
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 June 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.912166
A correction has been applied to this article in:
Corrigendum: H3K27M mutation doesn’t mean worse prognosis in old patients
Objective: Diffuse midline glioma (DMG), H3K27 altered is a new entity that has become widely recognized. However, studies concerning DMG in adult patients remain rare. We did a retrospective study covering the largest amount of patients to date to analyze the clinicopathological characteristics of diffuse glioma in midline structures of the adult.
Methods: We reviewed 108 cases of adult DMG, collected their clinical data, and pathological results including H3K27 mutation. Summarized their features and the connection with overall survival in different age groups.
Results: Among 108 cases, 79 tumors were located at the thalamus. 38 patients had H3K27M mutation, whose average age was 35.7 years. The median overall survival of H3K27M-mutant gliomas and the 70 H3K27M wild-type gliomas were both 17 months. For young patients (age ≤ 35), The median survival time of the H3K27M-mutant was 18 months, while that of the H3K27M wild-type was 37 months. For older patients (age>35), the median survival time of the H3K27M-mutant was 16 months, while that of the H3K27M wild-type was 13 months. Other clinicopathological factors including sex, tumor location, the approach of surgery, histological grade, ATRX, and P53 were statistically irrelevant to prognosis.
Conclusion: The DMG in adults mainly occurred in the thalamus. H3K27M mutations tend to happen more frequently in young adults, and this genetic alteration results in a worse outcome only in young patients (≤35). For old patients, age is the only independent prognostic factor. Patients who underwent different surgical operations including biopsy, subtotal resection, and total resection had similar prognoses.
The Diffuse Midline Glioma (DMG), H3K27 altered, is a new pathologic entity that has become widely recognized since it was first classified as a central nervous system neoplastic disorder by the WHO in 2016. As illustrated by its name, these gliomas are commonly located in midline structures of the brain, including the brainstem, thalamus, spinal cord, and cerebellum. These are defined as grade 4 gliomas and are correlated with worse prognoses (1). However, with more and more investigations being carried out on this tumor entity, several questions have been noticed by researchers. Firstly, due to this kind of glioma mainly involving children, cases in adult patients are seldom reported and their prognoses remain unclear. Whether the H3K27M mutation still indicates a poor prognosis is questionable. Secondly, carrying out successful neurosurgery to resect tumors in midline structures is a challenging task. As a result, the limitation on operations or genetic alterations that mainly contribute to the dismal outcome of DMG has been confused.
To figure out the prognosis of DMG in adult patients and explore the benefit that tumor resections offer patients, we have reviewed patients diagnosed with DMG in our hospital, collected their clinical and pathological data, and summarized its characteristics.
In this retrospective study, we reviewed our pathology database and picked out cases of patients diagnosed with glioma in the midline structures of their brains - including the thalamus, brainstem, cerebellum, and spinal cord - from January 2013 to December 2019. All the patients in our cohort were adults (age>18), and had to satisfy the following standards: 1. no previous medical history of glioma; 2. no severe complications during the perioperative period; 3. possess a complete medical history and have an accessible survival outcome. The tumor locations were verified via a radiologist through preoperative MR images. Pathologic sections were reassessed by two experienced pathologists according to the 2021 WHO classification of central nervous system tumors. To confirm the diagnosis, the H3K27M status was tested or reconfirmed via immunostaining and PCR tests. All the tumor samples underwent genetic tests for IDH1/2. Other important markers including ATRX, P53, and Ki67 were all re-evaluated via immunohistochemistry.
By looking up the patients’ detailed operation records in our hospital’s medical history system and consulting the related neurosurgeons, we obtained the approaches of operations and range of resections. And the range was further confirmed from postoperative MR images by radiologists. We divided the patients into 3 subgroups according to the range of tumor resections: biopsy, subtotal resection, and total resection.
All the tumor specimens were formalin fixed and paraffin embedded (FFPE). Immunohistochemistry was performed on 4um sections with the antibodies: H3K27M (ZSGB-BIO China, ZA-0321), H3K27me3 (ZSGB-BIO China, ZA-0327), IDH (ZSGB-BIO China, ZM-0447), ATRX (ZSGB-BIO China, ZA-0016), P53 (ZSGB-BIO China, ZM-0408), and Ki67 (Dako Denmark, GA626). For the PCR tests, DNA extractions were conducted with four 10mm sections from FFPE tissues. The enriched areas in tumor cells confirmed from HE sections were marked in advance. Tissues previously labeled for extraction were scraped off and placed on an Eppendorf tube for DNA isolation using the QIAamp DNA Mini Kit (Qiagen GmbH, Germany), the process for which was carried out according to the manufacturer’s protocol. A spectrophotometer (Biophotometer Eppendorf, Germany) was used for detecting the quality and concentration of DNA samples.
The sequencing reactions of Histone H3F3A and IDH1/2 were analyzed by direct sequencing of polymerase chain reaction–amplified products from tumor DNA using the primers designed according to literature (2, 3). Tests were performed using clean-up exonuclease ExoSAP-IT (Affymetrix, Santa Clara, CA) with a Big Dye Terminator Cycle Sequencing Kit and capillary electrophoresis on the automated sequencer ABI3730 (Applied Biosystems, Carlsbad, CA). The PCR products were then analyzed and purified. Exonic alterations of sense and nonsense sequences were detected using SeqScape v2.5 software (Applied Biosystems), before being compared with the National Center for Biotechnology Information reference sequences of H3F3A and IDH1/2.
We carried out statistical analysis using the STATA software (Stata Corp TEXAS V15.1). The Kaplan-Meier estimator and Log-Rank test were conducted to form a comparison of patients’ survival rates in different subgroups. We also performed univariate and multivariate COX models to evaluate the significance of related factors. Spearman test was done to verify the correlation between age and H3K27M mutation. The χ2 test, Fisher’s exact t-test, and Welch’s t-test were done to compare differences in clinicopathological factors in subgroups divided by H3K27M mutation. The OS was defined and counted from the date of surgery to the date of death from any causes. This is viewed as statistically significant when p<0.05.
We identified 108 patients with DMG. They were all adults (>18 years old) ranging from 18 to 74 years old, with an average age of 43.5. 61 were males and 47 were females. Their pathological and clinical characteristics are summarized in Table 1. 79 tumors were located in the thalamus, 15 tumors in the brainstem, 5 in the cerebellum, and 4 in the spinal cord. The remaining 5 tumors involved multiple sites mentioned above (Figure 1). Histologically, 19 corresponded to WHO grade 2, 23 were grade 3, and the remaining 66 cases were grade 4 (Figures 2A1, B1, C1).
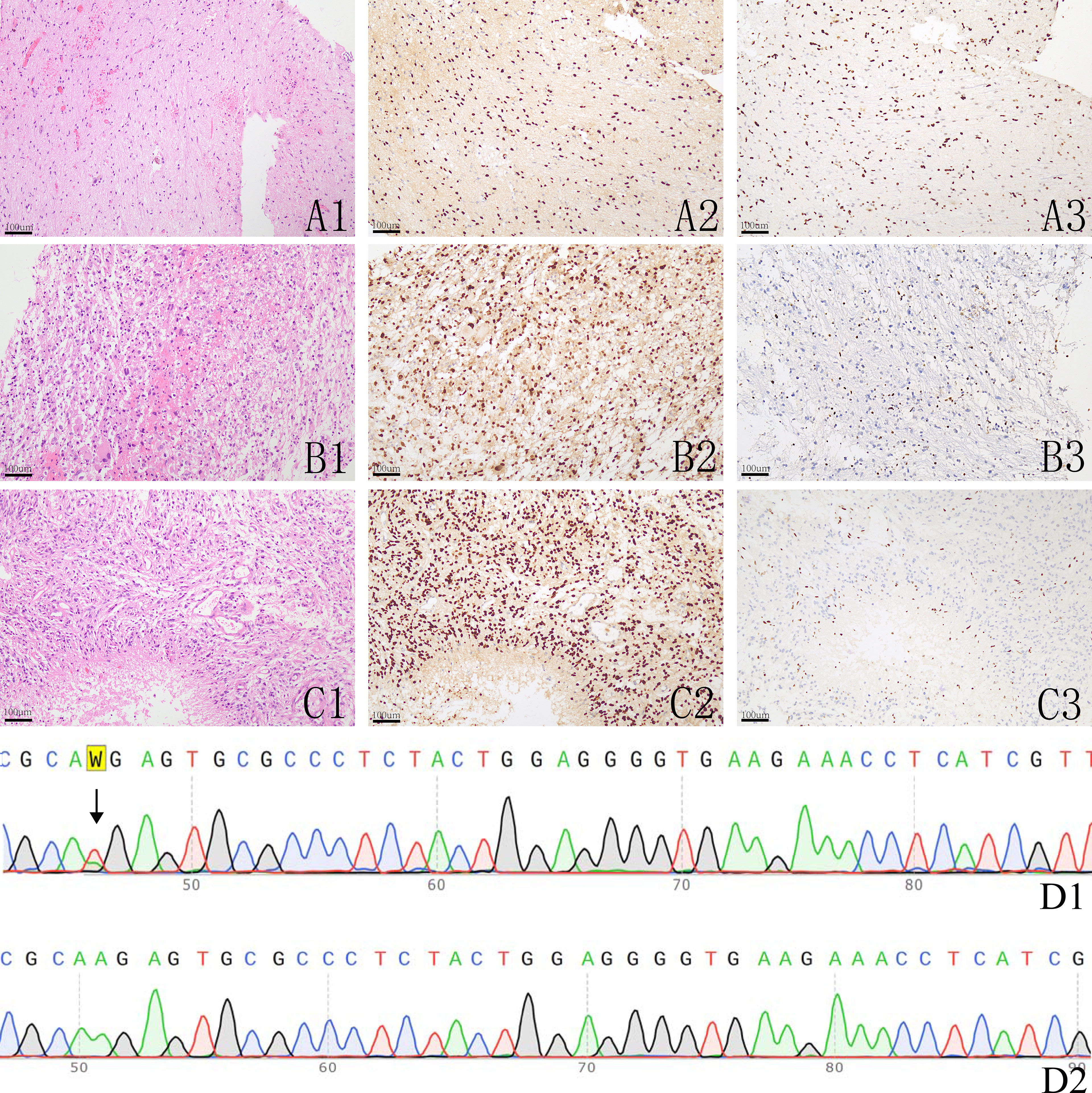
Figure 2 The histological image and immunohistochemistry results of H3K27M and H3K27me3, A1-A3: a histologically grade 2 glioma showing H3K27M expression (A2) and downregulation of H3K27me3 (A3); B1-B3: a histologically grade 3 glioma showing H3K27M expression (B2) and slightly downregulation of H3K27me3 (B3); C1-C3: a histologically grade 4 glioma showing H3K27M expression (C2) and loss the expression of H3K27me3 (C3); D1-D2: Sanger sequencing of H3F3A in one H3K27M mutant glioma showing A > T mutation (D1) and a H3K27M wild glioma (D2).
According to our IHC results, 38 patients were positive for H3K27M, and these tumors all demonstrated down regulation of H3K27me3. Out of the tumors that were negative for H3K27M, 70 samples were all diffusely positive for H3K27me3 (Figure 2). All tumor samples underwent PCR tests for H3.3 K27M, and all H3K27M immune-positive gliomas were genetically verified to be H3K27M mutants (Figure 2D). Those who were H3K27M negative were confirmed to be H3K27M wild-type. The mutation rate of H3K27M in the thalamus, brainstem, cerebellum, spinal cord and multiple locations was 34.2%, 53.3%, 20%, 25.0%, and 0% respectively. There were a total of 23 (23/38 50.5%) H3K27M mutant cases in the young adult group and 15 (15/70 21.4%) in the old group. The mutation rate of H3K27M was also included in Table 1. Spearman tests demonstrated that there existed a weak correlation between age and H3K27M mutation (p=0.394). G34R/V mutation was not detected in our cohort. We also conducted routine IDH1/2 tests for all tumor specimens. Only 3 gliomas were mutated at R132H of IDH-1, and the rest were all IDH wild-type. The 3 IDH mutant gliomas were in the cerebellum (n=1) and brainstem (n=2), and were all H3K27M wild-type. In our cohort, 58 gliomas (53.7%) showed overexpression of P53, and 33 (30.6%) gliomas had lost the expression of ATRX. Loss of ATRX was observed in 16 H3K27M mutant gliomas and 17 H3K27M wildtype gliomas.
Out of all the patients, 17 received a total tumor resection and 49 patients underwent a subtotal tumor resection; 42 patients received a stereotactic biopsy instead of a tumor resection. All patients received chemotherapy with temozolomide accompanied by radiotherapy.
The mean follow-up time was 19.4 months (range from 7 to 66 months). Our patients’ OS ranged from 2 months to 56 months with a median survival time of 17 months. The 1-year and 3-year survival rates were 55.7% and 14.8% respectively. The median survival time of the young adults was 22 months, while that of the older patients was 13 months (p=0.244). For 38 H3K27M mutant gliomas, the median OS was 17 months, while that of 70 H3K27M wild-type gliomas was also 17 months (p=0.986). The median OS of 19 histologically low-grade gliomas (grade 2) was 18 months and that of 89 high-grade gliomas (grade 3 and 4) was 17 months (p=0.353). It can therefore be seen that there is no statistical difference between the subgroups divided by H3K27M mutation or histological grade, the same results can also be observed when tumors were divided by locations (Figures 3D–F). When dividing the data according to approaches of operations they received, the median OS of patients who received biopsies, subtotal resections, and total resections were 17, 14, and 17 months respectively. There is no statistical difference between the three groups (p=0.303) (Figures 3G, H).
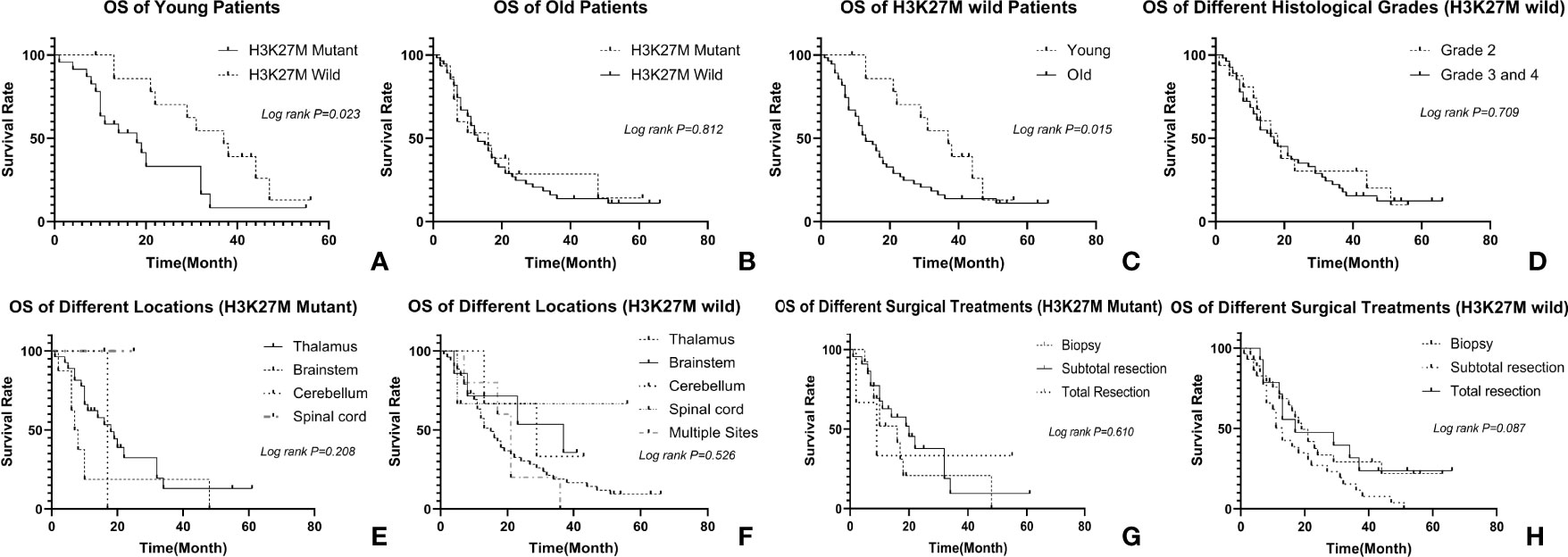
Figure 3 Relationship between clinicopathological factors and OS: (A) relationship between H3K27M and OS in young patients, the H3K27M mutant had a worse prognosis; (B) relationship between H3K27M and OS in old patients, there is no difference in prognosis of two different subgroups; (C) prognosis of different age groups in H3K27M wild patients, the young has a better prognosis. (D) relationship between histological grade and OS in H3K27M wild patients. There is no difference in different histological grades; (E, F) relationship between tumor location and OS in patients, tumors in different location have similar prognosis regardless of H3K27M mutation; (G, H) relationship between operation and OS in patients, patients underwent biopsy, subtotal resection or total resection have similar prognosis regardless of H3K27M mutation.
We then further explored the prognosis in different age groups. We tried different ages from 25 to 70 by every gap of 5 years to explore an appropriate cutoff value to define age subgroups. We finally found that 35 was a statistically significant cutoff age. As a result, we divided our cohorts into two subgroups depending on whether their age was >35, which formed the old group, or ≤35, which formed the young adult group. Out of all the patients, 38 were classified as young adults, and 70 were put in the old group. Although having a similar outcome in the long run, the young have a better prognosis than the old for those H3K27 wild gliomas (Figure 3C). In younger patients, the clinicopathological factors mentioned above, including tumor location (p=0.336), sex (p=0.694), ATRX (p=0.599), P53 (p=0.523), histological grades (p=0.348), and operations (p=0.062) had no statistical relevance with prognoses. The median survival time of the H3K27M-mutant was 18 months, while that of the H3K27M wild-type was 37 months (p=0.023) (Figure 3A). For older patients, H3K27M was not a significant factor that affected patients’ outcomes since the median survival time of the H3K27M-mutant was 16 months, while that of the H3K27M wild-type was 13 months (p=0.812) (Figure 3B).
The “DMG, H3K27M mutant” is a newly defined entity in the 2016 revision of the WHO classification of central nervous system tumors. In the 2021 WHO classification, it was renamed as “DMG, H3K27 altered” and classified into pediatric type high grade glioma (4). It is a high grade glioma that mainly occurs in children, and the mutation in H3 often results in a dismal outcome, with a median survival of around 1 year from the time of diagnosis in children (5). However, the characteristics of this tumor in adults remain unclear. We carried out a retrospective analysis on 108 adult patients which focused on their clinical and pathological characteristics and overall survival. To date, this is the largest cohort concerning DMG in adults in available literature
Unlike pediatric patients whose midline gliomas are mostly located at the brainstem and pons (6), our results revealed that the most common sites of DMG in adults are the thalamus as the finding of the study by Huy Gia et al. (7). For H3K27M wild-type gliomas, histologically low grade cases comprised more than H3K27M mutated gliomas. The average age of H3K27M-mutated glioma patients was younger than the H3K27M wild-type patients. There was no H3K27M mutation detected in patients older than 55. According to our analysis, there is a weak correlation between H3K27M mutations and the age of patients. All these factors prove that the H3K27M mutation tends to happen in younger patients among all age ranges. The older a patient is, the lower the chance they will develop a H3K27M mutation. Another mutation of H3F3A, H3G34R/V, was not founded in our research, partly because the H3G34 mutation are mostly founded in diffuse hemispheric gliomas (8). Genetic tests concerning HIST1H3B/C were not included in this study. However, we believe that this had little effect on our result since H3K27me3, the trimethylated lysine 27 of histone could be downregulated by mutation of K27M in both H3.3 and H3.1 (9), were evaluated in all tumor samples and the rate of H3.1 K27M mutation in adult patients were lower than that of children (10).
Compared with the 6% rate of adult GBM (11)and the 14-29% rate of pediatric high grade glioma (12), the observed rate of ATRX mutation in our study was 42.1% and 24.3% in the H3K27M mutated gliomas and the H3K27M wild-type gliomas, respectively. The ratio was higher than gliomas in non-midline structures, corresponding to the fact that H3K27M mutated gliomas seem to predominantly result from lengthening of telomeres through ATRX mutation (13). Consistent with former reports, P53 overexpression was easily observed in our research, especially in the H3K27M mutated groups (14).
The H3K27M mutation is a genetic alteration reportedly linked with dismal outcomes in pediatric diffuse intrinsic pontine gliomas (DIPG) (15). The same phenomenon was also found in some studies concerning H3K27M mutated DMG in adults (16, 17). From 30 cases combined with the 171 cases reported in literature, Toshiyuki et al. found that the status of H3K27M is not related to the prognosis in patients who are older than 40 (18). However, another piece of research reported that H3K27M does not correlate with the prognoses of adults (19). To date, our study covers the largest cohorts and found that H3K27M mutant gliomas tend to have poorer prognoses in patients younger than 35. For patients older than 35, the H3K27M mutation does not affect the patients’ outcomes. The contradictory results found in the literature are possibly due to some research not subdividing patients according to age. Our cohort’s cutoff value for age was 35 instead of 40 used in the literature mentioned above (18). More cases are needed to discover the potential link between H3K27M and patients’ ages.
It is challenging for neurosurgeons to conduct operations on midline structures. The development of technologies - including the integration of functional mapping data on the neuro navigation cortical and subcortical electrical stimulation and awake anesthesia - has made surgical intervention more widely accepted as an important part of DMG treatment (20, 21).
It is widely accepted that extensive resections of high grade gliomas are beneficial for patients, with more extensive resections providing greater advantages (22). For adult DMG, it was reported that patients could benefit from tumor resections (23, 24). Our work found that patients who underwent different operations had a similar outcome, regardless of whether H3K27M had mutated or not. Our research was limited by the fact that we did not include a quantitative analysis of the extent of resections, and we didn’t take postoperative morbidity along with life quality into consideration. However, this tells us that if it is not necessary, neurosurgeons don’t need to risk a total tumor resection. Biopsies are recommended so that the genetic phenotype can be accurately decided at a low cost of living quality.
With regards to pediatric DMG, the WHO histological grade is of no prognostic significance (25). From our observations of adult patients, the tumor grade does not influence on the prognosis either. According to the 2021 WHO classification, all of our H3K27M wild-type gliomas that used to be diagnosed as “astrocytoma, grade II” may be considered to be diagnosed as glioblastoma now since they are all adult-type glioma and IDH wild-type. Due to the limitation of tumor samples from biopsy, we didn’t perform tests concerning some relatively rare genetic alterations whose significance is unclear in adult patients. Clinical significance of other forms of H3K27 mutation, EGFR amplification or mutation, overexpression of EZHIP, and TERT mutation are needed to be explored in future studies on DMG in adults. Our follow-up shows that our cohorts share a similar prognosis with glioblastoma in other sites (26).
It is interesting of the H3K27M mutation’s low frequency and meaninglessness in old patients. Further studies with complete genetic records are needed to describe the characteristics of this kind of glioma and potentially obtain a more comprehensive understanding of the gene, H3K27.
In conclusion, this study covers the largest single-center series of gliomas in the midline structure of adult patients. It found that the DMG in adults mainly occurred in the thalamus. H3K27M mutations tend to happen more frequently in young adults (age<35), and this genetic alteration only results in a worse outcome in young patients. For old patients, DMG has a similar outcome as glioblastomas in cerebral hemisphere, and age is an independent prognostic factor, while H3K27M mutation has no prognostic value.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
XH contribute to investigation and writing the original draft; XN contributed to investigation and data collection; KX mainly contributed to data analysis and design experiments; YW modified the manuscript; FT provided the method; ZD contributed to study design; JX designed the project and revised the manuscript. All authors contributed to the article and approved the submitted version.
ZD was funded by Natural Science Foundation of China (Number: 82072692).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
2. Huang TY, Piunti A, Lulla RR, Qi J, Horbinski CM, Tomita T, et al. Detection of Histone H3 Mutations in Cerebrospinal Fluid-Derived Tumor DNA From Children With Diffuse Midline Glioma. Acta Neuropathol Commun (2017) 5:28. doi: 10.1186/s40478-017-0436-6
3. Perizzolo M, Winkfein B, Hui S, Krulicki W, Chan JA, Demetrick DJ. IDH Mutation Detection in Formalin-Fixed Paraffin-Embedded Gliomas Using Multiplex PCR and Single-Base Extension. Brain Pathol (2012) 22:619–24. doi: 10.1111/j.1750-3639.2012.00579.x
4. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
5. Lowe BR, Maxham LA, Hamey JJ, Wilkins MR, Partridge JF. Histone H3 Mutations: An Updated View of Their Role in Chromatin Deregulation and Cancer. Cancers (Basel) (2019) 11(5):660. doi: 10.3390/cancers11050660
6. Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse Midline Gliomas With Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol (2016) 26:569–80. doi: 10.1111/bpa.12336
7. Vuong HG, Le HT, Jea A, McNall-Knapp R, Dunn IF. Risk Stratification of H3 K27M–mutant Diffuse Midline Gliomas Based on Anatomical Locations: An Integrated Systematic Review of Individual Participant Data. J Neurosurg: Pediatr (2022) 29:1–8. doi: 10.3171/2022.3.PEDS2250
8. Wang L, Shao L, Li H, Yao K, Duan Z, Zhi C, et al. Histone H3.3 G34-Mutant Diffuse Gliomas in Adults. Am J Surg Pathol (2022) 46:249–57. doi: 10.1097/PAS.0000000000001781
9. Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, et al. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science (2013) 340:857–61. doi: 10.1126/science.1232245
10. Zheng L, Gong J, Yu T, Zou Y, Zhang M, Nie L, et al. Diffuse Midline Gliomas With Histone H3 K27M Mutation in Adults and Children: A Retrospective Series of 164 Cases. Am J Surg Pathol (2022) 46:863–71. doi: 10.1097/PAS.0000000000001897
11. Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The Somatic Genomic Landscape of Glioblastoma. Cell (2013) 155:462–77. doi: 10.1016/j.cell.2013.09.034
12. Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature (2012) 482:226–31. doi: 10.1038/nature10833
13. Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, et al. Paediatric and Adult Glioblastoma: Multiform (Epi)Genomic Culprits Emerge. Nat Rev Cancer (2014) 14:92–107. doi: 10.1038/nrc3655
14. Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, et al. H3F3A K27M Mutations in Thalamic Gliomas From Young Adult Patients. Neuro Oncol (2014) 16:140–6. doi: 10.1093/neuonc/not144
15. Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, et al. Histone H3F3A and HIST1H3B K27M Mutations Define Two Subgroups of Diffuse Intrinsic Pontine Gliomas With Different Prognosis and Phenotypes. Acta Neuropathol (2015) 130:815–27. doi: 10.1007/s00401-015-1478-0
16. Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, et al. Characteristics of H3 K27M-Mutant Gliomas in Adults. Neuro Oncol (2017) 19:1127–34. doi: 10.1093/neuonc/now274
17. Liu Y, Zhang Y, Hua W, Li Z, Wu B, Liu W. Clinical and Molecular Characteristics of Thalamic Gliomas: Retrospective Report of 26 Cases. World Neurosurg (2019) 126:e1169–e82. doi: 10.1016/j.wneu.2019.03.061
18. Enomoto T, Aoki M, Hamasaki M, Abe H, Nonaka M, Inoue T, et al. Midline Glioma in Adults: Clinicopathological, Genetic, and Epigenetic Analysis. Neurol Med Chir (Tokyo) (2020) 60:136–46. doi: 10.2176/nmc.oa.2019-0168
19. Ebrahimi A, Skardelly M, Schuhmann MU, Ebinger M, Reuss D, Neumann M, et al. High Frequency of H3 K27M Mutations in Adult Midline Gliomas. J Cancer Res Clin Oncol (2019) 145:839–50. doi: 10.1007/s00432-018-02836-5
20. Alemo S, Sayadipour A. Role of Intraoperative Neurophysiologic Monitoring in Lumbosacral Spine Fusion and Instrumentation: A Retrospective Study. World Neurosurg (2010) 73:72–6; discussion e7. doi: 10.1016/j.surneu.2009.04.024
21. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of Intraoperative Stimulation Brain Mapping on Glioma Surgery Outcome: A Meta-Analysis. J Clin Oncol (2012) 30:2559–65. doi: 10.1200/JCO.2011.38.4818
22. Tang S, Liao J, Long Y. Comparative Assessment of the Efficacy of Gross Total Versus Subtotal Total Resection in Patients With Glioma: A Meta-Analysis. Int J Surg (2019) 63:90–7. doi: 10.1016/j.ijsu.2019.02.004
23. Wu B, Tang C, Wang Y, Li Z, Hu S, Hua W, et al. High-Grade Thalamic Gliomas: Microsurgical Treatment and Prognosis Analysis. J Clin Neurosci (2018) 49:56–61. doi: 10.1016/j.jocn.2017.12.008
24. Dey M, Lin Y, Melkonian S, Lam S. Prognostic Factors and Survival in Primary Adult High Grade Brainstem Astrocytoma: A Population Based Study From 1973-2008. J Clin Neurosci (2014) 21:1298–303. doi: 10.1016/j.jocn.2013.12.011
25. Cohen KJ, Jabado N, Grill J. Diffuse Intrinsic Pontine Gliomas-Current Management and New Biologic Insights. Is There a Glimmer of Hope? Neuro Oncol (2017) 19:1025–34. doi: 10.1093/neuonc/nox021
Keywords: midline glioma, H3K27M, clinicopathological study, prognosis, age
Citation: Hu XM, Nie Xy, Xu Kl, Wang Y, Tang F, Du Zg and Xiong J (2022) H3K27M Mutation Doesn’t Mean Worse Prognosis in Old Patients. Front. Oncol. 12:912166. doi: 10.3389/fonc.2022.912166
Received: 04 April 2022; Accepted: 23 May 2022;
Published: 08 June 2022.
Edited by:
Pankaj Pathak, National Institutes of Health (NIH), United StatesReviewed by:
Prit Benny Malgulwar, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2022 Hu, Nie, Xu, Wang, Tang, Du and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zun guo Du, ZHV6dW5ndW9Ac2luYS5jb20uY24=; Ji Xiong, ZGFiZW54QDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.