
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 04 July 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.905976
This article is part of the Research Topic Meningioma: From Basic Research to Clinical Translational Study, Volume II View all 32 articles
Meningiomas are the most common primary brain tumors accounting for about 30% of all brain tumors. The vast majority of meningiomas are slow-growing and of benign histopathology rendering them curable by surgery alone. Symptomatic lesions depend on the location with signs of mass effect or neurological deficits. Seizures are the presenting symptoms in approximately 30% of cases, which negatively affect quality of life, limit independence, impair cognitive functioning, as well as increase the risk for psychiatric comorbidities including depression. Although surgical resection may offer seizure freedom in 60-90% of meningiomas, seizures persist after surgical resection in approximately 12-19% of patients. Anti-seizure medications (ASMs) are employed in management, however, are limited by adverse neurocognitive side-effects and inefficacy in some patients. The potential predictors of pre- and post-operative seizures in meningioma patients have been identified in the literature. Understanding various factors associated with seizure likelihood in meningioma patients can help guide more effective seizure control and allow for better determination of risk before and after surgery.
Meningioma accounts for about 30% of primary brain tumors and approximately 54% of primary benign ones (1–3). The vast majority of meningiomas are slow-growing and of benign histopathology (i.e., World Health organization (WHO) grade I tumors), rendering them curable by surgery alone (4, 5). Symptomatic lesions depend on the location with signs of mass effect or neurological deficits. Seizures are the presenting symptom in approximately 30% of cases, and in some studies, the percentage ranges from 13-60% (6–8). Although surgical resection can offer seizure freedom in 60-90% of meningiomas, seizures may persist after surgical resection in about 12-19% of patients (9, 10). Seizures can negatively affect the quality of life, hindering a patient’s independence, cognitive functions, and ability to drive safely (11–13). It puts patients at increased risk for different psychiatric comorbidities, including depression (14). Seizure control using various anti-seizure medications (ASMs) is usually offered despite adverse side effects on neurocognition and inefficacy in some patients (15).
Many theories have been postulated to explain the pathogenesis of brain tumor-related epilepsy (BTRE) in various brain tumors; however, unanswered questions remain regarding seizure control and management in meningioma patients, for example, the ability of surgical resections to cure seizures, when to start ASMs, duration of treatment as well as structured guidelines for patient selection for ASMs. Understanding and predicting seizures in meningioma can help guide seizure control and allow for better determination of at-risk patients before and after surgery. This review aims to summarize the pathogenesis of seizures in meningioma, pre- and post-operative predictors of seizures, surgical resection resulting in seizure freedom, the benefit of ASMs usage, intraoperative electrocorticography (ECoG) and electroencephalogram (EEG) monitoring in meningioma patients and proper patient selection.
The incidence of pre-operative seizures in meningioma was respectively reported to be 29% of 4709 patients (7) and 14% of 598 patients (16) with supratentorial meningioma. Seizure freedom was achieved in about 69% of patients after surgery with 12% of new seizures onset postoperatively (17). Chozick’s study reported 63/158 patients with meningioma had pre-operative seizures and 40 (63.5%) of the 63 patients had complete resolution of seizure after surgery within follow-up years of 7.3± 3.8. In this cohort, 100% of 63 patients were on anti-seizure medications anticonvulsant preoperatively and during the initial stage postoperatively. The authors did not report the exact portion of these 43 patients weaned from anti-seizure medication over time postoperatively. While some neurosurgeons tended to stop using the medication approximately 6 months after surgery if there was no evidence of seizures, the other neurosurgeons continued using the anticonvulsant medications prophylactically. They reported that eighty-five patients (53.8% of 158) were eventually weaned from anticonvulsant and 44.7% were not off anti-seizure medications at the last follow-up visit postoperatively. Seizures recurred in 1 patient during weaning off ASMs process, in 4 patients with subtherapeutic ASMs levels, in 6 patients who were not on ASMs, in 2 patients correlated with alcohol abuse, and 5 patients with tumor recurrence. Eight patients (5.1%) with no history of preoperative epilepsy developed postoperative seizures. Chozick et al. concluded that in their study only the extent of tumor removal was a significant predictor of postoperative seizures. However, a history of preoperative seizures, preoperative language disturbance, postoperative anti-seizure medications status, postoperative hydrocephalus, or parietal region location of tumor were also predictive factors of the occurrence of postoperative seizures (18). Wirsching reported 26.6% of postoperative seizures within median 67 months (95% CI: 63–72) of post-surgery follow-up (19). The incidence of de-novo seizures in seizure naïve patients ranges widely from 2.4 to 19.4% (7, 17–23).The wide variation of these studies can be contributed to lacking standardization of retrospectively collected data from patients with different demographics, different features/locations/type of meningiomas, different follow up periods, different age groups analysis between pediatric and adult patients, and different surgical skills and techniques at different institutions. The majority of postoperative seizures were experienced in the first week after surgery, but one-third of patients experienced seizures three months after surgery (17).
The pathophysiology of brain tumor related epilepsy is multifactorial and can be divided into morphologic, biochemical and metabolic causes. The morphologic changes in the peritumoral neocortex include the connection of the neurons and the connectivity and localization of the synaptic vesicles, causing higher concentration of voltage-dependent Na+ channel, Ca++ and Glutamate receptors with loss of inhibitory synapses and an increase of the excitatory synapses. Biochemically, there is an increase of Glutamatergic and reduction in GABAergic somatostatin immunoreactive neurons. At the ion level, there is a report of low Mg2+, high extracellular K+, high Fe3+, low neuron-specific K+/Cl− cotransporter-2 (KCC2). Extracellular peritumoral pH is thought to be slightly alkaline. Finally there are enzymatic, amino acid and immunologic changes with upregulation of Glutamatergic receptors for NMDA and AMPA neurotransmitters (24). More recently, the genetic drivers of epileptogenicity in meningiomas have been investigated. NF2 mutation was shown to be predictive marker for preoperative seizures, which was via an indirect mediation effect with atypical histology and edema (25). Meningioma originates from arachnoid cap cells and is usually a slow-growing tumor (1). Such slow growth can partially explain the peritumoral changes that lead to epileptogenicity (24, 26). The partial differentiation of cortical brain surface may produce an epileptogenic zone, thus causing denervation hypersensitivity (27). The morphologic changes that develop in the brain tissue adjacent to the lesion, like inefficient neuronal migration, synaptic vesicles, and glial gap-junction coupling alterations, are also thought to contribute to seizure generation (28). Although pediatric meningioma is rare, epilepsy was reported as one of common symptoms (29). Inefficient neuronal migration may serve as an additional peritumoral mechanism of epileptogenesis in this age group of patients.
The percentage of brain edema in patients with meningioma ranges between 30% to 60% (30–32). It is usually vasogenic and related to an increase in pial supply, angiogenesis, and increased expression of vascular endothelial growth factor (VEGF) (33, 34). Chemical changes in the peritumoral milieu and local hypoxia from local tumor compression are thought to be underlying mechanisms that decrease the threshold for seizures (26). Increased levels of glutamate in the peritumoral edema are often described as an instigating factor for the state of hyperexcitability and epilepsy (8, 26). Edema is strongly correlated with brain invasion (35), and may also be intimately associated with tumor location and more invasive and higher grades of meningioma (31, 32, 36). Notably Hess et al. reported a five-fold increase in edema volume in patients with brain invasion compared to those without, with a reported 20% increase of risk of brain invasion with each 1cm increase in peritumoral edema (35). Chernov, et al. reported a high incidence of peritumoral edema in macroscopically invasive meningiomas (37). Brain invasion and breakdown of the arachnoid layer distort and alter the peritumoral cortex, releasing amino acids and affecting the neurotransmitter pathway (35, 38).
For post-operative seizures onset, intraoperative strong adhesions, the need for microdissection, and possible injury to cortical surface and irritation can contribute to the generation, especially in seizure naïve patients (36). Retraction and manipulation, which are sometimes necessary to achieve total resection in skull base lesions, can also lead to further cortical damage and edema (39). Post-operative complications like infection, hematoma, and hydrocephalus can further increase cerebral edema and increase the risk of seizures (40).
Based on histopathological characteristics, the WHO grading system classifies meningiomas into grade I (benign), grade II (atypical), and grade III (anaplastic) (41). Hess et al. analyzed the brain invasion and risk of seizure retrospectively in a total of 176 patients with meningioma. There were 92 (52%) grade I, 79 (45%) grade II, and 5 (3%) grade III tumors. Grade I meningioma included 16 (17%) transitional, 4 (4%) secretory, 68 (74%) meningothelial, 3 (3%) fibrous, and 1 (1%) angiomatous subtypes. Preoperative seizures were present in 10 (11%) of 92 patients with grade I meningioma, 23 (29%) of 79 patients with grade II meningioma, and absence in patients with anaplastic meningioma. In grade I meningioma, histopathological subtype correlated significantly with the rate of preoperative epilepsy. Overall, the risk of preoperative seizures was significantly higher in patients with a grade II or III tumor than in those with a grade I tumor. Brain invasion was absent in all patients with a grade I meningioma, but it was present in 35 (44%) of those with an atypical and 3 (60%) with an anaplastic meningioma. Brain invasion was independent of tumor volume but strongly correlated with edema volume. Multivariate analyses showed the risk of preoperative seizures was increased distinctly in patients with brain-invasive meningioma over those with noninvasive meningioma (OR 5.26, 95% CI 1.52–18.15; p = 0.009). However, postoperative seizure-free rates were similar among patients with invasive and those with noninvasive meningioma. The incidence of postoperative epilepsy was correlated significantly with the increasing preoperative tumor volume (35). In another retrospective study, Gadot et al. reviewed the 384 patients who underwent meningioma resection. The significant association was not found between any histological subtype and worse postoperative seizure outcomes. However, there was an associative tendency between subtypes of higher grades (malignant, rhabdoid) with worse postoperative seizure outcomes. The subtypes of lower grades (fibrous, transitional) trended toward improved postoperative outcomes (p = 0.081) (25). There are no data in the medical literature for the incidental small meningioma, which are not part of the epileptogenic network.
In an attempt to better understand and predict seizures in patients with meningioma, several retrospective studies investigated the possible predictors of seizures both pre-operatively and post-operatively. Throughout the literature, peritumoral edema and location have been associated with seizures in meningioma. Peritumoral edema has been extensively studied and considered the strongest predictor of seizure in both pre- and post-operative periods (7, 8, 17, 20, 21, 26, 35). There is a less likelihood of achieving seizure freedom postoperatively in patients with with significant pre-operative edema (21, 42).
The preoperative predictors of epilepsy/seizures are summarized in Table 1.
In a retrospective study by Li et al., peritumoral edema of > 1cm was among the risk factors identified for preoperative seizures in meningioma patients (43). Tumor location in the temporal, parietal, and frontal (adjacent to neocortex) lobes are more likely to be associated with seizures (7, 18, 20, 21). Specifically, Lieu and Howng noted that tumor located in the temporal lobe increased the risk of pre-operative seizures than other lobes. The increased peritumoral edema noticed in convexity and parasagittal meningioma is thought to favor the likelihood of increased seizure frequency in affected individuals. Non-skull base meningiomas are suggested to be more aggressive with a high MIB-index (percentage of immunoreactive tumor cells) which favors brain invasion, edema, and seizure (20, 45). In another study, no consensus was found regarding the most epileptogenic cortical area (46).
Most studies suggest that bigger tumors are naturally associated with a higher risk of seizure preoperatively. Conceivably, larger tumors can cause more irritation and compression on surrounding brain tissue. Similar results reported by Chen et al. showed that tumors larger than 3 cm in size, of higher grade with peritumoral edema more than 1 cm are associated with preoperative seizures (20). In one study, no statistically significant correlation between tumor size and preoperative seizures could be found (43), while mean tumor diameter of 3.5 cm was used at cut-off to demonstrate an association with postoperative in-hospital seizures.
Interestingly meningiomas are more common in females, but males are more likely to present with seizures. Many studies have shown the male gender as a risk factor for developing preoperative seizures (7, 8, 20, 23, 43). There is a possible association of male gender with higher grade meningioma, larger size, and more edema (20). Younger age was a predictor (44), and a lower incidence of preoperative seizures was found in meningioma patients older than 55 years old (43).
Other factor like preoperative Karnofsky score (KPS) were also studied. A KPS <80 was positively associated with pre-operative seizures (40). Englot et al. reported a decreased incidence of preoperative seizures in patients presenting with cranial nerve deficits (7). However, there are limitations in symptom frequency studies. Prospective studies are needed to validate these potential predictors.
The postoperative predictors of epilepsy/seizures are summarized in Table 2.
The International League Against Epilepsy (ILAE) defined acute postoperative seizures as seizures happening within seven days of craniotomy (48). The late postoperative seizure is defined as on set of epilepsy beyond the first week of surgery (21, 49). In a retrospective study of 556 patients who underwent meningioma surgery, there were 74 patients with postoperative seizures, in which 43% was late seizures (49). Some studies categorized postoperative seizures into early, late, in-hospital, and post- hospital discharge. Identifying possible predictors of seizures postoperatively can help guide seizure control and minimize complications associated with ASMs long-term usage (13, 17, 20, 43, 47, 50).
Tumor location, size, grade, involvement of motor area and KPS have all been studied as predictors for postoperative seizures (21, 23). In one study, the occurrence of early in-hospital seizures was associated with involvement of motor cortex, post-operative KPS < 70, postoperative complications, and preoperative seizures (43). It was suggested that decreased threshold and the increased cortex sensitivity during the immediate postoperative period are important factors to be considered, and ASMs use may be justifiable in this period. The KPS < 80 was an independent predictor for postoperative seizures, with an almost threefold higher risk of having preoperative seizures (40). This further explains the impact of seizures on quality of life. Skull base lesions were associated with decreased incidence of seizures preoperatively, with an opposite trend and increased incidence in the postoperative period (40). Chen et al., in one study of 1033 patients, reported decreased incidence of seizure in non-skull base lesions (20). Skull base lesions require more brain retraction, further increasing brain edema (7, 51). Scott et al. noted an association of left-sided meningioma with greater risk for developing seizures (52), with higher rates of postoperative seizures reported on the left hemisphere (66.7%) compared to the right (23.3%) (17). In a radiological study analyzing 3D structural magnetic resonance imaging (MRI) of meningioma patients to identify hotspots for seizures, results showed a high likelihood of seizures when the lesion was located on the motor cortex of the frontal lobe (44).
Preoperative seizures were strong predictors of postoperative seizures, especially uncontrolled ones (13, 17, 20, 43). There is a contradiction in the literature regarding neurological deficits as presenting symptoms. In some studies, it was associated with less incidence of preoperative seizures (17, 20), and in others, it was found to be significantly associated with postoperative seizures before discharge (10, 19). On univariate analysis, Chen et al. found that a neurological deficit in the form of new weakness, pneumonia, hematoma, and infarction with edema were significantly associated with in-hospital seizures. In their study, weakness was a predictor for in-hospital but not pre-operative or post-discharge seizures (20). Interestingly, Wirsching et al. found that postoperative improvement and recovery from preoperative neurological deficits were associated with a lower risk of postoperative seizure and improved control (19).
Postoperative complications are independent predictors of postoperative seizures (20). In the immediate postoperative period, the brain is more sensitive with a decreased threshold for seizure (43). Any irritation to the highly sensitive and probably still edematous neocortex can aggravate seizures immediately after surgery. A positive correlation has been established between postoperative complications like hematoma, hydrocephalus, infection, and edema (40). Permanent new postoperative neurological deficits, especially in patients with vascular injury, increased the risk of seizures postoperatively significantly (47). Wirshing et al. specified major surgical complications like central nervous system infections, hydrocephalus, re-craniotomy, and symptomatic intracranial hemorrhage as risk factors for postoperative seizures (19).
For seizures after discharge, Li et al. identified tumor size > 3.5 cm, preoperative seizures, and tumor progression as strong predictors (43). In the same study, postoperative complications were associated with acute postoperative seizures, but no correlation with postoperative seizures on long-term follow-up. In another study, surgical complications were associated with in-hospital seizures and post-discharge seizures in seizure naïve patients (19, 53). Chen et al. did not find tumor recurrence or subtotal resection to be strong predictors for postoperative seizures (20). Englot et al. found a strong association of cranial nerve deficits with post-discharge seizure on univariate analysis (7).
Improved surgical techniques and earlier diagnosis of meningioma have affected the extent of resection with favorable outcomes. As previously reported, surgery offers seizure freedom in 70% of patients with rates ranging between 19% to 90% (7, 21). In some studies, the overall seizure freedom over a 5-year follow-up was 87% in patients with preoperative seizures and 59% in seizure naïve patients (4, 43). Lu et al. reported a 30-40% postoperative seizures in patients with seizure history preoperatively and 10-15% in seizure naïve patients (40). Komotar et al. showed a significant influence of gross total resection on seizure rates (54). These reports support surgical intervention and cytoreduction in patients with persistent seizures. In contrast, new postoperative seizures were reported to occur more frequently in patients with gross total resection (46). A possible explanation is that greater manipulation, dissection, and retraction of the brain to achieve gross total resection, can cause cortical injury, irritation, edema, and seizures. In one study, Simpson grade I resection was correlated with postoperative seizures (39). Most of these lesions were convexity meningiomas, which strongly correlate with seizures. Therefore, Simpson grading was not clinically relevant in that study. Similar results were reported by Hess et al., with no statistical significance noted between Simpson grade and postoperative seizures (35). Multiple studies showed an association between seizure and tumor recurrence/progression (23, 47). One postulated theory is that there is possible reactivation of previous epileptogenic focus or formation of a new one with tumor recurrence (40, 43). WHO grade I lesions have low recurrence rates, and with gross total resection, this can be a protective factor against postoperative seizures (4, 5).
Most of the data in the literature report seizure freedom after craniotomy and resection, with few studies discussing other treatment modalities like radiosurgery. Kondziolka et al. reported one case of mortality without further details (54). In Zada’s study of 116 patients undergoing Gamma knife for meningioma, there were zero seizure rates over 75 months of follow-up (55). Pollack et al. reported a 1.6% rate of new or worsened seizures after radiosurgery (56). Decreased seizure freedom rates have been reported after surgery in patients with intractable seizures preoperatively (40).
The American Academy of Neurology does not recommend the prophylactic use of ASMs in newly diagnosed brain tumors. In our institution we do not advocate for obtaining EEG pre-operatively to help in determining placing patient on ASMs. Yet some surgeons advocate for prophylactic use of ASMs in the immediate postoperative period to prevent de-novo seizures (57). In one study by Zheng et al., ASMs reduced the risk of early postoperative seizures (8, 58). ASMs can be used in patients with preoperative seizures as a temporizing measure until surgical resection. It is estimated that 40% of patients with well-controlled seizures before surgery could be weaned off ASMs over 27 months postoperatively, and only 22% remained with intractable seizures (8). For better patient selection and ASM use postoperatively, the STAMPE scoring system was an attempt to help guide epilepsy treatment in meningioma patients (19). They suggested a simple scoring system comprised of possible risk factors like sensorimotor deficit, tumor progression, age < 55 years, major surgical complication, preoperative seizures, post-operative EEG, and brain edema. Results were however not statistically significant and needed further validation.
Evaluation for epilepsy surgery for further resection after delineating the epileptogenic zone by intracranial EEG monitoring (grids, strips, or stereotactic electrodes) including intraoperative ECoG has been the gold standard approach in Level 4 epilepsy centers for patients with lesional epilepsies who have failed at least two adequately selected and dosed ASMs. EEG can be helpful for the assessment of seizure recurrence upon weaning or withdrawal of ASMs. Multiple studies suggested routine uses of EEG postoperatively to predict seizure recurrence. In one study of 340 patients, epileptiform discharge predicted postoperative seizures, advocating for routine EEG postoperative use (19). Intraoperative ECoG mapping and resection of secondary seizure focus in the peritumoral cortex can increase rates of seizure freedom postoperatively (23, 27). Postoperative EEG with epileptiform discharges is suggested as predictor for postoperative seizure occurrences (19, 59). However, the American academy of Neurology published a guideline practice in adult patients with epilepsy who achieved seizure freedom (though not specifically for meningioma), ordering EEG to detect interictal epileptiform discharges is not helpful to guide the decision of ASMs continuation. However, higher confidence of this approach exists in pediatric patients. An epileptiform potentials on EEG in pediatric patients increases the risk of seizure recurrence (60).
In our center we assess every patient with meningioma related epilepsy, particularly patients who continue to have uncontrolled postoperative seizures with stereotactic depth electrodes (S-EEG) implantation or by subdural grid/strip electrodes, and in cases where functional mapping is essential to rule out the involvement of eloquent cortex of the epileptogenic zone. S-EEG provides a safer option for patients who are planned to have a second surgery knowing the expected challenges from prior surgery complications like adhesions, infections, bleeding etc. Gross functional mapping can also be performed by S-EEG comparing to detailed functional mapping by grid/strip electrodes. In areas where there is room for safer resection outside eloquent cortices, S-EEG is helpful to encompass the surrounding edges of the lesion and for reaching remote areas of interest as well, like mesial temporal structures to rule out dual pathology.
The following case illustrates our own experience in post-operative seizure management after meningioma resection. A 36-year-old left-handed male who underwent left midfrontal parasagittal superior larger meningioma (6 x 7 cm) resection developed new-onset seizures 8-10 months postoperatively. His 3-month post-surgical MRI showed complete resection of the tumor. Approximately 11 months after the resection he developed his first ‘tonic-clonic’ seizure. It started with right-sided numbness, weakness and tingling of his back going down his mid-spine. He was subsequently started on Lamotrigine, but continued to experience repeated seizures which started with the same tingling sensations down to his spine, coupled with abnormal butterfly sensations in his abdomen, ultimately culminating in right foot shaking movements, with further spread to his right arm. Due to developing drug resistant epilepsy (DRE) including lamotrigine, lacosamide and levetiracetam, he underwent further epilepsy surgery evaluation, including scalp video EEG and intracranial EEG monitoring with stereotactic S-EEG intracranial monitoring, which resulted in greater delineation of the epileptogenic zone in the left central and paracentral frontal channels behind the posterior and mesial margins of the surgical cavity, likely with earlier onset on the mesial surface of left side of the interhemispheric fissure given early involvement of the right foot (Figure 1). Approximately 25 months after his initial surgery, he underwent a second scheduled left sided frontal craniotomy for resection of epileptogenic foci. He was continued on antiseizure medications postoperatively with subsequent self-reported improvement in seizure frequency. Since undergoing his second surgery, there has been notable reduction in seizure frequency from twice per week to twice per year from focal aware type triggered by medication reductions or alcohol consumption.
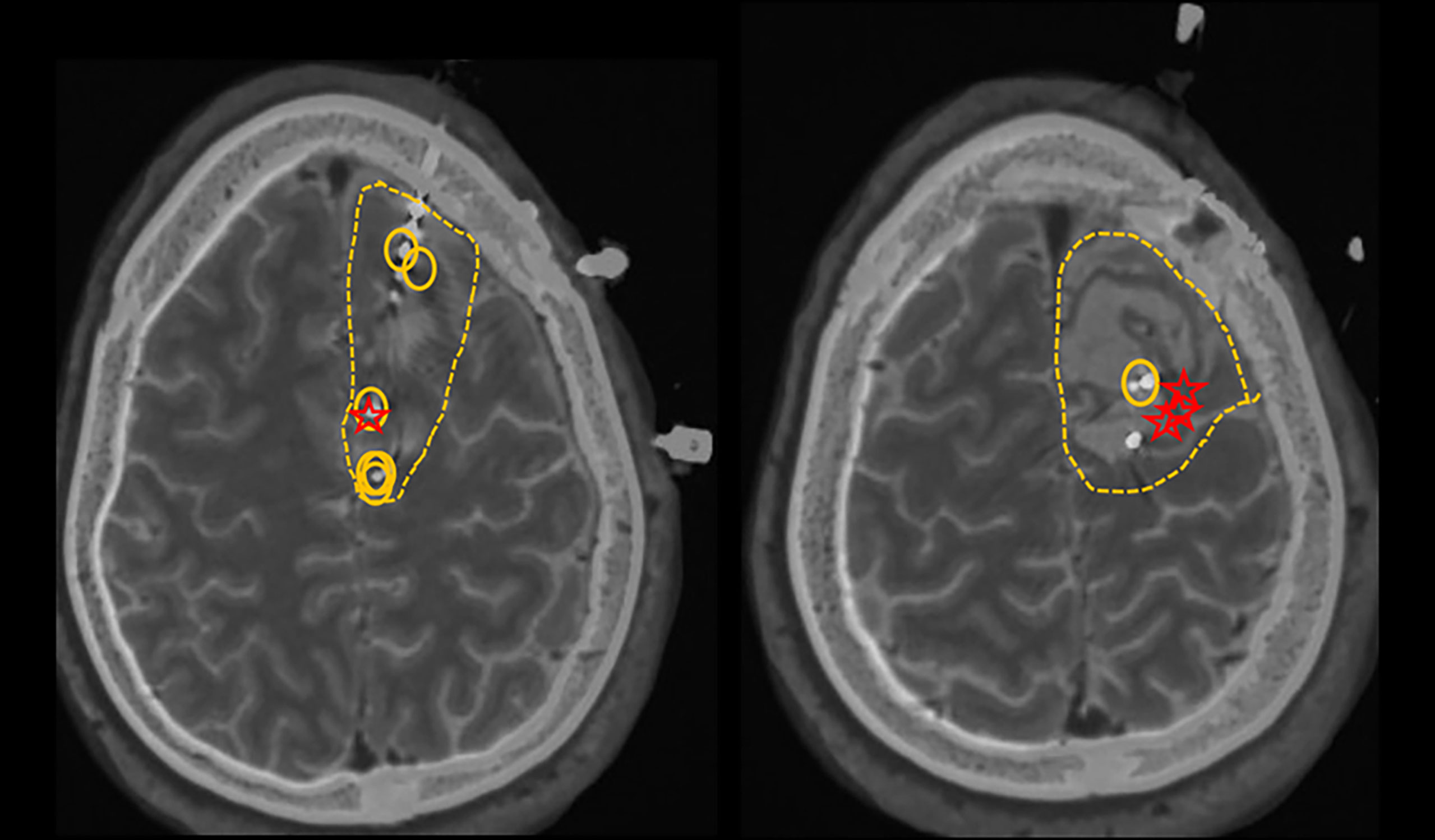
Figure 1 Circles represent active interictal epileptiform discharges (the irritative zones). Stars represent the first involved contacts at the ictal onset. Dashed lines represent the proposed resection zone.
The case report is used as example to show the complexity of management for meningioma patient underwent craniotomy surgery. A separate IRB-approved project will be performed to analyze retrospectively the success rate of such procedures in our Center.
When it comes to the medical treatment of primary brain tumors (PBTs) in general, there is no robust, randomized studies to support the choice of ASMs. Several factors should be taken into considerations including gender, age, cost, profession, cognition, common medication related side effects, neurological baseline related to tumor/surgery (in order to avoid additive drug adverse events), medications pharmacokinetics, drug-to-drug interactions, efficacy and comorbidities. Other considerations are interaction with chemotherapy treatment and radiation effect on the brain. Some type of tumors (like low grade tumors) are known to be resistant to treatment with ASMs due to several hypothesis like intrinsic severity of the underlying mechanism of epileptogenicity, altered expression of molecules which ASMs work on, or changing of the expression of transporters at the blood-brain barrier limiting drug penetration to the epileptogenic tissue (61). Newer ASMs (oxcarbazepine, topiramate, lamotrigine, levetiracetam, zonisamide, and lacosamide) have provided better tolerability and efficacy due to different aspects including non-enzyme inducing property, limited drug to drug interaction, pure renal excretion, and lesser side effects. Older generation ASMs like carbamazepine, phenytoin and phenobarbital are falling out of favor due to high protein-bound, medications interaction and hepatic P-450 induction. Adverse events to ASMs are reported to be higher in PBTs than in the general epilepsy population (24% vs 0.5-12%) (57). In PBTs ASMs adverse events directed to the brain function such as executive function, attention span, cognitive function are six fold higher than the adverse events related to the radiation of the brain (62). Overall, the best risk–benefit ratio of which ASM to use is based on the physician’s judgment. It is very important to mention that treatment should be started after a single seizure. Based on the American Academy of Neurology (AAN) guideline, there is no need for prophylactic treatment with ASMs in patients with brain tumor, without history of seizures. It is also suggesting that tapering and discontinuing ASMs after the first postoperative week is appropriate if there is no history of seizures (57). In summary, the strategy of drug selection for the management of BTRE should favor drugs with parenteral administration, the ASMs which don’t need slow titration, and should avoid enzyme inducing ASMs. If monotherapy fails, consider combination therapy, poor compliance, repeated surgery and tumor recurrence/progression.
There is a wide range of reported efficacy of each individual ASM: oxcarbazepine as a monotherapy: 62.9%; topiramate as a monotherapy: 55.6%; Gabapentin, pregabalin, tiagabine, zonisamide as an adjunctive therapy: 27.4-100%, levetiracetam both in monotherapy and as add-on: 47.4% to 88%; lacosamide as an add-on drug with 42.9% (63). Levetiracetam and valproic acid are the most widely studied medications in tumor related epilepsy. Levetiracetam was studied against Valproic acid and the failure to treat seizures in glioblastoma was 33% vs 50% perhaps due to its tolerability and property of enhancing p53-mediated inhibition of methylguanine-DNA methyltransferase in this patient population (64). The most attractive factors for levetiracetam popularity are its well tolerability, its ease to use without a need for titration, no interaction with other ASMs, not hepatically metabolized by CYP450, and thus the absence of interaction with some chemotherapy drugs used in certain cases of BTRE, and finally good insurance coverage.
In a recent published survey of ASMs prescription preference among the European neuro-oncologists, levetiracetam is considered the first choice for brain tumor patients with the presumed highest efficacy and least adverse effects (65). ASMs are different in the pharmacokinetics, treatment efficacy, and side effects, which were reviewed by Maschio in detail (63).
Management of seizures should extend beyond pharmacological options. Untreated seizures can put patients at a risk of catastrophic outcome such as sudden unexpected death in epilepsy patients. Furthermore, seizures can negatively affect patient lifestyle including work, employment, education and driving. The risk of physical injury or death is not restricted to the driver and passengers, but applies to pedestrians and people in other vehicles. Different American States have different laws to determine which group of patients with epilepsy can drive. Seizures can result in other physical injuries. Patients with intractable epilepsy should be treated in tertiary centers where they can receive medical, social, and behavioral support and more importantly evaluation for epilepsy surgery.
Despite advancements in understanding the pathophysiologic mechanisms and management of meningioma related epilepsy, important knowledge gaps remain. Pertinent questions include, “who are patients most at risk for seizures?” and “when to start ASMs and for how long?". The risk of persistent postoperative seizures underscores the need for further research on seizure control in meningioma patients. Long-term and arbitrary use of ASMs in meningioma patients emphasize the importance of guidelines for appropriate patient selection. Thus, prospective randomized trials are needed to guide ASMs selection and prescription. STOP ‘EM is an ongoing randomized controlled trial, with an end date of Sept 2027 (66). It aims at determining the need for ASMs postoperatively in seizure naïve patients. The study’s main goals are determining the efficacy of levetiracetam in seizure prevention over 12 months after surgery, the effect of starting levetiracetam on the ability to resume driving, quality of life, and cost-effectiveness.
Understanding and predicting seizures in meningioma can help guide seizures control and allow for better determination of patients at risk before and after surgery. The current medical literature provides limited data for postoperative seizure prediction and optimal management in patients with meningioma related epilepsy. In reference to the cohort of meningioma patients undergoing surgery stratified based on preoperative seizure status to postoperative seizure status, it is logical to identify four different groups: no seizures to no seizures, seizures to no seizures, no seizures to seizures, and seizures to seizures. The future effort on stratifying patients into these four groups including medications alone, surgery/ies alone, medications + surgery/ies will be able to predict surgical outcome and optimally treat patients with the most successful modalities.
RE, HT, LH, WB and FB contributed to conception and design of the review. RE wrote the first draft of the manuscript. AA wrote sections of the manuscript. HT, LH, AA, WB and FB contributed to manuscript revision. All authors approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was supported by the grant (No. 19140900105) from the Shanghai Committee of Science and Technology and Research Fund from Neurosurgery Department, Loma Linda University.
1. Wiemels J, Wrensch M, Claus EB. Epidemiology and Etiology of Meningioma. J Neurooncol (2010) 99(3):307–14. doi: 10.1007/s11060-010-0386-3
2. Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence Estimates for Primary Brain Tumors in the United States by Age, Gender, Behavior, and Histology. Neuro Oncol (2010) 12(6):520–7. doi: 10.1093/neuonc/nop066
3. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol (2015) 17 Suppl 4:iv1–iv62. doi: 10.1093/neuonc/nov189
4. Sughrue ME, Kane AJ, Shangari G, Rutkowski MJ, McDermott MW, Berger MS, et al. The Relevance of Simpson Grade I and II Resection in Modern Neurosurgical Treatment of World Health Organization Grade I Meningiomas. J Neurosurg (2010) 113(5):1029–35. doi: 10.3171/2010.3.JNS091971
5. Hasseleid BF, Meling TR, Ronning P, Scheie D, Helseth E. Surgery for Convexity Meningioma: Simpson Grade I Resection as the Goal: Clinical Article. J Neurosurg (2012) 117(6):999–1006. doi: 10.3171/2012.9.JNS12294
6. Erturk Cetin O, Isler C, Uzan M, Ozkara C. Epilepsy-Related Brain Tumors. Seizure (2017) 44:93–7. doi: 10.1016/j.seizure.2016.12.012
7. Englot DJ, Magill ST, Han SJ, Chang EF, Berger MS, McDermott MW. Seizures in Supratentorial Meningioma: A Systematic Review and Meta-Analysis. J Neurosurg (2016) 124(6):1552–61. doi: 10.3171/2015.4.JNS142742
8. Chaichana KL, Pendleton C, Zaidi H, Olivi A, Weingart JD, Gallia GL, et al. Seizure Control for Patients Undergoing Meningioma Surgery. World Neurosurg (2013) 79(3-4):515–24. doi: 10.1016/j.wneu.2012.02.051
9. Van Breemen MS, Wilms EB, Vecht CJ. Seizure Control in Brain Tumors. Handb Clin Neurol (2012) 104:381–9. doi: 10.1016/B978-0-444-52138-5.00026-8
10. Bauer R, Ortler M, Seiz-Rosenhagen M, Maier R, Anton JV, Unterberger I. Treatment of Epileptic Seizures in Brain Tumors: A Critical Review. Neurosurg Rev (2014) 37(3):381–8:discussion 8. doi: 10.1007/s10143-014-0538-6
11. Taphoorn MJ, Klein M. Cognitive Deficits in Adult Patients With Brain Tumours. Lancet Neurol (2004) 3(3):159–68. doi: 10.1016/S1474-4422(04)00680-5
12. Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. Patient-Validated Content of Epilepsy-Specific Quality-of-Life Measurement. Epilepsia (1997) 38(2):233–6. doi: 10.1111/j.1528-1157.1997.tb01102.x
13. Chaichana KL, Parker SL, Olivi A, Quinones-Hinojosa A. Long-Term Seizure Outcomes in Adult Patients Undergoing Primary Resection of Malignant Brain Astrocytomas. Clinical Article. J Neurosurg (2009) 111(2):282–92. doi: 10.3171/2009.2.JNS081132
14. Harden CL. The Co-Morbidity of Depression and Epilepsy: Epidemiology, Etiology, and Treatment. Neurology (2002) 59(6 Suppl 4):S48–55. doi: 10.1212/WNL.59.6_suppl_4.S48
15. Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse Effects of Antiepileptic Drugs: A Brief Overview of Important Issues. Expert Rev Neurother (2010) 10(6):885–91. doi: 10.1586/ern.10.71
16. Hamasaki T, Yamada K, Kuratsu J. Seizures as a Presenting Symptom in Neurosurgical Patients: A Retrospective Single-Institution Analysis. Clin Neurol Neurosurg (2013) 115(11):2336–40. doi: 10.1016/j.clineuro.2013.08.016
17. Seyedi JF, Pedersen CB, Poulsen FR. Risk of Seizures Before and After Neurosurgical Treatment of Intracranial Meningiomas. Clin Neurol Neurosurg (2018) 165:60–6. doi: 10.1016/j.clineuro.2018.01.002
18. Chozick BS, Reinert SE, Greenblatt SH. Incidence of Seizures After Surgery for Supratentorial Meningiomas: A Modern Analysis. J Neurosurg (1996) 84(3):382–6. doi: 10.3171/jns.1996.84.3.0382
19. Wirsching HG, Morel C, Gmur C, Neidert MC, Baumann CR, Valavanis A, et al. Predicting Outcome of Epilepsy After Meningioma Resection. Neuro Oncol (2016) 18(7):1002–10. doi: 10.1093/neuonc/nov303
20. Chen WC, Magill ST, Englot DJ, Baal JD, Wagle S, Rick JW, et al. Factors Associated With Pre- and Postoperative Seizures in 1033 Patients Undergoing Supratentorial Meningioma Resection. Neurosurgery (2017) 81(2):297–306. doi: 10.1093/neuros/nyx001
21. Lieu AS, Howng SL. Intracranial Meningiomas and Epilepsy: Incidence, Prognosis and Influencing Factors. Epilepsy Res (2000) 38(1):45–52. doi: 10.1016/S0920-1211(99)00066-2
22. Morsy MM, El-Saadany WF, Moussa WM, Sultan AE. Predictive Factors for Seizures Accompanying Intracranial Meningiomas. Asian J Neurosurg (2019) 14(2):403–9. doi: 10.4103/ajns.AJNS_152_18
23. Xue H, Sveinsson O, Bartek J Jr., Forander P, Skyrman S, Kihlstrom L, et al. Long-Term Control and Predictors of Seizures in Intracranial Meningioma Surgery: A Population-Based Study. Acta Neurochir (Wien) (2018) 160(3):589–96. doi: 10.1007/s00701-017-3434-3
24. Schaller B. Brain Tumor and Seizures: Pathophysiology and its Implications for Treatment Revisited (Epilepsia 2003; 44:1223-1232). Epilepsia (2006) 47(3):661; discussion. doi: 10.1111/j.1528-1167.2006.00484_1.x
25. Gadot R, Khan AB, Patel R, Goethe E, Shetty A, Hadley CC, et al. Predictors of Postoperative Seizure Outcome in Supratentorial Meningioma. J Neurosurg (2021), 1–10. doi: 10.3171/2021.9.JNS211738
26. Shamji MF, Fric-Shamji EC, Benoit BG. Brain Tumors and Epilepsy: Pathophysiology of Peritumoral Changes. Neurosurg Rev (2009) 32(3):275–84; discussion 84-6. doi: 10.1007/s10143-009-0191-7
27. Fang S, Zhan Y, Xie YF, Shi Q, Dan W. Predictive Value of Electrocorticography for Postoperative Epilepsy in Patients With Supratentorial Meningioma. J Clin Neurosci (2013) 20(1):112–6. doi: 10.1016/j.jocn.2012.02.021
28. van Diessen E, Diederen SJ, Braun KP, Jansen FE, Stam CJ. Functional and Structural Brain Networks in Epilepsy: What Have We Learned? Epilepsia (2013) 54(11):1855–65. doi: 10.1111/epi.12350
29. Mehta N, Bhagwati S, Parulekar G. Meningiomas in Children: A Study of 18 Cases. J Pediatr Neurosci (2009) 4(2):61–5. doi: 10.4103/1817-1745.57322
30. Simis A, Pires de Aguiar PH, Leite CC, Santana PA Jr., Rosemberg S, Teixeira MJ. Peritumoral Brain Edema in Benign Meningiomas: Correlation With Clinical, Radiologic, and Surgical Factors and Possible Role on Recurrence. Surg Neurol (2008) 70(5):471–7; discussion 7. doi: 10.1016/j.surneu.2008.03.006
31. Lobato RD, Alday R, Gomez PA, Rivas JJ, Dominguez J, Cabrera A, et al. Brain Oedema in Patients With Intracranial Meningioma. Correlation Between Clinical, Radiological, and Histological Factors and the Presence and Intensity of Oedema. Acta Neurochir (Wien) (1996) 138(5):485–93; discussion 93-4. doi: 10.1007/BF01411166
32. Kawaguchi T, Kameyama S, Tanaka R. Peritumoral Edema and Seizure in Patients With Cerebral Convexity and Parasagittal Meningiomas. Neurol Med Chir (Tokyo) (1996) 36(8):568–73; discussion 73-4. doi: 10.2176/nmc.36.568
33. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral Brain Edema Associated With Meningioma: Influence of Vascular Endothelial Growth Factor Expression and Vascular Blood Supply. Cancer (1999) 85(4):936–44. doi: 10.1002/(sici)1097-0142(19990215)85:4<936::aid-cncr23>3.0.co;2-j
34. Pistolesi S, Fontanini G, Camacci T, De Ieso K, Boldrini L, Lupi G, et al. Meningioma-Associated Brain Oedema: The Role of Angiogenic Factors and Pial Blood Supply. J Neurooncol (2002) 60(2):159–64. doi: 10.1023/a:1020624119944
35. Hess K, Spille DC, Adeli A, Sporns PB, Brokinkel C, Grauer O, et al. Brain Invasion and the Risk of Seizures in Patients With Meningioma. J Neurosurg (2018) 130(3):789–96. doi: 10.3171/2017.11.JNS172265
36. de Vries J, Wakhloo AK. Cerebral Oedema Associated With WHO-I, WHO-II, and WHO-III-Meningiomas: Correlation of Clinical, Computed Tomographic, Operative and Histological Findings. Acta Neurochir (Wien) (1993) 125(1-4):34–40. doi: 10.1007/BF01401825
37. Chernov MF, Kasuya H, Nakaya K, Kato K, Ono Y, Yoshida S, et al. (1)H-MRS of Intracranial Meningiomas: What it can Add to Known Clinical and MRI Predictors of the Histopathological and Biological Characteristics of the Tumor? Clin Neurol Neurosurg (2011) 113(3):202–12. doi: 10.1016/j.clineuro.2010.11.008
38. Spille DC, Hess K, Sauerland C, Sanai N, Stummer W, Paulus W, et al. Brain Invasion in Meningiomas: Incidence and Correlations With Clinical Variables and Prognosis. World Neurosurg (2016) 93:346–54. doi: 10.1016/j.wneu.2016.06.055
39. Islim AI, McKeever S, Kusu-Orkar TE, Jenkinson MD. The Role of Prophylactic Antiepileptic Drugs for Seizure Prophylaxis in Meningioma Surgery: A Systematic Review. J Clin Neurosci (2017) 43:47–53. doi: 10.1016/j.jocn.2017.05.020
40. Lu VM, Wahood W, Akinduro OO, Parney IF, Quinones-Hinojosa A, Chaichana KL. Four Independent Predictors of Postoperative Seizures After Meningioma Surgery: A Meta-Analysis. World Neurosurg (2019) 130:537–45 e3. doi: 10.1016/j.wneu.2019.06.063
41. Wilson TA, Huang L, Ramanathan D, Lopez-Gonzalez M, Pillai P, De Los Reyes K, et al. Review of Atypical and Anaplastic Meningiomas: Classification, Molecular Biology, and Management. Front Oncol (2020) 10:565582. doi: 10.3389/fonc.2020.565582
42. Tsuji M, Shinomiya S, Inoue R, Sato K. Prospective Study of Postoperative Seizure in Intracranial Meningioma. Jpn J Psychiatry Neurol (1993) 47(2):331–4. doi: 10.1111/j.1440-1819.1993.tb02094.x
43. Li X, Wang C, Lin Z, Zhao M, Ren X, Zhang X, et al. Risk Factors and Control of Seizures in 778 Chinese Patients Undergoing Initial Resection of Supratentorial Meningiomas. Neurosurg Rev (2020) 43(2):597–608. doi: 10.1007/s10143-019-01085-5
44. Hamasaki T, Yamada K, Yano S, Nakamura H, Makino K, Hide T, et al. Higher Incidence of Epilepsy in Meningiomas Located on the Premotor Cortex: A Voxel-Wise Statistical Analysis. Acta Neurochir (Wien) (2012) 154(12):2241–9. doi: 10.1007/s00701-012-1511-1
45. McGovern SL, Aldape KD, Munsell MF, Mahajan A, DeMonte F, Woo SY. A Comparison of World Health Organization Tumor Grades at Recurrence in Patients With non-Skull Base and Skull Base Meningiomas. J Neurosurg (2010) 112(5):925–33. doi: 10.3171/2009.9.JNS09617
46. Baumgarten P, Sarlak M, Baumgarten G, Marquardt G, Seifert V, Strzelczyk A, et al. Focused Review on Seizures Caused by Meningiomas. Epilepsy Behav (2018) 88:146–51. doi: 10.1016/j.yebeh.2018.09.002
47. Zheng Z, Chen P, Fu W, Zhu J, Zhang H, Shi J, et al. Early and Late Postoperative Seizure Outcome in 97 Patients With Supratentorial Meningioma and Preoperative Seizures: A Retrospective Study. J Neurooncol (2013) 114(1):101–9. doi: 10.1007/s11060-013-1156-9
48. Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a Definition of Acute Symptomatic Seizure. Epilepsia (2010) 51(4):671–5. doi: 10.1111/j.1528-1167.2009.02285.x
49. Baumgarten P, Sarlak M, Monden D, Spyrantis A, Bernatz S, Gessler F, et al. Early and Late Postoperative Seizures in Meningioma Patients and Prediction by a Recent Scoring System. Cancers (Basel) (2021) 13(3):450. doi: 10.3390/cancers13030450
50. Joiner EF, Youngerman BE, Hudson TS, Yang J, Welch MR, McKhann GM, et al. Effectiveness of Perioperative Antiepileptic Drug Prophylaxis for Early and Late Seizures Following Oncologic Neurosurgery: A Meta-Analysis. J Neurosurg (2018) 130(4):1–9. doi: 10.3171/2017.10.JNS172236
51. Raza SM, Gallia GL, Brem H, Weingart JD, Long DM, Olivi A. Perioperative and Long-Term Outcomes From the Management of Parasagittal Meningiomas Invading the Superior Sagittal Sinus. Neurosurgery (2010) 67(4):885–93; discussion 93. doi: 10.1227/NEU.0b013e3181ef2a18
52. Scott DF. Left and Right Cerebral Hemisphere Differences in the Occurrence of Epilepsy. Br J Med Psychol (1985) 58( Pt 2):189–92. doi: 10.1111/j.2044-8341.1985.tb02633.x
53. Islim AI, Ali A, Bagchi A, Ahmad MU, Mills SJ, Chavredakis E, et al. Postoperative Seizures in Meningioma Patients: Improving Patient Selection for Antiepileptic Drug Therapy. J Neurooncol (2018) 140(1):123–34. doi: 10.1007/s11060-018-2941-2
54. Komotar RJ, Raper DM, Starke RM, Iorgulescu JB, Gutin PH. Prophylactic Antiepileptic Drug Therapy in Patients Undergoing Supratentorial Meningioma Resection: A Systematic Analysis of Efficacy. J Neurosurg (2011) 115(3):483–90. doi: 10.3171/2011.4.JNS101585
55. Zada G, Pagnini PG, Yu C, Erickson KT, Hirschbein J, Zelman V, et al. Long-Term Outcomes and Patterns of Tumor Progression After Gamma Knife Radiosurgery for Benign Meningiomas. Neurosurgery (2010) 67(2):322–8; discussion 8-9. doi: 10.1227/01.NEU.0000371974.88873.15
56. Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Single-Fraction Radiosurgery for Presumed Intracranial Meningiomas: Efficacy and Complications From a 22-Year Experience. Int J Radiat Oncol Biol Phys (2012) 83(5):1414–8. doi: 10.1016/j.ijrobp.2011.10.033
57. Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, et al. Practice Parameter: Anticonvulsant Prophylaxis in Patients With Newly Diagnosed Brain Tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology (2000) 54(10):1886–93. doi: 10.1212/WNL.54.10.1886
58. Zhang B, Zhao G, Yang HF, Wang D, Yu JL, Huang HY. Assessment of Risk Factors for Early Seizures Following Surgery for Meningiomas Using Logistic Regression Analysis. J Int Med Res (2011) 39(5):1728–35. doi: 10.1177/147323001103900515
59. Harward SC, Rolston JD, Englot DJ. Seizures in Meningioma. Handb Clin Neurol (2020) 170:187–200. doi: 10.1016/B978-0-12-822198-3.00053-7
60. Gloss D, Pargeon K, Pack A, Varma J, French JA, Tolchin B, et al. Antiseizure Medication Withdrawal in Seizure-Free Patients: Practice Advisory Update Summary: Report of the AAN Guideline Subcommittee. Neurology (2021) 97(23):1072–81. doi: 10.1212/WNL.0000000000012944
61. Guerrini R, Rosati A, Giordano F, Genitori L, Barba C. The Medical and Surgical Treatment of Tumoral Seizures: Current and Future Perspectives. Epilepsia (2013) 54:84–90. doi: 10.1111/epi.12450
62. Klein M. Neurocognitive Functioning in Adult WHO Grade II Gliomas: Impact of Old and New Treatment Modalities. Neuro Oncol (2012) 14:17–24. doi: 10.1093/neuonc/nos161
63. Maschio M, Dinapoli L, Sperati F, Pace A, Fabi A, Vidiri A, et al. Effect of Pregabalin Add-on Treatment on Seizure Control, Quality of Life, and Anxiety in Patients With Brain Tumour-Related Epilepsy: A Pilot Study. Epileptic Disord (2012) 14(4):388–97. doi: 10.1684/epd.2012.0542
64. van der Meer PB, Dirven L, Fiocco M, Vos MJ, Kouwenhoven MCM, van den Bent MJ, et al. First-Line Antiepileptic Drug Treatment in Glioma Patients With Epilepsy: Levetiracetam vs Valproic Acid. Epilepsia (2021) 62(5):1119–29. doi: 10.1111/epi.16880
65. van der Meer PB, Dirven L, van den Bent MJ, Preusser M, Taphoorn MJB, Rudá R, et al. Prescription Preferences of Antiepileptic Drugs in Brain Tumor Patients: An International Survey Among EANO Members. Neuro-Oncol Pract (2021) 9(2):105–13. doi: 10.1093/nop/npab059
Keywords: meningiomas, seizure, epilepsy, risk factor, surgical resection, anti-seizure medications
Citation: Elbadry Ahmed R, Tang H, Asemota A, Huang L, Boling W and Bannout F (2022) Meningioma Related Epilepsy- Pathophysiology, Pre/postoperative Seizures Predicators and Treatment. Front. Oncol. 12:905976. doi: 10.3389/fonc.2022.905976
Received: 28 March 2022; Accepted: 06 June 2022;
Published: 04 July 2022.
Edited by:
Christine Marosi, Medical University of Vienna, AustriaReviewed by:
Robin Grant, Edinburgh Royal Infirmary, United KingdomCopyright © 2022 Elbadry Ahmed, Tang, Asemota, Huang, Boling and Bannout. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Warren Boling, V0JvbGluZ0BsbHUuZWR1; Firas Bannout, RkJhbm5vdXRAbGx1LmVkdQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.