
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 July 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.916650
This article is part of the Research Topic Precision Medicine in Gastrointestinal Cancers View all 24 articles
 Chalirmporn Atasilp1
Chalirmporn Atasilp1 Rinradee Lenavat1
Rinradee Lenavat1 Natchaya Vanwong2
Natchaya Vanwong2 Phichai Chansriwong3
Phichai Chansriwong3 Ekaphop Sirachainan3
Ekaphop Sirachainan3 Thanyanan Reungwetwattana3
Thanyanan Reungwetwattana3 Pimonpan Jinda4,5
Pimonpan Jinda4,5 Somthawin Aiempradit3
Somthawin Aiempradit3 Suwannee Sirilerttrakul3
Suwannee Sirilerttrakul3 Monpat Chamnanphon6
Monpat Chamnanphon6 Apichaya Puangpetch4,5
Apichaya Puangpetch4,5 Nipaporn Sankuntaw1
Nipaporn Sankuntaw1 Patompong Satapornpong7,8
Patompong Satapornpong7,8 Chonlaphat Sukasem4,5*
Chonlaphat Sukasem4,5*Background: The two common methylenetetrahydrofolate reductase (MTHFR) polymorphisms 677G>A and 1298A>C may have been affecting 5-FU toxicity in cancer patients for decades. Drug efficacy has also been shown by previous studies to be affected. In this study, we investigated the effects of these polymorphisms on 5-FU hematological toxicity and treatment efficacy, to provide enhanced pharmacological treatment for cancer patients.
Methods: This is a retrospective study involving 52 Thai colorectal cancer patients who were treated with 5-FU based therapy, using TaqMAN real-time PCR to genotype the MTHFR polymorphisms (677G>A and 1298A>C). The toxicity and response rate were assessed using standardized measures.
Results: Neutropenia was significantly more likely to be experienced (P=0.049, OR=7.286, 95% CI=0.697-76.181) by patients with the MTHFR 677G>A polymorphism, in the same way as leukopenia (P =0.036, OR=3.333, 95%CI=2.183-5.090) and thrombocytopenia (P<0.001, OR=3.917, 95%CI=2.404-6.382). The MTHFR 1298A>C polymorphism had no statistical association with hematological toxicity in 5-FU treatment. The response rate to 5-FU was not significantly affected by these two polymorphisms.
Conclusion: The MTHFR polymorphism 677G>A is a significant risk factor for developing leukopenia, neutropenia and thrombocytopenia as toxic effects of 5-FU therapy in cancer patients. Therefore, patients receiving 5-FU-based therapy should be aware of their polymorphisms as one risk factor for experiencing severe toxicity.
Data from GLOBOCAN 2018 reveals that colorectal cancer is the third most deadly and fourth most commonly diagnosed cancer in the world (1). When restricted to Thailand, a 2021 study shows that colorectal cancer is also the third most common cancer, contributing to 11% of the total national cancer burden (2).
The cell-cycle specific anti-metabolite 5-fluorouracil (5-FU) is one of the most commonly used drug regimens for the treatment of many cancers including colorectal cancer. It is a pyrimidine analog which acts to interfere with DNA synthesis. However, 69% of patients undergoing 5-FU therapy for colon cancer experience neutropenia (3). Therefore, for certain patients, this serious toxicity is the main limitation to its use, as increased susceptibility to infections can occur which is potentially life-threatening. The enzyme methylenetetrahydrofolate reductase (MTHFR) plays a key role in the metabolism of 5-FU. Fluorouracil is irreversibly reduced by MTHFR enzyme to the compound 5-methyltetrahydrofolate (5-MTHF), which is later used in DNA methylation through the conversion of homocysteine to methionine (4). This step normally involves the conversion of dUMP to dTMP by thymidylate synthase. As a result, DNA synthesis is directly disrupted, leading to cell damage and apoptosis. A decrease in MTHFR has been linked to an increase in 5,10-MTHF the substrate for MTHFR and thus an increase in 5-FU toxicity (5).
Genetics play an important role in individual differences among patients that have shown different levels of pharmacological toxicity. The MTHFR gene is located on chromosome 1p36.22 and is prone to polymorphisms. The two most common polymorphisms studied are G677A (alanine to valine) and A1298C (glutamine to alanine). Several studies have shown the effects of these two polymorphisms of the MTHFR gene in reduced enzymatic activity in the metabolism of 5-FU (6, 7). For the G677A polymorphism, homozygous TT individuals have 30% of expected enzyme activity, whilst heterozygous CT individuals have 65% of such activity, in comparison to the most common genotype CC (8).
The G677A single nucleotide polymorphism (SNP-rs1801133) of the MTHFR gene is most commonly linked with hematologic toxicity, and this correlation has been shown in the Chinese population (p = 0.005) (9). A previous study found that the 677 GG genotype is associated with toxicity (odds ratio = 1.83, P = 0.01) (10). On the other hand, another study concluded that the G677A genotype did not significantly affect the cytotoxic activity of 5-FU (11). As for the 1298A>C genotype, a study found it was linked to toxicity (4).
The variant 1298 A>C (rs1801131) has been demonstrated to increase 5-FU efficacy in patients with colorectal cancer, by increasing progression-free survival (PFS - time from operation to death or censorship), whereas 677 G>A showed no correlation (6, 11). However, in another study, the MTHFR G677A mutation was shown to increase chemosensitivity to 5-FU in colon and breast cancer (7). Another study showed a specific association of a good clinical response to FOLFOX therapy (leucovorin, fluorouracil, and oxaliplatin) with the two MTHFR polymorphisms 677G>A and 1298A>C (p=0.040) (12).
Hence, the results from previous studies are currently inconclusive and controversy still exists over whether these two SNPs of the MTHFR gene really lead to increased hematological toxicity and treatment efficacy (13, 14). Additionally, few studies have reported the use of 5-FU in Thai colorectal cancer patients.
The aim of this study was to investigate the effects of the specific SNPs of MTHFR polymorphism on the association with hematological toxicity as an adverse drug reaction of 5-FU in Thai colorectal cancer patients, along with the efficacy of the drug.
A total of 108 colorectal cancer patients were recruited between October 2020 and October 2021 from the Division of Oncology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Thailand. The clinical eligibility criteria to recruit patients were as follow: histologically or cytologically confirmed to be diagnosed with colorectal cancer, having not received fluorouracil before (first or second cycles of treatment), aged at least 18 years, Eastern Cooperative Oncology Group (ECOG) performance status 0-2, life expectancy > 3 months, neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 8 × 1010/L, serum creatinine ≤ 1.25, upper limit normal (ULN), total bilirubin ≤ 1.25 ULN, and alanine aminotransferase and aspartate aminotransferase ≤ 2.5 ULN. Fifty-two patients who had been treated with 5-FU-based-chemotherapy were analyzed for toxicity assessment. The flow chart for patient screening is shown in Figure 1. Patients who were excluded from the study had one or more of the following characteristics: liver or kidney disease, pregnancy, or did not consent to the study.
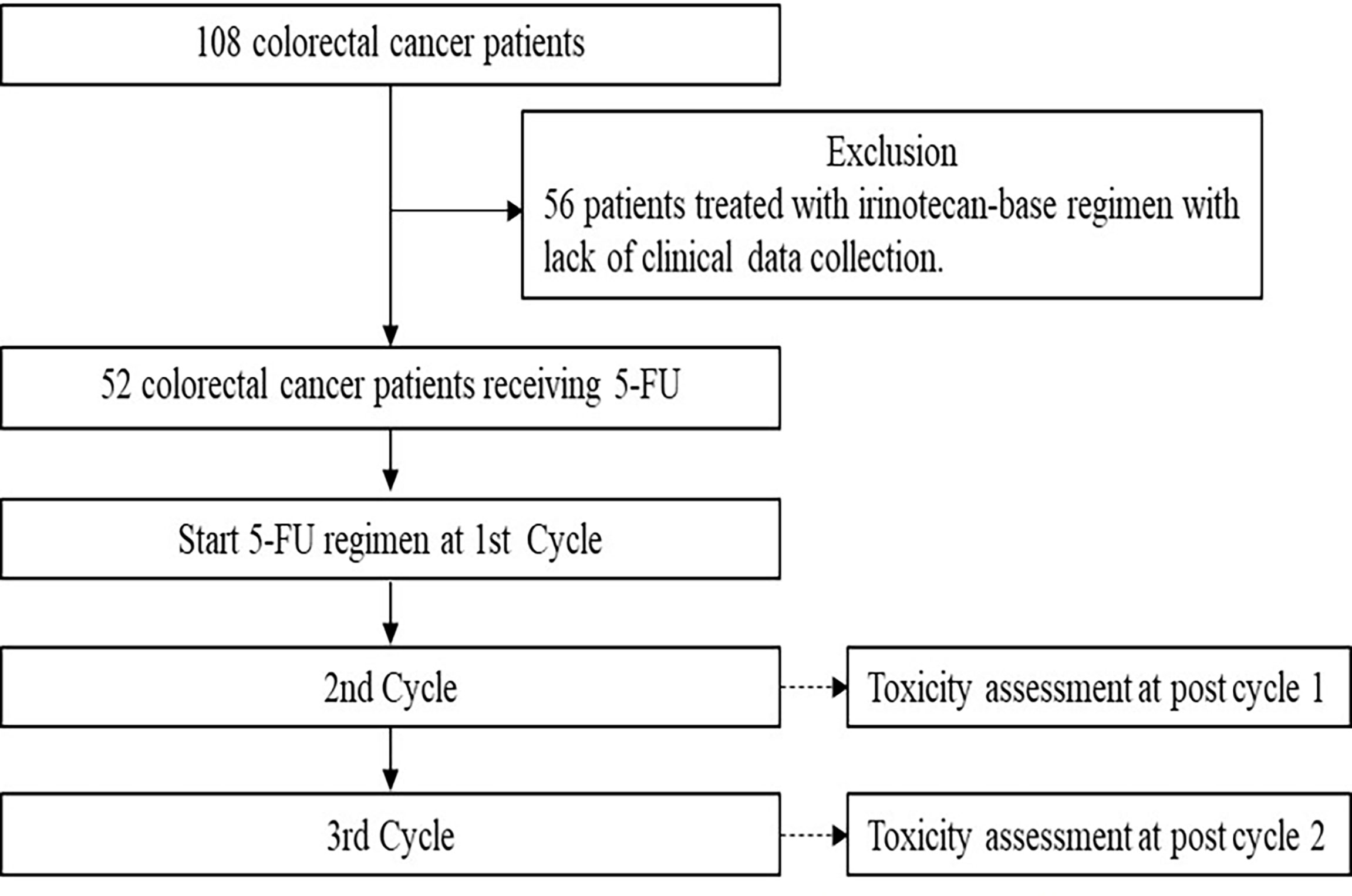
Figure 1 Flow chart for patient screening. A total of 108 metastatic colorectal cancer patients were genotyped for generic polymorphisms and 56 patients who did not treated with 5-flourouracil-based chemotherapy were excluded. Of the 52 patients treated with 5-flourouracil-based chemotherapy were included in this analysis.
This study was approved by the Ethics Review Committee on Human Research of the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand (MURA2020/1613) and was conducted in accordance with the Declaration of Helsinki. The study procedure was clearly explained to the patients before the study and written consent forms were issued accordingly.
Peripheral blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes. Firstly, the MagNA pure compact system (Roche, Manheim, Germany) was used to purify the DNA in the blood samples. Following this, nanodrop microvolume technology (Thermo Fisher Scientific, DE, USA) was used to check the purity of the DNA, relying on the surface tension qualities of the sample liquified into a column using a 260/280 ratio. A score of 1.80-2.00 was considered to represent of good purity. The methylenetetrahydrofolate reductase (MTHFR) polymorphisms were examined by TaqMAN real-time PCR, which involved amplifying and detecting targeted polymorphisms quantitatively. The two SNPs studied were 677G>A (C_1202883_20) and 1298A>C (C_850486_20).
As for the drug administration the most common drug or drug combination received was 5-fluorouracil + leucovorin (5-FU at 425 mg/m2/day, 5 days + leucovolin 30 mg), which 22 patients (42.3%) received. Another set of 20 patients received FOLFOX (intravenous oxaliplatin 85 mg/m2 on day 1 and a 2-hour infusion of leucovorin 200 mg/m2 followed by bolus 5-FU 400 mg/m2 and a 22-hour continuous infusion of 5-FU 600 mg/m2 for two consecutive days with treatment repeated every two weeks) (38.5%) while the following drugs were received by less than 10% of patients: modified FOLFOX (intravenous oxaliplatin 85 mg/m2 on day 1 and a 2-hour infusion of leucovorin 200 mg/m2 followed by bolus 5-FU 400 mg/m2 and a 22-hour continuous infusion of 5-FU 1200 mg/m2 for two consecutive days with this treatment repeated every two weeks) and FOLFOX + Avastin (Avastin 5–10 mg/kg intravenous infusion once every 2 weeks; intravenous oxaliplatin 85 mg/m2 on day 1 and a 2-hour infusion of leucovorin 200 mg/m2 followed by bolus 5-FU 400 mg/m2 and a 22-hour continuous infusion of 5-FU 600 mg/m2 for two consecutive days with this treatment repeated every two weeks) which 7 patients (13.5%) and 3 patients (5.8%) received, respectively.
Toxicity was assessed according to National Cancer Institute Common Toxicity Criteria for Adverse Events version 5.0 (CTCAE). Grades 1-4 were considered to be toxic. Grades 3–4 were considered severely toxic. The specific toxicity this study focused on was hematological toxicity.
The efficacy or response rate of cancer after drug administration was measured in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0, which comprises the following ratings: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).
Descriptive statistics were used to describe the clinical characteristics of the subjects. Data are reported as medians (interquartile range, IQR). Associations among the genetic polymorphisms (alleles and genotypes), adverse events (toxicity), response rates, and clinical characteristics (age and risk group) were evaluated with the χ2 test, or Fisher’s exact test. The odds ratio (OR) and 95% confidence interval (CI) were calculated from the contingency table. All statistics were calculated using SPSS software version 22 (Chicago, IL, USA), and the statistical significance was set at p < 0.05.
A total of 108 colorectal cancer patients receiving 5-fluorouracil based therapy were genotyped for two SNPs: MTHFR 677G>A and MTHFR 1298A>C. The genotype and allele frequencies are shown in Table 1. The prevalence of MTHFR 677G>A polymorphism is 0.17. Most of the patients (75/108; 69.4%) had the homozygous wild type (GG), while 26.9% (29/108) had the heterozygous variant (GA) and 3.7% (4/108) had the homozygous variant. As for the MTHFR 1298A>C polymorphism, the prevalence of the allele was 0.27. Most patients (55/108; 50.9%) had the homozygous wild type (AA), while 43.5% (47/108) had the heterozygous variant (AC) and 5.6% (6/108) had the homozygous variant (CC).
The clinical characteristics of the colorectal cancer patients are summarized in Table 2. Of those 108, 52 colorectal cancer patients who were treated with 5-FU-based-chemotherapy were included in the study. A total of 31 were male, 21 were female and the mean age of the sample was 60 years (range 47-73). The majority (69.2%) had an ECOG performance status of 0. The most common site of disease was the rectum (50%). The liver was the most common site of metastasis (47.7%). There were no statistically significant differences between clinical characteristics and hematological toxicity including neutropenia, leucopenia, thrombocytopenia, and anemia (data not shown).
The clinical association is summarized in Table 3. As for the first cycle of treatment, the MTHFR 677G>A polymorphism was statistically associated (P=0.036, OR=3.333, 95%CI = 2.183-5.090) with grade 3-4 leukopenia. For the second cycle, the MTHFR 677G>A polymorphism was statistically associated (P =0.049, OR=7.286, 95% CI=0.697-76.181) with grade 3-4 neutropenia. The MTHFR 677G>A polymorphism was significantly statistically associated with grade 1-4 thrombocytopenia (P <0.001, OR=3.917, 95%CI=2.404-6.382).
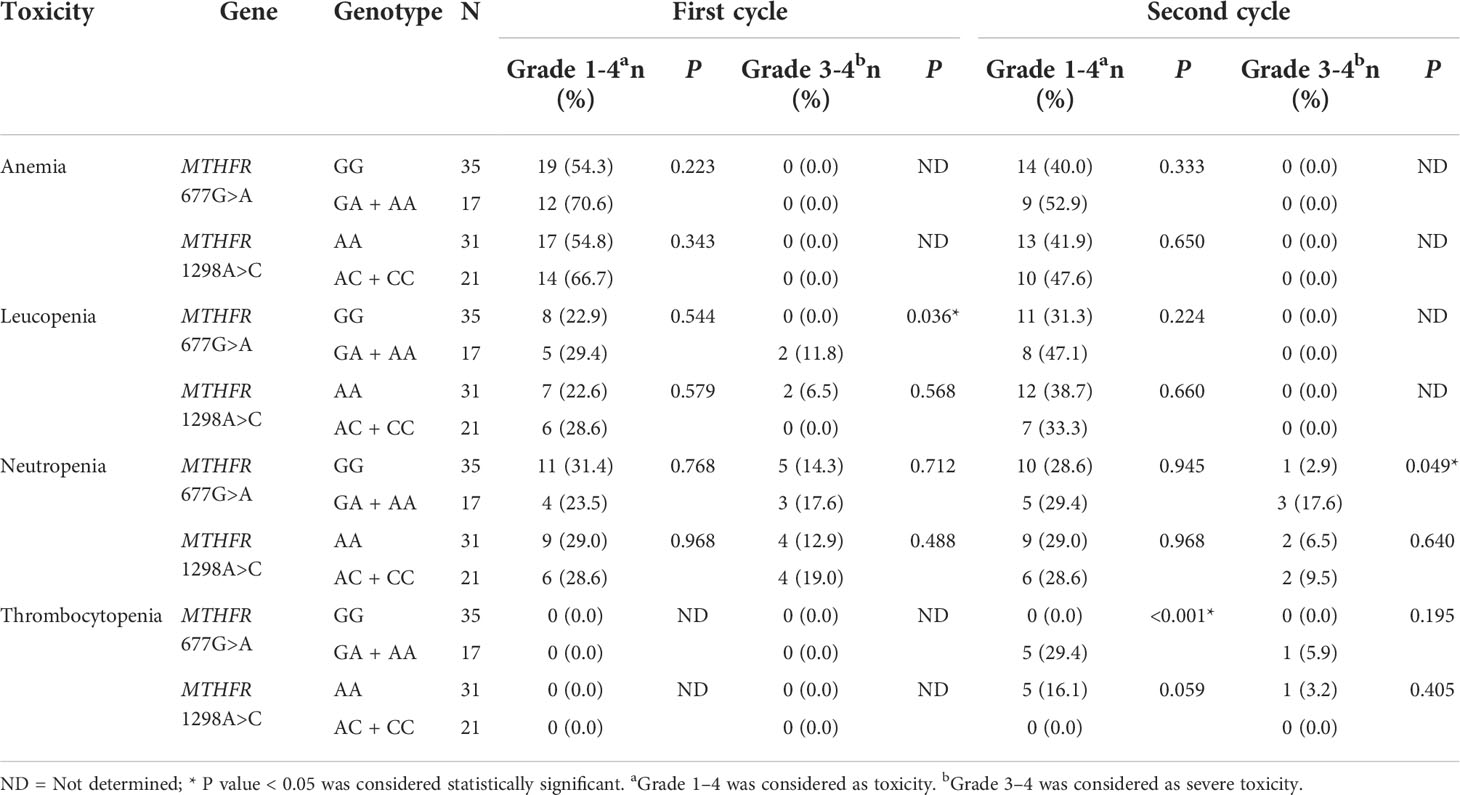
Table 3 Different grades of toxicities in first and second cycles (N=52) caused by 5-FU-based chemotherapy in the patients with different genotypes of MTHFR polymorphism.
Simultaneously, the MTHFR 677G>A polymorphism had no statistical significance in 5-FU treatment in conjunction with the first and second cycles for all grades of anemia toxicity, the first cycle of grade 1-4 leukopenia, the second cycle for all grades of leukopenia, the first cycle of all grades of neutropenia, or second cycle grade 1-4 neutropenia. For all grades of thrombocytopenia, the first cycle of treatment had no significant association and the second cycle had no association for severe toxicity.
The MTHFR 1298A>C polymorphism had no statistical association with either the first or second cycles of 5-FU treatment, or with any grade or types of hematological toxicity. Anemia as an effect of hematological toxicity caused by 5-FU treatment was not statistically associated with the two polymorphisms (MTHFR 677G>A and MTHFR 1298A>C) in either first or second cycles. There were no statistically significant differences between the combined MTHFR polymorphisms and hematological toxicities in the first or second cycles.
There was no statistical significance in the response rate of patients with MTHFR 677G>A and MTHFR 1298A>C polymorphisms. This clinical data is summarized in Table 4. There were no statistically significant differences between response rate and clinical characteristics in the first or second cycles.
To our knowledge, there is not yet another study on the association of MTHFR 677G>A and MTHFR 1298A>C polymorphisms with 5-FU treatment in Thai colorectal cancer patients. Our findings suggest that the MTHFR 677G>A polymorphism is a high-risk factor that contributes to hematologic toxicity associated with 5-FU-based therapy in both first and second cycles of treatment.
The prevalence of MTHFR 677A allele frequencies in the Chinese population found in other studies was much higher than that in this study, being 0.56 compared to 0.17, respectively (15). However, similar allele frequencies were found for MTHFR 1298C in a minority group of the Chinese population, with 0.26 compared to the 0.27 in this study (16). In comparison to other ethnic groups such as the Caucasian population, both allele frequencies 677A and 1298C for the polymorphisms were higher than those found in this study, with 0.33 and 0.38 respectively (17). Similarly, a study investigating the female Turkish population found both allele frequencies 677A and 1298C to be higher (0.26 and 0.37 respectively) than values found in the Thai population (18).
This present study found that having the MTHFR 677A allele increases the risk of severe neutropenia sevenfold as a symptom of hematological toxicity in the second cycle of 5-FU treatment (95% CI =0.697-76.181). This finding is in accordance with another study investigating the Chinese population which found that the 677A allele was closely associated with severe neutropenia (p=0.043) (19). Similarly, a study on Bangladeshi patients found that the 677A allele could predict grade 3 or 4 neutropenia as a result of 5-FU toxicity (9). A meta-analysis consisting mostly of Asians and Europeans also found that gastric cancer patients with the GG or GA genotype tended to experience less severe hematological toxicity than those with the AA genotype[(GG+GA)/TT OR=0.66, 95% CI: 0.48-0.91] (20). These findings are opposed to several studies that found that the 677A allele was not associated with hematological toxicity (11). As a matter of fact, one study actually found the 677GG genotype to be related to toxicity(odds ratio = 1.83, CI = 1.13-2.96, P = 0.01) (10).
In this study, leukopenia is three times (P=0.036, OR=3.333, 95%CI = 2.183-5.090) more likely for patients with the MTHFR 677G>A polymorphism as a result of toxicity caused by receiving 5-FU. In contrast, Matthias Schwab et al. found no significant association between the MTHFR 677G>A polymorphism and all grades of leukopenia (21).
One interesting finding is that patients with the MTHFR 677G>A polymorphism have around four times (P<0.001, OR=3.917, 95%CI=0.697-76.181) the risk of experiencing thrombocytopenia as a side effect of 5-FU therapy. From Ahmad, F, et al., and Franchini, M., et al., reported that MTHFR 677C/T was associated with an increased prothrombotic risk factor (22, 23). This result is in alignment with V. Adamo et al. who reported that one third of patients with the homozygous variant genotype (MTHFR 677 AA) faced grade 3 thrombocytopenia after the first cycle of treatment (24).
The MTHFR 1298A>C polymorphism was discovered to be not statistically associated with any hematological toxicity in this study. Previous studies have reported similar findings, as researchers also did not find any statistical significance correlated with the 1298C allele and 5-FU toxicity (10). This finding is further in agreement with another study on Indian patients, which also did not find any significant association between the 1298C allele and 5-FU toxicity (25). However, another study on the French population reported that 1298CC genotype was associated with toxicity(p = 0.0018), in the opposite manner to the current study (4). Furthermore, V. Adamo et al. found that one third of patients with homozygous wild genotype (MTHFR 1298 AA) experienced grade 3 granulocytopenia and grade 3 thrombocytopenia (24).
As for the response rate, although this study did find better response rates, patients with responders was low number 13.5% (7/52). Therefore, the difference regarding the association with MTHFR 677A and MTHFR 1298C alleles was not statistically significant.
Previous studies have reported similar results regarding drug response (10, 20). However, other studies have also reported the association of both alleles with a good clinical response (12). Some studies found the MTHFR 677A allele increased chemosensitivity in 5-FU response in colon and breast cancer patients (7). Reduced enzyme activity has also been associated with MTHFR 1298C polymorphism (7). In the same way, a study on Bangladeshi patients also found the MTHFR polymorphism to be associated with a good clinical 5-FU response (9). Another study found 5-FU sensitivity to be related to the MTHFR 1298C allele, with the 1298CC genotype being the most sensitive (11).
The main limitations to this study are the sample size and it being a retrospective study. The sample was small and was only drawn from one hospital in Bangkok, Thailand. Moreover, other non-hematological toxicities such as diarrhea were not considered in this study. Other genes, such as DPYD and TYMS polymorphisms that can affect toxicity were not studied. Further prospective studies with larger sample sizes need to be carried out to further validate these findings.
In conclusion, the MTHFR 677G>A polymorphism was statistically associated with grade 3-4 hematologic toxicity in both the first and second cycles of treatment of Thai colorectal cancer patients who received 5-FU-based therapy, whereas the MTHFR 1298A>C polymorphism had no significant association. The response to 5-FU treatment was not statistically associated with these two single nucleotide polymorphisms. These findings imply that the MTHFR 677G>A polymorphism may predict 5-FU toxicity. Therefore, patients receiving 5-FU-based therapy should be aware of their polymorphisms as risk factor for experiencing severe toxicity.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the ethics review committee on Human Research of the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand (MURA2020/1613). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CA and CS designed the research study. PC, ES, TR, SA, and SS collected samples. CA and NV collected clinical data. PJ, PS, MC, AP, and NS performed procedures. CA and NV analyzed data. RL, CA, and NV wrote original draft preparation. CA, NV, and CS contributed to the discussion and reviewed/edited the manuscript. All authors have read and agreed to the published version of the manuscript.
This study was supported by grants from the (1) The Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation No. RGNS 63–196, (2) The Health Systems Research Institute under Genomics Thailand Strategic Fund No. 64-099.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all the staffs of the Division of Pharmacogenomics and Personalized Medicine, Department of Pathology Faculty of Medicine Ramathibodi Hospital, Mahidol University, and Chulabhorn International College of Medicine, Thammasat University, Pathum Thani, Thailand.
1. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol (2019) 14:89–103. doi: 10.5114/pg.2018.81072
2. Lohsiriwat V, Chaisomboon N, Pattana-Arun J. Current colorectal cancer in thailand. Ann Coloproctol (2020) 36:78–82. doi: 10.3393/ac.2020.01.07
3. Garg MB, Lincz LF, Adler K, Scorgie FE, Ackland SP, Sakoff JA. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br J Cancer (2012) 107:1525–33. doi: 10.1038/bjc.2012.421
4. Capitain O, Boisdron-Celle M, Poirier AL, Abadie-Lacourtoisie S, Morel A, Gamelin E. The influence of fluorouracil outcome parameters on tolerance and efficacy in patients with advanced colorectal cancer. Pharmacogenomics J (2008) 8:256–67. doi: 10.1038/sj.tpj.6500476
5. Cohen V, Panet-Raymond V, Sabbaghian N, Morin I, Batist G, Rozen R. Methylenetetrahydrofolate reductase polymorphism in advanced colorectal cancer: a novel genomic predictor of clinical response to fluoropyrimidine-based chemotherapy. Clin Cancer Res (2003) 9:1611–5.
6. Pardini B, Kumar R, Naccarati A, Novotny J, Prasad RB, Forsti A, et al. 5-fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br J Clin Pharmacol (2011) 72:162–3. doi: 10.1111/j.1365-2125.2010.03892.x
7. Sohn KJ, Croxford R, Yates Z, Lucock M, Kim YI. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate. J Natl Cancer Inst (2004) 96:134–44. doi: 10.1093/jnci/djh015
8. Van Der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet (1998) 62:1044–51. doi: 10.1086/301825
9. Nahid NA, Apu MNH, Islam MR, Shabnaz S, Chowdhury SM, Ahmed MU, et al. DPYD*2A and MTHFR C677T predict toxicity and efficacy, respectively, in patients on chemotherapy with 5-fluorouracil for colorectal cancer. Cancer Chemother Pharmacol (2018) 81:119–29. doi: 10.1007/s00280-017-3478-3
10. Afzal S, Jensen SA, Vainer B, Vogel U, Matsen JP, Sorensen JB, et al. MTHFR polymorphisms and 5-FU-based adjuvant chemotherapy in colorectal cancer. Ann Oncol (2009) 20:1660–6. doi: 10.1093/annonc/mdp046
11. Etienne MC, Ilc K, Formento JL, Laurent-Puig P, Formento P, Cheradame S, et al. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphisms: relationships with 5-fluorouracil sensitivity. Br J Cancer (2004) 90:526–34. doi: 10.1038/sj.bjc.6601523
12. Etienne-Grimaldi MC, Milano G, Maindrault-Goebel F, Chibaudel B, Formento JL, Francoual M, et al. Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and FOLFOX response in colorectal cancer patients. Br J Clin Pharmacol (2010) 69:58–66. doi: 10.1111/j.1365-2125.2009.03556.x
13. Zhong L, He X, Zhang Y, Chuan JL, Chen M, Zhu SM, et al. Relevance of methylenetetrahydrofolate reductase gene variants C677T and A1298C with response to fluoropyrimidine-based chemotherapy in colorectal cancer: a systematic review and meta-analysis. Oncotarget (2018) 9:31291–301. doi: 10.18632/oncotarget.24933
14. De Mattia E, Toffoli G. C677T and A1298C MTHFR polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur J Cancer (2009) 45:1333–51. doi: 10.1016/j.ejca.2008.12.004
15. Zhu X, Li W, Zhu J, Chen H, Guan J, Zhou D, et al. Influence of MTHFR C677T and A1298C polymorphisms on the survival of pediatric patients with non-Hodgkin lymphoma. Leuk Lymphoma (2021) 62:2374–82. doi: 10.1080/10428194.2021.1927017
16. Wang X, Fu J, Li Q, Zeng D. Geographical and ethnic distributions of the MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in chinese populations: a meta-analysis. PLoS One (2016) 11:e0152414. doi: 10.1371/journal.pone.0152414
17. Nefic H, Mackic-Djurovic M, Eminovic I. The frequency of the 677C>T and 1298A>C polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene in the population. Med Arch (2018) 72:164–9. doi: 10.5455/medarh.2018.72.164-169
18. Bagheri M, Ardi Rad I. Frequency of the methylenetetrahydrofolate REDUCTASE 677CT and 1298AC mutations in an Iranian Turkish female population. Maedica (Bucur) (2010) 5:171–7.
19. Ludovini V, Antognelli C, Rulli A, Foglietta J, Pistola L, Eliana R, et al. Influence of chemotherapeutic drug-related gene polymorphisms on toxicity and survival of early breast cancer patients receiving adjuvant chemotherapy. BMC Cancer (2017) 17:502. doi: 10.1186/s12885-017-3483-2
20. Tang C, Yu S, Jiang H, Li W, Xu X, Cheng X, et al. A meta-analysis: methylenetetrahydrofolate reductase C677T polymorphism in gastric cancer patients treated with 5-fu based chemotherapy predicts serious hematologic toxicity but not prognosis. J Cancer (2018) 9:1057–66. doi: 10.7150/jca.23391
21. Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the german 5-FU toxicity study group. J Clin Oncol (2008) 26:2131–8. doi: 10.1200/JCO.2006.10.4182
22. Ahmad F, Kanna M, Yadav V, Biswas A, Saxena R. Impact of thrombogenic mutations on clinical phenotypes of von willebrand disease. Clin Appl Thromb Hemost (2010) 16:281–7. doi: 10.1177/1076029609351291
23. Franchini M, Veneri D, Poli G, Manzato F, Salvagno GL, Lippi G. High prevalence of inherited prothrombotic risk factors in 134 consecutive patients with von willebrand disease. Am J Hematol (2006) 81:465–7. doi: 10.1002/ajh.20623
24. Adamo V, Franchina T, Adamo B, Briguglio R, Restuccia E, Chiofalo G, et al. Role of MTHFR polymorphisms as predictive markers of acute toxicity during 5-fluorouracil based chemotherapy for colorectal cancer: Preliminary data. J Clin Oncol (2008) 26:15036–6. doi: 10.1200/jco.2008.26.15_suppl.15036
Keywords: 5-fluorouracil, colorectal cancer, MTHFR polymorphisms, toxicity, efficacy
Citation: Atasilp C, Lenavat R, Vanwong N, Chansriwong P, Sirachainan E, Reungwetwattana T, Jinda P, Aiempradit S, Sirilerttrakul S, Chamnanphon M, Puangpetch A, Sankuntaw N, Satapornpong P and Sukasem C (2022) Effects of polymorphisms in the MTHFR gene on 5-FU hematological toxicity and efficacy in Thai colorectal cancer patients. Front. Oncol. 12:916650. doi: 10.3389/fonc.2022.916650
Received: 09 April 2022; Accepted: 27 June 2022;
Published: 15 July 2022.
Edited by:
Afsaneh Barzi, City of Hope National Medical Center, United StatesReviewed by:
Larysa Sydorchuk, Bukovinian State Medical University, UkraineCopyright © 2022 Atasilp, Lenavat, Vanwong, Chansriwong, Sirachainan, Reungwetwattana, Jinda, Aiempradit, Sirilerttrakul, Chamnanphon, Puangpetch, Sankuntaw, Satapornpong and Sukasem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chonlaphat Sukasem, Y2hvbmxhcGhhdC5zdWtAbWFoaWRvbC5hYy50aA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.