
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 24 June 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.916804
 Zhongqin Gong1
Zhongqin Gong1 Shucai Yang2
Shucai Yang2 Minghui Wei3
Minghui Wei3 Alexander C. Vlantis1
Alexander C. Vlantis1 Jason Y. K. Chan1
Jason Y. K. Chan1 C. Andrew van Hasselt1
C. Andrew van Hasselt1 Dongcai Li4
Dongcai Li4 Xianhai Zeng4
Xianhai Zeng4 Lingbin Xue1
Lingbin Xue1 Michael C. F. Tong1*
Michael C. F. Tong1* George G. Chen1*†
George G. Chen1*†The incidence of thyroid cancer was predominant in women, indicating that the sex hormone may have a role in thyroid cancer development. Generally, the sex hormone exerts its function by binding to the correspondent nuclear receptors. Therefore, aberrant of these receptors may be involved in the development of thyroid cancer. Estrogen receptor alpha (ERα) and beta (ERβ), two main estrogen receptors, have been reported to have an important role in the pathogenesis of thyroid cancer. When the ERα and ERβ genes undergo the alternative RNA splicing, some ERα and ERβ isoforms with incomplete functional domains may be formed. To date, several isoforms of ERα and ERβ have been identified. However, their expression and roles in thyroid cancer are far from clear. In this review, we summarized the expressions and roles of ERα and ERβ isoforms in thyroid cancer, aiming to provide the perspective of modulating the alternative RNA splicing of ERα and ERβ against thyroid cancer.
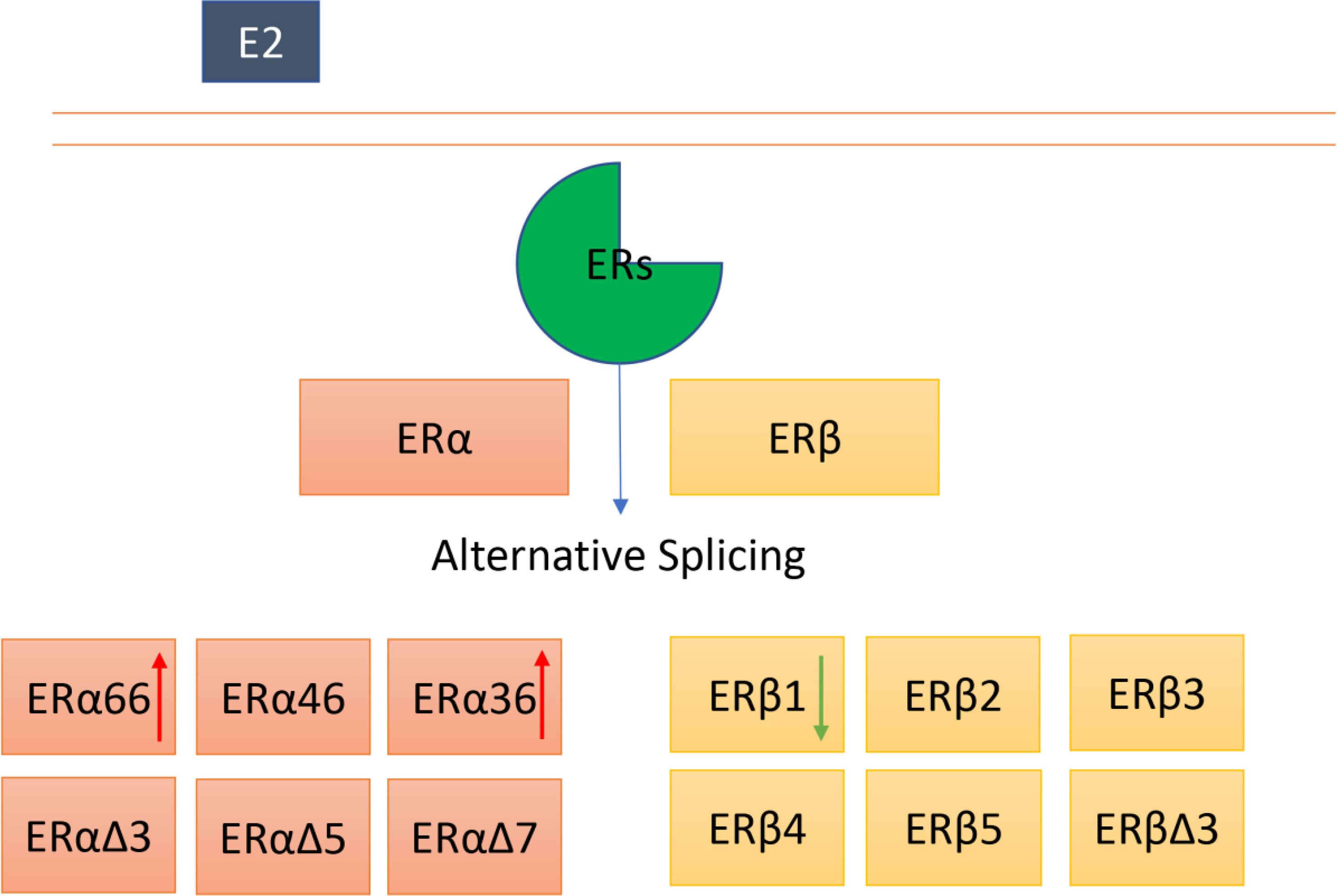
Graphical Abstract ERα and ERβ can undergo alternative splicing, and several isoforms of ERα and Erβ have been identified. The roles of ERα and ERβ full length in thyroid cancer appear to be controversial. Moreover, the exact functions of other isoforms in thyroid cancer remain largely unknown. The red arrow indicates an increased expression, the green arrow represents a deceased expression in thyroid cancer.
The morbidity of thyroid cancer has been rapidly increased during the past decades (1, 2). The increased rate in women was particularly pronounced (2–5). The biased occurrence of thyroid cancer between males and females suggests that the sex hormone may play a central role in the initiation of thyroid cancers or certain types of thyroid cancers. Traditionally, estrogen is the primary female sex hormone mainly responsible for the control of functions of the female reproductive system. In the genomic pathway, estrogen exerts its physiological functions by binding to specific nuclear receptors, the estrogen receptors (ERs), which activate transcriptional processes and/or signaling events and thus control the gene expression (6). ERs can express in both male and female organs/tissues. Therefore, the ERs are critical in the maintenance of health.
Numerous studies have shown that the critical and opposite roles of ERα and ERβ in the development and progression of thyroid cancer. For example, Maura Di Vito et al. reported that the mRNA and protein of ERα, but not ERβ, was upregulated in thyroid cancer, suggesting that ERα has a vital role in thyroid cancer (7). In addition, ERα positive and ERβ negative were associated with a more aggressive phenotype of T1 and T2 thyroid cancer (8, 9). Estrogen induced the metastatic potential of thyroid cancer through ERα and ERβ (10). Yanhong Huang et al. evaluated the expression of ERα and ERβ by immunohistochemical staining, and they reported that estrogen-activated ERα might mediate the stimulatory effect on thyroid cancer growth and progression (11). However, ERβ was negatively correlated with mutant P53, suggesting that ERβ has some inhibitory actions in thyroid cancer (11). ERα is significantly correlated with distant metastases and poorly differentiated thyroid cancer with multicentricity cases, whereas ERβ is significantly associated with lymph node metastases in follicular thyroid cancer (12). These studies have all suggested that ERα and ERβ play an important role in thyroid cancer.
Our previous studies have also illustrated the significance of ERs in thyroid cancer. We found crosstalk between ERs and peroxisome proliferator-activated receptor gamma (PPAR-γ). The interaction between PPAR-γ and ERβ inhibited the proliferation and migration of thyroid cancer (13). ERα induced prosurvival autophagy through generating the reactive oxygen species and activating ERK1/2 in thyroid cancer (14). In addition, we also reported that the differential role of ERα and ERβ in thyroid cancer mediated the production of endogenous PPAR-γ ligand (15). Upregulated ERα/ERβ ratio by PES1 will promote the occurrence and development of papillary thyroid cancer (PTC) (16).
The roles of ERα and ERβ in thyroid cancer appear to be convincing, and the signal pathway of estrogen and estrogen receptors in the development of thyroid cancer has been well reviewed (17). However, there is a controversial result showing the association of the expression of ERα with a good outcome in thyroid cancer. Giacomo sturniolo et al. (18) evaluated the expression of ERα in 203 PTC, and they observed an association between ERα expression and a favorable outcome in their cohort. The cause of such a controversial result remains unknown.
However, it is possible that controversial results are related to ERα antibodies used. For example, the antibody used by Giacomo sturniolo and colleagues is ERα SP1 clone, which was a synthetic peptide derived from the C-terminal of human estrogen receptor (18). The antibody used by Yanhong Huang and colleagues is ERα 1D5 clone, which is a recombinant human estrogen receptor protein (11). The antibodies recognized different regions of ERα protein might result in different expression patterns since several isoforms of ERα have been identified.
The signaling mechanism of ERs and their expression and roles in thyroid cancer have been well-reviewed (6, 17, 19, 20). Therefore, this review focused on the alternative splicing of ERs or isoforms of ERα and ERβ in thyroid cancer (Table 1).
Alternative splicing of protein-coding mRNAs is an essential regulatory mechanism in eukaryotic gene expression that controls the proper function of proteins. The alternative is a fundamental biological process that allows for considerable proteomics diversity and complexity from the limited approximately 20,000 genes (24). However, aberrant alternative splicing may lead to cancer development, and understanding aberrant alternative splicing can facilitate cancer diagnosis and therapy (25, 26). Overall, The abnormal regulation of alternative splicing that can produce multiple different isoforms and diversify protein expression may lead to development of tumors.
Alternative splicing events frequently occur in thyroid cancer. Zenghong Wu et al. found that 45150 alternative splicing events in 10446 thyroid cancer cells derived from 506 patients (27). Furthermore, they found that the alternative splicing signatures were significantly associated with thyroid cancer patients’ overall survival (27). Baoai Han et al. showed that abnormal alternative splicing events might play critical roles in the development and progression of thyroid cancer by participating in changes in molecular structure, homeostasis of the cell environment (28). To date, several isoforms of ERα and ERβ have been reported, given the significance of alternative splicing and ERs isoforms in thyroid cancer, indicating that the expression and role of ERα and ERβ isoforms in thyroid cancer are important. Therefore, in the following section, we would discuss the ERα and ERβ isoforms in thyroid cancer.
According to the national center for biotechnology information database (https://www.ncbi.nlm.nih.gov/), ERα is located in 6q25.1-q25.2. The ERα protein contains an N-terminal ligand-independent transactivation domain, a central DNA binding domain, a hinge domain, and a C-terminal ligand-dependent transactivation domain. The N-terminal ligand-independent transactivation domain encompassed a ligand-independent activation function (AF1) domain involved in the transcriptional activation of target genes. The DNA binding domain mediates sequence-specific binding of ERs to DNA sequences in the target gene denoted estrogen-responsive elements (EREs). The C-terminal ligand-dependent transactivation domain contains a ligand-dependent activation domain (AF2) (29, 30). The protein localizes to the nucleus, where it may form either a homodimer or a heterodimer with ERβ.
Several alternative splicing isoforms of ERα have been identified, including ERα wild type/full length (ERα66), ERα46, and ERα36 (Figure 1). The isoforms of ERα have incomplete function domains that may alter their roles in thyroid cancer. The expression and role of ERα66 in thyroid cancer have been described in the previous section. Therefore, this section would focus on the ERα46, ERα36 and exon-deleted ERα isoforms.
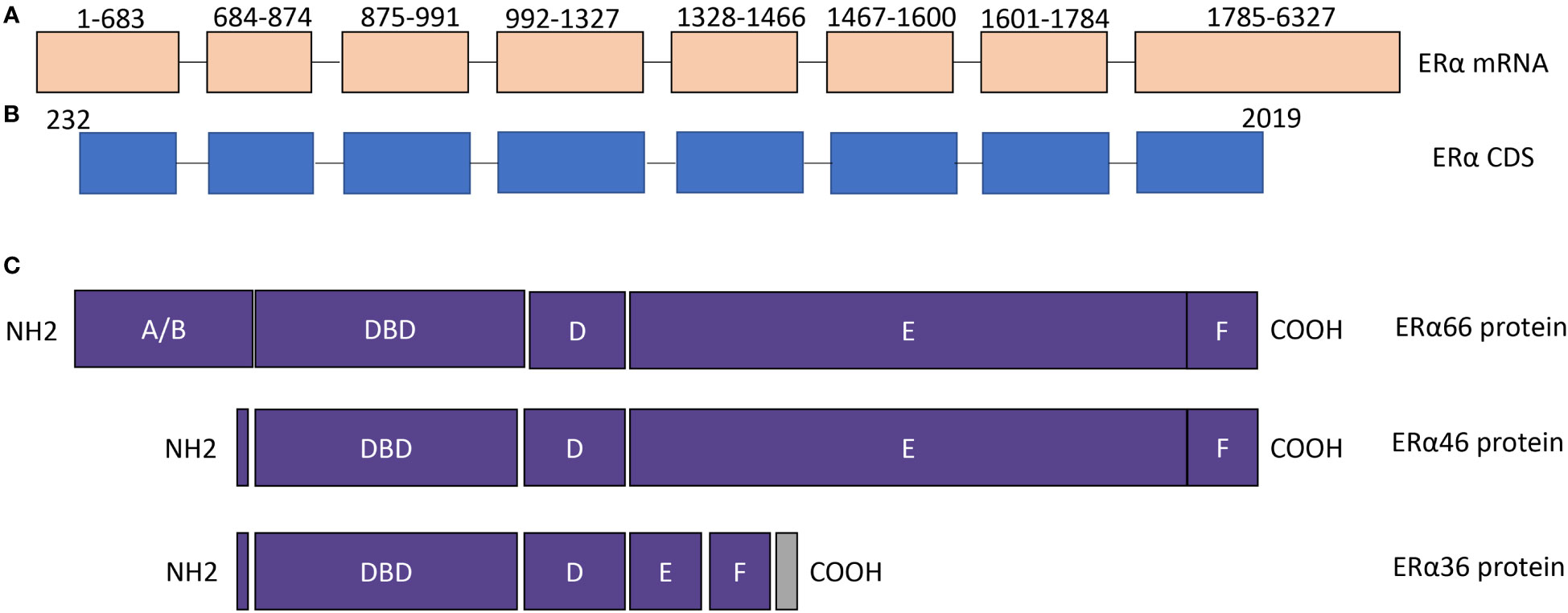
Figure 1 The structure of ERα isoforms. (A) ERα mRNA contains 8 exons. NCBI reference sequence: NM_000125.4, transcript variant 1. (B) ERα coding region. (C) The protein structure of ERα66, ERα46, and ERα36. The difference among ERα66, ERα 46, and ERα36 is mainly located in AF1 and AF2 domains. DBD, DNA binding domain.
ERα46 was first identified and characterized in osteoblasts (31). ERα46 is generated by alternative splicing of an ERα66 gene product, which results in exon 1 being skipped with a start codon in exon 2 used to initiate translation of the protein. Consequently, compared to ERα66, the ERα46 protein lacks amino acids 1-173, which codes N-terminal ligand-independent transactivation domain (AF1). Therefore, ERα46 has an incomplete AF1 domain.
Functional analysis revealed that ERα46 could heterodimerize with the ERα66 as well as the ERβ (31). However, the expression and role of ERα46 in thyroid cancer remain largely unknown. In another ER-related cancer, breast cancer, the expression of ERα46 was observed in over 70% of breast tumors among 116 ERα66 positive human breast tumors (32). In addition, ERα46 decreased the proliferation rate of breast cancer MCF7 cells in response to 17β estradiol (32). The data suggested that ERα46 inhibited tumor cell functions, which is different from ERα66.
Furthermore, the reduced expression of ERα46 was found in tamoxifen-resistant breast cancer cells, and the force overexpression of ERα46 in these tamoxifen-resistant breast cancer cells restored growth inhibition by tamoxifen (33). A study reported that the enhanced expression of ERα in breast cancer was associated with thyroid cancer occurrence, suggesting that ERα may have a role in the link between breast cancer and thyroid cancer (34). However, no data shows the expression and the role of ERα46 in thyroid cancer. Further studies are necessary.
ERα36 isofrom is shorter than ERα46. ERα36 lacks both AF-1 and C-terminal ligand-dependent transactivation domain (AF2), and the last 138 amino acids are replaced with a unique 22 amino acid sequence. It was first identified and cloned by Zhaoyi Wang and colleagues, and ERα36 is predicted to function as a dominant-negative effector of ERα66 mediated estrogen-responsive gene pathways and has the potential to trigger membrane-initiated mitogenic estrogen signaling (35, 36). Structurally, ERα36 has an incomplete AF1 domain and an AF2 domain. Therefore, understanding the role of ERα36 in thyroid cancer is vital for us to develop ERs as therapeutic targets.
There are limited studies on the ERα36 in thyroid cancer. The expression of ERα36 proteins was analyzed in 218 primary PTC by immunohistochemistry staining and it was found that its expression was upregulated in thyroid cancer (21). The functional study showed that upregulation of ERα36 by E2 enhanced the proliferation, invasion, and migration of PTC cells. The results suggested that increased expression of ERα36 is associated with aggressive thyroid cancer (21). Given the significance of ERα36 in cancer development and progression (37), further investigation of ERα36 in thyroid cancer may provide us with novel insight into the pathogenesis of thyroid cancer.
In addition to ERα46 and ERα36, several exon-deleted ERα isoforms have been reported in breast cancer, such as exon 3 deleted ERα (ERαΔ3), exon 5 deleted ERα (ERαΔ5), exon 7 deleted ERα (ERαΔ7) (38). As shown in Figure 1, exon 3 codes for the DNA binding domain, exon 5 and exon 7 codes for part of the AF2 domain. Therefore, each exon-deleted ERα isoform may alter the function of ERα due to the alteration in functional domains, and their roles in thyroid cancer is warrant further studying.
To date, the expression and function of ERα isoforms in thyroid cancer were far from clear. Further studies were warranted to investigate it.
According to the national center for biotechnology information database (https://www.ncbi.nlm.nih.gov/), the ERβ gene is located at 14q23.2-q23.3. The ERβ protein contains an N-terminal ligand-binding domain, DNA binding domain, and C-terminal ligand-binding domain. ERβ is classified as the nuclear receptor, and mainly located in the nucleus. However, The expression of ERβ also can be observed in the cytoplasm and mitochondrial (20, 39). The impact of subcellular localization on the ERβ function remains unclear.
Structurally, there is only a 16% similarity between the N-terminal ligand-binding domain of ERα and ERβ. In contrast, the DNA binding domain is highly conserved between ERα and ERβ with 97% amino acid identity. The C-terminal ligand-binding domains of ERα and ERβ show a 59% overall amino acid sequence identity (29).
Generally, the function of ERβ is opposite to ERα and it may act as a tumor suppressor in thyroid cancer (40). Downregulation of ERβ will decrease its inhibitory role in thyroid cancer. Our previous study has found that the methylation of the ERβ 5’-untranslated region will attenuate its inhibitory effect on ERα gene transcription and promote the initiation and progression of PTC (41). However, controversial results reported that the expression of ERβ was upregulated by lncRNA-H19 to promote cancer stem-like properties in thyroid cancer, suggesting that ERβ may exert its oncogenic role in thyroid cancer (22).
Similar to ERα, several isoforms of ERβ have been identified in human cells. In 1998, 5 isoforms of ERβ were cloned and characterized, and named from ERβ1 (Erβ full length) to ERβ5. All these five ERβ isoforms have novel C-terminus (42). Another splicing isoform of ERβ was identified in 2001, and exon 3 was deleted from ERβ, named ERβΔ3 (43). Missing exon 3 altered the subnuclear localization and capacity for transcriptional activation (43). Therefore, alternative splicing will change the function domain of ERβ (Figure 2), subsequently affecting its function in thyroid cancer. This section would discuss the expression and roles of ERβ and its isoforms in thyroid cancer.
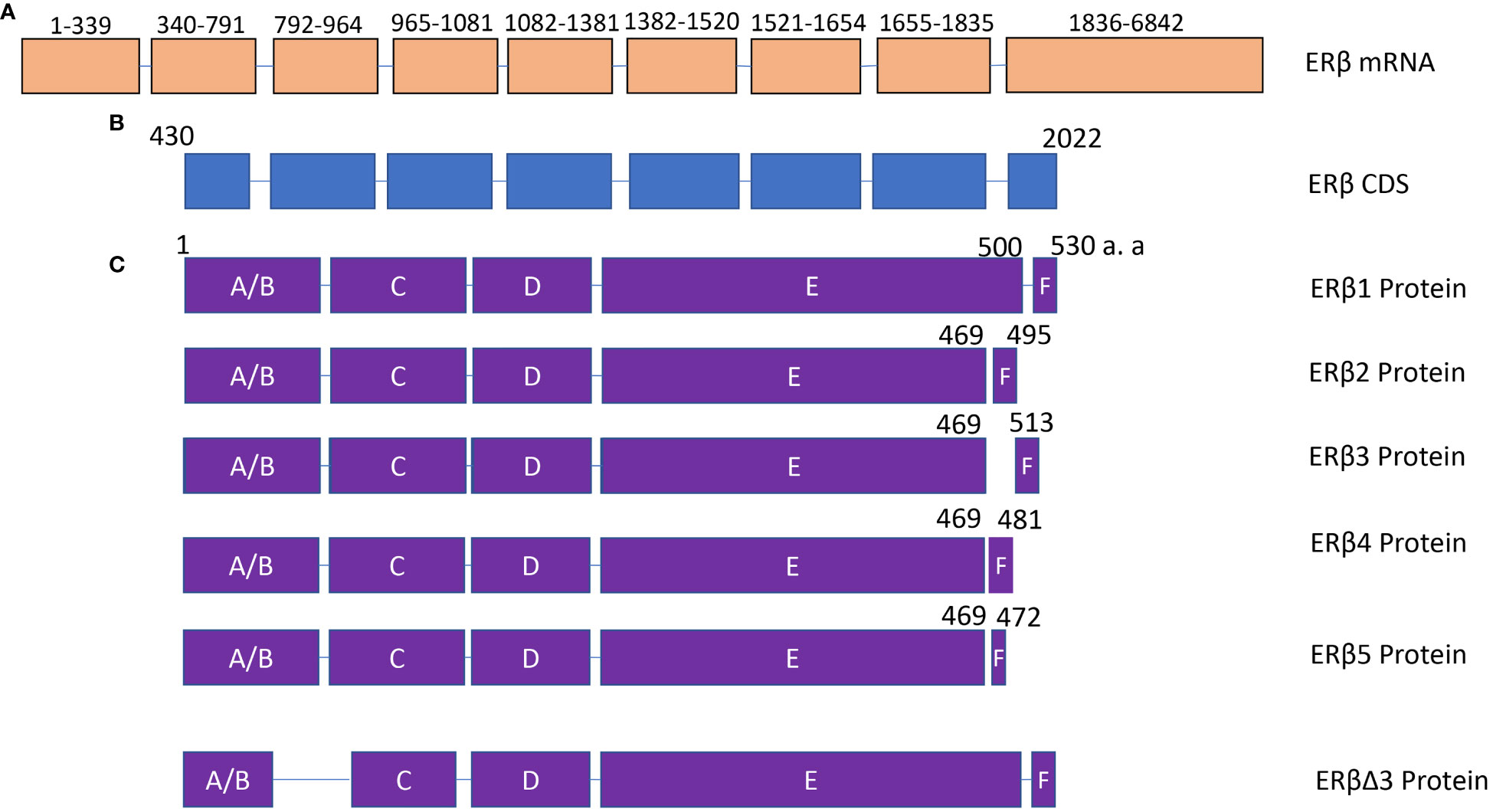
Figure 2 The structure of ERβ isoforms. (A) ERβ mRNA contains 9 exons. NCBI reference sequence: NM_001437.3, transcript variant a. (B) ERβ coding region. (C) The protein structure of ERβ1, ERβ2, ERβ3, ERβ4, ERβ5 and ERβΔ3. The difference among ERβ1, ERβ2, ERβ3, ERβ4, and ERβ5 is mainly located in the C-terminal domain. DBD, DNA binding domain.
Though several isoforms of ERβ have been identified for many years, limited studies have been performed to analyze ERβ isoforms in thyroid cancer. Wenwu Dong et al. (23) evaluated the expression of ERβ2 in 106 PTC tissues. They reported that the expression of ERβ2 was positively associated with Ki-67 expression in female patients with advanced reproductive age (>45 years, in low-estrogen status) and with VEGF expression in male PTC patients with reproductive age (18~45 years, in low-estrogen status) (P=0.005 and P=0.044, respectively). There was no association between ERβ2 expression and tumor size, extrathyroidal extension, and tumor-node-metastasis stage in PTC patients. In addition, the expression of ERβ2 was lower in female patients of reproductive age (18~45 years, in relatively high-estrogen status) with lymph node metastasis than in those patients without lymph node metastasis (P=0.035). The results suggested that the expression of ERβ2 in PTC is associated with the progression of the disease (23).
Overall, the role of ERβ in cancer is important. It has been proposed as a promising marker and potential target in cancer metastases (44). ERβ was also correlated with the tumor microenvironment (45). However, the expression and roles of ERβ isoforms remain largely unknown.
The functional domains of ERs will respond to different modulators and degraders (46, 47). Modulations of different ERs domains may have therapeutic impacts (48). The alternative splicing of ERs can result in an incomplete domain, thus affecting the treatment’s efficiency. Therefore, the investigation should focus on the isoforms of ERs in thyroid cancer.
The ERα and ERβ in thyroid cancer are multifaced and complicated. This review has focused on the ERα and ERβ isoforms in thyroid cancer. Given the significance of ERα and ERβ in the development of thyroid cancer and the perspective potential of estrogen receptor modulators and degraders in the treatment of thyroid cancer, the investigation of ERα and ERβ isoforms in the development and progression of thyroid cancer will provide us with a new avenue for the understanding and treatment of thyroid cancer.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was supported by grants from the National Natural Science Foundation of China (No.81972493), and the Research Grants Council of the Hong Kong Special Administrative Region (CUHK 14108921).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Du L, Li R, Ge M, Wang Y, Li H, Chen W, et al. Incidence and Mortality of Thyroid Cancer in China, 2008-2012. Chin J Cancer Res (2019) 31(1):144–51. doi: 10.21147/j.issn.1000-9604.2019.01.09
2. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid Cancer Incidence Trends by Histology in 25 Countries: A Population-Based Study. Lancet Diabetes Endocrinol (2021) 9:225–34. doi: 10.1016/s2213-8587(21)00027-9
3. Li M, Maso LD, Vaccarella S. Global Trends in Thyroid Cancer Incidence and the Impact of Overdiagnosis. Lancet Diabetes Endocrinol (2020) 8(6):468–70. doi: 10.1016/s2213-8587(20)30115-7
4. Kim J, Gosnell JE, Roman SA. Geographic Influences in the Global Rise of Thyroid Cancer. Nat Rev Endocrinol (2020) 16(1):17–29. doi: 10.1038/s41574-019-0263-x
5. Ding DC, Chen W, Wang JH, Lin SZ, Sung FC. Thyroid Cancer Risk in Women With Infertility and Association With Fertility Medications in Taiwan. Cancer (2019) 125(10):1701–8. doi: 10.1002/cncr.31964
6. Fuentes N, Silveyra P. Estrogen Receptor Signaling Mechanisms. Adv Protein Chem Struct Biol (2019) 116:135–70. doi: 10.1016/bs.apcsb.2019.01.001
7. Di Vito M, De Santis E, Perrone GA, Mari E, Giordano MC, De Antoni E, et al. Overexpression of Estrogen Receptor-Alpha in Human Papillary Thyroid Carcinomas Studied by Laser- Capture Microdissection and Molecular Biology. Cancer Sci (2011) 102(10):1921–7. doi: 10.1111/j.1349-7006.2011.02017.x
8. Magri F, Capelli V, Rotondi M, Leporati P, La Manna L, Ruggiero R, et al. Expression of Estrogen and Androgen Receptors in Differentiated Thyroid Cancer: An Additional Criterion to Assess the Patient's Risk. Endocr Relat Cancer (2012) 19(4):463–71. doi: 10.1530/ERC-11-0389
9. Magri F, Capelli V, Gaiti M, Villani L, Zerbini F, La Manna L, et al. Er-Alpha and Er-Beta Expression in Differentiated Thyroid Cancer: Relation With Tumor Phenotype Across the Tnm Staging and Peri-Tumor Inflammation. Endocrine (2015) 49(2):429–35. doi: 10.1007/s12020-014-0457-x
10. Dong W, Zhang H, Li J, Guan H, He L, Wang Z, et al. Estrogen Induces Metastatic Potential of Papillary Thyroid Cancer Cells Through Estrogen Receptor Alpha and Beta. Int J Endocrinol (2013) 2013:941568. doi: 10.1155/2013/941568
11. Huang Y, Dong W, Li J, Zhang H, Shan Z, Teng W. Differential Expression Patterns and Clinical Significance of Estrogen Receptor-α and β in Papillary Thyroid Carcinoma. BMC Cancer (2014) 14(1):1–10. doi: 10.1186/1471-2407-14-383
12. Mishra A, Kumari N, Jha CK, Bichoo RA, Mishra SK, Krishnani N, et al. Distribution and Prognostic Significance of Estrogen Receptor Alpha (Eralpha), Estrogen Receptor Beta (Erbeta), and Human Epidermal Growth Factor Receptor 2 (Her-2) in Thyroid Carcinoma. J Thyroid Res (2020) 2020:6935724. doi: 10.1155/2020/6935724
13. Chu R, van Hasselt A, Vlantis AC, Ng EK, Liu SY, Fan MD, et al. The Cross-Talk Between Estrogen Receptor and Peroxisome Proliferator-Activated Receptor Gamma in Thyroid Cancer. Cancer (2014) 120(1):142–53. doi: 10.1002/cncr.28383
14. Fan D, Liu SY, van Hasselt CA, Vlantis AC, Ng EK, Zhang H, et al. Estrogen Receptor Alpha Induces Prosurvival Autophagy in Papillary Thyroid Cancer Via Stimulating Reactive Oxygen Species and Extracellular Signal Regulated Kinases. J Clin Endocrinol Metab (2015) 100(4):E561–71. doi: 10.1210/jc.2014-3257
15. Yang S, Gong Z, Liu Z, Wei M, Xue L, Vlantis AC, et al. Differential Effects of Estrogen Receptor Alpha and Beta on Endogenous Ligands of Peroxisome Proliferator-Activated Receptor Gamma in Papillary Thyroid Cancer. Front Endocrinol (Lausanne) (2021) 12:708248. doi: 10.3389/fendo.2021.708248
16. Qiu YB, Liao LY, Jiang R, Xu M, Xu LW, Chen GG, et al. Pes1 Promotes the Occurrence and Development of Papillary Thyroid Cancer by Upregulating the Eralpha/Erbeta Protein Ratio. Sci Rep (2019) 9(1):1032. doi: 10.1038/s41598-018-37648-7
17. Liu J, Xu T, Ma L, Chang W. Signal Pathway of Estrogen and Estrogen Receptor in the Development of Thyroid Cancer. Front Oncol (2021) 11:593479. doi: 10.3389/fonc.2021.593479
18. Sturniolo G, Zafon C, Moleti M, Castellvi J, Vermiglio F, Mesa J. Immunohistochemical Expression of Estrogen Receptor-Alpha and Progesterone Receptor in Patients With Papillary Thyroid Cancer. Eur Thyroid J (2016) 5(4):224–30. doi: 10.1159/000452488
19. Chen GG, Zeng Q, Tse GM. Estrogen and Its Receptors in Cancer. Med Res Rev (2008) 28(6):954–74. doi: 10.1002/med.20131
20. Acconcia F, Fiocchetti M, Busonero C, Fernandez VS, Montalesi E, Cipolletti M, et al. The Extra-Nuclear Interactome of the Estrogen Receptors: Implications for Physiological Functions. Mol Cell Endocrinol (2021) 538:111452. doi: 10.1016/j.mce.2021.111452
21. Dai YJ, Qiu YB, Jiang R, Xu M, Liao LY, Chen GG, et al. Concomitant High Expression of Eralpha36, Grp78 and Grp94 Is Associated With Aggressive Papillary Thyroid Cancer Behavior. Cell Oncol (Dordr) (2018) 41(3):269–82. doi: 10.1007/s13402-017-0368-y
22. Li M, Chai HF, Peng F, Meng YT, Zhang LZ, Zhang L, et al. Estrogen Receptor Beta Upregulated by Lncrna-H19 to Promote Cancer Stem-Like Properties in Papillary Thyroid Carcinoma. Cell Death Dis (2018) 9(11):1120. doi: 10.1038/s41419-018-1077-9
23. Dong W, Li J, Zhang H, Huang Y, He L, Wang Z, et al. Altered Expression of Estrogen Receptor β2 Is Associated With Different Biological Markers and Clinicopathological Factors in Papillary Thyroid Cancer. Int J Clin Exp Pathol (2015) 8(6):7149.
24. Kim HK, Pham MHC, Ko KS, Rhee BD, Han J. Alternative Splicing Isoforms in Health and Disease. Pflügers Archiv - Eur J Physiol (2018) 470(7):995–1016. doi: 10.1007/s00424-018-2136-x
25. Dong X, Chen R. Understanding Aberrant Rna Splicing to Facilitate Cancer Diagnosis and Therapy. Oncogene (2020) 39(11):2231–42. doi: 10.1038/s41388-019-1138-2
26. Slusher AL, Kim JJ, Ludlow AT. The Role of Alternative Rna Splicing in the Regulation of Htert, Telomerase, and Telomeres: Implications for Cancer Therapeutics. Cancers (2020) 12(6):1514. doi: 10.3390/cancers12061514
27. Wu ZH, Tang Y, Zhou Y. Alternative Splicing Events Implicated in Carcinogenesis and Prognosis of Thyroid Gland Cancer. Sci Rep (2021) 11(1):4841. doi: 10.1038/s41598-021-84403-6
28. Han B, Yang M, Yang X, Liu M, Xie Q, Fan G, et al. Systematic Analysis of Survival-Associated Alternative Splicing Signatures in Thyroid Carcinoma. Front Oncol (2021) 11:561457. doi: 10.3389/fonc.2021.561457
29. Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen Receptor Alpha and Beta in Health and Disease. Best Pract Res Clin Endocrinol Metab (2015) 29(4):557–68. doi: 10.1016/j.beem.2015.04.008
30. Hewitt SC, Korach KS. Estrogen Receptors: New Directions in the New Millennium. Endocr Rev (2018) 39(5):664–75. doi: 10.1210/er.2018-00087
31. Denger S, Reid G, Koš M, Flouriot G, Parsch D, Brand H, et al. Erα Gene Expression in Human Primary Osteoblasts: Evidence for the Expression of Two Receptor Proteins. Mol Endocrinol (2001) 15(12):2064–77. doi: 10.1210/mend.15.12.0741
32. Chantalat E, Boudou F, Laurell H, Palierne G, Houtman R, Melchers D, et al. The Af-1-Deficient Estrogen Receptor Eralpha46 Isoform Is Frequently Expressed in Human Breast Tumors. Breast Cancer Res (2016) 18(1):123. doi: 10.1186/s13058-016-0780-7
33. Klinge CM, Riggs KA, Wickramasinghe NS, Emberts CG, McConda DB, Barry PN, et al. Estrogen Receptor Alpha 46 Is Reduced in Tamoxifen Resistant Breast Cancer Cells and Re-Expression Inhibits Cell Proliferation and Estrogen Receptor Alpha 66-Regulated Target Gene Transcription. Mol Cell Endocrinol (2010) 323(2):268–76. doi: 10.1016/j.mce.2010.03.013
34. Kim YA, Kim YA, Cho SW, Song YS, Min HS, Park IA, et al. Increased Expression of Thyroid Hormone Receptor Alpha and Estrogen Receptor Alpha in Breast Cancer Associated With Thyroid Cancer. Eur J Surg Oncol (2021) 47(6):1316–23. doi: 10.1016/j.ejso.2021.01.015
35. Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, Cloning, and Expression of Human Estrogen Receptor-Alpha36, a Novel Variant of Human Estrogen Receptor-Alpha66. Biochem Biophys Res Commun (2005) 336(4):1023–7. doi: 10.1016/j.bbrc.2005.08.226
36. Zou Y, Ding L, Coleman M, Wang Z. Estrogen Receptor-Alpha (Er-Alpha) Suppresses Expression of Its Variant Er-Alpha 36. FEBS Lett (2009) 583(8):1368–74. doi: 10.1016/j.febslet.2009.03.047
37. Pagano MT, Ortona E, Dupuis ML. A Role for Estrogen Receptor Alpha36 in Cancer Progression. Front Endocrinol (Lausanne) (2020) 11:506. doi: 10.3389/fendo.2020.00506
38. Al-Bader M, Ford C, Al-Ayadhy B, Francis I. Analysis of Estrogen Receptor Isoforms and Variants in Breast Cancer Cell Lines. Exp Ther Med (2011) 2(3):537–44. doi: 10.3892/etm.2011.226
39. Yang S-H, Liu R, Perez EJ, Wen Y, Stevens SM, Valencia T, et al. Mitochondrial Localization of Estrogen Receptor β. Proc Natl Acad Sci (2004) 101(12):4130–5. doi: 10.1073/pnas.0306948101
40. Mal R, Magner A, David J, Datta J, Vallabhaneni M, Kassem M, et al. Estrogen Receptor Beta (Erbeta): A Ligand Activated Tumor Suppressor. Front Oncol (2020) 10:587386. doi: 10.3389/fonc.2020.587386
41. Xu LW, Gou X, Yang JY, Jiang R, Jiang X, Chen GG, et al. Methylation of Erbeta 5'-Untranslated Region Attenuates Its Inhibitory Effect on Eralpha Gene Transcription and Promotes the Initiation and Progression of Papillary Thyroid Cancer. FASEB J Off Publ Fed Am Societies Exp Biol (2021) 35(4):e21516. doi: 10.1096/fj.202001467R
42. Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, et al. Cloning and Characterization of Human Estrogen Receptor β Isoforms. Biochem Biophys Res Commun (1998) 247(1):75–8. doi: 10.1006/bbrc.1998.8738
43. Price RH Jr., Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A Splice Variant of Estrogen Receptor β Missing Exon 3 Displays Altered Subnuclear Localization and Capacity for Transcriptional Activation. Endocrinology (2001) 142(5):2039–49. doi: 10.1210/endo.142.5.8130
44. Bozovic A, Mandusic V, Todorovic L, Krajnovic M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int J Mol Sci (2021) 22(4):1656. doi: 10.3390/ijms22041656
45. Rothenberger NJ, Somasundaram A, Stabile LP. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int J Mol Sci (2018) 19(2):611. doi: 10.3390/ijms19020611
46. Arao Y, Korach KS. The Physiological Role of Estrogen Receptor Functional Domains. Essays Biochem (2021) 65(6):867–75. doi: 10.1042/EBC20200167
47. Patel HK, Bihani T. Selective Estrogen Receptor Modulators (Serms) and Selective Estrogen Receptor Degraders (Serds) in Cancer Treatment. Pharmacol Ther (2018) 186:1–24. doi: 10.1016/j.pharmthera.2017.12.012
Keywords: ERα, ERβ, isoforms, splicing, thyroid cancer
Citation: Gong Z, Yang S, Wei M, Vlantis AC, Chan JYK, van Hasselt CA, Li D, Zeng X, Xue L, Tong MCF and Chen GG (2022) The Isoforms of Estrogen Receptor Alpha and Beta in Thyroid Cancer. Front. Oncol. 12:916804. doi: 10.3389/fonc.2022.916804
Received: 10 April 2022; Accepted: 27 May 2022;
Published: 24 June 2022.
Edited by:
Rengyun Liu, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Jorge Sastre-Serra, University of the Balearic Islands, SpainCopyright © 2022 Gong, Yang, Wei, Vlantis, Chan, van Hasselt, Li, Zeng, Xue, Tong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael C. F. Tong, bXRvbmdAZW50LmN1aGsuZWR1Lmhr; George G. Chen, Z2NoZW5AY3Voay5lZHUuaGs=
†ORCID: George G. Chen, orcid.org/0000-0001-7276-3830
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.