- 1Department of Vascular Surgery, Changhai Hospital, The People’s Liberation Army (PLA) Naval Medical University, Shanghai, China
- 2Department of Vascular Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of General Surgery, Jingling Hospital, Medical School of Nanjing University, Nanjing, China
Background: Carotid body tumor (CBT) is a rare paraganglioma located at the carotid bifurcation. The red blood cell count, hemoglobin, and hematocrit are indexes to be evaluated in blood routine tests. The purpose of this study was to clarify their predictive value for temporary postoperative complications in patients that had undergone CBT surgery.
Methods: This retrospective trial included data from 169 patients received surgical treatment for CBT from October 2008 to September 2018 in this retrospective study. Postoperative follow-up was conducted under the guidance of both vascular surgeon and neurologist. The symptoms existed less than 2 years postoperatively were regarded as temporary injuries. The red blood cell count, hemoglobin, and hematocrit were obtained from the complete blood count results of the participants. Analyses of multilevel multivariable regression and descriptive statistics were conducted.
Results: The baseline data showed no significant difference. Patients were predominantly women (53.8%), with a mean age of 42.6 years. The total incidence of temporary postoperative complications was 22 (13.0%), including transient ischemic attack (8, 4.7%), tongue bias (7, 4.1%), dysphagia (2, 1.2%), hoarseness (4, 1.8%), and eyelid ptosis (1, 2.4%). The univariate and multivariate regression analysis results revealed that the occurrence of temporary postoperative complications was increased with age [odd ratio (OR, 0.09; 95% CI (CI), 0.9–1.0; P = 0.014], length of operation time (OR, 1.0; 95% CI, 1.0–1.0; P = 0.005), Shamblin type II vs. I (OR, 0.1; 95% CI, 0.0–0.5; P = 0.008), red blood cell count postoperative (OR, 0.2; 95% CI, 0.1–0.8; P = 0.026), hemoglobin (OR, 0.9; 95% CI, 0.9–1.0; P = 0.011), and hematocrit (OR, 0.8; 95% CI, 0.7–1.0; P = 0.025). The smooth curve fitting showed that the trend of complications occurrence rate was reduced with the increase of patients’ postoperative red blood cell count, hemoglobin, and hematocrit. Gender, weight, length of operation, Shamblin type, postoperative red blood cell count, hemoglobin, and hematocrit were included in the risk model with AUC = 0.86.
Conclusion: These patients with CBT who received surgical resection with low postoperative red blood cell, hemoglobin, or hematocrit had a high risk of temporary postoperative complications. The risk prediction model established for predicting temporary postoperative complications showed satisfactory prediction effects.
Introduction
Carotid body tumors (CBTs) are paragangliomas that most commonly originating from the head and neck areas (1, 2). Because of the clinical symptoms and unpredictable malignant potential (systemic metastasis, local compression, erosion of the carotid artery and peripheral nerve tissue, tumor thrombus, etc.) of CBT, surgical management is the preferred treatment for CBT that is better to performed as soon as possible upon diagnosis (3). CBT resection is a relatively risky procedure because of the proximity of the surgical site to several important nerves and blood vessels; its perioperative period complication rate is from 30% to 40% (4, 5).
The two most common complications are cranial nerve injury and intraoperative bleeding (4). Patients with cranial nerve injury will often experience hoarse, dysphagia, and tongue deviation; these deficits that are related to injuries to the vagus nerves, the superior laryngeal nerve, and the hypoglossal nerve (6–9). Whereas some patients have those neurological symptoms for a long time or even permanently, in others, they are just occurring as a temporary phenomenon and most of patients recover before hospital discharge. Another significant complication is intraoperative bleeding, which might affect the normal cranial nerve function or the recovery of the damaged nerves (4). For years, researchers have focused on reducing the incidence of CBT resection complications (10–12). In this study, we found several routine indicators that can predict the occurrence of postoperative adverse events. Taking appropriate measures in a timely manner may reduce the incidence of complications.
Materials and methods
Data source
Patients underwent CBT surgical resection in our institution from October 2008 to September 2018 were recruited into this retrospective study and grouped by gender. Computed tomography (CT) was performed for each patient as a necessary routine preoperative examination. All these Shamblin type I, II, and III CBTs were included into this research. Patients were excluded in the following conditions: (1) CBT surgery failed, (2) history of CBT resection, (3) other head and neck radiotherapy or surgery history, (4) carotid artery replacements, and (5) lack of postoperative blood routine examination data.
Their hospital records were reviewed, including preoperative patient profile, intraoperative blood loss, and adverse symptoms after surgery. The postoperative outcome data were collected during 2-year follow-up. These symptoms that still exist 2 years postoperatively were regarded as permanent injuries and not included in the temporary postoperative complications. Temporary postoperative complications after CBT surgery included the following: (1) transient ischemic attack (dizziness, headache, and temporary blurred vision), (2) tongue bias, (3) dysphagia, (4) hoarseness, and (5) eyelid ptosis.
Every included patient’s blood sample was taken at the admission and the second day after CBT surgery. The complete blood routine was assessed by means of an automatic blood counter. Red blood cell count, hemoglobin, and hematocrit were obtained. This trial was approved by the ethics committee and all patients provided written informed consent.
Procedure
All these patients underwent CBT surgical resection with general anesthesia. The internal jugular vein was identified and the superior thyroid artery was exposed. In general, the vagus nerve accompanying the common carotid artery and the superior laryngeal nerve accompanying the superior thyroid artery can be observed. The hypoglossal nerve can also be seen during some operations. In principle, all nerves should be preserved. These common veins and several cutaneous nerves that would impede the progress of surgery were ligated and cutoff. The CBT was carefully dissected and successfully completely removed according to strict technical standards.
Analysis and statistics
Continuous variables were reported as means ± SD. Skewed variables were summarized as median and range, depending on distribution of the variables. Group comparisons were analyzed with Student’s t-test or Wilcoxon rank sum test for numeric variables and χ2 or Fisher’s exact test for categorical variables. Generalized linear models with a logit link were used to test the independent and combined effects of these indexes (including red blood cell count, hemoglobin, or hematocrit) and the incidence of temporary postoperative complications with crude or full model adjusted for age, weight, and gender. All analyses were performed using Empower(R) (www.empowerstats.com; X&Y Solutions, Inc., Boston MA) and R (http://www.R-project.org). A P-value of <0.05 was considered to be statistically significant.
Results
Patient characteristics
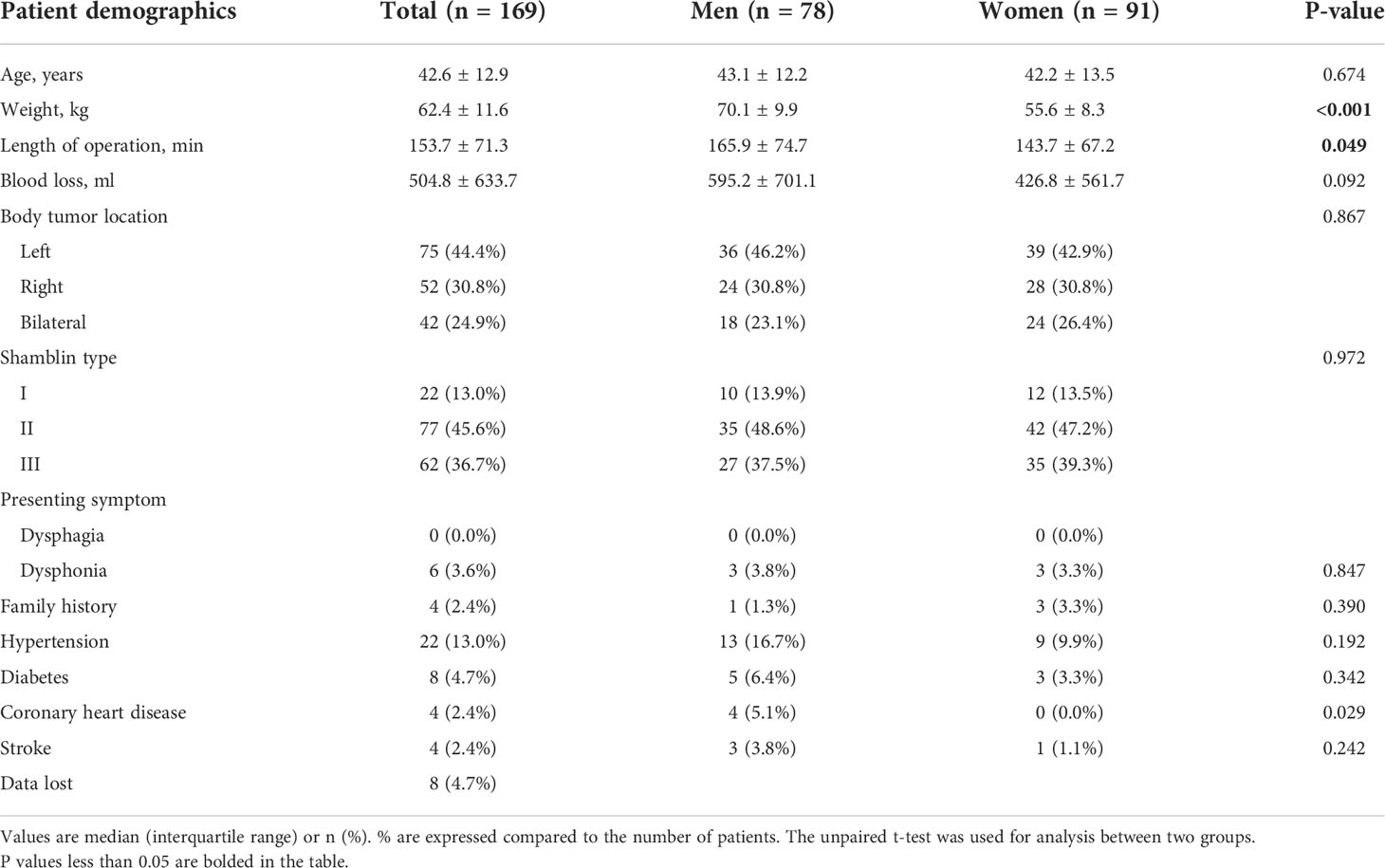
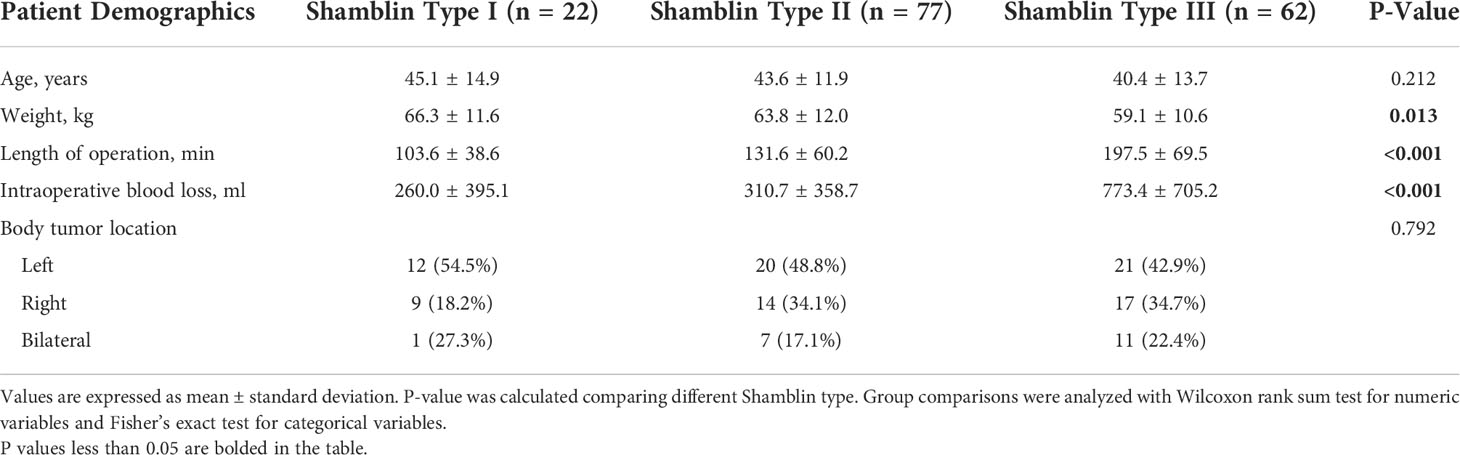
Between October 2008 and September 2018, 169 consecutive patients (78 men and 91 women) entered analysis. Mean patient age was 42.6 ± 12.9 years (range 17 to 75 years). Seventy-five tumors are located on the left side, 52 tumors are located on the right, and 42 patients had bilateral tumors (surgery site, 96; left, 67, right). Length of operation in total was 153.7 ± 71.3 min, a little longer in men (165.9 ± 74.7 min) than in women (143.7 ± 67.2 min), P = 0.049. Intraoperative blood loss shows no significance difference between these two groups, 504.8 ± 633.7 ml in total (Table 1). According to the Shamblin classification, tumors were identified: Shamblin type I (n = 22), Shamblin type II (n = 77), Shamblin type III (n = 62), and eight patients’ data lost (Table 2).
Analysis of preoperative indicators
Red blood cell count, hemoglobin, and hematocrit before surgery in different gender of patients showed significance difference. Red blood cell count in men was 4.7 ± 0.6 × 1012/L but 4.2 ± 0.5 × 1012/L in women, P < 0.001. Hemoglobin in men was 144.7 ± 31.1 g/L but 120.3 ± 17.9 g/L in women, P < 0.001. Hematocrit in men was 42.2 ± 3.9% but 36.6 ± 4.6% in women, P < 0.001. Although there was intraoperative bleeding during CBT resection, no significant change could be observed, except red blood cell count, in blood routine at the second day after surgery compared with preoperative. Red blood cell count before surgery in total was 4.5 ± 0.6 × 1012/L, compared with 4.3 ± 0.6 × 1012/L after surgery, P = 0.022. However, after grouping by gender, there was no significant difference in red blood cell count between preoperative and postoperative (Table 3). The statistics of temporary postoperative complications are shown in Table 4. No remarkable differences were shown between male and female patients. The total incidence of temporary postoperative complications was 22 (13.0%), including transient ischemic attack (8, 4.7%), tongue bias (7, 4.1%), dysphagia (2, 1.2%), hoarseness (4, 1.8%), and eyelid ptosis (1, 2.4%). Only nine (5.3%) patients with permanent complications could be observed until the end of follow-up, including 1 (0.6%) ipsilateral stroke, 2 (1.2%) tongue bias, and 6 (3.6%) hoarseness.
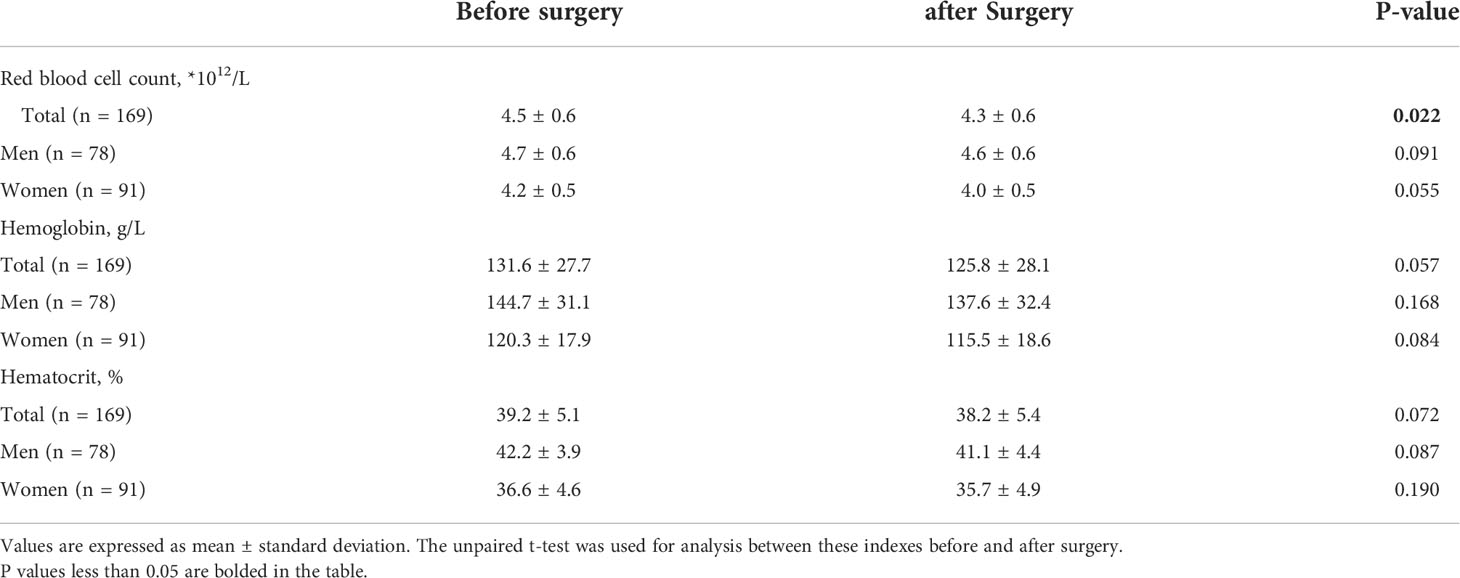
Table 3 Red blood cell count, hemoglobin, and hematocrit before and after surgery in patients with carotid body tumors.
Analysis of postoperative indicators
In the univariate regression analysis, a significant association with a higher risk of temporary postoperative complications in age (OR, 0.09; 95% CI, 0.9–1.0; P = 0.008), length of operation time (OR, 1.0; 95% CI, 1.0–1.0; P < 0.001), blood loss (OR, 1.0; 95% CI, 1.0–1.0; P = 0.003), Shamblin type II vs. I (OR, 0.2; 95% CI, 0.0–0.9; P = 0.035), and red blood cell count postoperative (OR, 0.5; 95% CI, 0.2–1.0; P = 0.043). Further multivariate analyses were performed to screen important independent factors. In addition, the following were adjusted in this model: age, gender, weight, length of operation, blood loss, body tumor location, and Shamblin type. Except for blood loss, the occurrence of temporary postoperative complications was still accompanied with an increase of age (OR, 0.09; 95% CI, 0.9–1.0; P = 0.014), length of operation time (OR, 1.0; 95% CI, 1.0–1.0; P = 0.005), Shamblin type II vs. I (OR, 0.1; 95% CI, 0.0–0.5; P = 0.008), and red blood cell count postoperative (OR, 0.2; 95% CI, 0.1–0.8; P = 0.026). Otherwise, hemoglobin (OR, 0.9; 95% CI, 0.9–1.0; P = 0.011) and hematocrit (OR, 0.8; 95% CI, 0.7-1.0; P = 0.025) after adjusted were also found to be independent factors for temporary postoperative complications (Table 5).
After adjusting for possible confounding factors, the smooth curve fitting was performed to explore the relationships (Figure 1). An SD increase in red blood cell count postoperative was associated with an 80% reduction in the adjusted risk of temporary postoperative complications (OR, 0.2; 95% CI, 0.1–0.8; P = 0.026), not statistically significant in male patients but still obvious in female patients (OR, 0.0; 95% CI, 0.0–0.5; P = 0.018). An SD increase in hemoglobin was associated with a 10% reduction in the adjusted risk of temporary postoperative complications (OR, 0.9; 95% CI, 0.9–1.0; P = 0.011), still not statistically significant in male patients but remarkable in female patients (OR, 0.9; 95% CI, 0.9–1.0; P = 0.030). The relationship between hematocrit and the incidence of temporary postoperative complications was also statistically significant (OR, 0.8; 95% CI, 0.7–1.0; P = 0.025), still remarkable in female patients (OR, 0.7; 95% CI, 0.5–0.9; P = 0.022). Although there was no significant correlation between these three indicators (red blood cell count, hemoglobin, and hematocrit) and the incidence of temporary postoperative complications in male patients, the trend of complications occurrence rate was still reduced with their increase. In patients with CBT with different Shamblin types, the association between blood routine index and temporary postoperative complications was almost the same (Figure 2).
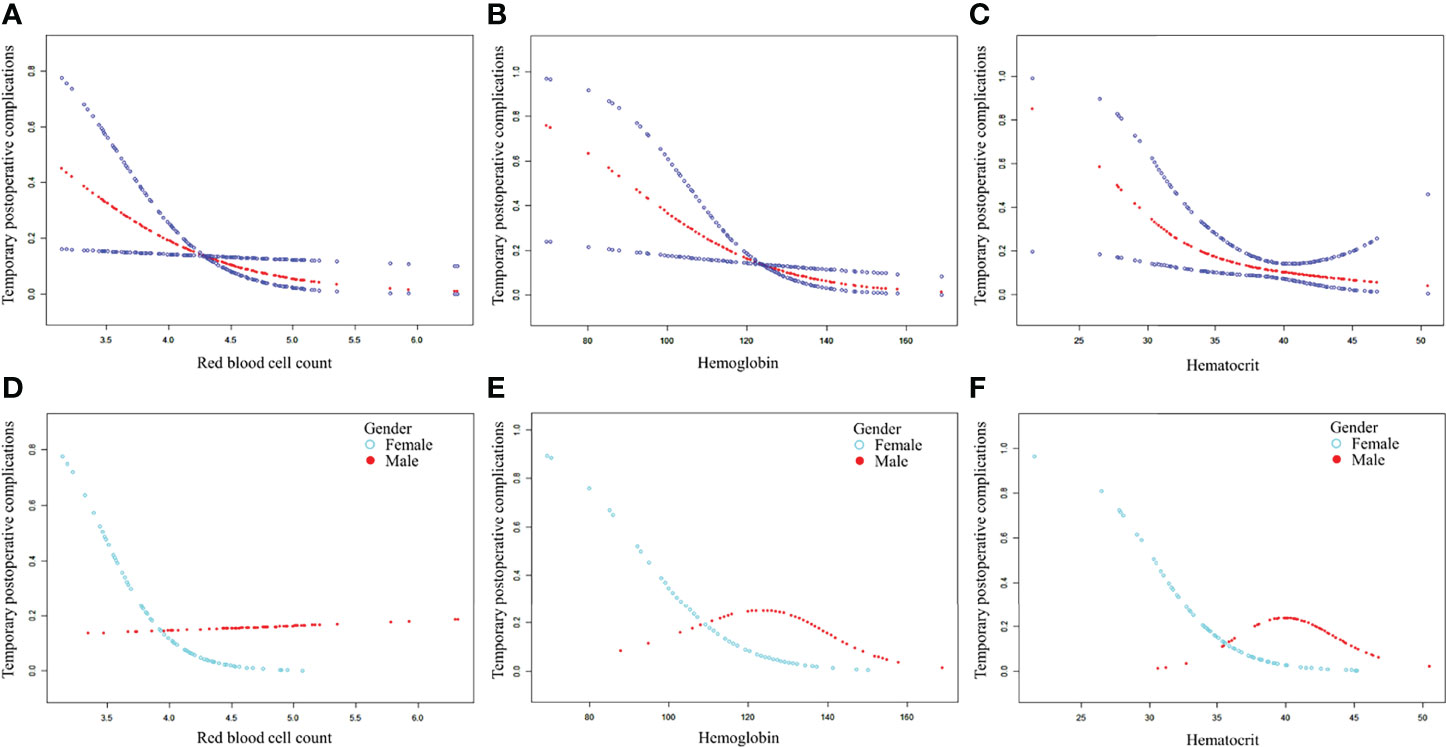
Figure 1 The association between blood routine indexes and temporary postoperative complications. Adjusted for age, gender, weight, length of operation, blood loss, body tumor location, and Shamblin type. (A–C) The association between postoperative red blood cell, hemoglobin, or hematocrit and temporary postoperative complications in total patients after CBT surgery. (D–F) The association between postoperative red blood cell, hemoglobin, or hematocrit and temporary postoperative complications in male and female patients after CBT surgery. CBT, carotid body tumor.
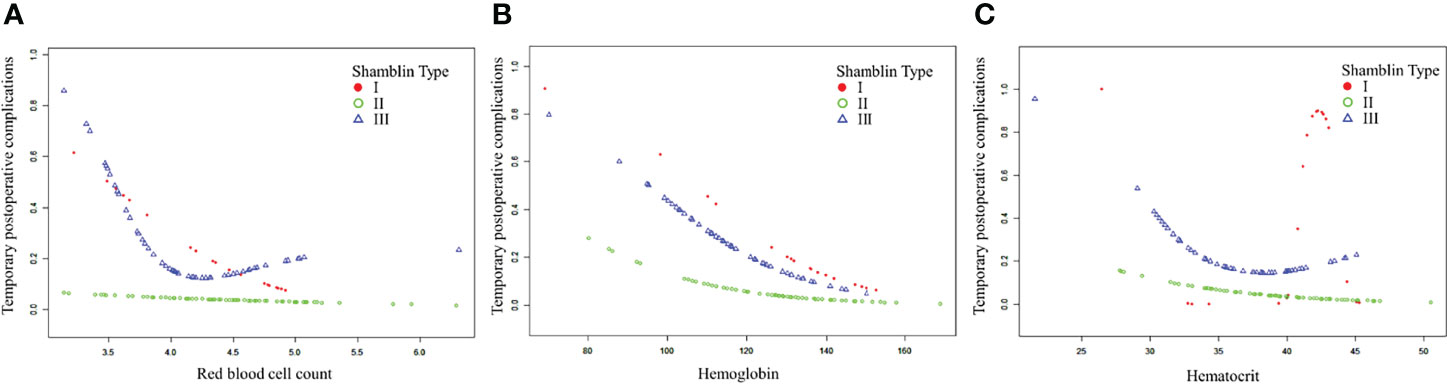
Figure 2 The association between blood routine indexes and temporary postoperative complications in patients with CBT with different Shamblin type. CBT, carotid body tumor. (A–C). The association between postoperative red blood cell, hemoglobin or hematocrit and temporary postoperative complications in CBT patients with different Shamblin type.
Risk prediction model and ROC analysis
In total, seven parameters were included in the risk model: gender, weight, length of operation, Shamblin type, postoperative red blood cell count, hemoglobin, and hematocrit. With the receiver operating characteristic (ROC) curves, the area under ROC curve (AUC) of this risk prediction model was 0.86 (95% CI = 0.77–0.95) in predicting temporary postoperative complications. On the basis of Youden’s index algorithm in the ROC curve, the optimal cutoff value was 2.18, with sensitivity of 95.0%, specificity of 73.13%, PPV of 34.55%, and NPV of 98.99% (Figure 3 and Table 6).
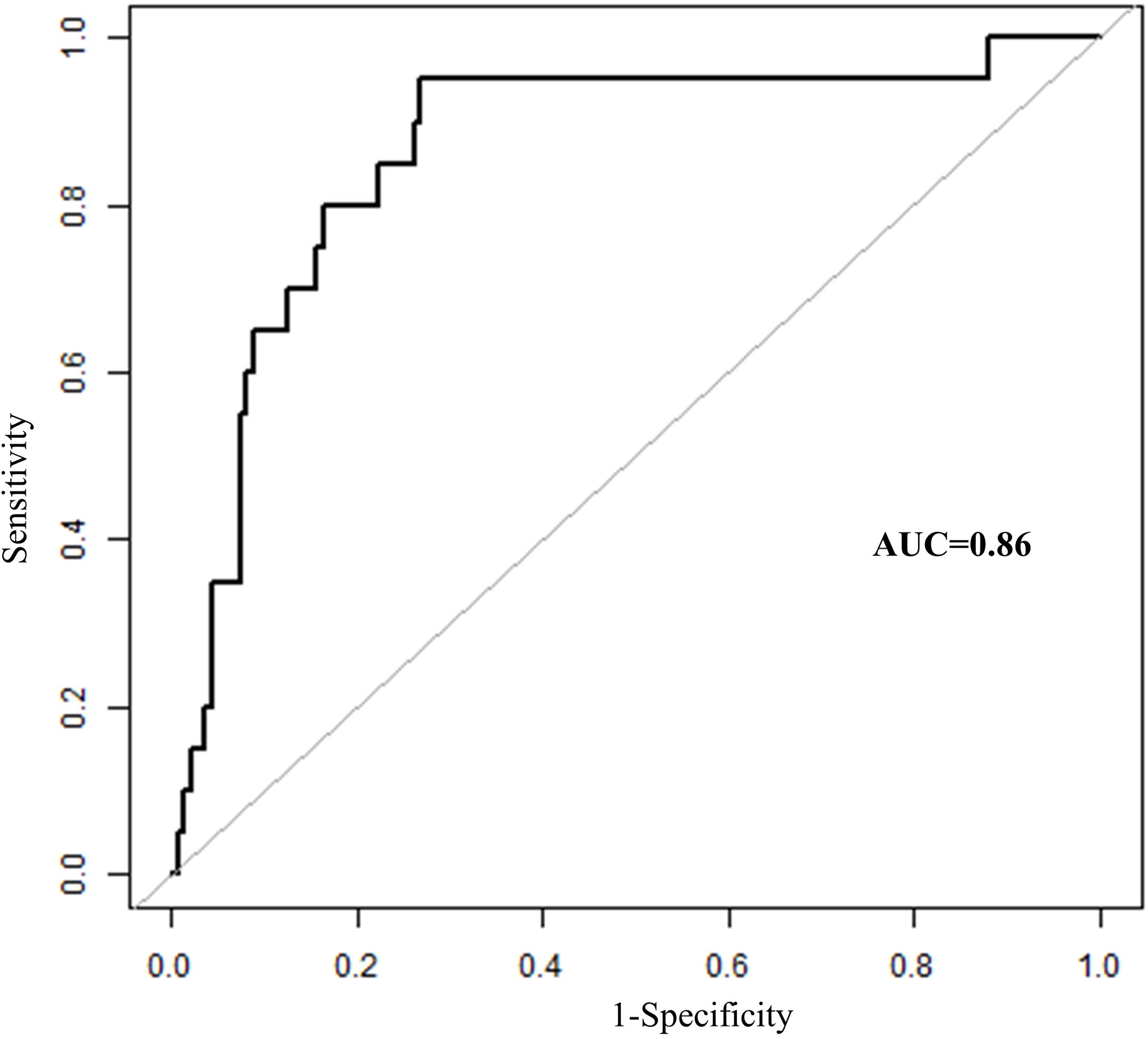
Figure 3 The receiver operating characteristics curves of risk prediction model in predicting temporary postoperative complications after surgical treatment in patients with CBT. CBT, carotid body tumor; AUC, area under ROC curve.
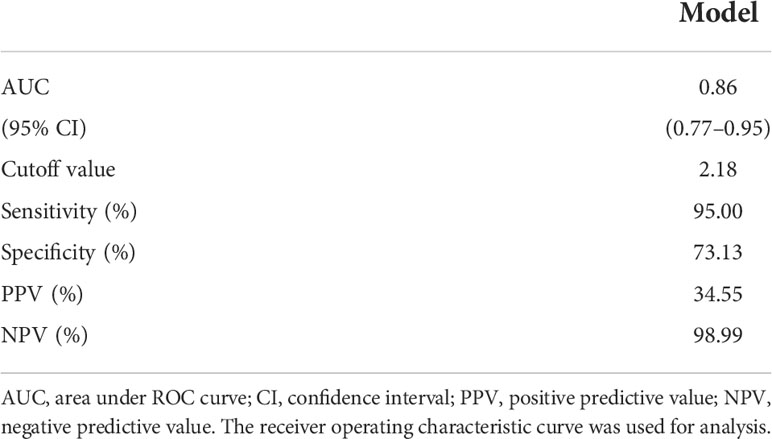
Table 6 The predictive performance of the risk model in predicting temporary postoperative adverse symptoms after CBT surgery treatment.
Discussion
CBTs originate mostly from the paraganglia in the head and neck region (13, 14). They always arise at the carotid bifurcation below the mandibular angle and appear as slow-growing and painless masses (11, 15–17). Some patients with CBTs might present hoarseness or dysphagia because of the tumors’ progressive growth, which compress cranial nerves. Partial cranial nerve function can be recovered or compensated after tumor removal, and the symptoms can also disappear. The previous study indicated that CN XII (hypoglossal nerve, 21.9%), CN X (vagus nerve, 20.3%), and recurrent laryngeal nerve (18.8%) were three most commonly injured cranial nerves after surgical resection of CBT (1). Despite being a rare, mostly benign tumor, CBTs have unpredictable malignant potential (18). Thus, early and complete surgical resection is recommended (7, 19). However, the resection of CBTs often adjacent to or surrounded by many carotid arteries and cranial nerves is rather difficult. Moreover, many unpredictable postoperative complications frequently occur (7, 20). Nevertheless, some symptoms disappear over time, including tongue bias, hoarseness, dizziness, headache, and eyelid ptosis.
These temporary postoperative complications in patients with CBTs after surgical resection were caused by nerve injury or cerebral ischemia/reperfusion. The symptoms of some nerve injuries can be surgically recovered, but other symptoms are permanent. Currently, we do not know how these injured cranial nerves are compensated and repaired. No remarkable differences between men and women in the incidence of temporary postoperative complications in patients after CBT surgery was found in our study. The most common temporary symptom in patients after resection of CBTs in our center is transient ischemic attack, followed by tongue bias and hoarseness. These trends indicate that bleeding is the most important factor affecting the occurrence of temporary symptoms.
Abundant evidence has suggested that preoperative embolization in patients with CBTs can reduce intraoperative bleeding. Significant blood loss was associated with the occurrence of severe complications (8, 21). However, relatively few studies have been performed on determining the mechanisms to reduce the incidence of postoperative complications. For example, Bozan et al. have compared the mean platelet volume, red cell distribution width, and neutrophil-to-lymphocyte ratio before and after surgery in patients with CBTs (22). However, the differences in all these indexes were no statistically significant. Moreover, this study did not consider the occurrence of postoperative adverse events and thus did not analyze their association with the parameters investigated.
Intraoperative hemorrhage can decrease the number of red blood cells, as well as the levels of hemoglobin and hematocrit. On the basis of the amount of bleeding in the patient’s CBT surgery, the vascular surgeon decides whether to perform blood transfusion or not. The red blood cell counts of all patients in our study were reduced after CBT surgery, although the differences between male and female patients before and after the surgery were not obvious. Postoperative hemoglobin and postoperative hematocrit were also decreased, but with no statistical significance. Nonetheless, a significant inverse association was established between these three postoperative indexes and the incidence of temporary postoperative complications, especially in female patients. The analysis of the clinical data revealed several potential reasons for this outcome. The red blood cell count in male patients was significantly higher than that in female patients both before and after the surgery. This physiological difference might have retarded the recovery of women’s cranial nerve function. In addition, part of the nerve injury diagnoses was based on patients’ subjective reports, such as transient amaurosis, headache, or dizziness. Women might be more sensitive than men. Smooth curves between postoperative red blood cell count, hemoglobin, or hematocrit and the incidence of temporary postoperative complications stratified by gender are consistent with the smooth curve of all patients. On the basis of the analysis results of the data, we speculated that the incidence of temporary postoperative complications might be reduced with the increase of the postoperative red blood cell, hemoglobin, or hematocrit level of patients with CBT.
A few temporary postoperative complications might develop into permanent injuries. Reducing postoperative complications to minimize or prevent such occurrences could improve patient prognosis. Thus, the early identification of high-risk individuals was very important. This established risk prediction model was easy to implement and would help clinicians in changing subsequent treatment strategies. For these patients with a high risk of temporary postoperative complications, the appropriate amount of iron supplements or blood transfusion was perhaps necessary. Limited by the small sample size and the low incidence of CBT, a prospective research design was difficult to conduct. Our research only revealed several independent factors associated with the occurrence of temporary postoperative complications and provided a risk prediction model. A randomized controlled trial (RCT) or case-control study is required to evaluate this finding.
Conclusion
The incidence of temporary postoperative complications might be reduced with the increase of the postoperative red blood cell, hemoglobin, or hematocrit counts of patients with CBT, especially in women. The risk prediction model with satisfactory prediction effects could be used for predicting temporary postoperative complications.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statement
This study was reviewed and approved by the Ethics Committee of Naval Military Medical University. Written approval was waived.
Author contributions
Conception and design: TH, SW, JZho, and ZZ. Analysis and interpretation: TH, JZhu, and XW. Data collection: TH, YS, and YX. Writing the article: TH, SW, and JZhu. Critical revision of the article: YS, YX, XW, JZho, and ZZ. Statistical analysis: TH and YS. Obtained funding: XW and ZZ. Overall responsibility: ZZ. All authors contributed to the article and approved the submitted version.
Funding
This study was financed by the Shanghai Clinical Research Youth Project (20184Y0313) and National Natural Science Foundation of China (81770482).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tang H, Jiang X, Xue S, Fu W, Tang X, Guo D. Long-term surgical outcomes of carotid body tumors with pathological fibrosis: A cohort study. Front Oncol (2021) 7:684600(11). doi: 10.3389/fonc.2021.684600
2. Cerecer-Gil NY, Figuera LE, Llamas FJ, Lara M, Escamilla JG, Ramos R, et al. Mutation of SDHB is a cause of hypoxia-related high-altitude paraganglioma. Clin Cancer Res (2010) 16(16):4148–54. doi: 10.1158/1078-0432.CCR-10-0637
3. Smith JJ, Passman MA, Dattilo JB, Guzman RJ, Naslund TC, Netterville JL. Carotid body tumor resection: Does the need for vascular reconstruction worsen outcome? Ann Vasc Surg (2006) 20(4):435–9. doi: 10.1007/s10016-006-9093-0
4. Kruger AJ, Walker PJ, Foster WJ, Jenkins JS, Boyne NS, Jenkins J. Important observations made managing carotid body tumors during a 25-year experience. J Vasc Surg (2010) 52(6):1518–23. doi: 10.1016/j.jvs.2010.06.153
5. Kim GY, Lawrence PF, Moridzadeh RS, Zimmerman K, Munoz A, Luna-Ortiz K, et al. New predictors of complications in carotid body tumor resection. J Vasc Surg (2017) 65(6):1673–9. doi: 10.1016/j.jvs.2016.12.124
6. Abu-Ghanem S, Yehuda M, Carmel NN, Abergel A, Fliss DM. Impact of preoperative embolization on the outcomes of carotid body tumor surgery: A meta-analysis and review of the literature. Head Neck (2016) 38(Suppl 1):E2386–94. doi: 10.1002/hed.24381
7. Hallett JW Jr., Nora JD, Hollier LH, Cherry KJ Jr. Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: A fifty-year experience with 153 tumors. J Vasc Surg (1988) 7(2):284–91. doi: 10.1016/0741-5214(88)90147-4
8. Maxwell JG, Jones SW, Wilson E, Kotwall CA, Hall T, Hamann S. Carotid body tumor excisions: Adverse outcomes of adding carotid endarterectomy. J Am Coll Surg (2004) 198(1):36–41. doi: 10.1016/j.jamcollsurg.2003.08.024
9. Schneider R, Elwerr M, Lorenz K, Plontke S, Dralle H, Ukkat J. [Surgical treatment options for cervical paragangliomas]. Chirurg (2018) 90(1):29–36. doi: 10.1007/s00104-018-0734-y
10. Tamura A, Nakasato T, Izumisawa M, Nakayama M, Ishida K, Shiga K, et al. Same-day preventive embolization and surgical excision of carotid body tumor. Cardiovasc Intervent Radiol (2018) 41(6):979–82. doi: 10.1007/s00270-018-1894-3
11. Liu J, Li Y, Yang L, Cai H. Surgical resection of carotid body tumors with versus without preoperative embolization: A retrospective case-control study. Head Neck (2018) 40(12):2590–5. doi: 10.1002/hed.25387
12. Pacheco-Ojeda LA. Carotid body tumors: Surgical experience in 215 cases. J Craniomaxillofac Surg (2017) 45(9):1472–7. doi: 10.1016/j.jcms.2017.06.007
13. Williams MD. Paragangliomas of the head and neck: An overview from diagnosis to genetics. Head Neck Pathol (2017) 11(3):278–87. doi: 10.1007/s12105-017-0803-4
14. Ma D, Liu M, Yang H, Ma X, Zhang C. Diagnosis and surgical treatment of carotid body tumor: A report of 18 cases. J Cardiovasc Dis Res (2010) 1(3):122–4. doi: 10.4103/0975-3583.70905
15. Pellitteri PK, Rinaldo A, Myssiorek D, Gary Jackson C, Bradley PJ, Devaney KO, et al. Paragangliomas of the head and neck. Oral Oncol (2004) 40(6):563–75. doi: 10.1016/j.oraloncology.2003.09.004
16. Lozano-Corona R, Anaya-Ayala JE, Martinez-Martinez R, Lopez-Rocha S, Rivas-Rojas MA, Torres-Machorro A, et al. Usefulness of preoperative three-dimensional volumetric analysis of carotid body tumors. Neuroradiology (2018) 60(12):1281–6. doi: 10.1007/s00234-018-2095-0
17. Hinojosa CA, Ortiz-Lopez LJ, Anaya-Ayala JE, Orozco-Sevilla V, Nunez-Salgado AE. Comparison of retrocarotid and caudocranial dissection techniques for the surgical treatment of carotid body tumors. J Vasc Surg (2015) 62(4):958–64. doi: 10.1016/j.jvs.2015.05.001
18. Luo T, Zhang C, Ning YC, Gu YQ, Li JX, Wang ZG. Surgical treatment of carotid body tumor: Case report and literature review. J Geriatr Cardiol (2013) 10(1):116–8. doi: 10.3969/j.issn.1671-5411.2013.01.018
19. Knight TT Jr., Gonzalez JA, Rary JM, Rush DS. Current concepts for the surgical management of carotid body tumor. Am J Surg (2006) 191(1):104–10. doi: 10.1016/j.amjsurg.2005.10.010
20. Shamblin WR, ReMine WH, Sheps SG, Harrison EG Jr. Carotid body tumor (Chemodectoma). clinicopathologic analysis of ninety cases. Am J Surg (1971) 122(6):732–9. doi: 10.1016/j.amjsurg.2005.10.010
21. Li YH, Wang JS, Yao C, Chang GQ, Yin HH, Li SQ, et al. [Risk factors of rupture of internal carotid artery during surgical resection of carotid body tumor]. Zhonghua yi xue za zhi (2017) 97(22):1724–8. doi: 10.3760/cma.j.issn.0376-2491.2017.22.010
Keywords: carotid body tumor, temporary postoperative complications, red blood cell, hemoglobin, hematocrit, risk prediction model
Citation: Han T, Wang S, Zhu J, Sun Y, Xie Y, Wei X, Zhou J and Zhao Z (2022) Low red blood cell predicts high risk of temporary postoperative complications after carotid body tumor surgical resection. Front. Oncol. 12:906048. doi: 10.3389/fonc.2022.906048
Received: 28 March 2022; Accepted: 24 June 2022;
Published: 25 July 2022.
Edited by:
Jun Itami, Shinmatsudo Central General Hospital, JapanReviewed by:
Vincenzo Lizzi, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, ItalyKaichuang Ye, Shanghai Jiao Tong University, China
Copyright © 2022 Han, Wang, Zhu, Sun, Xie, Wei, Zhou and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolong Wei, ZHJ3ZWl4bEBxcS5jb20=; Jian Zhou, emhvdWppYW4xLTJAMTYzLmNvbQ==; Zhiqing Zhao, emhhb3pxeHVlZ3VhbkAxMjYuY29t
†These authors have contributed equally to this work
 Tonglei Han1,2†
Tonglei Han1,2† Jian Zhou
Jian Zhou Zhiqing Zhao
Zhiqing Zhao