- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Urological Department, Peking University Cancer Hospital & Institute, Beijing, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Renal Cancer and Melanoma, Peking University Cancer Hospital & Institute, Beijing, China
Purpose: To investigate the association between preoperative systemic immune-inflammation index (SII) and neutrophil–lymphocyte ratio (NLR) and oncological outcomes in localized prostate cancer (PCa) patients after radical prostatectomy (RP).
Methods: Between January 2014 and December 2019, 291 patients with pathologically confirmed localized PCa who underwent RP were included in this study. The threshold values of SII and NLR for biochemical recurrence (BCR) were calculated according to Youden’s index based on the receiver operating characteristic (ROC) curve, then the patients were divided into two groups by the threshold values of SII and NLR, and the clinicopathological outcomes were analyzed and compared between groups, respectively. The binary logistic regression model was used to evaluate the association between SII, NLR, and pathological outcomes including Gleason score (GS) and pathological T (pT) stage. Kaplan–Meier curves and univariable and multivariable Cox regression models were used to determine the association between high SII, high NLR, and BCR-free survival, respectively.
Results: The median follow-up time was 48 months (IQR 36–62), and 114 (39.18%) patients developed BCR. The AUC of SII for BCR was 0.813 (P < 0.001), with a threshold value of 528.54, a sensitivity of 72.9%, and a specificity of 76.3%; the AUC of NLR for BCR was 0.824 (P < 0.001), with a threshold value of 2.62, a sensitivity of 71.2%, and a specificity of 81.6%. Patients were divided into two groups according to the threshold values of SII and NLR, respectively. Patients in the high SII group had higher tPSA, GS, pT stage, and BCR rate than patients in the low SII group (P = 0.004, 0.04, 0.007, and <0.001, respectively), and patients in the high NLR group had higher tPSA, GS, pT stage, and BCR rate than patients in the low NLR group (P = 0.04, 0.02, 0.006, and <0.001, respectively). Multivariable logistic regression analysis revealed that high SII was significantly correlated with adverse pathological outcomes of GS (HR, 1.656; 95% CI, 1.00–2.742, P = 0.042) and pT stage (HR, 1.478; 95% CI, 0.972–3.64, P = 0.028); there was no association between high NLR and pathological events. Kaplan–Meier analysis showed significantly poorer BCR-free survival in patients with high SII or high NLR (P < 0.001 and <0.001, respectively). By using the multivariable Cox regression model, high SII (HR, 4.521; 95% CI, 2.262–9.037, P < 0.001) and high NLR (HR, 4.787; 95% CI, 2.339–9.798, P < 0.001) were both significant predictors of BCR after RP.
Conclusion: High SII was significantly related to unfavorable clinicopathological outcomes. High preoperative SII and NLR were related to higher BCR rate in localized PCa after RP, and they were all independent risk factors associated with shorter BCR-free survival. These two factors might provide promising and inexpensive methods for predicting clinical outcomes in patients with RP.
Introduction
For localized prostate cancer (PCa), the most useful treatment method is radical prostatectomy. Unfortunately, approximately 30%–50% of patients will experience biochemical recurrence (BCR) after radical prostatectomy (RP), which is closely associated with tumor recurrence and metastasis (1). Many factors may influence the prostate-specific antigen (PSA) level after RP, and a retrospective study demonstrated that smoking status might be one of the most important factors (2). Meanwhile, several factors including Gleason score (GS) and clinical or pathological stage may be used for predicting BCR after RP as proven by several studies, but they lack the accuracy to guide the following therapeutic approach (2, 3). Therefore, reliable, easily accessible, and inexpensive markers are needed for assessing clinical outcomes in patients with localized PCa after RP.
The association between inflammation and PCa has been proven by literature (4). Neutrophil–lymphocyte ratio (NLR) is a well-known inexpensive and effective representative marker of an inflammatory condition. It has been proven to be positively associated with prognosis in various kinds of malignant tumors (5, 6). Regarding PCa, NLR was revealed to be an independent predictor for overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) (7). Recently, one retrospective study has shown a significantly worse prognosis in metastasis-free and OS of localized PCa patients with high NLR after radiotherapy (8). However, there is a paucity of studies about the association between NLR and clinical and pathological outcomes in localized PCa after RP.
In addition, another novel inflammatory marker, systemic immune-inflammation index (SII) which combines components of NLR and platelet–lymphocyte ratio (PLR), has been proven to be a more powerful method of predicting occurrence and progression in several kinds of tumors (9–11). In terms of PCa, it was firstly described in 2016 and was considered a powerful marker for predicting the prognosis of mCRPC (12). However, there are no data on the predictive value of SII on BCR in the setting of localized PCa after RP.
Thus, in this retrospective study, we aimed to evaluate the values of preoperative NLR and SII in predicting BCR after RP and detected their association with clinicopathological outcomes.
Material and Methods
This retrospective study was carried out at Peking University Cancer Hospital & Institute and got the approval of the Medical Ethics Review Committee of Peking University Cancer Hospital & Institute (protocol code 2020KT30).
Patients
Two hundred and ninety-one patients with localized PCa who underwent RP, consisting of 287 with laparoscopic RP and 4 with open RP, between January 2014 and December 2019z were reviewed. Among these patients, no one received neoadjuvant therapy before RP and adjuvant therapy after RP until the detection of BCR. In patients with smoking status, smoking was recommended to be ceased 1 month before RP. Preoperative clinical characteristics including age, serum total PSA (tPSA) value, total prostate volume (TPV), body mass index (BMI), and complete blood count (CBC)-based parameters as well as postoperative pathological and BCR outcomes were collected and compared according to the level of NLR and SII, respectively. Data of risk factors related to BCR including GS, pT stage, NLR, and SII were collected, and their associations with BCR-free survival time were analyzed. A single preoperative CBC with differential was performed as part of the routine assessment testing 1–2 days before RP simultaneously with the tPSA value. The CBC-based parameters including NLR and SII were used in this study. To ensure the CBCs were not affected by other factors, patients who met one of the following criteria were excluded: any surgical intervention within 1 month, non-steroidal anti-inflammatory drugs used within 1 month, acute or chronic infection, malignant tumors in other organs, and systemic inflammatory disease.
Procedure
Ultrasound-guided 13-core transrectal prostate biopsy was performed in patients with PSA >4 ng/ml at our institute. The results of serum tPSA value and CBC-based parameters were collected just 1–2 days before the RP surgery and at least 3 weeks after the prostate biopsy to minimize the effect of the prostate biopsy. MRI, emission computed tomography (ECT), or CT was performed before surgery to confirm no bone, lymph node, or distant organ metastasis. Laparoscopic RP or open RP was performed in patients with PCa at least 30 days after the biopsy. Extrafascial radical prostatectomy through an extraperitoneal approach was performed by skilled and experienced surgeons in our institute according to the technique of Walsh et al., and standard pelvic lymph node dissection was performed in all patients (13). All specimens were assessed by a sophisticated pathologist at our institute, and serum tPSA value was detected every 1–3 months after RP.
Variables
The prostate was measured in 3-dimensional aspects, and its volume was estimated with the modified ellipsoid formulation in cm3 (0.523 [length × width × height]) after surgery. Pathologic GSs were recorded and patients were staged according to the 2010 American Joint Committee on Cancer system (AJCC, pathologic stages T1–T4) (14). Tumors were classified into low (GS ≤ 6), intermediate (GS = 7), and high grade (GS ≥ 8) according to the D’Amico risk classification (15). NLR and SII were calculated by using the numbers of blood cell count-based systemic markers of inflammation. The NLR and SII were calculated as follows: NLR = neutrophil count/lymphocyte count; SII = platelet count × neutrophil count/lymphocyte count. SII was presented as a combination of NLR and PLR (9, 16). Body mass index (BMI) = weight (kg)/height (meter)2. BCR was defined as at least two consecutive serum tPSA ≥0.2 ng/ml according to the guidelines of the American Urological Association (17), and data of time free from BCR were collected.
Statistical Analysis
Measurement data confirming normal distribution analyzed by the Shapiro–Wilk test are presented as mean ± SD. The independent sample t-test was used to evaluate the differences between continuous variables, while chi-square tests were performed to examine categorical variables. To determine the optimal cutoff value of NLR and SII for BCR, Youden’s index was calculated using the receiver operating characteristic curve (ROC), and the corresponding specificity–sensitivity levels were provided. Youden’s index was defined as YI(C) = max c [Se(C) + SP(C) − 1]. The binary logistic regression model (univariate and multivariate analysis) was used to evaluate the association between NLR, SII, and adverse pathological events, which were all compared with the reference group (Ref). Kaplan–Meier analyses were performed for BCR-free survival according to NLR and SII using the log-rank test, and the survival curves were described. The univariable and multivariable Cox regression models were used to identify the co-variables that influence BCR. The software used to run the analysis was IBM-SPSS version 20. All tests were two-sided. P <0.05 was considered to be the threshold for statistically meaningful differences.
Results
Patients’ Clinicopathologic Characteristics and the Cutoff Values of SII and NLR for BCR
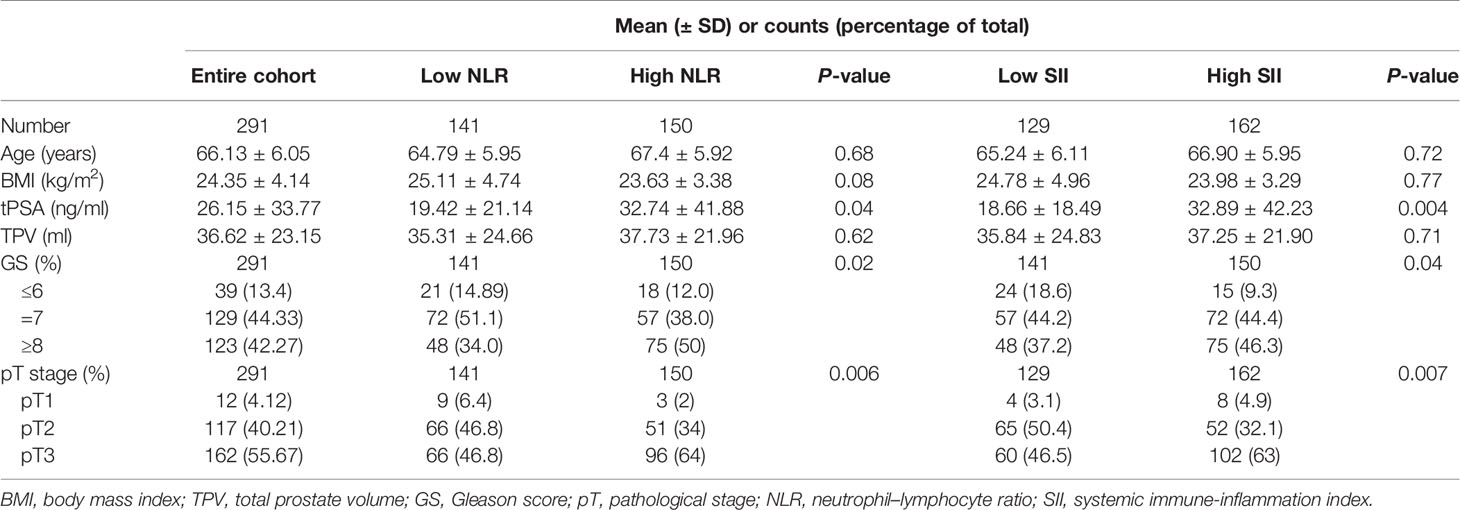
A total of 291 patients with localized PCa were enrolled in the study. The median values of clinical factors were 66.13 ± 6.05 years for age, 24.35 ± 4.14 for BMI, 36.62 ± 23.15 ml for TPV, and 26.15 ± 33.77 ng/ml for tPSA. Twelve patients (4.12%) were pT1, 117 (40.21%) were pT2, and 162 (55.67%) were pT3. Thirty-nine patients (13.4%) were of low risk (GS ≤ 6), 129 (44.33%) were of intermediate risk (GS = 7), and 123 (42.27%) were of high risk (GS ≥ 8). Twenty patients (6.87%) were with pelvic lymph node metastases. The ROC of SII and NLR for BCR were analyzed to determine the optimal cutoff values for SII and NLR (Figure 1). The AUC for SII was 0.813, which was significantly lower than 0.05 (P < 0.001), with a threshold value of 528.54, a sensitivity of 72.9%, and a specificity of 76.3%; the AUC for NLR was 0.824, which was significantly lower than 0.05 (P < 0.001), with a threshold value of 2.62, a sensitivity of 71.2%, and a specificity of 81.6%; therefore, according to the threshold values of NLR and SII, the patients were divided into low-level and high-level groups, respectively. The patients’ clinicopathologic demographics are summarized in Table 1.
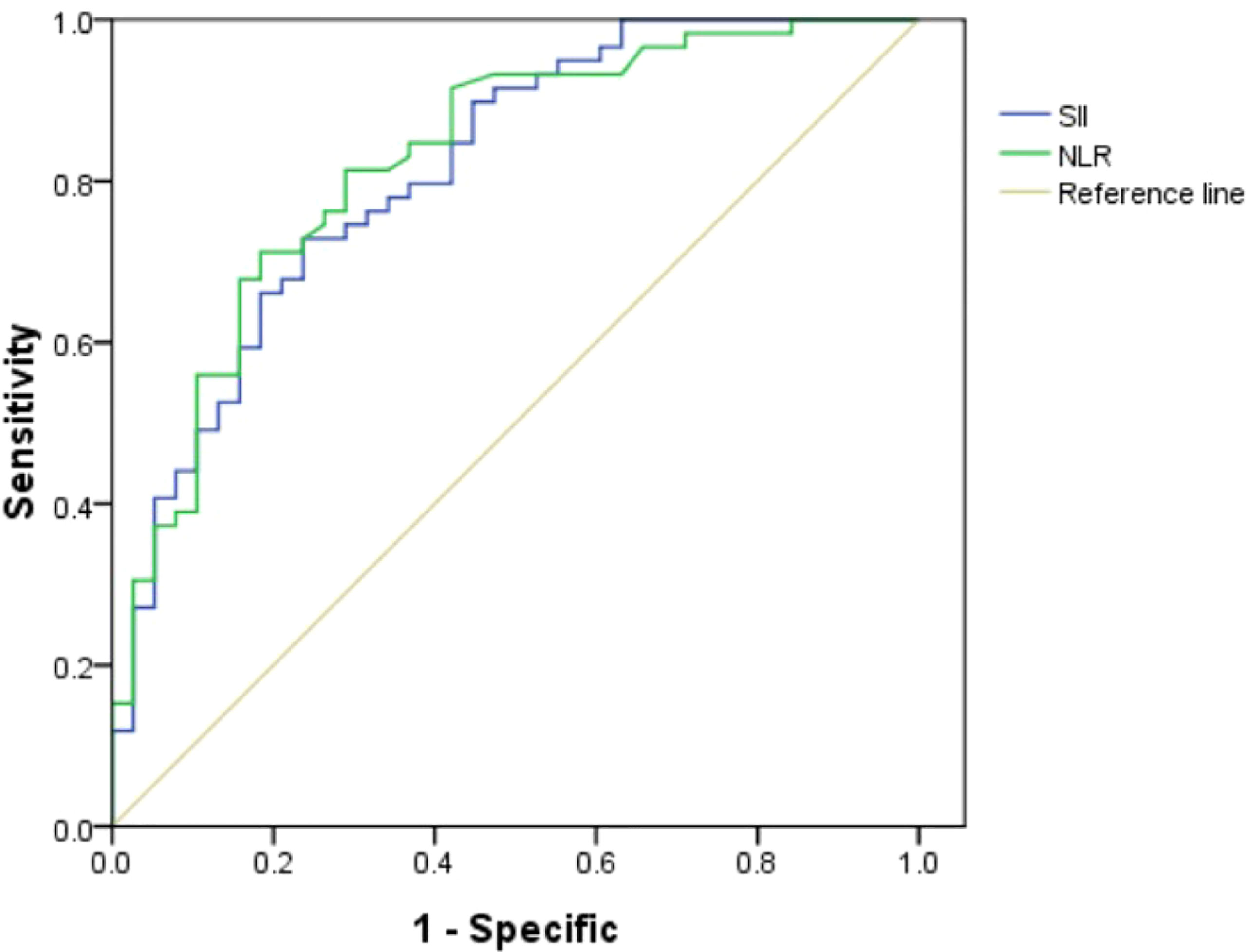
Figure 1 Role of the systemic immune-inflammation index (SII) and neutrophil–lymphocyte ratio (NLR) in predicting biochemical recurrence (BCR) after radical prostatectomy (RP) by ROC curve analysis. The AUC for NLR was 0.824 with P-value <0.001, and the AUC for SII was 0.813 with P-value <0.001.
Clinicopathological Characteristics in the Low and High NLR Groups
Initially, the distribution of clinicopathological characteristics was compared between groups according to the threshold value of NLR. The high NLR group showed unfavorable features compared with the low NLR group. In the high NLR group, preoperative serum tPSA (P = 0.04), GS (P = 0.02), and pT stage (P = 0.006) were significantly higher compared with those in the low NLR group, but the distribution of age, BMI, and TPV did not show any significant differences as shown in Table 1.
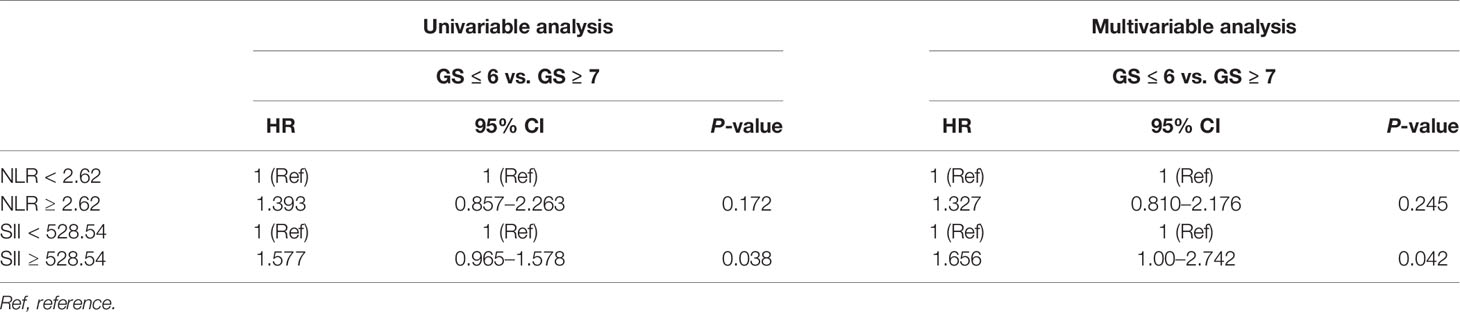
Then, univariable and multivariable logistic regression models were used to evaluate the association between NLR and several adverse pathological events. The results showed that there was no association between high NLR and pathological events including pT stage and GS as shown in Tables 2, 3.
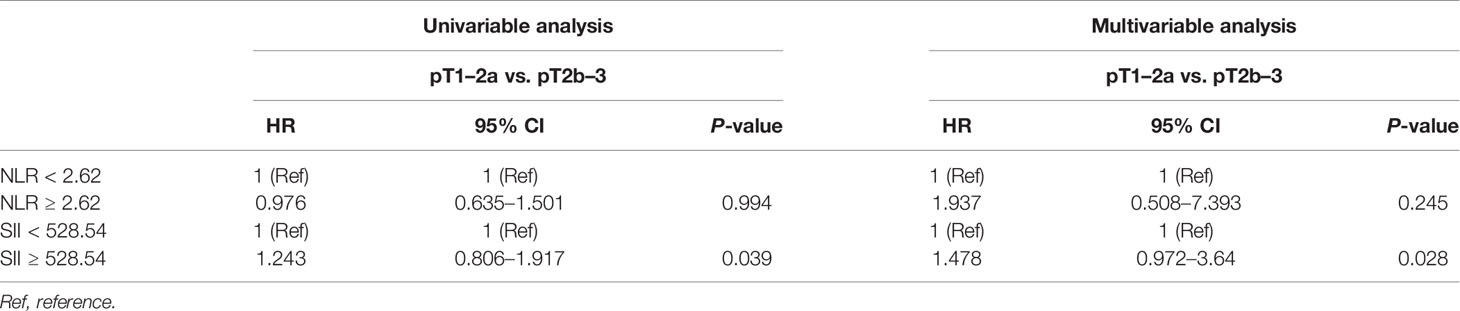
Table 2 Univariable and multivariable analyses of the impact of NLR and SII on pathological T stage.
Clinicopathological Characteristics in the Low and High SII Groups
The distribution of clinicopathological characteristics was compared between groups according to the threshold value of SII. The high SII group showed unfavorable features compared with the low SII group. In the high SII group, preoperative serum tPSA (P = 0.004), GS (P = 0.04), and pT stage (P = 0.007) were significantly higher compared with those in the low SII group, but the distribution of age, BMI, and TPV did not show any significant differences as shown in Table 1.
Then, univariable and multivariable logistic regression models were used to evaluate the association between SII and adverse pathological events. In the univariable analysis, SII ≥528.54 was a risk factor associated with higher pT stage (HR, 1.243; 95% CI, 0.806–1.917, P = 0.039) and higher GS (HR, 1.577; 95% CI, 0.965–1.578, P = 0.038); in the multivariable analysis, SII ≥528.54 was an independent risk factor strongly associated with higher pT stage (HR, 1.478; 95% CI, 0.972–3.64, P = 0.028) and higher GS (HR, 1.656; 95% CI, 1.00–2.742, P = 0.042) as shown in Tables 2, 3.
The Association Between NLR, SII, and BCR-Free Survival
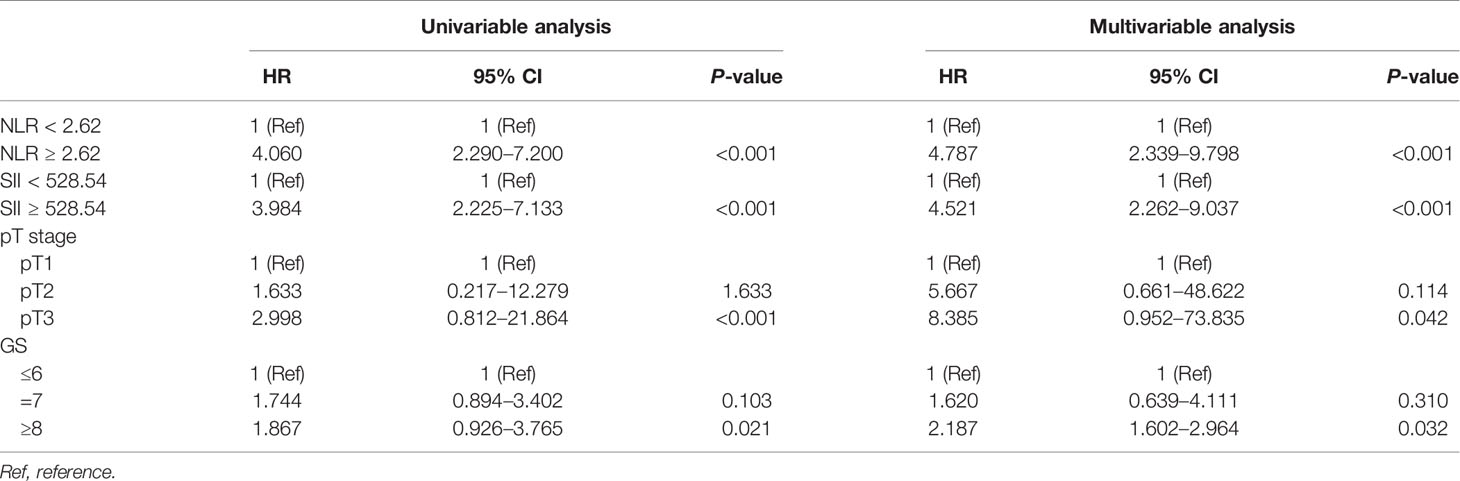
The median follow-up time was 48 months (IQR 36–62) and 114 (39.18%) patients developed BCR. Thirty-eight (26.95%) and 76 (50.67%) patients developed BCR in the low and high NLR groups (P < 0.001). Kaplan–Meier analysis showed that BCR-free survival was significantly shorter in the high NLR group than in the low NLR group as shown in Figure 2 (P < 0.001). By using the multivariable Cox regression model, it was revealed that NLR ≥2.62 (HR, 4.787; 95% CI, 2.339–9.798, P < 0.001) was a significant independent factor associated with BCR after RP as shown in Table 4.
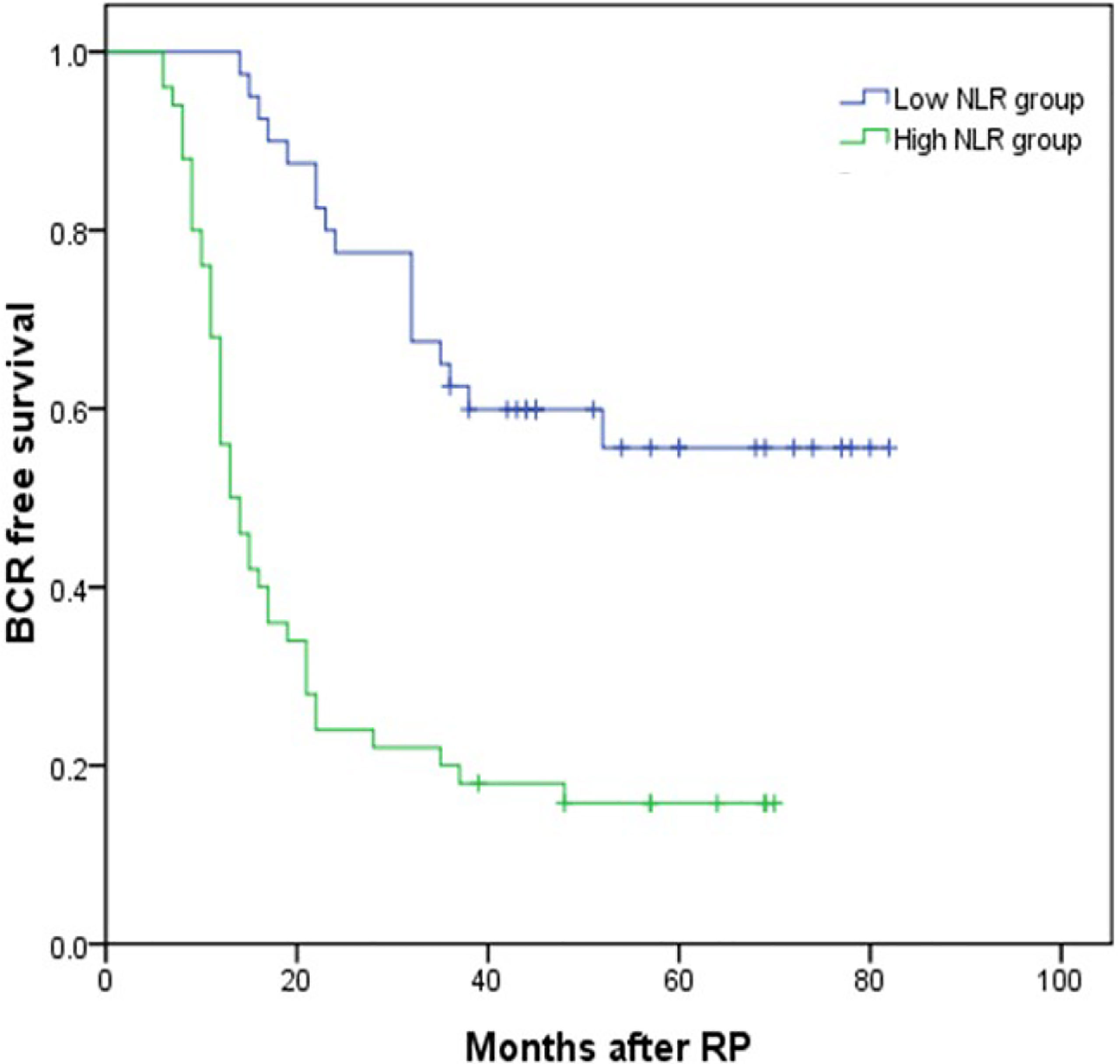
Figure 2 Kaplan–Meier curves for BCR-free survival according to NLR level. BCR-free survival of patients with NLR <2.62 was significantly longer than that of patients with NLR ≥2.62 (P < 0.001 by log-rank test).
Thirty-five (27.13%) and 79 (48.77%) patients developed BCR in the low and high SII groups (P < 0.001). Kaplan–Meier analysis showed the BCR-free survival was significantly shorter in the high SII group than in the low SII group as shown in Figure 3 (P < 0.001). By using the multivariable Cox regression model, it was revealed that SII ≥528.54 (HR, 4.521; 95% CI, 2.262–9.037, P < 0.001) was a significant independent factor associated with BCR after RP as shown in Table 4.
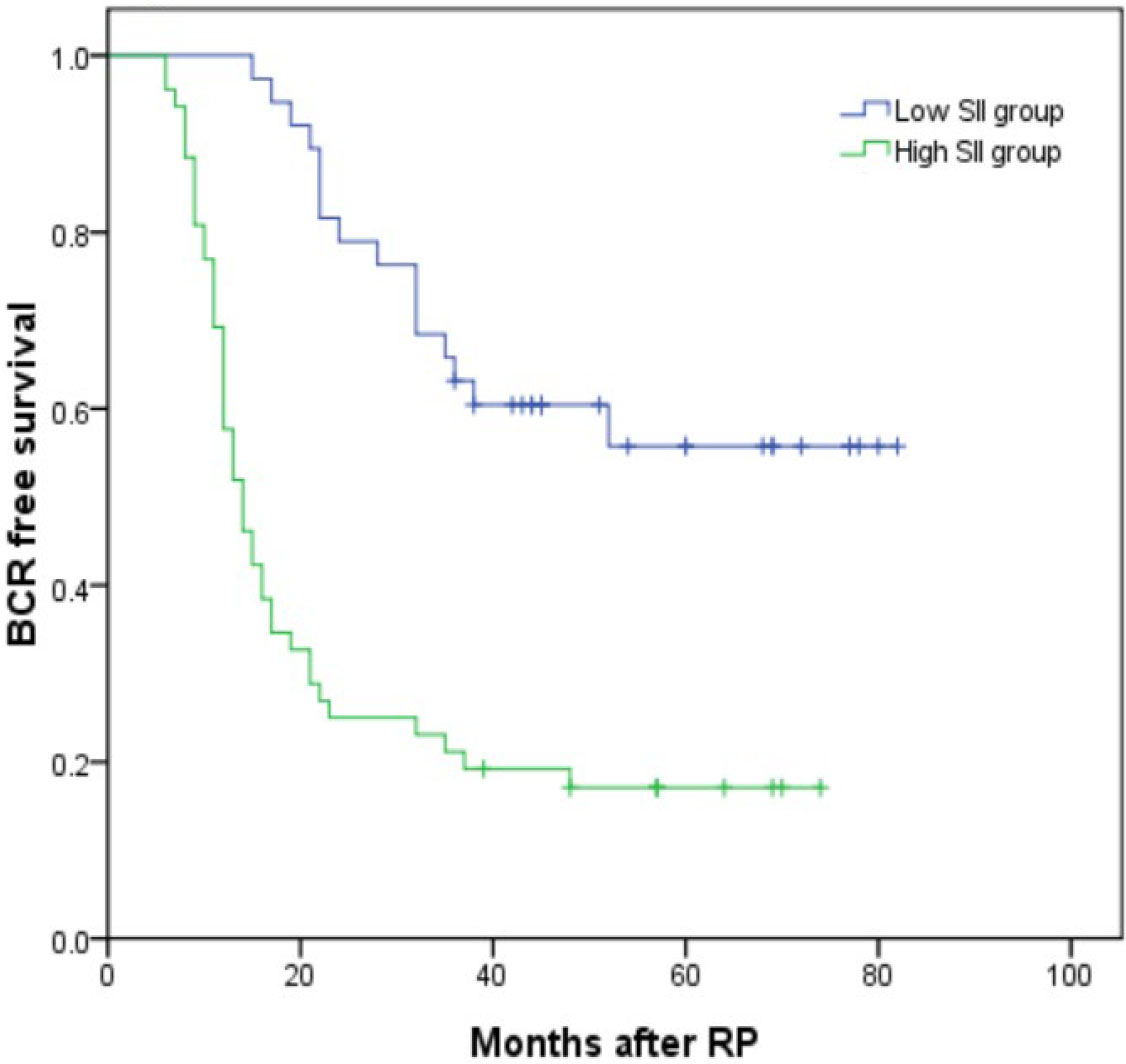
Figure 3 Kaplan–Meier curves for BCR-free survival according to SII level. BCR-free survival of patients with SII <528.54 was significantly longer than that of patients with SII ≥528.54 (P < 0.001 by log-rank test).
Meanwhile, the multivariable Cox regression model revealed that GS ≥8 (HR, 2.187; 95% CI, 1.602–2.964, P = 0.032) and pT3 stage (HR, 8.385; 95% CI, 0.952–73.835, P = 0.042) were also significant independent factors associated with BCR after RP as shown in Table 4.
Discussion
In China, the incidence of PCa has been increasing in recent years. Among PCa patients after RP, BCR is one of the most important factors associated with the poor prognosis of patients (18). In a previous study, we investigated the association between NLR, SII, and the occurrence of PCa and revealed that high SII and NLR were all independent factors predicting PCa. SII seemed to be a more powerful tool compared with NLR (19). In this study, we further investigated the relationship between inflammatory factors and clinicopathological outcomes in localized PCa patients after RP and demonstrated that high SII and NLR were significantly associated with higher BCR rate and shorter BCR-free survival; meanwhile, high SII was strongly associated with higher GS and pT stage. To the best of our knowledge, this is the first study that investigated the relationship between SII and BCR in localized PCa patients after RP.
The relationship between inflammation and various kinds of malignant tumors has been reported by many studies (20, 21). The NLR based on the calculation of neutrophil to lymphocyte counts has been proposed as an indicator of general immune response to various stress stimuli and the host inflammatory status. An elevated NLR may be associated with both an increased neutrophil-dependent systemic inflammatory response and a lower lymphocyte-mediated antitumor immune response, reflecting a favorable immune microenvironment for tumor development and metastasis (22). In urological malignant tumors, inflammatory parameters have been considered important biomarkers for predicting bladder cancer progression (23). The relationship between NLR and clinical outcomes in PCa has been reported by many studies. Zhang et al. indicated that NLR ≥2.36 increased the risk of involvement of lymph nodes and was associated with higher GS (24). Another study revealed that NLR ≥2.5 was positively associated with GS, pT stage, and extracapsular extension (25). But only a few studies have investigated the role of NLR in predicting BCR after RP in localized PCa (26, 27). One study investigating the clinical outcomes in localized PCa patients after RP revealed that high NLR was significantly correlated with poor OS, CSS (cancer-specific survival), and BCR (27). Lee et al. demonstrated that NLR ≥2.5 was significantly related to unfavorable clinicopathological outcomes and worse BCR-free survival (25). Another study obtained the opposite conclusion: the study analyzed the data of 327 PCa patients who underwent robot-assisted RP and found that there was no correlation between NLR and PLR with BCR (28). However, the relationship between NLR and BCR remained controversial. In our study, we revealed that the threshold value of NLR for BCR was 2.62, which was similar to those reported by Lee et al. and Zhao et al. (25, 29). NLR ≥2.62 was significantly associated with poorer BCR-free survival according to Kaplan–Meier analysis and Cox regression analysis, but not associated with clinicopathological outcomes. We believed that NLR was an effective factor in predicting BCR in localized PCa patients after RP.
Recently, besides neutrophils and lymphocytes (30), the role of platelets has also been well-established in tumor occurrence and metastasis (31). SII, a novel inflammatory index that combines components of neutrophils, lymphocytes, and platelets, has been considered to reflect the systemic inflammatory responses more comprehensively than other inflammatory indexes. High SII suggested an elevated non-specific inflammatory status and a weak adaptive immune response in patients, which might promote the occurrence and progression of the tumor (32, 33). Several studies on inflammatory markers analyzed their predictive values in the PCa setting with various conclusions, but only a few of them included SII (34, 35). Our previous study demonstrated that high SII was an independent predictor for PCa, and it was one of the few studies detecting the role of SII in PCa (18). Recently, Rajwa et al. have evaluated the role of SII in non-metastatic PCa patients after RP and demonstrated that high preoperative SII ≥620 was independently associated with extracapsular extension, non-organ confined disease, and upgrading at RP (36). Another study evaluated the prognostic role of SII and NLR in mCRPC patients treated with abiraterone and revealed that SII ≥535 and NLR ≥3 were all independent predictors associated with shorter OS (12). Fan et al. obtained the same results and concluded that high SII could be used as a predictor for OS in mCRPC patients treated with abiraterone (37). However, none of these studies investigated the association between SII and BCR-free survival in localized PCa after RP. In our study, for the first time in the literature, the role of SII in predicting BCR-free survival was analyzed and the results indicated that high SII was significantly associated with shorter BCR-free survival. The cutoff value of SII for BCR was determined to be 528.54, and we also demonstrated that SII was associated with high BCR rate, pT stage, and GS, which was consistent with the conclusion of previous literature (36). For NLR, we failed to detect the association between NLR and pathological outcomes. Furthermore, both SII and NLR could represent the novel predictive markers for BCR in PCa patients after RP, and SII seemed more favorable for it was also associated with aggressive pathological outcomes.
This study still has some limitations. First, this was a single-center, retrospective study. Second, the biomarker was measured at a single time point, and it can be strengthened by collecting different preoperative sets of blood samples. Third, because of the relatively small sample size, more samples are needed in further studies.
Conclusion
High preoperative SII was associated with higher GS and pT stage. High preoperative SII and NLR were related to higher BCR rate in localized PCa after RP, and they were all independent risk factors associated with shorter BCR-free survival. These two factors might provide promising and inexpensive methods predicting clinical outcomes in patients with RP. However, additional well-organized and large prospective studies are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This retrospective study was carried out at Peking University Cancer Hospital & Institute and got the approval of the Medical Ethics Review Committee of Peking University Cancer Hospital & Institute (protocol code 2020KT30).
Author Contributions
PD, XS, and SW designed the study. SW, XY, ZY, YC, JM, PD, XS, XQY, and YY performed the study and analyzed the data. PD, XS, SW, XY, and ZY wrote the manuscript draft and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Capital’s Funds for Health Improvement and Research (2022-1G-1021) and the National Nature Science Foundation of China (82172604).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Broeck T, Bergh RCN, Arfi N, Gross T, Moris L, Biers E, et al. Prognostic Value of Biochemical Recurrence Following Treatment With Curative Intent for Prostate Cancer: A Systematic Review. Eur Urol (2019) 75:967–87. doi: 10.1016/j.eururo.2018.10.011
2. Tarantino G, Crocetto F, Di Vito C, Martino R, Pandolfo SD, Creta M, et al. Clinical Factors Affecting Prostate Specific Antigen Levels in Prostate Cancer Patients Undergoing Radical Prostatectomy: A Retrospective Study. Future Sci OA (2021) 7:FSO643. doi: 10.2144/fsoa-2020-0154
3. Shariat SF, Semjonow A, Lilja H, Caroline S, Andrew JV, Bjartell A, et al. Tumor Markers in Prostate Cancer I: Blood Based Markers. Acta Oncol (2011) 50:61–75. doi: 10.3109/0284186X.2010.542174
4. Andrew GR, Sudha MS, Dhananjay AC, Nilesh SG, Sean RW, Olesandr NK, et al. Racial Differences in the Systemic Inflammatory Response to Prostate Cancer. PloS One (2021) 16:e0252951. doi: 10.1371/journal.pone.0252951
5. Sarraf KM, Belcher E, Raevsky E, Andrew GN, Peter G, Lim E, et al. Neutrohphil/lymphocyte Ratio and its Association With Survival After Compete Resection in non-Small Cell Lung Cancer. J Thorac Cardiovasc Surg (2009) 137:425–8. doi: 10.1016/j.jtcvs.2008.05.046
6. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NK. Neutrophil-Lymphocyte Ratio as a Prognostic Factor in Colorectal Cancer. J Surg Oncol (2005) 91:181–4. doi: 10.1002/jso.20329
7. Sonpavde G, Pond GR, Armstrong AJ, Clarke SJ, Vardy JL, Templeton AJ, et al. Prognostic Impact of the Neutrophil to Lymphocyte Ratio in Men With Metastatic Castration Resistant Prostate Cancer. Clin Genitourin Cancer (2014) 12:317–24. doi: 10.1016/j.clgc.2014.03.005
8. Langsenlehner T, Thurner EM, Krenn-Pilko S, Langsenlehner U, Stojakvoic T, Gerger A, et al. Validation of the Neutrophil to Lymphocyte Ratio as a Prognostic Factor in a Cohort of European Prostate Cancer Patients. World J Urol (2015) 33:1661–7. doi: 10.1007/s00345-015-1494-7
9. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic Immune Inflammation Index Predicts Prognosis of Patients After Curative Resection for Hepatocellular Carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
10. Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, et al. Systemic Immune Inflammation Index Predicts the Clinical Outcome in Patients With Metastatic Renal Cell Cancer Treated With Sunitinib. Oncotarget (2014) 7:54564–71. doi: 10.18632/oncotarget.10515
11. Passardi A, Scarpi E, Cavanna L, Monia DA, Tassinari D, Silvana L, et al. Inflammatory Indexes as Predictors of Prognosis and Bevacizumab Efficacy in Patients With Metastatic Colorectal Cancer. Oncotarget (2016) 22:33210–9. doi: 10.18632/oncotarget.8901
12. Lolli C, Caffo O, Scarpi E, Aieta M, Conteduca V, Maines F, et al. Systemic Immune Inflammation Index Predicts the Clinical Outcome in Patients With mCRPC Treated With Abiraterone. Front Pharmacol (2016) 13:376. doi: 10.3389/fphar.2016.00376
13. Walsh PC, Retik AB, Vaughan ED, Roger RD, Louis RK, Craig AP, et al. Anatomic Radical Retropubic Prostatectomy. In: Campbell’s Urology, Ed 8, vol. 4. Philadephia: Elsevier (2002). p. 3107–29.
14. Frederick L, Page DL, Fleming ID, Abedin J, Abeliovich H, Abraham AA, et al. AJCC Cancer Staging Manual. New York, NY, USA: Springer Science & Business Media (2002).
15. D’ Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA (1998) 280:969–74. doi: 10.1001/jama.280.11.969
16. Chovanec N, Cierna Z, Miskovska V, Machalekova K, Kalavska K, Rejlekova K, et al. Systemic Immune-Inflammation Index is Prognostic in Testicular Germ Cell Tumors With PD-L1 Expressing Tumor Infiltrating Lymphocytes. J Clin Oncol (2017) 35:e16042. doi: 10.1200/JCO.2017.35.15_suppl.e16042
17. Cookson MS, Aus G, Burnett AL, Edith DC, Anthony VD, Roger RD, et al. Variation in the Definition of Biochemical Recurrence in Patients Treated for Localized Prostate Cancer: The American Urological Association Prostate Guidelines for Localized Prostate Update Panel Report and Recommendations for a Standard in the Reporting of Surgical Outcomes. J Urol (2007) 177:540–5. doi: 10.1016/j.juro.2006.10.097
18. Fan Y, Ye L, Liang L, Li QH, Zhang J, Ma M, et al. Analysis of Risk Factors for Clinical Cure and Biochemical Recurrence in Patients After Radical Prostatectomy. Chin J Urol (2021) 9:644–9. doi: 10.3760/cma.j.cn112330-20210720-00379
19. Wang S, Ji Y, Chen Y, Du P, Cao Y, Yang X, et al. The Values of Systemic Immune-Inflammation Index and Neutrophil-Lymphocyte Ratio in Predicting the Localized Prostate Cancer: A Retrospective Study. Front Oncol (2022) 11:812319. doi: 10.3389/fonc.2021.812319
20. Gomez D, Morris-stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of Systemic Inflammation on Outcome Following Resection for Intrahepatic Cholangiocarcinoma. J Surg Oncol (2008) 97:513–8. doi: 10.1002/jso.21001
21. Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, et al. The Association of Pre-Treatment Neutrophil to Lymphocyte Ratio With Response Rate, Progression Free Survival and Overall Survival of Patients Treated With Sunitinib for Metastatic Renal Cell Carcinoma. Eur J Cancer (2012) 48:202–8. doi: 10.1016/j.ejca.2011.09.001
22. Piccard H, Muschel RJ, Opdenakker G. Neutrophil-Mediated Tumor Angiogenesis: Subversion of Immune Response to Promote Tumour Growth. Semin Cancer Biol (2013) 23:149–58. doi: 10.1016/j.semcancer.2013.02.003
23. Ferro M, Caputo VF, Barone B, Imbimbo C, de Cobelli O, Crocetto F. Lymphocyte to Monocyte Ratio: A New Independent Prognostic Factor in Bladder Cancer Progression. Front Oncol (2021) 11:754649. doi: 10.3389/fonc.2021.754649
24. Zhang GM, Zhu Y, Ma XC, Qin XJ, Wan FN, Dai B, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio: A Predictor of Advanced Prostate Cancer and Biochemical Recurrence in Patients Receiving Radical Prostatectomy. Medicine (Baltimore) (2015) 94:e1473. doi: 10.1097/MD.0000000000001473
25. Lee H, Jeong SJ, Hong SK, Byun SS, Lee SE, Qh JJ, et al. High Preoperative Neutrophil-Lymphocyte Ratio Predicts Biochemical Recurrence in Patients With Localized Prostate Cancer After Radical Prostatectomy. World J Urol (2016) 34:821–7. doi: 10.1007/s00345-015-1701-6
26. Minardi D, Scartozzi M, Montesi L, Santoni M, Burattini L, Bianconi M, et al. Neutrophil-To-Lymphocyte Ratio may be Associated With the Outcome in Patients With Prostate Cancer. Springerplus (2015) 4. doi: 10.1186/s40064-015-1036-1
27. Jang WS, Cho KS, Kim KH, Yoon CY, Kang YJ, Lee JY, et al. Prognostic Impact of Preoperative Neutrophil-to-Lymphocyte Ratio After Radical Prostatectomy in Localized Prostate Cancer. Prostate Cancer Prostatic Dis (2016) 19:298–304. doi: 10.1038/pcan.2016.20
28. Zanaty M, Ajib K, Alnazari M, Elie EB, Fouad A, Kevin CZ, et al. Prognostic Utility of Neutrophil-to-Lymphocyte and Platelets-to-Lymphocyte Ratio in Predicting Biochemical Recurrence Post Robotic Prostatectomy. Biomark Med (2018) 12:841–8. doi: 10.2217/bmm-2017-0321
29. Cao Z, Ji J, Zhang C, Wang F, Xu H, Yu Y, et al. The Preoperative Neutrophil-to-Lymphocyte Ratio is Not a Marker of Prostate Cancer Characteristics But is an Independent Predictor of Biochemical Recurrence in Patients Receiving Radical Prostatectomy. Cancer Med (2019) 8:1004–12. doi: 10.1002/cam4.1984
30. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in Cancer: Neutral No More. Nat Rev Cancer (2016) 16:431–46. doi: 10.1038/nrc.2016.52
31. Riedj J, Pabinger I, Ay C. Platelets in Cancer and Thrombosis. Hamostaseologie (2014) 34:54–62. doi: 10.5482/HAMO-13-10-0054
32. Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren H, et al. Prognostic Value of Systemic Immune Inflammation Index in Patients With Gastric Cancer. Clin J Cancer (2017) 36:75. doi: 10.1186/s40880-017-0243-2
33. Man Y, Chen Y. Systemic Immune-Inflammation Index, Serum Albumin, and Fibrinogen Impact Prognosis in Castration Resistant Prostate Cancer Patients Treated With First Line Docetaxel. Int Urol Nephrol (2019) 12:2189–99. doi: 10.1007/s11255-019-02265-4
34. Ozsoy M, Moschini M, Fajkovic H, Soria F, Seitz C, Klatte T, et al. Elevated Preoperative Neutrophil-Lymphocyte Ratio Predicts Upgrading at Radical Prostatectomy. Prostate Cancer Prognostic Dis (2018) 21:100–5. doi: 10.1038/s41391-017-0015-8
35. Kwon YS, Han CS, Yu JW, Kim S, Modi P, Davis R, et al. Neutrophil and Lymphocyte Counts as Clinical Markers for Stratifying Low-Risk Prostate Cancer. Clin Genitourin Cancer (2016) 14:e1–8. doi: 10.1016/j.clgc.2015.07.018
36. Rajwa P, Schuettfort VM, D'Andrea D, Quhal F, Mori K, Katayama S, et al. Impact of Systemic Immune-Inflammation Index on Oncologic Outcomes in Patients Treated With Radical Prostatectomy for Clinically Nonmetastatic Prostate Cancer. Urol Oncol (2021) 39:785.e19–785.e27. doi: 10.1016/j.urolonc.2021.05.002
Keywords: prostate cancer, systemic immune-inflammation index, neutrophil–lymphocyte ratio, inflammatory markers, biochemical recurrence
Citation: Wang S, Yang X, Yu Z, Du P, Sheng X, Cao Y, Yan X, Ma J and Yang Y (2022) The Values of Systemic Immune-Inflammation Index and Neutrophil–Lymphocyte Ratio in Predicting Biochemical Recurrence in Patients With Localized Prostate Cancer After Radical Prostatectomy. Front. Oncol. 12:907625. doi: 10.3389/fonc.2022.907625
Received: 29 March 2022; Accepted: 04 May 2022;
Published: 02 June 2022.
Edited by:
Takeshi Yuasa, Japanese Foundation for Cancer Research, JapanCopyright © 2022 Wang, Yang, Yu, Du, Sheng, Cao, Yan, Ma and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Du, ZHVwZW5nOTAwMEAxMjYuY29t; Xinan Sheng, ZG9jdG9yX3NoZW5nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Shuo Wang
Shuo Wang Xiao Yang1†
Xiao Yang1† Ziyi Yu
Ziyi Yu Peng Du
Peng Du