
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 June 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.914299
 Ji Ha Lim1
Ji Ha Lim1 Woo Yong Lee2*
Woo Yong Lee2* Seong Hyeon Yun2
Seong Hyeon Yun2 Hee Cheol Kim2
Hee Cheol Kim2 Yong Beom Cho2
Yong Beom Cho2 Jung Wook Huh2
Jung Wook Huh2 Yoon Ah Park2
Yoon Ah Park2 Jung Kyong Shin2
Jung Kyong Shin2Introduction: Obstruction in colon cancer is a well-known risk factor for worse oncologic outcomes. However, studies on differences in survival of patients with incomplete obstructive colon cancer (IOCC) by tumor location are insufficient. Thus, the aim of this study was to compare oncologic outcomes between IOCC and non-obstructive colon cancer (NOCC) according to tumor location.
Methods: From January 2010 to December 2015, a total of 2,004 patients diagnosed with stage II or stage III colon adenocarcinoma who underwent elective colectomy were included (IOCC, n = 405; NOCC, n = 1,599). Incomplete obstruction was defined as a state in which colonoscopy could not pass through the cancer lesion but did not require emergent surgery, stent insertion, or stoma formation because the patient was asymptomatic without problem in bowel preparation. Kaplan–Meier method and log-rank tests were used to compare survival between IOCC and NOCC. Multivariable analysis was performed to determine which factors affected survivals.
Results: Stage III IOCC patients showed significantly lower overall survival (OS) and recurrence-free survival (RFS). Stage II IOCC patients and stage III NOCC patients had similar survival curves. IOCC patients with tumors on the right side showed worse OS than other patients. In multivariable analysis, incomplete obstruction was an independent risk factor for worse OS and RFS in all stages. Tumor located at the right side in stage III was an independent risk factor for RFS (HR: 1.40, p = 0.030).
Conclusions: Patients with IOCC showed significantly worse survival outcomes than those with NOCC. Stage II IOCC patients and stage III NOCC patients showed similar survival. Patients with stage III IOCC located at the right side showed significantly worse oncologic outcomes than those located at the left side. These results confirm that prognosis is different depending on the presence of incomplete obstruction and the location of the tumor, even in the same stage.
Obstruction is presented in approximately 10% to 29% of colorectal cancer cases. It is the most common cause of large intestinal obstruction (1–3). Patients with acute complete obstructive colon cancer (OCC) need emergent colectomy with or without anastomosis, colonic stent insertion, or primary stoma formation. Surgery under this emergent condition is associated with increased morbidity and mortality, affecting survival outcomes (4–6). Moreover, complete obstruction by colorectal cancer is associated with worse survival (3, 7–9). However, studies so far have only analyzed or included an acute obstructive condition that needs instant treatment. Studies on survival of patients with incomplete obstructive colon cancer (IOCC), which does not need instant treatment for resolving obstruction, are insufficient. Studies comparing oncologic outcomes between right-sided and left-sided OCC are also scarce. Some studies have reported that right-sided OCC has a worse prognosis than left-sided OCC (10–12). However, other studies have shown no differences in oncologic outcome between left-side and right-side OCCs (13, 14). Accordingly, the aim of this study was to compare oncologic outcomes of IOCC patients with those of non-obstructive colon cancer (NOCC) patients. Differences in outcomes between right-sided and left-sided IOCC were also determined.
From January 2010 to December 2015, 7,288 patients who underwent surgery for primary colorectal cancer in Samsung Medical Center (SMC) were identified. Patients who were diagnosed with rectal cancer; who were not diagnosed with adenocarcinoma, stage II and III; who had hereditary colon diseases and multiple colon cancer; who underwent preoperative chemotherapy and emergent surgery; who had cancer peroration; and who had colonic stent placed or formed a stoma for obstructive colon cancer before radical colectomy were excluded. A total of 2,004 patients were included and analyzed in this study (Figure 1).
Information on age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, preoperative erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9) level, and tissue samples after colectomy was collected. All patients underwent colectomy without stoma formation. Several pathologists evaluated all specimens. Stages were classified according to the American Joint Committee on Cancer (AJCC) 8th guidelines (15). Data on any postoperative complications were collected. Among them, data on Clavien–Dindo classification (CDC) (16, 17) grade 3 (needing surgical, endoscopic, or radiological intervention) or higher were collected with anastomosis leakage data separately. Anastomosis leakage was defined as any condition showing the presence of an abscess around the anastomosis or anastomosis site dehiscence identified on imaging examination. Readmission data within 30 days after surgery were also collected.
Adjuvant chemotherapy was performed based on the National Comprehensive Cancer Network (NCCN) guidelines. If patients refused, routine follow-up was performed. Follow-up examinations included serum CEA level, abdomen and chest CT, and colonoscopy. Usually, follow-up started 2 weeks after the discharge date and was performed every 3 months for the first 3 years and every 6 months until 5 years after that. However, if patients wanted to revisit after 5 years of follow-up, additional follow-up was performed. Moreover, if recurrence was suspected, follow-up could be shortened and further evaluation was performed.
Group was initially divided into IOCC and NOCC. Incomplete obstruction was defined as a state in which colonoscopy could not pass through the cancer lesion but did not require emergent surgery, stent insertion, or stoma formation because the patient was asymptomatic without problem in bowel preparation. Subgroup analysis was performed by tumor location (left colon vs. right colon). The ascending colon to the mid-transverse colon was included in the right colon. The distal transverse colon to the rectosigmoid colon was included in the left colon.
All statistical analyses were performed using SPSS version 27.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant. Except for the preoperative CRP (missing value of 12.7%) and ESR (missing value of 11.3%), all other data missing values were less than 2%, and any missing data were omitted and the remaining data were analyzed. Comparison of baseline characteristics was performed using χ2 test or Fisher’s exact test for categorical variables. Continuous variables were compared using Student’s t-test and Mann–Whitney test after checking normality of data with the Shapiro–Wilk test. Survival was analyzed with the Kaplan–Meier method and log-rank test. Univariable and multivariable analyses were performed using Cox proportional-hazards (PH) regression analysis to determine which factors affected survival. Variables that showed significance in univariable Cox PH regression analysis were entered into a multivariable Cox PH regression analysis using the backward elimination method. This study was approved by the Institutional Review Board of SMC (Approval number: SMC 2021-06-041).
A total of 2,004 patients pathologically diagnosed with stage II or III colon cancer who underwent elective colectomy for IOCC or NOCC from 2010 to 2015 were included in this study (IOCC, n = 405; NOCC, n = 1,599). Baseline characteristics of subjects with IOCC or NOCC are shown in Table 1. Preoperative median ESR and CRP levels were higher in the IOCC group [ESR: 34 (range, 2–120) mm/h vs. 25 (range, 2–120) mm/h, p < 0.001; CRP: 0.28 (range, 0.03–13.53) mg/dl vs. 0.12 (range, 0.02–34.12) mg/dl, p < 0.001]. More patients showed elevated CEA (over 5 ng/ml) and CA 19-9 (over 37 ng/ml) levels in the IOCC group (elevated CEA: 30.4% vs. 16.8%, p < 0.001; elevated CA: 19-9, 15.3% vs. 7.8%, p < 0.001). The IOCC group had more open surgery cases (23.2% vs. 11.5%, p < 0.001), longer mean operative time (150.79 ± 55.32 min vs. 143.63 ± 46.70 min, p = 0.017), and more blood loss (115.55 ± 108.00 ml vs. 97.48 ± 82.54 ml, p = 0.002). Although the proportion of left colon cancer was higher in the IOCC group than in the NOCC group, the difference was not statistically significant (60.5% vs. 57.5%, p = 0.281, Table 1).
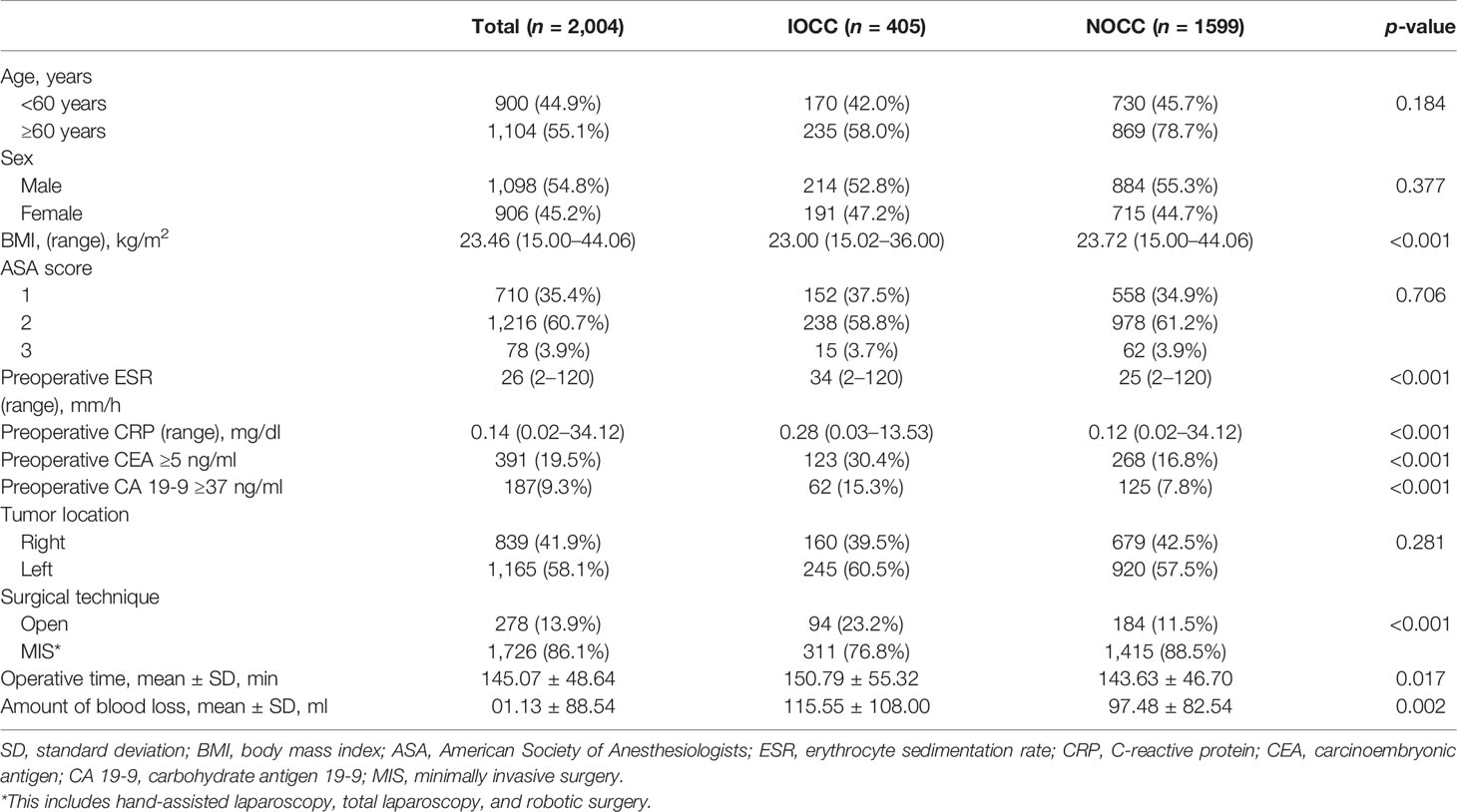
Table 1 Baseline characteristics of incomplete obstructive colon cancer (IOCC) and non-obstructive colon cancer (NOCC).
In pathologic results, patients with IOCC showed bigger tumor size (5.96 ± 2.12 cm vs. 4.54 ± 2.09 cm, p < 0.001), more serosal exposure tumors (T4: 27.2% vs. 14.9%, p < 0.001), and more moderately (MD) and poorly differentiated (PD) adenocarcinomas (MD+PD: 83.0% vs. 76.5%, p = 0.006). However, there was no statistical significance in nodal status (p = 0.139) or tumor-nodal-metastasis (TNM) stage (p = 0.713) between the two groups. Lymphatic invasion and high microsatellite instability (MSI) status were not significantly different between IOCC and NOCC groups (both p > 0.05), but perineural invasion (40.7% vs. 30.3%, p < 0.001), vascular invasion (18.3% vs. 13.1%, p = 0.007), and positive tumor budding (62.0% vs. 55.7%, p = 0.022) were more common in IOCC than in NOCC. Any morbidity (18.8% vs. 14%, p = 0.017) and morbidity ≥ CDC grade 3 (6.7% vs. 3.4%, p = 0.003) were more common in IOCC. There was no significant difference in anastomotic leakage (p = 0.596) between the two groups. Furthermore, there was no significant difference in the proportion of patients receiving adjuvant chemotherapy (overall: 67.9% vs. 68.3%; stage II: 43.6% vs. 40.5%; stage III: 87.2% vs. 91.2%, all p > 0.05). However, the recurrence rate was higher in the IOCC group (20.2% vs. 10.4%, p < 0.001), although there was no statistically significant difference in the recurrence pattern (p = 0.621, Table 2).
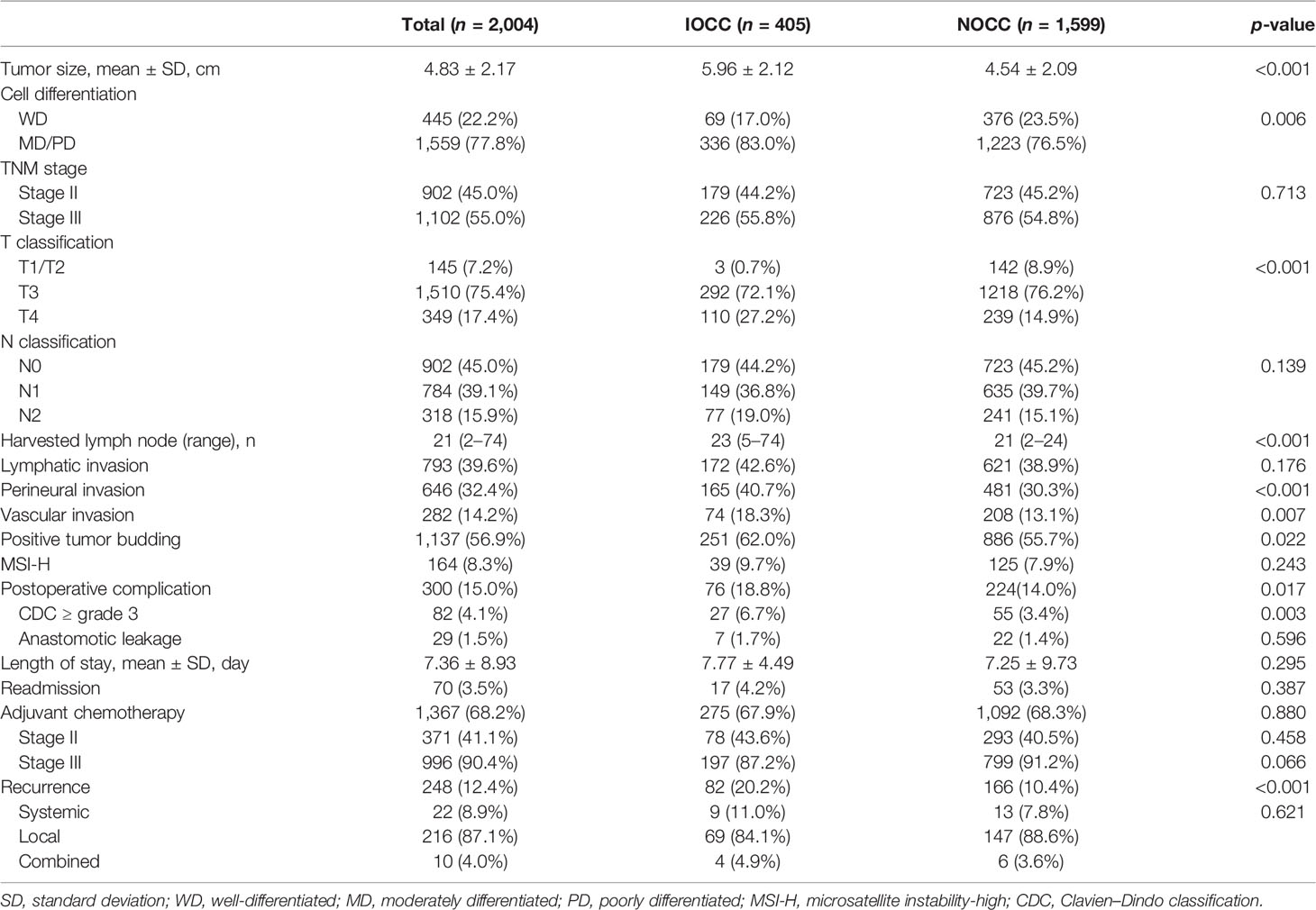
Table 2 Postoperative outcomes of incomplete obstructive colon cancer (IOCC) and non-obstructive colon cancer (NOCC).
Of 405 IOCC patients, 245 had left colon cancer and 160 had right colon cancer. Preoperative ESR and CRP were higher in the right colon cancer group [ESR: 40 (range, 3–120) mm/h vs. 28.5 (range, 2–120) mm/h, p < 0.001; CRP: 0.41 mg/dl vs. 0.23 mg/dl, p = 0.023]. More patients showed elevated CA 19-9 level (over 37 ng/ml) in the right IOCC group (23.8% vs. 9.8%, p < 0.001). However, the proportion of patients who showed elevated CEA level (over 5 ng/ml) did not differ between left and right colon cancer groups (p = 0.216, Table 3). Results of comparing postoperative outcomes between right and left IOCC groups are shown in Table 4. Right colon cancer showed bigger tumor size (6.61 ± 2.36 cm vs. 5.54 ± 1.83 cm, p < 0.001), more T4 cancer (35.0% vs. 22.0%, p = 0.006), more MSI high tumor (18.4% vs. 4.1%, p < 0.001), and higher recurrence rate (26.3% vs. 16.3%, p = 0.015). However, there were no significant differences in nodal status, stage, or risk factors such as lymphatic invasion, vascular invasion, perineural invasion, or positive tumor budding (all p > 0.05, Table 4).
The total median follow-up period was 68.6 (range, 0.1–128.8) months, and follow-up loss occurred in 18.6% of patients. Stage III IOCC patients showed significantly lower survival, while stage II IOCC patients and stage III NOCC patients had similar survival curves (5-year OS: 93.0% for stage II IOCC, 96.0% for stage II NOCC, 79.2% for stage III IOCC, 91.2% for stage III NOCC, p < 0.001, Figure 2A). In a sub-analysis by tumor location, there was no significant difference in survival for stage II (p = 0.181, Figure 3A). However, for stage III, right IOCC patients showed worse overall survival than other patients (5-year OS: 65.2% for right IOCC, 88.9% for left IOCC, 89.1% for right NOCC, 92.5% for left NOCC, p < 0.001, Figure 3B).
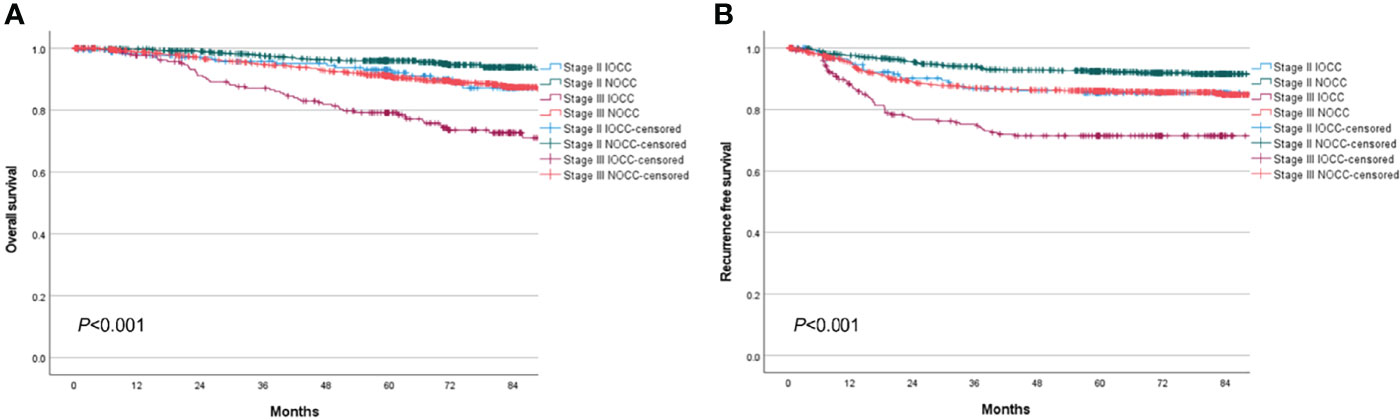
Figure 2 Overall survival and recurrence-free survival between IOCC and NOCC by stage. (A) Overall survival by obstruction status and stage. (B) Recurrence-free survival by obstruction status and stage.

Figure 3 Overall survival and recurrence-free survival between stage II and stage III IOCC and NOCC by tumor location. (A) Overall survival of stage II colon cancer by obstruction status and tumor location. (B) Overall survival of stage III colon cancer by obstruction status and tumor location. (C) Recurrence-free survival of stage II colon cancer by obstruction status and tumor location. (D) Recurrence-free survival of stage III colon cancer by obstruction status and tumor location.
In multivariable Cox regression analysis of stage II colon cancer, incomplete obstruction was an independent risk factor for worse OS (HR: 1.86, 95% CI: 1.03–3.37, p = 0.040). Elevated CEA levels and postoperative complication were also independent risk factors (CEA level, HR: 2.02, 95% CI: 1.10–3.70, p = 0.023; morbidity, HR: 2.73, 95% CI: 1.54–4.83, p = 0.001). Age over 60 years was also a strong risk factor (HR: 12.87, 95% CI: 4.00–41.44, p < 0.001).
For stage III, incomplete obstruction was also an independent risk factor for worse OS (HR: 1.51, 95% CI: 1.06–2.14, p = 0.022). Age over 60 years (HR: 2.79, 95% CI: 1.87–4.16, p < 0.001), elevated CA 19-9 level (HR: 2.23, 95% CI: 1.51–3.31, p < 0.001), N2 nodal status (HR: 1.48, 95% CI: 1.03–2.13, p = 0.035), moderately differentiated feature (HR: 2.62, 95% CI: 1.21–5.71, p = 0.015), poorly differentiated feature (HR: 2.63, 95% CI: 1.02–6.78, p = 0.045), vascular invasion (HR: 1.81, 95% CI: 1.25–2.64, p = 0.002), and not undergoing or completing adjuvant chemotherapy (HR: 3.82, 95% CI: 2.53–5.77, p < 0.001) were other risk factors of overall survival (Table 6). However, tumor location was not an independent factor affecting overall survival for any stage (Tables 5, 6).
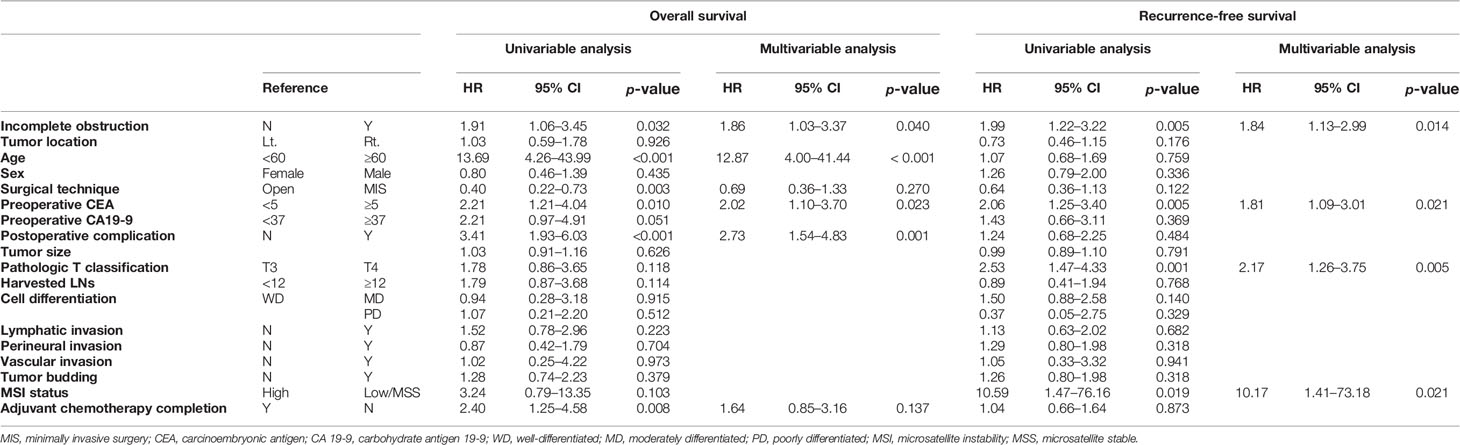
Table 5 Univariable and multivariable Cox regression analysis of overall survival and recurrence-free survival of stage II colon cancer.
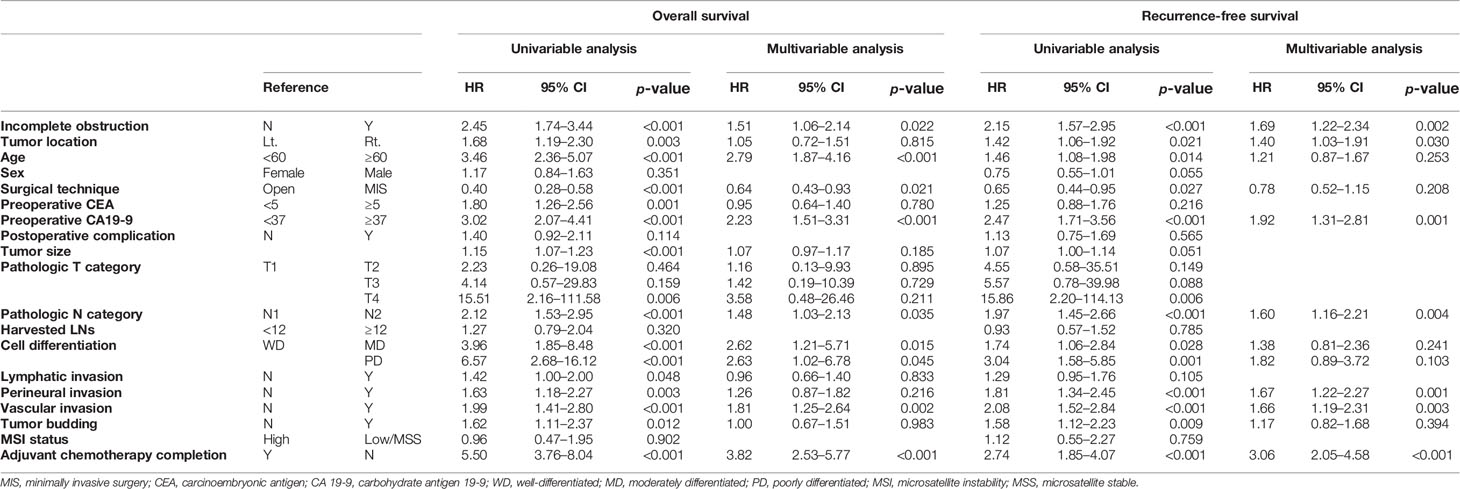
Table 6 Univariable and multivariable Cox regression analysis of overall survival and recurrence-free survival of stage III colon cancer.
The RFS curve showed a similar pattern to the OS curve. Stage III IOCC patients had significantly lower RFS than other patients, while similar survival was found for stage II IOCC and stage III NOCC (5-year RFS: 85.4% for stage II IOCC, 92.5% for stage II NOCC, 71.5% for stage III IOCC, 86.0% for stage III NOCC, p < 0.001, Figure 2B). In a sub-analysis by tumor location, there was a difference in RFS according to the presence of incomplete obstruction, not the location of the tumor, in patients with stage II cancer (5-year RFS: 85.6% for right IOCC, 85.3% for left IOCC, 93.5% for right NOCC, 91.5% for left NOCC, p = 0.023, Figure 3C). For stage III, right IOCC patients showed significantly worse RFS (5-year RFS: 60.4% for right IOCC, 78.9% for left IOCC, 85.0% for right NOCC, and 86.7% for left NOCC, p < 0.001, Figure 3D).
In multivariable Cox regression analysis of stage II colon cancer, incomplete obstruction was an independent risk factor for worse RFS (HR: 1.84, 95% CI: 1.13–2.99, p = 0.014). Elevated CEA level (HR: 1.81, 95% CI: 1.09–3.01, p = 0.021), T4 adenocarcinoma (HR: 2.17, 95% CI: 1.26–3.75, p = 0.005), and MSI low/microsatellite stable (MSS) tumors (HR: 10.17, 95% CI: 1.41–73.18, p = 0.021) were also independent risk factors for worse RFS. However, tumor location did not independently affect RFS (Table 5).
Incomplete obstruction was also an independent risk factor for worse RFS in stage III (HR: 1.69, 95% CI: 1.22–2.34, p = 0.002). Elevated CA 19-9 level (HR: 1.92, 95% CI: 1.31–2.81, p = 0.001), N2 nodal status (HR: 1.60, 95% CI: 1.16–2.21, p = 0.004), perineural invasion (HR: 1.67, 95% CI: 1.22–2.27, p = 0.001), vascular invasion (HR: 1.66, 95% CI: 1.19–2.31, p = 0.003), and not undergoing or completing adjuvant chemotherapy (HR: 3.06, 95% CI: 2.05–4.58, p < 0.001) were other risk factors. For stage III RFS, right side tumor location was an independent risk factor (HR: 1.40, 95% CI: 1.03–1.91, p = 0.030, Table 6).
Instant management is needed for acute complete OCC (COCC). Recommended treatments are different according to tumor location (1, 2, 4–6, 18, 19). According to 2017 WSES guidelines on colon cancer obstruction, for right COCC, right colectomy with primary anastomosis is a preferred treatment option (18). However, for left COCC, colectomy with or without primary anastomosis, endoscopic colonic stent insertion by self-expanding metallic stents (SEMS), tube decompression, and stoma formation are all possible options. The present study only analyzed patients with incomplete obstruction who did not require emergent management. All patients had undergone elective colectomy with primary anastomosis. Overall postoperative complications and CDC grade 3 or higher complications were more frequent in IOCC than in NOCC. However, there was no mortality case. There were no significant differences in readmission within 30 days or length of hospital stays between the two groups. This meant that there was no significant increase in hospitalization, readmission, or mortality, although the IOCC group had a high rate of postoperative complications. Moreover, anastomosis leakage rates did not differ significantly between the two groups. Therefore, for IOCC, regardless of the tumor location, colectomy with primary anastomosis can be an appropriate treatment procedure without increasing mortality or severe complications caused by primary anastomosis such as anastomotic leakage.
Many studies have shown that obstructive colon cancer has a poor prognosis (3, 7–9). According to NCCN guidelines (20), obstruction in stage II colon cancer is a high-risk factor and an indication of adjuvant chemotherapy. In our study, stage II IOCC showed similar overall and recurrence-free survival curves to stage III NOCC, with stage III IOCC showing a significantly lower survival rate. Furthermore, incomplete colon obstruction at all stages was an independent risk factor for worse OS and RFS in multivariable analysis. Our study showed that incomplete obstruction was a definitive factor affecting prognosis. Patients with incomplete obstruction had worse prognoses in stages 2 and 3 than those without incomplete obstruction. However, since COCC patients were not included in this study, it was not confirmed whether COCC and IOCC had the same survival rate, making it difficult to say whether they should be regarded as the same risk factors. Moreover, in this study, we could not find out whether chemotherapy helped improve survival of patients with stage II IOCC because we did not analyze the difference in survival following chemotherapy administration for stage II IOCC without other risk factors due to the small sample size. Therefore, further study on this is needed. Nevertheless, results of our study confirmed that the prognosis of IOCC was poor. This is meaningful in that it can provide more accurate information on prognosis of IOCC patients.
We also sub-analyzed characteristics and survivals according to tumor location to find any differences by tumor side. Some studies have investigated whether there are oncologic differences between left-sided and right-sided obstructive colon cancer. Frago et al. have compared proximal and distal obstructive colon cancer and reported that tumor location does not influence the prognosis after curative surgery (13). However, Mege et al. have reported that patients with right-sided OCC have significantly lower 5-year OS and RFS than those with left-sided OCC (OS: 43% for right-sided OCC vs. 53% for left-sided OCC, p < 0.0001; RFS: 36% for right-sided OCC vs. 46% for left-sided OCC, p = 0.001) (11). However, the present study did not show a significant difference in OS or RFS by tumor location for stage II. On the other hand, for stage III colon cancer patients, right-sided IOCC showed significantly lower survival than left-sided IOCC. In multivariable analysis, right side tumor location was also an independent risk factor for RFS, but not OS, in stage III colon cancer. These results indicate that the location of the tumor might have a sufficient impact on the survival of IOCC patients with lymph node metastasis, showing worse outcomes for right-sided tumors than for left-sided tumors.
This study has some limitations. First, there may be bias in the analysis because there were relatively fewer IOCC patients (n = 405) included compared to NOCC patients (n = 1599). Second, as mentioned above, it did not include complete obstruction, making it hard to know differences in survival between complete obstruction and incomplete obstruction. Liu et al. (21) have compared short-term and long-term outcomes between incomplete and complete left-sided malignant obstruction cases. There was no significant difference in survival between IOCC and COCC groups in their study. However, since their study only included left-sided tumors, it was difficult to assess whether survival was different for those with right-sided colon cancer. Further studies that compare survivals between right-sided IOCC and COCC are needed. Third, since our hospital is a tertiary hospital, most patients who visited our hospital usually received colonoscopy at a primary or secondary healthcare hospital. In addition, various physicians performed colonoscopy. Due to these characteristics, it is challenging to evaluate obstruction objectively as many physicians who have performed colonoscopy have different abilities. Their ability to pass through obstruction might also be different. Moreover, the degree of difficulty of performing colonoscopy may vary depending on the location of the tumor, such as hepatic flexure colon cancer, which may be a limitation in evaluating the oncologic outcomes according to the tumor location. However, the strength of this study was that it only included non-emergent obstructive colon cancer cases. Thus, morbidities in an emergent situation would not affect survival (4–6).
In conclusion, patients with IOCC showed significantly worse survival outcomes than those with NOCC. In addition, stage III right-sided IOCC patients showed significantly worse oncologic outcomes than left-sided colon cancer patients or right-sided NOCC patients. Based on these results, we can inform patients that the prognosis is different depending on the presence of incomplete obstruction and the location of the tumor, even in the same stage. However, further studies are needed to determine whether chemotherapy can help improve the prognosis of patients with IOCC, especially stage II.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Samsung Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JL and WL contributed to the conception and design of the study. JL, WL, JH, SY, HK, YC, YP, and JS organized the database. JL performed the statistical analysis. JL and WL wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kim YJ. Surgical Treatment of Obstructed Left-Sided Colorectal Cancer Patients. Ann Coloproctol (2014) 30:245. doi: 10.3393/ac.2014.30.6.245\
2. Boeding JRE, Ramphal W, Rijken AM, Crolla RMPH, Verhoef C, Gobardhan PD, et al. A Systematic Review Comparing Emergency Resection and Staged Treatment for Curable Obstructing Right-Sided Colon Cancer. Ann Surg Oncol (2021) 28(7):3545–55. doi: 10.1245/s10434-020-09
3. Wang HS, Lin JK, Mou CY, Lin TC, Chen WS, Jiang JK, et al. Long-Term Prognosis of Patients With Obstructing Carcinoma of the Right Colon. Am J Surg (2004) 187(4):497–500. doi: 10.1016/j.amjsurg.2003.12.028
4. Ramos RF, Dos-Reis LCS, Teixeira BEB, Andrade IM, Sulzbach JS, Leal RA. Colon Cancer Surgery in Patients Operated on an Emergency Basis. Rev do Colégio Brasileiro Cirurgiões (2017) 44:465–70. doi: 10.1590/0100-69912017005007
5. Alvarez JA, Baldonedo RF, Bear IG, Truán N, Pire G, Alvarez P. Presentation, Treatment, and Multivariate Analysis of Risk Factors for Obstructive and Perforative Colorectal Carcinoma. Am J Surg (2005) 190(3):376–82. doi: 10.1016/j.amjsurg.2005.01.045
6. Alvarez JA, Baldonedo RF, Bear IG, Truán N, Pire G, Alvarez P. Obstructing Colorectal Carcinoma: Outcome and Risk Factors for Morbidity and Mortality. Dig Surg (2005) 22(3):174–81. doi: 10.1159/000087436
7. Chen T-M, Huang Y-T, Wang G-C. Outcome of Colon Cancer Initially Presenting as Colon Perforation and Obstruction. World J Surg Oncol (2017) 15(1):164. doi: 10.1186/s12957-017-1228-y
8. Dahdaleh FS, Sherman SK, Poli EC, Vigneswaran J, Polite BN, Sharma MR, et al. Obstruction Predicts Worse Long-Term Outcomes in Stage III Colon Cancer: A Secondary Analysis of the N0147 Trial. Surgery (2018) 164:1223–9. doi: 10.1016/j.surg.2018.06.044
9. Chin C-C, Wang J-Y, Changchien C-R, Huang W-S, Tang R. Carcinoma Obstruction of the Proximal Colon Cancer and Long-Term Prognosis—Obstruction Is a Predictor of Worse Outcome in TNM Stage II Tumor. Int J Colorectal Dis (2010) 25:817–22. doi: 10.1007/s00384-010-0904-y
10. Wolmark N, Wieand HS, Rockette HE, Fisher B, Glass A, Lawrence W, et al. The Prognostic Significance of Tumor Location and Bowel Obstruction in Dukes B and C Colorectal Cancer. Findings From the NSABP Clinical Trials. Ann Surg (1983) 198(6):743–52. doi: 10.1097/00000658-198312000-00013
11. Mege D, Manceau G, Beyer L, Bridoux V, Lakkis Z, Venara A, et al. Right-Sided vs. Left-Sided Obstructing Colonic Cancer: Results of a Multicenter Study of the French Surgical Association in 2325 Patients and Literature Review. Int J Colorectal Dis (2019) 34:1021–32. doi: 10.1007/s00384-019-03286-2
12. Faucheron J-L, Paquette B, Trilling B, Heyd B, Koch S, Mantion G. Emergency Surgery for Obstructing Colonic Cancer: A Comparison Between Right-Sided and Left-Sided Lesions. Eur J Trauma Emergency Surg (2018) 44:71–7. doi: 10.1007/s00068-017-0766-x
13. Frago R, Biondo S, Millan M, Kreisler E, Golda T, Fraccalvieri D, et al. Differences Between Proximal and Distal Obstructing Colonic Cancer After Curative Surgery. Colorectal Dis (2011) 13:e116–22. doi: 10.1111/j.1463-1318.2010.02549.x
14. Sugiura K, Seo Y, Aoki H, Onishi Y, Nishi Y, Kishida N, et al. Bridge to Surgery for Obstructing Colonic Cancer: A Comparison Between Right- and Left-Sided Lesions. J Anus Rectum Colon (2021) 5:34–9. doi: 10.23922/jarc.2020-046
15. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th Ed. New York: Springer (2017).
16. Clavien PA, Sanabria JR, Strasberg SM. Proposed Classification of Complications of Surgery With Examples of Utility in Cholecystectomy. Surgery (1992) 111:518–26.
17. Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
18. Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, et al. 2017 WSES Guidelines on Colon and Rectal Cancer Emergencies: Obstruction and Perforation. World J Emergency Surg (2018) 13:36. doi: 10.1186/s13017-018-0192-3
19. Veld JV, Amelung FJ, Borstlap WAA, Van Halsema EE, Consten ECJ, Siersema PD, et al. Comparison of Decompressing Stoma vs Stent as a Bridge to Surgery for Left-Sided Obstructive Colon Cancer. JAMA Surg (2020) 155:206. doi: 10.1001/jamasurg.2019.5466
20. National Comprehensive Cancer Network. Colon Cancer (Version2.2021) . Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (Accessed May 25, 2021).
Keywords: incomplete obstruction, colon cancer, stage, sidedness, survival
Citation: Lim JH, Lee WY, Yun SH, Kim HC, Cho YB, Huh JW, Park YA and Shin JK (2022) Comparison of Oncologic Outcomes Between Incomplete Obstructive Colon Cancer and Non-Obstructive Colon Cancer by Tumor Location. Front. Oncol. 12:914299. doi: 10.3389/fonc.2022.914299
Received: 06 April 2022; Accepted: 09 May 2022;
Published: 06 June 2022.
Edited by:
Igor Kiss, Masaryk Memorial Cancer Institute (MMCI), CzechiaReviewed by:
Caroline Nordenvall, Karolinska Institutet (KI), SwedenCopyright © 2022 Lim, Lee, Yun, Kim, Cho, Huh, Park and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woo Yong Lee, bHd5NTU1QHNra3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.