- 1Department of Liver Surgery and Transplantation, Key Laboratory of Carcinogenesis and Cancer Invasion, Ministry of Education, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Nuclear Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 4Shanghai Medical College and Zhongshan Hospital Immunotherapy Technology Transfer Center, Fudan University, Shanghai, China
Brain metastasis from intrahepatic cholangiocarcinoma (iCCA) is extremely rare, and no standard therapeutic strategy has been established. Camrelizumab is a programmed cell death protein 1 (PD-1) inhibitor that has been widely studied in treating liver cancer. Combined immunotherapy and targeted therapy are a promising approach for treating advanced iCCA. Despite that immune checkpoint inhibitor (ICI)-based neoadjuvant therapy on iCCA has shown a significant response rate and resection rate, few reports have shown the therapeutic efficacy of immunotherapy in treating brain metastasis from iCCA. Although PD-1 inhibitors such as pembrolizumab, nivolumab, or camrelizumab are increasingly applied in clinic practice to treat multiple malignancies, to the best of our knowledge, we report the first case of an iCCA patient with brain metastasis successfully treated with a combined immunotherapy and targeted therapy. The patient is a 54-year-old man with metastatic iCCA in brain treated though camrelizumab plus lenvatinib therapy with a complete response (CR). By the time of writing, he has had a progression-free survival of 17.5 months and did not experience any severe side effects related to this therapy. Camrelizumab plus lenvatinib therapy showed favorable efficacy and manageable toxicity for this patient with advanced iCCA and could be of interest for more prospective randomized trials to further verify the potential clinical benefits.
Introduction
Cholangiocarcinoma (CCA) is the second most common primary liver cancer arising from the hepatic biliary system (1). It is a rare malignancy accounting for around 10%–20% of all hepatic malignancies and has poor prognosis as patients usually have metastatic diseases when being diagnosed (2). Regional lymph nodes and adjacent organs are the most common metastatic sites for CCA (3). However, CCA with brain metastasis is extremely rare and the prognosis is very poor (4). In treating these patients, no treatment guidelines exist to date and common therapeutic strategies include surgical excision, radiation, and chemotherapy (1).
Advanced CCA remains a difficult-to-treat disease, and therapy remains palliative. Although chemotherapy with gemcitabine and cisplatin is considered as the standard first-line treatment for unresectable patients with advanced biliary tract cancer, the median overall survival is less than 1 year (5). Targeted therapies such as using isocitrate dehydrogenase 1 or 2 (IDH1 or 2) inhibitors and fibroblast growth factor receptor (FGFR) 2 have also been rapidly translated into promising therapeutic strategies in treating advanced CCA (6, 7). As a multitargeted kinase inhibitor, lenvatinib (E7080) can target PDGFR-b, VEGFR1-3, FGFR1–4, KIT, and RET (8). The published phase II clinical trials include lenvatinib with sorafenib in hepatocellular carcinoma (NCT01761266) and lenvatinib as monotherapy for treating unresectable biliary cancer (NCT02579616).
Treating biliary tract cancer with immune checkpoint inhibitors (ICIs) is the largest area of ongoing research among all immunotherapy strategies. ICIs combined with other therapies including chemotherapy, additional immunotherapies, and targeted therapies have all been approaches to increase response rates and improve outcomes (9). A recent study of Lin et al. demonstrated that pembrolizumab combined with lenvatinib is potentially effective and tolerable as a systemic therapy for patients with refractory bile tract carcinoma (10). Camrelizumab is a humanized monoclonal antibody against PD-1 which can block the PD-1/PD-L1 interaction and thus suppress the immune evasion of malignant cancer (11). Phase I trials have demonstrated positive antitumor effects of camrelizumab among patients with advanced solid tumor (12, 13). Here, we report the first case of a patient with intrahepatic cholangiocarcinoma (iCCA) brain metastasis successfully treated by camrelizumab plus lenvatinib combination therapy.
Case report
A 54-year-old male patient with a history of chronic hepatitis B for more than 30 years was referred for a liver lesion detected by abdominal ultrasound because of abdominal pain. Enhanced abdominal magnetic resonance imaging (MRI) presented one tumor lesion (with a size of 6.5 × 6 × 5 cm) in segments V of liver (Figures 1A, B). Additionally, positron emission tomography-computed tomography (PET-CT) presented no extrahepatic metastasis of this patient. Laboratory tests showed that the tumor markers alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen (CA) 19.9 were negative and physical examination showed no remarkable findings. After a discussion with the medical team, our team performed special segmental hepatectomy combined with cholecystectomy and skeletal dissection of hepatoduodenal ligament together in July 2020. Tumor biopsies from resected liver lesions revealed a poorly differentiated intrahepatic sarcomatoid cholangiocarcinoma (ISSC), which is a rare histopathology classification of iCCA (Figure 1C). Moreover, histochemistry staining showed positivity for cytokeratin (CK), CK19, CK7, CD34, Ki67 (50% positive rate, Figure 1D), and β-catenin (Figure 1E) and negativity for AFP and hepatocyte specific antigen (Hep-Par-1). Chemotherapy with capecitabine was initiated in the second month after surgery, and no recurrence of the primary tumor had been found at the last follow-up in February 2022 (Figure 1F).
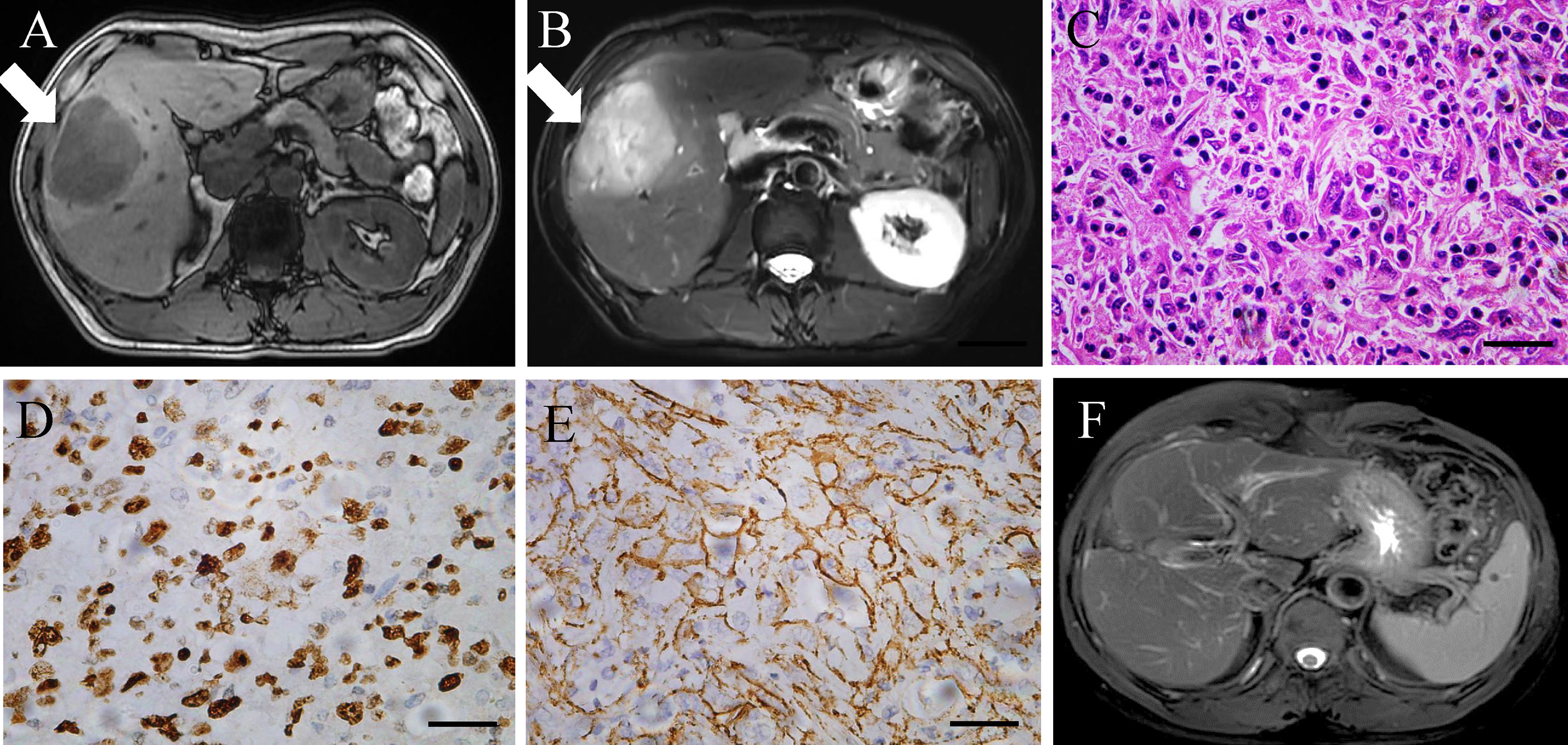
Figure 1 (A, B) Enhanced MRI scan of the liver lesion before surgery. (C–E) Histopathological finding (hematoxylin and eosin staining, Ki67, and β-catenin), poorly differentiated intrahepatic sarcomatoid cholangiocarcinoma. Magnification, ×400. Scale bar: 50 µm. (F) MRI scan of the liver lesion after surgery in February 2022.
Three months after surgery in October 2021, this patient complained weakness of left upper limb and left lower limb. Intracranial hypertension was diagnosed for this patient, and mannitol was applied to treat the increased intracranial pressure. In July 2021 before the liver surgery, no extrahepatic metastasis was found in the brain of this patient (Figure 2A). However, on this presentation, PET-CT revealed a 1.1 cm × 0.6 cm mass in his right frontal lobe (Figure 2B). As a result, our multidisciplinary team (MDT) diagnosed that the patient had brain metastasis of iCCA. According to the whole-exome sequencing (WES) performed on the resected tumor tissue, the tumor genomics of this patient exhibited mutations in KIT, TP53, and PDGFR. Considering no considerable evidence proving the effectiveness of operation and radiotherapy in treating patients with brain metastasis from iCCA, we decided to initiate the systemic treatment with camrelizumab (200 mg every 3 weeks) plus lenvatinib (8 mg orally once daily) therapy in October 2020.
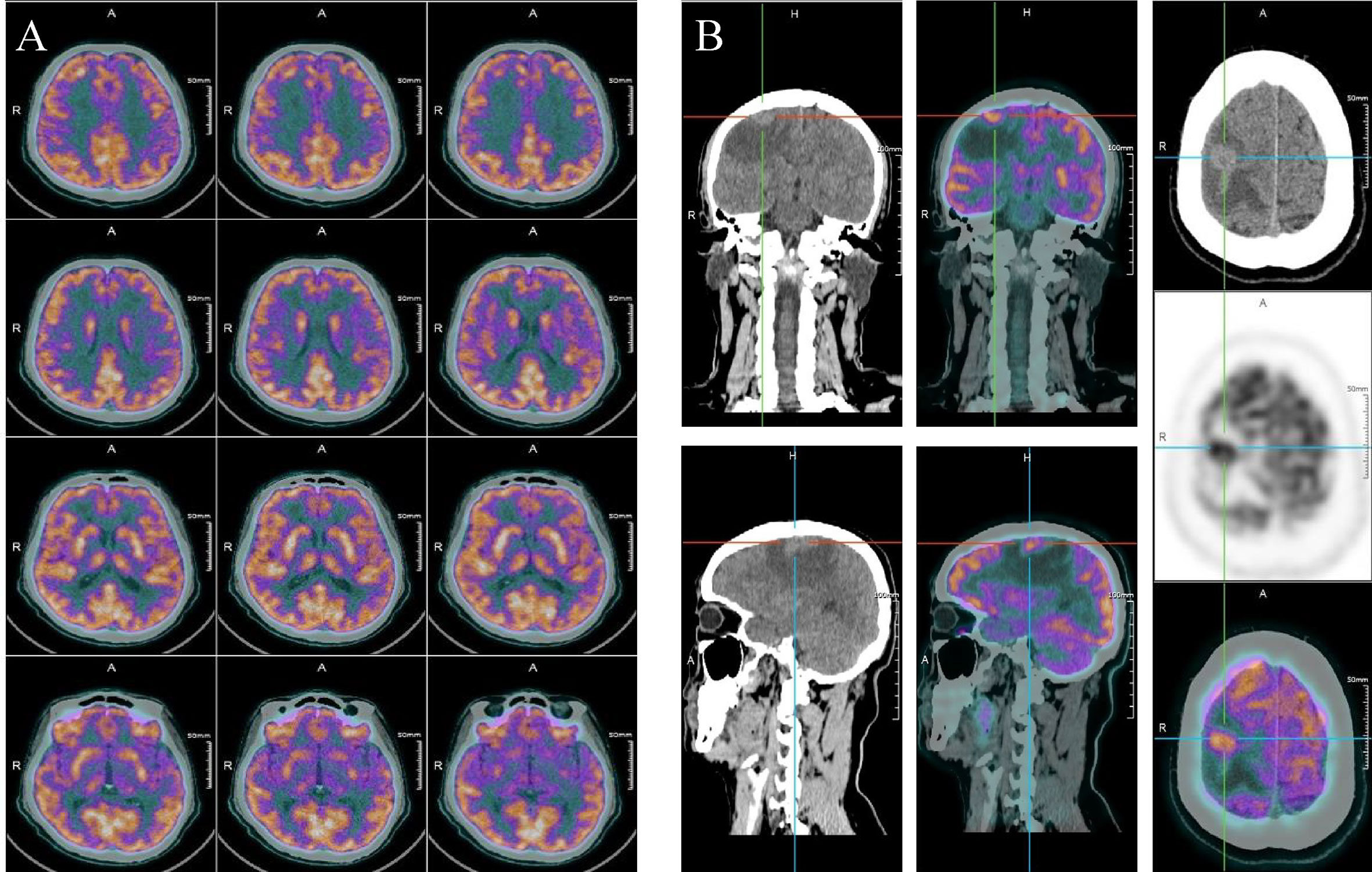
Figure 2 (A) PET-CT scan of the brain before liver surgery. (B) PET-CT scan of the brain 3 months after liver surgery.
After the first three-cycle treatment (approximately 2 months), the general condition of this patient remained stable, and the brain MRI presented a 0.8 × 0.7 mass in the right frontal lobe and little amount of surrounding edema (Figure 3A). In the following treatment cycles, this patient was followed up regularly. During this period, the previous metastatic lesions in the brain regressed gradually until complete regression was examined after 16-cycle treatments in September 2021 (Figure 3B). Additionally, abdominal and brain MRI presented no recurrence of the primary tumor and no occurrence of new focus. Overall, from June 2020 to February 2022, this patient admitted to our hospital twice. Although during the first admission, we successfully excised his liver tumor through surgery, the brain metastatic lesion was first found after 3 months. Fortunately, immunotherapy plus targeted therapy was effective and well-tolerated until the tumor lesion in the brain totally disappeared (Figures 3C, D).
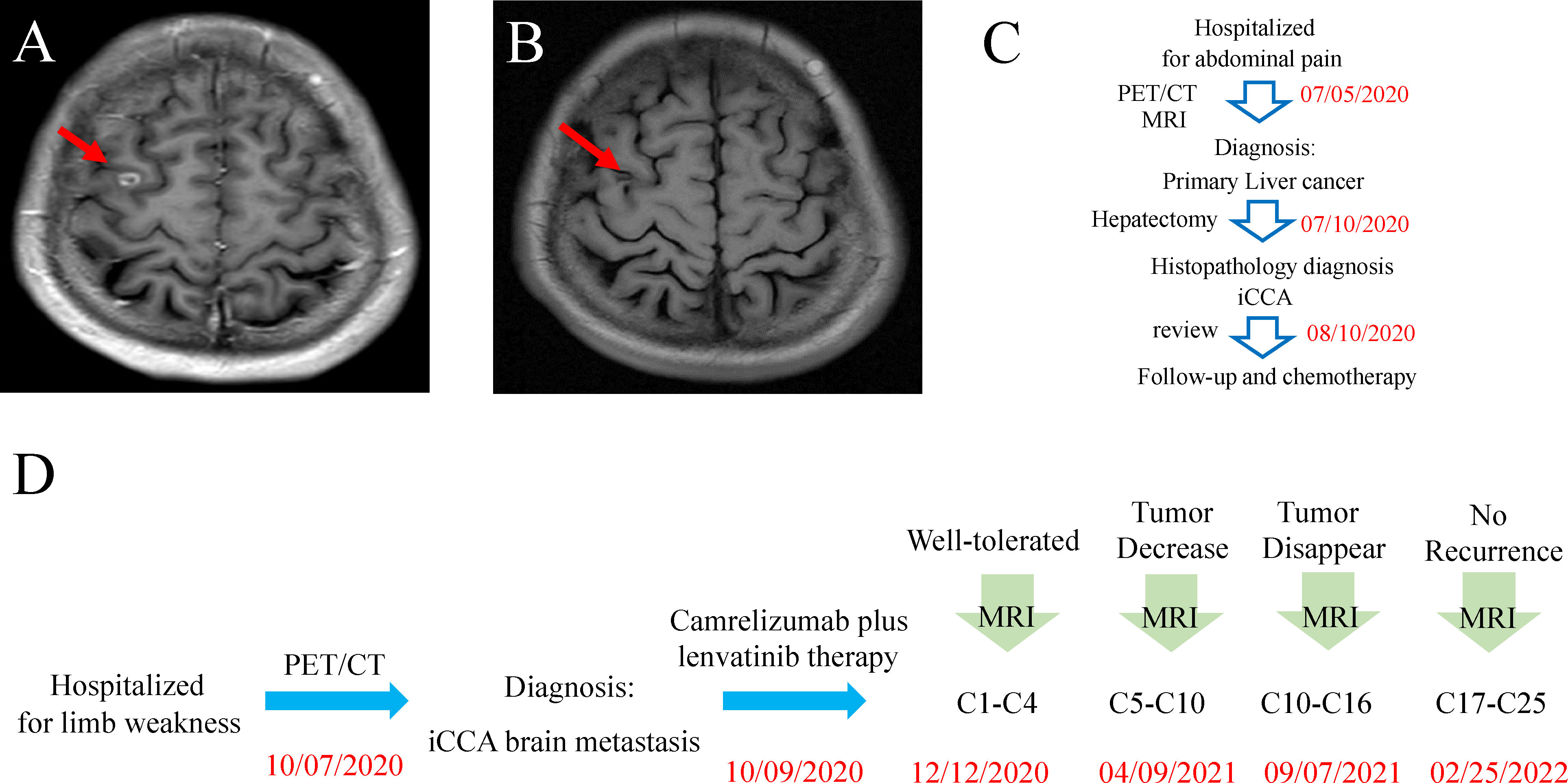
Figure 3 (A) Brain MRI scan after treatment with camrelizumab and lenvatinib for 4 cycles. (B) Brain MRI scan after treatment with camrelizumab and lenvatinib for 16 cycles. (C) Timeline of the disease course and treatment before brain metastasis. (D) Timeline of the disease course and treatment after brain metastasis.
By the time of writing, the patient had achieved a progression-free survival of 17.5 months since initiating the combination therapy. During this period, the patient remained a relatively stable physical state throughout the treatment and no evidence of active disease and clinically relevant adverse event was found.
Discussion
In our article, we report a rare case with brain metastasis from iCCA and the metastatic lesion in the brain had a complete response to PD1 inhibitor camrelizumab plus lenvatinib combination therapy. After the 16-cycle PD1 inhibitor treatment, the metastatic lesion in the brain disappeared totally and the condition of this patient remained stable. Considering the low incidence of brain metastasis in iCCA patients, more of these patients are expected to be included in the future clinical trials to explore more effective and reliable therapeutic strategies. Thus, our report will supplement the existing literatures and provide valuable evidence for PD1 inhibitors in treating iCCA patients with brain metastasis.
Cholangiocarcinoma (CCA) consists of various malignancies occurring in the biliary tree. iCCA is a subtype of CCA and accounts for around 10%–20% of all CCAs (14). The prognosis for patients with iCCA is very poor, and the average 5-year overall survival (OS) rate is between 5% and 10% for patients with advanced iCCA (15). One of the most important reasons for causing the poor prognosis is the limited efficacy of traditional systemic and locoregional treatments such as chemotherapy and radiotherapy (16). To treat patients with advanced iCCA, gemcitabine plus cisplatin is considered as the first-line systemic therapy regimen recommended in most treatment guidelines (NCCN, CSCO, and ESMO) (17). However, traditional systemic chemotherapy has limited therapeutic efficacy in treating metastatic brain tumor because of poor penetration of the blood–brain barrier (BBB) (18). At present, the most effective way to treat brain metastasis is surgery, and whole-brain radiation therapy (WBRT) can be considered for those not suitable for surgery (19). Additionally, targeted therapy also showed some value for treating brain metastasis in some kinds of tumor such as osimertinib in epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) (20) and lapatinib in patients with HER2-positive breast cancer brain metastases (21, 22).
Immune therapy through ICIs in treating brain metastasis has also shown some promising therapeutic efficacy, examples including brain metastases from melanoma and lung cancer (23, 24). Ipilimumab was the first ICI in treating brain metastasis from melanoma. In an open-label, multicenter, phase II study, the rate of intracranial clinical benefit was 57% with nivolumab plus ipilimumab treatment for melanoma patients with brain metastasis (24). Another phase II study also showed that ipilimumab has therapeutic effects in some patients with advanced melanoma and brain metastases, particularly when metastases are asymptomatic and small (25). Moreover, a monoclonal antibody called pembrolizumab was investigated in a group of patients with asymptomatic brain metastases, and researchers found that 26% of patients had a response both intracranially and extracranially in a year (26). There are also some studies that focused on the treatment efficacy of ICIs in lung cancer. In a phase II trial, 42 patients were treated with pembrolizumab and researchers found that it has activity in brain metastases from NSCLC and can result in prolonged survival in a group of patients (27). In another large study, 155 patients had brain metastasis and 89 of them had NSCLC. Researchers found that patients treated with stereotactic radiosurgery (SRS) and ICIs together had better response rates and response durability compared with those treated with SRS and delayed ICIs (28).
However, one question which has to be considered when treating brain metastasis with ICIs or with most of other anticancer agents is the fact that several barriers in the central nervous system (CNS) can limit the access of monoclonal antibodies (mAbs). The BBB, the blood–tumor barrier (BTB), and the blood–cerebrospinal fluid (CSF) barrier are the three major barriers limiting systemic drug delivery. The BBB can selectively prevent certain substances from entering the brain from blood so that large anticancer agents such as mAbs cannot cross a normal BBB. Although the superior therapeutic efficacy of some targeted agents and ICIs has been reported in some clinical trials, limited information showed the capacity to cross CNS barriers and exert their pharmacodynamic effect. This fact hampers the further investigation about the reasons for the failure or success of these drugs applied in clinical settings (18). Although the brain is believed to be secluded from peripheral immune activity for decades, this perception was revised recently with the findings that higher functions, homeostasis, and repair of the CNS are supported by peripheral innate and adaptive immune cells (29). Actually, the mechanisms related to the priming and recruitment of T cells to the CNS are only partially understood. One would ask whether ICI-treated CD8+ T cells can be recruited to the brain metastasis and killed the tumor cells. This is a conceivable scenario because CNS-reactive T cells might be activated in the peripheral immune compartment by non-CNS antigens that from peripheral sites (30).
In this case, our patient with iCCA brain metastasis was treated with camrelizumab 200 mg intravenously every 3 weeks plus lenvatinib (8 mg orally once daily) and this treatment showed favorable efficacy. The rationale for combining lenvatinib with PD1 inhibitors is based on the ability of lenvatinib to inhibit the proneoangiogenic and immunosuppressive effects of tumor microenvironments; such inhibition would improve the clinical benefit of PD-1 antibodies by enhancing the antitumor immune response (31, 32). In a phase Ib study accessing the efficacy and safety of lenvatinib in combination with pembrolizumab in 13 evaluable patients with unresectable HCC (33), no new adverse event was identified, with a partial response (PR) rate of 46% (6/13). Notably, Chen et al. reported the first use of nivolumab plus lenvatinib to successfully treat recurrent, progressive, metastatic cholangiocarcinoma (34). Additionally, pembrolizumab, nivolumab, and three PD-1 antibodies manufactured in China (camrelizumab, sintilimab, and toripalimab) have been approved by the National Medical Products Administration (NMPA) of China. The price of the three PD-1 antibodies manufactured by local pharmaceutical companies is about one-third that of nivolumab or pembrolizumab (less than 2,000 US dollars per month). Clinical trials have accessed the efficacy and safety of camrelizumab in treating primary liver cancer patients (35–37). In this way, camrelizumab might be a good choice for patients who need immunotherapy. However, brain metastasis from iCCA is extremely rare. To the best of our knowledge, the efficacy of PD-1 inhibitors in treating solid central nervous system metastasis from iCCA is seldom reported. In our report, this patient had a symptomatic brain metastasis and the focus was recognized 3 months after liver surgery. After discussion of our MDT, we initiated immunotherapy combined with targeted therapy to treat this patient. Intriguingly, this patient is well-tolerated to this therapy and after 16 cycles’ treatment, the metastatic tumor in the brain disappeared totally. Until the last follow-up in February 2022, no new metastatic lesions and recurrence was found. This case provides a proof of principle that brain metastatic lesions from iCCA are accessible to the action of immunotherapy and that immunotherapy combined with targeted therapy may have a promising effect.
One limitation of this case is that we proceeded with treating the brain lesion as a presumptive metastasis without acquiring a histological verification. The time interval between iCCA diagnosis and brain tumor lesion occurrence was only within 3 months. As a general rule, brain metastases should be suspected in any patient with known systemic cancer in whom neurological findings develop (38). On the basis of the imaging characteristics of PET/CT and brain MRI, the new tumor lesion appearing in this period can be identified as liver cancer brain metastasis, but not specific enough for definitive diagnosis because histopathological analysis of tissue harvested at surgical resection remains the gold standard for diagnosis (38, 39). Compared with the invasive surgical resection strategy, our MDT considered PD1 inhibitor plus targeted drug combination therapy might be the best choice.
Solid central nervous system (CNS) metastasis from iCCA is rare, and together with this case, there are currently only 20 cases having been reported in literature (Table 1) (40–48). The ages of these patients range from 46 to 75 (mean 61.1 years), and the male: female ratio is 1:1.9. Among these 20 cases, the median overall survival was 5.7 months, which was much shorter than that of brain metastasis in other malignancies (49). Additionally, the time interval from the diagnosis of iCCA to the discovery of brain metastasis extended up to 3.5 years (median 8 months). Apart from three patients who did not receive any treatment, the therapeutic strategies in these cases were distinct. Generally, 12 of these patients underwent tumor resection of liver and only four of them further accepted craniotomy whereas nearly all of them (11 cases) underwent adjuvant therapy such as WBRT, chemotherapy, targeted therapy, or immunotherapy. For these 12 cases, the median overall survival was 6.2 months, longer than that in all of these 20 cases. Further, the five left cases who just accepted adjuvant therapy such as chemotherapy, WBRT, or laser interstitial thermal therapy (LITT) only achieved moderated therapeutic efficacy. Notably, although among all of these 20 cases, only two patients used the combined immunotherapy and targeted therapy, the survival time of these two patients was both longer than 16 months and the follow-up of them was still continue at the time of reporting. However, more evidence is expected to identify the efficacy of combined immunotherapy and targeted therapy in treating iCCA brain metastasis in future clinical trials.

Table 1 Case reports summary of the iCCA patients with brain metastasis (iCCA: intrahepatic cholangiocarcinoma).
Generally, our clinical case describes a patient with a complete regression of the metastatic brain tumor from iCCA after surgical resection of the primary focus and combination therapy with camrelizumab and lenvatinib. In treating brain metastasis from iCCA, we suggest it important to prevent the recurrence of the primary tumor with early employment of immunotherapy-based combination therapy to keep the activation of the surveillance of immune system.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was funded by the National Nature Science Foundation of China (82102959, 82172739, 81871924, 82072666, 81802893, and 81972829), Shanghai Natural Science Foundation (21ZR1481900), Beijing Xisike Clinical Oncology Research Foundation (Y-Roche2019/2-0037), Beijing iGandan Foundation (GDXZ-08-06), and Zhongshan Talent Development Program (2021ZSYQ11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Novegno F, Umana G, Granaroli P, Borri F, Orlandi A, Lunardi P. Current management of central nervous system metastasis from cholangiocarcinoma: the neurosurgical perspective. literature review. Br J Neurosurg (2020) 34:575–83. doi: 10.1080/02688697.2019.1639614
2. Alsaleh M, Leftley Z, Barbera TA, Sithithaworn P, Khuntikeo N, Loilome W, et al. Cholangiocarcinoma: a guide for the nonspecialist. Int J Gen Med (2019) 12:13–23. doi: 10.2147/IJGM.S186854
3. Chindaprasirt P, Promsorn J, Ungareewittaya P, Twinprai N, Chindaprasirt J. Bone metastasis from cholangiocarcinoma mimicking osteosarcoma: a case report and review literature. Mol Clin Oncol (2018) 9:532–4. doi: 10.3892/mco.2018.1720
4. Kilbourn KJ, Aferzon J, Menon M. Isolated brain metastasis in cholangiocarcinoma: a case report and review of literature. Conn Med (2014) 78:161–2.
5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
6. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72:353–63. doi: 10.1016/j.jhep.2019.10.009
7. Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase ii study of bgj398 in patients with fgfr-altered advanced cholangiocarcinoma. J Clin Oncol (2018) 36:276–82. doi: 10.1200/JCO.2017.75.5009
8. Montella L, Palmieri G, Addeo R, Del PS. Hepatocellular carcinoma: will novel targeted drugs really impact the next future? World J Gastroenterol (2016) 22:6114–26. doi: 10.3748/wjg.v22.i27.6114
9. Jakubowski CD, Azad NS. Immune checkpoint inhibitor therapy in biliary tract cancer (cholangiocarcinoma). Chin Clin Oncol (2020) 9:2. doi: 10.21037/cco.2019.12.10
10. Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr (2020) 9:414–24. doi: 10.21037/hbsn-20-338
11. Markham A, Keam SJ. Camrelizumab: first global approval. Drugs. (2019) 79:1355–61. doi: 10.1007/s40265-019-01167-0
12. Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose shr-1210, an anti-pd-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. (2018) 119:538–45. doi: 10.1038/s41416-018-0100-3
13. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-pd-1 antibody shr-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res (2019) 25:515–23. doi: 10.1158/1078-0432.CCR-18-2484
14. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the european network for the study of cholangiocarcinoma (ens-cca). Nat Rev Gastroenterol Hepatol (2016) 13:261–80. doi: 10.1038/nrgastro.2016.51
15. Al MA, Bouvier V, Menahem B, Bazille C, Fohlen A, Alves A, et al. Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the french population. Eur J Gastroenterol Hepatol (2019) 31:678–84. doi: 10.1097/MEG.0000000000001337
16. Banales JM, Marin J, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol (2020) 17:557–88. doi: 10.1038/s41575-020-0310-z
17. Kruger D. Responses to health, safety survey; top management plays crucial role. Occup Health Saf. (1987) 56:57–60.
18. Soffietti R, Ahluwalia M, Lin N, Ruda R. Management of brain metastases according to molecular subtypes. Nat Rev Neurol (2020) 16:557–74. doi: 10.1038/s41582-020-0391-x
19. Xiao L, Lin C, Liu Y, Wu Y, Wang J. Case report: immune checkpoint inhibitors successfully controlled asymptomatic brain metastasis in esophageal squamous cell carcinoma. Front Immunol (2022) 13:746869. doi: 10.3389/fimmu.2022.746869
20. Akamatsu H, Toi Y, Hayashi H, Fujimoto D, Tachihara M, Furuya N, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with egfr t790m-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: west japan oncology group 8715l phase 2 randomized clinical trial. JAMA Oncol (2021) 7:386–94. doi: 10.1001/jamaoncol.2020.6758
21. Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in her2-positive metastatic breast cancer previously treated with >/= 2 her2-directed regimens: phase iii nala trial. J Clin Oncol (2020) 38:3138–49. doi: 10.1200/JCO.20.00147
22. Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from her2-positive metastatic breast cancer (landscape): a single-group phase 2 study. Lancet Oncol (2013) 14:64–71. doi: 10.1016/S1470-2045(12)70432-1
23. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol (2016) 17:976–83. doi: 10.1016/S1470-2045(16)30053-5
24. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med (2018) 379:722–30. doi: 10.1056/NEJMoa1805453
25. Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol (2012) 13:459–65. doi: 10.1016/S1470-2045(12)70090-6
26. Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase ii trial. J Clin Oncol (2019) 37:52–60. doi: 10.1200/JCO.18.00204
27. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with nsclc and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21:655–63. doi: 10.1016/S1470-2045(20)30111-X
28. Kotecha R, Kim JM, Miller JA, Juloori A, Chao ST, Murphy ES, et al. The impact of sequencing pd-1/pd-l1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol (2019) 21:1060–8. doi: 10.1093/neuonc/noz046
29. Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol (2021) 22:1083–92. doi: 10.1038/s41590-021-00994-2
30. Korn T, Kallies A. T Cell responses in the central nervous system. Nat Rev Immunol (2017) 17:179–94. doi: 10.1038/nri.2016.144
31. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
32. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-pd-1 antibody combination treatment activates cd8+ t cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PloS One (2019) 14:e212513. doi: 10.1371/journal.pone.0212513
33. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
34. Chen WX, Li GX, Hu ZN, Zhu P, Zhang BX, Ding ZY. Significant response to anti-pd-1 based immunotherapy plus lenvatinib for recurrent intrahepatic cholangiocarcinoma with bone metastasis: a case report and literature review. Med (Baltimore). (2019) 98:e17832. doi: 10.1097/MD.0000000000017832
35. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol (2020) 21:571–80. doi: 10.1016/S1470-2045(20)30011-5
36. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (rescue): a nonrandomized, open-label, phase ii trial. Clin Cancer Res (2021) 27:1003–11. doi: 10.1158/1078-0432.CCR-20-2571
37. Wang D, Yang X, Long J, Lin J, Mao J, Xie F, et al. The efficacy and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: a prospective clinical study. Front Oncol (2021) 11:646979. doi: 10.3389/fonc.2021.646979
38. Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. (2019) 5:5. doi: 10.1038/s41572-018-0055-y
39. Usinskiene J, Ulyte A, Bjornerud A, Venius J, Katsaros VK, Rynkeviciene R, et al. Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology. (2016) 58:339–50. doi: 10.1007/s00234-016-1642-9
40. Gudesblatt MS, Sencer W, Sacher M, Lanzieri CF, Song SK. Cholangiocarcinoma presenting as a cerebellar metastasis: case report and review of the literature. J Comput Tomogr. (1984) 8:191–5. doi: 10.1016/0149-936x(84)90059-6
41. Miyamoto J, Tatsuzawa K, Sasajima H, Mineura K. Metastatic skull tumor from cholangiocarcinoma. case report. Neurol Med Chir (Tokyo). (2007) 47:132–5. doi: 10.2176/nmc.47.132
42. Mimatsu K, Oida T, Kawasaki A, Kano H, Fukino N, Kida K, et al. Long-term survival after resection of mass-forming type intrahepatic cholangiocarcinoma directly infiltrating the transverse colon and sequential brain metastasis: report of a case. Surg Today (2011) 41:1410–3. doi: 10.1007/s00595-010-4500-0
43. Chindaprasirt J, Sookprasert A, Sawanyawisuth K, Limpawattana P, Tiamkao S. Brain metastases from cholangiocarcinoma: a first case series in thailand. Asian Pac J Cancer Prev (2012) 13:1995–7. doi: 10.7314/apjcp.2012.13.5.1995
44. Mirrakhimov AE, Nwankwo N, Zdunek T, Bucher N. Cholangiocarcinoma and brain lesions: an extremely rare finding. BMJ Case Rep (2013) 2013:bcr2013009235. doi: 10.1136/bcr-2013-009235
45. Frega G, Garajova I, Palloni A, Barbera MA, Trossello PM, Faccioli L, et al. Brain metastases from biliary tract cancer: a monocentric retrospective analysis of 450 patients. Oncology. (2018) 94:7–11. doi: 10.1159/000479929
46. Fujimoto K, Kuroda J, Makino K, Hasegawa Y, Kuratsu J. Skull metastasis from intrahepatic cholangiocarcinoma: report of 3 cases and review of the literature. Neurol Med Chir (Tokyo). (2013) 53:717–21. doi: 10.2176/nmc.cr2012-0237
47. Tan SK, Luther E, Eichberg D, Shah A, Khan K, Jamshidi A, et al. Complete regression of a solitary cholangiocarcinoma brain metastasis following laser interstitial thermal therapy. World Neurosurg (2020) 144:94–8. doi: 10.1016/j.wneu.2020.08.122
48. Zeng JT, Zhang JF, Wang Y, Qing Z, Luo ZH, Zhang YL, et al. Intrahepatic cholangiocarcinoma is more complex than we thought: a case report. World J Clin Cases. (2021) 9:1469–74. doi: 10.12998/wjcc.v9.i6.1469
Keywords: brain metastasis, advanced intrahepatic cholangiocarcinoma (iCCA), immune checkpoint inhibitors (ICIs), PD1, immunotherapy
Citation: Xie P, Guo L, Zhang B, Xu Y, Song Q, Shi H, Ye Q, Li H and Xiao Y (2022) Case report: immunotherapy successfully treated brain metastasis in intrahepatic cholangiocarcinoma and literature review. Front. Oncol. 12:911202. doi: 10.3389/fonc.2022.911202
Received: 19 April 2022; Accepted: 05 July 2022;
Published: 03 August 2022.
Edited by:
Dooil Jeoung, Kangwon National University, South KoreaReviewed by:
Ugur Sener, Mayo Clinic, United StatesMarion Tonneau, University of Montreal Hospital Centre (CRCHUM), Canada
Copyright © 2022 Xie, Guo, Zhang, Xu, Song, Shi, Ye, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongsheng Xiao, eGlhby55b25nc2hlbmdAenMtaG9zcGl0YWwuc2guY24=; Hui Li, bGkuaHVpMUB6cy1ob3NwaXRhbC5zaC5jbg==
 Peiyi Xie1
Peiyi Xie1 Lei Guo
Lei Guo Bo Zhang
Bo Zhang Yongfeng Xu
Yongfeng Xu Qinghai Ye
Qinghai Ye Hui Li
Hui Li