
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 June 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.915103
This article is part of the Research Topic Kinesiophobia – Psychological Aspects of Physical Activity in Breast Cancer Patients View all 5 articles
Objective: The aim of this study was to develop and validate the breast cancer scale among the system of quality-of-life instruments for cancer patients (QLICP-BR V2.0).
Methods: Programmed decision procedures and theories on instrument development were applied to develop QLICP-BR V2.0. A total of 246 breast cancer inpatients were investigated using QLICP-BR V2.0 from hospital admission until discharge. The reliability, validity, and responsiveness of the QLICP-BR V2.0 scale were evaluated by using the classical test theory combined with the generalizability theory (GT), including correlation analysis, multi-trait scaling analysis, factor analyses, t-tests, and also multivariate generalizability theory analysis.
Results: The test–retest reliability of the total scale is 0.79, the Cronbach coefficient is 0.85, and the intra-class correlations coefficient is 0.88. The item–domain correlation analysis showed that the correlation coefficient between items and their own domain is greater than that with other domains except of item GSO4. The exploratory factor analysis showed that three principal components are obtained in the specific module. The outcome of the factor analysis coincides substantially with our theoretical conception. The score difference of each domain of the scale and the total scale before and after treatment is statistically significant (P < 0.05), with the standardized response mean of the total scale being 0.61. According to GT, the generalization coefficient of the scores in the 5 domains is between 0.626 and 0.768, and the reliability index is between 0.557 and 0.695.
Conclusion: QLICP-BR V2.0 exhibited reasonable degrees of validity, reliability, and responsiveness according to classical test and the generalizability theory. The number of items in the scale is appropriate.
Breast cancer is one of the most common malignant tumors in women and an important obstacle to women’s health (1). In China, its morbidity and mortality are increasing year by year, accounting for 7 to 10% of various malignant tumors in the whole body. A recent report estimated that there were 4.3 million new cancer cases and 2.8 million cancer-related deaths in China in 2015, with breast cancer as the most common (estimated at 268,000 new cases) among women (2, 3).
With social and economic changes, the medical model has become a multi-dimensional concept containing multiple meanings of biology, psychology, and society. When we evaluate the treatment effect of breast cancer patients, we should no longer simply confine ourselves to survival time but should focus on whether patients receive adequate physical and psychological care during treatments (4). Many studies have shown that a scale can integrate the patient’s own feelings with clinical practice, and it is the core method for evaluating the health of patients, so many quality-of-life (QOL) assessment scales for breast cancer patients have been produced, including the following: European Organization for Cancer Research and Treatment Quality of Life Questionnaire-Breast Cancer Module (EORTC QLQ-BR23) (5), Functional Assessment of Cancer Therapy- Breast Cancer (FACT-B) (6), Hopwood Body Image Scale (7), which also focuses on the non-surgical treatment of breast cancer patients, and Body Image Questionnaire for Breast Cancer Patients (BIBCQ) (7), which does not solve the esthetic problems after breast reconstruction. Among them, EORTC QLQ-BR23 and FACT-B are the most widely used, both for surgical treatment and/or non-surgical treatment of breast cancer patients, and are disease-specific rather than surgery-specific measurement tools. However, most scales are mainly suitable for European and American environments for QOL cultural dependence. Although the Chinese versions of QLQ-BR53 (8) (QLQ-C30 and QLQ-BR23) and FACT-B (9) can be used for Chinese patients, they are lacking Chinese cultural backgrounds to some extent considering their original use in English-speaking patients—for example, the QOL scales developed abroad are constructed mainly under the Western cultural system, which are more concerned about the two aspects of religious belief and sexual life (10).
In China, Peng et al. (11) compiled a questionnaire for evaluating the quality of life of breast cancer patients, including 64 items in 4 dimensions of physical, psychological, symptom, and social function. However, the development of this scale has not been updated for a long time and not based on modern test theory. Zhang et al. (12) formulated the (Patient Reported Outcome scale of Chinese medicine after breast cancer surgery, but this scale has not been tested for test–retest reliability and may not achieve long-term efficacy evaluation. Moreover, the responsiveness of the scale (the differences before and after treatments) needs to be investigated further. It is necessary to develop Chinese-specific QOL instruments systematically. In response to this need, our QOL team started the research focusing on the development of the quality-of-life scales for cancer patients since 1997. The Chinese QOL instrument system called Quality-of-Life Instruments for Cancer Patients (QLICP) was developed by module approach. This system includes a general module (QLICP-GM) which can be used with all types of cancer and specific modules for different cancers, with each module being used for only the relevant cancer. The first version of the system has been completed in 2013, with 13 scales being developed, including the QLICP-GM and the 12 cancer-specific QOL instruments such as those for lung cancer, head and neck cancer, colorectal cancer, etc. (13–16). The first version of the breast cancer scale QLICP-BR V1.0 is an important one of this system and has been put into use after it has been developed (17, 18).
However, QLICP-BR V1.0 also exposed some problems during long-term use. Firstly, the specific module mainly describes the specific adverse reactions of breast cancer, which need to be distinguished from other diseases. There may be insufficient item expression, and the structure of the scale may need to be adjusted. Secondly, with the improvement of medical technology, there may be new changes in the specific response of the disease in the specific module, and items need to be added or deleted. Thirdly, theoretical support needs to be updated. The theoretical basis for the development and validation of the first version of the system is mainly classical test theory (CTT), and it still has some shortcomings. The modern test theory should be fully combined with the scale development.
Therefore, we have started the second version of the system QLICP V2.0 since 2010 based on V1.0 and in accordance with classical test theory and modern test theories such as generalizability theory (GT). QLICP V2.0 includes the general scale (module) QLICP-GM V2.0 and 22 cancer-specific scales such as those for brain cancer, bladder cancer, prostate cancer, cervical cancer, leukemia and lymphoma, etc. Up to now, most scales of the QLICP V2.0 have been developed and put into use (19). This paper is aimed to report the developmental process and validation of QLICP-BR V2.0, which will assist in management and decision making (19) and has also wide practical applications because patients with breast cancer account for a large proportion of cancer cases in China and also in the world.
This study is based on inpatients with breast cancer clinical diagnosis and diagnosed by pathological examination in the Affiliated Hospital of Guangdong Medical University and Central Hospital of Guangdong Nongken. The inclusion and exclusion criteria are as follows:
–Inclusion criteria: (1) patients with a clear diagnosis, that is, those diagnosed as breast cancer by pathological examination; (2) good reading and presentation skills and able to fill out questionnaires by themselves; and (3) volunteered to participate in the survey—no mental illness or disturbance of consciousness.
–Exclusion criteria: (1) cognitive and consciousness dysfunction; (2) those who refuse to participate in the research or those with a low degree of education; (3) combined with other primary cancers, other serious diseases, mental illnesses, etc.; and (4) multiple metastases of malignant tumors.
The scale adopts the modular approach by combining the general module with the specific module for breast cancer. The methodology is similar with that of the first version (17, 18), and the main steps to form the final QLICP-BR V2.0 are presented in Figure 1.
The general module QLICP-GM V2.0 has been successfully developed and was confirmed to have good reliability, validity, and responsiveness (20) in 2015 using classical test theory, item response theory, and generalization theory. It includes 4 domains and 32 items: physical, psychological, social, and the common symptoms/side effects.
The specific module of QLICP-BR V2.0 is developed in strict accordance with the following steps:
(1) Establish a research team, which includes experts and scholars in the domains of quality of life, statistics, public health, psychology, and breast cancer.
(2) The conceptual framework of the specific module of breast cancer patients is presented according to the definition of QOL, which can be classified into 3 facets: clinical symptoms, treatment side effects, and specific psychological effects.
(3) The formation of the item pool of the scale is mainly based on the decomposition of the multidimensional QOL concept of breast cancer, the search of literature, the reference of domestic and foreign mature scales, and the clinical experience of breast cancer. As a result, 24 items in the item pool are proposed under the abovementioned three facets.
(4) Selection and determination of items: Through discussions by the experts of the research group, the items that have no significant impact on the quality of life of breast cancer patients were deleted to form a preliminary scale. During this process, this study conducted two rounds of discussions, and finally 13 items were kept to determine the specific module, as shown in Figure 1, phrase I.
(5) Pre-survey and item selection. Using the preliminary scale, a questionnaire survey of patients was conducted, and item selection was conducted by statistical analysis and also experts’ clinical experience. After the discussion by experts at this stage, 11 items were selected to form a test version of the scale, as shown in Figure 1, phrase II.
(6) Item selection again based on a survey using a test version. A questionnaire survey using a test version of the scale was conducted, and an item was screened again by statistical analysis and also experts’ discussions. As a result, 10 items were selected to determine the official version of the scale, as shown in Figure 1, phrase III.
(7) Evaluation of the formal version of the scale: This study uses classical test theory and generalization theory to evaluate the reliability and validity of the scale.
The specific module of the breast cancer was combined with the general module to form the complete QLICP-BR V2.0. A large-scale questionnaire survey was conducted among eligible breast cancer patients to validate the QLICP-BR V2.0.
The investigator (doctors, nurses, and medical postgraduate students) briefly explained the content and purpose of the investigation. After obtaining the consent of the patient and the signed informed consent form, the investigator sent the QLICP-BR V2.0 to the patients to fill out by themselves. The first questionnaire survey was conducted on the first day after admission, the same questionnaire was used for the retest survey on the second or third day after admission to evaluate the test–retest reliability, and the third survey was conducted before discharge in order to evaluate responsiveness.
Firstly, the raw scores (RS) of items, domains, and overall scale were calculated according to the unified scoring rules on the scale. Each item of QLICP-BR V2.0 is rated in a five-level scoring system, namely, not at all, a little bit, somewhat, quite a bit, and very much. Therefore, the positively stated items directly obtain scores from 1 to 5 points, and the negatively stated items are reversed. Each domain score is obtained by adding its own item score together, and the overall scale score is the sum of five domain scores.
Secondly, the corresponding standard score (SS) for all domains and the overall were linearly converted to a 0–100 scale using the formula: SS = (RS - Min) × 100/R, where SS, RS, Min, and R represent the standardized score, raw score, minimum score, and range of scores, respectively.
After scoring, classical test theory and modern test theory were used to evaluate the validity, reliability, and responsiveness of QLICP-BR V2.0.
We evaluate the reliability of the scale by calculating the test–retest reliability, Cronbach’s α coefficient, and intra-class correlation coefficient (ICC) and its corresponding 95% confidence interval (21).
The validity of content was evaluated by means of expert evaluation. Construct validity was evaluated by calculating the Pearson correlation coefficient, r, among items and domains as well as factor analysis. Exploratory factor analysis was used to examine whether the scale structure is consistent with the theoretical conception (22). In this study, the Chinese version of FACT-B was selected as the criterion for assessing the criterion-related validity, and the correlation coefficient between the domain scores of QLICP-BR V2.0 and FACT-B (V4.0) was calculated.
The average scores between the first and third assessments (before and after treatments) were compared by paired t-test, with the standardized response mean (SRM) being calculated, which is the ratio of the difference before and after treatment to its standard deviation.
GT is a modern measurement theory that introduces irrelevant variables or factors that interfere with test scores into the measurement model and analyzes the impact of these factors and the interaction between factors and factors on the measurement scores through statistical techniques. It is applied in quantitative research to analyze the influence of patients, items, and interactions between patients and items on the total score of the scale. GT provides a comprehensive and unifying framework that goes beyond the CTT model of a single error term by allowing for the simultaneous analysis of the main and interaction effect source of error variance (23, 24). GT subsumes other forms of reliability approaches (e.g., internal consistency reliability, inter-rater reliability, and intra-class correlation) and provides a comprehensive and unifying framework for assessing the measurement reliability, especially for complex measurement situations. The application of GT includes the univariate generalizability theory method and the multivariate generalizability theory (MGT) method. The MGT was initially proposed by Cronbach based on the multivariate analysis of variance, and it is appropriate for multidimensional and complicated measurement situations (24).
The GT-based scale assessment includes G-study and D-study: (1) G study, also known as generalizability study, has the main task to find out various potential sources of measurement errors in the research design as much as possible in the universe of admissible observations and to estimate the variance components of these error sources; and (2) D-study, also known as decision research, has the main task which is based on G-study by adjusting various relationships in the measurement process to explore how to control and adjust measurement errors. Its indicators are generalization coefficient and reliability index (25, 26).
In this study, SPSS25.0 was used to calculate the reliability, validity, and responsiveness, and mGENOVA was used for generalizability theory analysis.
A total of 246 breast cancer patients were investigated in this study, all of which were women. Moreover, these patients range in age from 17 to 77, with a mean age of 50.07 ± 10.25, and 96.3% (237 cases) were of Han ethnicity. The household economy is mostly medium, accounting for 67.9% of the total population. In terms of occupation, workers accounted for 8.1% (20 cases) and 45.5% (112) were farmers. Furthermore, 97.2% were married. A total of 148 cases (60.2%) finished middle school or high school, while 65 (26.4%) completed primary school and 33 (13.4%) had a college/university degree. In addition, 226 cases (91.9%) used medical insurance, while 20 cases (8.1%) used self-paid/private insurance. On the basis of clinical stage, 53 cases (21.5%) were in stage I, 86 cases (35%) were in stage II, 54 (22.0%) were in stage III, and 27 (11.0%) were in stage IV.
In this study, the test questionnaires on the day of admission of the breast cancer patients and the second or third day of admission were tested for test–retest reliability. The results show that the test–retest reliability of each domain is greater than 0.8 (the ideal value is greater than 0.7 (27)), and the test–retest reliability of each facet is between 0.7 and 0.8. Table 1 shows a summary of the test–retest reliability.
The internal consistency reliability of the scale was assessed through Cronbach’s α coefficient (Cronbach’s α) and ICC. The result shows that Cronbach’s α coefficient of the total scale is 0.85 (the ideal value is greater than 0.7 (17). At the same time, the Cronbach’s α coefficients of various domains of the general module and the specific module are all around 0.7. In addition, the ICC values for these five domains were higher than 0.85. Table 1 shows a record of the details of Cronbach’s α and ICC.
QLICP-BR V2.0 is based on a large amount of literature review and many discussions by experts in the subject group. It involves physical function, psychological function, social function, common symptoms and side effects to cancer patients, and the specific symptoms and special psychological changes of breast cancer patients. Through rigorous procedures and methods, the items are also screened and analyzed. These insure good content validity.
The construct validity was evaluated by item–domain Pearson’s correlation coefficient r. As shown in Table 2, with the exception of item GSO4, there is a strong correlation between the items and their domain (mostly above 0.40). However, the relationship between the item and the other domains is weak.
In this study, an exploratory factor analysis was carried out on the general and the specific module of the scale, and the results showed that the KMO of the general module and the specific module are 0.819 and 0.752, respectively. There is a strong partial correlation between variables, and Bartlett’s spherical test for both was P <0.001, suggesting that the variables are not independent of each other and that factor analysis is suitable for data analysis.
In factor analysis of the general module, the principal component method is used to extract the common factors whose characteristic roots are greater than 1. Ten principal components were extracted with the cumulative contribution rate of variance being 71.29%. After maximum variance rotation, it can be seen that the construct of the general module set by the extracted principal components is basically consistent with the original theoretical assumption.
In factor analysis of the specific module, the principal component method is used to extract the common factors whose characteristic roots are greater than 1. Three principal components are obtained, and the cumulative contribution rate of the variance is 64.17%. After maximum variance rotation, it can be seen that the characteristic root of the first principal component is 3.24, which mainly reflects the related symptoms of breast cancer, involving items SBR1, SRB2, SBR3, SBR4, and SBR5, and the variance contribution rate is 32.41%. The second principal component characteristic root is 1.70, which mainly reflects the side effects of disease treatment prognosis, involving items SBR8, SBR9, and SBR10, and the variance contribution rate is 17.01%. The characteristic root of the third principal component is 1.47, which mainly reflects the unique psychological changes of breast cancer patients, involving items SBR6 and SBR7, and the variance contribution rate is 14.74%. It is basically consistent with the breast cancer-specific module framework proposed in advance.
From the results mentioned above, theoretical construct was confirmed by data analysis, and good construct validity was shown.
The correlation coefficients between the QLICP-BR V2.0 and FACT-B (V4.0) domain scores indicate that the correlation between the same and similar domains (bold in the table) is usually higher than that with different or dissimilar domains—for example, the correlation coefficients between the physiological status domain (PWB) and functional status domain (FWB) of the FACT-B (V4.0) and the PHD of the QLICP-BR V2.0 are 0.39 and 0.44, respectively. The correlation coefficients between the emotional status (EWB) and functional status (FWB) domains of the FACT-B (V4.0) and the PSD of the QLICP-BR V2.0 are 0.60 and 0.67, respectively. The correlation coefficient between the emotional status (EWB) of the FACT-B (V4.0) and the SOD of the QLICP-BR V2.0 is 0.59. On the other hand, the correlation coefficients between the additional focus domain (AC) of the FACT-B (V4.0) and the SSD and SPD of the QLICP-BR V2.0 are 0.61 and 0.48, respectively.
The data from 246 patients who completed the questionnaire after treatments were used to assess responsiveness. The paired t-test and the response index SRM were used to check the average score change of each domain/facet of QLICP-BR V2.0 before and after treatments. The results are shown in Table 3. It can be seen that all domains/facets and overall scale have undergone major changes (P < 0.01). The SRM of the total scale is 0.61, and the SRM of all the domains are greater than 0.40, with the exception of PHD. It can be considered that QLICP-BR V2.0 scales have good responsiveness.
In the PHD, PSD, SOD, SSD, and SPD domains, the variation components of the interaction between the subject and the item are 0.624, 0.558, 0.521, 0.670, and 0.626, respectively. The variance components of the subjects in the five domains are between 0.109 and 0.205, and the variance components of the items are between 0.121 and 0.253, indicating that the variance of the scale score is mainly due to the interaction between the subject and the scale items, and other factors lead to the smaller variance in the patients’ scale scores. Detailed results are shown in Table 4.
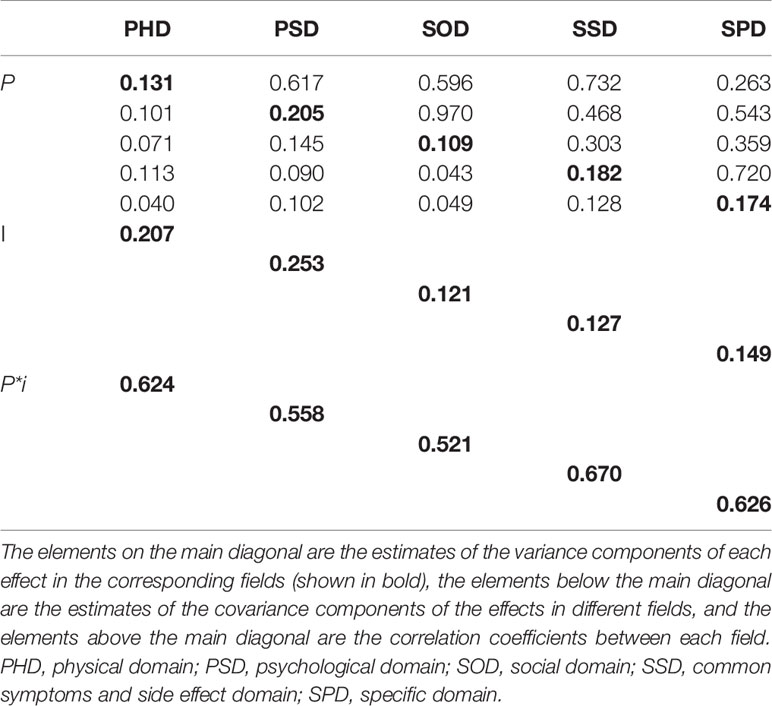
Table 4 Estimation of variance and covariance components in various domains in the p•× i°-designed G-study (n = 246).
According to D-study, the generalization coefficients (G coefficient) of scores in the 5 domains are between 0.626 and 0.768, the reliability index (Ф coefficient) is between 0.557 and 0.695, and all domains fluctuate around 0.6. The relative error variance is within 0.1, and the absolute error variance is within 0.2, indicating that the reliability of these five domains is relatively high, which is basically consistent with the results of the classical test theory cited above.
In the case of 10 items of breast cancer-specific modules, the generalization coefficient and the reliability index of the specific module are 0.735 and 0.692, respectively. When the number of patients is fixed and the general module remains the same, the number of items increases from 5 to 15, the absolute error and the relative error decrease in sequence, and the generalization coefficient and the reliability index increase in sequence. The results of the D-study of QLICP-BR V2.0 are shown in Table 5.
This study is mainly aimed at breast cancer patients, which is part of the cancer patient reporting outcome measurement scale system. By learning from the experience in the development of mature scales at home and abroad, adopting the advanced model of combining general and specific modules in structure, following the rules and procedures of scale formulation, and combining with the actual situation in China, QLICP-BR V2.0 was developed.
In this study, the test–retest reliability, Cronbach coefficient, and ICC have been calculated to confirm good reliability. In addition, the item–domain correlation analysis, exploratory factor analysis, FACT-B (V4.0) as a criterion to calculate the criterion-related validity, etc., have confirmed that the scale has good validity. Furthermore, the paired t-test and the calculation of SRM indicators confirm that the scale has a good degree of responsiveness. The results of the GT of this scale show that the main source of scale score variation is the interaction between patients and items. The purpose of this scale is to measure the quality of life of patients, so the source of variation in the scale is more reasonable. In the D-study of this research, the G and Ф coefficients for the current number of items and the recommended number of items according to the G and Ф coefficients after fixing the subjects are also presented. The standard for G and Ф coefficients is 0.6. When these two indicators are greater than 0.6, the scale is considered reliable. According to Table 5, this study shows that, when the number of specific module entries is 5, it is not satisfactory. As the number of items increases, the G and Ф coefficients are increasing, but when the number of items is greater than 10, the magnitude of the increase begins to decrease. Therefore, the number of items of the specific module was finally determined to be 10 (28).
The development of QLICP-BR V2.0 is based on QLICP-BR (V1.0). In terms of psychometric characteristics, the second version of the scale adopts FACT-B as the criterion scale. Compared with the first version (QLQ-BR53 as the criterion scale), the division of domains in QLICP-BR V2.0 is clearer, the correlation coefficient in the same domain is larger, and it has better criterion-related validity. In addition, the responsiveness of the second version of the scale is more obvious, and it is statistically significant in all domains when evaluating the effect of the treatment plan, while the first version of the scale is not the case. Finally, the QLICP-BR V2.0 development process adopted the multivariate generalization theory, which is an indicator of measurement reliability developed by organically integrating true score theory and variance analysis. The random error is further divided into different source components, their respective proportions are examined, and their indicators are calculated to reflect the accuracy and stability of the measurement results. The G-study can randomly sample from a clearly defined range, and it is not limited by observable results, and can provide evidence of validity based on the test content (29).
The QLICP-BR V2.0 scale can be applied in many aspects of clinical research, such as evaluating the effectiveness of treatment measures and the feasibility of intervention programs, exploring factors affecting the quality of life in breast cancer, accurately capturing changes in patients’ symptoms, evaluating and improving the quality of medical services, optimizing benefit evaluation of health resource investment, etc. (30). In the 1970s, the MAPI Institute in Lyon, France, established the PROQOLID database, which used the network to push the patient-reported outcome scale and the quality-of-life scale to the relevant medical staff (31). Fisher et al. conducted a 20-year follow-up survey of breast cancer patients undergoing mastectomy and breast-conserving surgery and found that women who underwent breast-conserving surgery were more satisfied with their body image and had better functional status and fewer symptoms (32, 33).
There are also some related studies on breast cancer scales at home and abroad, such as EORTC QLQ-BR53 (QLQ-C30 and QLQ-BR23), FACT-B, SLDS-BC, BIBCQ, HIBS, BREAST-Q, BCTOS, and so on, but they are lacking Chinese cultural backgrounds to some extent considering their original use in English-speaking patients. Some scales, such as those specific to breast cancer surgery, have not been rigorously assessed (7).
In this study, some improvements are necessary before QLICP-BR V2.0 can be used as a practical instrument to measure and assess the QOL of Chinese breast cancer patients. The survey of this study is limited to inpatients. In the future, the survey should be extended to outpatient or community patients, and IRT methods should be used to obtain more representative survey results.
Given what has been discussed above, QLICP-BR V2.0 exhibited reasonable degrees of validity, reliability, and responsiveness according to classical test and generalizability theories.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The study protocol and the written informed consent form were approved by the IRB (institutional review board) of the affiliated hospital of Guangdong Medical University (PJ2012052, YJYS2019010). The respondents were voluntary and provided written informed consent for participation.
CW designed the study. FL, ZY, JZ, QL, WL, and HC performed the data collection. FL performed the data analyses and drafted the manuscript. CW revised the manuscript intensively. All authors contributed to the article and approved the submitted version.
This study is supported by the National Natural Science Foundation of China (71974040 and 81273185) and the Features Innovative Projects of Key Platform and Major Scientific Research Project of Universities in Guangdong Province (2017KZDXM040 and 2018KZDXM037).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
In carrying out this research project, we have received substantial assistance from Prof. Gary Lyman of Hutchinson Institute for Cancer Outcomes Research and Prof. David Cella, Benjamin J. Arnold, and Hiramatsu Toshiko of the Center on Outcomes, Research, and Education as well as the many staffs at the Central Hospital of Guangdong Nongken. We sincerely acknowledge all the support.
QLICP-BR, Quality of Life Instruments system in breast cancer patients; QOL, quality of life; EORTC, European Organization for Research and Treatment; GT, Generalizability theory; CTT, classical test theory; MTT, modern test theory; IRT, item response theory; RS, raw scores; SS, standard score; SRM, standardized response mean; MGT, multivariate generalizability theory; UGT, univariate generalizability theory; CGD, core/general module; SPD, specific domain; TOT, total; ICC, intra-class correlation confident; SBR, specific module of breast cancer; PHD, physical domain; PSD, psychological domain; SOD, social domain; SSD, common symptoms and side effect domain; FWB, functional status domain; PWB, physiological status domain; EWB, emotional status; AC, additional focus domain; G coefficient, generalization coefficients; Ф coefficient, reliability index
1. Feng YX, Spezia M, Huang SF, Yuan CF, Zeng ZY, Zhang LH, et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis (2018) 5(2):77–106. doi: 10.1016/j.gendis.2018.05.001
2. Chen WQ, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast Cancer in Young Women: An Overview. Update Surg (2017) 69(3):313–7. doi: 10.1007/s13304-017-0424-1
4. Fahad Ullah M. Breast Cancer: Current Perspectives on the Disease Status. Adv Exp Med Biol (2019) 1152:51–64. doi: 10.1007/978-3-030-20301-6_4
5. Zawisza K, Tobiasz-Adamczyk B, Nowak W, Kulig J, Jedrys J. Validity and Reliability of the Quality of Life Questionnaire (EORTC QLQ C30) and its Breast Cancer Module (EORTC QLQ Br23). Ginekol Pol (2010) 81(4):262–7.
6. Matthies LM, Taran FA, Keilmann L, Schneeweiss A, Simoes E, Hartkopf AD, et al. An Electronic Patient-Reported Outcome Tool for the FACT-B (Functional Assessment of Cancer Therapy-Breast) Questionnaire for Measuring the Health-Related Quality of Life in Patients With Breast Cancer: Reliability Study. J Med Internet Res (2019) 21(1):e10004. doi: 10.2196/10004
7. Kanatas A, Velikova G, Roe B, Horgan K, Ghazali N, Shaw RJ, et al. Patient-Reported Outcomes in Breast Oncology: A Review of Validated Outcome Instruments. Tumori (2012) 98(6):678–88. doi: 10.1700/1217.13489
8. Wan C, Tang X, Tu X, Feng C, Messing S, Meng Q, et al. Psychometric Properties of the Simplified Chinese Version of the EORTC QLQ-BR53 for Measuring Quality of Life for Breast Cancer Patients. Breast Cancer Res Treat (2007) 105(2):187–93. doi: 10.1007/s10549-006-9443-1
9. Wan C, Zhang D, Yang Z, Tu X, Tang W, Feng C, et al. Validation of the Simplified Chinese Version of the FACT-B for Measuring Quality of Life for Patients With Breast Cancer. Breast Cancer Res Treat (2007) 106(3):413–8. doi: 10.1007/s10549-007-9511-1
10. Mancha RG, Muñoz M, de la Cruz-Merino L, Calvo L, Cruz J, Baena-Cañada JM, et al. Development and Validation of a Sexual Relations Satisfaction Scale in Patients With Breast Cancer - "SEXSAT-Q". Health Qual Life Outcome (2019) 17(1):143. doi: 10.1186/s12955-019-1197-7
11. Peng GY, Li LJ, Jiang BN. Research on Assessment Tools of Quality of Life for Breast Cancer. Natl Med J China (1999) 23(4):161–2. doi: CNKI:SUN:HYXZ.0.1999-04-009
12. Zhang J, Yao YF, Zha XM, Pan LQ, Bian WH, Tang JH. Development and Evaluation of a Patient-Reported Outcome (PRO) Scale for Breast Cancer. Asian Pacific J Cancer Prev Apjcp (2015) 16(18):8573–8. doi: 10.7314/apjcp.2015.16.18.8573
13. Wan CH, Yang Z, Meng Q, Feng CY, Wang HY, Tang XL. Development and Validation of the General Module of the System of Quality of Life Instruments for Cancer Patients (QLICP-Gm). Int J Cancer (2008) 122(1):190–6. doi: 10.1002/ijc.23036
14. Wan CH, Lu YB, Tang W, Tu X, Zhang CZ, Li GF. Development and Validation of the System of Quality of Life Instruments for Cancer Patients: Lung Cancer (QLICP-Lu). Lung Cancer (2008) 60(1):105–12. doi: 10.1016/j.lungcan.2007.09.006
15. Xu CZ, Yang Z, Tan JF, Meng Q, Cun YL, Tang XL, et al. Development and Validation of the System of Quality of Life Instruments for Cancer Patients: Colorectal Cancer (QLICP-Cr). Cancer Invest (2012) 30(10):732–40. doi: 10.3109/07357907.2012.727933
16. Yang Z, Luo JH, Meng Q, Li GF, Li XJ, Ding YL, et al. Development and Validation of the System of Quality of Life Instruments for Cancer Patients: Head and Neck Cancer (QLICP-Hn). Oral Oncol (2012) 48(8):737–46. doi: 10.1016/j.oraloncology.2012.01.025
17. Wan CH, Yang Z, Meng Q, Feng CY, Wang HY, Tang XL, et al. Development and Validation of the General Module of the System of Quality of Life Instruments for Cancer Patients. Int J Cancer (2010) 122(1):190–6. doi: 10.1002/ijc.23036
18. Wan CH, Yang Z, Tang XL, Zou TN, Chen DD, Zhang DM, et al. Development and Validation of the System of Quality of Life Instruments for Cancer Patients: Breast Cancer (QLICP-Br). Support Care Cancer (2009) 17(4):359–66. doi: 10.1007/s00520-008-0478-1
19. Wan CH. Research Status on the Second Version of the System of Quality of Life Instruments for Cancer Patients QLICP V2.0. J Guangdong Med Univ (2020) 38(5):511–7. doi: 10.3969/j.issn.1005-4057.2020.05.001
20. Yang Z. Development of the General Module of the System of Quality of Life Instruments for Cancer Patients V2.0 and Estimation of its Minimal Clinically Important Difference. Guangzhou: Southern Medical University (2015). doi: 10.7666/d.Y2910625
21. Tsai YS, Fang TP, Chi CC. A Scale for Measuring Evidence-Searching Capability: A Development and Validation Study. J Eval Clin Pract (2019) 25(4):676–81. doi: 10.1111/jep.13153
22. Shilling V, Starkings R, Jenkins V, Cella D, Fallowfield L. Development and Validation of the Patient Roles and Responsibilities Scale in Cancer Patients. Qual Life Res (2018) 27(11):2923–34. doi: 10.1007/s11136-018-1940-2
23. Hamrick LR, Haney AM, Kelleher BL, Lane SP. Using Generalizability Theory to Evaluate the Comparative Reliability of Developmental Measures in Neurogenetic Syndrome and Low-Risk Populations. J Neurodev Disord (2020) 12(1):16. doi: 10.1186/s11689-020-09318-1
24. Meng Q, Yang Z, Wu Y, Xiao YY, Gu XZ, Zhang MX, et al. Reliability Analysis of the Chinese Version of the Functional Assessment of Cancer Therapy - Leukemia (FACT-Leu) Scale Based on Multivariate Generalizability Theory. Health Qual Life Outcome (2017) 15(1):93. doi: 10.1186/s12955-017-0664-2
25. Wang TJ. An Explanation of the Conceptual Framework and Methods of Generalizability Theory. J Nanyang Inst Technol (2019) 11(2):112–7. doi: CNKI:SUN:NYLG.0.2019-02-023
26. Bilgic E, Watanabe Y, McKendy KM, Ito Y, Vassiliou MC. Reliable Assessment of Performance in Surgery: A Practical Approach to Generalizability Theory. J Surg Educ (2015) 72(5):774–5. doi: 10.1016/j.jsurg.2015.04.020
27. Holtmann G, Chassany O, Devault KR, Schmitt H, Gebauer U, Doerfler H, et al. International Validation of a Health-Related Quality of Life Questionnaire in Patients With Erosive Gastro-Oesophageal Reflux Disease. Aliment Pharmacol Ther (2019) 29(6):615–25. doi: 10.1111/j.1365-2036.2008.03922.x
28. Wan CH, Li HZ, Fan XJ, Yang RX, Pan JH, Chen WR, et al. Development and Validation of the Coronary Heart Disease Scale Under the System of Quality of Life Instruments for Chronic Diseases QLICD-CHD: Combinations of Classical Test Theory and Generalizability Theory. Health Qual Life Outcome (2014) 12:82. doi: 10.1186/1477-7525-12-82
29. Wasserman RH, Levy KN, Loken E. Generalizability Theory in Psychotherapy Research: The Impact of Multiple Sources of Variance on the Dependability of Psychotherapy Process Ratings. Psychother Res (2009) 19(4-5):397–408. doi: 10.1080/10503300802579156
30. Dong AP, Han LH. Progress in Measurement Scale of Quality of Life for Patients With Breast Cancer and its Application. Tumor (2017) 37(01):107–16. doi: 10.3781/j.issn.1000-7431.2017.55.579
31. Emery MP, Perrier LL, Acquadro C. Patient-Reported Outcome and Quality of Life Instruments Database (PROQOLID): Frequently Asked Questions. Health Qual Life Outcome (2005) 3:12. doi: 10.1186/1477-7525-3-12
32. Rosenberg SM, Tamimi RM, Gelber S, Ruddy KJ, Kereakoglow S, Borges VF, et al. Body Image in Recently Diagnosed Young Women With Early Breast Cancer. Psychooncology (2013) 22(8):1849–55. doi: 10.1002/pon.3221
Keywords: breast cancer, quality of life, classical test theory, generalizability theory, scale
Citation: Li F, Zhou J, Wan C, Yang Z, Liang Q, Li W and Chen H (2022) Development and Validation of the Breast Cancer Scale QLICP-BR V2.0 Based on Classical Test Theory and Generalizability Theory. Front. Oncol. 12:915103. doi: 10.3389/fonc.2022.915103
Received: 07 April 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Zbigniew Waśkiewicz, Jerzy Kukuczka Academy of Physical Education in Katowice, PolandCopyright © 2022 Li, Zhou, Wan, Yang, Liang, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chonghua Wan, d2FuY2hoQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.