
43. Li H, Wang H, Shao S, Gu Y, Yao J, Huang J. Pretreatment albumin-to-Fibrinogen ratio independently predicts chemotherapy response and prognosis in patients with locally advanced rectal cancer undergoing total mesorectal excision after neoadjuvant chemoradiotherapy. Onco Targets Ther (2020) 13:13121–30. doi: 10.2147/OTT.S288265
44. Okugawa Y, Toiyama Y, Oki S, Ide S, Yamamoto A, Ichikawa T, et al. Feasibility of assessing prognostic nutrition index in patients with rectal cancer who receive preoperative chemoradiotherapy. JPEN J Parenter Enteral Nutr (2018) 42(6):998–1007. doi: 10.1002/jpen.1041
45. Xiao WW, Zhang LN, You KY, Huang R, Yu X, Ding PR, et al. A low lymphocyte-to-Monocyte ratio predicts unfavorable prognosis in pathological T3N0 rectal cancer patients following total mesorectal excision. J Canc (2015) 6(7):616–22. doi: 10.7150/jca.11727
46. Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today (2012) 42(6):532–5. doi: 10.1007/s00595-011-0061-0
47. Dayde D, Tanaka I, Jain R, Tai MC, Taguchi A. Predictive and prognostic molecular biomarkers for response to neoadjuvant chemoradiation in rectal cancer. Int J Mol Sci (2017) 18(3):573. doi: 10.3390/ijms18030573
Keywords: locally advanced rectal cancer, neoadjuvant chemoradiotherapy, pathological complete response, blood biomarkers, magnetic resonance imaging, prognosis
Citation: Shi X, Zhao M, Shi B, Chen G, Yao H, Chen J, Wan D, Gu W and He S (2022) Pretreatment blood biomarkers combined with magnetic resonance imaging predict responses to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Front. Oncol. 12:916840. doi: 10.3389/fonc.2022.916840
Received: 10 April 2022; Accepted: 19 July 2022;
Published: 09 August 2022.
Edited by:
Yingli Yang, UCLA Health System, United StatesReviewed by:
Marta Zerunian, Sapienza University of Rome, ItalyCopyright © 2022 Shi, Zhao, Shi, Chen, Yao, Chen, Wan, Gu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songbing He, Y2FwdGFpbl9oc2JAMTYzLmNvbQ==
†These authors contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.