
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 29 June 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.909064
 Giulia Pasello1,2*
Giulia Pasello1,2* Martina Lorenzi1,2
Martina Lorenzi1,2 Giulia Pretelli1,2
Giulia Pretelli1,2 Giovanni Maria Comacchio3
Giovanni Maria Comacchio3 Federica Pezzuto4
Federica Pezzuto4 Marco Schiavon3
Marco Schiavon3 Alessandra Buja5
Alessandra Buja5 Stefano Frega2
Stefano Frega2 Laura Bonanno2
Laura Bonanno2 Valentina Guarneri1,2
Valentina Guarneri1,2 Fiorella Calabrese4†
Fiorella Calabrese4† Federico Rea3†
Federico Rea3†Background: Osimertinib is considered the standard-of-care for previously-untreated EGFR mutant advanced non-small cell lung cancer (NSCLC). Oncogene driver screening in early NSCLC is not standard practice. A real-world study has been designed in order to investigate the optimal testing frequency and timing for EGFR mutations in early NSCLC in clinical practice.
Patients and Methods: The present observational, retrospective study evaluated the real-world diagnostic-therapeutic pathway and clinical outcomes of 225 patients with stage I-III NSCLC, with particular reference to the EGFR-mutant subgroup.
Results: Prior to surgery, 101 patients had undergone a diagnostic biopsy; EGFR mutational analysis was available in 56 (55%) patients and 12 patients (21%) had a cancer harboring an EGFR mutation. Among surgical specimens, reflex EGFR test was performed in 181 (80%) of 225 and 35 cases (19%) were EGFR mutant. The majority of patients had not received adjuvant chemotherapy (N=174, 77%) or adjuvant radiotherapy (N=201, 89%). Of 49 (22%) patients experiencing disease relapse, 26 (53%) received first-line systemic treatment. All EGFR-mutant relapsed patients (N=6, 12.2%) received an EGFR-TKI. Median overall survival (OS) and relapse-free survival for the entire population were not reached. Multivariate analysis for OS confirmed a significant correlation with age, female gender, EGFR status, necrosis score, perineural invasion, and relapsed disease. EGFR test costs represented 1.6-2.4% of the total costs of management per patient (€34,340).
Conclusions: Our results suggest that the frequency of EGFR mutations in early stage (I-III) NSCLC is similar to that of advanced stages. Reflex EGFR testing in all early-stage NSCLC at diagnosis or after surgery appears to be a valid tool to give patients the chance to benefit from targeted adjuvant treatment.
Overall, roughly one-third of non-small cell lung cancers (NSCLC) have a mutation in the epidermal growth factor receptor (EGFR) gene, although distinct geographical differences have been reported, with the lowest in Europe and the highest in Asia (1). The majority of mutations in EGFR comprise deletion of exon 19 and point mutations consisting of L858R, residing in Exon 21 (2, 3), with the remainder being uncommon mutations (4, 5). The increasing use of next-generation sequencing (NGS) and other methods in routine practice has led to an enhanced ability to detect both common and rare variants (6). Improved knowledge of the mutational spectrum of EGFR in NSCLC has also allowed for better targeting of the available tyrosine kinase inhibitors to the individual patient (6).
EGFR-tyrosine kinase inhibitors (EGFR-TKIs) are among the front-line agents used for EGFR-mutant NSCLC, and several first-, second-, and third-generation EGFR-TKIs have been developed, starting from gefitinib (7, 8). Compared with first-generation EGFR-TKIs, second- and third generation EGFR-TKIs were demonstrated to add clinical benefits in this setting Significant improvement in overall survival (OS) was seen with osimertinib in the FLAURA trial (9–11).
Osimertinib is a 3rd-generation EGFR-TKI that is associated with lasting response in patients with NSCLC harboring the most frequent EGFR mutations (10, 12).
Based on the initial results from the Phase 3 ADAURA trial, osimertinib was approved for the treatment of resectable EGFR-mutated NSCLC after surgical removal of the tumor. In ADAURA, 90% of patients with stage II-IIIA disease receiving osimertinib were alive and free of cancer at 2 years, compared with 44% of those receiving placebo (13). The ADAURA study is still ongoing and the definitive results, including overall survival, are expected to be released in the future. Osimertinib is also being studied in the Phase III NeoADAURA trial (NCT04351555), which is assessing neoadjuvant osimertinib with or without chemotherapy versus chemotherapy alone prior to surgery, in patients with resectable stage II-IIIB N2 EGFR mutation-positive NSCLC (14).
At present, in daily practice, real-world approaches to NSCLC differ greatly, especially in terms of testing frequency of EGFR that can be used to drive clinical decisions (15, 16). In Italy, reflex EGFR molecular assessment in surgical samples from resected patients is not standard practice and this may have a significant impact on the treatment pathway and outcomes.
The aim of the present study was to characterize the real-world diagnostic-therapeutic pathway and clinical outcomes of early-stage NSCLC, with particular reference to the EGFR-mutant subgroup, at a reference thoracic oncology unit in the Veneto Region. A final evaluation of costs for cancer care in this setting was also carried out.
This is an observational study including a retrospective series of patients with resected non-squamous NSCLC patients referred, between January 2017 and March 2019, to the Thoracic Surgery Unit of the Department of Cardio-Thoracic and Vascular Sciences, University Hospital of Padova and to the Medical Oncology Unit of the Comprehensive Cancer Center Veneto Institute of Oncology (IOV). Main inclusion criteria were: age > 18 years, histologically confirmed diagnosis of NSCLC, chemo-naïve early stage or recurrent disease patients (stage I-IIIB according to 8th edition of the TNM Classification of Malignant Tumors) who underwent radical surgical resection. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The study was approved by the IOV Ethical Committee and all patients signed a specific Informed Consent Form, according to Regulation (EU) 2016/679 of the European Parliament and the Council on personal data protection.
Clinical characteristics included age, gender, residence, smoking status, occupational exposure, symptoms at diagnosis, and family history for neoplasms. Pathological data included histological tumor pattern, vascular and perineural invasion, spread through air spaces (STAS), fibrosis, necrosis, inflammation score, number of mitoses, proliferative index (Ki-67), mucinous secretion, pathological disease stage, and molecular profile.
The diagnostic-therapeutic pathway was tracked, registered, and measured using specific indicators (Supplementary Table 1). The process of biopsy sampling, if present, along with specimen handling and pathological procedures for histological classification and molecular assessment were collected. Moreover, we collected systemic and locoregional treatment approaches in the adjuvant setting. At disease relapse, we recorded the site of relapse, further treatments, and relative response, when available. Finally, patient status and date of death or last follow-up was recorded.
In addition, administrative data from a subset of patients with a diagnosis of early-stage NSCLC, were extracted in order to track diagnostic-therapeutic procedures and costs of the overall path.
The main objective of the study was to describe the diagnostic-therapeutic pathway of non-squamous NSCLC patients who underwent radical surgical resection through specific indicators (Supplementary Table 1). In particular, our primary objective was to evaluate the following data: a) the proportion of EGFR mutation analyses performed autonomously by the pathologist (reflex test); b) turnaround time (TAT) expressed in calendar days between the date of biopsy/specimen acceptance at the pathology unit and histologic report (including EGFR mutation test); c) systemic treatment at the time of disease relapse. The secondary objectives of the study were to evaluate, in a real-world setting, survival and treatment outcomes in the overall population and in patients with mutated EGFR, in terms of: a) median relapse-free survival (mRFS), measured as the time from surgery to first evidence of disease relapse; b) median overall survival (mOS), measured as the time between surgery and death for any cause. A description of treatment received at relapse was also reported. An exploratory objective was to assess the influence of clinical-pathological features on outcomes in order to identify independent predictive and prognostic factors.
Finally, a cost analysis for the management of NSCLC patients undergoing radical surgery, including molecular analysis, was also performed in a subset of patients.
EGFR mutations in exons 18-21 were tested at diagnosis on tumor biopsy/cell blocks or on tumor specimens. EGFR rare/uncommon mutations are defined as alterations with the exception of common sensitizing exon 19 deletion, L858R point mutation and T790M mutation, accounting for about 15% to 20% of all EGFR mutations. Complex or compound mutations are usually defined as the presence of two or more different EGFR mutations in the same tumor sample. For analyses of tissue samples, tumor DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tumor slices using the QIAamp DNA FFPE kit (Qiagen, Hilden, Germany), and quantified with a Nanodrop spectrophotometer (NanoVue, GE Healthcare, Milwaukee, WI, USA); DNA sequencing was carried out with Sanger sequencing, pyrosequencing, polymerase chain reaction (PCR)-based methods (easy EGFR kit, Diatech Pharmacogenetics, Jesi, Italy; EGFR mutation analysis kit EntroGen, EntroGen, USA), and mass spectrometry-based methods (Myriapod lung status kit, Sequenom MassARRAY, Diatech Pharmacogenetics, Jesi, Italy) (17).
The cost analysis was performed on anonymized aggregate data from patients referring to our center in 2017. Data on drug prescriptions, use of medical devices, hospital admissions, visits to outpatient clinics and the emergency room, and hospice admissions were drawn from the administrative databases. In particular, costs were drawn from the reimbursement rates established by the Veneto Regional Authority for each procedure or medical action, as previously described (17). We assessed overall costs within 2 years of follow-up after NSCLC was diagnosed. The average per-patient, stage-specific, real-world costs were calculated. Moreover, methods for EGFR mutation detection adopted at the referral center within the time-frame of interest were collected and costs of each analysis were calculated on a per-patient basis according to reimbursement rates established by the Veneto Regional Authority.
All statistical analyses were performed with SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY), mRFS and mOS were estimated using the Kaplan-Meier method, and the log-rank test was used to compare survival between groups. Hazard ratios (HRs) and 95% CI were calculated with the Cox proportional hazard model; these analyses were applied to identify the impact of each clinical-pathological feature on outcome as mentioned above. Statistical significance was set at p<0.05.
The characteristics of the study population are detailed in Table 1. The cohort included 225 patients, all of whom had adenocarcinoma, with a median age of 70 years of whom 57% were male. Among those screened (223) 44 patients (20%) had adenocarcinoma harboring EGFR mutations (Table 1). Most patients had stage I at diagnosis (IA, 20%; IB, 39%). Lobectomy was the most common surgical procedure used and was performed in 163 patients (72%). Pathological features are listed in Table 2. Most cases (N=155, 69%) had prevalent acinar histology and a combination of lepidic/acinar and acinar/acinar pattern (N=217, 96.4%). Nodal, vascular, perineural, alveolar, and pleural invasion was present in 49 (22%), 99 (44%). 13 (6%), 117 (52%), and 145 cases (64%), respectively.
Details on the diagnostic procedures are depicted in Figure 1. No patient received neoadjuvant systemic treatment.
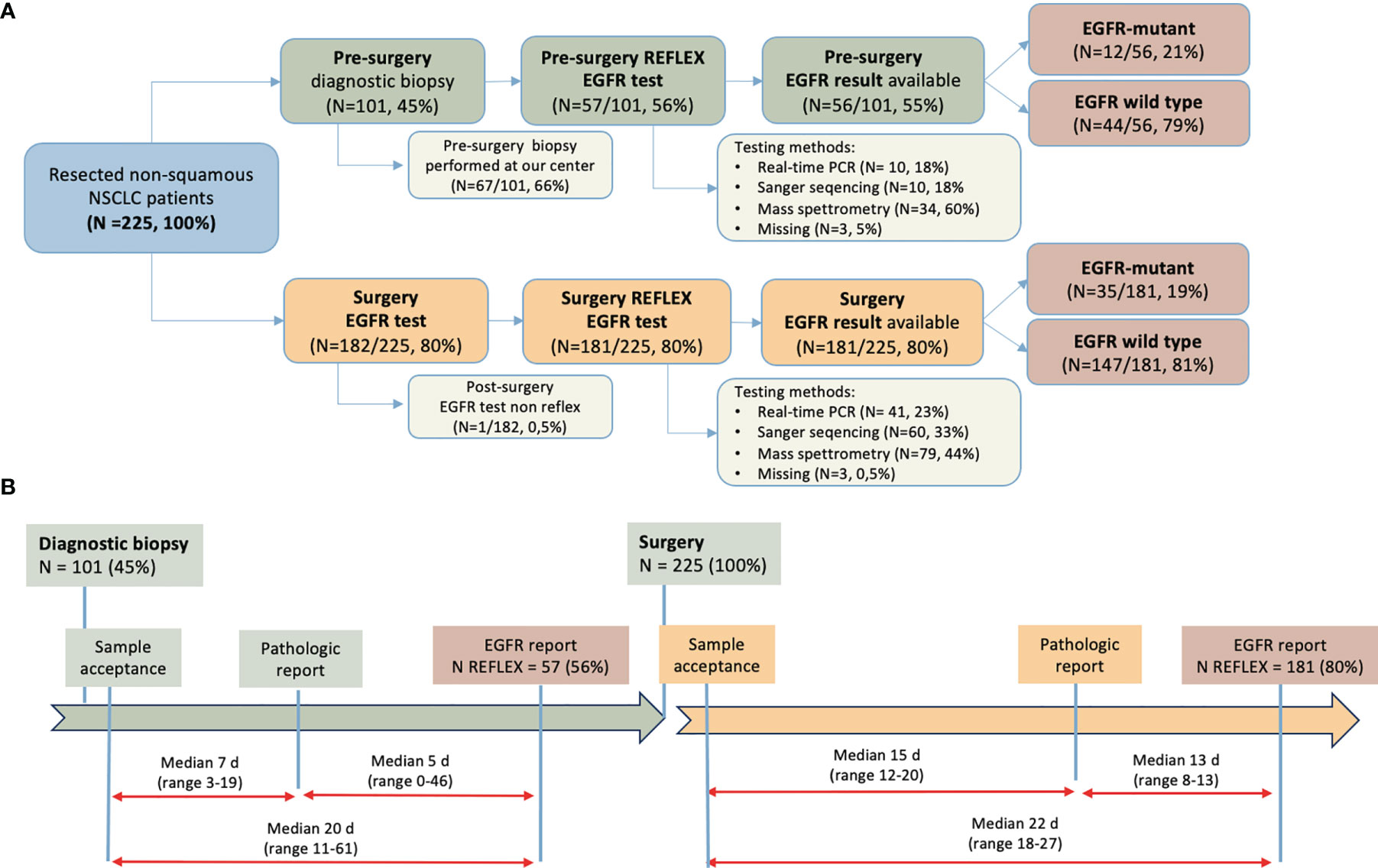
Figure 1 Diagnostic-therapeutic pathway of patients enrolled. (A) Patient flow according to EGFR-testing time, material, methods used, and result; (B) Turnaround time between diagnostic procedure and pathological and molecular report, pre- and post-surgery. NSCLC, non-small-cell lung cancer; EGFR, epidermal growth factor receptor; N, number; PCR, polymerase chain reaction; d, days.
Prior to surgery, 101 patients had undergone diagnostic biopsy; 67 of the 101 (66%) patients with diagnostic biopsy had undergone the procedure at the same center in Padua. Prior to surgery, EGFR testing was performed on 57 patients (56%) who had undergone diagnostic biopsy, although EGFR mutational results were only available in 56 (55%) patients because of one case of failed analysis. An EGFR mutation was found in 12 cases (21%). Among those analyzed, a mass spectrometry-based method was most frequently used method (34 of 57, 60%). After surgery, a reflex EGFR test was performed in 181 (80%) of 225 cases. Overall, a reflex EGFR test (pre- or post-surgery) was performed in almost all samples (221 of 225; 98%) with the surgical specimen used in most cases (74%). Of note, in 14 cases an EGFR test was performed both pre- and post-surgery. Among surgical specimens, EGFR was mutated in 35 cases (19%) and was wild-type in 147 cases (81%).
The median TAT for key pathological analyses is shown in Figure 1B. The median time from pre-surgical biopsy sample registration to pathological report was 7 calendar days (range 3-19 days), and from pathological report to EGFR report an additional 5 days (range 0-46). Following surgery, the definitive pathological report was available after a median of 15 days (range 12-20), and another median of 13 days (range 8-13) was needed for the EGFR report. Median time from surgery to EGFR report was 22 calendar days (range 18-27).
Median follow-up from the date of surgery to the last follow-up or death was 36 months. Adjuvant chemotherapy or adjuvant radiotherapy was administered in 51(23%) and 24(11%) patients, respectively (Table 1). Treatments administered at disease relapse are reported in Supplementary Table 2. Of 49 patients experiencing a disease relapse (21.7% of all population), 6 (12.2%) patients had an EGFR mutant adenocarcinoma and 43 (87.8%) were EGFR wild type.
Twenty-six (53%) received a first-line systemic treatment: 8 (31%) chemotherapy, 7 (27%) a single-agent immune checkpoint inhibitor, 7 (27%) a tyrosine kinase inhibitor (TKI) and 2 (8%) chemo-immunotherapy. In two cases (7.7%), the type of treatment was unknown. All EGFR-mutant relapsed patients (N=6, 12.2%) received an EGFR-TKI: 3 (6.1%) osimertinib, one (2%) gefitinib, one (2%) afatinib. In one EGFR-mutant relapsed patient, the specific drug was unknown. One (2%) patient received a KRAS TKI (sotorasib) as first line treatment at the time of disease relapse.
At disease relapse, 20 patients (43%) received locoregional treatments: 13 (65%) radiotherapy and 7 (35%) a surgical procedure. 10 patients (20.4%) received only locoregional treatments at relapse. In 12 cases (24.5%), no data on systemic and locoregional treatments was available.
Regarding survival outcomes, mOS and mRFS for the entire population were not reached (Figure 2). Among variables explored in univariate analysis (Supplementary Table 3), OS was significantly longer in patients with age < 70 years (p=0.040) (Figure 3A), females (p=0.006) (Figure 3B), patients harboring an EGFR mutation (p= 0.044) (Figure 3C), not undergoing pneumonectomy (p=0.001), without a solid growth pattern (p=0.008), necrosis score ≤30% (p<0.0001), a combination of lepidic/acinar and acinar/acinar patterns (p=0.003), stage I-II (p=0.001) (Figure 3C), no nodal involvement (p=0.006), no vascular invasion (p=0.04), no perineural invasion (p<0.0001), and without relapse (p<0.0001). In particular, two-year survival was 100% in stage I, and decreased to 90% and 64% for stages II and III, respectively.
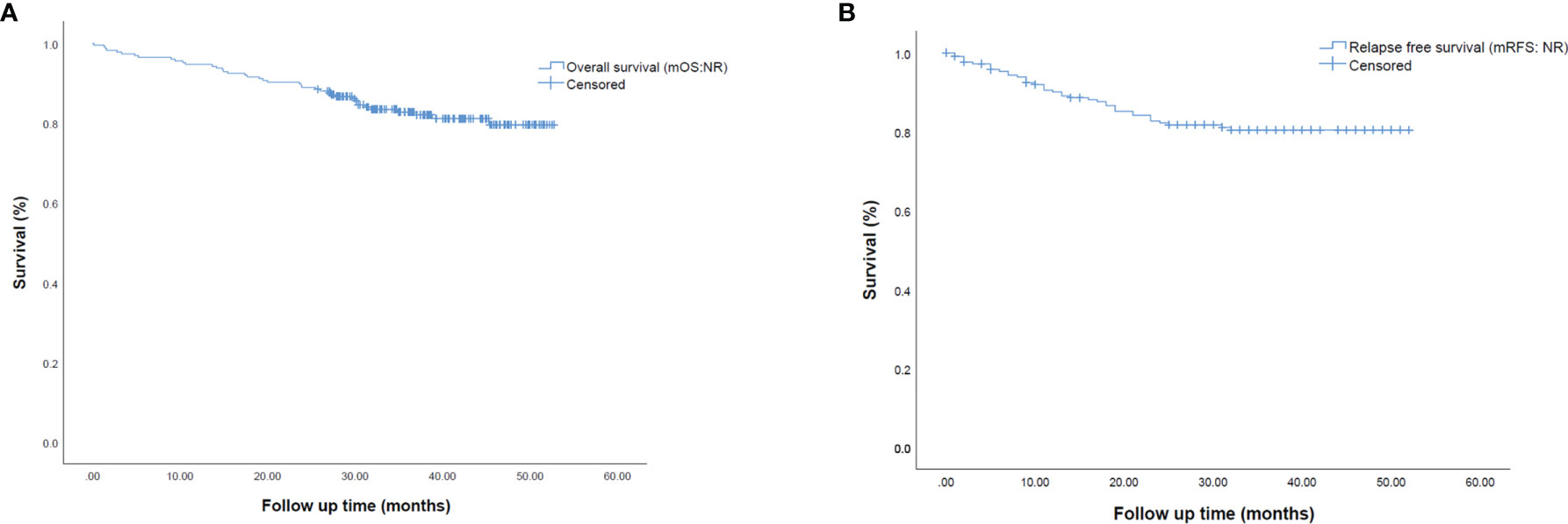
Figure 2 Kaplan-Meier survival curves representing (A) median overall survival (mOS) and (B) median relapse-free survival (mRFS) in the overall population of early-stage resected NSCLC patients.

Figure 3 Kaplan-Meier survival curves representing median overall survival (mOS) in early-stage resected NSCLC patients according to (A) median age, (B) gender, (C) EGFR status and (D) stage at diagnosis. EGFR, epidermal growth factor receptor; WT, wild-type.
Multivariate analysis for OS confirmed the correlation with age (p=0.035; HR 1.052, 95% CI 1.004-1.104), female gender (p=0.008, HR 0.032; 95% CI 0.125-0.727), EGFR status (p=0.032, HR 0.190; 95% CI 0.042-0.866), necrosis score (p=0.12, HR 3.299, 95% CI, 1.294-8.414), perineural invasion (p=0.034, HR 2.989, 95% CI, 1.086-8.222), and relapsed disease (p=0.004, HR 2.856, 95% CI 1.396-5.841) (Supplementary Table 3). As far as the impact on EGFR mutation on survival is concerned, univariate and multivariate analyses showed its predictive role and impact of the targeted treatment at the time of disease relapse on survival.
Univariate and multivariate analyses for mRFS are reported in Supplementary Table 4. We found significantly longer mRFS in patients without a solid growth pattern (p=0.007), necrosis score ≤30% (p<0.0001), a combination of lepidic/acinar and acinar/acinar patterns (p=0.019), stage I or II (p<0.0001) (Figure 4D), and no vascular invasion (p=0.017). No difference in mRFS was seen for age, gender, or EGFR status. (Figures 4A–C). Multivariate analysis for mRFS (Supplementary Table 2) confirmed the correlation within necrosis score (p=0.001; HR 4.579, 95% CI 1.927-10.880), stage at diagnosis (p=0.047, HR 2.272, 95% CI 1.012-5.102), and occupational exposure (p=0.013, HR 2.696, 95% CI, 1.232-5.901).
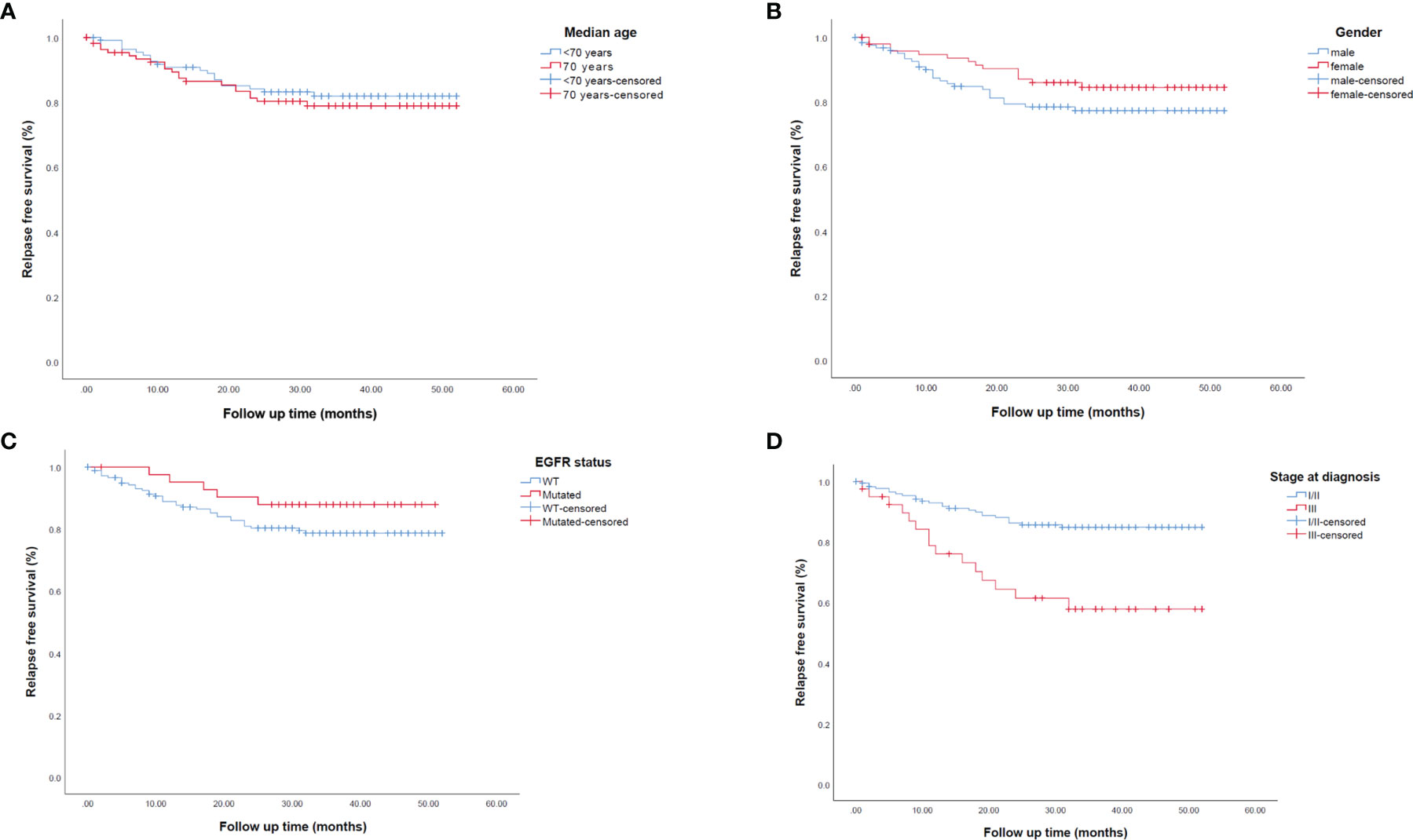
Figure 4 Kaplan-Meier survival curves representing median relapse-free survival (mRFS) in early-stage resected NSCLC patients according to (A) median age, (B) gender, (C) EGFR status and (D) stage at diagnosis. EGFR, epidermal growth factor receptor; WT, wild-type.
A cost analysis from administrative data flows showed average costs of € 34,340.40 (range 28,423.20 - 40,257.50) for the management of 2017 early NSCLC cases undergoing radical surgery (2 years after diagnosis), with a 2-year survival of 79.6%. When overall costs were subdivided according to stage, we observed a medium cost of €25,784.20 (16,016.44 - 35,551.96) in stage I; €29,842.20 (22,033.91 - 37,650.49) in stage II; €39,608.65 (29,243.86 - 49,973.44) in stage III. Mean costs of real time (RT)-PCR and Sanger sequencing was €544.55 and €814.70 per patient, respectively, according to reimbursement rates established by the Veneto Regional Authority.
The present real-world analysis in patients with early-stage NSCLC evaluated baseline characteristics, diagnostic and treatment patterns, and TAT of pathological analyses, as well as outcomes and costs. Median age is in line with previously published observational studies (18, 19). While one-fifth of patients had mutations in EGFR, over 60% had no detectable mutations. Lobectomy was performed in the majority of patients.
At disease relapse, almost a quarter of patients received only locoregional treatment and all EGFR-mutant patients received an EGFR-TKI (20, 21), as described in previous similar series of EGFR mutant NSCLC, eventually followed by a variety of combination therapies (22, 23).
Tissue rebiopsy at the time of disease relapse after radical surgery was not the standard of care to drive treatment decisions, considering that this procedure is mostly invasive and sometimes not feasible. Currently, the regional document about the diagnostic-therapeutic pathway of lung cancer patients, recommend tissue rebiopsy in this setting after multidisciplinary team discussion and consideration of the time interval between the primary treatment and disease relapse. A prospective study aiming at evaluating the concordance of the molecular status between diagnostic biopsy, surgical specimen and relapsed biopsy, should be warranted.
In the overall population, median OS was not reached, with small differences observed in different subgroups of patients. Most real-world studies have been performed on patients with advanced NSCLC, and thus comparison of OS in early-stage tumors in the present study is difficult. For example, in the study by Arriola et al. in advanced EGFR-positive NSCLC, OS was 21.4 months in those with exon 19 deletions and 11.1 months in those with rare mutations (22).
Regarding the diagnostic-therapeutic pathway, EGFR-reflex test was performed on virtually all surgical samples using a variety of methods; it is of interest that EGFR status was available in only 55% of patients undergoing biopsy prior to surgery and that in 14 cases (6%) the test was repeated post-surgery. The repetition of molecular analyses on surgery specimen is regulated in our center according to a specific standard operation procedure (SOP) to improve allocation of resources and avoid unneeded tests (e.g. the molecular analysis is repeated when the percentage of neoplastic cells is <20%).
As far as the timing of diagnostic procedures is concerned, TAT from pathologic report of biopsy samples to EGFR report (5 days) is in line with another real-world study from our group, including EGFR-mutant advanced NSCLC patients (24). In contrast, TAT from post-surgery pathological reported for the EGFR test was longer (13 days). This could be due to the different molecular testing methods that are preferably used. Indeed, in a subset of surgical specimens (cases of 2017 and many of 2018) Sanger sequencing was more frequently used than RT-PCR. Sanger sequencing can be used in cases with a higher number of neoplastic cells as is usually the case in surgical specimens, but is labor intensive and time consuming.
The importance of testing NSCLC for EGFR mutations at earlier stages of management is further reinforced by the recent results of the ADAURA trial which enrolled patients with NSCLC harboring EGFR exon 19 deletions or exon 21 L858R mutations (13). Early testing would allow for earlier identification of patients eligible to adjuvant osimertinib. Recent results from CTONG1104 have shown that adjuvant treatment with gefitinib is associated with superior disease-free survival, reduced toxicity, and improved quality of life, although the benefit did not translate into an advantage in OS (25). The importance of testing should also be considered in light of the ongoing NEOADAURA trial (NCT04351555) (14), where patient selection on the presence of EGFR mutation is required. Indeed, the option of a targeted treatment in the adjuvant setting may have an impact on a sensitive detection of disease relapse by scheduling monthly clinical control visits in patients under treatment. This could imply a new characterization of the tumor at the time of disease relapse by using tissue re-biopsy or liquid biopsy to tailor treatment with new targeted drugs.
Finally, we reported a cost analysis assessed in the real-world. Mean costs of the management of early NSCLC patients progressively increased from stage I to stage III due to greater need of medical assistance. Depending on the method used, EGFR test costs represented 1.6-2.4% of the total cost of management per patient (€34,340.40). In the ADAURA trial, an 83% reduction in risk of recurrence or death with adjuvant osimertinib in stage II/IIIA disease (HR: 0.17; P<0.0001) was reported (13). More recently, Buja and Pasello showed a reduction in 2-year survival for patients in stage II from 2015 to 2017 in the Veneto Region (84.6% in 2015 compared with 83.3% in 2017), underling the need to improve treatment in this setting (26).
One strength of the present study is the comprehensive analysis of the diagnostic and treatment pathway carried out in all stages of resectable NSCLC. This study however has some limitations. First, this is a single-center retrospective design, although it does include patients who underwent surgery in the Thoracic Surgery Unit at the Department of Cardio-Thoracic and Vascular Sciences of the University of Padua, a referral center also for patients outside the Veneto Region. This allowed a standardized treatment and a uniform collection of clinical data.
Second, the study recruited a relatively limited number of patients. Nevertheless, very few data are reported in the literature on the real-world diagnostic pathway including cost-consequences of EGFR testing in early-stage NSCLC patients (27). This data would be useful for different stakeholders (clinician, patients, policymakers, and payers) to assess the benefit and sustainability of interventions in daily clinical practice.
The real-world diagnostic-therapeutic pathway of early-stage NSCLC demonstrated a similar occurrence of EGFR mutations to advanced tumors. Reflex EGFR testing in all early-stage NSCLC at diagnosis or after surgery may be a sustainable approach to give patients the best chance to benefit from targeted adjuvant treatment and prevent disease relapse.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by IOV Ethical Committee. The patients/participants provided their written informed consent to participate in this study.
GPa: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation, Writing - original draft; Writing - review and editing. ML and GPr: Data curation; Formal analysis; Methodology; Writing - original draft GC, FP, MS, SF, and LB: Data curation, Investigation, Validation; AB: Data curation; Formal analysis; Methodology; Writing - review and editing. FC, VG, and FR: Conceptualization, Supervision; Validation, Writing review and editing. All authors contributed to the article and approved the submitted version.
Support from AstraZeneca for development of this manuscript is acknowledged. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
GPa: Advisory Boards/Honoraria/Speakers’ fee/Consultant for: AstraZeneca, BMS, Boehringer Ing., Eli Lilly; MSD, Novartis, Pfizer, Roche, Takeda; VG: personal fees from Eli Lilly, Roche, Novartis, MSD, GSK, Gilead, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Medical writing support in the preparation of this article was provided by Patrick Moore, PhD on behalf of Edra S.p.A., and unconditionally funded by AstraZeneca.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.909064/full#supplementary-material
1. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The Prevalence of EGFR Mutation in Patients With Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncotarget (2016) 7(48):78985–93. doi: 10.18632/oncotarget.12587
2. Vyse S, Huang PH. Targeting EGFR Exon 20 Insertion Mutations in non-Small Cell Lung Cancer. Signal Transduct Target Ther (2019) 4:5. doi: 10.1038/s41392-019-0038-9
3. Zappa C, Mousa SA. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl Lung Cancer Res (2016) 5(3):288–300. doi: 10.21037/tlcr.2016.06.07
4. Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, et al. EGFR Exon 20 Insertion Mutations in Lung Adenocarcinomas: Prevalence, Molecular Heterogeneity, and Clinicopathologic Characteristics. Mol Cancer Ther (2013) 12(2):220–9. doi: 10.1158/1535-7163.MCT-12-0620
5. Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, et al. Natural History and Molecular Characteristics of Lung Cancers Harboring EGFR Exon 20 Insertions. J Thorac Oncol (2013) 8(2):179–84. doi: 10.1097/JTO.0b013e3182779d18
6. Russo A, Franchina T, Ricciardi G, Battaglia A, Picciotto M, Adamo V. Heterogeneous Responses to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) in Patients With Uncommon EGFR Mutations: New Insights and Future Perspectives in This Rar Clinical Scenario. Int J Mol Sci (2019) 20(6):1431. doi: 10.3390/ijms20061431
7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib Versus Standard Chemotherapy as First-Line Treatment for European Patients With Advanced EGFR Mutation-Positive non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2012) 13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X
9. Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib Versus Gefitinib as First-Line Treatment of Patients With EGFR Mutation-Positive non-Small-Cell Lung Cancer (LUX-Lung 7): A Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol (2016) 17(5):577–89. doi: 10.1016/S1470-2045(16)30033-X
10. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
11. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib Versus Gefitinib as First-Line Treatment for Patients With EGFR-Mutation-Positive non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(11):1454–66. doi: 10.1016/S1470-2045(17)30608-3
12. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall Survival With Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
13. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med (2020) 383(18):1711–23. doi: 10.1056/NEJMoa2027071
14. Tsuboi M, Weder W, Escriu C, Blakely C, He J, Dacic S, et al. Neoadjuvant Osimertinib With/Without Chemotherapy Versus Chemotherapy Alone for EGFR-Mutated Resectable non-Small-Cell Lung Cancer: NeoADAURA. Future Oncol (2021) 17(31):4045–55. doi: 10.2217/fon-2021-0549
15. Chang N, Duan J, Wang L, Dong Z, Liu Z. Patients With Advanced non-Small Cell Lung Cancer With EGFR Mutations in Addition to Complex Mutations Treated With Osimertinib Have a Poor Clinical Outcome: A Real-World Data Analysis. Oncol Lett (2020) 20(3):2266–72. doi: 10.3892/ol.2020.11801
16. Winfree KB, Sheffield KM, Cui ZL, Sugihara T, Feliciano J. Study of Patient Characteristics, Treatment Patterns, EGFR Testing Patterns and Outcomes in Real-World Patients With EGFRm(+) non-Small Cell Lung Cancer. Curr Med Res Opin (2021) 38(1):91–9. doi: 10.1080/03007995.2021.1983530
17. Sheikine Y, Rangachari D, McDonald DC, Huberman MS, Folch ES, VanderLaan DA, et al. EGFR Testing in Advanced Non–Small-Cell Lung Cancer, A Mini-Review. Clin Lung Cancer (2016) 17:483–92. doi: 10.1016/j.cllc.2016.05.016
18. Chi A, Fang W, Sun Y, Wen S. Comparison of Long-Term Survival of Patients With Early-Stage Non-Small Cell Lung Cancer After Surgery vs Stereotactic Body Radiotherapy. JAMA Netw Open (2019) 2(11):e1915724. doi: 10.1001/jamanetworkopen.2019.15724
19. Evers J, Hendriks LEL, De Jaeger K, Wijsman R, De Ruysscher D, Terhaard C, et al. Trends and Variations in the Treatment of Stage I-III Small Cell Lung Cancer From 2008 to 2019: A Nationwide Population-Based Study From the Netherlands. Lung Cancer (2021) 162:61–70. doi: 10.1016/j.lungcan.2021.10.011
20. Nadler E, Pavilack M, Espirito JL, Clark J, Fernandes A. Observational Study of Treatment Patterns in Patients With Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer After First-Line EGFR-Tyrosine Kinase Inhibitors. Adv Ther (2020) 37(2):946–54. doi: 10.1007/s12325-020-01221-4
21. Soo RA, Seto T, Gray JE, Thiel E, Taylor A, Sawyer W, et al. Treatment Patterns in Patients With Locally Advanced or Metastatic Non-Small-Cell Lung Cancer Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors: Analysis of US Insurance Claims Databases. Drugs Real World Outcomes (2021) 9(1):31–41. doi: 10.1007/s40801-021-00272-5
22. Arriola E, Garcia Gomez R, Diz P, Majem M, Martinez Aguillo M, Valdivia J, et al. Clinical Management and Outcome of Patients With Advanced NSCLC Carrying EGFR Mutations in Spain. BMC Cancer (2018) 18(1):106. doi: 10.1186/s12885-018-4004-7
23. Gelatti ACZ, Drilon A, Santini FC. Optimizing the Sequencing of Tyrosine Kinase Inhibitors (TKIs) in Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer (NSCLC). Lung Cancer (2019) 137:113–22. doi: 10.1016/j.lungcan.2019.09.017
24. Lorenzi M, Ferro A, Cecere F, Scattolin D, Del Conte A, Follador A, et al. First-Line Osimertinib in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer: Outcome and Safety in the Real World: FLOWER Study. Oncologist (2021) 27(2):87–e115. doi: 10.1002/onco.13951
25. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei YC, et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial. J Clin Oncol (2021) 39(7):713–22. doi: 10.1200/JCO.20.01820
26. Buja A, Pasello G, De Luca G, Bortolami A, Zorzi M, Rea F, et al. Non-Small-Cell Lung Cancer: Real-World Cost Consequence Analysis. JCO Oncol Pract (2021) 17(8):e1085–e93. doi: 10.1200/OP.20.00863
27. Sauter JL, Butnor KJ. Clinical and Cost Implications of Universal Versus Locally Advanced-Stage and Advanced-Stage-Only Molecular Testing for Epidermal Growth Factor Receptor Mutations and Anaplastic Lymphoma Kinase Rearrangements in Non-Small Cell Lung Carcinoma: A Tertiary Academic Institution Experience. Arch Pathol Lab Med (2016) 140(4):358–61. doi: 10.5858/arpa.2015-0147-OA
Keywords: NSCLC, early-stage, real-world, EGFR, costs
Citation: Pasello G, Lorenzi M, Pretelli G, Comacchio GM, Pezzuto F, Schiavon M, Buja A, Frega S, Bonanno L, Guarneri V, Calabrese F and Rea F (2022) Diagnostic-Therapeutic Pathway and Outcomes of Early Stage NSCLC: a Focus on EGFR Testing in the Real-World. Front. Oncol. 12:909064. doi: 10.3389/fonc.2022.909064
Received: 31 March 2022; Accepted: 31 May 2022;
Published: 29 June 2022.
Edited by:
Marco Lucchi, University of Pisa, ItalyReviewed by:
Hiromi Wada, Kyoto University, JapanCopyright © 2022 Pasello, Lorenzi, Pretelli, Comacchio, Pezzuto, Schiavon, Buja, Frega, Bonanno, Guarneri, Calabrese and Rea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Pasello, Z2l1bGlhLnBhc2VsbG9AaW92LnZlbmV0by5pdA==
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.