
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 16 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.911906
This article is part of the Research Topic Reviews in Thoracic Oncology View all 17 articles
Immune checkpoint inhibitors (ICIs) have shown definite therapeutic effects in various types of cancers, especially non-small cell lung cancer (NSCLC). However, ICIs have unique side effects, called immune-related adverse events (irAEs), which can occur in various systems throughout the body. Among such irAEs, immune checkpoint inhibitor-related pneumonitis (ICI-P) is a fatal adverse reaction. In this review, we discussed the risk factors, pathogenesis, clinical characteristics, radiological manifestations, pathological features, diagnosis, grading, and management of ICI-P in NSCLC and the relationship between ICI-P and the efficacy of ICI therapy. In addition, we discussed the predictive factors for ICI-P. This review will play a crucial role in the prediction, evaluation, and management of ICI-P for widespread application of immunotherapy.
Lung cancer is one of the most common malignant tumours worldwide, with non-small cell lung cancer (NSCLC) accounting for 85% of cases (1, 2). According to the recent population-based cancer incidence and mortality data reported by the American Cancer Society, which is compiled every year, the incidence of cancer is gradually decreasing, and the decline in the number of lung cancer cases is particularly pronounced (3). In addition, the mortality rate of lung cancer has declined significantly, which is related to improved management.
Immune checkpoint inhibitors (ICIs) exert significant clinical therapeutic effects and have accelerated the treatment of advanced cancer in the new era of immunotherapy. The use of ICIs has shown great success in improving the overall survival (OS) and progression-free survival (PFS) rates of NSCLC (4–7). ICIs have been approved as a first-line treatment for advanced driver gene-negative NSCLC owing to their superior efficacy and no evident side effects compared with conventional chemotherapy (8). However, they can result in systemic reactions called immune-related adverse events (irAEs) (9) that are completely different from adverse reactions resulting from conventional chemotherapy.
IrAEs can affect all organs of the body, including the skin, gastrointestinal tract, liver, kidneys, lungs, endocrine organs, and the central nervous system (9–11). Among irAEs, immune checkpoint inhibitor-related pneumonitis (ICI-P) is a rare but fatal reaction (12, 13). ICI-P is defined as the development of dyspnoea and/or other respiratory symptoms and the appearance of a new infiltrative shadow on chest imaging after the patient has been treated with ICI, except for clinical conditions such as lung infection or tumour progression. According to the data of clinical trials, the incidence of ICI-P is 3%—6.3% in NSCLC, and the mortality rate is <1% (14–16). However, in previous epidemiological studies, the incidence of ICI-P varied greatly, ranging from 2.7% to 19% in NSCLC (17–20) (Table 1). Patients with lung cancer are more likely to develop ICI-P than patients with other types of cancer (17). The onset of ICI-P is earlier in patients with lung cancer (78 days) than in patients with non-lung cancer (186 days) (21). Recent real-world statistical data show that in clinical practice, the incidence of ICI-P is higher than that reported in pivotal trials, leading to the approval of programmed death-(ligand) 1 (PD-[L]1) inhibitors (22) by the United States Food and Drug Administration. High-grade ICI-P is extremely dangerous and often threatens the lives of patients. Therefore, clinicians should make rapid and accurate decisions for providing reasonable and effective treatment for ICI-P.
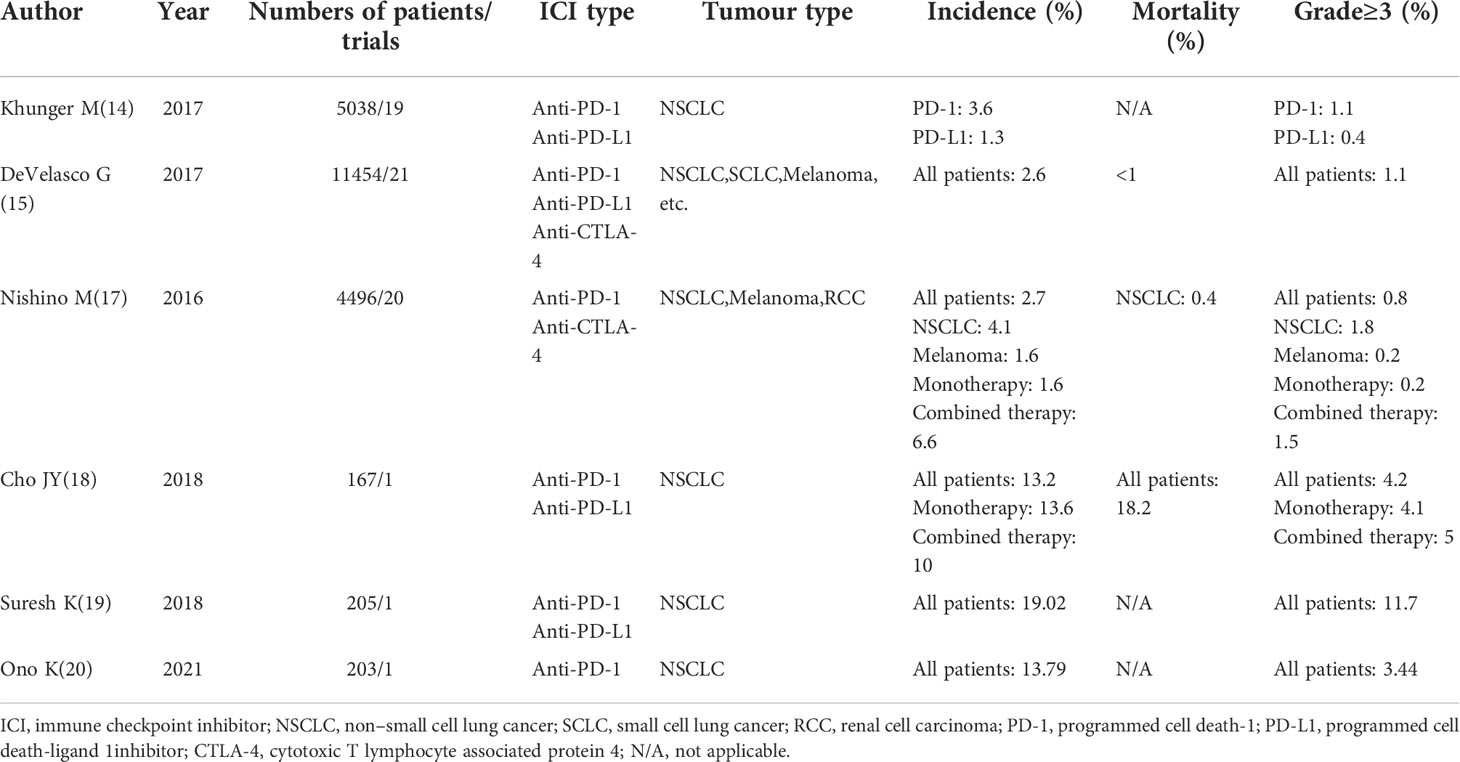
Table 1 Published meta-analysis and clinical trials on immune checkpoint inhibitor-related pneumonitis.
Many potential factors can increase the incidence of ICI-P in NSCLC patients treated with ICIs. Among the 97 patients who participated in the subgroup analysis of the Keynote-001 trial, OS was significantly longer after pembrolizumab administration in patients who had previously received radiotherapy than in patients who had not received radiotherapy. However, 3 (3/24, 13%) patients who underwent thoracic radiotherapy had developed ICI-P, whereas only 1 (1/72, 1%) patient who did not receive thoracic radiotherapy had developed ICI-P. Therefore, the probability of treatment-related pulmonary toxicity is higher in patients who have received radiotherapy than in patients who have not received radiotherapy (23). Other clinical studies have reported that patients who have received thoracic radiotherapy are more likely to have ICI-P (24–26) and respiratory failure (24). According to several retrospective studies, pre-existing history of interstitial pneumonitis is also associated with the incidence of ICI-P (27, 28). The development of ICI-P is independently associated with the presence of baseline fibrosis on computed tomography (CT) of the chest, which is a composite measure of obstructive lung disease (29). Cho et al. reported the same phenomenon, indicating that a pre-existing pulmonary disease associated with a significantly higher incidence of ICI-P, which could explain why ICI-P is more common in lung cancer patients than in other cancer types (18).
According to a recent meta-analysis of ICI clinical trials, the total incidence of ICI-P after single and combination therapy is 1.6% and 6.6%, respectively, suggesting that the risk of ICI-P is higher after combination therapy than after single therapy (17). In another study, a higher incidence of ICI-P was seen with the risk ratio of all grades of ICI-P increasing up to 2.92 after combination therapy (30). According to a meta-analysis, ICI-P was the most common cause of anti-PD-1/PD-L1-related fatalities (35%). In addition, toxicity-related fatality rates were higher in patients who received combination therapy of PD-1/PD-L1 plus CTLA-4 (1.23%) than in those who received single therapy with anti-PD-1 (0.36%), anti-PD-L1 (0.38%), or anti-CTLA-4 (1.08%) (31). The incidence of ICI-P was higher in patients treated with sequential therapy with targeted agents (18.8%) within 8 weeks of ICI treatment than in patients treated with cytotoxic agents (7.4%) and in patients not treated with chemotherapy (5.1%). The onset of ICI-P was earlier in patients who received sequential therapy with targeted agents after immunotherapy than in those who received sequential therapy with cytotoxic drugs (35 days versus 62 days, respectively). Patients who received targeted agents within 8 weeks of immunotherapy had a higher chance (100%) of developing ≥grade 3 ICI-P than those treated with cytotoxic agents (0%). Among 23 patients with ICI-P, 16 patients (69.6%) required intravenous steroids. Despite receiving high-dose systemic intravenous steroids, 1 patient with grade 4 pneumonitis recovered, whereas 6 (26.1%) patients died (32). Some findings showed that PD-1 inhibitors were associated with a higher incidence of ICI-P compared with PD-L1 inhibitors (immune monotherapy) (33–35). In a study by Khunger, compared with PD-L1 inhibitors (1.3%), PD-1 inhibitors were associated with a higher risk of ICI-P (3.6%). In addition, the incidence of >grade3 ICI-P was higher in patients receiving anti-PD-1 therapy (1.1%) than in those receiving PD-L1 therapy (0.4%) (14).
A retrospective study enrolling 1826 patients with cancer reported that ICI-P occurred more frequently in men and former or current smokers (64 [3.5%] patients) (36). Nakahama reported that tumour invasion in the central airway (TICA) was associated with an increased risk of ICI-P. Patients with TICA had a higher risk of ICI-P than patients with a history of radiotherapy, which is a well-known risk factor for ICI-P (37). Based on the conclusions of these two studies, the incidence of ICI-P is higher in NSCLC (especially squamous cell lung cancer) than in melanoma (38, 39). Furthermore, a study involving 837 patients showed that 354 (42.3%) patients aged ≥ 65 years had a significantly increased risk of developing ICI-P, compared with 483 (57.7%) patients aged < 65 years, with a risk ratio of 2.12 (40).
In conclusion, patients with the following characteristics: male, former or current smoker, ≥65 years old, previous chest radiotherapy, previous lung disease, combination therapy, and TICA are predisposed to ICI-P after immunotherapy (Table 2). Clinicians should be cautious while using ICIs to treat the aforementioned susceptible populations and keep patients under careful observation after ICI therapy.
The mechanisms of ICI-P remain unclear; however, some theories based on the mechanism of action of ICIs and related studies are described below. Activated T cells, B cells, NK T cells and myeloid cells express PD1 (41). Tumour cells express PD-L1, which is upregulated in macrophages, dendritic cells, fibroblasts and activated T cells (42). The interaction between PD-L1 and PD1 inhibits the function, differentiation and survival of T cell (41). Anti-PD(L)-1 improves the anti-tumour immune response by activating T cells and relieving the inhibition of associated signalling pathways. CTLA-4 is an inhibitory receptor belonging to the CD28 immunoglobulin subfamily, expressed mainly by T-cells, which inhibits binding of CD28 on T cells, to B7 proteins on antigen-presenting cells (APCs), thereby impairing the costimulatory effect of T cells the costimulation on T cells (43, 44). In addition, CTLA4 can also interferes with Treg cell function (45). In a study on a knockout mouse model, mice lacking the CTLA-4 gene died of lymphoproliferation, whereas those lacking PD-1 developed lupus-like autoimmune diseases (46, 47). The occurrence of ICI-P may be associated with excessive T cell activation and tumour microenvironment disturbance. Although ICIs promote lymphocyte activation against tumours, activated T cells can damage alveolar cells, leading to ICI-P (48, 49). A study by Suresh reported a marked increase in the number of lymphocytes, especially CD4+ T cells, in the bronchoalveolar lavage (BAL) of patients with ICI-P (50). The tumour microenvironment includes both immune cells and associated cytokines. Disturbance in the tumour microenvironment owing to ICI use may also contribute to the development of ICI-P. A study by Catacchio highlighted the significance of the tumour microenvironment (44). ICIs are immunotherapeutic agents with a specific target. Off-target toxicity is a specific mechanism by which targeted therapy causes negative effects (51). CD8+ cytotoxic T lymphocyte-mediated cell lysis induces the release of neoantigens, tumour antigens, and autoantigens from normal tissues. The immune tolerance of normal tissues is reduced as a result of this phenomenon known as “epitope spreading,”, which may lead to the development of ICI-P (52).
ICI-P is a unique toxic reaction that occurs after immunotherapy. Although it is relatively rare, it is one of the most important causes of death caused by ICIs in patients with NSCLC. A meta-analysis by Mizuki Nishino (17) showed that the overall incidence of ICI-P after PD-1 inhibitor monotherapy was 2.7% for all-grades of ICI-P and 0.8% for ≥grade 3 ICI-P. However, the incidence was higher among patients with NSCLC than with melanoma and kidney cancer, for all-grade (4.1%) and ≥grade 3 ICI-P (1.8%). The extent of involvement of the lungs in ICI-P is highest in the lower lungs, followed by the middle and upper lungs. In a clinical study by Myriam Delaunay (53), the average time required for the onset of ICI-P after introducing immunotherapy was 2.3 (0.2-27.4) months. A majority (42.2%) of patients developed ICI-P within < 2 months of introducing immunotherapy; the time to development of ICI-P was 2-4 months in 26.6%, 4-6 months in 17.2% and > 6 months in 14.1% of patients. Another study also showed that the onset of all grades of ICI-P was early, usually within 6 months of initiating immunotherapy, with higher-grade ICI-P occurring earlier than lower-grade ICI-P (19).
ICI-P is non-infectious pneumonitis, and its clinical manifestations are different from ordinary pneumonitis (54). A study by Myriam Delaunay showed that the most common symptoms of ICI-P were dyspnoea (80.3%) and cough (52.5%). Fever (32.8%) and asymptomatic conditions (6.6%) were less common (53). Another clinical study by Tomomi W Nobashi reported that one of the major symptoms of ICI-P was fever, lasting for a few days or more. They found that the symptoms with a higher incidence were fever (30%), dyspnoea (26%), low oxygen saturation (15%) and cough (15%), whereas those with a relatively low incidence were malaise (7%), rash (4%) and anorexia (4%). In addition, thyroid dysfunction and rashes were common in patients with and without ICI-P; however, the frequency of incidence was significantly higher in patients with ICI-P (21).
When ICI-P is suspected, clinicians should make accurate and rapid decisions, because ICI-P has characteristics that demand urgency in treatment. However, the clinical manifestations of ICI-P are diverse, and it is difficult to predict the occurrence of ICI-P before initiating treatment. CT of the chest plays a significant role in the diagnosis of ICI-P. Understanding the features of CT of the chest in ICI-P is important for prompt treatment. At present, the imaging classifications are mainly divided into the following categories: organising pneumonitis (OP), non-specific interstitial pneumonitis (NSIP), hypersensitivity pneumonitis (HP) and diffuse alveolar damage (DAD). DAD is also called acute respiratory distress syndrome (ARDS). The severity of these conditions is graded as follows: DAD>NSIP/HP>OP. In terms of incidence rate, OP has the highest incidence (65%), NSIP has a lower incidence (15%) and HP and DAD have the lowest incidence (10%) (55, 56). The radiological features of cryptogenic organising pneumonitis (COP) may be a sign of enhanced efficacy of ICIs (57). In addition, signs of ground-glass opacity (GGO), consolidations, traction bronchiectasis, nodular lesions, and reversed halo can be observed on CT. Among the five major types of signs, GGO is observed in a majority of patients, followed by consolidations. Although the reversed halo sign is rare, it is a typical finding in OP (18).
Pathological methods are becoming increasingly essential for the diagnosis of ICI-P. They can be used to rule out infectious pneumonitis and tumour progression. However, BAL and lung biopsy are not routinely performed in patients with ICI-P. In a retrospective study by Brandon T on 9 patients with ICI-P (58), OP was the most common histological pattern (7 patients). Among the 9 patients, 3 had concomitant ambiguous non-necrotising granulomas in the airway, and 2 presented with more acute symptoms, with histological changes indicating severe acute lung injury. In addition, 1 patient showed a pattern of acute fibrinous pneumonitis, and 1 patient with acute respiratory failure showed a pattern of acute and organising DAD. All 9 patients showed patchy accumulation of foamy macrophages in the airway and vacuolisation of type II pneumocytes. BAL has been used in a few studies on patients with NSCLC with ICI-P. Sabino Strippoli (59) analysed the characteristics of BAL in patients with melanoma with ICI-P and showed that cellular analysis using BAL revealed typical and homogeneous features with increased lymphoid population, relevant enrichment of CD8 + T cells and consequent inversion of the CD4/CD8 ratio. Moreover, the proportion of activated CD3 + HLA-DR + T cells was associated with the grading of adverse events. It has been reported that a major feature of BAL analysis in ICI-related NSCLC is an increase in the proportion of lymphocytes (60). The proportion of BAL lymphocytes, mainly CD4 + T cells, increases in ICI-P. An increase in the number of BAL central memory T (Tcm) cells, evidence of type I polarisation, and decreased expression of CTLA-4 and PD-1 in BAL Tregs indicate both activation of pro-inflammatory subpopulations and a weakened inhibitory phenotype. In a study, the myeloid immune population in BAL supernatants in ICI-P showed increased expression of IL-1β and decreased counter-regulation of IL-1RA expression, with increased levels of Tcm chemoattractants. These dysregulated immune cell subsets may represent possible targets for the treatment of pathological irAEs (50). Bronchoscopy plays an important role in the diagnosis of acute lung injury and fibrosis (61).
The diagnosis of ICI-P requires a comprehensive consideration of the clinical symptoms, as well as general bloodwork, CT imaging, and invasive evaluation (BAL or lung biopsy). Exclusion diagnosis is also an important strategy. Infectious pneumonitis, radiation pneumonitis (RP), tumour progression, carcinomatous lymphangitis, and pulmonary oedema caused by heart failure or myocarditis are common differential diagnoses. Establishing a diagnosis of ICI-P requires the exclusion of diseases mentioned in Table 3 (62–64). Among them, the presentation of RP and ICI-P is similar to that of interstitial pneumonitis. Therefore, it is difficult to distinguish clinically RP from ICI-P in patients who have undergone both radiotherapy to the chest and immunotherapy. However, there are some differences in terms of CT location distribution between the two types of pneumonitis. On CT of the chest, RP usually shows sharp margins; thin, dense plaques or streak-like changes in the lung ipsilateral to a lesion consistent with the extent of irradiation and to a lesion that is not consistent with the normal lung tissue structure (not distributed based on lung field or lung segment) (65). However, ICI-P is usually bilateral, involves multiple lung lobes and shows no sharp borders on CT, and the ICI-P area usually does not cross the lung fissures (66).
According to the latest National Comprehensive Cancer Network (NCCN) guidelines, ICI-P is divided into three levels and four grades as follows: mild (G1), moderate (G2) and severe (G3-4) (Table 4). Grade G1 refers to asymptomatic disease; ICI-P is confined to one lobe or < 25% of the lung parenchyma. Grade G2 marks the appearance of new symptoms such as shortness of breath, cough, chest pain, fever, and hypoxia; with the involvement of multiple lung lobes, affecting 25%-50% of the lung parenchyma; It affects daily life and requires drug intervention. Grade G3 refers to the appearance of serious new symptoms. It involves all lung lobes or > 50% of the lung parenchyma. Patients with G3 ICI-P have limited self-care ability and require oxygen supplementation. Grade G4 refers to life-threatening respiratory system damage, such as acute respiratory distressyndrome (ARDS), which requires emergency care (67).
Steroid therapy is a routine strategy for the management of ICI-P. Regular and sufficient use of steroids can help to treat 70—80% of patients with ICI-P (16). According to the current consensus on ICI-P treatment, steroid therapy should be initiated after a confirmed diagnosis of ≥G2 ICI-P. Patients with G1 ICI-P can be temporarily observed, and the use of ICIs should be suspended (1-2 weeks). However, if there are signs of progress of ICI-P, steroid therapy should be initiated. For patients with G2 ICI-P, prednisone/methylprednisolone at a dose of 1—2 mg/kg/day (Treatment until symptoms improve to ≤ grade 1, then taper over 4-6 weeks) is usually administered. For patients with G3-4, methylprednisolone at a dose of 1—2 mg/kg/day (taper over ≥6 weeks) is usually administered (67). Some patients are not sensitive to steroid therapy (no improvement after 48 hours for G3-4 ICI-P). This condition is usually called steroid-refractory pneumonitis (68), the criterion of which is mainly based on clinical symptoms and chest CT. In this case, the following treatments can be considered: 1) Intravenous administration of infliximab at a dose of 5 mg/kg, which can be repeated after 14 days at the discretion of a physician; 2) Intravenous injection of immunoglobulin; 3) Mycophenolate mofetil at a dose of 1-1.5 g twice daily (BID); the dosage can be gradually decreased over time (67, 69, 70). During treatment, the efficacy should be continuously monitored. If the infection has not been completely ruled out, empirical use of antibiotics should be considered. In terms of supportive treatment, clinicians should provide corresponding respiratory and systemic support to patients and actively deal with their complications. For reinitiating ICI treatment, a cohort study showed that after re-challenge with the same ICI, the recurrence rate of the same irAE associated with the discontinuation of ICI therapy was 28.8%. In such cases, clinicians can consider resuming ICI treatment for selected patients, who should be monitored appropriately (71, 72).
Some scholars have compared a large amount of research data and concluded that the occurrence of irAEs is directly proportional to the prognosis (73). The occurrence of irAEs indicates that immunotherapy has activated the immune system of patients. Patients with greater toxicity to immune drugs can attain better curative effects, leading to prolonged PFS and OS (20). In a meta-analysis (74), irAEs, especially endocrine, dermatologic and low-grade irAEs, were significantly associated with better ICI outcomes in patients with cancer. In addition, the development of irAEs was associated with the beneficial effects of treatment on survival in patients with cancer treated with PD-1 inhibitors but not in those treated with CTLA-4 inhibitors. Patients receiving immune monotherapy have more significant benefits than patients receiving combination therapy. Syed Hussaini reported a similar conclusion (75). In patients treated with ICIs, the occurrence of irAEs is positively correlated with objective response rate (ORR), PFS and OS but is not associated with the site of disease, type of ICIs and irAEs. Patients with ≥grade 3 ICI-P have better ORR but worse OS. However, some studies have reported more positive results, indicating that the OS of patients with multiple irAEs is significantly better than that of patients with a single irAE (76). Although studies have shown that endocrine, skin, and low-grade irAEs are associated with the efficacy of immunotherapy, some studies have reported that ICI-P can significantly improve recurrence-free survival (RFS) (77). Studies have also shown that the ORR and PFS of patients with ICI-P are significantly better than those of patients with irAE-non-ICI-P and non-irAEs (27). In a study by Shankar B, the PFS and OS of the ICI-P group were better than those of the non-ICI-P group (76). The incidence of low-level ICI-P can prolong PFS and OS, and increase ORR. High-grade ICI-P does not benefit OS but can help in achieving better ORR (75) (Table 5)
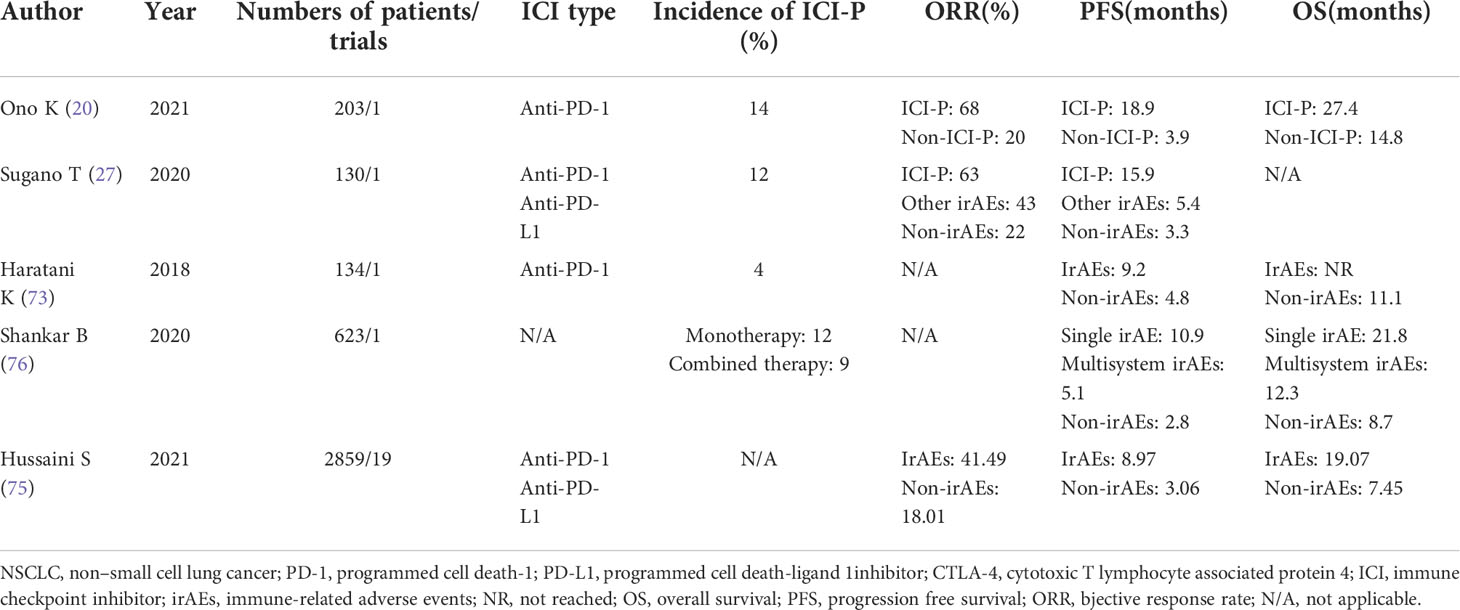
Table 5 Published researches on association between the occurrence of ICI-P and the outcome of immunotherapy in NSCLC.
The incidence of serious ICI-P after immunotherapy adversely affects the survival and quality of life of patients. The discovery and optimisation of predictive factors through laboratory tests can help clinicians to predict the occurrence of ICI-P, discontinue the use of ICIs in time or administer steroids early and may help in prolonging the survival of patients. A study showed that the expression of PD-L2 may be related to the incidence of irAEs in patients with NSCLC treated with PD-1 inhibitors. In addition, pre-existing autoimmunity markers such as the rheumatoid factor have been identified as independent predictors of skin reactions caused by ICIs (78). Thyroid dysfunction is more common in patients with anti-thyroid antibodies (79). However, to the best of our knowledge, studies have not reported the specific predictors of ICI-P. Therefore, relevant fundamental research is warranted to guide the application of clinical immunotherapy.
Clinicians should be cognizant of adverse reactions caused by ICIs, especially ICI-P. It is important to make an assessment before administering medications and pay attention to high-risk groups. After the administration of immunotherapeutic drugs, clinicians should pay close attention to changes in the condition of patients. Based on the combination of clinical manifestations, imaging data, and pathological characteristics of patients, ICI-P can be easily diagnosed, considering that infectious pneumonitis is excluded. ICI-P is mild in most cases and can be cured by appropriate treatment such as discontinuing immunotherapy or using steroids. If severe ICI-P occurs, immunotherapy should be promptly discontinued, and steroids and immunosuppressive therapy should be administered. Early intervention has a great impact on the survival and quality of life of patients. More studies are required for steroid-refractory pneumonitis, because, at present, an effective standard treatment plan is not available. In addition, further investigation is warranted to identify the predictors of ICI-P in the future.
All authors planned and wrote the manuscript and contributed to the article and approved of the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet (London England) (2010) 375(9722):1267–77. doi: 10.1016/s0140-6736(10)60059-1
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proc (2008) 83(5):584–94. doi: 10.4065/83.5.584
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
4. Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn MJ, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med (2019) 7(4):347–57. doi: 10.1016/s2213-2600(18)30500-9
5. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. New Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948
6. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. New Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
7. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/s0140-6736(16)32517-x
8. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J Natl Compr Cancer Network JNCCN (2019) 17(12):1464–72. doi: 10.6004/jnccn.2019.0059
9. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
10. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ (Clinical Res ed) (2018) 360:k793. doi: 10.1136/bmj.k793
11. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front Pharmacol (2017) 8:730. doi: 10.3389/fphar.2017.00730
12. Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer (2019) 10(10):2006–12. doi: 10.1111/1759-7714.13187
13. Ikeda S, Kato T, Kenmotsu H, Ogura T, Iwasawa S, Sato Y, et al. A phase 2 study of atezolizumab for pretreated NSCLC with idiopathic interstitial pneumonitis. J Thorac Oncol (2020) 15(12):1935–42. doi: 10.1016/j.jtho.2020.08.018
14. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest (2017) 152(2):271–81. doi: 10.1016/j.chest.2017.04.177
15. De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res (2017) 5(4):312–8. doi: 10.1158/2326-6066.Cir-16-0237
16. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: Benefits and pulmonary toxicities. Chest (2018) 154(6):1416–23. doi: 10.1016/j.chest.2018.08.1048
17. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol (2016) 2(12):1607–16. doi: 10.1001/jamaoncol.2016.2453
18. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/j.lungcan.2018.09.015
19. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors. J Thorac Oncol (2018) 13(12):1930–9. doi: 10.1016/j.jtho.2018.08.2035
20. Ono K, Ono H, Toi Y, Sugisaka J, Aso M, Saito R, et al. Association of immune-related pneumonitis with clinical benefit of anti-programmed cell death-1 monotherapy in advanced non-small cell lung cancer. Cancer Med (2021) 10(14):4796–804. doi: 10.1002/cam4.4045
21. Nobashi TW, Nishimoto Y, Kawata Y, Yutani H, Nakamura M, Tsuji Y, et al. Clinical and radiological features of immune checkpoint inhibitor-related pneumonitis in lung cancer and non-lung cancers. Br J radiology (2020) 93(1115):20200409. doi: 10.1259/bjr.20200409
22. Cathcart-Rake EJ, Sangaralingham LR, Henk HJ, Shah ND, Riaz IB, Mansfield AS. A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer (2020) 21(5):421–7.e2. doi: 10.1016/j.cllc.2020.04.003
23. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/s1470-2045(17)30380-7
24. Shaverdian N, Beattie J, Thor M, Offin M, Shepherd AF, Gelblum DY, et al. Safety of thoracic radiotherapy in patients with prior immune-related adverse events from immune checkpoint inhibitors. Ann Oncol (2020) 31(12):1719–24. doi: 10.1016/j.annonc.2020.09.016
25. Nakahama K, Tamiya A, Isa SI, Taniguchi Y, Shiroyama T, Suzuki H, et al. Association between imaging findings of airway obstruction adjacent to lung tumors and the onset of interstitial lung disease after nivolumab. In Vivo (Athens Greece) (2018) 32(4):887–91. doi: 10.21873/invivo.11324
26. Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-Small-Cell lung cancer. Clin Lung Cancer (2019) 20(4):e470–e9. doi: 10.1016/j.cllc.2019.02.018
27. Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer (2020) 11(4):1052–60. doi: 10.1111/1759-7714.13364
28. Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E, et al. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer (2021) 12(2):153–64. doi: 10.1111/1759-7714.13736
29. Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: Real-world incidence, risk factors, and management practices across six health care centers in north Carolina. Chest (2021) 160(2):731–42. doi: 10.1016/j.chest.2021.02.032
30. Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int immunopharmacol (2018) 63:292–8. doi: 10.1016/j.intimp.2018.08.014
31. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
32. Jung J, Kim HY, Kim DG, Park SY, Ko AR, Han JY, et al. Sequential treatment with an immune checkpoint inhibitor followed by a small-molecule targeted agent increases drug-induced pneumonitis. Cancer Res Treat (2021) 53(1):77–86. doi: 10.4143/crt.2020.543
33. Balasubramanian A, Onggo J, Gunjur A, John T, Parakh S. Immune checkpoint inhibition with chemoradiotherapy in stage III non-small-cell lung cancer: A systematic review and meta-analysis of safety results. Clin Lung Cancer (2021) 22(2):74–82. doi: 10.1016/j.cllc.2020.10.023
34. Chen X, Zhang Z, Hou X, Zhang Y, Zhou T, Liu J, et al. Immune-related pneumonitis associated with immune checkpoint inhibitors in lung cancer: a network meta-analysis. J Immunother Cancer (2020) 8(2):e001170. doi: 10.1136/jitc-2020-001170
35. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
36. Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the fleischner society. Chest (2021) 159(3):1107–25. doi: 10.1016/j.chest.2020.11.027
37. Moda M, Saito H, Kato T, Usui R, Kondo T, Nakahara Y, et al. Tumor invasion in the central airway is a risk factor for early-onset checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Thorac Cancer (2020) 11(12):3576–84. doi: 10.1111/1759-7714.13703
38. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. New Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
39. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. New Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
40. Li M, Spakowicz D, Zhao S, Patel SH, Johns A, Grogan M, et al. Brief report: inhaled corticosteroid use and the risk of checkpoint inhibitor pneumonitis in patients with advanced cancer. Cancer Immunol Immunother (2020) 69(11):2403–8. doi: 10.1007/s00262-020-02674-w
41. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
42. Soria JC, Marabelle A, Brahmer JR, Gettinger S. Immune checkpoint modulation for non-small cell lung cancer. Clin Cancer Res (2015) 21(10):2256–62. doi: 10.1158/1078-0432.Ccr-14-2959
43. Van Coillie S, Wiernicki B, Xu J. Molecular and cellular functions of CTLA-4. Adv Exp Med Biol (2020) 1248:7–32. doi: 10.1007/978-981-15-3266-5_2
44. Catacchio I, Scattone A, Silvestris N, Mangia A. Immune prophets of lung cancer: The prognostic and predictive landscape of cellular and molecular immune markers. Trans Oncol (2018) 11(3):825–35. doi: 10.1016/j.tranon.2018.04.006
45. Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, et al. CTLA-4 blockade drives loss of t(reg) stability in glycolysis-low tumours. Nature (2021) 591(7851):652–8. doi: 10.1038/s41586-021-03326-4
46. Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in ctla-4. Science (1995) 270(5238):985–8. doi: 10.1126/science.270.5238.985
47. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity (1999) 11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8
48. Liang J, Wang H, Ding W, Huang J, Zhou X, Wang H, et al. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci Adv (2020) 6(35):eabc3646. doi: 10.1126/sciadv.abc3646
49. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. New Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
50. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest (2019) 129(10):4305–15. doi: 10.1172/jci128654
51. Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Trans Med (2013) 5(215):215ra172. doi: 10.1126/scitranslmed.3006597
52. Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. [Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment]. Bull du cancer (2018) 105(11):1033–41. doi: 10.1016/j.bulcan.2018.07.005
53. Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J (2017) 50(2):1700050. doi: 10.1183/13993003.00050-2017
54. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
55. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin Cancer Res (2016) 22(24):6051–60. doi: 10.1158/1078-0432.Ccr-16-1320
56. Lin X, Deng H, Chen L, Wu D, Chen X, Yang Y, et al. Clinical types of checkpoint inhibitor-related pneumonitis in lung cancer patients: a multicenter experience. Transl Lung Cancer Res (2021) 10(1):415–29. doi: 10.21037/tlcr-20-1258
57. Cui P, Huang D, Wu Z, Tao H, Zhang S, Ma J, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol (2020) 12:1758835920922033. doi: 10.1177/1758835920922033
58. Larsen BT, Chae JM, Dixit AS, Hartman TE, Peikert T, Roden AC. Clinical and histopathologic features of immune checkpoint inhibitor-related pneumonitis. Am J Surg pathology (2019) 43(10):1331–40. doi: 10.1097/pas.0000000000001298
59. Strippoli S, Fucci L, Negri A, Putignano D, Cisternino ML, Napoli G, et al. Cellular analysis of bronchoalveolar lavage fluid to narrow differential diagnosis of checkpoint inhibitor-related pneumonitis in metastatic melanoma. J Transl Med (2020) 18(1):473. doi: 10.1186/s12967-020-02650-z
60. Naidoo J, Cottrell TR, Lipson EJ, Forde PM, Illei PB, Yarmus LB, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer (2020) 8(1):e000840. doi: 10.1136/jitc-2020-000840
61. Nishiyama O, Shimizu S, Haratani K, Isomoto K, Tanizaki J, Hayashi H, et al. Clinical implications of bronchoscopy for immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. BMC pulmonary Med (2021) 21(1):155. doi: 10.1186/s12890-021-01523-5
62. Clark AL, Cleland JG. Causes and treatment of oedema in patients with heart failure. Nat Rev Cardiol (2013) 10(3):156–70. doi: 10.1038/nrcardio.2012.191
63. Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, Muñoz-Montaño W, Nuñez-Baez M, Arrieta O. Radiation-induced lung injury: current evidence. BMC pulmonary Med (2021) 21(1):9. doi: 10.1186/s12890-020-01376-4
64. Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet (2021) 398(10303):906–19. doi: 10.1016/s0140-6736(21)00630-9
65. Ghaye B, Wanet M, El Hajjam M. Imaging after radiation therapy of thoracic tumors. Diagn interventional imaging (2016) 97(10):1037–52. doi: 10.1016/j.diii.2016.06.019
66. Pozzessere C, Lazor R, Jumeau R, Peters S, Prior JO, Beigelman-Aubry C. Imaging features of pulmonary immune-related adverse events. J Thorac Oncol (2021) 16(9):1449–60. doi: 10.1016/j.jtho.2021.05.017
67. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Cancer Network JNCCN (2020) 18(3):230–41. doi: 10.6004/jnccn.2020.0012
68. Balaji A, Hsu M, Lin CT, Feliciano J, Marrone K, Brahmer JR, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer (2021) 9(1):e001731. doi: 10.1136/jitc-2020-001731
69. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/jco.2017.77.6385
70. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
71. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol (2020) 6(6):865–71. doi: 10.1001/jamaoncol.2020.0726
72. Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol (2019) 5(9):1310–7. doi: 10.1001/jamaoncol.2019.1022
73. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-Small-Cell lung cancer. JAMA Oncol (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
74. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? a systematic review and meta-analysis. BMC Med (2020) 18(1):87. doi: 10.1186/s12916-020-01549-2
75. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - a systematic review and meta-analysis. Cancer Treat Rev (2021) 92:102134. doi: 10.1016/j.ctrv.2020.102134
76. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol (2020) 6(12):1952–6. doi: 10.1001/jamaoncol.2020.5012
77. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol (2020) 6(4):519–27. doi: 10.1001/jamaoncol.2019.5570
78. Takamori S, Takada K, Toyokawa G, Azuma K, Shimokawa M, Jogo T, et al. PD-L2 expression as a potential predictive biomarker for the response to anti-PD-1 drugs in patients with non-small cell lung cancer. Anticancer Res (2018) 38(10):5897–901. doi: 10.21873/anticanres.12933
Keywords: immune checkpoint inhibitor, immune checkpoint inhibitor-related pneumonitis, immune-related adverse events, immunotherapy, non-small cell lung cancer
Citation: Hao Y, Zhang X and Yu L (2022) Immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer: A review. Front. Oncol. 12:911906. doi: 10.3389/fonc.2022.911906
Received: 03 April 2022; Accepted: 19 July 2022;
Published: 16 August 2022.
Edited by:
Kohei Fujita, National Hospital Organization Kyoto Medical Center, JapanReviewed by:
Luke Mantle, Princess Margaret Cancer Centre, University Health Network, Toronto, CanadaCopyright © 2022 Hao, Zhang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yu, eXVsaW1haWwzNjlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.