
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 May 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.913254
This article is part of the Research Topic Improving our Understanding of the Management and Pathogenesis of Rare and Neglected Tumors of the Central and Peripheral Nervous System View all 23 articles
 Songshan Feng1,2,3,4
Songshan Feng1,2,3,4 Jing Li5
Jing Li5 Fan Fan1
Fan Fan1 Zeyu Wang1
Zeyu Wang1 Qian Zhang6
Qian Zhang6 Hao Zhang1
Hao Zhang1 Ziyu Dai1
Ziyu Dai1 Xun Zhang1
Xun Zhang1 Peng Luo7
Peng Luo7 Zaoqu Liu8
Zaoqu Liu8 Jian Zhang7
Jian Zhang7 Zhuoyi Liu6*
Zhuoyi Liu6* Quan Cheng1,2,9*
Quan Cheng1,2,9*Objective: Malignant meningioma (MM) is a relatively rare disease with poor survival. Few studies had focused on MM in the elderly population. This study aims to explore the prognostic factors and optimal therapeutic strategy in elderly patients with MM.
Methods: We took advantage of the Surveillance, Epidemiology, and End Results (SEER) database to include 275 adult patients with histologically confirmed MM between 2011 and 2018. The Kaplan–Meier curves were plotted by different covariates to reveal the survival probability. Univariate and multivariable Cox proportional hazard regression analyses were applied to identify prognostic factors for cancer-specific survival (CSS).
Results: The multivariable analysis in the elderly group revealed that when compared with patients receiving gross total resection (GTR), patients receiving biopsy had significantly worse CSS (HR = 3.72; 95% CI: 1.35–10.21; P = 0.011), whereas patients receiving subtotal resection (STR) had nearly the same CSS (HR = 0.83; 95% CI: 0.37–1.86; P = 0.653). Meanwhile, postoperative radiotherapy (PORT) showed no significant association with CSS in the elderly patient group (HR = 0.94; 95% CI: 0.42–2.12; P = 0.888).
Conclusion: Surgical resection is recommended for elderly patients with MM in the absence of surgical contraindications, but GTR does not present survival benefit in the elderly patients compared with STR. Additional large-scale clinical studies are needed to explore the survival benefit of PORT applied in patients with MM.
Meningioma is the most common primary neoplasm of the central nervous system, accounting for 38.3% of all brain tumors (1). According to the most recent report from the Central Brain Tumor Registry of the United States, malignant meningioma (MM) composes 1.04% of all meningiomas with an incidence of 0.09 per 100,000 people (1). There is evidence that age-specific incidence rates of meningiomas increase in both men and women, with a median age at diagnosis of 65 and 66 years old for malignant and non-malignant meningiomas, respectively (1, 2). As far as we know, most studies on MMs did not take the elderly (≥65 years old) as an independent patient group to describe (3). There were reports revealing that older age was associated with worse patient survival (4–7). Several studies suggested that craniotomy for resection of meningioma in the elderly patients carried higher risk of mortality and morbidity compared with younger patients (8, 9). Other studies reported that no significant difference was detected in the mortality rate after surgery for elderly versus non-elderly patients, but more elderly patients presented postoperative complications and neurological deterioration (10–12). At present, there is still a lack of consensus on the surgical outcome of elderly patients with MM, and the specific treatment strategies need to be further explored. Furthermore, it is expected that the average human life expectancy continues to increase and more elderly patients with MM will be diagnosed (10). Therefore, we conducted this study aiming to explore the prognostic factors and figure out the optimal therapeutic strategy, especially in elderly patients with MM.
Given the low incidence of MM, we took advantage of the Surveillance, Epidemiology, and End Results (SEER) database and retrospectively analyzed 275 patients diagnosed with histologically confirmed MM between 2011 and 2018. The subgroup analysis for elderly and younger patients was performed with respect to extent of surgical resection (EOR), postoperative radiotherapy (PORT), and their influence on long-term patient survival. All records of intracranial MM with positive histology between 2004 and 2018 were initially extracted from the SEER database, which provides patient demographics, tumor characteristics, treatment methods, and survival status with de-identified records. WHO grade 3 meningioma was defined as MM, which included the ICD-O-3 histology and behavior records of 9530/3 (Meningioma, malignant), 9531/3 (Meningiothelial meningioma, malignant), 9532/3 (Fibrous meningioma, malignant), 9534/3 (Angiomatous meningioma, malignant), 9535/3 (Hemangioblastic meningioma, malignant), 9537/3 (Transitional meningioma, malignant), 9538/3 (Papillary meningioma), and 9539/3 (Meningeal sarcomatosis) according to existing studies (13, 14). Patients with unknown information of marital status, race, tumor size, laterality, cancer-specific survival (CSS) status, and age<18 years old were excluded. Patients with recurrent MM were also excluded, which had at least one prior record of WHO grade 1 or WHO grade 2 meningioma in the SEER database. The detailed protocol was provided by the SEER*Stat tutorial naming “case listing exercise 1b: view patient histories” (https://seer.cancer.gov/seerstat/tutorials/case-listings.html). Surgery code 0 (no surgery of primary site; autopsy only), code 10 (no specimen sent to pathology), code 22 (resection of tumor of spinal cord or nerve), and code 90 (surgery, not otherwise specified) were excluded. In addition, the small part of patients treated with radiotherapy prior to surgery, intraoperative radiotherapy, radioactive implants, radioisotopes, and unknown method were excluded. Supplementary Table 1 showed that the records of surgery code changed significantly since 2011, which revealed the advancement in surgical techniques. To provide the most up-dated evidence, the patient diagnosed before 2011 were excluded, and little parts of patients with surgery code 40 (partial resection of lobe of brain) and 55 (gross total resection of lobe of brain) were also excluded (n = 20). The final study population included 275 adult patients diagnosed between 2011 and 2018 recorded as surgery code 20 (local excision, biopsy), 21 [subtotal resection (STR) of tumor], and 30 [radical, total, gross resection of tumor (GTR)] (Figure 1).
The following demographic information was included for analysis: age group (<65 and ≥65 years), gender (male and female), race (other, black, and white), and marital status (single, divorced, married, and widowed). The following tumor characteristics were analyzed: tumor site (cerebral meninges and other), laterality (unilateral, bilateral, and midline), histology (9530/3 and other), tumor size (≥4.9 and <4.9 cm, the best cutoff was defined by x-tile software) (15), and other tumor(s) (before MM, and after MM, defined by the record of “sequence number” in SEER*Stata). EOR includes code 20 (biopsy), code 21 (STR), and code 30 (GTR). Concerning adjuvant therapy, PORT
(no/unknown and beam radiation), and chemotherapy (no/unknown and yes) were included for analysis. CSS was defined as the event of interest in this study.
The distribution of the baseline characteristics between different age groups was compared by the chi-squared test (categorical variables with all cell counts>5) or the Fisher’s exact test (categorical variables with cell counts ≤5). The Kaplan–Meier curves in the entire cohort were plotted by all covariates to reveal the CSS probability of different groups, which were compared by log-rank test. Univariate and multivariable Cox proportional hazard regression analyses were applied to identify prognostic factors from all covariates for CSS. The baseline characteristics between groups receiving different EOR were compared by the chi-squared test, Fisher’s exact test, or one-way ANOVA test (continuous variable). The Kaplan–Meier curves by EOR and PORT were plotted in elderly and younger patient group. Furthermore, univariate and multivariable Cox proportional hazard regression analyses were also applied to assess the survival benefits provided by EOR and PORT for younger and elderly patients, respectively. P < 0.05 was considered statistically significant. All statistical analyses were performed in R version 3.5.1 (http://www.r-project.org/).
The median age was 62 years old, and median survival time was 28 months. At the time of data collected, 183 cases were alive, 56 cases died of MM, and 36 cases died of other causes. The 1-, 2-, and 5-year CSS rates were 88.5%, 80.7%, and 52.1%, respectively. The baseline characteristics were compared between age groups in Table 1. The marital status showed a significant difference, whereas race and gender showed no difference between age groups. The majority of patients had tumor larger than 4.9 cm, tumor located in cerebral meninges, unilateral tumor, tumor with histology 9530/3, tumor without metastasis, and no other tumor(s). Tumor characteristics including tumor size, site, laterality, histology, metastasis, and other tumor(s) showed no significant difference between different age groups (P > 0.05). Concerning treatment methods, the results revealed that the GTR rate was 52.4% in the entire cohort, 51.3% in the younger group, and 53.7% in the elderly group. Compared with patients in the younger group, more patients received biopsy only and fewer patients received STR in the elderly group. A total of 149 patients received PORT and 12 patients received postoperative chemotherapy, which showed no significant difference between age groups.
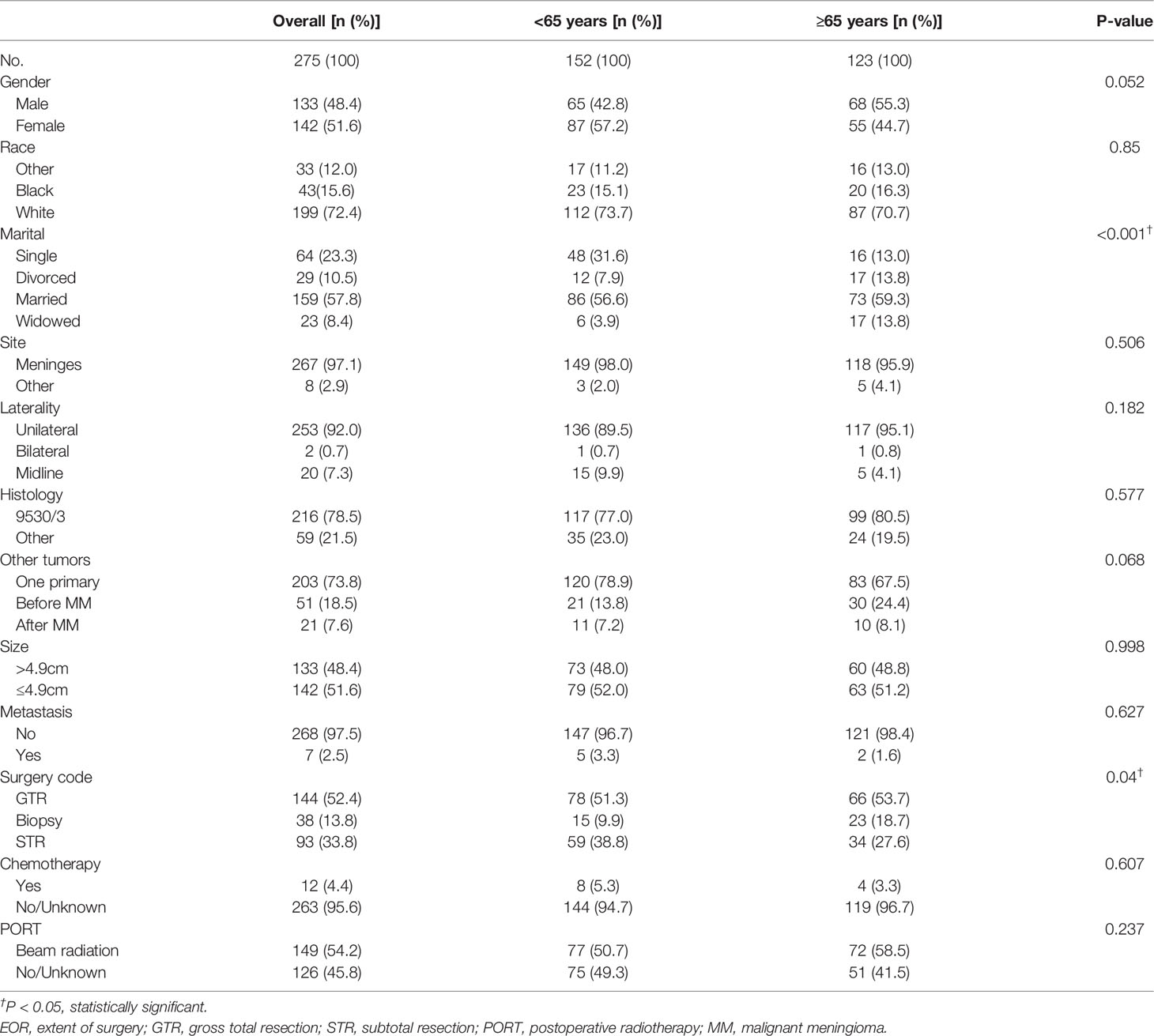
Table 1 Patient demographics, tumor characteristics, and treatment options of 275 patients with MM from 2011 to 2018 in different age groups.
Kaplan–Meier curves indicated that patients in the elderly group, with tumor size>4.9 cm, receiving biopsy only, and receiving chemotherapy had significantly worse survival probability. In addition, the Kaplan–Meier curves by PORT, gender, race, marital status, histology, tumor site, laterality, metastasis, and other tumor(s) showed no significant difference (Figure 2 and Supplementary Figure 1). The results of univariate analysis revealed that patients in elderly group (HR = 2.73; 95% CI: 1.57–4.74; P = 3.56e−4), with tumor size>4.9 cm (HR = 1.77; 95% CI: 1.04–3.04; P = 0.036), receiving biopsy only (HR = 2.62; 95% CI: 1.29–5.31; P = 0.007), and receiving chemotherapy (HR = 3.69; 95% CI: 1.75–7.81; P = 6.3e−4) presented significantly worse CSS. PORT, gender, race, marital status, histology, tumor site, laterality, metastasis, and other tumor(s) were not significantly associated with CSS (P > 0.05) (Table 2 and Supplementary Table 2). Consistently, the results of the multivariable analysis revealed that patients in the elderly group (HR = 3.41; 95% CI: 1.86–6.23; P = 6.81e−5), with tumor size>4.9 cm (HR = 1.78; 95% CI: 1.01–3.16; P = 0.048), receiving biopsy only (HR = 3.03; 95% CI: 1.43–6.44; P = 0.004), and receiving chemotherapy (HR = 4.19; 95% CI: 1.77–9.90; P = 0.001) showed significant worse CSS. Meanwhile, PORT, gender, race, marital status, histology, tumor site, laterality, metastasis, and other tumor(s) were not significantly associated with CSS (P > 0.5) (Table 2 and Supplementary Table 2).
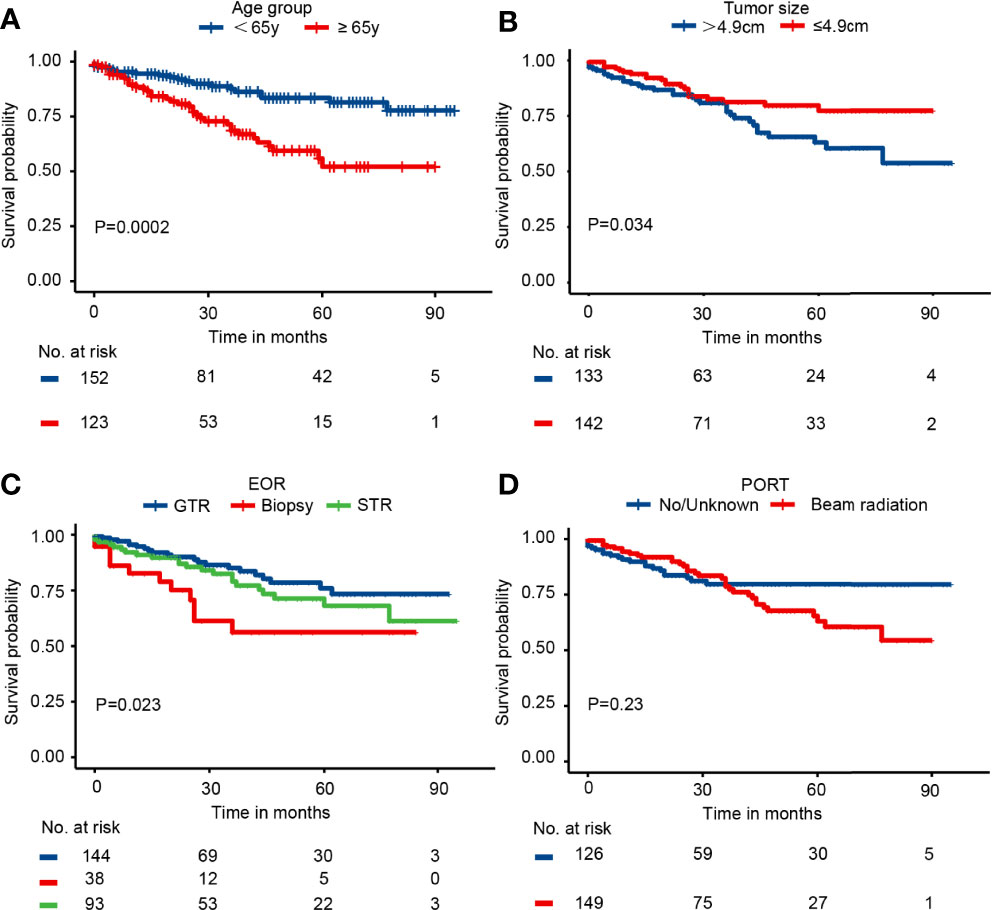
Figure 2 The Kaplan–Meier curves by (A) age group, (B) tumor size, (C) EOR, and (D) PORT in the entire cohort.
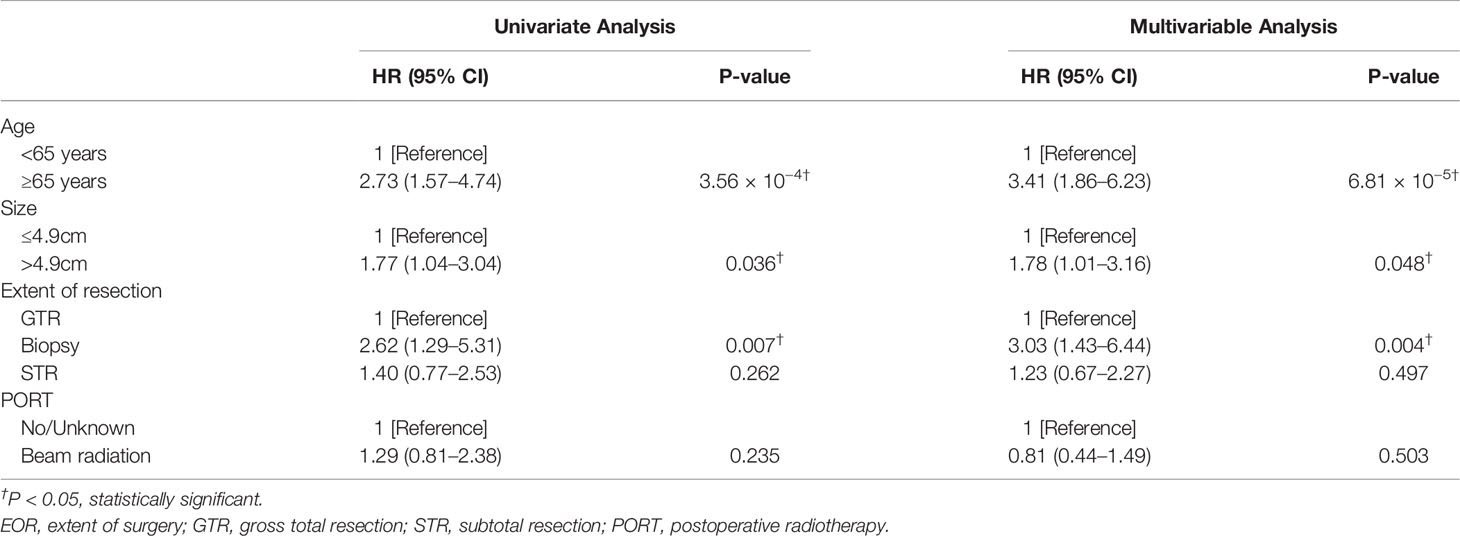
Table 2 Results of univariate and multivariable Cox proportional regression analysis of age group, tumor size, EOR, and PORT in the entire study population.
The subgroup analysis of elderly and younger patients was conducted to assess the survival benefits of EOR and PORT. First, the Kaplan–Meier curves in the younger group indicated that patients receiving biopsy presented the worst survival probability, and patients receiving GTR had a slightly better survival probability than that receiving STR (P = 0.055). The Kaplan–Meier curves in the elderly group showed that the survival probability of patients receiving different EOR had no significant difference (P = 0.22). The Kaplan–Meier curves in both age groups suggested that PORT did not affect survival probability (Figure 3). The univariate analysis in the younger group showed that when compared with patients receiving GTR, patients receiving biopsy had significantly worse CSS (HR = 4.23; 95% CI: 1.13–15.81; P = 0.032) and patients receiving STR had slightly worse CSS (HR = 2.66; 95% CI: 0.93–7.69; P = 0.069). Meanwhile, the univariate analysis in the elderly group illustrated that when compared with patients receiving GTR, patients receiving biopsy had slightly worse CSS (HR = 2.07; 95% CI: 0.89–4.82; P = 0.091), but patients receiving STR possessed nearly the same CSS (HR = 1.09; 95% CI: 0.51–2.35; P = 0.808). The results of univariate analysis revealed that PORT presented no significant association with CSS in both younger group and elderly group (Table 3). Consistently, the multivariable analysis in the younger group suggested that when compared with patients receiving GTR, patients receiving biopsy had significantly worse CSS (HR = 6.47; 95% CI: 1.42–29.44; P = 0.018) and patients receiving STR had slightly worse CSS (HR = 2.77; 95% CI: 0.81–9.48; P = 0.103). The multivariable analysis in the elderly group revealed that when compared with patients receiving GTR, patients receiving biopsy had significantly worse CSS (HR = 3.72; 95% CI: 1.35–10.21; P = 0.011) and patients receiving STR had nearly the same CSS (HR = 0.83; 95% CI: 0.37–1.86; P = 0.653). At the same time, the results of multivariable analysis illustrated that PORT showed no significant association with CSS in both younger group and elderly group (Table 3). The results of univariate and multivariable analyses of gender, race, marital, tumor size, histology, site, laterality, metastasis, other tumor(s), and chemotherapy in different age groups were presented in Supplementary Table 3 (<65 years) and Supplementary Table 4 (≥65 years), respectively.
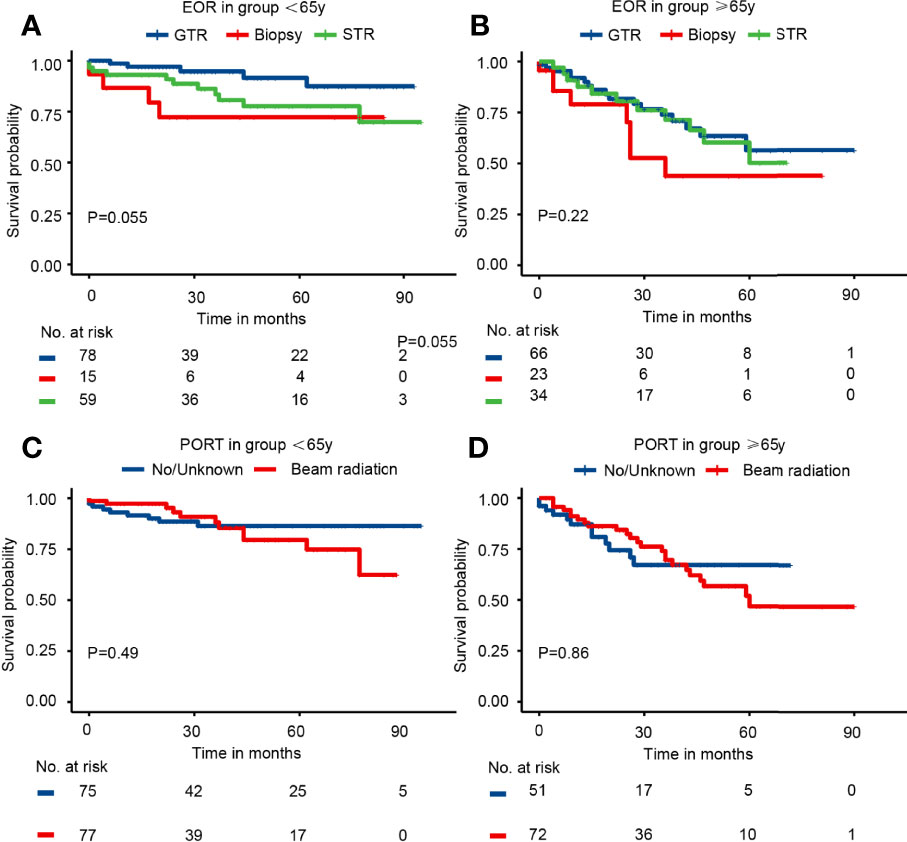
Figure 3 The Kaplan–Meier curves by EOR and PORT in different age groups. (A) EOR in group <65 years. (B) EOR in group ≥65 years. (C) PORT in group <65 years. (D) PORT in group ≥65 years.
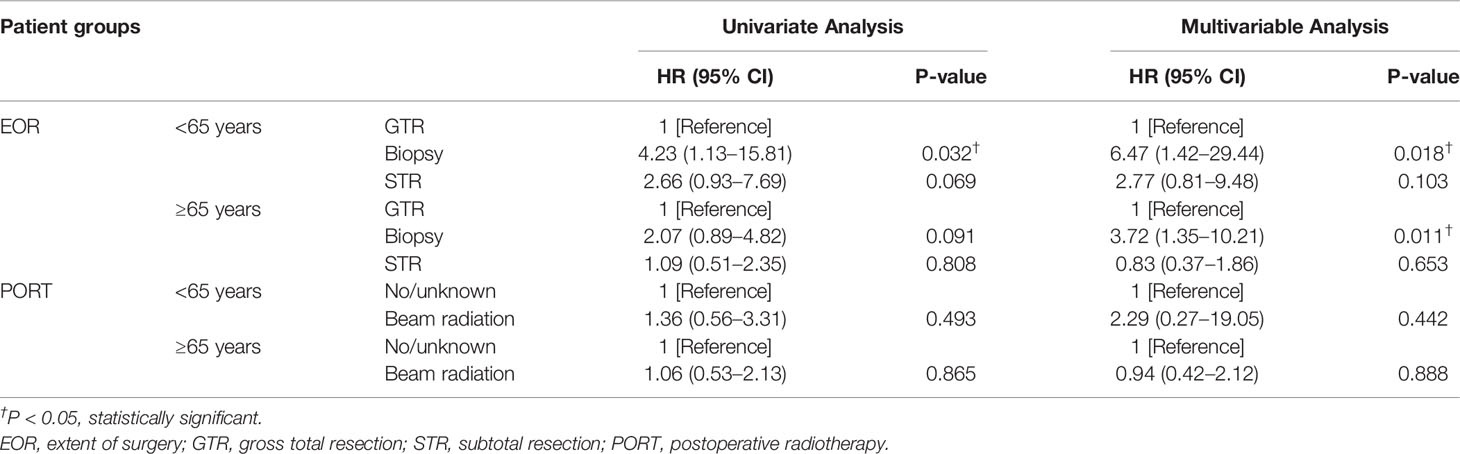
Table 3 Results of univariate and multivariable Cox proportional regression analysis of EOR and PORT in different age groups.
The baseline characteristics of patients were compared between groups receiving different EOR in Supplementary Table 5 (<65 years) and Supplementary Table 6 (≥65 years), respectively. The results suggested that the patient demographics and tumor characteristics such as age, gender, tumor size, and tumor location presented no significant difference between elderly patients receiving different EOR.
Because of the prolongation of life expectancy, the treatment strategy of meningiomas in elderly patients has become a more and more important issue. Thus, we utilized the SEER database and retrospectively analyzed 275 patients diagnosed as MM with long-term outcome results, aiming to explore the prognostic factors and figure out the optimal therapeutic strategy for this specific population.
Elderly patients are more likely to be accompanied by other diseases, resulting in poor physical condition before surgery. Considering the risk of surgery and the corresponding surgical morbidity and mortality, conservative treatment may be a reasonable choice for elderly patients. However, it was reported that elderly patients who received conservative treatment had increased tumor-related mortality compared with patients who underwent surgical resection (16). Furthermore, both the univariate and multivariable analysis in our study suggested that biopsy was significantly associated with worse CSS in elderly patients. The European Association of Neuro-Oncology guidelines suggested surgical resection followed by PORT for the treatment of patients with MM (17). However, the specific surgical benefits and the choice of surgical patterns need to be further discussed.
There were studies reporting that meningioma surgery in elderly patients presented a higher risk of mortality and morbidity compared to intracranial tumor surgery in general (8, 18). Steinberger et al. revealed in their study that old age was an independent predictor of morbidity and mortality in patients undergoing craniotomy for resection of meningioma (9). Ferroli et al. reported in their retrospective cohort study that postoperative complications and surgical complexity could significantly influence the early outcome in elderly patients undergoing brain tumor surgery, and postoperative complications was the only factor with a strong correlation to postoperative worsening at the 3-month follow-up (19). In another study, the authors reported that no significant difference was discovered regarding the 30-day mortality rate for elderly versus non-elderly patients, whereas elderly patients had a significantly higher complication rate compared with non-elderly patients (10). Boviatsis et al. also revealed that the mortality rate between the elderly group and the younger group was not significant, but more elderly patients were discharged having deteriorated neurologically in comparison to their preoperative status (11). Hence, although there is dispute on whether the surgical resection would increase the mortality rate or not, it is generally recognized that the incidence of postoperative complications is higher in elderly patients.
Regarding the EOR and its influence on the long-term patient survival, several studies revealed that GTR was a favorable factor for patient survival in the general population (20, 21). Other studies indicated that GTR was not significantly associated with patient survival (22, 23). One particular study suggested that the overall survival of patients treated with near total resection was better than patients treated with GTR (24). Taking age into consideration, Brokinkel et al. reported that the progression-free interval of patients undergoing GTR was distinctly prolonged as compared with STR and emphasized the importance of achieving maximum safe resection in elderly patients (25). Another study also reported that the EOR had no influence on the functional outcome of elderly patients (26). However, D’Andrea et al. indicated that radical resection could increase postoperative morbidity in elderly patients (27). In another study, Chen et al. suggested that the aggressive resection of meningiomas in elderly patients could increase the morbidity and mortality, and survival with residual tumor was acceptable in this specific population (28). Our results revealed that GTR only improved CSS in younger patients compared with STR but did not present survival benefit in elderly patients. Therefore, we believe that surgeons should take into account the factors that the elderly are more prone to surgical complications when formulating surgical strategy for this special patient group, and a more balanced choice should be made in the pursuit of GTR and preservation of neurological function, so as to improve the postoperative functional status and survival of elderly patients.
Generally, PORT is recommended after tumor resection for the treatment of MM (17). There was supporting evidence revealing that PORT improved the survival of patients with MM (29, 30). Orton reported that PORT improved the overall survival of patients with MM undergoing both GTR and STR (4). However, another study illustrated that patients with MM did not benefit from PORT (20). For elderly patients with MM specifically, Zhou et al. and Achey et al. both suggested that PORT could not provide survival benefits after GTR (6, 31). The results of univariate analysis and multivariable analysis in our study showed that PORT exhibited no significant association with CSS in both younger group and elderly group. However, there may be a selection bias that those patients considered to possess a higher risk of recurrence or with more aggressive tumors are more likely to receive PORT. In addition, the information about PORT is not complete in the SEER database, which may affect the accuracy of the conclusion. We believe that additional large-scale clinical studies are needed to explore the survival benefit of PORT applied in patients with MM.
We are aware of the limitations of this study. The patients extracted from the SEER database may not represent the general patient cohort. For the elderly population, the concomitant disease before surgery, the complications, and functional status after surgery are important factors and may affect patient survival largely, which could not be obtained through the SEER database. Furthermore, the records of Simpson grades of resection and the exact radiotherapy information are also not available. Moreover, the insufficient number of patients may affect the analysis results and lead to selection bias.
Surgical resection is recommended for elderly patients with MM in the absence of surgical contraindications, but GTR do not present survival benefit in the elderly patients compared with STR. Meanwhile, PORT exhibits no significant association with CSS in elderly group. Additional large-scale clinical studies are needed to explore the survival benefit of PORT applied in patients with MM. Despite several limitations, we believe that this study would help clinicians evaluate the prognosis of patients with MM and optimize treatment strategies for elderly patients specifically.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
SF, ZYL, and QC made substantial contributions to the design of this study. SF, JL, FF, ZW, ZQL, and QC carried out the analysis and interpreted the data. SF, ZD, HZ, and JL made contributions to the drafting of the manuscript. ZD, QZ, PL, JZ, and XZ made contributions to the review of previous literature. SF, ZYL, and QC contributed substantially to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. 82102848, 82073893, and 81703622), China Postdoctoral Science Foundation (No. 2018M633002), Hunan Provincial Natural Science Foundation of China (Nos. 2018JJ3838 and 2020JJ8111), and Xiangya Hospital Central South University postdoctoral foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
All authors thank SEER database for providing the worthy patient cohort for research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.913254/full#supplementary-material
1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol (2020) 22(12 Suppl 2):iv1–96. doi: 10.1093/neuonc/noaa200
2. Wiemels J, Wrensch M, Claus EB. Epidemiology and Etiology of Meningioma. J Neurooncol (2010) 99:307–14. doi: 10.1007/s11060-010-0386-3
3. Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, et al. Quality of Care, Process, and Outcomes in Elderly Patients With Pneumonia. JAMA (1997) 278:2080–4. doi: 10.1001/jama.1997.03550230056037
4. Orton A, Frandsen J, Jensen R, Shrieve DC, Suneja G. Anaplastic Meningioma: An Analysis of the National Cancer Database From 2004 to 2012. J Neurosurg (2018) 128:1684–9. doi: 10.3171/2017.2.JNS162282
5. Champeaux C, Jecko V, Houston D, Thorne L, Dunn L, Fersht N, et al. Malignant Meningioma: An International Multicentre Retrospective Study. Neurosurgery (2019) 85:E461–9. doi: 10.1093/neuros/nyy610
6. Achey RL, Gittleman H, Schroer J, Khanna V, Kruchko C, Barnholtz-Sloan JS. Nonmalignant and Malignant Meningioma Incidence and Survival in the Elderly, 2005-2015, Using the Central Brain Tumor Registry of the United States. Neuro Oncol (2019) 21:380–91. doi: 10.1093/neuonc/noy162
7. Barthelemy E, Loewenstern J, Konuthula N, Pain M, Hall J, Govindaraj S, et al. Primary Management of Atypical Meningioma: Treatment Patterns and Survival Outcomes by Patient Age. J Cancer Res Clin Oncol (2018) 144:969–78. doi: 10.1007/s00432-018-2618-4
8. Konglund A, Rogne SG, Lund-Johansen M, Scheie D, Helseth E, Meling TR. Outcome Following Surgery for Intracranial Meningiomas in the Aging. Acta Neurol Scand (2013) 127:161–9. doi: 10.1111/j.1600-0404.2012.01692.x
9. Steinberger J, Bronheim RS, Vempati P, Oermann EK, Ladner TR, Lee NJ, et al. Morbidity and Mortality of Meningioma Resection Increases in Octogenarians. World Neurosurg (2018) 109:e16–23. doi: 10.1016/j.wneu.2017.09.021
10. Connolly ID, Cole T, Veeravagu A, Popat R, Ratliff J, Li G. Craniotomy for Resection of Meningioma: An Age-Stratified Analysis of the MarketScan Longitudinal Database. World Neurosurg (2015) 84:1864–70. doi: 10.1016/j.wneu.2015.08.018
11. Boviatsis EJ, Bouras TI, Kouyialis AT, Themistocleous MS, Sakas DE. Impact of Age on Complications and Outcome in Meningioma Surgery. Surg Neurol (2007) 68:407–11; discussion 411. doi: 10.1016/j.surneu.2006.11.071
12. Poon MT, Fung LH, Pu JK, Leung GK. Outcome Comparison Between Younger and Older Patients Undergoing Intracranial Meningioma Resections. J Neurooncol (2013) 114:219–27. doi: 10.1007/s11060-013-1173-8
13. Moreau JT, Hankinson TC, Baillet S, Dudley RWR. Individual-Patient Prediction of Meningioma Malignancy and Survival Using the Surveillance, Epidemiology, and End Results Database. NPJ Digit Med (2020) 3:12. doi: 10.1038/s41746-020-0219-5
14. Dolecek TA, Dressler EV, Thakkar JP, Liu M, Al-Qaisi A, Villano JL. Epidemiology of Meningiomas Post-Public Law 107-206: The Benign Brain Tumor Cancer Registries Amendment Act. Cancer (2015) 121:2400–10. doi: 10.1002/cncr.29379
15. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
16. Arienta C, Caroli M, Balbi S. Intracranial Meningiomas in Patients Over 70 Years Old. Follow-Up in Operated and Unoperated Cases. Aging (Milano) (1992) 4:29–33. doi: 10.1007/BF03324059
17. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO Guidelines for the Diagnosis and Treatment of Meningiomas. Lancet Oncol (2016) 17:e383–91. doi: 10.1016/S1470-2045(16)30321-7
18. Eksi MS, Canbolat C, Akbas A, Ozmen BB, Akpinar E, Usseli MI, et al. Elderly Patients With Intracranial Meningioma: Surgical Considerations in 228 Patients With a Comprehensive Analysis of the Literature. World Neurosurg (2019) 132:e350–65. doi: 10.1016/j.wneu.2019.08.150
19. Ferroli P, Vetrano IG, Schiavolin S, Acerbi F, Zattra CM, Schiariti M, et al. Brain Tumor Resection in Elderly Patients: Potential Factors of Postoperative Worsening in a Predictive Outcome Model. Cancers (Basel) (2021) 13(10):2320. doi: 10.3390/cancers13102320
20. Choi Y, Lim DH, Jo K, Nam DH, Seol HJ, Lee JI. Efficacy of Postoperative Radiotherapy for High Grade Meningiomas. J Neurooncol (2014) 119:405–12. doi: 10.1007/s11060-014-1507-1
21. Zhang GJ, Zhang YS, Zhang GB, Li D, Zhang LW, Wu Z, et al. Prognostic Factors and the Management of Anaplastic Meningioma. Clin Neurol Neurosurg (2018) 170:13–9. doi: 10.1016/j.clineuro.2018.03.028
22. Champeaux C, Dunn L. World Health Organization Grade II Meningioma: A 10-Year Retrospective Study for Recurrence and Prognostic Factor Assessment. World Neurosurg (2016) 89:180–6. doi: 10.1016/j.wneu.2016.01.055
23. Rosenberg LA, Prayson RA, Lee J, Reddy C, Chao ST, Barnett GH, et al. Long-Term Experience With World Health Organization Grade III (Malignant) Meningiomas at a Single Institution. Int J Radiat Oncol Biol Phys (2009) 74:427–32. doi: 10.1016/j.ijrobp.2008.08.018
24. Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and Survival Following Primary and Repeat Surgery for World Health Organization Grade III Meningiomas. J Neurosurg (2010) 113:202–9. doi: 10.3171/2010.1.JNS091114
25. Brokinkel B, Holling M, Spille DC, Hess K, Sauerland C, Bleimuller C, et al. Surgery for Meningioma in the Elderly and Long-Term Survival: Comparison With an Age- and Sex-Matched General Population and With Younger Patients. J Neurosurg (2017) 126:1201–11. doi: 10.3171/2016.2.JNS152611
26. Cohen-Inbar O, Soustiel JF, Zaaroor M. Meningiomas in the Elderly, the Surgical Benefit and a New Scoring System. Acta Neurochir (Wien) (2010) 152:87–97; discussion 97. doi: 10.1007/s00701-009-0552-6
27. D'Andrea G, Roperto R, Caroli E, Crispo F, Ferrante L. Thirty-Seven Cases of Intracranial Meningiomas in the Ninth Decade of Life: Our Experience and Review of the Literature. Neurosurgery (2005) 56:956–61; discussion 956-61. doi: 10.1227/01.NEU.0000158303.28823.E9
28. Chen ZY, Zheng CH, Tang L, Su XY, Lu GH, Zhang CY, et al. Intracranial Meningioma Surgery in the Elderly (Over 65 Years): Prognostic Factors and Outcome. Acta Neurochir (Wien) (2015) 157:1549–57; discussion 1557. doi: 10.1007/s00701-015-2502-9
29. Modha A, Gutin PH. Diagnosis and Treatment of Atypical and Anaplastic Meningiomas: A Review. Neurosurgery (2005) 57:538–50; discussion 538-50. doi: 10.1227/01.NEU.0000170980.47582.A5
30. Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, et al. Malignant Meningioma: An Indication for Initial Aggressive Surgery and Adjuvant Radiotherapy. J Neurooncol (1998) 37:177–88. doi: 10.1023/A:1005853720926
Keywords: malignant meningioma, elderly patient, treatment strategy, SEER, patient prognosis
Citation: Feng S, Li J, Fan F, Wang Z, Zhang Q, Zhang H, Dai Z, Zhang X, Luo P, Liu Z, Zhang J, Liu Z and Cheng Q (2022) Prognostic Factors and Treatment Strategies for Elderly Patients with Malignant Meningioma: A SEER Population-Based Study. Front. Oncol. 12:913254. doi: 10.3389/fonc.2022.913254
Received: 05 April 2022; Accepted: 14 April 2022;
Published: 13 May 2022.
Edited by:
Ignazio Gaspare Vetrano, Carlo Besta Neurological Institute Foundation (IRCCS), ItalyReviewed by:
Manuela Caroli, Fondazione Politecnico di Milano, ItalyCopyright © 2022 Feng, Li, Fan, Wang, Zhang, Zhang, Dai, Zhang, Luo, Liu, Zhang, Liu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Cheng, Y2hlbmdxdWFuQGNzdS5lZHUuY24=; Zhuoyi Liu, emh1b3lpbGl1QGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.