
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 July 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.916442
 Dao-Xing Deng1†
Dao-Xing Deng1† Shuang Fan1†
Shuang Fan1† Xiao-Hui Zhang1
Xiao-Hui Zhang1 Lan-Ping Xu1
Lan-Ping Xu1 Yu Wang1
Yu Wang1 Chen-Hua Yan1
Chen-Hua Yan1 Huan Chen1
Huan Chen1 Yu-Hong Chen1
Yu-Hong Chen1 Wei Han1
Wei Han1 Feng-Rong Wang1
Feng-Rong Wang1 Jing-Zhi Wang1
Jing-Zhi Wang1 Xu-Ying Pei1
Xu-Ying Pei1 Ying-Jun Chang1
Ying-Jun Chang1 Kai-Yan Liu1
Kai-Yan Liu1 Xiao-Jun Huang1,2,3
Xiao-Jun Huang1,2,3 Xiao-Dong Mo1,3*
Xiao-Dong Mo1,3*We aimed to identify the characteristics of immune reconstitution (IR) in patients who recovered from steroid-refractory acute graft-versus-host disease (SR-aGVHD) after basiliximab treatment. A total of 179, 124, 80, and 92 patients were included in the analysis for IR at 3, 6, 9, and 12 months, respectively, after haploidentical donor hematopoietic stem cell transplantation (HID HSCT). We observed that IR was fastest for monocytes and CD8+ T cells, followed by lymphocytes, CD3+ T cells, and CD19+ B cells and slowest for CD4+ T cells. Almost all immune cell subsets recovered comparably between patients receiving <5 doses and ≥5 doses of basiliximab. Most immune cell subsets recovered comparably between SR-aGVHD patients who recovered after basiliximab treatment and event-free HID HSCT recipients. Patients who recovered from SR-aGVHD after basiliximab treatment experienced satisfactory IR, which suggested that basiliximab may not have prolonged the negative impact on IR in these patients.
Human leukocyte antigen (HLA) haploidentical donor (HID) has become one of the most important donors for allogeneic hematopoietic stem cell transplantation (allo-HSCT) (1), which accounts for the proportion at 60% among all of the allo-HSCTs in China (2). However, although many strategies [e.g., anti-thymocyte globulin (ATG)] are used to prevent acute graft-versus-host disease (aGVHD), there are still 40%–50% of patients suffering from grade II–IV aGVHD, which remains one of the major causes of early mortality after HID HSCT (3).
Corticosteroids are the first-line treatment for aGVHD, but their response rate can only reach nearly 50% (4, 5). In addition, Liu et al. (6) reported that the complete response (CR) rate of steroid treatment for grade II–IV aGVHD was only 34.1% in HID HSCT recipients. Thus, steroid-refractory (SR)-aGVHD is common; however, there is no consensus on the best therapeutic options for HID HSCT recipients (7). Considering that interleukin-2 (IL-2) plays a critical role in aGVHD pathogenesis, IL-2 receptor blockade (e.g., basiliximab) is important for SR-aGVHD therapy (8–10). Several studies have identified the efficacy of basiliximab in SR-aGVHD patients (11–14). In addition, 2 cohorts with large samples of HID HSCT recipients reported that the overall response rate (ORR) of basiliximab therapy was approximately 80% in SR-aGVHD patients and the long-term survival could achieve more than 60% (15, 16). Thus far, basiliximab has been the most important treatment for SR-aGVHD in China (17).
Immune reconstitution (IR) was important for long-term survivors after allo-HSCT, and patients with poor IR were associated with a higher risk of infections and non-relapse mortality (NRM) (18, 19). Active aGVHD could influence IR (20). In addition, SR-aGVHD patients would receive intensive immunosuppressants, which might show a prolonged negative impact on IR even in those who recovered after therapies. Because more and more SR-aGVHD patients could be cured and achieve long-term survival after second-line or third-line therapies, it was necessary to identify the characteristics of IR in these patients. However, to the best of our knowledge, no study had identified the characteristics of IR in patients who recovered from SR-aGVHD and achieved GVHD-free survival.
Particularly, SR-aGVHD mostly occurs in HID HSCT recipients (15). Although some authors reported the characteristics of IR in event-free HID HSCT recipients (21, 22), patients with aGVHD were excluded and whether patients who recovered from SR-aGVHD had the same characteristics of IR was unclear.
Thus, we aimed to identify the characteristics of IR in SR-aGVHD patients who recovered from SR-aGVHD with the help of basiliximab treatment after HID HSCT.
Consecutive HID HSCT recipients who recovered from SR-GVHD after basiliximab therapy at the Peking University Institute of Hematology (PUIH) from January 2016 to December 2018 were enrolled in this retrospective study. In order to reflect the true rule of IR in patients who recovered from SR-aGVHD after basiliximab, we excluded patients who did not achieve an ORR after basiliximab treatment. In addition, patients who had other situations that could influence IR, including aGVHD recurrence, serious infection [i.e., serious bacterial infections, invasive fungal infection (IFI), and posttransplant lymphoproliferative disorders (PTLDs)], moderate to severe chronic GVHD (cGVHD), relapse, NRM, receiving donor lymphocyte infusion (DLI), receiving second allo-HSCT, were all excluded. Patients who did not monitor IR after HSCT were also excluded (Figure 1). Considering that most of the patients recovered from SR-aGVHD within 3 months after HSCT, we focused on the data of IR at 3, 6, 9, and 12 months following HID HSCT. The last follow-up for survivors was conducted on September 30, 2021. To further identify the IR in patients who recovered from SR-aGVHD, two historical cohorts (event-free HID HSCT recipients: n = 85 and healthy donors: n = 41) reported by Pei et al. (21) were also included as control. The study protocol was approved by the institutional review board of Peking University People’s Hospital and was conducted in accordance with the Declaration of Helsinki.
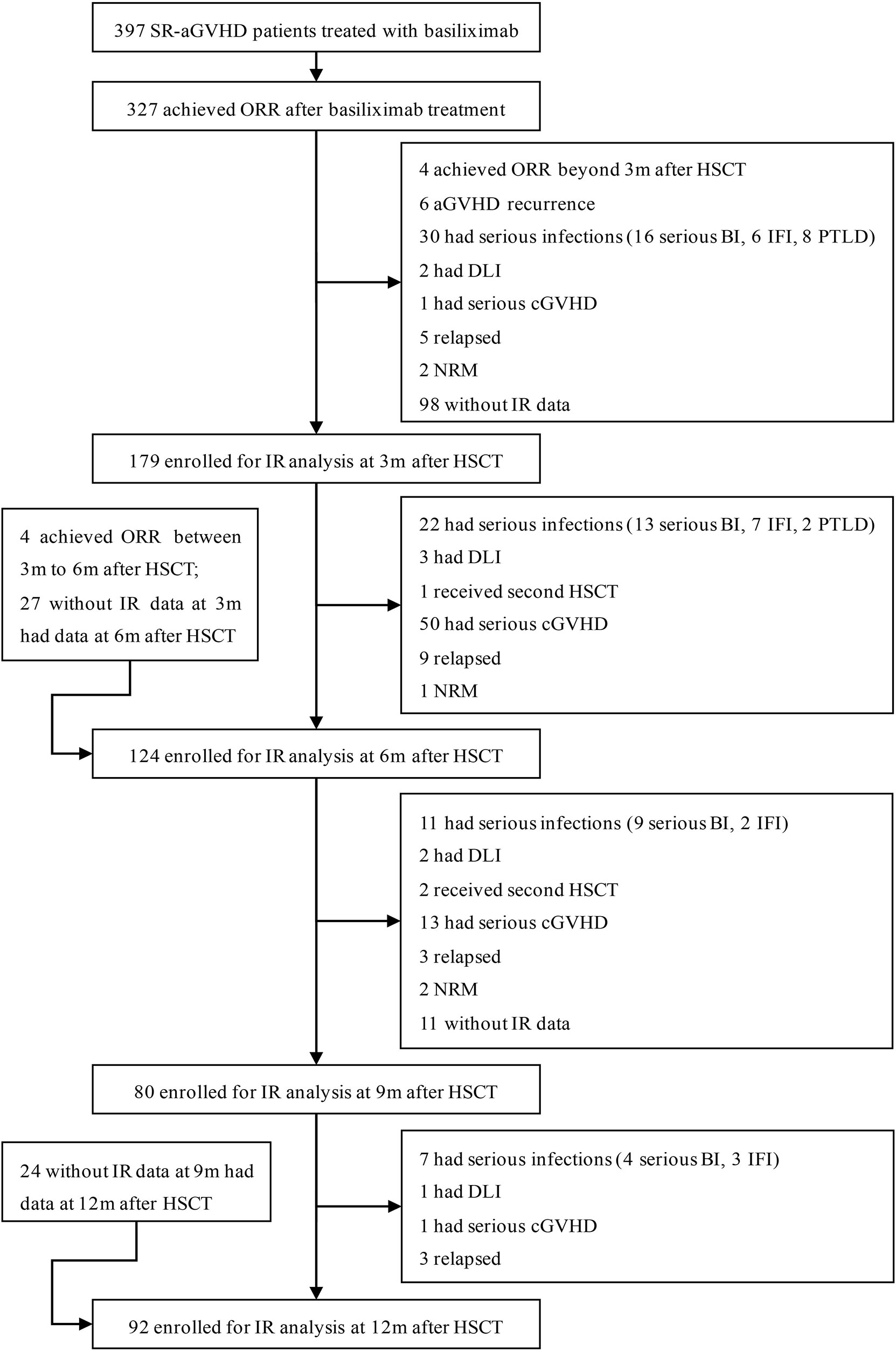
Figure 1 Diagram of patients enrolled. A total of 327 SR-aGVHD patients who showed an ORR to basiliximab treatment were eligible, and 179, 124, 80, and 92 patients were included in the final analysis for IR at 3, 6, 9, and 12 months, respectively, after HID HSCT. HID HSCT, haploidentical donor hematopoietic stem cell transplantation; IR, immune reconstitution; NRM, non-relapse mortality; ORR, overall response rate; SR-aGVHD, steroid-refractory acute graft-versus-host disease.
For patients with hematologic malignancies, the preconditioning regimen mainly included cytarabine, busulfex, cyclophosphamide, simustine, and rabbit anti-thymocyte globulin (ATG) (23, 24). For patients with severe aplastic anemia, the preconditioning regimen mainly included busulfex, cyclophosphamide, and ATG (25). We used cyclosporine A (CSA), mycophenolate mofetil (MMF), and methotrexate (MTX) to prevent GVHD (26, 27). The protocol of basiliximab (Simulect; Novartis Pharma AG, Basel, Switzerland) treatment was shown in the Supplementary methods (15, 28).
The total lymphocyte and monocyte counts were detected by peripheral blood cell analysis at 1, 2, 3, 6, 9, and 12 months following HID HSCT. Immune cell subsets were recognized and measured by multiparameter flow cytometry (MFC) at 1, 2, 3, 6, 9, and 12 months following allo-HSCT, as described previously (Supplementary methods) (22). Serum IgA, IgG, and IgM levels were detected by immunonephelometry.
Good IR was defined as at least 3 types of major immune cells (i.e., monocytes, lymphocytes, CD19+ B cells, CD3+ T cells, CD4+ T cells, and CD8+ T cells) meeting or exceeding the median value of event-free HID HSCT cohort. cGVHD was diagnosed and categorized according to accepted international criteria (29). The definition of relapse, NRM, disease-free survival (DFS), and overall survival (OS) were shown in the Supplementary methods.
Continuous variables were compared using the Mann–Whitney U test; categorical variables were compared using χ2 and Fisher’s exact tests. Survival was estimated by the Kaplan–Meier method. Competing risk analysis was used for estimating relapse and NRM, and the Gray’s test was applied for comparisons between subgroups. NRM was the competing event for relapse and vice versa. P values were two-sided, and P < 0.05 was considered to be statistically significant. SPSS 26 (SPSS Inc./IBM, Armonk, NY, USA) and the R software package (version 4.0.0; http://www.r-project.org) were used for data analysis.
Among 327 patients achieving ORR after basiliximab treatment, 265 patients achieved DFS at 1 year after HSCT. A total of 231 SR-aGVHD patients were included in the analysis for IR because they offered at least once IR data at 3, 6, 9, or 12 months after HSCT, and their characteristics were shown in Table 1. Among them, 188 and 43 patients received <5 doses and ≥5 doses of basiliximab treatments, respectively. The median time from allo-HSCT to basiliximab treatment was 24 (range, 12–95) days, the median interval from the occurrence of aGVHD to basiliximab treatment was 5 (range, 3–25) days, and the median doses of basiliximab were 3 (range, 2–11) doses. A total of 179, 124, 80, and 92 patients were included in the analysis for IR at 3, 6, 9, and 12 months, respectively (Figure 1), and their characteristics were compared in Tables S1–S4.
Among a total of 231 SR-aGVHD patients included in the analysis for IR, 180 and 151 patients had IR data available at 1 and 2 months following HSCT, respectively. The CD4+ T cells (P = 0.035), CD4+naïve T cells (CD4+CD45RA+ T cells) (P = 0.017), and CD4+CD28+ T cells (P = 0.042) were higher in patients receiving ≥5 doses of basiliximab compared with those receiving <5 doses of basiliximab at 1 month after HID HSCT (Table 2; Figure 2). But they were comparable between patients receiving <5 doses and ≥5 doses of basiliximab at 2 months after HID HSCT (Table 2; Figure 2). The monocytes, lymphocytes, CD19+ B cells, CD3+ T cells, CD8+ T cells, CD4+ memory T cells (CD4+CD45RO+ T cells), CD4+CD25+ T cells and CD8+CD28+ T cells were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab at 1 and 2 months after HID HSCT, which were also observed in both grade II and grade III–IV aGVHD groups (Table 2; Figures 2–4).
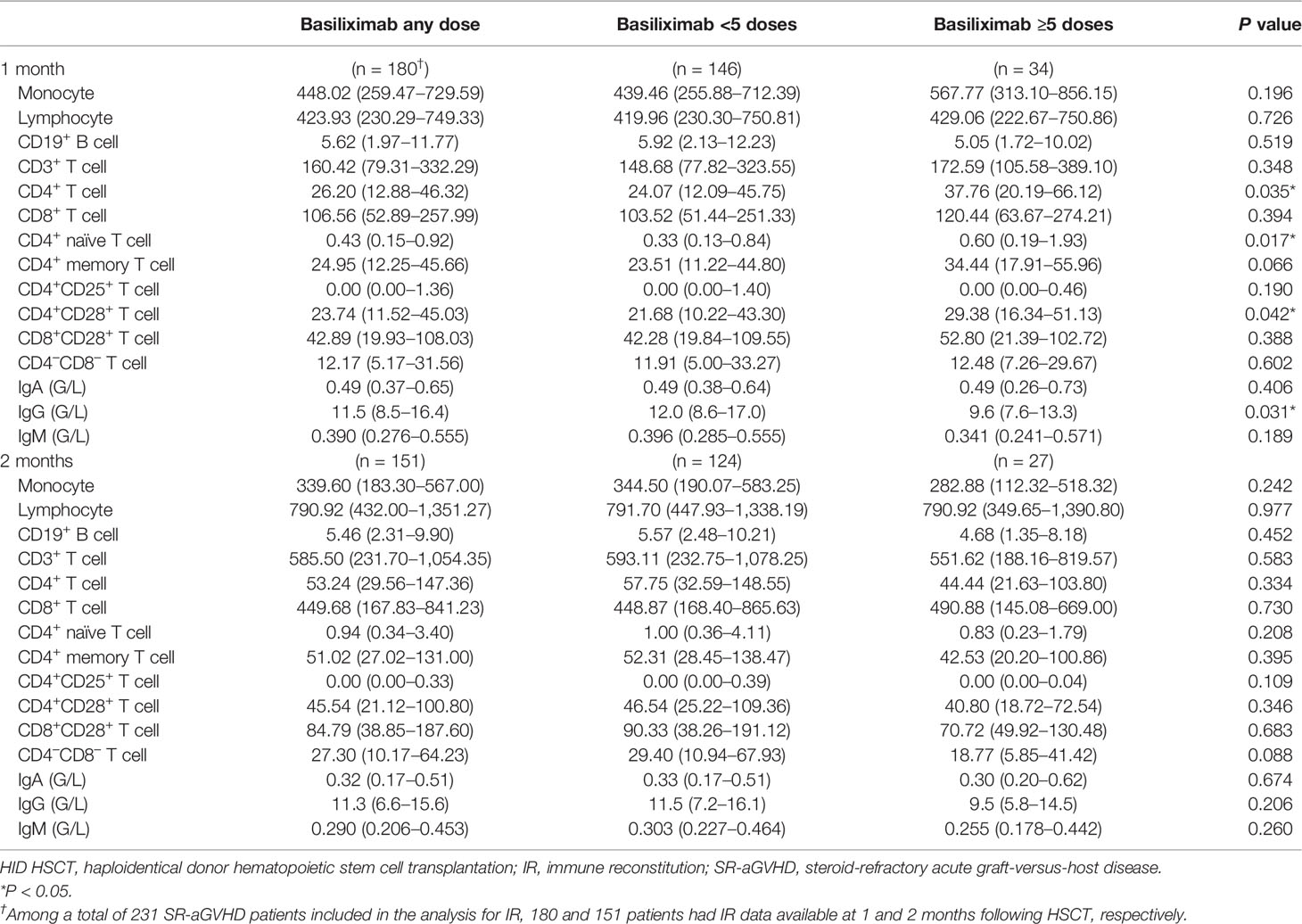
Table 2 The values of immune cell subset counts at 1 and 2 months after HID HSCT [median (25th–75th)].
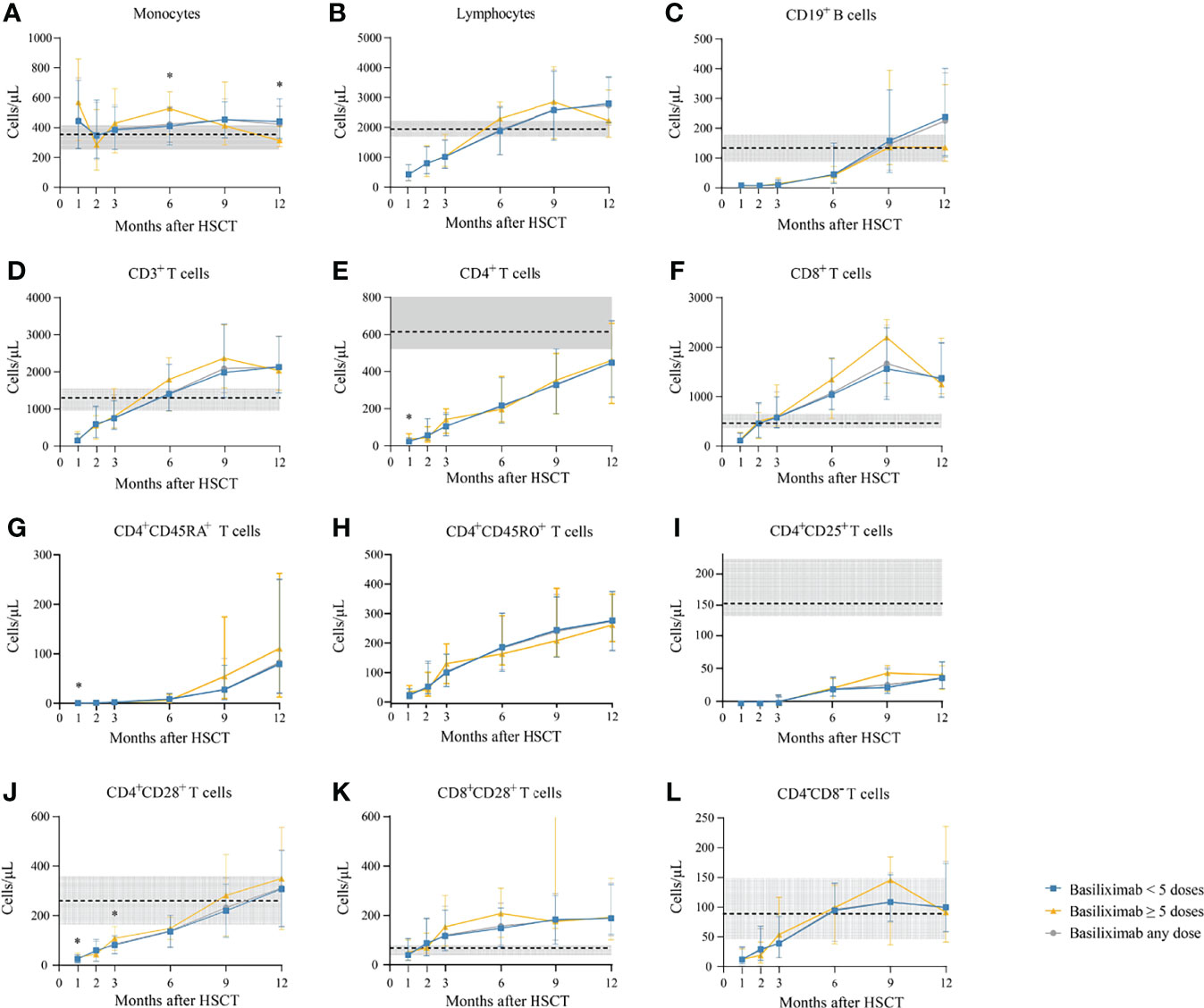
Figure 2 (A-L) Kinetics of immune reconstitution in the total population that showed an ORR after basiliximab treatment. Immune cell subsets are compared between basiliximab <5 doses and basiliximab ≥5 doses. Because of lack of data about CD4+CD45RA+ T cells and CD4+CD45RO+ T cells in healthy donors, the values of these two immune cell subsets in healthy donors are not shown. Data are shown as median absolute counts with error bars indicating the 25th–75th percentiles. The horizontal dotted lines represent the median value of healthy cohorts, and the gray areas represent the 25th–75th percentiles for the healthy cohorts. *P < 0.05, basiliximab <5 doses vs. basiliximab ≥5 doses. ORR, overall response rate.
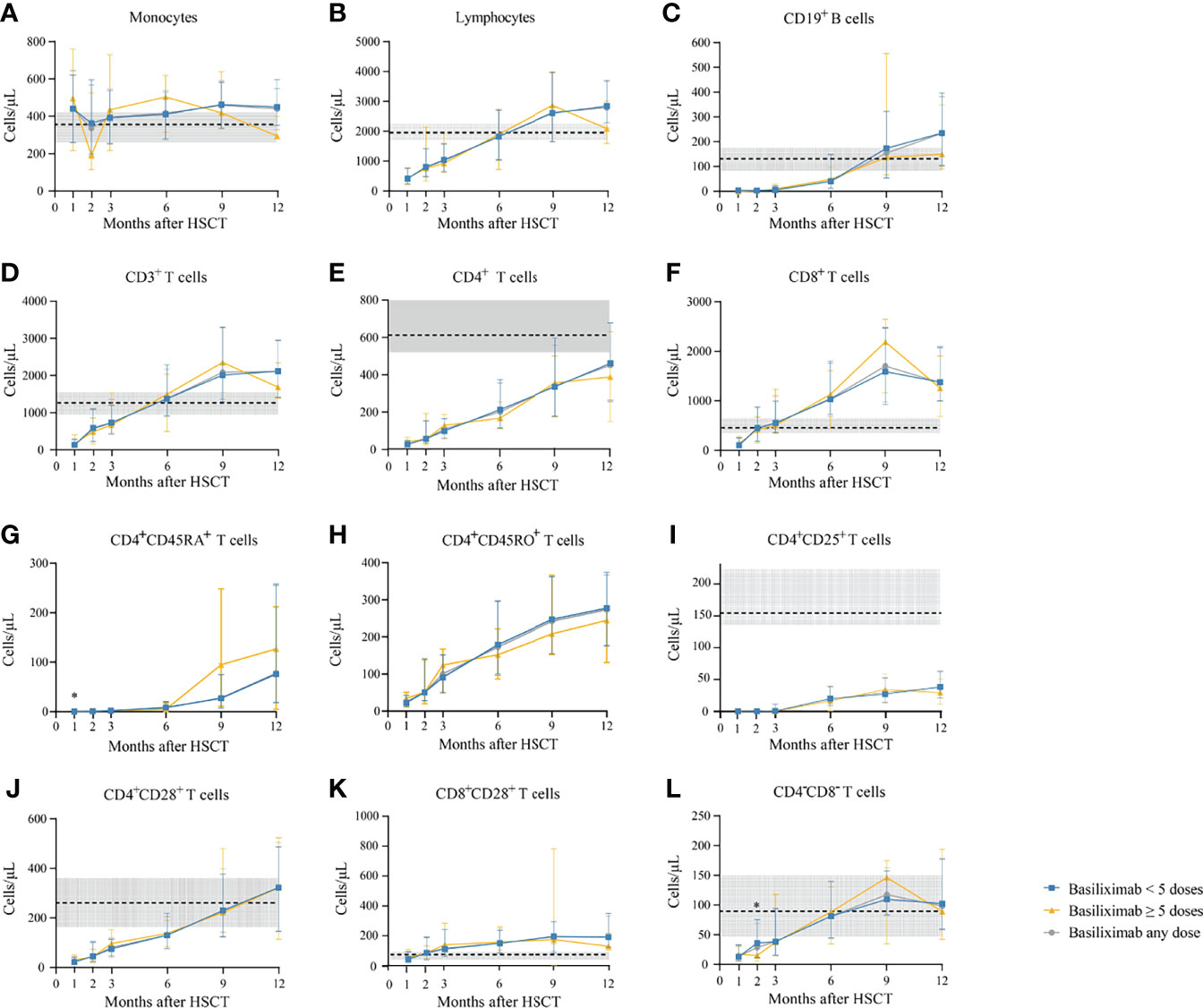
Figure 3 (A-L) Kinetics of immune reconstitution in grade II SR-aGVHD patients who showed an ORR after basiliximab treatment. Because of lack of data about CD4+CD45RA+ T cells and CD4+CD45RO+ T cells in healthy donors, the values of these two immune cell subsets in healthy donors are not shown. Data are shown as median absolute counts with error bars indicating the 25th–75th percentiles. The horizontal dotted lines represent the median value of healthy cohorts, and the gray areas represent the 25th–75th percentiles for the healthy cohorts. *P < 0.05, basiliximab <5 doses vs. basiliximab ≥5 doses. ORR, overall response rate; SR-aGVHD, steroid-refractory acute graft-versus-host disease.
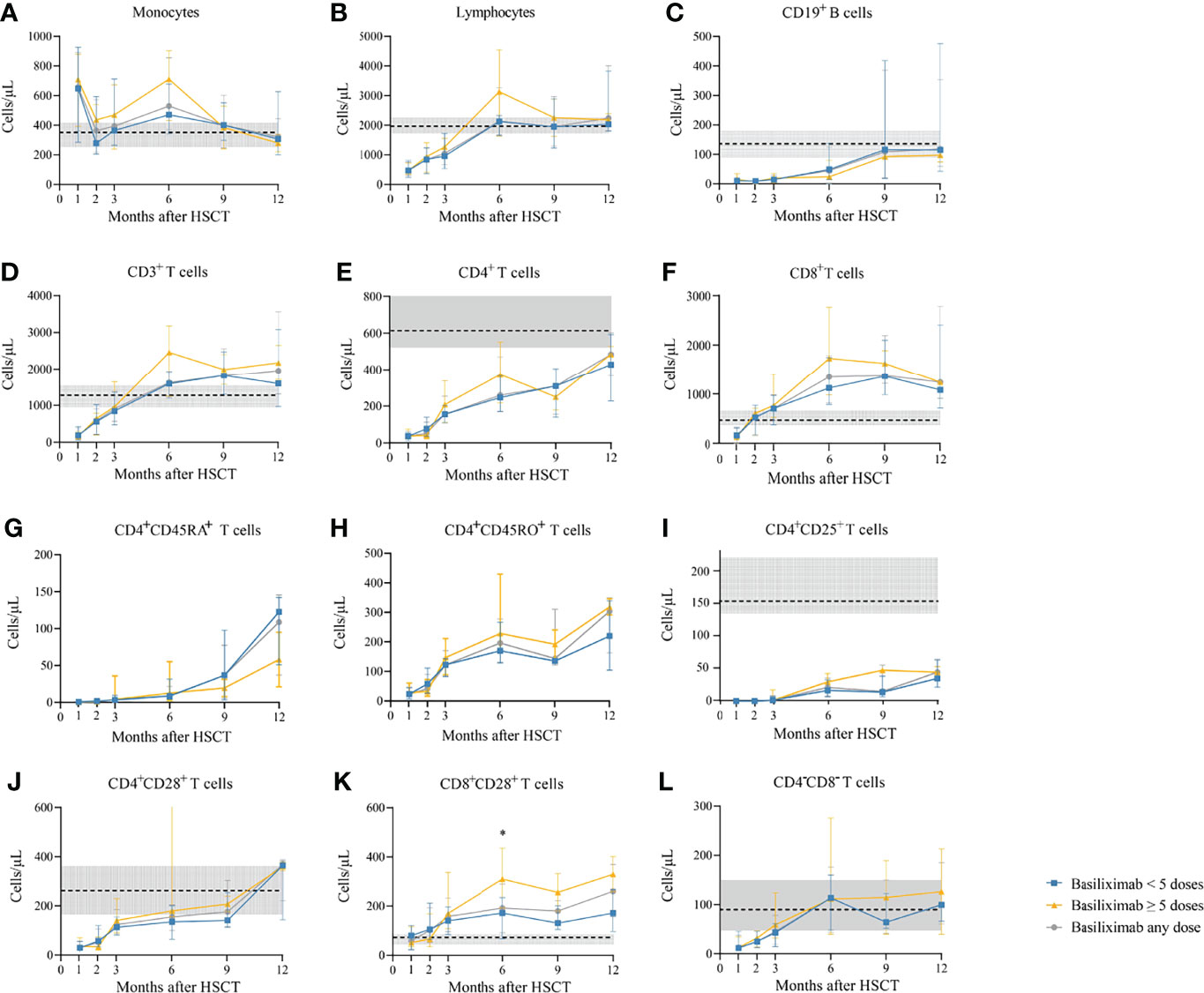
Figure 4 (A-L) Kinetics of immune reconstitution in grade III–IV SR-aGVHD patients who showed an ORR after basiliximab treatment. Because of lack of data about CD4+CD45RA+ T cells and CD4+CD45RO+ T cells in healthy donors, the values of these two immune cell subsets in healthy donors are not shown. Data are shown as median absolute counts with error bars indicating the 25th–75th percentiles. The horizontal dotted lines represent the median value of healthy cohorts, and the gray areas represent the 25th–75th percentiles for the healthy cohorts. *P < 0.05, basiliximab <5 doses vs. basiliximab ≥5 doses. ORR, overall response rate; SR-aGVHD, steroid-refractory acute graft-versus-host disease.
Serum IgG was lower in patients receiving ≥5 doses of basiliximab compared with those receiving <5 doses of basiliximab at 1 month after HID HSCT (P = 0.031) (Table 2; Figure 5). However, IgG at 2 months after HID HSCT was comparable between patients receiving <5 doses and ≥5 doses of basiliximab (Table 2, Figure 5B), which was also observed in both grade II and grade III–IV aGVHD groups (Figures 5E, H). Serum IgA and IgM at 1 and 2 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab (Table 2, Figures 5A, C), which were also observed in both grade II and grade III–IV aGVHD groups (Figures 5D, F, G, I).
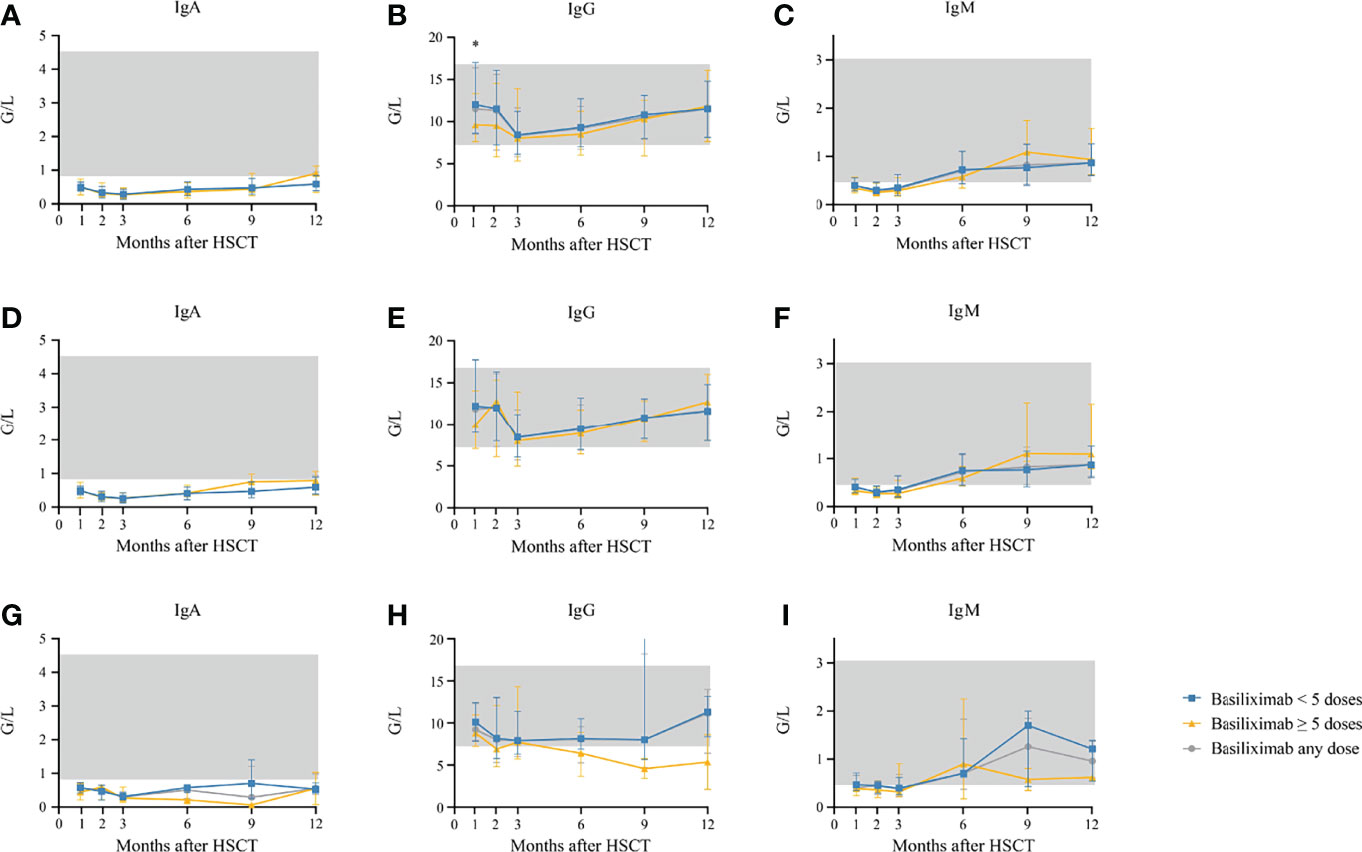
Figure 5 (A–C) Kinetics of IgA, IgG, and IgM in the total population who showed an ORR after basiliximab treatment; (D–F) Kinetics of IgA, IgG, and IgM in grade II SR-aGVHD patients who showed an ORR after basiliximab treatment; (G–I) Kinetics of IgA, IgG, and IgM in grade III–IV SR-aGVHD patients who showed an ORR after basiliximab treatment. IgA, IgG, and IgM are compared between basiliximab <5 doses and basiliximab ≥5 doses. Data are shown as median absolute counts with error bars indicating the 25th–75th percentiles. The gray areas represent the normal range. *P < 0.05, basiliximab <5 doses vs. basiliximab ≥5 doses. ORR, overall response rate; SR-aGVHD, steroid-refractory acute graft-versus-host disease.
The absolute counts of the immune cells at 3, 6, 9, and 12 months after HID HSCT were shown in Table 3 and Figures 2–4.
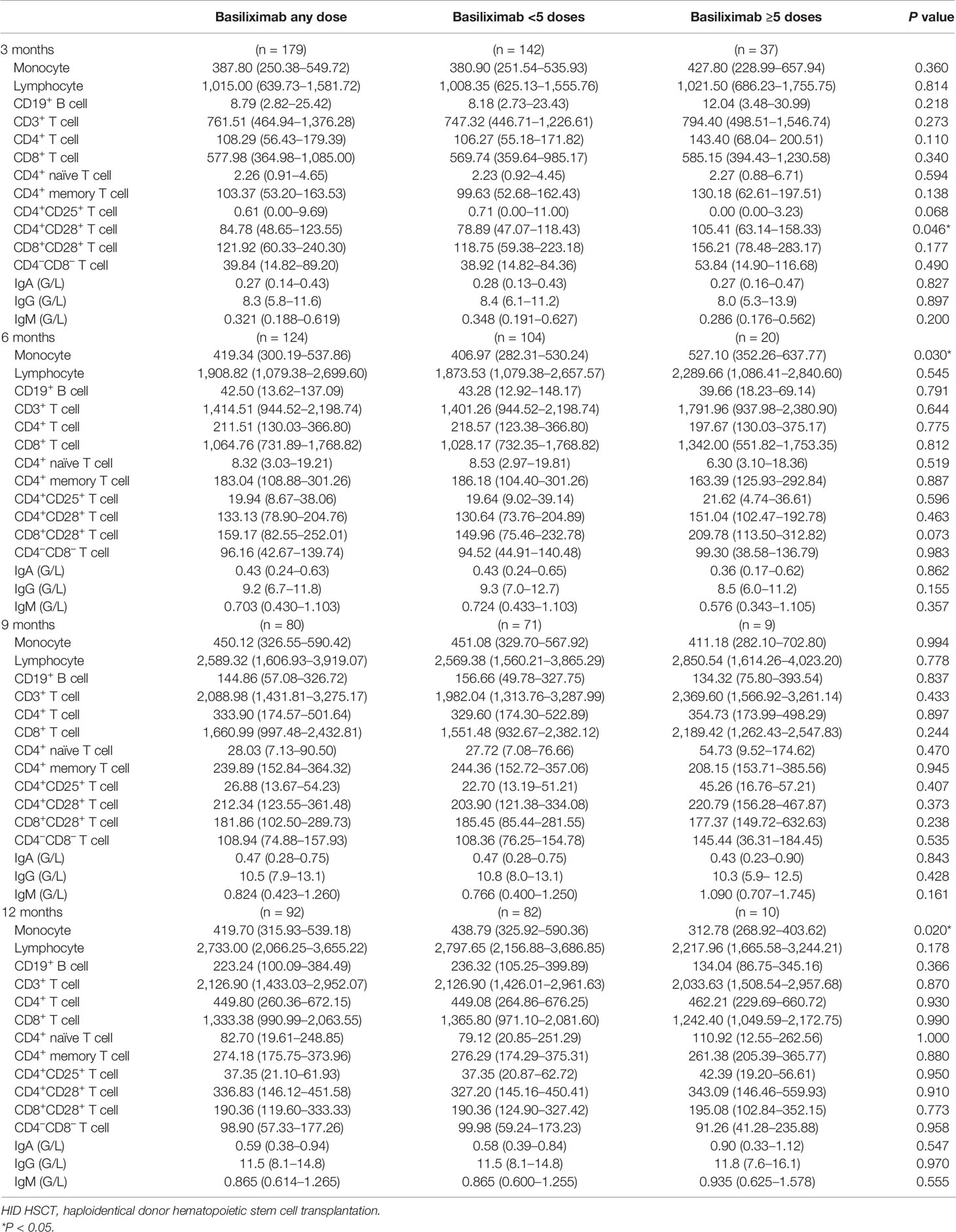
Table 3 The values of immune cell subset counts at 3, 6, 9, and 12 months after HID HSCT [median (25th–75th)].
The monocyte counts achieved a comparable level in healthy controls since the third month after HID HSCT (Figure 2A). The monocyte absolute counts were comparable between patients receiving <5 doses and ≥5 doses of basiliximab at 3 and 9 months after HID HSCT (Figure 2A); however, it was higher in patients receiving ≥5 doses of basiliximab compared with those receiving <5 doses of basiliximab at 6 months after HID HSCT (P = 0.03). In addition, the monocyte absolute counts were lower in patients receiving ≥5 doses of basiliximab compared with those receiving <5 doses of basiliximab at 12 months after HID HSCT (P = 0.02). The monocyte absolute counts at all monitoring points were comparable between patients receiving <5 doses and ≥5 doses of basiliximab in both grade II and grade III–IV aGVHD groups (Figures 3A, 4A).
The lymphocyte absolute counts achieved a comparable level in healthy controls since the sixth month after HID HSCT (Figure 2B). The lymphocyte absolute counts at 3, 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab (Figure 2B), which were also observed in both grade II and grade III–IV aGVHD groups (Figures 3B, 4B).
The absolute counts of CD19+ B cells achieved comparable levels in healthy controls since the ninth month after HID HSCT (Figure 2C). The absolute counts of CD19+ B cells at 3, 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab, which were also observed in both grade II and grade III–IV aGVHD groups (Figures 3C, 4C).
The absolute counts of CD3+ T cells achieved comparable levels in healthy controls since the sixth month after HID HSCT (Figure 2D). The absolute counts of CD3+ T cells at 3, 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab (Figure 2D), which were also observed in both grade II and grade III–IV aGVHD groups (Figures 3D, 4D).
The absolute counts of CD4+ T cells and CD4+CD25+ T cells did not achieve comparable levels in healthy controls within 1 year after HID HSCT (Figures 2E, I). The absolute counts of CD4+CD28+ T cells achieved comparable levels in healthy controls since the ninth month after HID HSCT (Figure 2J).
The absolute counts of CD4+ T cells (Figure 2E), CD4+CD45RA+ T cells (Figure 2G), CD4+CD45RO+ T cells (Figure 2H), and CD4+CD25+ T cells (Figure 2I) at all monitoring points and CD4+CD28+ T cells (Figure 2J) at 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab, which were also observed in both grade II and grade III–IV aGVHD groups (Figures 3E, G–J, 4E, G–J). The absolute counts of CD4+CD28+ T cells of patients receiving ≥5 doses of basiliximab were higher than those receiving <5 doses of basiliximab at 3 months after HID HSCT (P = 0.046) (Figure 2J), but they were comparable between patients receiving <5 doses and ≥5 doses of basiliximab in either grade II or grade III–IV aGVHD groups (Figures 3J, 4J).
The absolute counts of CD8+ T cells (Figure 2F) and CD8+CD28+ T cells (Figure 2K) both achieved comparable levels in healthy controls since the third month after HID HSCT. The absolute counts of CD8+ T cells (Figure 2F) and CD8+CD28+ T cells (Figure 2K) at 3, 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab, which were also observed in both grade II and grade III–IV aGVHD groups (Figures 3F, K, 4F, K), except that the absolute count of CD8+CD28+ T cells at 6 months after HID HSCT was lower in patients receiving <5 doses compared with that of those receiving ≥5 doses of basiliximab in grade III–IV aGVHD groups (P = 0.02) (Figure 4K).
The absolute counts of CD4–CD8– T cells achieved comparable levels in healthy controls since the sixth month after HID HSCT (Figure 2L). The absolute counts of CD4–CD8– T cells at 3, 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab (Figure 2L), which were also observed in both grade II and grade III–IV aGVHD groups (Figures 3L, 4L).
Serum IgA (normal range: 0.82–4.53 G/L) did not reach the normal range within 1 year after HID HSCT (Figure 5A). Serum IgG (normal range: 7.2–16.8 G/L) and IgM (normal range: 0.460–3.040 G/L) recovered to the normal range since the first and sixth month after HID HSCT, respectively (Figures 5B, C). The levels of serum IgA, IgG, and IgM at 3, 6, 9, and 12 months after HID HSCT were all comparable between patients receiving <5 doses and ≥5 doses of basiliximab (Table 3; Figures 5A–C), which were also observed in both grade II and grade III–IV aGVHD groups (Figures 5D–I).
The characteristics of IR at 3, 6, and 12 months after HID HSCT between patients who recovered from SR-aGVHD after basiliximab therapy and event-free HID HSCT patients in the historical cohort were shown in Figure 6. We observed that the evolution of CD19+ B cells, CD8+ T cells, and CD8+CD28+ T cells was all comparable between these two cohorts at each time point. Similarly, the evolution of monocytes, lymphocytes, CD3+ T cells, and CD4+ T cells was basically comparable between these two cohorts. SR-aGVHD patients who recovered after basiliximab treatment showed a slower recovery of CD4+CD25+ T cells and a faster recovery of CD4+CD28+ T cells at each time point compared with those of event-free HID HSCT recipients.
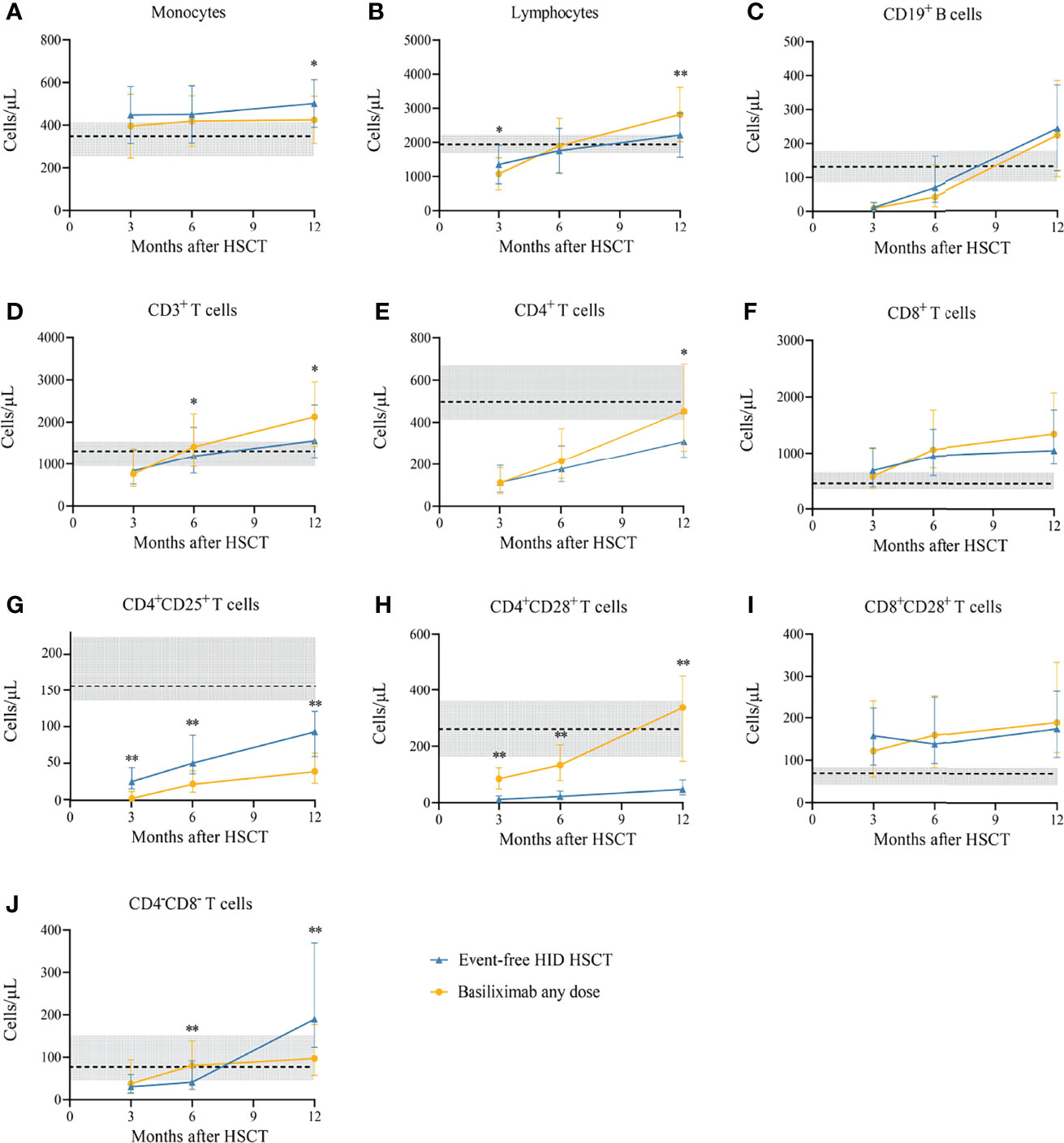
Figure 6 (A-J) Immune reconstitution between SR-aGVHD patients who showed an ORR after basiliximab treatment and event-free HID HSCT recipients. Data are shown as median absolute counts with error bars indicating the 25th–75th percentiles. The horizontal dotted lines represent the median value of healthy cohorts, and the gray areas represent the 25th–75th percentiles for the healthy cohorts. *P < 0.05, **P < 0.01, basiliximab any dose vs. event-free HID HSCT. HID HSCT, haploidentical donor hematopoietic stem cell transplantation; ORR, overall response rate; SR-aGVHD, steroid-refractory acute graft-versus-host disease.
The infection events of SR-aGVHD patients who recovered after basiliximab treatment were shown in Tables S5–S10. At 3 months after HID HSCT, patients with good IR showed a lower rate of any infection, viral infection, and serious bacterial infection than those with poor IR. However, at 6 and 12 months after HID HSCT, the rates of infection were all comparable between patients with and without good IR. The incidences of cGVHD, NRM, DFS, and OS were all comparable between patients with and without good IR (Tables S11–S13). In addition, at 3 months after HID HSCT, patients with good IgM IR showed a lower rate of any infection than those with poor IgM IR. However, the infections were all comparable between patients with and without good IR about IgM at 6 and 12 months after HID HSCT, as well as IgA and IgG at any monitoring point after HID HSCT (Tables S14–S22).
In the present study, we firstly evaluated the kinetics of IR in patients who recovered from SR-aGVHD after basiliximab treatment following HID HSCT. We observed that 1) IR was fastest for monocytes and CD8+ T cells, followed by lymphocytes, CD3+ T cells, and CD19+ B cells, and slowest for CD4+ T cells; 2) Almost all immune cell subsets recovered comparably between patients receiving <5 doses and ≥5 doses of basiliximab; 3) Most immune cell subsets recovered comparably between patients who recovered from SR-aGVHD after basiliximab treatment and event-free patients; 4) All immune cell subsets except CD4+ T cells and CD4+CD25+ T cells achieved comparable levels in healthy donors within 1 year after HID HSCT. To the best of our knowledge, this was the first study to identify the IR in patients who recovered from SR-aGVHD, and our results firstly demonstrated that basiliximab might not have prolonged negative effect on IR in SR-aGVHD survivors.
We observed that in patients who recovered from SR-aGVHD after basiliximab therapy, CD8+ T cells rapidly achieved similar levels compared with those of healthy donors and event-free HID HSCT recipients. Basiliximab selectively inhibited activated T lymphocytes, and it did not significantly influence the total peripheral lymphocyte or lymphocyte subset counts (30). Basiliximab did not downregulate IL-2 receptor expression on circulating T lymphocytes either. The terminal elimination half-life of basiliximab was reported to be 13.4 days in adults and 9.4 days in children (31, 32), and the duration of IL-2 receptor saturation with basiliximab was 4–6 weeks and 29 days, respectively, in adults and children (30, 33). Thus, although basiliximab could inhibit the rapid clonal expansion of activated T lymphocytes, it did not show prolonged inhibition on IR of CD8+ T cells after the end of basiliximab treatment. Several studies observed that CD8+ T cell recovery was associated with improved transplant outcomes. Tian et al. (34) reported that rapid recovery of CD8+ T cells was associated with better prognosis. In addition, Pei et al. (21) reported that an extremely high CD8+ T cell count (684 cells/µl) was observed in event-free patients at 3 months after HID HSCT. Our results showed that basiliximab did not influence the IR of CD8+ T cells in SR-aGVHD survivors, which may contribute to good prognosis of our patients.
In addition, we also observed that monocytes of SR-aGVHD survivors rapidly achieved similar levels compared with those of healthy donors and event-free HID HSCT recipients at most time points. It was because basiliximab did not inhibit the monocyte (35). On the contrary, some second-line therapies, such as ruxolitinib, could significantly inhibit the monocyte (36). Monocyte recovery was important for infection prophylaxis and better survival after allo-HSCT. Podgorny et al. (37) reported that low counts of monocytes (total and inflammatory) were associated with increased viral infections after HSCT. Storek et al. (38) also observed that the counts of the monocytes on day 80 were significantly inversely correlated with the rates of any infection, severe infections, and viral infections between days 100 and 365 after allo-HSCT. In addition, DeCook et al. (39) reported that the absolute count of monocytes in peripheral blood >0.3 × 109/L at day +100 was associated with improved relapse-free survival, and monocyte recovery at day +100 was an independent prognostic factor for improved survival in multivariate analysis. Basiliximab did not inhibit the IR of monocytes, which may partly contribute to the favorable outcomes of SR-aGVHD survivors.
Poorer B cell reconstitution was associated with increased infections after allo-HSCT (38). Corre et al. (40) reported that patients with a slower B-cell recovery at 12 months after HSCT experienced higher rates of late infection. Storek et al. (38) reported that the counts of total B cells on day 80 were significantly inversely correlated with the rates of any infection, viral infections, bacterial infections, and fungal infections between days 100 and 365 after allo-HSCT. Basiliximab treatment did not inhibit the B cells, and we observed that the IR of CD19+ B cells in patients who recovered from SR-aGVHD was similar to that in event-free HID HSCT recipients, which may help to prevent the late infections in SR-aGVHD survivors.
Our results showed that serum IgA did not recover to the normal range within 1 year after HID HSCT, while serum IgG and IgM returned to the normal range since the first and sixth month after HID HSCT, respectively. Previous studies showed that IgG and IgM achieved the normal level 3–4 months after HSCT (41–44). Norlin et al. (44) reported that serum IgG reached the normal range since the third month after HSCT in a cohort of 179 recipients. HID HSCT recipients routinely received immunoglobulin (400 mg/kg) on days 1, 11, 21, and 31 after transplantation at our center (27). In addition, serum IgG had a long half-life of approximately 3 weeks (45). The above two points may account for normal IgG levels at 1 and 2 months after HSCT in the present study. Acute GVHD had a negative effect on serum IgG (46). Nevertheless, SR-aGVHD HSCT recipients included in the present study achieved an ORR within 3 months after HSCT and did not experience serious complications, such as serious infections and cGVHD, which may account for normal IgG levels since the third month after HSCT.
We observed that the IR of CD4+ T cells, particularly the CD4+CD25+ T cells, was relatively slow after basiliximab treatment. However, the recovery of CD4+ T cells was comparable between patients who recovered from SR-aGVHD after basiliximab treatment and event-free HID HSCT recipients. Pei et al. (21) reported that CD4+ T cells recovered slower in HID HSCT recipients compared with those receiving HLA matched donor HSCT, which may be due to the use of ATG for GVHD prophylaxis (47, 48). Therefore, the slower IR of CD4+ T cells in our study might not be fully attributed to basiliximab treatment.
CD28-mediated costimulation was very important in T-cell activation; CD4+CD28+ T cells and CD8+CD28+ T cells mediated graft-versus-leukemia (GVL) effect to prevent relapse, and CD4+CD28+ T cells also mediated protection against infections (49, 50). Poor CD4+CD25+ T cell reconstitution increased the risk of infections (51). We observed that patients who recovered from SR-aGVHD after basiliximab treatment had a slower CD4+CD25+ T-cell recovery compared with event-free HID HSCT recipients, which was supported by previous studies (52, 53). Chakupurakal et al. (53) observed significant depletion of CD4+CD25+ T cells in SR-aGVHD patients treated with basiliximab, and Vondran et al. (54) reported that basiliximab downregulated the expression of CD25 on T cells in vitro. However, the relatively slow IR of CD4+CD25+ T cells did not seem to have a negative effect on clinical outcomes after basiliximab treatment in the present study.
Liu et al. (15) reported that SR-aGVHD patients receiving ≥5 doses of basiliximab were more likely to experience infections. Therefore, patients were divided in <5 dose group and ≥5 dose group in the present study.
In this study, the median interval from the occurrence of aGVHD to basiliximab treatment was 5 days. According to the European Society for Blood and Marrow Transplantation (EBMT), the first-line steroid treatment should be continued for 7 days (7). However, some studies showed that 5 days of steroids helped to distinguish patients with a high risk of NRM (55, 56). In addition, the British Committee for Standards in Haematology (BCSH) and the British Society for Bone Marrow Transplantation (BSBMT) also considered no response after 5 days of steroid as SR-aGVHD (57). Therefore, the definition of SR-aGVHD was different, which should be evaluated by prospective studies in the future. Finally, it might be reasonable for patients failing to respond after 5 days of steroids to receive basiliximab treatment because of poor outcomes of SR-aGVHD patients and the relative safety of basiliximab treatment.
There were several limitations in the study. First, we did not routinely detect the function of immune cell subsets. Thus, we could not identify whether basiliximab treatment would affect the function recovery of the immune cells. Second, in this retrospective study, we did not use MFC to monitor the IR of monocytes; however, we could get the absolute count of monocytes from the blood routine examination. Third, we did not monitor the count and function of natural killer (NK) cells regularly in the present study. Fourth, due to lack of CD127 and Forkhead box P3 (FOXP3) in our routine panels for IR monitoring, we were unable to identify the IR of regulatory T cells (Tregs). However, previous studies showed that basiliximab treatment could decrease the counts of Treg, but it did not seem to have a negative impact on the function of Tregs (52–54). Fifth, because the IR monitoring was not performed in real time after basiliximab treatment, we could not explore whether basiliximab induced a transient lymphodepletion and whether IR could be considered as a sign of aGVHD control. Regardless, IR data at 1 and 2 months after HSCT might provide some information for these issues. Sixth, because we wanted to identify the real rules of IR after basiliximab treatment, HSCT recipients with serious complications that may have a negative impact on IR, such as serious infections and cGVHD, were excluded; however, this reflected a relatively selective subgroup of patients, and the IR of the other survivors after basiliximab treatment should be further identified. Finally, the patients’ heterogeneity such as transplant regimen, primary disease, and donor type may have an impact on the results of the study. Further studies enrolling uniform cohorts may help to further identify the IR after basiliximab treatment.
In conclusion, IR of most of the immune cells was rapid in SR-aGVHD patients who recovered after basiliximab treatment, which was similar to those of event-free HID HSCT recipients. Thus, basiliximab treatment may not seriously impact the IR in SR-aGVHD survivors.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The institutional review board of Peking University People’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
X-DM designed the study. X-HZ, L-PX, YW, C-HY, HC, Y-HC, WH, F-RW, J-ZW, X-YP, Y-JC, and K-YL collected the data. X-DM, D-XD and SF analyzed the data and drafted the manuscript. X-DM and X-JH reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Program of the National Natural Science Foundation of China (grant number 82170208), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2019-I2M-5-034), the Key Program of the National Natural Science Foundation of China (grant number 81930004), and the Fundamental Research Funds for the Central Universities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.916442/full#supplementary-material
allo-HSCT, allogeneic hematopoietic stem cell transplantation; ATG, anti-thymocyte globulin; CR, complete response; CSA, cyclosporine A; DFS, disease-free survival; DLI, donor lymphocyte infusion; GVHD, graft-versus-host disease; HID, haploidentical donor; HLA, human leukocyte antigen; IFI, invasive fungal infection; IL-2, interleukin-2; IR, immune reconstitution; MFC, multiparameter flow cytometry; MMF, mycophenolate mofetil; MTX, methotrexate; NRM, non-relapse mortality; ORR, overall response rate; OS, overall survival; PTLD, posttransplant lymphoproliferative disorder; SR-aGVHD, steroid-refractory acute graft-versus-host disease.
1. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The Consensus From The Chinese Society of Hematology on Indications, Conditioning Regimens and Donor Selection for Allogeneic Hematopoietic Stem Cell Transplantation: 2021 Update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2
2. Xu LP, Lu PH, Wu DP, Sun ZM, Liu QF, Han MZ, et al. Hematopoietic Stem Cell T Ransplantation Activity in China 2019: A Report From the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant (2021) 56(12):2940–2947. doi: 10.1038/s41409-021-01431-6
3. Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Controlled, Randomized, Open-Label Trial of Risk-Stratified Corticosteroid Prevention of Acute Graft-Versus-Host Disease After Haploidentical Transplantation. J Clin Oncol (2016) 34(16):1855–63. doi: 10.1200/JCO.2015.63.8817
4. Garnett C, Apperley JF, Pavlů J. Treatment and Management of Graft-Versus-Host Disease: Improving Response and Survival. Ther Adv Hematol (2013) 4(6):366–78. doi: 10.1177/2040620713489842
5. Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and Second-Line Systemic Treatment of Acute Graft-Versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005
6. Liu WB, Sun YQ, Zhang XH, Xu LP, Wang Y, Yan CH, et al. [Risk Factors Analysis for Steroid-Resistant Acute Graft Versus Host Disease After Haploidentical Hematopoietic Stem Cell Transplantation]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi (2020) 41(2):106–11. doi: 10.3760/cma.j.issn.0253-2727.2020.02.004
7. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and Management of Graft Versus Host Disease After Stem-Cell Transplantation for Haematological Malignancies: Updated Consensus Recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematology (2020) 7(2):e157–67. doi: 10.1016/S2352-3026(19)30256-X
8. Berger M, Biasin E, Saglio F, Fagioli F, et al. Innovative Approaches to Treat Steroid-Resistant or Steroid Refractory GVHD. Bone Marrow Transplant (2008) 42 Suppl 2:S101–5. doi: 10.1038/bmt.2008.294
9. Shen MZ, Li JX, Zhang XH, Xu LP, Wang Y, Liu KY, et al. Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.749266
10. Malard F, Huang XJ and Sim JPY. Treatment and Unmet Needs in Steroid-Refractory Acute Graft-Versus-Host Disease. Leukemia (2020) 34(5):1229–40. doi: 10.1038/s41375-020-0804-2
11. Massenkeil G, Rackwitz S, Genvresse I, Rosen O, Dörken B, Arnold R. Basiliximab is Well Tolerated and Effective in the Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2002) 30(12):899–903. doi: 10.1038/sj.bmt.1703737
12. Schmidt-Hieber M, Fietz T, Knauf W, Uharek L, Hopfenmüller W, Thiel E, et al. Efficacy of the Interleukin-2 Receptor Antagonist Basiliximab in Steroid-Refractory Acute Graft-Versus-Host Disease. Br J Haematol (2005) 130(4):568–74. doi: 10.1111/j.1365-2141.2005.05631.x
13. Funke VA, de Medeiros CR, Setúbal DC, Ruiz J, Bitencourt MA, Bonfim CM, et al. Therapy for Severe Refractory Acute Graft-Versus-Host Disease With Basiliximab, a Selective Interleukin-2 Receptor Antagonist. Bone Marrow Transplant (2006) 37(10):961–5. doi: 10.1038/sj.bmt.1705306
14. Wang JZ, Liu KY, Xu LP, Liu DH, Han W, Chen H, et al. Basiliximab for the Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease After Unmanipulated HLA-Mismatched/Haploidentical Hematopoietic Stem Cell Transplantation. Transplant Proc (2011) 43(5):1928–33. doi: 10.1016/j.transproceed.2011.03.044
15. Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Prognostic Factors and Long-Term Follow-Up of Basiliximab for Steroid-Refractory Acute Graft-Versus-Host Disease: Updated Experience From a Large-Scale Study. Am J Hematol (2020) 95(8):927–36. doi: 10.1016/j.bbmt.2019.10.031
16. Tang FF, Cheng YF, Xu LP, Zhang XH, Yan CH, Han W, et al. Basiliximab as Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease in Pediatric Patients After Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26(2):351–7. doi: 10.1016/j.bbmt.2019.10.031
17. [Chinese Consensus of Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Disease (III) -Acute Graft-Versus-Host Disease (2020)]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi (2020) 41(7):529–36. doi: 10.3760/cma.j.issn.0253-2727.2020.07.001
18. Bondanza A, Ruggeri L, Noviello M, Eikema DJ, Bonini C, Chabannon C, et al. Beneficial Role of CD8+ T-Cell Reconstitution After HLA-Haploidentical Stem Cell Transplantation for High-Risk Acute Leukaemias: Results From a Clinico-Biological EBMT Registry Study Mostly in the T-Cell-Depleted Setting. Bone Marrow Transplant (2019) 54(6):867–76. doi: 10.1038/s41409-018-0351-x
19. Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Luo X, Curtsinger J, et al. Delayed Immune Reconstitution After Allogeneic Transplantation Increases the Risks of Mortality and Chronic GVHD. Blood Adv (2018) 2(8):909–22. doi: 10.1182/bloodadvances.2017014464
20. Elmariah H, Brunstein CG, Bejanyan N. Immune Reconstitution After Haploidentical Donor and Umbilical Cord Blood Allogeneic Hematopoietic Cell Transplantation. Life (Basel, Switzerland) (2021) 11(2):102. doi: 10.3390/life11020102
21. Pei X, Zhao X, Wang Y, Xu L, Zhang X, Liu K, et al. Comparison of Reference Values for Immune Recovery Between Event-Free Patients Receiving Haploidentical Allografts and Those Receiving Human Leukocyte Antigen-Matched Sibling Donor Allografts. Front Med (2018) 12(2). doi: 10.1007/s11684-017-0548-1
22. Chang YJ, Zhao XY, Huo MR, Xu LP, Liu DH, Liu KY, et al. Immune Reconstitution Following Unmanipulated HLA-Mismatched/Haploidentical Transplantation Compared With HLA-Identical Sibling Transplantation. J Clin Immunol (2012) 32(2):268–80. doi: 10.1007/s10875-011-9630-7
23. Shen MZ, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Preemptive Interferon-α Therapy Could Protect Against Relapse and Improve Survival of Acute Myeloid Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation: Long-Term Results of Two Registry Studies. Front Immunol (2022) 13:757002. doi: 10.3389/fimmu.2022.757002
24. Fan S, Shen MZ, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Preemptive Immunotherapy for Minimal Residual Disease in Patients With T(8;21) Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. Front Oncol (2022) 11:773394. doi: 10.3389/fonc.2021.773394
25. Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, et al. A Novel Protocol for Haploidentical Hematopoietic SCT Without In Vitro T-Cell Depletion in the Treatment of Severe Acquired Aplastic Anemia. Bone marrow Transplant (2012) 47(12):1507–12. doi: 10.1038/bmt.2012.79
26. Shen MZ, Liu XX, Qiu ZY, Xu LP, Zhang XH, Wang Y, et al. Efficacy and Safety of Mesenchymal Stem Cells Treatment for Multidrug-Resistant Graft-Versus-Host Disease After Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation. Ther Adv Hematol (2022) 13:20406207211072838. doi: 10.1177/20406207211072838
27. Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical Hematopoietic Stem Cell Transplantation Without In Vitro T-Cell Depletion for the Treatment of Hematological Malignancies. Bone Marrow Transplant (2006) 38(4):291–7. doi: 10.1038/sj.bmt.1705445
28. Mo XD, Hong SD, Zhao YL, Jiang EL, Chen J, Xu Y, et al. Basiliximab for Steroid-Refractory Acute Graft-Versus-Host Disease: A Real-World Analysis. Am J Hematol (2022) 97(4):458–69. doi: 10.1002/ajh.26475
29. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood marrow Transplant (2015) 21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001
30. Onrust SV, Wiseman LR. Basiliximab. Drugs (1999) 57(2):207–13. doi: 10.2165/00003495-199957020-00006
31. Kovarik JM, Rawlings E, Sweny P, Fernando O, Moore R, Griffin PJ, et al. Prolonged Immunosuppressive Effect and Minimal Immunogenicity From Chimeric (CD25) Monoclonal Antibody SDZ CHI 621 in Renal Transplantation. Transplant Proc (1996) 28(2):913–4.
32. Kovarik J, Mentser M, Broyer P, Loirat C, Crocker J, Offner G, et al. DISPOSITION OF BASILIXIMAB, A CHIMERIC IL-2 RECEPTOR (CD25) MONOCLONAL ANTIBODY, IN PEDIATRIC RENAL TRANSPLANT PATIENTS. Transplantation (1998) 65(Supplement):142. doi: 10.1097/00007890-199805131-00252
33. Amlot PL, Rawlings E, Fernando ON, Griffin PJ, Heinrich G, Schreier MH, et al. Prolonged Action of a Chimeric Interleukin-2 Receptor (CD25) Monoclonal Antibody Used in Cadaveric Renal Transplantation. Transplantation (1995) 60(7):748–56. doi: 10.1097/00007890-199510150-00023
34. Tian DM, Wang Y, Zhang XH, Liu KY, Huang XJ, Chang YJ. Rapid Recovery of CD3+CD8+ T Cells on Day 90 Predicts Superior Survival After Unmanipulated Haploidentical Blood and Marrow Transplantation. PloS One (2016) 11(6):e0156777. doi: 10.1371/journal.pone.0156777
35. Pascual J, Marcén R, Ortuño J. Anti-Interleukin-2 Receptor Antibodies: Basiliximab and Daclizumab. Nephrology Dialysis Transplant (2001) 16(9):1756–60. doi: 10.1093/ndt/16.9.1756
36. Ryu DB, Lim JY, Kim TW, Shin S, Lee SE, Park G, et al. Preclinical Evaluation of JAK1/2 Inhibition by Ruxolitinib in a Murine Model of Chronic Graft-Versus-Host Disease. Exp Hematol (2021) 98:36–46. doi: 10.1016/j.exphem.2021.03.004
37. Podgorny PJ, Pratt LM, Liu Y, Dharmani-Khan P, Luider J, Auer-Grzesiak I. Low Counts of B Cells, Natural Killer Cells, Monocytes, Dendritic Cells, Basophils, and Eosinophils are Associated With Postengraftment Infections After Allogeneic Hematopoietic Cell Transplantation. Biol Blood marrow Transplant (2016) 22(1):37–46. doi: 10.1016/j.bbmt.2015.09.003
38. Storek J, Espino G, Dawson MA, Storer B, Flowers ME, Maloney DG. Low B-Cell and Monocyte Counts on Day 80 are Associated With High Infection Rates Between Days 100 and 365 After Allogeneic Marrow Transplantation. Blood (2000) 96(9):3290–3. doi: 10.1182/blood.V96.9.3290
39. DeCook LJ, Thoma M, Huneke T, Johnson ND, Wiegand RA, Patnaik MM. Impact of Lymphocyte and Monocyte Recovery on the Outcomes of Allogeneic Hematopoietic SCT With Fludarabine and Melphalan Conditioning. Bone Marrow Transplant (2013) 48(5):708–14. doi: 10.1038/bmt.2012.211
40. Corre E, Carmagnat M, Busson M, de Latour RP, Robin M, Ribaud P, et al. Long-Term Immune Deficiency After Allogeneic Stem Cell Transplantation: B-Cell Deficiency is Associated With Late Infections. Haematologica (2010) 95(6):1025–9. doi: 10.3324/haematol.2009.018853
41. Halterman RH, Graw RG Jr, Fuccillo DA, Leventhal BG. Immunocompetence Following Allogeneic Bone Marrow Transplantation in Man. Transplantation (1972) 14(6):689–97. doi: 10.1097/00007890-197212000-00004
42. Fass L, Ochs HD, Thomas ED, Mickelson E, Storb R, Fefer A. Studies of Immunological Reactivity Following Syngeneic or Allogeneic Marrow Grafts in Man. Transplantation (1973) 16(6):630–40. doi: 10.1097/00007890-197312000-00015
43. Small TN, Keever CA, Weiner-Fedus S, Heller G, O'Reilly RJ, Flomenberg N. B-Cell Differentiation Following Autologous, Conventional, or T-Cell Depleted Bone Marrow Transplantation: A Recapitulation of Normal B-Cell Ontogeny. Blood (1990) 76(8):1647–56. doi: 10.1182/blood.V76.8.1647.1647
44. Norlin AC, Sairafi D, Mattsson J, Ljungman P, Ringdén O, Remberger M. Allogeneic Stem Cell Transplantation: Low Immunoglobulin Levels Associated With Decreased Survival. Bone Marrow Transplant (2008) 41(3):267–73. doi: 10.1038/sj.bmt.1705892
45. Grevys A, Frick R, Mester S, Flem-Karlsen K, Nilsen J, Foss S, et al. Antibody Variable Sequences Have a Pronounced Effect on Cellular Transport and Plasma Half-Life. iScience (2022) 25(2):103746. doi: 10.1016/j.isci.2022.103746
46. Koh S, Koh H, Nanno S, Okamura H, Nakashima Y, Nakamae M, et al. Kinetics of IgG Subclasses and Their Effects on the Incidence of Infection After Allogeneic Hematopoietic Stem Cell Transplantation. Transpl Immunol (2021) 67:101413. doi: 10.1016/j.trim.2021.101413
47. Nishihori T, Al-Kadhimi Z, Hamadani M, Kharfan-Dabaja MA. Antithymocyte Globulin in Allogeneic Hematopoietic Cell Transplantation: Benefits and Limitations. Immunotherapy (2016) 8(4):435–47. doi: 10.2217/imt.15.128
48. Cherkassky L, Lanning M, Lalli PN, Czerr J, Siegel H, Danziger-Isakov L, et al. Evaluation of Alloreactivity in Kidney Transplant Recipients Treated With Antithymocyte Globulin Versus IL-2 Receptor Blocker. Am J Transplant (2011) 11(7):1388–96. doi: 10.1111/j.1600-6143.2011.03540.x
49. Barrett AJ. Mechanisms of the Graft-Versus-Leukemia Reaction. Stem Cells (1997) 15(4):248–58. doi: 10.1002/stem.150248
50. Arens R, Loewendorf A, Her MJ, Schneider-Ohrum K, Shellam GR, Janssen E, et al. B7-Mediated Costimulation of CD4 T Cells Constrains Cytomegalovirus Persistence. J Virol (2011) 85(1):390–6. doi: 10.1128/JVI.01839-10
51. Zhou JR, Shi DY, Wei R, Wang Y, Yan CH, Zhang XH, et al. Co-Reactivation of Cytomegalovirus and Epstein-Barr Virus Was Associated With Poor Prognosis After Allogeneic Stem Cell Transplantation. Front Immunol (2021) 11. doi: 10.3389/fimmu.2020.620891
52. López-Abente J, Martínez-Bonet M, Bernaldo-de-Quirós E, Camino M, Gil N, Panadero E, et al. Basiliximab Impairs Regulatory T Cell (TREG) Function and Could Affect the Short-Term Graft Acceptance in Children With Heart Transplantation. Sci Rep (2021) 11(1):827. doi: 10.1038/s41598-020-80567-9
53. Chakupurakal G, García-Márquez MA, Shimabukuro-Vornhagen A, Theurich S, Holtick U, Hallek M, et al. Immunological Effects in Patients With Steroid-Refractory Graft-Versus-Host Disease Following Treatment With Basiliximab, a CD25 Monoclonal Antibody. Eur J Haematol (2016) 97(2):121–7. doi: 10.1111/ejh.12691
54. Vondran FW, Timrott K, Tross J, Kollrich S, Schwarz A, Lehner F, et al. Impact of Basiliximab on Regulatory T-Cells Early After Kidney Transplantation: Down-Regulation of CD25 by Receptor Modulation. Transplant Int (2010) 23(5):514–23. doi: 10.1111/j.1432-2277.2009.01013.x
55. Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scimé R, Locatelli F, et al. Early Treatment of Acute Graft-Versus-Host Disease With High- or Low-Dose 6-Methylprednisolone: A Multicenter Randomized Trial From the Italian Group for Bone Marrow Transplantation. Blood (1998) 92:2288–93.
56. Van Lint MT, Milone G, Leotta S, Uderzo C, Scimè R, Dallorso S, et al. Treatment of Acute Graft-Versus-Host Disease With Prednisolone: Significant Survival Advantage for Day +5 Responders and No Advantage for Nonresponders Receiving Anti-Thymocyte Globulin. Blood (2006) 107:4177–81. doi: 10.1182/blood-2005-12-4851
Keywords: Immune reconstitution, basiliximab, steroid-refractory, acute graft-versus-host disease, haploidentical, allogeneic hematopoietic stem cell transplantation
Citation: Deng D-X, Fan S, Zhang X-H, Xu L-P, Wang Y, Yan C-H, Chen H, Chen Y-H, Han W, Wang F-R, Wang J-Z, Pei X-Y, Chang Y-J, Liu K-Y, Huang X-J and Mo X-D (2022) Immune Reconstitution of Patients Who Recovered From Steroid-Refractory Acute Graft-Versus-Host Disease After Basiliximab Treatment. Front. Oncol. 12:916442. doi: 10.3389/fonc.2022.916442
Received: 09 April 2022; Accepted: 15 June 2022;
Published: 15 July 2022.
Edited by:
Jean El Cheikh, American University of Beirut Medical Center, LebanonReviewed by:
Suparno Chakrabarti, Narayana Health, IndiaCopyright © 2022 Deng, Fan, Zhang, Xu, Wang, Yan, Chen, Chen, Han, Wang, Wang, Pei, Chang, Liu, Huang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Dong Mo, bXhkNDUzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.