- 1Department of Medicine and Rehabilitation, Tung Wah Eastern Hospital, Hong Kong, Hong Kong SAR, China
- 2Department of Surgery, The Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, China
- 3School of Biomedical Sciences, The Chinese, University of Hong Kong, Hong Kong, Hong Kong SAR, China
Breast cancer is one of the leading causes of mortality in females. Over the past decades, intensive efforts have been made to uncover the pathogenesis of breast cancer. Interleukin-6 (IL-6) is a pleiotropic factor which has a vital role in host defense immunity and acute stress. Moreover, a wide range of studies have identified the physiological and pathological roles of IL-6 in inflammation, immune and cancer. Recently, several IL-6 signaling pathway-targeted monoclonal antibodies have been developed for cancer and immune therapy. Combination of IL-6 inhibitory antibody with other pathways blockage drugs have demonstrated promising outcome in both preclinical and clinical trials. This review focuses on emerging studies on the strong linkages of IL-6/IL-6R mediated regulation of inflammation and immunity in cancer, especially in breast cancer.
Introduction
Breast cancer is one of the leading diagnosed cancers in women with high mortality. According to International Agency for Research on Cancer (IARC), there were 2,261,419 women diagnosed with breast cancer in 2020 worldwide. It is a common cause of cancer-related death especially in less developed countries. Despite the recent advanced technique in breast cancer screening and early diagnosis, the high morbidity and mortality rates urge the need of investigation into the molecular mechanism of breast cancer.
Genome wide analyses have recently demonstrated thousands of mutations accumulated in breast cancer cells (1). In addition, as a multifactorial disease, the etiologies of breast cancer include not only distinct inherent factors such as genetic status, but also environmental factors such as obesity, lifestyle, and chronic inflammation (2).
Accumulating studies have been performed on the relationship between inflammation and cancer (3). It is well-accepted that inflammatory diseases could increase the risk of cancer development during tumor initiation, promotion, progression, and metastasis (3–6).
As one of the best-characterized pro-tumorigenic cytokines, IL-6 has been studied extensively for its central role in both physiological and pathological processes (7). Previous studies indicated that IL-6 regulate the pro-inflammatory and enhance monocyte infiltration at the inflammatory site during chronic inflammation (8). IL-6 responsive tissues would become resistant gradually during chronic inflammation, which correlated with high basal level of IL-6 (9, 10). IL-6 was also elevated in many solid tumors including breast cancer (11–13), which correlated with poor prognosis and metastasis (14, 15). The current review will further discuss the intricate relationship between IL-6, inflammation, and breast cancer.
The IL-6 Signaling Pathways and Functions
The Il-6 Signaling Pathway
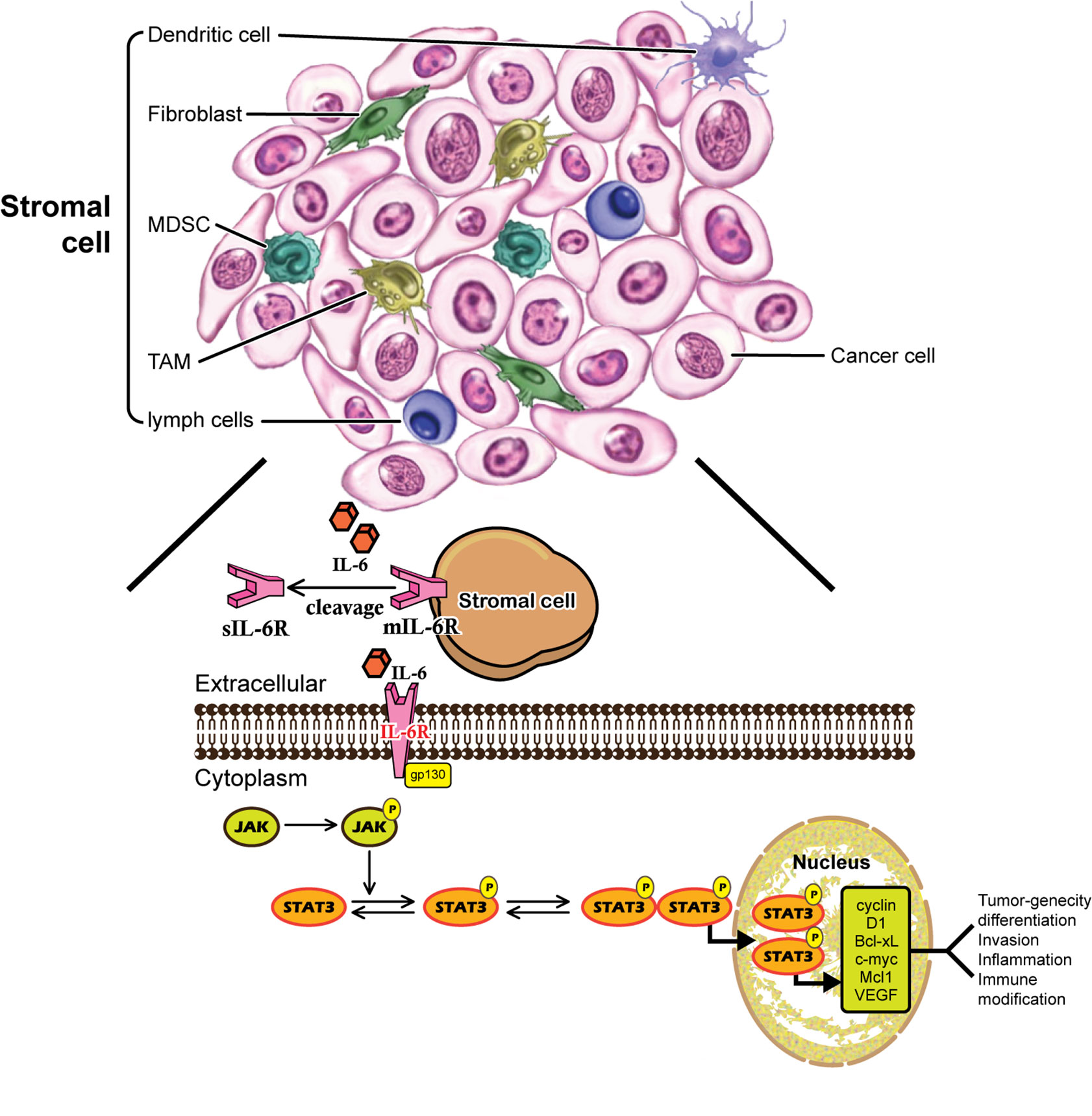
Human IL-6 is a 26 kDa glycoprotein known as a B-cell differentiation regulator (16) which is secreted by a number of cells (17). IL-6 is a multifunctional cytokine that plays both pro-inflammatory and anti-inflammatory roles in humans (18). IL-6 is a single chain phosphorylated glycoprotein consisting of four helix bundles (A-D), with A and B run in one direction while C and D run in the opposite direction. IL-6 transmits its signals through a cell-surface type-I receptor complex, which consists of the membrane-bound IL-6 receptor (IL-6R) and a signal-transducing component gp130 homodimer (19). IL-6R is expressed on a limited number of cell types, such as macrophages, B cells and subtypes of T cells (20, 21). IL-6R is 80 kDa α-chain and is also called as CD126 consisting of three domains namely D1, D2 and D3. Besides the membrane bound receptor (mIL-6R) as previously mentioned, soluble (sIL-6R) is the other form of IL-6R, which is expressed mainly in hepatocytes, neutrophils, monocytes, and T-cells (22). IL-6 selectively activates different signaling pathways, the classical signaling pathway through mIL-6R, and the trans-signaling pathway through sIL-6R. In both the cases, IL-6 binds to the receptor and then to gp130, but elicits different biological effects depending upon the receptor form (23). Cytokine IL-6 triggers the anti-inflammatory responses through classic signaling by binging to mIL-6R and gp130, while in contrast, trans-signaling can be manifested in all gp130-expressing cells, and leads to pro-inflammatory responses (24). The sIL-6R can be found at circulation with concentration from 25 to 35 ng/ml in human, which is generated by proteolytic cleavage of the membrane bound form IL-6R and by proteolytic cleavage of metalloproteinases gene family members, or by alternative splicing of IL-6R mRNA (25). There are three routes of the IL-6 signaling pathway. In route 1, Janus kinase (JAK) is phosphorylated and activated, subsequently activates dimerization of signal transducer and transcription-3 (STAT3) (26). In route 2, JAK activates Ras/Raf pathway, causing hyperphosphorylation of mitogen activated protein kinases (MAPK) and incudes its serine/threonine kinase activity (23). The third route involves the activation of phosphoinositol-3 kinase (PI3K)-protein kinase B (PKB)/Akt pathway (27).
IL-6 and Immunity
IL-6 is secreted by largely plasmacytoid dendritic cells (pDCs), which is critical for differentiation from B cells to plasma cells (28). This cytokine is also a vital modulator to maintain dynamic balance between Th1 and Th2 immune cells (29). For example, IL-6 is necessary during the differentiation from Th1 to Th2 cells (30). The process was proved to interfere with IFN-γ production via up-regulation of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in CD4+T cells (31). Meanwhile, together with transforming growth factor-β (TGF-β), IL-6 could promote the differentiation of Th17 cells via activating both retinoic acid-related orphan receptor γt (RORγt) and RORα (32). It was reported that STAT3 mediated the effectiveness of IL-6 on Th17 differentiation and this cytokine could inhibit the activity of Treg cells (33). Therefore, IL-6 is regarded as the main regulator of Treg/Th17 equilibrium (34).
IL-6 also plays a vital role in early differentiation of T follicular helper cells (Tfh), the main T helper cell subtype provides support for germinal center formation, affinity maturation, and immune cells’ generation. Early BCl6+/CXCR5+/Tfh differentiation would be mostly interfered in the case of IL-6 absence which was proved to mediate by STAT1 and STAT3 (35).
Novel agents against the IL-6/IL-6R signaling pathway have been proved to be effective for some inflammatory diseases. Preclinical studies have demonstrated that IL-6 has crucial functions in inflammatory cells recruitment (36). Tumor-associated macrophages (TAMs) secreted IL-6 and plays critical role in carcinogenesis and differentiation of myeloid-derived suppressor cells (MDSCs), which gives rise to intra-tumoral inflammatory processes (37, 38). A previous study demonstrated that inhibition of NF-κB decreased the stem cell compartment, which in turn reduced blood vessel formation in breast cancer (39). In addition, high expression of IL-6R on liver cells led to recruitment of acute phase proteins (40). High expression levels of acute phase proteins including CRP, fibrinogen and serum amyloid protein A were identified during both acute and chronic disease (41, 42). Interestingly, clinical observation found that CRP levels in patients with severe bacterial infections were not elevated when IL-6 was absent (43). Further studies demonstrated that blocking IL-6 signaling by neutralizing antibody may reverse low serum level of CRP (44). However, the application of IL-6/IL-6R blockers as anti-cancer agents has not been proved intensively in cancers including breast cancer.
IL-6 and Stem Cell
IL-6 family cytokines play an important role in generation and maintenance of stem/progenitor cells including cancer stem cells (CSCs) (45). As a member in IL-6 family, leukemia inhibitory factor (LIF) has an crucial role in both embryonic stem (ES) cells and cancer development (46), which is necessary to maintain mouse ES cells in an undifferentiated condition via STAT3 activation (47). Active LIF was detected in a wide range of malignancies including lung, breast, stomach, colon, liver, gallbladder, and pancreatic carcinoma (48). Once activated, STAT3 may induce gene expression including c-Myc, which contribute to the maintenance of undifferentiated state in mouse ES cells (49). It is also reported that IL-6 increased pluripotent stem (iPS) cell population by inducing c-Myc and Pim1 (50). The transcription factor C/EBPδ, was reported to be pro-tumorigenic in breast cancer cell lines by directly targeting IL-6R, leading to cancer progression with cancer stem cells activation (51). The IL-6-JAK1-STAT3 pathway has a vital function in the transition from non-CSCs into CSCs by regulating OCT4 in human breast cancer cell lines (52). In lung cancer CSCs, IL-6Rα was detected in CSCs (53), whereas STAT3 was necessary for proliferation and survival in colon cancer-initiating cells (54, 55). It was reported that constitutive activation of STAT3 and NF-κB signaling in glioblastoma CSCs regulate Notch pathway, which played a key role in CSC maintenance and cell survival (56). STAT3 activation by IL-6 from adipose-derived stem cells could promote endometrial carcinoma proliferation and metastasis (57).
IL-6 is also crucial for epigenetic modification in stem cells (58, 59). NF-κB and STAT3 were identified as key regulators in epigenetic switch in inflammation (60, 61). Recently, a positive feedback loop involving microRNA let-7 has been demonstrated for maintaining chronic inflammatory status in malignant cells (60). Interestingly, this feedback loop regulated by IL-6 signaling could in turn activate NF-κB pathway and its downstream targets such as let-7 and Lin-28. Similarly, IL-6 was proved to be essential in keeping inflammatory loop in breast cancer CSCs (60, 61). In summary, IL-6 signaling plays a regulatory role in controlling cancer cell growth, CSC renewal and metastasis (62).
IL-6 and Tumor Microenvironment
Tumor microenvironment contributes significantly towards potentiating the stemness and metastasis properties of cancer cells. Solid tumors, including breast cancer cells were reported to have intense interaction with stromal cells such as mesenchymal stem cells (MSCs), adipocytes, cancer associated fibroblasts (CAFs), endothelial cells and immune cells in tumor microenvironment (63). Majority of these stromal cells within tumor microenvironment could secrete both IL-6 and IL-8 (63, 64). Mesenchymal cells could be either recruited from bone marrow (65) or normal breast stroma (66). In breast tumor cells, it has been identified that MSCs could be selectively recruited to the sites of growing carcinoma through cytokine such as IL-6 and CXCL7, where they interact with breast cancer CSCs (65, 66). In addition, MSCs are capable to differentiate into CAFs as well as adipocytes, which also interact with cancer cells (67).
CAFs have been demonstrated to have the ability to support tumorigenesis by stimulating angiogenesis, cell proliferation and invasion (68). CAFs in breast tumors expressed high levels of IL-6 (68, 69), which mediated epithelial-stromal interactions and promoted tumorigenesis (70). CAFs were reported to induce trastuzumab resistance in HER2 positive breast cancer cells (71). More importantly, IL-6 could in turn reactivate breast stromal fibroblasts through STAT3-dependent manner (72). CAFs could affect intra tumoral CD8+ and FoxP3+ T cells via IL-6 in tumor microenvironment (73). Recent findings also indicated miR-149’s role in the crosstalk between tumor cells and CAFs, which highlighted the potential therapeutic strategy using interfering miRNAs (74). There was growing evidence support that CAFs promote stem cell-like properties of hepatocellular carcinoma via IL-6/STAT3/Notch signaling pathway (75).
In a recent study, a novel developed liposomal nanoparticle loaded with anti-IL6R antibody which deliver to tumor microenvironment achieved a significant effect in inhibiting the metastasis of breast cancer cells in mouse models (76).
Obesity has been recently identified as a negative prognostic factor in breast cancer (77, 78), which appears to be independent of menopausal status, tumor stage, and hormone-related factors (79). According to the reported literature, adipocytes produced inflammatory cytokines such as IL-6 in obesity individuals (80). IL-6 was reported to mediate crosstalk between preadipocytes and breast ductal carcinoma in situ cells which may lead to progression of early-stage breast cancer (81). In addition, adipose-derived stem cells (ADSCs) promoted tumor initiation and accelerated tumor growth through IL-6 production (82). Obesity was suggested to induce resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 (83).
IL-6’s Functional Role in Breast Cancer Development
Experimental Studies
The predominant role of IL-6 in cancer is its key promotion of tumour growth. It has been demonstrated that deregulated IL-6 signaling pathway plays important roles in proliferation, migration, and adhesion among tumors (84–87). High level of IL-6 in breast cancer tissues stimulated Jagged-1 expression to promote cell growth and maintain the aggressive phenotype (88). High level of IL-6 secretion may facilitate tumor cell growth via suppressing apoptosis and promoting angiogenesis (89). High expression of IL-6Rα was also demonstrated to induce apoptosis resistance in breast cancer (90). In metastatic lesions of breast cancer patients, upregulated IL-6 was identified which may lead to chemotherapy resistance such as paclitaxel (91). The crosstalk between adipocytes and breast cancer cells in cancer progression has attracted much attention in recent years. The adipocyte-derived IL-6 was reported to promote breast cancer metastasis by inducing PLOD2 expression through activating the JAK/STAT3 and PI3K/AKT signaling pathways (92). In a recent study on triple-negative breast cancers (TNBCs), restraining of IL-6 and IL-8 expressions prominently suppressed both in vitro and in vivo cancer cell proliferation (93).
IL-12, which is produced by activated antigen presenting cells including dendritic cells and macrophages, was reported to inhibit tumor development (94). Some studies suggested that high expression level of IL-12 receptor were found to significantly increase breast cancer patients’ survival, especially in the more aggressive subtypes (95). It is also critical to initiate the differentiation of naive CD4+ T cells to T helper type 1 (Th-1) cells (96). However, the correlation between IL-6 and IL-12 remains elusive in breast cancer. According to the reported literature, the Th-1/Th-2 imbalance plays important role in the development of breast cancer (97). And circulating Th-1 and Th-2 levels and their ratios are associated with ER-negative and TNBC, suggesting their contribution in breast cancers (98). IL-6 played dual functions on Th-1/Th-2 differentiation by promoting Th-2 differentiation and inhibiting Th-1 polarization simultaneously (29).
IL-6 is a vital player during acute inflammation, controlling not only the inflammatory response but also tissue metabolism (99). Under chronic inflammation circumstance, IL-6 may induce cachexia through cytokines production and metabolism change in both lipids and proteins (100). Over-expression of IL-6 has been proved to be related with atrophy by promoting muscle protein metabolism (101). Cachexia and its related diseases account for approximately one third of all cancer-related deaths (102). Inflammatory breast cancer (IBC) describes a highly aggressive form of breast cancer of diverse molecular subtypes and clonal heterogeneity. The signature of IBC is recognized by its inflammation feature which is associated with IL-6 expression. A recent study published in May 2022 revealed that IL-6 signaling stimulate cell proliferation in IL-6R and HER2-expressing responsive sub-clones in IBC, and this effect was abrogated by the IL-6R neutralizing antibody Tocilizumab (103).
IL-6 is able to diffuse through cells structures and tissues in tumor microenvironment due to its low molecular weight (104). Tumor microenvironment-associated inflammation, mainly regulated by cytokines including IL-6, has been well-documented to contribute to every stage of cancer progression (105–108). Accumulating evidence has proved the significance of senescent cells in the microenvironment of cancer cells, of which pro-inflammatory IL-6 and IL-8 are consistently present. In this study, IL6 was reported to induce a self-reinforced senescence/inflammatory milieu responsible for the epithelial plasticity and stemness features which prone to a more aggressive phenotype in breast cancer (109).
Despite significant therapeutic achievements have been made in recent years, breast cancer is still one of the most common cancers with high mortality in women worldwide. Estrogen receptor (ER) α-positive breast cancers account for more than two thirds of all the category and endocrine therapies such as selective and aromatase inhibitors remain the standard adjuvant therapy for these tumors. However, majority of patients will develop drug resistance after treatment for several years and alternative hormone therapy is needed afterwards (110, 111). Interestingly, IL6/STAT3 signaling was suggested to drive metastasis in ER positive breast cancer independent of ER, decoupling IL6/STAT3 and ER oncogenic pathways could sensitize some hormonal resistant patients (112). In another study, similar conclusion was reported that Tocilizumab, an antibody that binds to IL-6R, could robustly reverse tamoxifen resistance (113). In compliance with this result, clinical breast cancer samples analysis confirmed that IL-6R expression was significantly associated with tamoxifen resistance in breast cancer tissues, with high IL-6R expression correlated with poor survival (113). Apart from the role in ER positive breast cancer, IL-6 was identified to trigger the migration and invasion of ER negative breast cancer cells via activation of YAP signals (114).
IL-6 could upregulate circulating VEGF in breast cancer patients, which was confirmed to promote angiogenesis and metastasis (115). Downregulation of IL-6 was related to the better response to breast cancer therapy (11, 116). Ligation of IL-6 with IL-6R activates Janus kinase (JAK) tyrosine kinases leading to phosphorylation of signal transducer and activator of transcription 3 (STAT3), which is a well-studied cancer signaling pathway. Moreover, the expression level of IL-6 was higher in aggressive tumors with multi-drug resistance and is negatively related to the expression of estrogen receptor in breast cancer patients (117, 118). Recently, the fact that IL-6-mediated Jagged1/Notch signaling pathway enhanced the ability for breast cancer cells metastasis has been demonstrated (119). All the evidence suggested that IL-6 and its receptor as attractive therapeutic targets.
Clinical Studies
In many preclinical models, IL-6 has been demonstrated to promote carcinogenicity, angiogenesis and metastasis (88, 118, 120, 121). IL-6 has been implicated in resistance to trastuzumab treatment in HER2 positive patients. The induction of IL-6 inflammatory feedback loop leads to the expanded population of CSCs, which lead to high levels of this cytokine secretion. The addition of tocilizumab, an anti-IL-6R antibody, was reported to be capable for the interruption against this feedback loop (122). Based on this finding, a Phase I clinical trial started from 2017 with combined treatment including trastuzumab and tocilizumab for patients with metastatic trastuzumab-resistant HER2+ breast cancer was carried out (NCT03135171). According to the reported literature, IL-6 signaling is a major determinant of TNBC cell proliferation and viability (123), and this chemotherapy-associated inflammatory cytokine may promote resistance mechanisms in TNBC cells as well (124). A Phase Ib/II, open-label, multicenter, randomized umbrella study is being carried out to evaluate the efficacy and safety of multiple immunotherapy-based treatment combinations including tocilizumab in patients with metastatic or inoperable locally advanced TNBC (NCT03424005).
The Prognostic Significance of IL-6 and Its Correlation With Survival
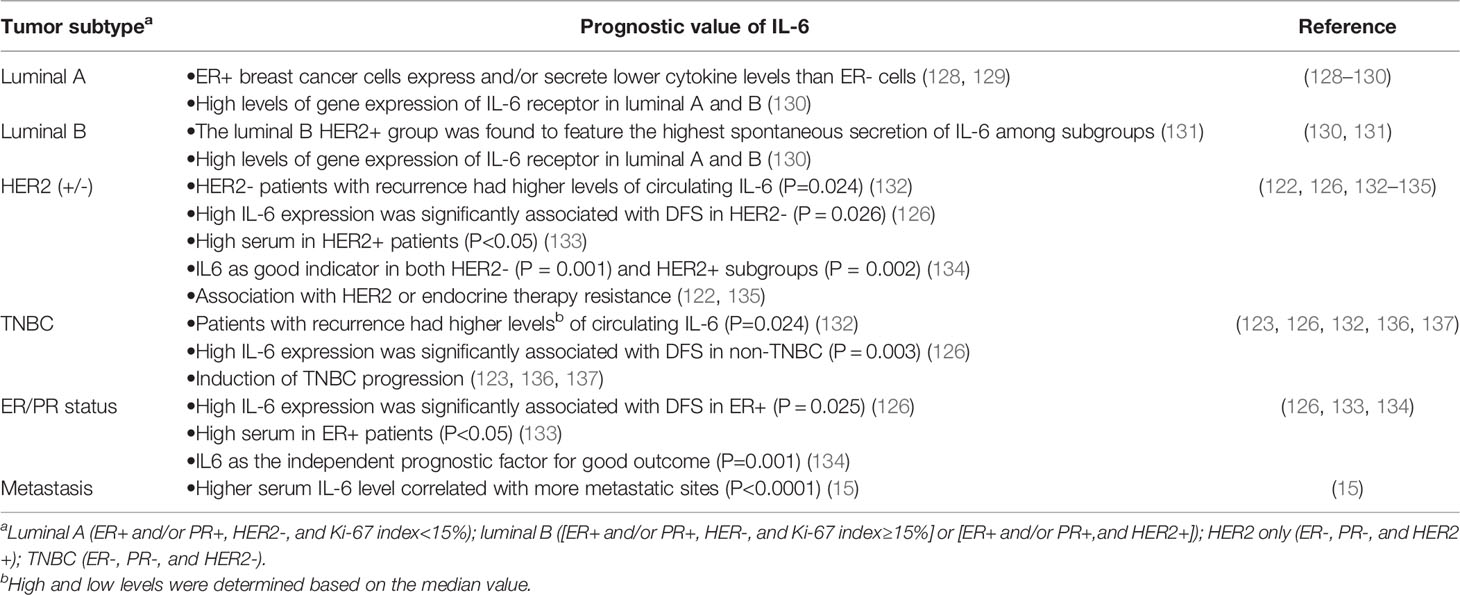
The prognostic impacts of preoperative IL-6 expression levels in patients with breast cancer remain controversial. In a meta-analysis extracted from thirteen articles containing 3,224 breast cancer patients showed that IL-6 expression was not associated with lymph node metastasis, tumor size, or histologic grade. Moreover, there was no correlation between IL-6 expression and disease-free survival. However, the combined hazard ratio for OS was 2.15 (125). Another study included 1,380 patients with early-stage invasive breast cancer revealed that high IL-6 expression is associated with better disease-free survival and breast cancer specific survival (126). However, anther investigation involving 55 female patients with invasive breast cancer demonstrated that the individuals with IL-6 ≥10.0 pg/ml had poorer overall survival compared with those with IL-6 <10.0 pg/ml (127). Similarly, it was reported that high level of serum IL-6 secreted by metastatic breast cancer cells were correlated with poor survival (15). Regarding the roles of IL-6 in ER positive breast cancers as previously described, we further summarized the prognostic value of IL-6 among different subtypes of breast cancer patients (Table 1). For example, in a prospective study included 240 patients who underwent surgery for management of newly diagnosed breast cancer, the associations between plasma concentration of IL-6 and breast cancer recurrence during a six-year follow-up period were examined. The result showed that patients with recurrence had higher levels of circulating IL-6 only among those with HER2 negative tumors. Results of survival analyses revealed an association of high levels of IL-6 with poor recurrence-free survival in patients with HER2 negative and TNBC patients (132).
The approximate percentage of HER2 gene amplified in human breast cancer is 25%, which is characterized by a more aggressive phenotype (138). Trastuzumab, as one of the targeted therapeutic agents for HER2+ breast cancer patients, has totally changed the treatment course. Although many patients benefit from the HER2 targeted therapy, nearly half of them will develop drug resistance after one to two years of treatment (139). Evidence showed that overexpression of HER2 in breast CSCs increased IL-6 production, which could promote CSC self-renewal. The fact that HER2 targeted therapy could prominently activate the IL-6 inflammatory loop and expand the CSC population, signified the cause of IL-6 in Herceptin resistance (122). In ER-negative breast cancer, findings demonstrated that IL-66/Stat3/NF-κB inflammatory loop was activated (140). And it has been proved that leptin-induced STAT3 is partially cross activated through SK1-mediated IL-6 secretion and gp130 activation, suggesting the potential significance of this pathway (141).
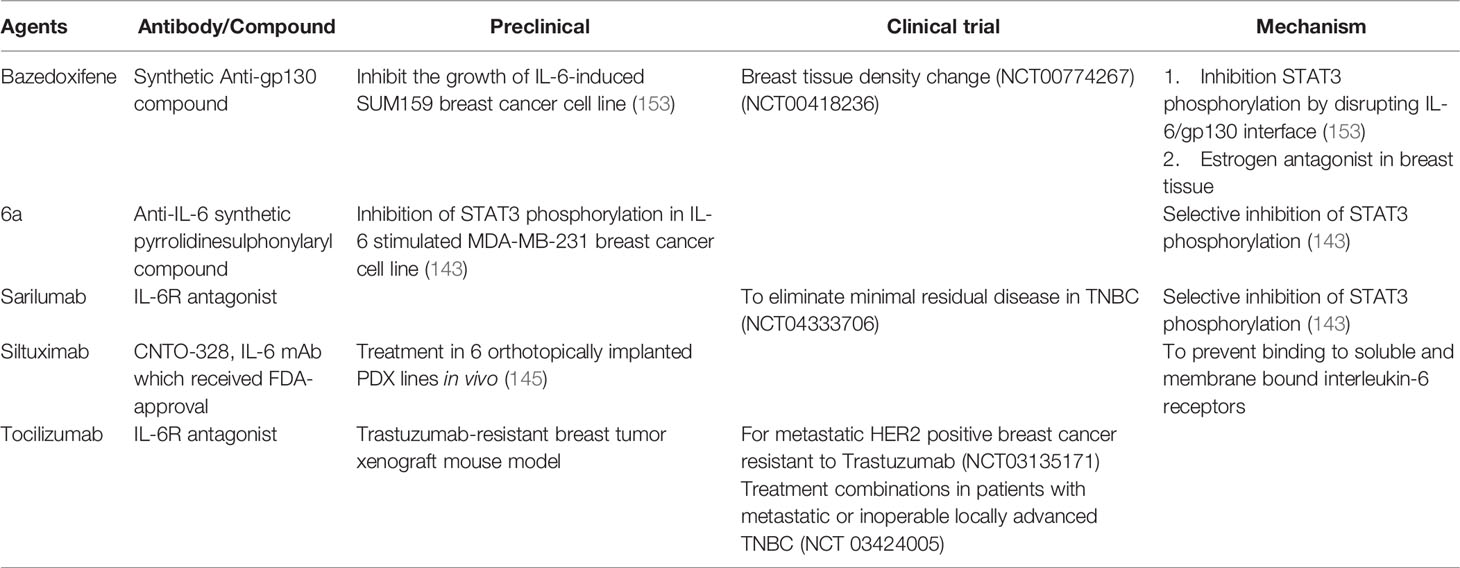
A growing body of evidence indicated Bazedoxifene, which is a synthetic anti-gp130 compound, could effectively disrupt the IL-6R/gp130 interactions thus inhibit cell viability, and overall cell survive, proliferation as well as cell migration in TNBC (142). A novel in-house prepared IL-6 pathway inhibitor namely 6a, which is capable of selectively inhibiting STAT3 activation following IL-6 stimulation in MDA-MB-231 breast cancer (143). Sarilumab, an FDA-approved anti-IL-6R antibody for rheumatoid arthritis, which blocks both mIL-6R and sIL-6R, is currently under clinical studies for breast cancer (144). Siltuximab, which is a neutralizing anti-IL-6 antibody, delayed engraftment of MCF-7 humanized xenograft tumors and elicited tumor xenograft regression in tumors (145). The anti-IL-6 receptor antibody, Tocilizumab, is effective in the treatment of various autoimmune diseases such as rheumatoid arthritis (RA) (146). Experimental results demonstrates that IL-6 pathway targeted drugs may have additional benefit in HER2+ breast cancer (122). It has been proved that IL-6 receptor inhibitor suppressed bone metastases in a breast cancer cell line (147). Another study showed that IL-6R antagonist Tocilizumab significantly decreases breast cancer stem cell and inhibits tumor growth in Notch3-expressing breast cancers (148). The high level of IL-6R expression in spindle-shaped stromal cells such as CAF was not associated with the vasculature but could be used as prognostic determinant of early breast cancer (149). CAFs in tumor microenvironment played a vital role in developing trastuzumab resistance by magnifying CSCs bulge and activating multiple pathways (150). Regarding this, combination of anti-IL-6 antibody, or multiple pathway inhibitors with trastuzumab maybe novel strategy to reverse drug resistance in HER2+ breast cancer (71). Genotype of IL-6 was prominently related to early events among patients bearing with ER-negative tumors (151). The IL-6 signaling loop mediated drug resistance to PI3K inhibitors via inducing epithelial-mesenchymal transition (EMT) and CSCs expansion in human breast cancer cells (152). In summary, IL-6 signaling pathway may be potential treatment target for breast cancer patients in the future. The previously mentioned agents targeting the IL-6/IL-6R signaling for breast cancer therapy were listed in Table 2.
IL-6 could promote the response of acute phase inflammatory via increasing the production of acute inflammatory proteins. IL-6 was also correlated with elevated CRP in different kinds of cancers including breast cancer (154), renal cancer (155), lung cancer (156), and colorectal cancer (157). Although breast cancers rarely are characterized by inflammation, a growing body of evidence nevertheless suggests that inflammatory process also play an important role in breast cancer progression (158, 159). Based on the reported literature, the results from epidemiologic studies in different centres are conflicting, with some showing significant association between elevated CRP levels and poor prognosis in breast cancers while others show no association (160–162). In a study consisted of 700 women with early-stage breast cancer found that elevated levels of CRP measured 2.5 years after diagnosis were associated with reduced DFS and OS (163). Similarly, another investigation included 2,910 women for up to seven years after invasive breast cancer diagnosis revealed elevated CRP levels were significantly associated with reduced DFS and OS (164). Preoperative CRP level was indicated as a more accurate prognostic factor compared with other factors, such as histological grade, tumor factor and node factor (127).
Conclusions
IL-6 is a pleiotropic cytokine in the regulations of various physiological and pathological processes. IL-6 causes uncontrolled inflammatory responses resulting in chronic inflammation and even carcinoma. IL-6 expression is associated with poor prognosis for breast cancer. The interaction network of IL-6 in breast cancer cells/stromal cells is listed as Figure 1. The IL-6 signal transduction pathway including IL-6, IL-6R, sIL-6R, gp130, JAK, and STAT3 has been suggested as promising therapeutic targets for breast cancer. Several antibodies for IL-6/IL-6R have been developed, either as single drug or combined with other traditional chemotherapy, have demonstrated dramatical outcome in both preclinical and clinical trials. In addition to the critical roles of IL-6/JAK/STAT3 signaling in breast cancer, hyperactivation of this pathway has also been implicated in suppressing anti-tumor immune responses in tumor microenvironment. Treatments targeting the IL-6/JAK/STAT3 pathway have provided benefit for patients with breast cancer by directly inhibiting tumor cell growth and activating anti-tumor immunity. Taken together, strategy targeting the IL-6/JAK/STAT3 signaling pathway, which has already been shown to be beneficial in certain cancers including breast cancer, has proven to be effective. Combination of IL-6 signaling pathway inhibitor and other targets blockage drugs may serve as novel strategy to treat IL-6 mediated immune disease and human cancers.
Author Contributions
Conception or design of the work, JC and YW. Data collection, JWC, WY, QH, YC, and KZ. Drafting the article, JC and JWC. Critical revision of the article, YW. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Yao Huang for reviewing the article.
References
1. Cancer Genome Atlas N. Comprehensive Molecular Portraits of Human Breast Tumours. Nature (2012) 490(7418):61–70. doi: 10.1038/nature11412
2. Pedersen BK. The Diseasome of Physical Inactivity–and the Role of Myokines in Muscle–Fat Cross Talk. J Physiol (2009) 587(Pt 23):5559–68. doi: 10.1113/jphysiol.2009.179515
3. Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-Mediated Promotion of Invasion and Metastasis. Cancer Metastasis Rev (2010) 29(2):243–8. doi: 10.1007/s10555-010-9227-2
4. Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of Breast Cancer in Asian Women. World J Surg (2007) 31(5):1031–40. doi: 10.1007/s00268-005-0585-9
5. Agarwal G, Ramakant P. Breast Cancer Care in India: The Current Scenario and the Challenges for the Future. Breast Care (Basel) (2008) 3(1):21–7. doi: 10.1159/000115288
6. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter Pylori Infection and the Development of Gastric Cancer. New Engl J Med (2001) 345(11):784–9. doi: 10.1056/NEJMoa001999
7. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. Il-6/Il-6 Receptor System and Its Role in Physiological and Pathological Conditions. Clin Sci (2011) 122(4):143–59. doi: 10.1042/cs20110340
8. Gabay C. Interleukin-6 and Chronic Inflammation. Arthritis Res Ther (2006) 8(2):S3. doi: 10.1186/ar1917
9. Scheele C, Nielsen S, Kelly M, Broholm C, Nielsen AR, Taudorf S, et al. Satellite Cells Derived From Obese Humans With Type 2 Diabetes and Differentiated Into Myocytes in Vitro Exhibit Abnormal Response to Il-6. PloS One (2012) 7(6):e39657. doi: 10.1371/journal.pone.0039657
10. Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma Levels of Interleukin-6 and C-Reactive Protein Are Associated With Physical Inactivity Independent of Obesity. Scandinavian J Med Sci sports (2007) 17(5):580–7. doi: 10.1111/j.1600-0838.2006.00602.x
11. Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 Signaling Pathway in Targeted Therapy for Cancer. Cancer Treat Rev (2012) 38(7):904–10. doi: 10.1016/j.ctrv.2012.04.007
12. Liu X, Ma Y, Yang W, Wu X, Jiang L, Chen X. Identification of Therapeutic Targets for Breast Cancer Using Biological Informatics Methods. Mol Med Rep (2015) 12(2):1789–95. doi: 10.3892/mmr.2015.3565
13. Zubor P, Hatok J, Moricova P, Kapustova I, Kajo K, Mendelova A, et al. Gene Expression Profiling of Histologically Normal Breast Tissue in Females With Human Epidermal Growth Factor Receptor 2positive Breast Cancer. Mol Med Rep (2015) 11(2):1421–7. doi: 10.3892/mmr.2014.2863
14. Zhang GJ, Adachi I. Serum Interleukin-6 Levels Correlate to Tumor Progression and Prognosis in Metastatic Breast Carcinoma. Anticancer Res (1999) 19(2B):1427–32.
15. Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating Interleukin-6 Predicts Survival in Patients With Metastatic Breast Cancer. Int J Cancer (2003) 103(5):642–6. doi: 10.1002/ijc.10833
16. Kishimoto T. Interleukin-6: Discovery of a Pleiotropic Cytokine. Arthritis Res Ther (2006) 8 Suppl 2:S2. doi: 10.1186/ar1916
17. Lotz M. Interleukin-6: A Comprehensive Review. Cancer Treat Res (1995) 80:209–33. doi: 10.1007/978-1-4613-1241-3_8
18. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim Biophys Acta (2011) 1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034
19. Varghese JN, Moritz RL, Lou M-Z, Donkelaar Av, Ji H, Ivancic N, et al. Structure of the Extracellular Domains of the Human Interleukin-6 Receptor α-Chain. Proc Natl Acad Sci (2002) 99(25):15959–64. doi: 10.1073/pnas.232432399
20. Scheller J, Rose-John S. Interleukin-6 and Its Receptor: From Bench to Bedside. Med Microbiol Immunol (2006) 195(4):173–83. doi: 10.1007/s00430-006-0019-9
21. Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 Biology Is Coordinated by Membrane-Bound and Soluble Receptors: Role in Inflammation and Cancer. J Leukoc Biol (2006) 80(2):227–36. doi: 10.1189/jlb.1105674
22. Briso EM, Dienz O, Rincon M. Cutting Edge: Soluble Il-6r Is Produced by Il-6r Ectodomain Shedding in Activated Cd4 T Cells. J Immunol (2008) 180(11):7102–6. doi: 10.4049/jimmunol.180.11.7102
23. Bousoik E, Montazeri Aliabadi H. "Do We Know Jack" About Jak? A Closer Look at Jak/Stat Signaling Pathway. Front Oncol (2018) 8:287. doi: 10.3389/fonc.2018.00287
24. Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The Complex Pattern of Cytokines in Serum From Patients With Meningococcal Septic Shock. Association Between Interleukin 6, Interleukin 1, and Fatal Outcome.+. J Exp Med (1989) 169(1):333–8. doi: 10.1084/jem.169.1.333
25. Honda M, Yamamoto S, Cheng M, Yasukawa K, Suzuki H, Saito T, et al. Human Soluble Il-6 Receptor: Its Detection and Enhanced Release by Hiv Infection. J Immunol (1992) 148(7):2175–80.
26. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of Interleukin (Il)-6-Type Cytokine Signalling and Its Regulation. Biochem J (2003) 374(Pt 1):1–20. doi: 10.1042/BJ20030407
27. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the Pi3k/Akt Pathway for Cancer Drug Discovery. Nat Rev Drug Discovery (2005) 4(12):988–1004. doi: 10.1038/nrd1902
28. Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid Dendritic Cells Induce Plasma Cell Differentiation Through Type I Interferon and Interleukin 6. Immunity (2003) 19(2):225–34. doi: 10.1016/S1074-7613(03)00208-5
29. Diehl S, Rincon M. The Two Faces of Il-6 on Th1/Th2 Differentiation. Mol Immunol (2002) 39(9):531–6. doi: 10.1016/s0161-5890(02)00210-9
30. Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, et al. Il-6 Is Required for Airway Mucus Production Induced by Inhaled Fungal Allergens. J Immunol (2009) 183(3):1732–8. doi: 10.4049/jimmunol.0802923
31. Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, et al. Inhibition of Th1 Differentiation by Il-6 Is Mediated by Socs1. Immunity (2000) 13(6):805–15. doi: 10.1016/S1074-7613(00)00078-9
32. Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals Mediated by Transforming Growth Factor-Beta Initiate Autoimmune Encephalomyelitis, But Chronic Inflammation Is Needed to Sustain Disease. Nat Immunol (2006) 7(11):1151–6. doi: 10.1038/ni1391
33. Ghoreschi K, Laurence A, Yang X-P, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of Pathogenic Th17 Cells in the Absence of Tgf-Β Signalling. Nature (2010) 467(7318):967–71. doi: 10.1038/nature09447
34. Kimura A, Kishimoto T. Il-6: Regulator of Treg/Th17 Balance. Eur J Immunol (2010) 40(7):1830–5. doi: 10.1002/eji.201040391
35. Choi HI, Chung KJ, Yang HY, Ren L, Sohn S, Kim PR, et al. Peroxiredoxin V Selectively Regulates Il-6 Production by Modulating the Jak2-Stat5 Pathway. Free Radical Biol Med (2013) 65:270–9. doi: 10.1016/j.freeradbiomed.2013.06.038
36. Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, et al. Il-6 Regulates Neutrophil Trafficking During Acute Inflammation Via Stat3. J Immunol (2008) 181(3):2189–95. doi: 10.4049/jimmunol.181.3.2189
37. Gabrilovich DI, Nagaraj S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat Rev Immunol (2009) 9(3):162–74. doi: 10.1038/nri2506
38. Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-Derived Suppressor Cell Heterogeneity and Subset Definition. Curr Opin Immunol (2010) 22(2):238–44. doi: 10.1016/j.coi.2010.01.021
39. Liu M, Sakamaki T, Casimiro MC, Willmarth NE, Quong AA, Ju X, et al. The Canonical Nf-Kappab Pathway Governs Mammary Tumorigenesis in Transgenic Mice and Tumor Stem Cell Expansion. Cancer Res (2010) 70(24):10464–73. doi: 10.1158/0008-5472.CAN-10-0732
40. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the Acute Phase Response. Biochem J (1990) 265(3):621–36. doi: 10.1042/bj2650621
41. Gulhar R, Ashraf MA, Jialal I. Physiology, Acute Phase Reactants. Treasure Island (FL: Statpearls (2020).
43. Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. Il-1beta/Il-6/Crp and Il-18/Ferritin: Distinct Inflammatory Programs in Infections. PloS Pathog (2016) 12(12):e1005973. doi: 10.1371/journal.ppat.1005973
44. Nanki T, Onoue I, Nagasaka K, Takayasu A, Ebisawa M, Hosoya T, et al. Suppression of Elevations in Serum C Reactive Protein Levels by Anti-Il-6 Autoantibodies in Two Patients With Severe Bacterial Infections. Ann rheumatic Dis (2013) 72(6):1100–2. doi: 10.1136/annrheumdis-2012-202768
45. Wei H. Interleukin 6 Signaling Maintains the Stem-Like Properties of Bladder Cancer Stem Cells. Trans Cancer Res (2019) 8(2):557–66. doi: 10.21037/tcr.2019.03.16
46. Kuphal S, Wallner S, Bosserhoff AK. Impact of Lif (Leukemia Inhibitory Factor) Expression in Malignant Melanoma. Exp Mol Pathol (2013) 95(2):156–65. doi: 10.1016/j.yexmp.2013.06.012
47. Mathieu ME, Saucourt C, Mournetas V, Gauthereau X, Theze N, Praloran V, et al. Lif-Dependent Signaling: New Pieces in the Lego. Stem Cell Rev (2012) 8(1):1–15. doi: 10.1007/s12015-011-9261-7
48. Kamohara H, Sakamoto K, Ishiko T, Mita S, Masuda Y, Abe T, et al. Human Carcinoma Cell Lines Produce Biologically Active Leukemia Inhibitory Factor (Lif). Res Commun Mol Pathol Pharmacol (1994) 85(2):131–40. doi: 10.1002/stem.110
49. Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, et al. Novel Stat3 Target Genes Exert Distinct Roles in the Inhibition of Mesoderm and Endoderm Differentiation in Cooperation With Nanog. Stem Cells (2009) 27(8):1760–71. doi: 10.1002/stem.110
50. Brady JJ, Li M, Suthram S, Jiang H, Wong WH, Blau HM. Early Role for Il-6 Signalling During Generation of Induced Pluripotent Stem Cells Revealed by Heterokaryon Rna-Seq. Nat Cell Biol (2013) 15(10):1244–52. doi: 10.1038/ncb2835
51. Balamurugan K, Mendoza-Villanueva D, Sharan S, Summers GH, Dobrolecki LE, Lewis MT, et al. C/Ebpdelta Links Il-6 and Hif-1 Signaling to Promote Breast Cancer Stem Cell-Associated Phenotypes. Oncogene (2019) 38(20):3765–80. doi: 10.1038/s41388-018-0516-5
52. Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, et al. Role of the Il-6-Jak1-Stat3-Oct-4 Pathway in the Conversion of Non-Stem Cancer Cells Into Cancer Stem-Like Cells. Cell signalling (2013) 25(4):961–9. doi: 10.1016/j.cellsig.2013.01.007
53. Yi H, Cho HJ, Cho SM, Jo K, Park JA, Kim NH, et al. Blockade of Interleukin-6 Receptor Suppresses the Proliferation of H460 Lung Cancer Stem Cells. Int J Oncol (2012) 41(1):310–6. doi: 10.3892/ijo.2012.1447
54. Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. Stat3 Is Necessary for Proliferation and Survival in Colon Cancer-Initiating Cells. Cancer Res (2011) 71(23):7226–37. doi: 10.1158/0008-5472.CAN-10-4660
55. Lin S, Li S, Chen Z, He X, Zhang Y, Xu X, et al. Formation, Recognition and Bioactivities of a Novel G-Quadruplex in the Stat3 Gene. Bioorg med Chem Lett (2011) 21(19):5987–91. doi: 10.1016/j.bmcl.2011.07.121
56. Garner JM, Fan M, Yang CH, Du Z, Sims M, Davidoff AM, et al. Constitutive Activation of Signal Transducer and Activator of Transcription 3 (Stat3) and Nuclear Factor Kappab Signaling in Glioblastoma Cancer Stem Cells Regulates the Notch Pathway. J Biol Chem (2013) 288(36):26167–76. doi: 10.1074/jbc.M113.477950
57. Chu Y, Wang Y, Peng W, Xu L, Liu M, Li J, et al. Stat3 Activation by Il-6 From Adipose-Derived Stem Cells Promotes Endometrial Carcinoma Proliferation and Metastasis. Biochem Biophys Res Commun (2018) 500(3):626–31. doi: 10.1016/j.bbrc.2018.04.121
58. D'Anello L, Sansone P, Storci G, Mitrugno V, D'Uva G, Chieco P, et al. Epigenetic Control of the Basal-Like Gene Expression Profile Via Interleukin-6 in Breast Cancer Cells. Mol Cancer (2010) 9:300. doi: 10.1186/1476-4598-9-300
59. Hodge DR, Hurt EM, Farrar WL. The Role of Il-6 and Stat3 in Inflammation and Cancer. Eur J Cancer (2005) 41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016
60. Iliopoulos D, Hirsch HA, Struhl K. An Epigenetic Switch Involving Nf-Kappab, Lin28, Let-7 Microrna, and Il6 Links Inflammation to Cell Transformation. Cell (2009) 139(4):693–706. doi: 10.1016/j.cell.2009.10.014
61. Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. Stat3 Activation of Mir-21 and Mir-181b-1 Via Pten and Cyld Are Part of the Epigenetic Switch Linking Inflammation to Cancer. Mol Cell (2010) 39(4):493–506. doi: 10.1016/j.molcel.2010.07.023
62. Dethlefsen C, Hojfeldt G, Hojman P. The Role of Intratumoral and Systemic Il-6 in Breast Cancer. Breast Cancer Res Treat (2013) 138(3):657–64. doi: 10.1007/s10549-013-2488-z
63. Waugh DJ, Wilson C. The Interleukin-8 Pathway in Cancer. Clin Cancer Res (2008) 14(21):6735–41. doi: 10.1158/1078-0432.CCR-07-4843
64. Fujisaki K, Fujimoto H, Sangai T, Nagashima T, Sakakibara M, Shiina N, et al. Cancer-Mediated Adipose Reversion Promotes Cancer Cell Migration Via Il-6 and Mcp-1. Breast Cancer Res Treat (2015) 150(2):255–63. doi: 10.1007/s10549-015-3318-2
65. Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. Breast Cancer Stem Cells Are Regulated by Mesenchymal Stem Cells Through Cytokine Networks. Cancer Res (2011) 71(2):614–24. doi: 10.1158/0008-5472.CAN-10-0538
66. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal Stem Cells Within Tumour Stroma Promote Breast Cancer Metastasis. Nature (2007) 449(7162):557–63. doi: 10.1038/nature06188
67. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science (1999) 284(5411):143–7. doi: 10.1126/science.284.5411.143
68. Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer Associated Fibroblasts Express Pro-Inflammatory Factors in Human Breast and Ovarian Tumors. Biochem Biophys Res Commun (2013) 437(3):397–402. doi: 10.1016/j.bbrc.2013.06.089
69. Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell (2011) 19(2):257–72. doi: 10.1016/j.ccr.2011.01.020
70. Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, et al. Interleukin-6 Mediates Epithelial-Stromal Interactions and Promotes Gastric Tumorigenesis. PloS One (2013) 8(4):e60914. doi: 10.1371/journal.pone.0060914
71. Mao Y, Zhang Y, Qu Q, Zhao M, Lou Y, Liu J, et al. Cancer-Associated Fibroblasts Induce Trastuzumab Resistance in Her2 Positive Breast Cancer Cells. Mol Biosyst (2015) 11(4):1029–40. doi: 10.1039/c4mb00710g
72. Hendrayani SF, Al-Khalaf HH, Aboussekhra A. The Cytokine Il-6 Reactivates Breast Stromal Fibroblasts Through Transcription Factor Stat3-Dependent Up-Regulation of the Rna-Binding Protein Auf1. J Biol Chem (2014) 289(45):30962–76. doi: 10.1074/jbc.M114.594044
73. Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, et al. Cancer-Associated Fibroblasts Affect Intratumoral Cd8(+) and Foxp3(+) T Cells Via Interleukin 6 in the Tumor Microenvironment. Clin Cancer Res (2018) 24(19):4820–33. doi: 10.1158/1078-0432.CCR-18-0205
74. Li P, Shan JX, Chen XH, Zhang D, Su LP, Huang XY, et al. Epigenetic Silencing of Microrna-149 in Cancer-Associated Fibroblasts Mediates Prostaglandin E2/Interleukin-6 Signaling in the Tumor Microenvironment. Cell Res (2015) 25(5):588–603. doi: 10.1038/cr.2015.51
75. Xiong S, Wang R, Chen Q, Luo J, Wang J, Zhao Z, et al. Cancer-Associated Fibroblasts Promote Stem Cell-Like Properties of Hepatocellular Carcinoma Cells Through Il-6/Stat3/Notch Signaling. Am J Cancer Res (2018) 8(2):302–16.
76. Guo C, Chen Y, Gao W, Chang A, Ye Y, Shen W, et al. Liposomal Nanoparticles Carrying Anti-Il6r Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models. Theranostics (2017) 7(3):775–88. doi: 10.7150/thno.17237
77. Calle EE, Thun MJ. Obesity and Cancer. Oncogene (2004) 23(38):6365–78. doi: 10.1038/sj.onc.1207751
78. Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is Obesity an Independent Prognosis Factor in Woman Breast Cancer? Breast Cancer Res Treat (2008) 111(2):329–42. doi: 10.1007/s10549-007-9785-3
79. Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the Obesity and Breast Cancer Links: A Role for Cancer-Associated Adipocytes? Endocr Dev (2010) 19:45–52. doi: 10.1159/000316896
80. Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, et al. Adipocyte Lipolysis-Stimulated Interleukin-6 Production Requires Sphingosine Kinase 1 Activity. J Biol Chem (2014) 289(46):32178–85. doi: 10.1074/jbc.M114.601096
81. Kim HS, Jung M, Choi SK, Woo J, Piao YJ, Hwang EH, et al. Il-6-Mediated Cross-Talk Between Human Preadipocytes and Ductal Carcinoma in Situ in Breast Cancer Progression. J Exp Clin Cancer Res (2018) 37(1):200. doi: 10.1186/s13046-018-0867-3
82. Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 Secreted From Adipose Stromal Cells Promotes Migration and Invasion of Breast Cancer Cells. Oncogene (2009) 28(30):2745–55. doi: 10.1038/onc.2009.130
83. Incio J, Ligibel JA, McManus DT, Suboj P, Jung K, Kawaguchi K, et al. Obesity Promotes Resistance to Anti-Vegf Therapy in Breast Cancer by Up-Regulating Il-6 and Potentially Fgf-2. Sci Transl Med (2018) 10(432):1–30. doi: 10.1126/scitranslmed.aag0945
84. Santer FR, Malinowska K, Culig Z, Cavarretta IT. Interleukin-6 Trans-Signalling Differentially Regulates Proliferation, Migration, Adhesion and Maspin Expression in Human Prostate Cancer Cells. Endocrine-related Cancer (2010) 17(1):241–53. doi: 10.1677/ERC-09-0200
85. Suchi K, Fujiwara H, Okamura S, Okamura H, Umehara S, Todo M, et al. Overexpression of Interleukin-6 Suppresses Cisplatin-Induced Cytotoxicity in Esophageal Squamous Cell Carcinoma Cells. Anticancer Res (2011) 31(1):67–75.
86. Grivennikov S, Karin M. Autocrine Il-6 Signaling: A Key Event in Tumorigenesis? Cancer Cell (2008) 13(1):7–9. doi: 10.1016/j.ccr.2007.12.020
87. Leslie K, Gao SP, Berishaj M, Podsypanina K, Ho H, Ivashkiv L, et al. Differential Interleukin-6/Stat3 Signaling as a Function of Cellular Context Mediates Ras-Induced Transformation. Breast Cancer Res (2010) 12(5):R80. doi: 10.1186/bcr2725
88. Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. Il-6 Triggers Malignant Features in Mammospheres From Human Ductal Breast Carcinoma and Normal Mammary Gland. J Clin Invest (2007) 117(12):3988–4002. doi: 10.1172/JCI32533
89. Naugler WE, Karin M. The Wolf in Sheep's Clothing: The Role of Interleukin-6 in Immunity, Inflammation and Cancer. Trends Mol Med (2008) 14(3):109–19. doi: 10.1016/j.molmed.2007.12.007
90. Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Il-6, Its Receptors and Its Relationship With Bcl-2 and Bax Proteins in Infiltrating and in Situ Human Breast Carcinoma. Histopathology (2005) 47(1):82–9. doi: 10.1111/j.1365-2559.2005.02178.x
91. Rincon M, Broadwater G, Harris L, Crocker A, Weaver D, Dressler L, et al. Interleukin-6, Multidrug Resistance Protein-1 Expression and Response to Paclitaxel in Women With Metastatic Breast Cancer: Results of Cancer and Leukemia Group B Trial 159806. Breast Cancer Res Treat (2006) 100(3):301–8. doi: 10.1007/s10549-006-9251-7
92. He JY, Wei XH, Li SJ, Liu Y, Hu HL, Li ZZ, et al. Adipocyte-Derived Il-6 and Leptin Promote Breast Cancer Metastasis Via Upregulation of Lysyl Hydroxylase-2 Expression. Cell Commun Signal (2018) 16(1):100. doi: 10.1186/s12964-018-0309-z
93. Fu S, Lin J. Blocking Interleukin-6 and Interleukin-8 Signaling Inhibits Cell Viability, Colony-Forming Activity, and Cell Migration in Human Triple-Negative Breast Cancer and Pancreatic Cancer Cells. Anticancer Res (2018) 38(11):6271–9. doi: 10.21873/anticanres.12983
94. Jafarzadeh A, Minaee K, Farsinejad AR, Nemati M, Khosravimashizi A, Daneshvar H, et al. Evaluation of the Circulating Levels of Il-12 and Il-33 in Patients With Breast Cancer: Influences of the Tumor Stages and Cytokine Gene Polymorphisms. Iran J Basic Med Sci (2015) 18(12):1189–98.
95. Nunez-Marrero A. Assessing the Role of the Interleukin-12/Stat4 Axis in Breast Cancer by a Bioinformatics Approach. Int J Sci Basic Appl Res (2019) 48(2):38–52.
96. Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE Jr., et al. Interleukin 12 Signaling in T Helper Type 1 (Th1) Cells Involves Tyrosine Phosphorylation of Signal Transducer and Activator of Transcription (Stat)3 and Stat4. J Exp Med (1995) 181(5):1755–62. doi: 10.1084/jem.181.5.1755
97. Green VL, Alexandropoulou A, Walker MB, Walker AA, Sharp DM, Walker LG, et al. Alterations in the Th1/Th2 Balance in Breast Cancer Patients Using Reflexology and Scalp Massage. Exp Ther Med (2010) 1(1):97–108. doi: 10.3892/etm_00000018
98. Hong CC, Yao S, McCann SE, Dolnick RY, Wallace PK, Gong Z, et al. Pretreatment Levels of Circulating Th1 and Th2 Cytokines, and Their Ratios, Are Associated With Er-Negative and Triple Negative Breast Cancers. Breast Cancer Res Treat (2013) 139(2):477–88. doi: 10.1007/s10549-013-2549-3
99. Tanaka T, Narazaki M, Kishimoto T. Il-6 in Inflammation, Immunity, and Disease. Cold Spring Harb Perspect Biol (2014) 6(10):a016295. doi: 10.1101/cshperspect.a016295
100. de Matos-Neto EM, Lima JD, de Pereira WO, Figueredo RG, Riccardi DM, Radloff K, et al. Systemic Inflammation in Cachexia - Is Tumor Cytokine Expression Profile the Culprit? Front Immunol (2015) 6:629. doi: 10.3389/fimmu.2015.00629
101. Barton BE. Il-6-Like Cytokines and Cancer Cachexia: Consequences of Chronic Inflammation. Immunol Res (2001) 23(1):41–58. doi: 10.1385/IR:23:1:41
102. Penet MF, Bhujwalla ZM. Cancer Cachexia, Recent Advances, and Future Directions. Cancer J (2015) 21(2):117–22. doi: 10.1097/PPO.0000000000000100
103. Morrow RJ, Allam AH, Yeo B, Deb S, Murone C, Lim E, et al. Paracrine Il-6 Signaling Confers Proliferation Between Heterogeneous Inflammatory Breast Cancer Sub-Clones. Cancers (Basel) (2022) 14(9):2292–309. doi: 10.3390/cancers14092292
104. Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating Il-6 Biology Into Effective Treatments. Nat Rev Rheumatol (2020) 16(6):335–45. doi: 10.1038/s41584-020-0419-z
105. Coussens LM, Werb Z. Inflammation and Cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
106. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
107. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
108. Piotrowski I, Kulcenty K, Suchorska W. Interplay Between Inflammation and Cancer. Rep Pract Oncol Radiother (2020) 25(3):422–7. doi: 10.1016/j.rpor.2020.04.004
109. Ortiz-Montero P, Londono-Vallejo A, Vernot JP. Senescence-Associated Il-6 and Il-8 Cytokines Induce a Self- and Cross-Reinforced Senescence/Inflammatory Milieu Strengthening Tumorigenic Capabilities in the Mcf-7 Breast Cancer Cell Line. Cell Commun Signal (2017) 15(1):17. doi: 10.1186/s12964-017-0172-3
110. Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and Molecular Pharmacology of Antiestrogen Action and Resistance. Pharmacol Rev (2001) 53(1):25–71.
111. Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, et al. Antiestrogen Resistance in Breast Cancer and the Role of Estrogen Receptor Signaling. Oncogene (2003) 22(47):7316–39. doi: 10.1038/sj.onc.1206937
112. Siersbaek R, Scabia V, Nagarajan S, Chernukhin I, Papachristou EK, Broome R, et al. Il6/Stat3 Signaling Hijacks Estrogen Receptor Alpha Enhancers to Drive Breast Cancer Metastasis. Cancer Cell (2020) 38(3):412–23 e9. doi: 10.1016/j.ccell.2020.06.007
113. Tsoi H, Man EPS, Chau KM, Khoo US. Targeting the Il-6/Stat3 Signalling Cascade to Reverse Tamoxifen Resistance in Estrogen Receptor Positive Breast Cancer. Cancers (Basel) (2021) 13(7):1511–32. doi: 10.3390/cancers13071511
114. Hou L, Xie S, Li G, Xiong B, Gao Y, Zhao X, et al. Il-6 Triggers the Migration and Invasion of Oestrogen Receptor-Negative Breast Cancer Cells Via Regulation of Hippo Pathways. Basic Clin Pharmacol Toxicol (2018) 123(5):549–57. doi: 10.1111/bcpt.13042
115. Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum Interleukin 6, Plasma Vegf, Serum Vegf, and Vegf Platelet Load in Breast Cancer Patients. Clin Breast Cancer (2002) 2(4):311–5. doi: 10.3816/CBC.2002.n.008
116. Shibayama O, Yoshiuchi K, Inagaki M, Matsuoka Y, Yoshikawa E, Sugawara Y, et al. Association Between Adjuvant Regional Radiotherapy and Cognitive Function in Breast Cancer Patients Treated With Conservation Therapy. Cancer Med (2014) 3(3):702–9. doi: 10.1002/cam4.174
117. Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, et al. Oestrogen Receptor Negative Breast Cancers Exhibit High Cytokine Content. Breast Cancer Res (2007) 9(1):R15. doi: 10.1186/bcr1648
118. Conze D, Weiss L, Regen PS, Bhushan A, Weaver D, Johnson P, et al. Autocrine Production of Interleukin 6 Causes Multidrug Resistance in Breast Cancer Cells. Cancer Res (2001) 61(24):8851–8.
119. Sethi N, Dai X, Winter CG, Kang Y. Tumor-Derived Jagged1 Promotes Osteolytic Bone Metastasis of Breast Cancer by Engaging Notch Signaling in Bone Cells. Cancer Cell (2011) 19(2):192–205. doi: 10.1016/j.ccr.2010.12.022
120. Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the Egfr Kinase Domain Mediate Stat3 Activation Via Il-6 Production in Human Lung Adenocarcinomas. J Clin Invest (2007) 117(12):3846–56. doi: 10.1172/JCI31871
121. Ara T, Declerck YA. Interleukin-6 in Bone Metastasis and Cancer Progression. Eur J Cancer (2010) 46(7):1223–31. doi: 10.1016/j.ejca.2010.02.026
122. Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, et al. Activation of an Il6 Inflammatory Loop Mediates Trastuzumab Resistance in Her2+ Breast Cancer by Expanding the Cancer Stem Cell Population. Mol Cell (2012) 47(4):570–84. doi: 10.1016/j.molcel.2012.06.014
123. Jin K, Pandey NB, Popel AS. Simultaneous Blockade of Il-6 and Ccl5 Signaling for Synergistic Inhibition of Triple-Negative Breast Cancer Growth and Metastasis. Breast Cancer Res (2018) 20(1):54. doi: 10.1186/s13058-018-0981-3
124. Chung AW, Kozielski AJ, Qian W, Zhou J, Anselme AC, Chan AA, et al. Tocilizumab Overcomes Chemotherapy Resistance in Mesenchymal Stem-Like Breast Cancer by Negating Autocrine Il-1a Induction of Il-6. NPJ Breast Cancer (2022) 8(1):30. doi: 10.1038/s41523-021-00371-0
125. Lin S, Gan Z, Han K, Yao Y, Min D. Interleukin-6 as a Prognostic Marker for Breast Cancer: A Meta-Analysis. Tumori (2015) 101(5):535–41. doi: 10.5301/tj.5000357
126. Ahmad N, Ammar A, Storr SJ, Green AR, Rakha E, Ellis IO, et al. Il-6 and Il-10 Are Associated With Good Prognosis in Early Stage Invasive Breast Cancer Patients. Cancer Immunol Immunother (2018) 67(4):537–49. doi: 10.1007/s00262-017-2106-8
127. Shimura T, Shibata M, Gonda K, Murakami Y, Noda M, Tachibana K, et al. Prognostic Impact of Interleukin-6 and C-Reactive Protein on Patients With Breast Cancer. Oncol Lett (2019) 17(6):5139–46. doi: 10.3892/ol.2019.10183
128. Chiu JJ, Sgagias MK, Cowan KH. Interleukin 6 Acts as a Paracrine Growth Factor in Human Mammary Carcinoma Cell Lines. Clin Cancer Res (1996) 2(1):215–21.
129. Liu H, Liu K, Bodenner DL. Estrogen Receptor Inhibits Interleukin-6 Gene Expression by Disruption of Nuclear Factor Kappab Transactivation. Cytokine (2005) 31(4):251–7. doi: 10.1016/j.cyto.2004.12.008
130. Fertig EJ, Lee E, Pandey NB, Popel AS. Analysis of Gene Expression of Secreted Factors Associated With Breast Cancer Metastases in Breast Cancer Subtypes. Sci Rep (2015) 5:12133. doi: 10.1038/srep12133
131. Autenshlyus A, Davletova K, Varaksin N, Marinkin I, Lyakhovich V. Cytokines in Various Molecular Subtypes of Breast Cancer. Int J Immunopathol Pharmacol (2021) 35:20587384211034089. doi: 10.1177/20587384211034089
132. Cho YA, Sung MK, Yeon JY, Ro J, Kim J. Prognostic Role of Interleukin-6, Interleukin-8, and Leptin Levels According to Breast Cancer Subtype. Cancer Res Treat (2013) 45(3):210–9. doi: 10.4143/crt.2013.45.3.210
133. Ma Y, Ren Y, Dai ZJ, Wu CJ, Ji YH, Xu J. Il-6, Il-8 and Tnf-Alpha Levels Correlate With Disease Stage in Breast Cancer Patients. Adv Clin Exp Med (2017) 26(3):421–6. doi: 10.17219/acem/62120
134. Milovanovic J, Todorovic-Rakovic N, Radulovic M. Interleukin-6 and Interleukin-8 Serum Levels in Prognosis of Hormone-Dependent Breast Cancer. Cytokine (2019) 118:93–8. doi: 10.1016/j.cyto.2018.02.019
135. Masjedi A, Hashemi V, Hojjat-Farsangi M, Ghalamfarsa G, Azizi G, Yousefi M, et al. The Significant Role of Interleukin-6 and Its Signaling Pathway in the Immunopathogenesis and Treatment of Breast Cancer. BioMed Pharmacother (2018) 108:1415–24. doi: 10.1016/j.biopha.2018.09.177
136. Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of Triple-Negative Breast Cancer Cells Relies Upon Coordinate Autocrine Expression of the Proinflammatory Cytokines Il-6 and Il-8. Cancer Res (2013) 73(11):3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T
137. Liang S, Chen Z, Jiang G, Zhou Y, Liu Q, Su Q, et al. Activation of Gper Suppresses Migration and Angiogenesis of Triple Negative Breast Cancer Via Inhibition of Nf-Kappab/Il-6 Signals. Cancer Lett (2017) 386:12–23. doi: 10.1016/j.canlet.2016.11.003
138. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human Breast Cancer: Correlation of Relapse and Survival With Amplification of the Her-2/Neu Oncogene. Science (1987) 235(4785):177–82. doi: 10.1126/science.3798106
139. Lan KH, Lu CH, Yu D. Mechanisms of Trastuzumab Resistance and Their Clinical Implications. Ann New York Acad Sci (2005) 1059:70–5. doi: 10.1196/annals.1339.026
140. Kim G, Ouzounova M, Quraishi AA, Davis A, Tawakkol N, Clouthier SG, et al. Socs3-Mediated Regulation of Inflammatory Cytokines in Pten and P53 Inactivated Triple Negative Breast Cancer Model. Oncogene (2015) 34(6):671–80. doi: 10.1038/onc.2014.4
141. Alshaker H, Wang Q, Frampton AE, Krell J, Waxman J, Winkler M, et al. Sphingosine Kinase 1 Contributes to Leptin-Induced Stat3 Phosphorylation Through Il-6/Gp130 Transactivation in Oestrogen Receptor-Negative Breast Cancer. Breast Cancer Res Treat (2015) 149(1):59–67. doi: 10.1007/s10549-014-3228-8
142. Tian J, Chen X, Fu S, Zhang R, Pan L, Cao Y, et al. Bazedoxifene Is a Novel Il-6/Gp130 Inhibitor for Treating Triple-Negative Breast Cancer. Breast Cancer Res Treat (2019) 175(3):553–66. doi: 10.1007/s10549-019-05183-2
143. Zinzalla G, Haque MR, Basu BP, Anderson J, Kaye SL, Haider S, et al. A Novel Small-Molecule Inhibitor of Il-6 Signalling. Bioorg Med Chem Lett (2010) 20(23):7029–32. doi: 10.1016/j.bmcl.2010.09.117
144. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat Rev Drug Discovery (2019) 18(1):41–58. doi: 10.1038/nrd.2018.168
145. Casneuf T, Axel AE, King P, Alvarez JD, Werbeck JL, Verhulst T, et al. Interleukin-6 Is a Potential Therapeutic Target in Interleukin-6 Dependent, Estrogen Receptor-Alpha-Positive Breast Cancer. Breast Cancer (Dove Med Press) (2016) 8:13–27. doi: 10.2147/BCTT.S92414
146. Biggioggero M, Crotti C, Becciolini A, Favalli EG. Tocilizumab in the Treatment of Rheumatoid Arthritis: An Evidence-Based Review and Patient Selection. Drug Des Devel Ther (2019) 13:57–70. doi: 10.2147/DDDT.S150580
147. Wakabayashi H, Hamaguchi T, Nagao N, Kato S, Iino T, Nakamura T, et al. Interleukin-6 Receptor Inhibitor Suppresses Bone Metastases in a Breast Cancer Cell Line. Breast Cancer (2018) 25(5):566–74. doi: 10.1007/s12282-018-0853-9
148. Wang D, Xu J, Liu B, He X, Zhou L, Hu X, et al. Il6 Blockade Potentiates the Anti-Tumor Effects of Gamma-Secretase Inhibitors in Notch3-Expressing Breast Cancer. Cell Death Differ (2018) 25(2):330–9. doi: 10.1038/cdd.2017.162
149. Labovsky V, Martinez LM, Calcagno ML, Davies KM, Garcia-Rivello H, Wernicke A, et al. Interleukin-6 Receptor in Spindle-Shaped Stromal Cells, a Prognostic Determinant of Early Breast Cancer. Tumour Biol (2016) 37(10):13377–84. doi: 10.1007/s13277-016-5268-7
150. Chan T-S, Shaked Y, Tsai KK. Targeting the Interplay Between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers. Front Oncol (2019) 9:688. doi: 10.3389/fonc.2019.00688
151. Markkula A, Simonsson M, Ingvar C, Rose C, Jernstrom H. Il6 Genotype, Tumour Er-Status, and Treatment Predicted Disease-Free Survival in a Prospective Breast Cancer Cohort. BMC Cancer (2014) 14:759. doi: 10.1186/1471-2407-14-759
152. Yang L, Han S, Sun Y. An Il6-Stat3 Loop Mediates Resistance to Pi3k Inhibitors by Inducing Epithelial-Mesenchymal Transition and Cancer Stem Cell Expansion in Human Breast Cancer Cells. Biochem Biophys Res Commun (2014) 453(3):582–7. doi: 10.1016/j.bbrc.2014.09.129
153. Li H, Xiao H, Lin L, Jou D, Kumari V, Lin J, et al. Drug Design Targeting Protein-Protein Interactions (Ppis) Using Multiple Ligand Simultaneous Docking (Mlsd) and Drug Repositioning: Discovery of Raloxifene and Bazedoxifene as Novel Inhibitors of Il-6/Gp130 Interface. J Med Chem (2014) 57(3):632–41. doi: 10.1021/jm401144z
154. Ravishankaran P, Karunanithi R. Clinical Significance of Preoperative Serum Interleukin-6 and C-Reactive Protein Level in Breast Cancer Patients. World J Surg Oncol (2011) 9:18. doi: 10.1186/1477-7819-9-18
155. Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, et al. Interleukin-6, Tumour Necrosis Factor Alpha and Interleukin-1beta in Patients With Renal Cell Carcinoma. Br J Cancer (2002) 86(9):1396–400. doi: 10.1038/sj.bjc.6600257
156. McKeown DJ, Brown DJ, Kelly A, Wallace AM, McMillan DC. The Relationship Between Circulating Concentrations of C-Reactive Protein, Inflammatory Cytokines and Cytokine Receptors in Patients With Non-Small-Cell Lung Cancer. Br J Cancer (2004) 91(12):1993–5. doi: 10.1038/sj.bjc.6602248
157. Chung YC, Chang YF. Serum Interleukin-6 Levels Reflect the Disease Status of Colorectal Cancer. J Surg Oncol (2003) 83(4):222–6. doi: 10.1002/jso.10269
158. Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. Cxcr1 Blockade Selectively Targets Human Breast Cancer Stem Cells in Vitro and in Xenografts. J Clin Invest (2010) 120(2):485–97. doi: 10.1172/JCI39397
159. Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer Associated Fibroblasts Promote Tumor Growth and Metastasis by Modulating the Tumor Immune Microenvironment in a 4t1 Murine Breast Cancer Model. PloS One (2009) 4(11):e7965. doi: 10.1371/journal.pone.0007965
160. Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an Inflammation-Based Prognostic Score (Gps) in Patients With Metastatic Breast Cancer. Br J Cancer (2006) 94(2):227–30. doi: 10.1038/sj.bjc.6602922
161. Al Murri AM, Wilson C, Lannigan A, Doughty JC, Angerson WJ, McArdle CS, et al. Evaluation of the Relationship Between the Systemic Inflammatory Response and Cancer-Specific Survival in Patients With Primary Operable Breast Cancer. Br J Cancer (2007) 96(6):891–5. doi: 10.1038/sj.bjc.6603682
162. Pasanisi P, Venturelli E, Morelli D, Fontana L, Secreto G, Berrino F. Serum Insulin-Like Growth Factor-I and Platelet-Derived Growth Factor as Biomarkers of Breast Cancer Prognosis. Cancer Epidemiol Biomarkers Prev (2008) 17(7):1719–22. doi: 10.1158/1055-9965.EPI-07-0654
163. Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated Biomarkers of Inflammation Are Associated With Reduced Survival Among Breast Cancer Patients. J Clin Oncol (2009) 27(21):3437–44. doi: 10.1200/JCO.2008.18.9068
Keywords: breast cancer, interleukin-6, inflammation, immune, target therapy
Citation: Chen J, Wei Y, Yang W, Huang Q, Chen Y, Zeng K and Chen J (2022) IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 12:903800. doi: 10.3389/fonc.2022.903800
Received: 24 March 2022; Accepted: 20 June 2022;
Published: 18 July 2022.
Edited by:
Monther Al-Alwan, King Faisal Specialist Hospital and Research Centre, Saudi ArabiaReviewed by:
Cheryl Lynn Jorcyk, Boise State University, United StatesTomoki Nakamura, Mie University Hospital, Japan
Copyright © 2022 Chen, Wei, Yang, Huang, Chen, Zeng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanghui Wei, d2VpeWgyOUBtYWlsLnN5c3UuZWR1LmNu; Jiawei Chen, Y2hlbmp3Mjg4QG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
 Juan Chen1†
Juan Chen1† Jiawei Chen
Jiawei Chen