- 1Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Key Laboratory of Gene Editing Screening and Research and Development (R&D) of Digestive System Tumor Drugs, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Purpose: To develop a novel criterion based on the response evaluation criteria in solid tumors (RECIST) 1.1 and alpha fetoprotein (AFP) and evaluate its performance in tumor response for patients with unresectable hepatocellular carcinoma (uHCC) receiving conversion-radiotherapy before hepatectomy.
Method: From June 2012 to December 2020, a total of 39 patients with uHCC, who received intensity-modulated radiotherapy (IMRT) before hepatectomy, were retrospectively included in this study. Pre- and post-treatment contrast-enhanced magnetic resonance imaging (CE-MRI) scans were performed in all patients. Eight modified criteria were developed with the combination of RECIST 1.1, modified RECIST (mRECIST), and the percentage change of AFP, baseline AFP. The endpoint events were recurrence-free survival (RFS).
Results: The median RFS and OS was 26.5 (IQR, 15.7-43.1), 38.8 (IQR, 18.4-53.6) months. An optimal revised evaluation criterion named α-RECIST (alpha fetoprotein-RECIST 1.1) was developed by combining the RECIST 1.1 with the AFPΔ (cut-off value, 76%). Patients defined as responders by α-RECIST showed significantly better RFS and OS than those defined as non-responders (p = 0.035, 0.048). The other criteria (RECIST 1.1, mRECIST, αΔ-mRECIST, α&Δ-RECIST, α&Δ-mRECIST, αBL-RECIST, αBL-mRECIST, α&BL-RECIST, α&BL-mRECIST) all failed to identify responders from non-responders (p = 0.405, 0.201, 0.773, 0.424, 0.266, 0.060, 0.721, 0.644, 0.910, respectively) when correlated with RFS. Responders according to α-RECIST showed significant better RFS compared to non-responders [HR, 0.31 (95% CI: 0.10, 0.98); p=0.046], but no statistical significance was observed in terms of OS [HR, 0.33 (95% CI: 0.11, 1.05); p = 0.06].
Conclusions: Patients identified as responders by α-RECIST provided significant better RFS. The α-RECIST criteria might be a promising tool for identifying tumor response of conversion-radiotherapy for unresectable hepatocellular carcinoma before hepatectomy.
Highlights
1. Conventional RECIST 1.1, mRECIST failed to correlate responders over non-responders with survival benefit for uHCC patients receiving conversion-radiotherapy before hepatectomy.
2. The novel criterion (a-RECIST) is based on RECIST 1.1 combining with the percentage change of AFP (AFPΔ) for identifying tumor response of conversion-radiotherapy for uHCC patients before hepatectomy.
3. Patients identified as responders by α-RECIST provided significant better RFS. The α-RECIST criteria might be a promising tool for identifying tumor response of conversion-radiotherapy for uHCC patients before hepatectomy.
Introduction
Liver cancer is the sixth most common cancer and the fourth leading cause of cancer-related deaths worldwide (1), in which hepatocellular carcinoma (HCC) is the most common histological subtype (2). Surgical therapy is the first-line curative therapeutic method for early-stage HCC (2). However, more than 70% of patients are diagnosed as unresectable HCC (uHCC) due to local vascular invasion or inadequate baseline hepatic function (3, 4). Conversion therapy, including regional and systemic therapies, originally aimed at palliation, has recently been reported to convert tumors from unresectable to resectable status and receive curative resection (5, 6), from which radiotherapy (RT) is considered a promising conversion therapy for uHCC with the development of radiotherapeutic technology (4, 7). Intensity-modulated radiotherapy (IMRT) can selectively deliver radiation to tumor regions with high precision and exquisitely conformal dose distribution (7–9).
Tumor response criteria for HCC mainly includes Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), modified Response Evaluation Criteria in Solid Tumors (mRECIST), and the European Association for the Study of Liver Diseases (EASL). RECIST 1.1 only considers tumor size and may underestimate therapeutic response (10). EASL and mRECIST may not be accurate for the identification of responders due to “pseudo-progression”, that is, persistent enhancement after radiotherapy did not necessarily indicate viable neoplasm (10–12). Currently, no clear-cut criterion has been established for tumor response of radiotherapy, especially conversion-radiotherapy (13). A reliable criterion for response evaluation of conversion-radiotherapy is crucial and urgent.
Besides the radiological characteristics, oncological characteristics such as serum α-fetoprotein (AFP) has also been reported as potential response indicators of HCC. A high level of serum AFP is an indicator of poor prognosis across all stages of HCC (14, 15). Decreased AFP level has been reported to correlate with prolonged progression free survival (PFS) and overall survival (OS) after systemic therapy (15, 16).
In this study, we aim to explore the optimal modified criteria based on radiological criteria (RECIST 1.1, mRECIST) and oncological characteristics (e.g., baseline AFP and AFP change) to assess tumor response for uHCC receiving conversion-radiotherapy before hepatectomy based on recurrence-free survival (RFS).
Materials and Methods
Patients
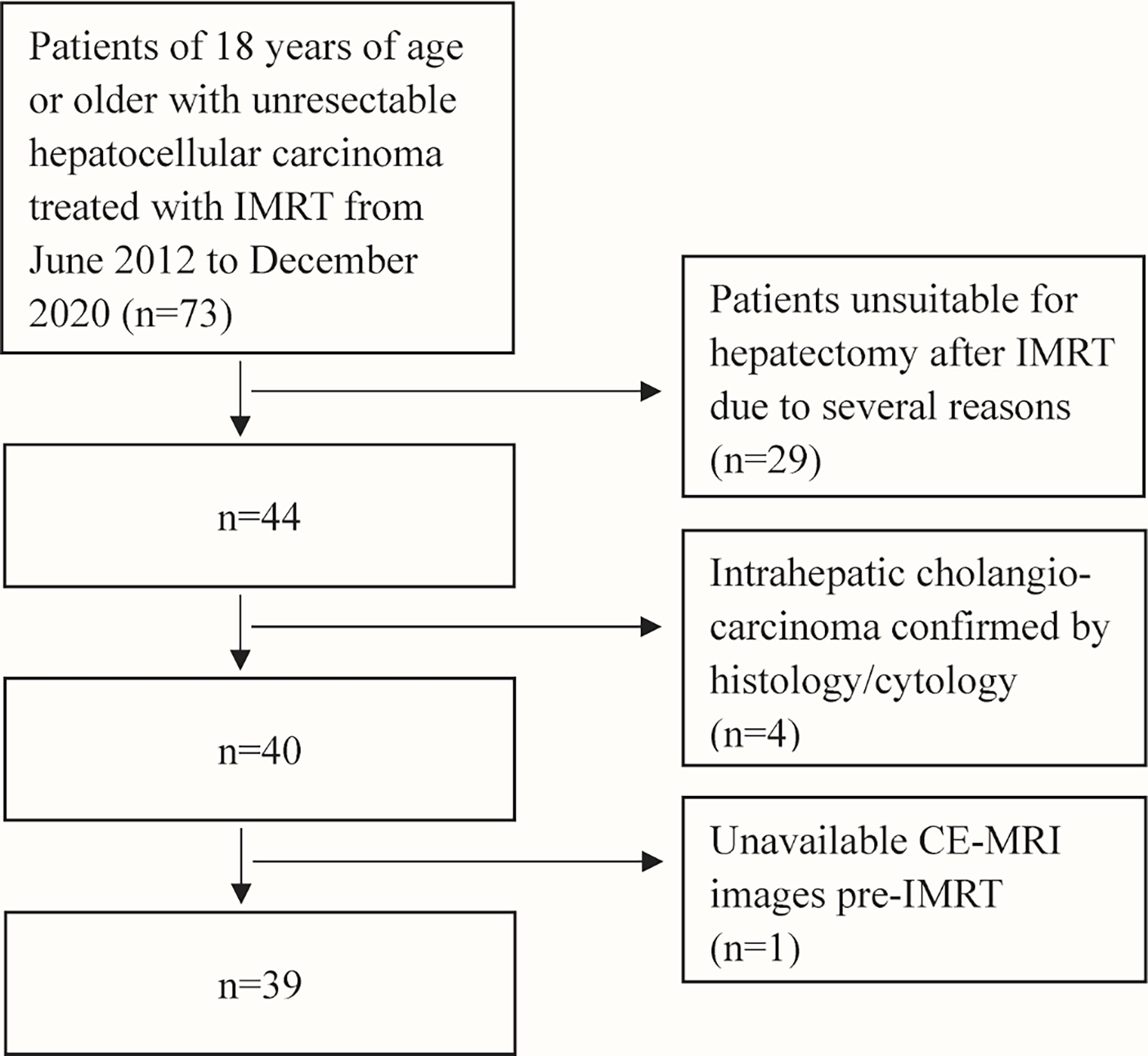
After searching the institutional medical database, sixty-nine patients with uHCC treated with IMRT from June 2012 to December 2020 were retrospectively included in this study. Inclusion criteria were: 1) patients with uHCC confirmed by liver biopsy; 2) patients without previous systemic therapy for HCC; 3) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1 and Child-Pugh score ≤6 points; 4) patients with both the contrast-enhanced magnetic resonance imaging (CE-MRI) of the abdomen within 1 month before IMRT as baseline and a follow-up CE-MRI scan after IMRT; 5) patients who had successful conversion-radiotherapy and subsequent hepatectomy. Exclusion criteria were: 1) patients unsuitable for hepatectomy after IMRT due to several reasons (such as inadequate hepatic functions); 2) baseline or follow-up CE-MRI scans were not within the predefined interval. A flowchart of the study population selection is shown in Figure 1. This study was approved by the Institutional Review Boards of National Cancer Center/Cancer Hospital and informed consent was waived for its retrospective design.
IMRT
Preoperative IMRT was performed within 3 days after completion of all preoperative investigations and careful evaluation by the radiation clinicians. The gross tumor volume was defined as the tumor volume that was enhanced in the arterial phase on preoperative CE-MRI scans using the Pinnacle3 9.0 treatment planning systems (Philips Healthcare, Andover, MA, USA). The clinical tumor volume (CTV) was drawn by adding 5–10 mm to the gross tumor volume. The planning target volume (PTV) was generated by adding 5mm in the anterior–posterior and left–right directions and 10mm in the cranial–caudal direction from the CTV considering respiratory liver motion and set-up errors. The prescription dose to 95% of the PTV was planned at 50-60 Gy in 25-30 fractions over 5-6 weeks. The final prescription dose was determined according to dose constraints for organs at risk.
Hepatectomy
After completion of IMRT, the evaluation of liver function and MRI scans were performed before hepatectomy. Resection ranges were decided by clinical surgeons based on individual detailed status of each patient according to the tumor size, number, location, and relationship with the major vascular structures.
Imaging Acquisition
MRI scans of the abdomen at baseline and follow-up of all patients were performed on three scanners: GE Discovery MR 750 3.0T, GE Pioneer 3.0T, and Siemens Prisma 3.0T. Routine liver MRI protocol included non-fat suppressed coronal single-shot fast/turbo spin-echo T2WI (SS-FSE/HASTE), axial fat suppressed fast/turbo spin echo (FSE/TSE) T2WI, T1WI in- and out-of- phase, DWI and dynamic CE-T1WI images. For dynamic CE-T1WI, unenhanced, early and late arterial phases (using fluoro trigger or carebolus technique), portal venous phase (60s), late venous phase (180s) were obtained using a 3D T1WI breath-hold fat-suppressed spoiled gradient-recall echo sequence (LAVA or VIBE) before and after intravenous administration of gadodiamide (0.5 mmol/ml, GE Healthcare) at a dose of 0.2 ml/kg body weight and an injection rate of 2ml/s. Respiratory-triggered or diaphragm-navigated DWI with an axial single-shot spin echo, echo-planar imaging (EPI) sequence with DW gradients (b value 0/50 and 800 s/mm2) was applied in three orthogonal directions (slice thickness/space: 6.0/1.0 mm, FOV: 34−38 cm, matrix size: 128×128, NEX: 4).
Definitions of Modified Criteria
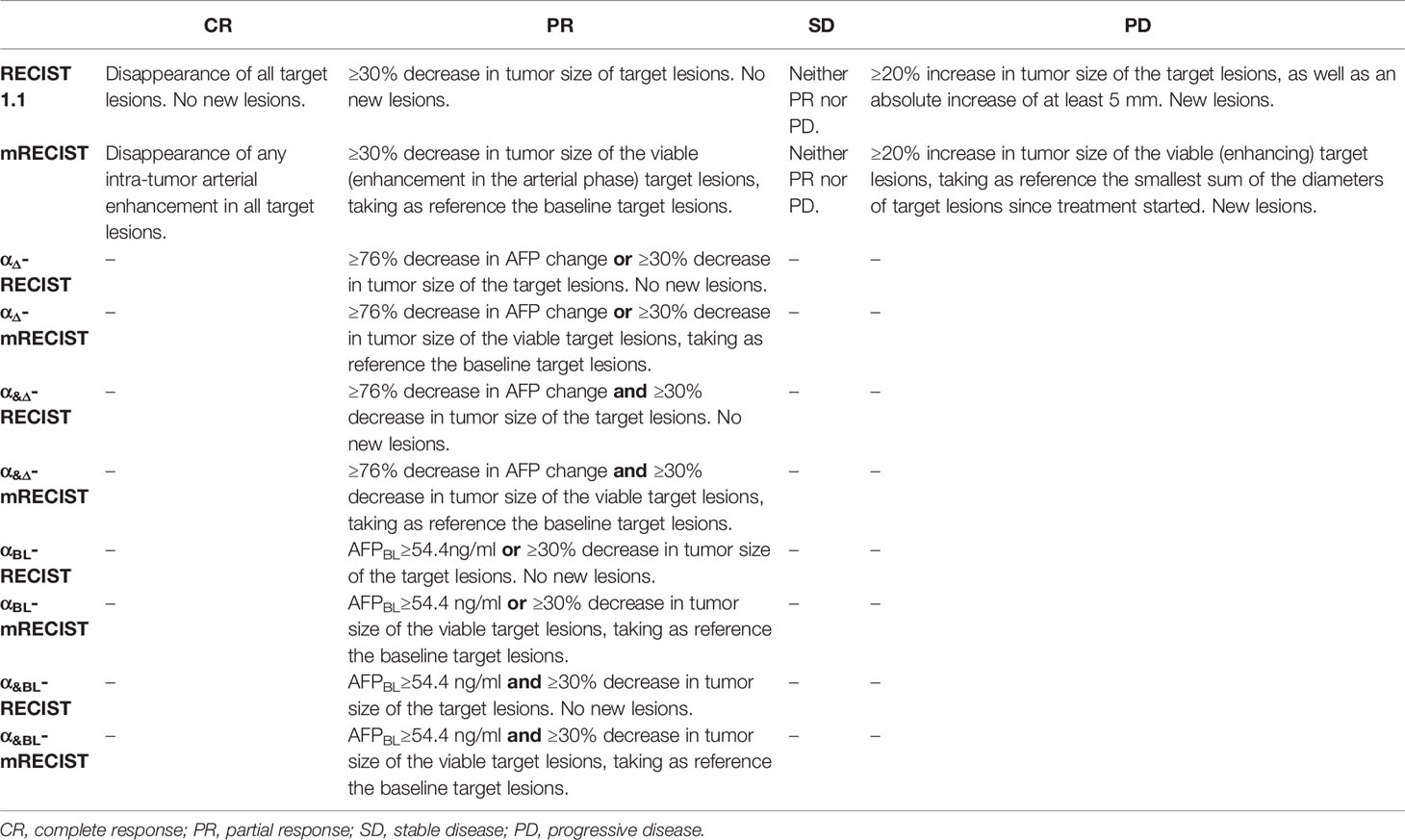
The baseline and follow-up AFP levels were calculated within one week before (AFPBL) and after (AFPFU) the IMRT. The percentage change from AFPBL to the AFPFU was calculated as the following formula: AFPΔ = (AFPFU-AFPBL)/AFPBL. The optimal cutoff values of AFPΔ and AFPBL were determined according to RFS. Eight modified radiological criteria by combining RECIST, mRECIST with AFPΔ, AFPBL were as follows, αΔ-RECIST, αΔ-mRECIST, α&Δ-RECIST, α&Δ-mRECIST, αBL-RECIST, αBL-mRECIST, α&BL-RECIST, α&BL-mRECIST (Table 1). The optimal criterion would be selected as alpha fetoprotein-RECIST (α-RECIST) at last.
Image Analysis and Radiological Evaluation
Baseline and follow-up CE-MRI images were analyzed on Advantage Workstation (GE Medical Systems, USA). On baseline CE-MRI images, target lesions should be at least 1.0 cm for the longest diameter according to RECIST 1.1. For each patient, a maximum of two lesions per organ and five lesions in total were selected (17). The arterial phase was chosen to measure the sum of the longest diameter (SLD) of target lesions and the SLD of viable (enhancing) target lesions due to the fact that HCC is abundant with blood supply and shows obvious enhancement in the arterial phase (18–20). We calculated the relative changes of the SLD and the SLD of viable (enhancing) areas of all target lesions from baseline to the first follow-up CE-MRI evaluation for each patient, respectively. Non-target lesions were also evaluated according to RECIST 1.1 and mRECIST and the final evaluation results were based on both the target lesions and non-target lesions. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were determined by RECIST1.1, mRECIST and 8 modified radiological criteria. Responders were defined as patients with CR or PR, while non-responders were defined as patients with SD or PD.
Radiological evaluation was performed by two radiologists (Radiologist 1: L.L. M.D with 10 years of experience in abdominal radiology; Radiologist 2: Y.X. M.D with 5 years of experience in abdominal radiology) on baseline and the first follow-up CE-MRI scans. The discrepancy between the two radiologists was adjudicated by a third senior radiologist (Radiologist 3: F.Y. M.D with 18-years of experience in abdominal radiology) to reach a consensus among the three radiologists. All the three radiologists were blinded to the patients’ clinical data and outcome.
Pathologic Response Evaluation
The per-lesion-based matching between each lesion on MRI and pathologic specimens was performed by two pathologists and a third pathologist was assigned to reach a consensus among the three pathologists when there was discrepancy. All the three pathologists were blinded to the imaging response results. A minimum of one slide per centimeter of each tumor was evaluated. The percentage of viable tumor region relative to the total tumor region was determined by the pathologists based on hematoxylin and eosin staining slides. Accordingly, the target lesions were categorized as pathologic complete response (no residual cancer cells), pathologic major response (1% to 49% residual cancer cells), and minor response (≥50% residual cancer cells) (21).
Endpoint of Study
The primary endpoint of this study was RFS, which was calculated from the date of radical hepatectomy to recurrence or the last follow-up. The secondary endpoint was OS which was defined as the time from hepatectomy until death by any cause or the last follow-up. For survival analyses, the last follow-up was completed on August 7th, 2021. Recurrence included intrahepatic metastases assessed using MRI of the upper abdomen and extrahepatic metastases detected using chest-abdomen-pelvis CT following hepatectomy.
Statistical Analysis
The RFS and OS curves of different criteria were prepared using the Kaplan-Meier method with the log-rank test. Univariate Cox regression analysis was performed to calculate the hazard ratios of responders to non-responders for RECIST 1.1 and α-RECIST criteria. The relationship of α-RECIST criteria and pathologic response were evaluated according to the Spearman’s correlation analysis. All the above statistical analyses were performed by SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The Waterfall plots and the histogram were conducted with GraphPad Prism v.6 (GraphPad Software, La Jolla, CA, USA). X-tile 3.6.1 software (22) (Yale University, New Haven, CT, USA) was used to determine the optimal cut-off values for AFPΔ and AFPBL based on RFS. Duration of follow-up was calculated by the reverse Kaplan-Meier estimate of RFS and OS (23). Weighted k statistics were used to evaluate the inter-reader variability of tumor response between two radiologists, respectively. The weighted k coefficients were stratified as follows: 0.81-1.00, almost perfect; 0.61-0.80, substantial; 0.41-0.60, moderate; 0.21-0.40, fair; ≤0.20, poor (24). A two-tailed p value <0.05 was considered statistically significant.
Results
Baseline Characteristics
The percentage rate of successful conversion-radiotherapy was 58.0% (39/69). Thirty-nine patients with 41 target lesions were finally included in this study. The median duration of IMRT was 1.1 months [interquartile range (IQR), 1.0-1.2]. The median time from IMRT completion to the first response evaluation was 1.1 months (IQR 0.9-1.3). The median time from IMRT completion to hepatectomy was 2.3 months (IQR 1.7-3.4). More baseline characteristics of patients included were shown in Table 2.
Tumor Response Evaluation
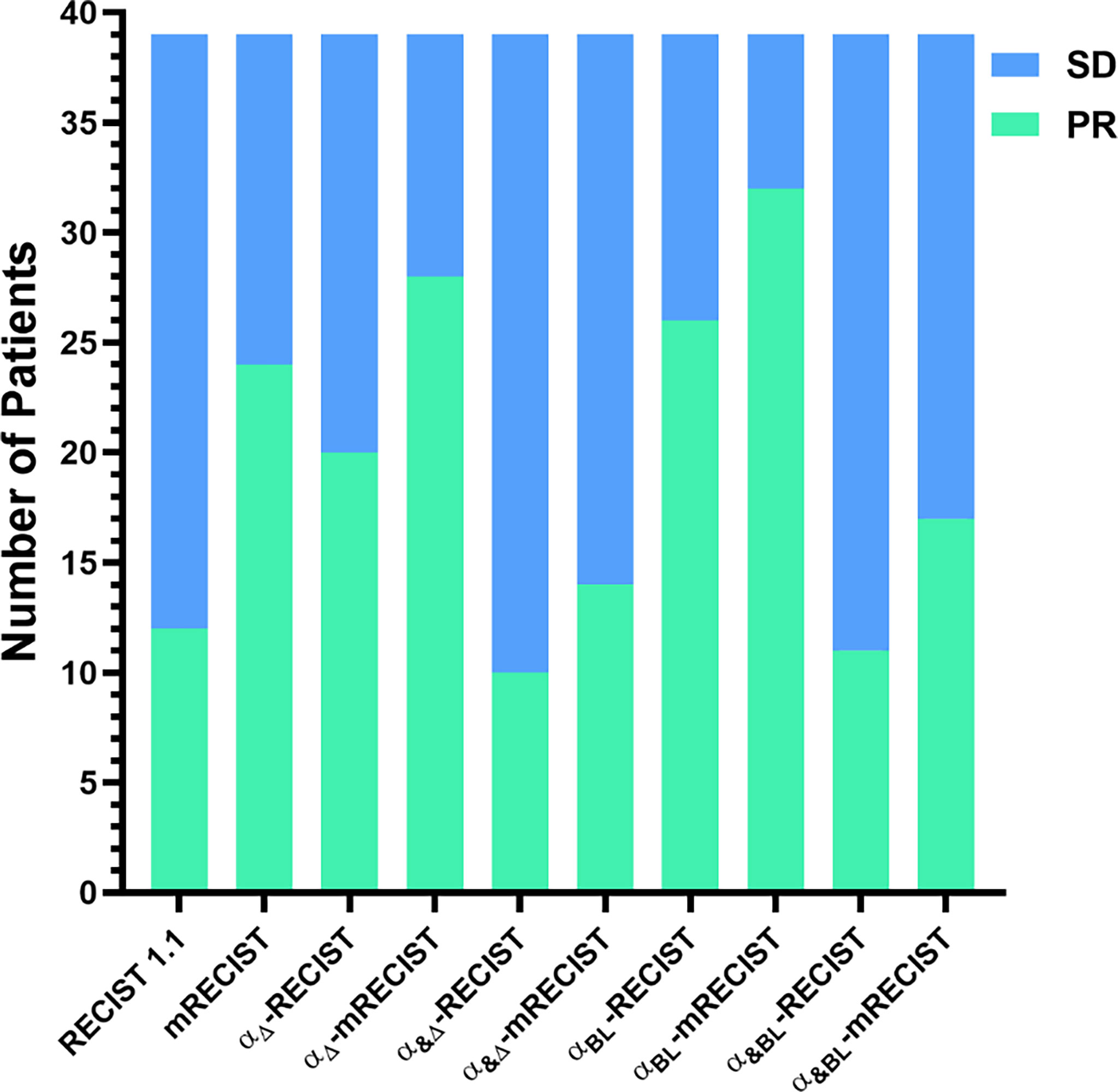
In the first tumor response evaluation, 12 (30.8%), 24 (61.5%), 20 (51.3%), 28 (71.8%), 10 (25.6%), 14 (35.9%), 26 (66.7%), 32 (82.1%), 11 (28.2%), 17 (43.6%) patients were diagnosed as PR and 27 (69.2%), 15 (38.5%), 19 (48.7%), 11 (28.2%), 29 (74.4%), 25 (64.1%), 13 (33.3%), 7 (17.9%), 28 (71.8%), 22 (56.4%) patients were diagnosed as SD according to RECIST 1.1, mRECIST, αΔ-RECIST, αΔ-mRECIST, α&Δ-RECIST, α&Δ-mRECIST, αBL-RECIST, αBL-mRECIST, α&BL-RECIST, α&BL-mRECIST, respectively. None of the patients were identified as CR or PD. The demonstration of evaluation categories among the different criteria are shown in Figure 2. For each patient, the changes of the SLD, the SLD of viable (enhancing) areas of all the target lesions on the first follow-up CE-MRI after IMRT are presented in Figure 3 and the AFPΔ is presented as Figure 4, respectively.
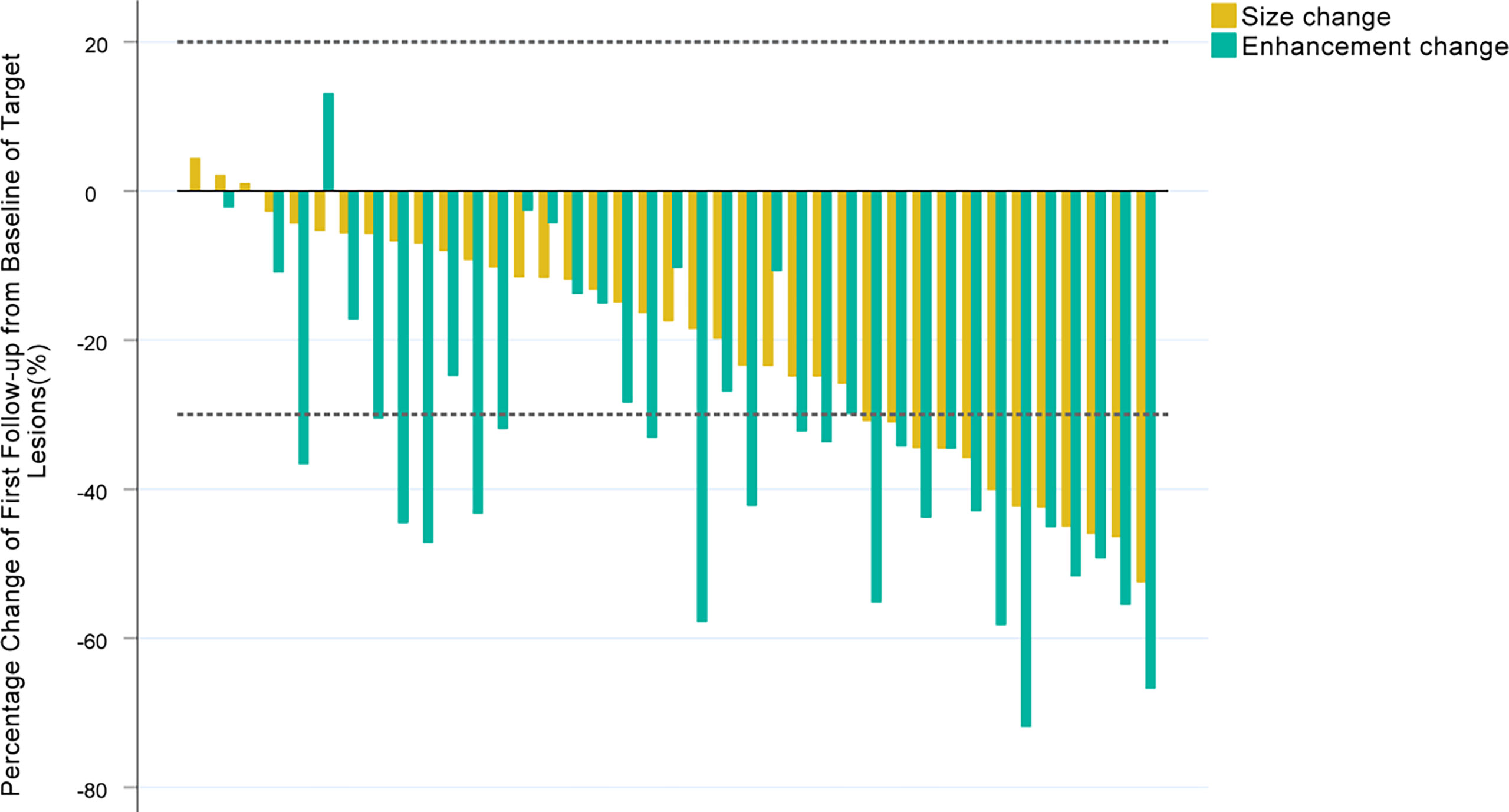
Figure 3 Waterfall plots summarizing the maximum percent change from baseline in the SLD, the SLD of viable (enhancing) areas of all the target lesions on the first follow-up CE-MRI scan after IMRT for each patient. The two adjacent bars in each group represent one patient. The dashed lines indicated size thresholds (+20%, -30%) for RECIST 1.1 and mRECIST criteria.
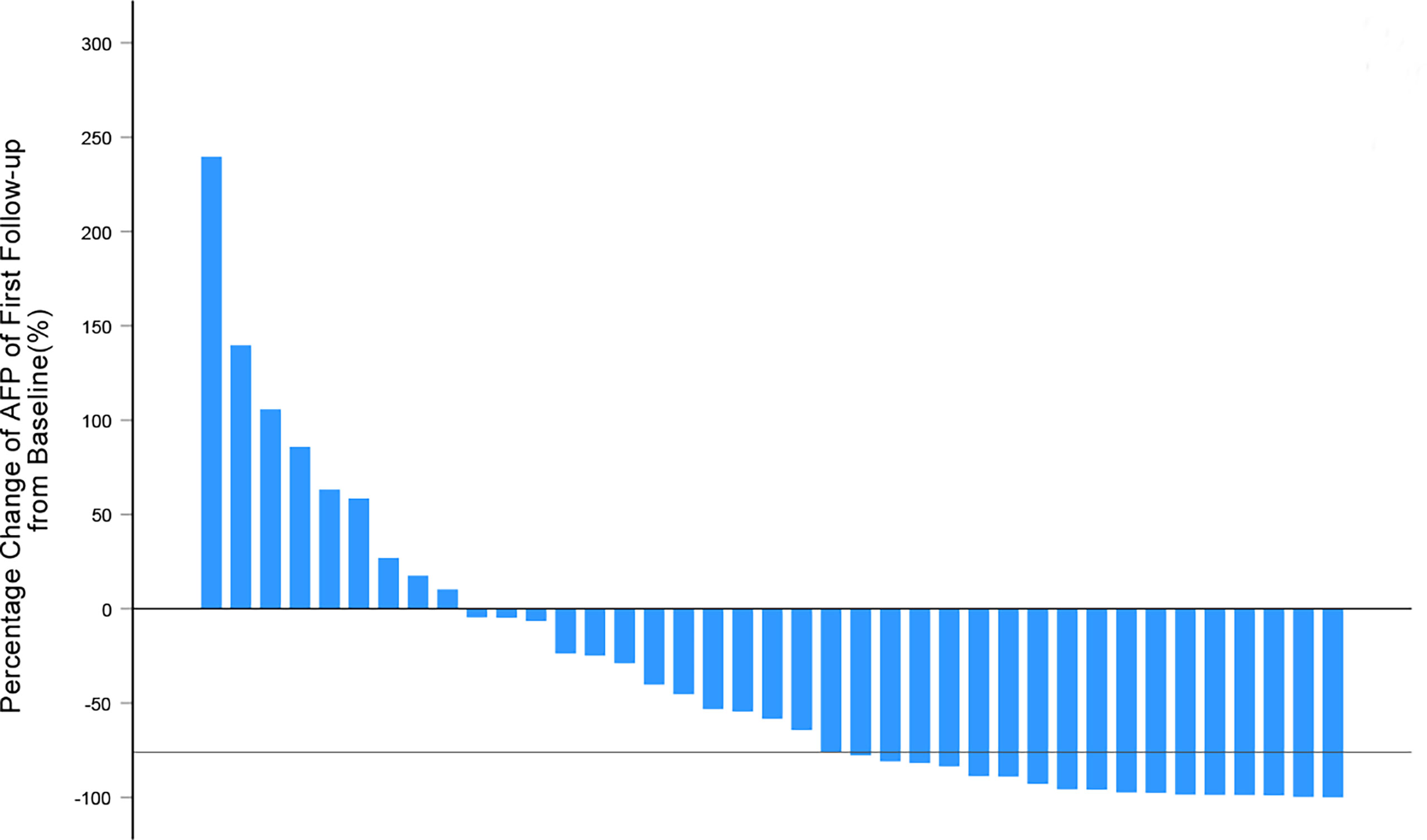
Figure 4 Waterfall plots summarizing the maximum percentage change of AFP from baseline on the first follow-up after IMRT for each patient. The solid line indicated AFP change thresholds (-76%) for α-RECIST criteria.
Correlations of Different Criteria With RFS and OS
Seventeen (43.6%) patients occurred recurrence and 16 (41.0%) patients died during a median follow-up duration of 52.8 months (95% CI 43.8-61.8 months). The median RFS and OS time was 26.5 (IQR, 15.7-43.1 months), 38.8 months (IQR, 18.4-53.6 months). The 1-year, 2-year and 3-year RFS rates were 82.1%, 70.6%, 59.4%, respectively.
In terms of RFS, responders according to RECIST 1.1 and mRECIST showed no significant difference compared with non-responders (p = 0.405 and 0.201, respectively, Supplemental Materials Figures 1A, B). Patients with no less than 76% decrease in AFPΔ showed significantly better RFS than those who did not (p = 0.042, Supplemental Materials Figure 1C). Patients with no less than 54.4ng/ml in AFPBL showed a trend with better RFS than those who did not (p = 0.138, Supplemental Materials Figure 1D). Patients defined as PR by αΔ-RECIST showed significantly better RFS than those defined as SD (p = 0.035, Figure 5A). Responders according to the other criteria (αΔ-mRECIST, α&Δ-RECIST, α&Δ-mRECIST, αBL-RECIST, αBL-mRECIST, α&BL-RECIST, and α&BL-mRECIST) showed no significant difference in terms of RFS compared with non-responders (p = 0.773, 0.424, 0.266, 0.060, 0.721, 0.644, 0.910, Figures 5B–D and Supplemental Materials Figures 2A–D, respectively).
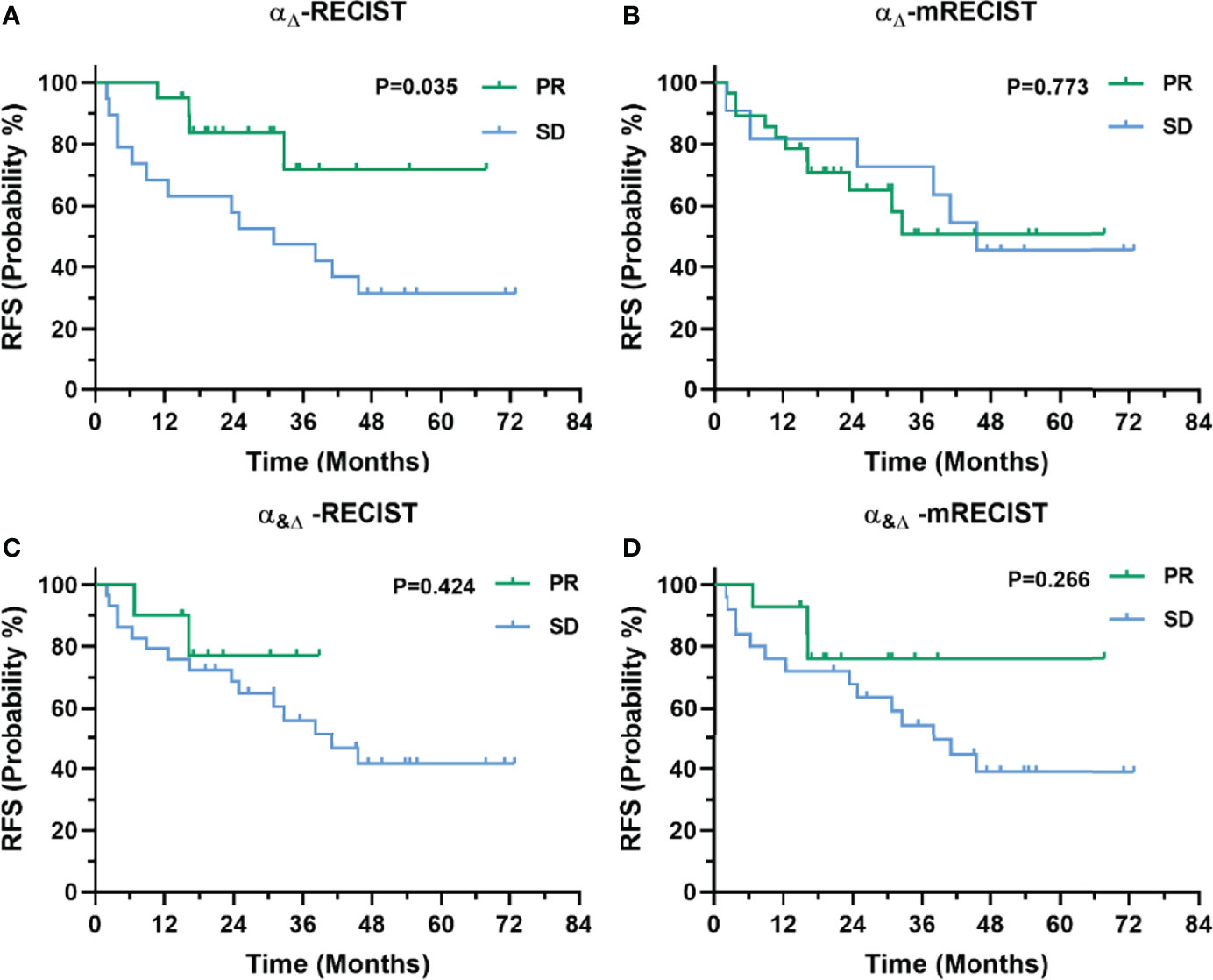
Figure 5 Kaplan-Meier curves for RFS of 39 patients with uHCC undergoing IMRT before hepatectomy as categorized by the αΔ-RECIST (A), αΔ-mRECIST (B), α&Δ-RECIST (C), and α&Δ-mRECIST (D) criteria.
In terms of OS, responders according to RECIST 1.1 and mRECIST showed no significant difference compared with non-responders (p = 0.508, 0.103, Supplemental Materials Figures 3A, B). Patients defined as PR by αΔ-RECIST showed significantly better OS than those defined as SD (p = 0.048, Supplemental Materials Figure 3C). Patients with no less than a 76% decrease in AFPΔ showed a trend with better OS than those who did not, though statistical significance was not detected (p = 0.180, Supplemental Materials Figure 3D).
Correlations of Pathologic Response With RFS
Of the 39 patients receiving hepatectomy, 15 and 24 patients had pathologic major and minor response, respectively. None of the patients had pathologic complete response. Patients with pathologic major response showed no significant difference in terms of RFS compared with those with minor response (p = 0.798, Supplemental Materials Figure 4).
Correlations of α-RECIST Evaluation With Pathologic Response
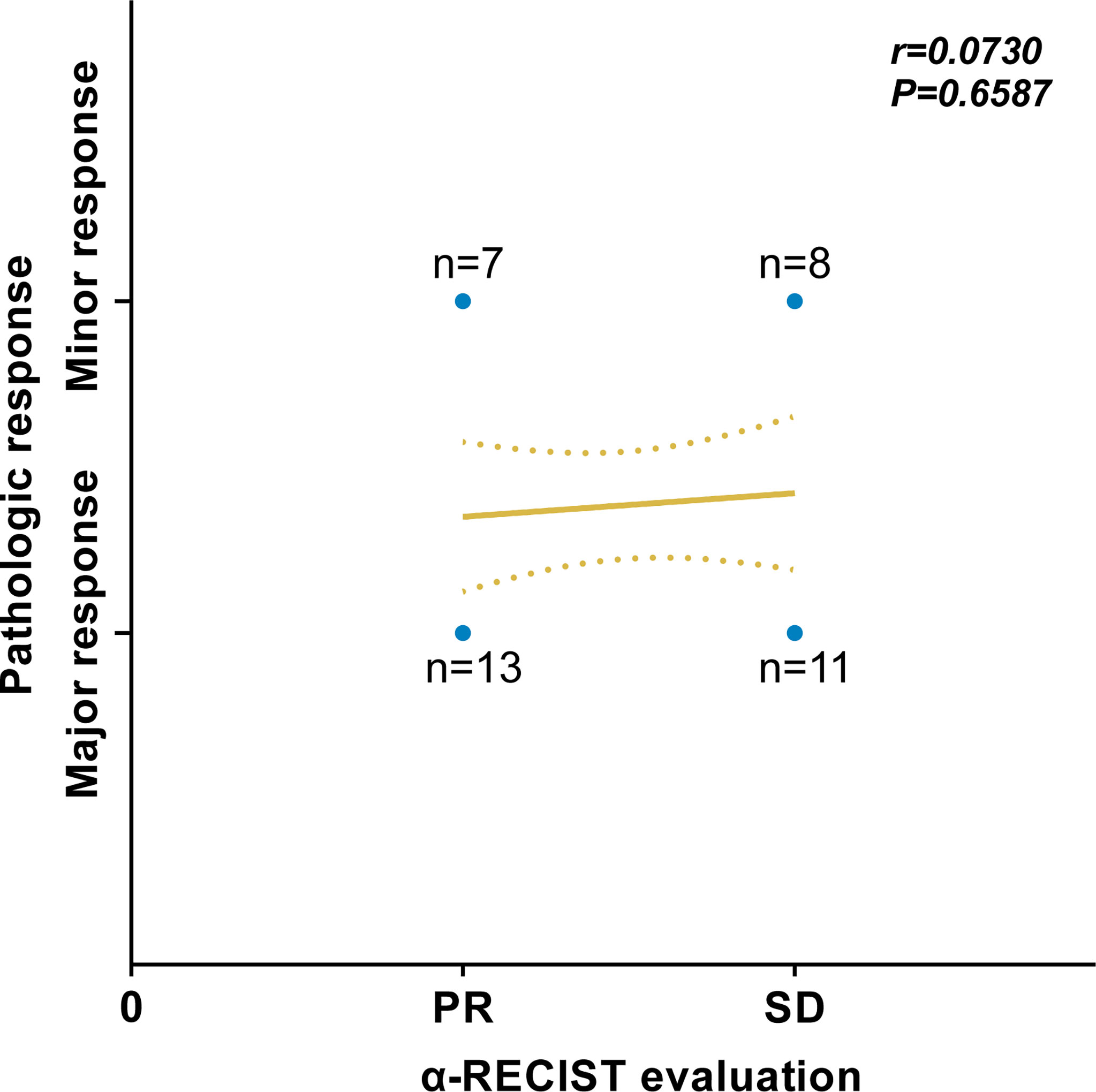
Of all 20 patients defined as PR by α-RECIST, 13 and 7 patients had pathologic major and minor response, respectively. Nineteen patients were defined as SD by α-RECIST, 11 and 8 patients had pathologic major and minor response, respectively. According to the Spearman’s correlation analysis, no significant correlation was detected between α-RECIST evaluation and pathologic response evaluation (r = 0.0730, 95% CI: -0.2573 to 0.3880, P = 0.6587, Figure 6).
The Superiority of α-RECIST Criteria
Above all, αΔ-RECIST could enable identification of responders and significantly correlate with both RFS and OS. As a result, αΔ-RECIST was defined as the α-RECIST criteria. As is shown in Figure 7, α-RECIST criteria could identify three patterns of PR: fulfilling both PR defined by RECIST 1.1 and by changes of AFP, by fulfilling PR defined by RECIST 1.1 alone, and by fulfilling changes of AFP alone. Responders, according to α-RECIST, showed significantly better RFS compared to non-responders [HR, 0.31 (95% CI: 0.10, 0.98); p = 0.046] and no statistical significance was observed in terms of OS [HR, 0.33 (95% CI: 0.11, 1.05); p = 0.06] (Table 3). RECIST 1.1 failed to correlate responders with survival benefit [RFS: HR, 0.59 (95% CI: 0.17, 2.11), p = 0.419; OS: HR, 0.65 (95% CI: 0.18, 2.33), p = 0.512]. The cumulative 1-year, 2-year, and 3-year RFS rates were 95.0%, 83.8%, 71.8%, respectively, in responders and 68.4%, 57.9%, 47.4%, respectively, in non-responders by α-RECIST criteria (p = 0.035). The cumulative 1-year, 2-year, and 3-year OS rates were 100.0%, 94.4%, 80.4%, respectively, in responders and 78.9%, 63.2%, 57.9%, respectively, in non-responders by α-RECIST criteria (p=0.048).
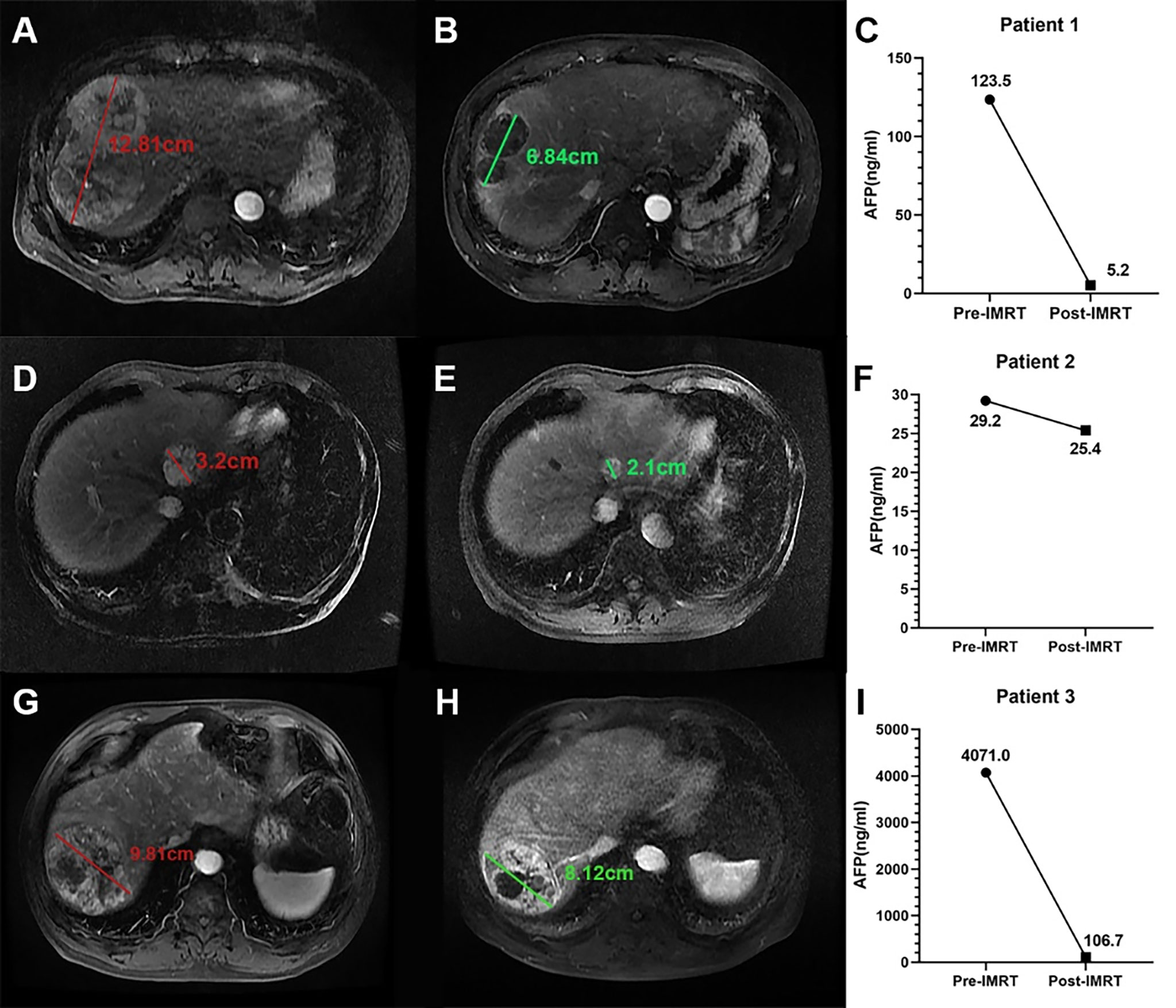
Figure 7 Three example cases of PR defined by the α-RECIST. (A, B) present the SLD on arterial phase images of pre- and post-IMRT CE-MRI scans for patient 1, and the size change is -42.0%; (C) presents the serum AFP levels before and after IMRT for patient 1, and the AFPΔ is -95.8%. (D, E) present the SLD on arterial phase images of pre- and post-IMRT CE-MRI scans for patient 2, and the size change is -34.0%; (F) presents the serum AFP levels before and after IMRT for patient 2, and the AFPΔ is -15.0%. (G, H) present the SLD on arterial phase images of pre- and post-IMRT CE-MRI scans for patient 3, and the size change is -17.0%; (I) presents the serum AFP levels before and after IMRT for patient 3, and the AFPΔ is -97.4%. For patient 1, both the size decrease and the AFP decrease degree exceed the threshold values; For patient 2, only the size decrease degree exceed the threshold value; For patient 3, only the AFP decrease degree exceed the threshold value. All the three patients are defined as PR by α-RECIST.
Inter-Reader Variability
The inter-reader agreements for RECIST 1.1, mRECIST, and α-RECIST criteria were 94.9%, 97.4%, 100.0%, respectively. The weighted k coefficients were 0.89 (0.74-1.04), 0.95 (0.85-1.05), 1.00 (1.00-1.00), respectively, as shown in Table 4.
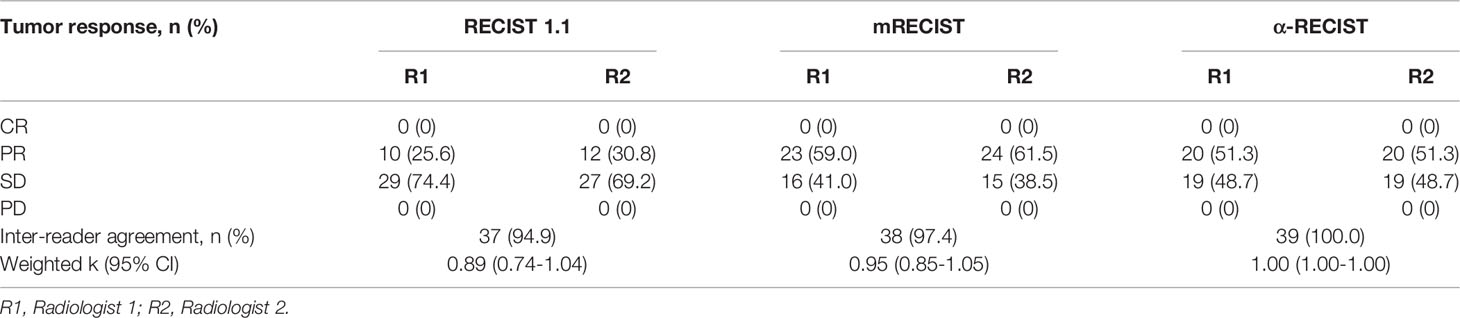
Table 4 Response rates according to evaluation criteria for two independent radiologists with inter-reader agreement (n=39).
Discussion
Currently, no clear-cut criterion has been established for tumor response of patients with uHCC treated with IMRT. In this study, we investigated different modified versions of tumor response criteria by combining AFPΔ, AFPBL with RECIST 1.1, mRECIST. It was demonstrated that responders identified by α-RECIST were associated with significant longer RFS (HR, 0.31 [95% CI: 0.10, 0.98]; p = 0.046), superior than the other evaluation criteria including RECIST 1.1, mRECIST, and pathologic response evaluation. The cumulative 1-year, 2-year, and 3-year RFS rates were 95.0%, 83.8%, 71.8%, respectively, in responders and 68.4%, 57.9%, 47.4%, respectively, in non-responders by α-RECIST criteria.
The most widely used criteria for tumor response in patients with HCC was RECIST 1.1 (4). In our study, responders detected by RECIST 1.1 showed a trend for longer RFS than non-responders, while no statistical significance was reached (p = 0.405). Since proposed in 2009 (25), mRECIST has been widely utilized in response evaluation of HCC after various treatment, including chemotherapy, targeted therapy, and immunotherapy (26–28). Currently, mRECIST has also been used in several clinical trials for tumor response in HCC treated with radiation (29–31). In our study, mRECIST failed to identify responders from non-responders with RFS benefit (p = 0.201). Park et al. (11) demonstrated a proportion of 46% patients showed arterial hypervascularity in parenchyma surrounding the original tumor at 3 months post radiotherapy, which was termed as “pseudo-progression” which probably resulted from subtotal collagenous occlusion of small hepatic vein branches and subsequent impaired outflow of blood and hyperemia (32). Thus, the persistent enhancement after radiotherapy did not necessarily indicate viable neoplasm, which could explain the limited value of mRECIST in tumor response for patients with uHCC treated with IMRT.
RECIST “or” changes of AFP gave the best separation of curves, while RECIST and changes of AFP were not predictive of RFS. We think it may be explained by the following reasons based on unidimensional diameter, RECIST 1.1 may underestimate tumor response and has been reported to have poor correlations with clinical outcomes in patients with HCC after systemic therapies (19, 33). After reviewing many radiological tumor response evaluations in clinical trials of our institution, tumor response usually occurs with minimal size shrinkage, which is usually insufficient to meet RECIST-defined response threshold (30%); meanwhile, AFP decrease quickly and distinctly after radiotherapy. As a result, some responders with minimal size shrinkage will be detected by the α-RECIST (RECIST or changes of AFP). As for RECIST 1.1 “and” changes of AFP, it seems too strict to define PR and it reclassified 10 α-RECIST-defined responders as non-responders. As a result, it may not detect the early response of responders with minimal size shrinkage. We noticed the insensitivity of the unidimensional diameter by RECIST 1.1 and tried to establish a novel criterion combined with the biochemical index AFP. The α-RECIST criteria reclassified eight RECIST-defined non-responders as responders and did not reclassify any patients who were RECIST-defined responders, which seemed to promote the detection sensitivity of responders. It seemed that α-RECIST increased the survival benefit of responders over non-responders compared with RECIST 1.1 [HR, 0.31 (95% CI: 0.10, 0.98); p = 0.046]. The α-RECIST criteria might be a promising tool for identifying tumor response of conversion-radiotherapy for uHCC before hepatectomy.
Pathologic response evaluation showed no significant correlation with neither RFS nor with α-RECIST evaluation. The pathologic response evaluation might only reflect the instant treatment response to radiotherapy, which may not translate into long-term survival benefit.
Several limitations should be stressed regarding this study. First, the limited sample size was the main limitation. As a result, generalization of our conclusions should be interpreted with caution and prospective studies with larger sample size are urgently needed. Second, the primary endpoint was RFS after hepatectomy rather than OS in this study because OS may not reflect the survival benefit of IMRT and could be influenced by the subsequent therapies. Various therapies were utilized once recurrence occurred after initial hepatic resection (secondary resection, local ablation therapy, hepatic artery infusion chemotherapy, or systemic therapy).
Conclusions
An optimal revised criterion (α-RECIST) was developed by combining the RECIST 1.1 with the AFPΔ (cutoff value, 76%) to evaluate tumor response for uHCC receiving conversion-radiotherapy before hepatectomy. It was demonstrated that patients identified as responders by α-RECIST showed better RFS than those defined as non-responders. The α-RECIST criteria might be a promising tool for identifying tumor response of conversion-radiotherapy for uHCC before hepatectomy.
Data Availability Statement
The datasets underlying this study are available on request to the corresponding authors. Requests to access these datasets should be directed to ZHJfZmVuZ3llX25jY0AxNjMuY29t.
Ethics Statement
This study involving human participants was reviewed and approved by Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and was conducted in compliance with the 1975 Declaration of Helsinki, Good Clinical Practice guidelines and local regulatory requirements. Written informed consent was waived by the Institutional Review Board.
Author Contributions
Study concept and design (YX, YY, and LL), acquisition of data (FY), analysis and interpretation of data (YX, YY, and LL), drafting of the manuscript (YX, YY, LL, and FY), critical revision of the manuscript for important intellectual content (FY), critical funding (MXZ and LL), administrative, technical, or material support, study supervision (FY and MXZ). All the authors have read and approved the manuscript and had access to the study data.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81971589) and the Youth Project of Beijing Hope Run Special Fund (No. LC2021B17).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.905260/full#supplementary-material
Abbreviations
AFP, alpha fetoprotein; α-RECIST, alpha fetoprotein-RECIST; CE-MRI, contrast-enhanced magnetic resonance imaging; CR, complete response; CTV, clinical tumor volume; EASL, European Association for the Study of Liver Diseases; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EPI, echo-planar imaging; HCC, hepatocellular carcinoma; HR, hazard ratio; IMRT, intensity-modulated radiotherapy; IQR, interquartile range; mRECIST, Modified Response Evaluation Criteria in Solid Tumors; OS, overall survival; PD, progressive disease; PFS, progression free survival; PR, partial response; PTV, planning target volume; RECIST 1.1, Response Evaluation Criteria in Solid Tumors, version 1.1; RFS, recurrence-free survival; RT, radiotherapy; SD, stable disease; SLD, sum of the longest diameter; uHCC, unresectable hepatocellular carcinoma; VEGF, vascular endothelial growth factor.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular Carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
3. Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and Adjuvant Therapy for Resectable Hepatocellular Carcinoma: Review of the Randomised Clinical Trials. Lancet Oncol (2002) 3(10):593–603. doi: 10.1016/s1470-2045(02)00873-2
4. Tanguturi SK, Wo JY, Zhu AX, Dawson LA, Hong TS. Radiation Therapy for Liver Tumors: Ready for Inclusion in Guidelines? Oncologist (2014) 19(8):868–79. doi: 10.1634/theoncologist.2014-0097
5. Lau WY, Lai EC. Salvage Surgery Following Downstaging of Unresectable Hepatocellular Carcinoma–a Strategy to Increase Resectability. Ann Surg Oncol (2007) 14(12):3301–9. doi: 10.1245/s10434-007-9549-7
6. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma With Tyrosine Kinase Inhibitor and Anti-Pd-1 Antibody Combinations. Liver Cancer (2021) 10(4):320–9. doi: 10.1159/000514313
7. Bae SH, Jang WI, Park HC. Intensity-Modulated Radiotherapy for Hepatocellular Carcinoma: Dosimetric and Clinical Results. Oncotarget (2017) 8(35):59965–76. doi: 10.18632/oncotarget.19219
8. Choi SH, Seong J. Strategic Application of Radiotherapy for Hepatocellular Carcinoma. Clin Mol Hepatol (2018) 24(2):114–34. doi: 10.3350/cmh.2017.0073
9. Mendiratta-Lala M, Masch WR, Shampain K, Zhang A, Jo AS, Moorman S, et al. Mri Assessment of Hepatocellular Carcinoma After Local-Regional Therapy: A Comprehensive Review. Radiol Imaging Cancer (2020) 2(1):e190024. doi: 10.1148/rycan.2020190024
10. Kellock T, Liang T, Harris A, Schellenberg D, Ma R, Ho S, et al. Stereotactic Body Radiation Therapy (Sbrt) for Hepatocellular Carcinoma: Imaging Evaluation Post Treatment. Br J Radiol (2018) 91(1085):20170118. doi: 10.1259/bjr.20170118
11. Park MJ, Kim SY, Yoon SM, Kim JH, Park SH, Lee SS, et al. Stereotactic Body Radiotherapy-Induced Arterial Hypervascularity of Non-Tumorous Hepatic Parenchyma in Patients With Hepatocellular Carcinoma: Potential Pitfalls in Tumor Response Evaluation on Multiphase Computed Tomography. PLos One (2014) 9(2):e90327. doi: 10.1371/journal.pone.0090327
12. Mendiratta-Lala M, Masch W, Shankar PR, Hartman HE, Davenport MS, Schipper MJ, et al. Magnetic Resonance Imaging Evaluation of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy: Long Term Imaging Follow-Up. Int J Radiat Oncol Biol Phys (2019) 103(1):169–79. doi: 10.1016/j.ijrobp.2018.09.004
13. Mastrocostas K, Jang HJ, Fischer S, Dawson LA, Munoz-Schuffenegger P, Sapisochin G, et al. Imaging Post-Stereotactic Body Radiation Therapy Responses for Hepatocellular Carcinoma: Typical Imaging Patterns and Pitfalls. Abdom Radiol (NY) (2019) 44(5):1795–807. doi: 10.1007/s00261-019-01901-y
14. Montal R, Andreu-Oller C, Bassaganyas L, Esteban-Fabro R, Moran S, Montironi C, et al. Molecular Portrait of High Alpha-Fetoprotein in Hepatocellular Carcinoma: Implications for Biomarker-Driven Clinical Trials. Br J Cancer (2019) 121(4):340–3. doi: 10.1038/s41416-019-0513-7
15. Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and Significance of Alpha-Fetoprotein in Hepatocellular Carcinoma. Liver Int (2019) 39(12):2214–29. doi: 10.1111/liv.14223
16. Chau I, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Alpha-Fetoprotein Kinetics in Patients With Hepatocellular Carcinoma Receiving Ramucirumab or Placebo: An Analysis of the Phase 3 Reach Study. Br J Cancer (2018) 119(1):19–26. doi: 10.1038/s41416-018-0103-0
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised Recist Guideline (Version 1.1). Eur J Cancer (Oxford Engl 1990) (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
18. Kaneko S, Tsuchiya K, Yasui Y, Inada K, Kirino S, Yamashita K, et al. Early Radiological Response Evaluation With Response Evaluation Criteria in Solid Tumors 1.1 Stratifies Survival in Hepatocellular Carcinoma Patients Treated With Lenvatinib. JGH Open (2020) 4(6):1183–90. doi: 10.1002/jgh3.12420
19. Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, et al. Alternative Response Criteria (Choi, European Association for the Study of the Liver, and Modified Response Evaluation Criteria in Solid Tumors [Recist]) Versus Recist 1.1 in Patients With Advanced Hepatocellular Carcinoma Treated With Sorafenib. Oncologist (2014) 19(4):394–402. doi: 10.1634/theoncologist.2013-0114
20. Kaneko S, Tsuchiya K, Kurosaki M, Kirino S, Inada K, Yamashita K, et al. Three Criteria for Radiological Response on Survival in Patients With Hepatocellular Carcinoma Treated With Lenvatinib. Hepatol Res (2020) 50(1):137–43. doi: 10.1111/hepr.13416
21. Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic Response to Preoperative Chemotherapy: A New Outcome End Point After Resection of Hepatic Colorectal Metastases. J Clin Oncol (2008) 26(33):5344–51. doi: 10.1200/JCO.2008.17.5299
22. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713
23. Schemper M, Smith TL. A Note on Quantifying Follow-Up in Studies of Failure Time. Control Clin Trials (1996) 17(4):343–6. doi: 10.1016/0197-2456(96)00075-x
24. Svanholm H, Starklint H, Gundersen HJ, Fabricius J, Barlebo H, Olsen S. Reproducibility of Histomorphologic Diagnoses With Special Reference to the Kappa Statistic. APMIS (1989) 97(8):689–98. doi: 10.1111/j.1699-0463.1989.tb00464.x
25. Lencioni R, Llovet JM. Modified Recist (Mrecist) Assessment for Hepatocellular Carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132
26. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
27. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic Arterial Infusion of Oxaliplatin Plus Fluorouracil/Leucovorin Vs. Sorafenib for Advanced Hepatocellular Carcinoma. J Hepatol (2018) 69(1):60–9. doi: 10.1016/j.jhep.2018.02.008
28. Kudo M, Ueshima K, Chiba Y, Ogasawara S, Obi S, Izumi N, et al. Objective Response by Mrecist Is an Independent Prognostic Factor for Overall Survival in Hepatocellular Carcinoma Treated With Sorafenib in the Silius Trial. Liver Cancer (2019) 8(6):505–19. doi: 10.1159/000503032
29. Chen B, Wu JX, Cheng SH, Wang LM, Rong WQ, Wu F, et al. Phase 2 Study of Adjuvant Radiotherapy Following Narrow-Margin Hepatectomy in Patients With Hepatocellular Carcinoma. Hepatology (2021) 74(5):2595–604. doi: 10.1002/hep.31993
30. Kim JW, Kim DY, Han KH, Seong J. Phase I/Ii Trial of Helical Imrt-Based Stereotactic Body Radiotherapy for Hepatocellular Carcinoma. Dig Liver Dis (2019) 51(3):445–51. doi: 10.1016/j.dld.2018.11.004
31. Li X, Ye Z, Lin S, Pang H. Predictive Factors for Survival Following Stereotactic Body Radiotherapy for Hepatocellular Carcinoma With Portal Vein Tumour Thrombosis and Construction of a Nomogram. BMC Cancer (2021) 21(1):701. doi: 10.1186/s12885-021-08469-1
32. Maturen KE, Feng MU, Wasnik AP, Azar SF, Appelman HD, Francis IR, et al. Imaging Effects of Radiation Therapy in the Abdomen and Pelvis: Evaluating "Innocent Bystander" Tissues. Radiographics (2013) 33(2):599–619. doi: 10.1148/rg.332125119
Keywords: hepatocellular carcinoma, radiotherapy, hepatectomy, tumor response, RECIST 1.1
Citation: Xu Y, Yang Y, Li L, Ye F and Zhao X (2022) The α-RECIST (RECIST 1.1 Combined With Alpha Fetoprotein): A Novel Tool for Identifying Tumor Response of Conversion-Radiotherapy for Unresectable Hepatocellular Carcinoma Before Hepatectomy. Front. Oncol. 12:905260. doi: 10.3389/fonc.2022.905260
Received: 26 March 2022; Accepted: 19 April 2022;
Published: 24 May 2022.
Edited by:
Long Jiang Zhang, Nanjing General Hospital of Nanjing Military Command, ChinaReviewed by:
Chi-Leung Chiang, The University of Hong Kong, Hong Kong SAR, ChinaChai Hong Rim, Korea University, South Korea
Copyright © 2022 Xu, Yang, Li, Ye and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ye, ZHJfZmVuZ3llX25jY0AxNjMuY29t; Xinming Zhao, eGlubWluZ3pobmNjQDE2My5jb20=
†These authors have contributed equally to this work
 Ying Xu1†
Ying Xu1† Yi Yang
Yi Yang Feng Ye
Feng Ye