
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 September 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.914076
The survival rate for colon cancer after radical surgery has been the focus of extensive debate. To assess the postoperative survival and prognostic factors for overall survival (OS), we collected clinicopathological information for 2,655 patients. The survival time and potential risk factors for OS were analyzed by using Kaplan–Meier curves, Cox proportional hazards models, best subset regression (BSR), and least absolute shrinkage and selection operator (LASSO). The 5-year survival rates of stage I–IV colon cancer were 96.6%, 88.7%, 69.9%, and 34.3%, respectively. Adjuvant chemotherapy improved the survival rate (90.4% vs. 82.4%, with versus without adjuvant chemotherapy, respectively) in stage II patients with high-risk factors. Elevated preoperative carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were significantly associated with worse OS compared with patients without these elevations. Less than 12 versus more than 12 harvested lymph nodes (LNs) affected prognosis (84.6% vs. 89.7%, respectively). Regarding the lymph node ratio (LNR), the 5-year OS rate was 89.2%, 71.5%, 55.8%, and 34.5% in patients with LNR values of 0, 0.3, 0.3–0.7, and >0.7, respectively. We constructed a nomogram comprising the independent factors associated with survival to better predict prognosis. On the basis of these findings, we propose that stage II colon cancer patients without high-risk factors and with both elevated preoperative CEA and CA199 should receive adjuvant therapy. Furthermore, the LNR could complement TNM staging in patients with <12 harvested LNs. Our nomogram might be useful as a new prognosis prediction system for colon cancer patients.
Colon cancer is one of the most prevalent malignancies and the second leading cause of cancer-related death in the world (1, 2). According to the latest statistics, there are approximately 520,000 new cases and 250,000 deaths in 2018 in China (3). Currently, the main treatment for colon cancer is radical surgical resection and adjuvant chemotherapy. The 5-year overall survival (OS) rate after radical surgery for colon cancer is approximately 60% in China (4), and the mortality rate remains high. Various factors might be associated with 5-year survival, such as stage, differentiation, histology, tumor location, age, and sex.
Accurate pathological diagnosis, radical surgery, and adequate postoperative adjuvant chemotherapy are critical for colon cancer patients. There have been extensive studies evaluating how to predict the postoperative OS of colon cancer; however, the results are controversial. Therefore, we retrospectively reviewed the clinicopathological data of 2,655 colon cancer patients after radical surgery between January 2011 and December 2016 at Harbin Medical University Cancer Hospital. A nomogram was developed based on clinical and histopathological high-risk factors to determine the OS of colon cancer patients undergoing curative resection.
The clinical data of 2,655 colon cancer patients treated between January 2011 and December 2016 were derived from the Department of Colorectal Surgery of Harbin Medical University Cancer Hospital. Patients who received palliative surgery, had multiple primary cancers, or received neoadjuvant chemotherapy were excluded; patients lost to follow-up were also excluded (Supplementary Figure 1). All patients were randomly divided into a training cohort and a testing cohort at a ratio of 70% to 30%. This study was approved by the Harbin Medical University Cancer Hospital ethics committee (KY2017-19), and written informed consent was obtained from the patients.
We evaluated the following baseline covariates: sex, age, pathological tumor (pT) stage, pathological lymph node (pN) stage, pathological metastasis (pM) stage, tumor location, tumor size, histology, differentiation, examined lymph nodes (LNs), metastatic lymph node ratio (LNR), perineural invasion, lymphatic invasion, preoperative carcinoembryonic antigen (CEA) level, preoperative carbohydrate antigen 199 (CA199) concentration, and adjuvant chemotherapy. Cancers of the proximal splenic flexure (cecum and ascending colon) were classified as right colon cancer. Cancers in the distal splenic flexure (descending colon and sigmoid colon) were classified as left colon cancer. Cancer in the middle of the transverse colon was classified as transverse cancer. Regarding adjuvant therapy, patients were classified into two groups (i.e., with vs. without). Patients were also categorized into three groups by their age at the time of the primary tumor resection, as follows: <40 years, 40–70 years, and >70 years. CEA level was classified as ≤5 ng/ml or >5 ng/ml based on the upper normal limit (5 u/ml), and CA199 level was classified as ≤37 U/ml or >37 U/ml based on the upper normal limit (37 U/ml). Tumor size was categorized as <5 cm or ≥5 cm. All patients in this study were reclassified in accordance with the 8th TNM classification of colon cancer.
All statistical analyses were performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). OS analyses were performed using the Kaplan–Meier (KM) method, and the results were compared using the log-rank test. Clinical and pathological variables associated with survival were assessed on the basis of clinical importance, scientific evidence, and predictors identified in previously published articles (5, 6). The variables screened by univariate Cox regression, best subset regression (BSR), and least absolute shrinkage and selection operator (LASSO) were included in a multivariate Cox regression model, and the final model of the three methods was determined using backward stepwise selection with minimum Akaike’s information criterion (AIC) values (Supplementary Table 1). Candidate variables with p-values < 0.2 in the univariate analysis were included in the multivariable model. Risk factors were measured as [hazard ratio (HR), 95% confidence interval (CI)]. All reported statistical significance levels were two-sided, with statistical significance set at 0.05. The predictive nomogram was developed based on a multivariate analysis using the rms package in R, version 4.1.2 (www.r-project.org). Calibration plots, Harrell’s concordance index (C-index), area under the curve (AUC) values, and decision curve analysis (DCA) were used to evaluate calibrating ability.
The baseline clinicopathological characteristics of the patients are shown in Table 1. More than half of the patients had tumors larger than 5.0 cm (N = 1,740, 65.5%). Regarding the differentiation grade of the primary site, approximately 9.0%, 81.5%, 5.2%, and 4.3% of the colon cancer patients had well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated tumors, respectively. The proportions of stage T1, T2, T3, and T4 cancer were 2.0%, 6.2%, 73.4%, and 18.2%, respectively. Stages II and III were more prevalent (N = 2,411, 90.8%) than stages I and IV, and 62.8% of the patients were classified as N0 (Figure 1). Regarding the LNs, approximately 70% of the patients had sufficient LNs examined (≥12; N = 1995, 75.1%), and the average number of LNs examined was 18. At the end of the follow-up, the mortality rate was 20.6%, and the median survival was 67.94 months.
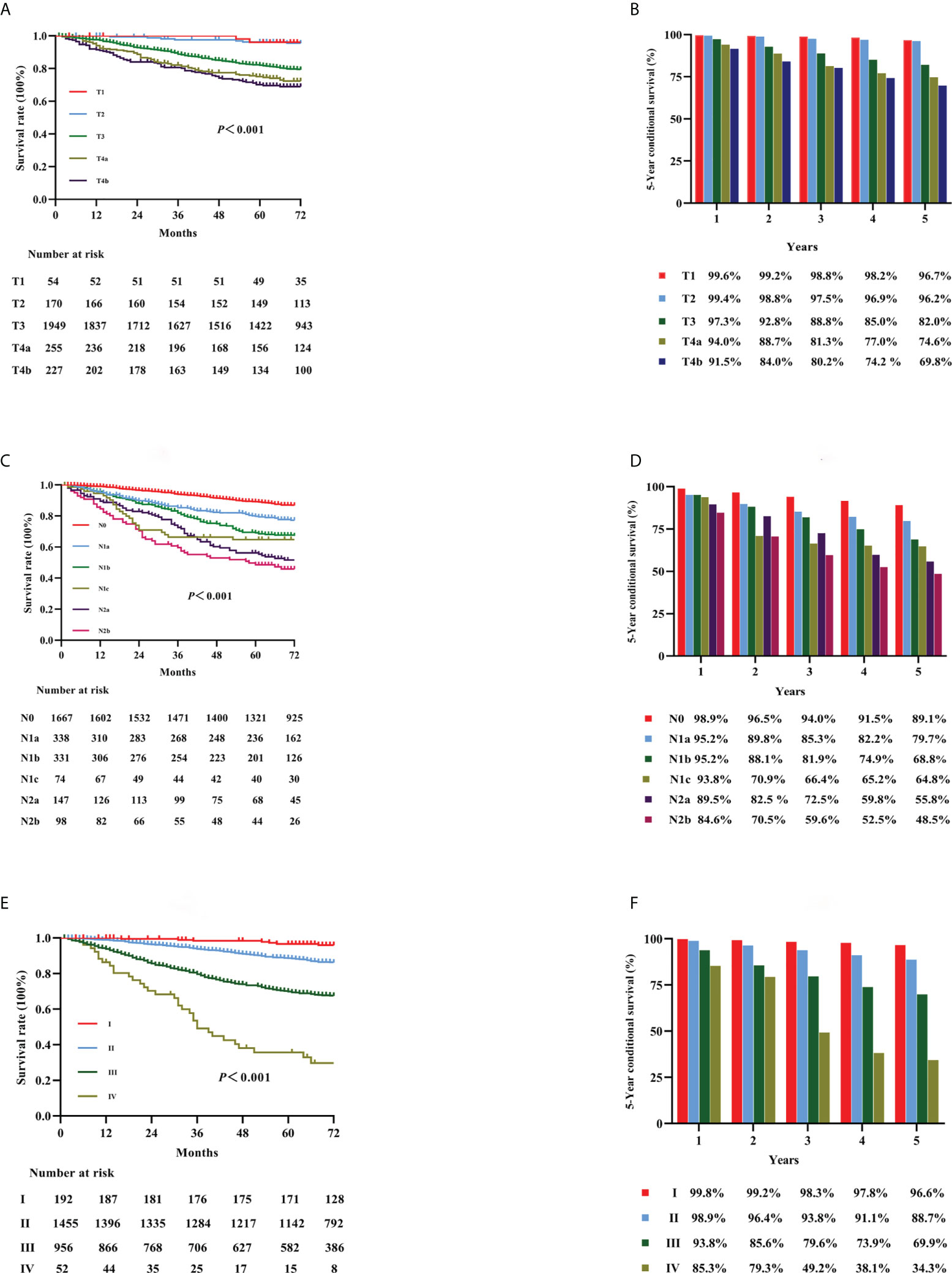
Figure 1 Kaplan–Meier curves for survival analysis in colon cancer patients. The OS according to T stage (A), N stage (C), and the TNM staging system (E). The 5-year OS rates of the three classifications [T stage, N stage, and TNM staging: (B, D, F), respectively]. OS, overall survival; T, tumor; N, node; TNM, tumor-node-metastasis.
Univariate and multivariate regression analyses were performed to determine the prognostic factors in colon cancer patients after curative surgical resection (Figures 2A, B). Age, preoperative CEA and CA199 levels, tumor size, histology, differentiation, pT stage, pN stage, M stage, number of harvested LNs, LNR, perineural invasion, vascular invasion, and adjuvant chemotherapy were identified as significant factors correlated with OS in the univariate analysis (Figure 2A). The multivariate analysis showed that age, preoperative CEA and CA199 levels, histology, differentiation, pT stage, pN stage, M stage, LNR, perineural invasion, and adjuvant chemotherapy were independent prognostic factors (Figure 2B).
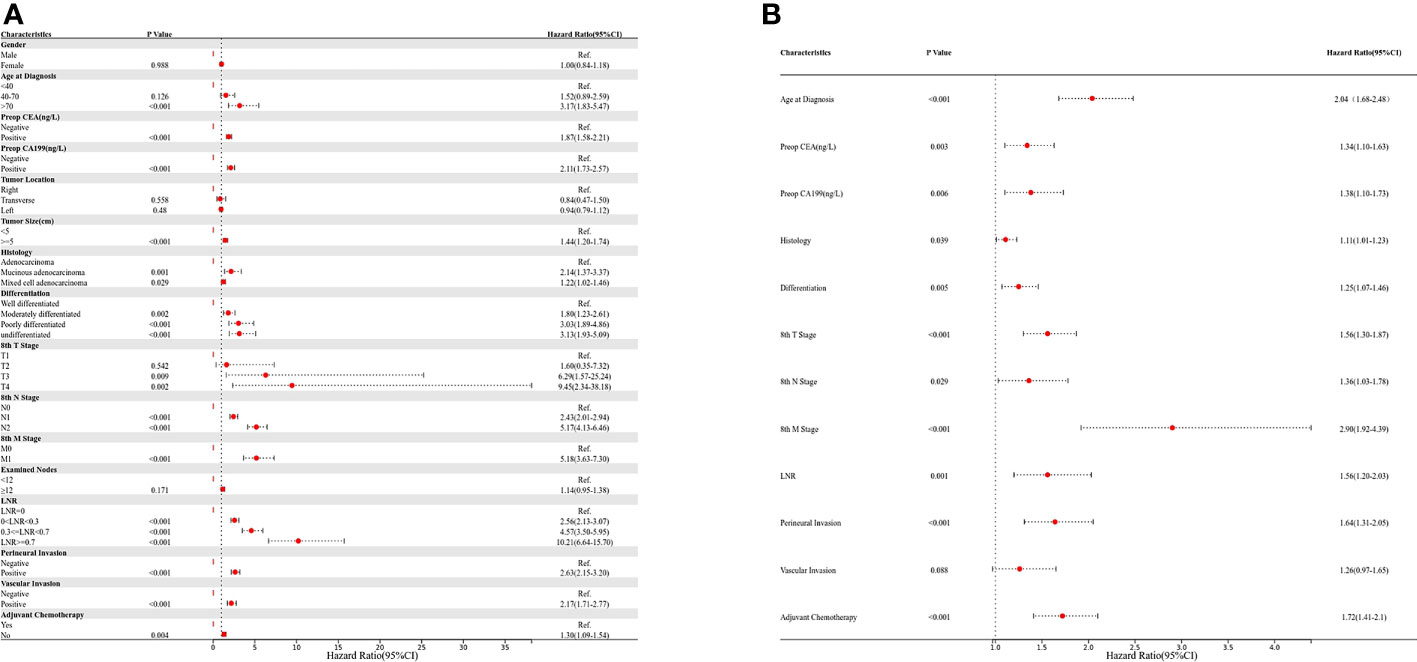
Figure 2 Univariate and multivariate Cox regression analyses; (A) univariate analysis; (B) multivariate analysis.
KM curves for stages T1, T2, T3, T4a, and T4b in all patients are shown in Figure 1A (p < 0.001). The OS rates for stages T1, T2, T3, T4a, and T4b were 96.7%, 96.2%, 82.0%, 74.6%, and 69.8%, respectively (Figure 1B). KM curves for stages N0, N1a, N1b, N1c, N2a, and N2b for all patients are shown in Figure 1C (p < 0.001). The OS rates for stages N0, N1a, N1b, N1c, N2a, and N2b were 89.1%, 79.7%, 68.8%, 64.8%, 55.8%, and 48.5%, respectively (Figure 1D). KM curves in accordance with the 8th edition TNM staging for stage I, II, III, and IV cancer are shown in Figure 1E (p < 0.001). The OS rates for cancer stages I, II, III, and IV were 96.6%, 88.7%, 69.9%, and 34.3%, respectively (Figure 1F).
For all patients, elevated preoperative CEA (5-year OS: 85.5% vs. 74.6%; p < 0.001) level or CA19-9 (5-year OS: 84.2% vs. 68.0%; p < 0.001) level was significantly associated with worse OS versus no elevation in either of these markers, respectively (Figures 3A–D). Furthermore, patients with both elevated CEA and CA199 (N = 286, 10.8%) had the worst 5-year OS compared with patients with other groups (5-year OS: 64.8%; p < 0.001) (Figures 3E, F).
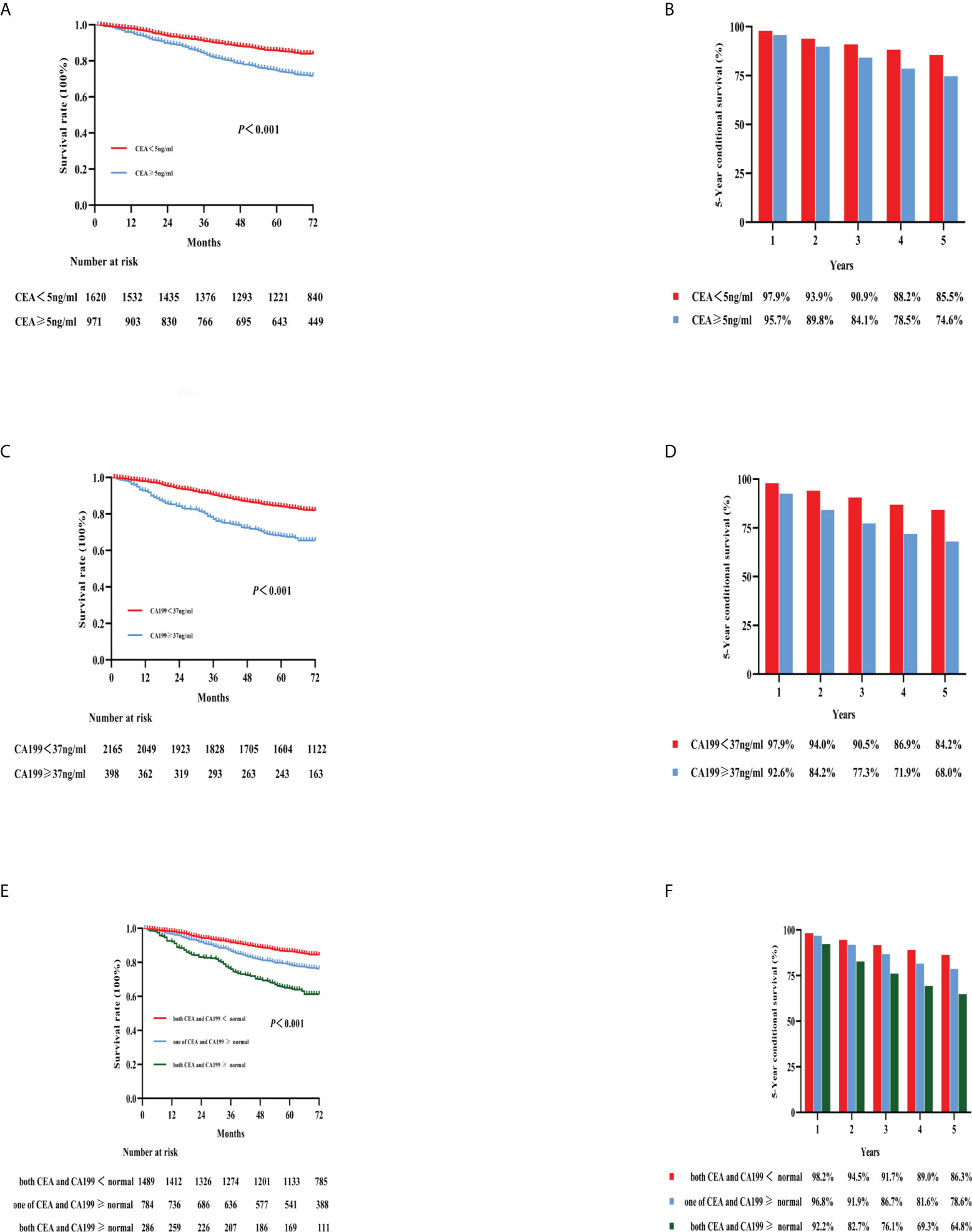
Figure 3 Kaplan–Meier curves for the survival analysis according to the level of preoperative CEA (A), CA199 (C), and combined CEA and CA199 (E). The 5-year OS rates of the three categories are shown in (B, D, F): CEA, CA199, and combined CEA and CA199, respectively. CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199; OS, overall survival.
In this study, stage II patients accounted for more than half of the cases (N = 1,455, 54.8%). The baseline clinicopathological characteristics of the patients with stage II colon cancer are shown in Supplementary Table S2. These patients were divided into three groups based on the number of risk factors (7, 8), which were stage pT4, bowel obstruction or tumor perforation, high tumor grade, vascular or perineural invasion, and <12 LNs examined. Accordingly, patients were classified as without risk factors (N = 782), only one risk factor (N = 502), or two or more risk factors (N = 171), and the 5-year OS rates were 91.2%, 87.3%, and 80.2%, respectively (Figure 4A). The 5-year survival of patients with deficient mismatch repair (dMMR) colon cancer was better than that in patients with proficient MMR (pMMR) without high-risk factors (5-year OS: 96.5% vs. 88.6%, respectively; p = 0.040) (Supplementary Figure 2A). The same trend was not observed in patients with high-risk factors (5-year OS: 88.0% vs. 87.6%, dMMR vs. dMMR, respectively; p = 0.687) (Supplementary Figure 2B). Additionally, in this study, we analyzed the effect of adjuvant chemotherapy on the 5-year OS rate of stage II colon cancer in different risk factor groups. In the group with only one risk factor, adjuvant chemotherapy improved OS (5-year OS: 91.5% vs. 84.6%, with versus without adjuvant chemotherapy, respectively; p < 0.001). Similar findings were observed for patients with two or more risk factors (5-year OS: 84.2% vs. 73.5%, with versus without adjuvant chemotherapy, respectively; p < 0.001) (Figure 4B). Preoperative CEA and CA199 levels had effects on survival and prognosis in patients with stage II colon cancer (Figures 4C, D). Elevated preoperative CEA and CA199 levels were associated with the worst 5-year OS rates (81.5% for both; p < 0.001) (Figure 4E). Adjuvant chemotherapy significantly improved the survival rate of patients without high-risk factors and with both elevated preoperative CEA and CA199 (p = 0.018) (Figure 4F).

Figure 4 Kaplan–Meier curves for the survival analysis in stage II colon cancer patients. The figures show OS according to different risk factors (A), adjuvant chemotherapy with different risk factors (B), preoperative CEA and CA199 levels (C–E), and adjuvant chemotherapy combined with elevated CEA and CA199 (F). OS, overall survival; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199.
The National Comprehensive Cancer Network (NCCN) guidelines recommend that at least 12 LNs be examined after surgery (9). Our study analyzed how the number of harvested LNs affected 5-year OS. The 5-year OS rate was associated with a significantly different prognosis in patients with stage II colon cancer with different numbers of examined LNs (Figures 5A, B; 5-year OS: LNs > 12: 89.7%, LNs < 12: 84.6%; p < 0.001). However, there was no significant difference for patients with stage III colon cancer regarding the number of examined LNs (Figures 5C, D; 5-year OS: 69.9% vs. 69.7%, >12 LNs vs. <12 LNs, respectively; p = 0.72).
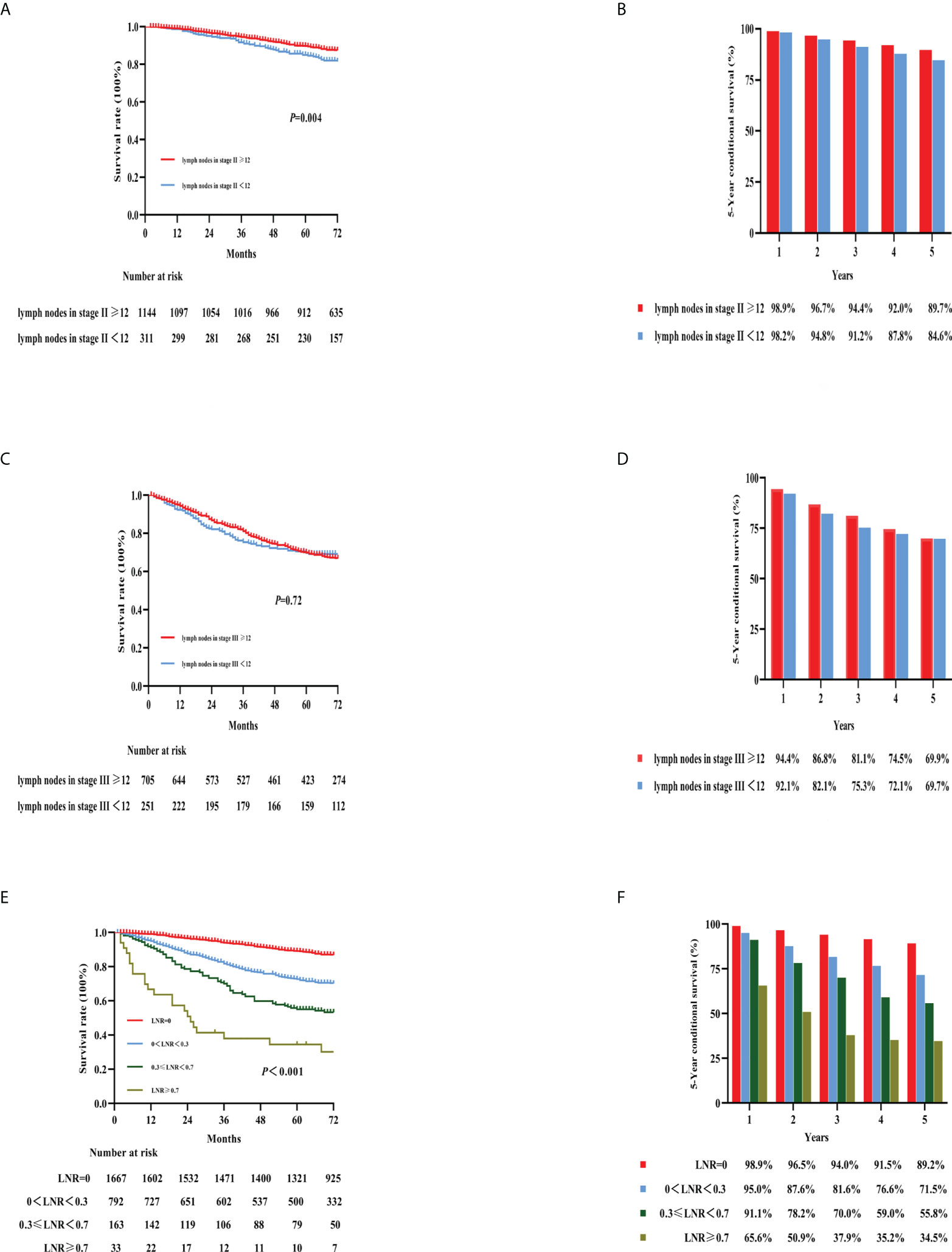
Figure 5 Kaplan–Meier curves for the survival analysis according to the number of examined lymph nodes. The figures show OS in stage II colon cancer patients (A), stage III colon cancer patients (C), LNRs (E), and the 5-year OS rates of the three categories [(B, D, F), respectively]. OS, overall survival; LNR, lymph node ratio.
LNR was defined as the proportion of the number of positive LNs to the number of harvested LNs. In our previous study (10), 0.3 and 0.7 were determined as the best cutoff values for LNR. Therefore, we divided the patients in this study into four groups with cutoff values of 0, 0.3, 0.3–0.7, and 0.7; the respective 5-year OS rates were 89.2%, 71.5%, 55.8%, and 34.5% (Figures 5E, F). Furthermore, no clear survival difference was observed between adequate examined LNs (≥12 LNs) and inadequate examined LNs (<12 LNs) in the same LNR group (Supplementary Figure 3).
On the basis of the stepwise regression results, the model containing age, CEA, CA199, histology, pT stage, pM stage, LNR, perineural invasion, and adjuvant chemotherapy had the minimal AIC value in the training cohort. A nomogram for colon cancer was constructed based on the results of the final multivariable model. Figure 6A shows an example of using the nomogram to predict the survival probability of a given patient. In the training cohort, the AUC was 0.783 at 5 years (Supplementary Figure 4A), and the C-index was 0.759. The OS nomogram was validated internally, which indicated an AUC at 5 years of 0.713 (Supplementary Figure 4B) and a C-index of 0.703. Supplementary Figure 5 shows the bootstrapped calibration plots in the training and testing cohorts. The discrimination ability indicated that in the training as well as testing sets, the nomogram was better than the TNM 8th staging classification for predicting survival. In both the training and testing cohorts (Figures 6B, C), the time-dependent AUC was >0.7 for the prediction of OS within 5 years. The time-dependent C-index was >0.7 for the prediction of OS within 5 years in both the training and validation cohorts (Supplementary Figure 6). DCA curves showed that the nomogram was better at predicting 5-year OS compared with the TNM 8th staging classification (Figures 6D, E).
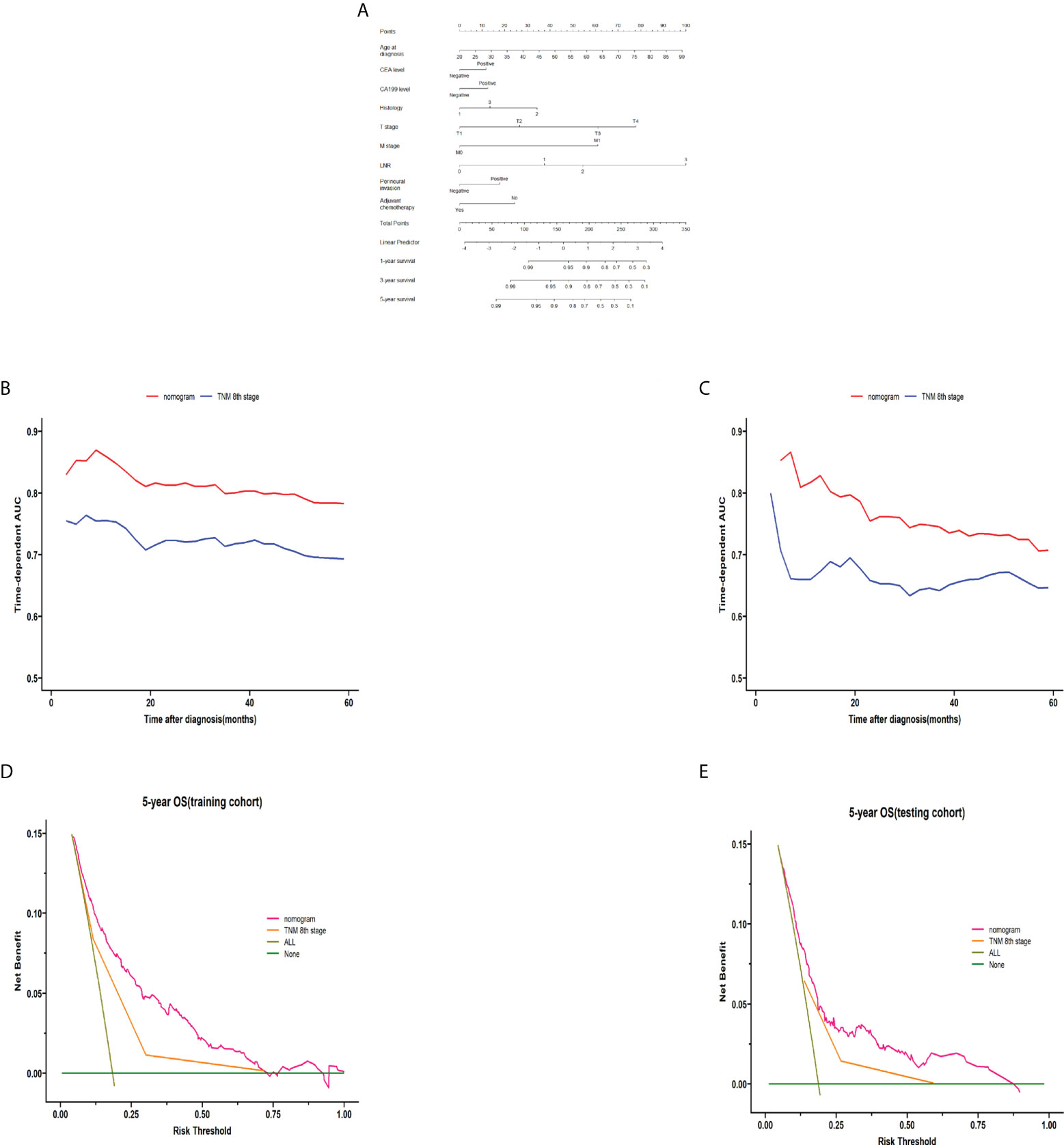
Figure 6 Construction and validation of the nomogram. The nomogram for predicting 1-, 3-, and 5-year survival in colon cancer (A). A web-based tool based on the formulated nomogram is available at https://coloncancernomogram20220118.shinyapps.io/DynNomapp/. The time-dependent AUCs using the nomogram to predict 5-year overall survival probability in both the training and testing cohorts are shown in panels (B, C), respectively. Decision curve analysis of the nomogram and TNM 8th edition staging for the survival prediction of patients with colon cancer are also shown. The 5-year survival benefit in both the training and testing cohorts is shown in panels (D, E), respectively. For LNR, 0 indicates LNR = 0, 1 indicates LNR = 0–0.3, 2 indicates LNR = 0.3–0.7, and 2 indicates LNR >0.7. For the histology, 1 indicates adenocarcinoma, 2 indicates mucinous adenocarcinoma, and 3 indicates mixed cell adenocarcinoma. AUC, area under the curve; TNM, tumor-node-metastasis; LNR, lymph node ratio.
In our study, the OS rates for stage I, II, III, and IV colon cancer were 96.6%, 88.7%, 69.9%, and 34.3%, respectively. Stage I and II survival rates were significantly higher than those for stage III and IV, which was consistent with previous studies (11). This difference might be related to tumor invasion and cell infiltration (12–14). To improve the survival of colon cancer patients, it is necessary to focus on prevention and early detection and treatment.
The key prognostic factors for colon cancer, especially the high-risk factor of stage II disease, have been a hot topic of research. The poor prognosis for stage II colon cancer has been confirmed to be associated with high-risk factors (15, 16). In our study, the presence of a higher number of high-risk factors was confirmed to be associated with worse OS based on the risk factor grouping. Additionally, adjuvant chemotherapy improved the survival rate in stage II patients with high-risk factors in our study. This is consistent with the results of earlier studies, including the QUASAR trial (17) and some retrospective analyses (18, 19). The recommendation that patients with stage II colon cancer with high-risk factors should receive postoperative adjuvant chemotherapy is also consistent with the current guidelines (20). Previous studies have shown that stage II patients with dMMR have a better prognosis than stage II patients with pMMR. However, another study indicated that pMMR should not be the only risk factor considered when determining whether to administer adjuvant chemotherapy in patients with stage II colon cancer or not (21).
The prognostic role of CEA and CA199 in predicting survival in colon cancer patients has been frequently studied; however, there is currently no reliable evidence confirming this role (22). Our study found that preoperative CEA and CA199 levels were independent prognostic factors in colon cancer. Elevated preoperative CEA and CA19-9 levels were significantly associated with worse OS. Furthermore, patients with both elevated CEA and CA199 levels had the worst 5-year OS rate. The same results were seen in stage II colon cancer patients. In a recent study, researchers found that CEA and CA199 were independent predictors of colon cancer recurrence and survival (23). However, there is no randomized controlled trial to evaluate whether adjuvant chemotherapy should be recommended for stage II patients without high-risk factors and with both elevated preoperative CEA and CA199. Our findings suggested that high preoperative levels of CEA and CA199 significantly affected the prognosis and survival of stage II colon cancer patients and that these factors can be high-risk factors for the need for chemotherapy in stage II colon cancer. This finding agrees with the results of a retrospective analysis by Thirunavukarasu et al. (24). There are also other clinical or pathological factors that significantly affect prognosis and survival in stage II patients.
Another issue that we evaluated in this study was the number of LNs examined. Pathological N staging reflects a strong association between the number of positive LNs and survival (6, 25). Notably, in this study, the 5-year OS rate for stage II patients with an insufficient number of harvested LNs (< 12) was 84.6%, while the 5-year OS rate for patients with a sufficient number of harvested LNs was 89.7%. However, there was no similar trend regarding LNs in stage III patients. This means that an insufficient number of LNs were examined, resulting in undetected positive LNs, especially in patients with stage II colon cancer. To avoid missing positive LNs, the NCCN guidelines recommend that the number of LNs collected should be more than 12 (9), which was further validated in our study.
A growing number of studies have shown that LNR is associated with prognosis, and that this ratio can be used as an independent prognostic tool to assess the prognosis of colon cancer patients (26, 27). However, currently, the optimal cutoff value for LNR is controversial. In our previous study, an information gain method was developed to redefine the cutoff value for LNR. The thresholds were 0, 0–0.3, 0.3–0.7, and >0.7. The study was based on a large sample of the Surveillance, Epidemiology and End Results database (SEER) to redefine the cutoff value for LNR (10). The thresholds of LNR were proved to be better than the previously reported studies (28, 29). Based on thresholds of 0, 0.3, and 0.7, the respective 5-year OS rates were 89.2%, 71.5%, 55.8%, and 34.5% in our study. There were different pN stages in each LNR group. In the LNR (0.3–0.7) group, we found that patients with pN1 stage colon cancer had worse 5-year OS than patients with pN2 stage cancer. Further observations showed that less than 12 LNs examined led to an underestimation of the pN stage. Moreover, the predictive effect of LNR was independent of the number of LNs examined, and other studies have found similar results (30, 31).
In this study, we developed and validated a nomogram for the postoperative individualized prediction of survival in colon cancer patients. The nomogram incorporates age, CEA, CA199, histology, pT stage, pM stage, LNR, perineural invasion, and adjuvant chemotherapy. Integrating the clinical and pathological risk factors into a convenient nomogram helps predict postoperative survival. It is important to perform an accurate risk stratification for patients with cancers such as colon cancer because the prognosis may be heterogeneous (32, 33). Nomograms may afford a more individualized method to provide prognostic information for patients compared with the TNM staging system. Compared with the TNM staging system, our nomogram had better performance for risk stratification of the prognosis of patients undergoing radical colon cancer surgery, with better AUC values and C-index.
TNM staging is indispensable for postoperative survival assessment of colon cancer and for determining whether postoperative adjuvant therapy is needed. Our results showed that elevated preoperative CEA and CA199 levels are poor prognostic factors in patients with colon cancer. Preoperative CEA and CA199 elevations should be considered as risk factors in stage II colon cancer patients. We propose that stage II colon cancer patients without high-risk factors and with both ele-vated preoperative CEA and CA199 should receive adjuvant therapy. Future randomized controlled trials to evaluate the role of adjuvant chemotherapy in these patients are needed. To avoid missing positive LNs and subsequent inadequate staging of LNs metastases, it is recommended that at least 12 lymph nodes be examined. LNR can be used as a supplement to pN staging when the number of LNs examined is insufficient. The nomogram described in this study predicted the survival of colon cancer patients better than that when using the TNM system.
Our study was a retrospective study performed in a single center. Although the study involved a large data sample, a study of colon cancer diagnosis, surgery, and postoperative treatment from a single center has limitations that may lead to selection bias. Hopefully, more data from multiple centers and larger samples will lead to more convincing conclusions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Harbin Medical University Cancer Hospital ethics committee (KY2017-19). The patients/participants provided their written informed consent to participate in this study.
HL and JL contributed to the study conception and design. XP, BX, RW, and XW collected the data. XP and BX analyzed the data. XP, BX, JS, and ST interpreted the data. XP and BX drafted the manuscript. HL and JL contributed to critical revision of the manuscript. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (grant nos. U20A20376 and 61972116) Beijing Award Foundation (grant nos. YXJL-2020-0818-0478) Wu Jieping Medical Foundation (grant no. 320.6750.2020-19-20) Heilongjiang Province Postdoctoral Science Foundation(grant no. LBHZ21189) Harbin Medical University Innovative Science Research Funded Project (grant no. 31041220028) China Postdoctoral Science Foundation(grant no. 2022MD713747).
We thank Jane Charbonneau, DVM, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.914076/full#supplementary-material
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Zhou J, Zheng R, Zhang S, Zeng H, Wang S, Chen R, et al. Colorectal cancer burden and trends: Comparison between China and major burden countries in the world. Chin J Cancer Res (2021) 33(1):1–10. doi: 10.21147/j.issn.1000-9604.2021.01.01
4. Li X, Zhou Y, Luo Z, Gu Y, Chen Y, Yang C, et al. The impact of screening on the survival of colorectal cancer in shanghai, China: A population based study. BMC Public Health (2019) 19(1):1016. doi: 10.1186/s12889-019-7318-8
5. Nagata H, Ishihara S, Oba K, Tanaka T, Hata K, Kawai K, et al. Development and validation of a prediction model for organ-specific recurrences after curative resection of colon cancer. Dis Colon Rectum (2019) 62(9):1043–54. doi: 10.1097/dcr.0000000000001430
6. Weiser MR, Hsu M, Bauer PS, Chapman WC Jr., González IA, Chatterjee D, et al. Clinical calculator based on molecular and clinicopathologic characteristics predicts recurrence following resection of stage I-III colon cancer. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39(8):911–9. doi: 10.1200/jco.20.02553
7. Straker RJ 3rd, Heo DHJ, Shannon AB, Fraker DL, Shanmugan S, Schneider CJ, et al. Predictive risk-score model for selection of patients with high-risk stage II colon cancer for adjuvant systemic therapy. Surgery (2022) 171(6):1473–9. doi: 10.1016/j.surg.2021.10.066
8. Iveson TJ, Sobrero AF, Yoshino T, Souglakos I, Ou FS, Meyers JP, et al. Duration of adjuvant doublet chemotherapy (3 or 6 months) in patients with high-risk stage II colorectal cancer. J Clin Oncol (2021) 39(6):631–41. doi: 10.1200/jco.20.01330
9. Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, et al. NCCN clinical practice guidelines in oncology: Colon cancer. J Natl Compr Cancer Netw (2009) 7(8):778–831. doi: 10.6004/jnccn.2009.0056
10. Wang L, Su M, Zhang M, Zhao H, Wang H, Xing J, et al. Accurate prediction of prognosis by integrating clinical and molecular characteristics in colon cancer. Front Cell Dev Biol (2021) 9:664415. doi: 10.3389/fcell.2021.664415
11. Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med (2020) 9(1):361–73. doi: 10.1002/cam4.2673
12. Baguena G, Pellino G, Frasson M, Roselló S, Cervantes A, García-Granero A, et al. Prognostic impact of pT stage and peritoneal invasion in locally advanced colon cancer. Dis Colon Rectum (2019) 62(6):684–93. doi: 10.1097/dcr.0000000000001367
13. Almeida N, Carrara G, Palmeira CM, Fernandes AS, Parsons M, Smith GL, et al. Stimulation of cell invasion by the golgi ion channel Gaap/Tmbim4 via an H2O2-dependent mechanism. Redox Biol (2020) 28:101361. doi: 10.1016/j.redox.2019.101361
14. Morishita K, Watanabe K, Ichijo H. Cell volume regulation in cancer cell migration driven by osmotic water flow. Cancer Sci (2019) 110(8):2337–47. doi: 10.1111/cas.14079
15. Taieb J, Karoui M, Basile D. How I treat stage II colon cancer patients. ESMO Open (2021) 6(4):100184. doi: 10.1016/j.esmoop.2021.100184
16. Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, et al. Adjuvant therapy for stage II colon cancer: ASCO guideline update. J Clin Oncol (2022) 40(8):892–910. doi: 10.1200/jco.21.02538
17. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet (London England) (2007) 370(9604):2020–9. doi: 10.1016/s0140-6736(07)61866-2
18. Teufel A, Gerken M, Fürst A, Ebert M, Hohenthanner I, Klinkhammer-Schalke M. Benefit of adjuvant chemotherapy in high-risk colon cancer: A 17-year population-based analysis of 6131 patients with union for international cancer control stage II T4N0M0 colon cancer. Eur J Cancer (Oxford Engl 1990) (2020) 137:148–60. doi: 10.1016/j.ejca.2020.06.036
19. Babaei M, Balavarca Y, Jansen L, Lemmens V, van Erning FN, van Eycken L, et al. Administration of adjuvant chemotherapy for stage II-III colon cancer patients: An European population-based study. Int J Cancer (2018) 142(7):1480–9. doi: 10.1002/ijc.31168
20. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer V.1.2022. In: © National Comprehensive Cancer Network, Inc. 2022. All rights reserved [Accessed September 8, 2022]. Available at: https://www.nccn.org/.
21. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol (2010) 28(20):3219–26. doi: 10.1200/jco.2009.27.1825
22. You W, Yan L, Cai Z, Xie L, Sheng N, Wang G, et al. Clinical significances of positive postoperative serum CEA and post-preoperative CEA increment in stage II and III colorectal cancer: A multicenter retrospective study. Front Oncol (2020) 10:671. doi: 10.3389/fonc.2020.00671
23. Zhu J, Hao J, Ma Q, Shi T, Wang S, Yan J, et al. A novel prognostic model and practical nomogram for predicting the outcomes of colorectal cancer: Based on tumor biomarkers and log odds of positive lymph node scheme. Front Oncol (2021) 11:661040. doi: 10.3389/fonc.2021.661040
24. Thirunavukarasu P, Talati C, Munjal S, Attwood K, Edge SB, Francescutti V. Effect of incorporation of pretreatment serum carcinoembryonic antigen levels into AJCC staging for colon cancer on 5-year survival. JAMA Surg (2015) 150(8):747–55. doi: 10.1001/jamasurg.2015.0871
25. Pyo JS, Shin YM, Kang DW. Prognostic implication of metastatic lymph node ratio in colorectal cancers: Comparison depending on tumor location. J Clin Med (2019) 8(11):1812. doi: 10.3390/jcm8111812
26. Jiang C, Wang F, Guo G, Dong J, Liu S, He W, et al. Metastatic lymph node ratio as a prognostic indicator in patients with stage IV colon cancer undergoing resection. J Cancer (2019) 10(11):2534–40. doi: 10.7150/jca.29216
27. Walker R, Wood T, LeSouder E, Cleghorn M, Maganti M, MacNeill A, et al. Comparison of two novel staging systems with the TNM system in predicting stage III colon cancer survival. J Surg Oncol (2018) 117(5):1049–57. doi: 10.1002/jso.25009
28. Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: A single-center analysis of 3,026 patients over a 25-year time period. Ann Surg (2008) 248(6):968–78. doi: 10.1097/SLA.0b013e318190eddc
29. Shinto E, Ike H, Hida JI, Kobayashi H, Hashiguchi Y, Kajiwara Y, et al. Marked impact of tumor location on the appropriate cutoff values and the prognostic significance of the lymph node ratio in stage III colon cancer: A multi-institutional retrospective analysis. J Gastroenterol (2019) 54(7):597–607. doi: 10.1007/s00535-018-01539-5
30. Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg (2010) 251(6):1070–8. doi: 10.1097/SLA.0b013e3181d7789d
31. Zhang CH, Li YY, Zhang QW, Biondi A, Fico V, Persiani R, et al. The prognostic impact of the metastatic lymph nodes ratio in colorectal cancer. Front Oncol (2018) 8:628. doi: 10.3389/fonc.2018.00628
32. Cheng E, Ou F, Ma C, Spiegelman D, Zhang S, Zhou X, et al. Diet- and lifestyle-based prediction models to estimate cancer recurrence and death in patients with stage III colon cancer (Calgb 89803/Alliance). J Clin Oncol (2022) 40(7):740–51. doi: 10.1200/jco.21.01784
33. Long C, Feng M, Wang S, Ji H, Li Z, Zheng J, et al. A Clinicopathological feature-based nomogram for predicting the likelihood of D3 lymph node metastasis in right-sided colon cancer patients. Diseases of the colon and rectum [Preprint] (2021). Available at: https://journals.lww.com/dcrjournal/Abstract/9000/A_Clinicopathological_Feature_Based_Nomogram_for.99238.aspx (Accessed September 8, 2022).
Keywords: colon cancer, radical surgery, survival rate, nomogram, prognostic factors
Citation: Pang X, Xu B, Lian J, Wang R, Wang X, Shao J, Tang S and Lu H (2022) Real-world survival of colon cancer after radical surgery: A single-institutional retrospective analysis. Front. Oncol. 12:914076. doi: 10.3389/fonc.2022.914076
Received: 06 April 2022; Accepted: 26 August 2022;
Published: 16 September 2022.
Edited by:
Rocco Ricciardi, Massachusetts General Hospital and Harvard Medical School, United StatesCopyright © 2022 Pang, Xu, Lian, Wang, Wang, Shao, Tang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Lu, bHVoYWlib0BocmJtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.