
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Oncol. , 12 April 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.902929
Parts of this article's content have been modified or rectified in:
Erratum: Corrigendum: Efficacy and Safety of Tumor Treating Fields (TTFields) in Elderly Patients With Newly Diagnosed Glioblastoma: Subgroup Analysis of the Phase 3 EF-14 Clinical Trial
By Ram Z, Kim C-Y, Hottinger AF, Idbaih A, Nicholas G and Zhu J-J (2021) Front. Oncol. 11:671972. doi: 10.3389/fonc.2021.671972
In the original article, there was a mistake in Figure 1 as published. An entry was labelled as “No adherence” rather than “Disease progression”. The corrected Figure 1 appears below.
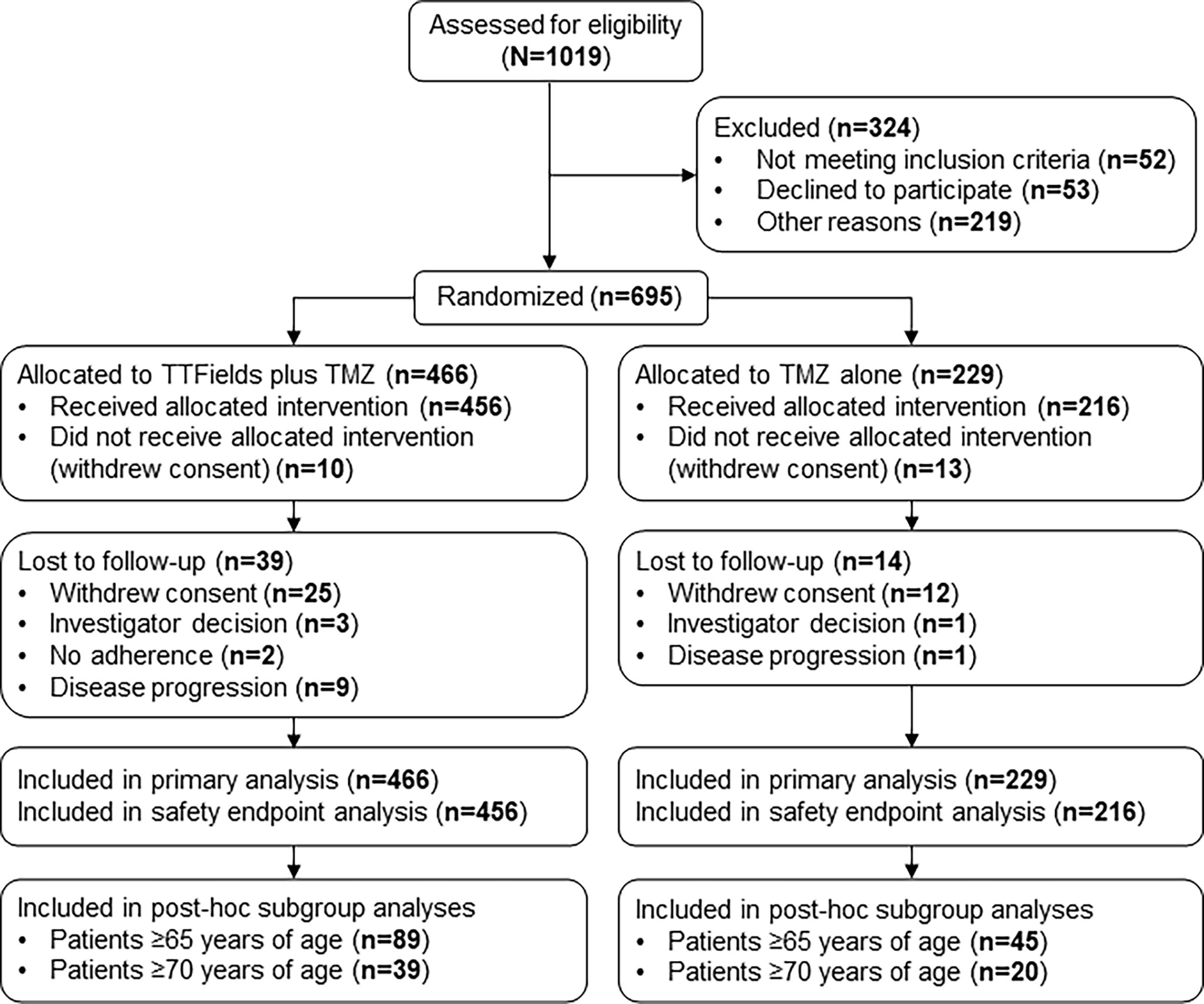
Figure 1 CONSORT Diagram. Updated from Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma a randomized clinical trial. JAMA. (2017) 318:2306–16 TMZ, temozolomide; TTFields, Tumor Treating Fields.
In the original article, there was also a mistake in Table 2 as published. Incorrect data appears for Duration of TTFields therapy, months, median (range) and TTFields daily usage ≥75%, n (%) for TTFields plus TMZ. The corrected Table 2 appears below.
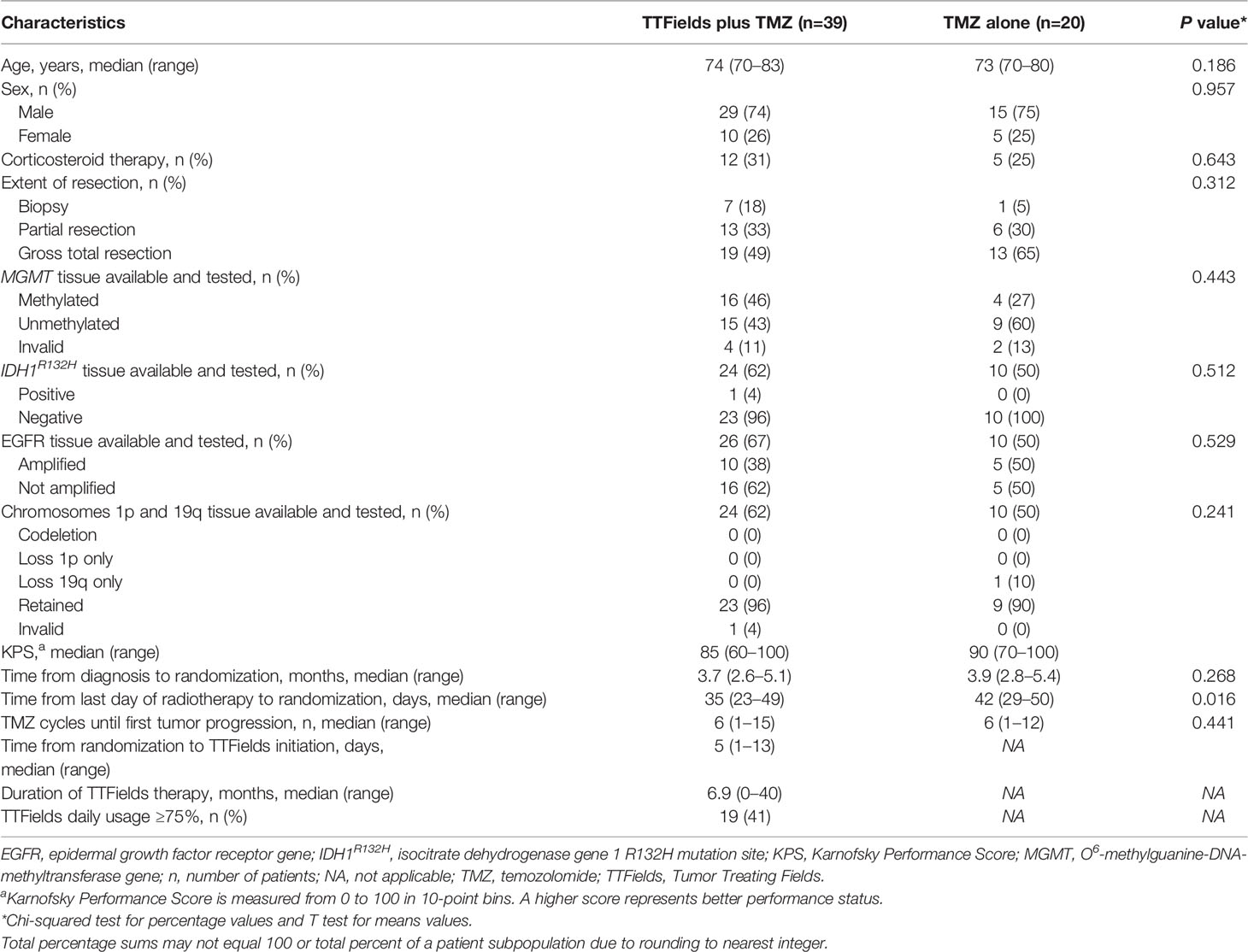
Table 2 Baseline characteristics in TTFields (200 kHz) plus TMZ combination versus TMZ monotherapy groups for patients ≥70 years of age.
In the original article, there was a further mistake in Figure 3C as published. There was an error in KPS, median (range) for TTFields (200 kHz) ≥75% Daily Usage. The corrected Figure 3 appears below.
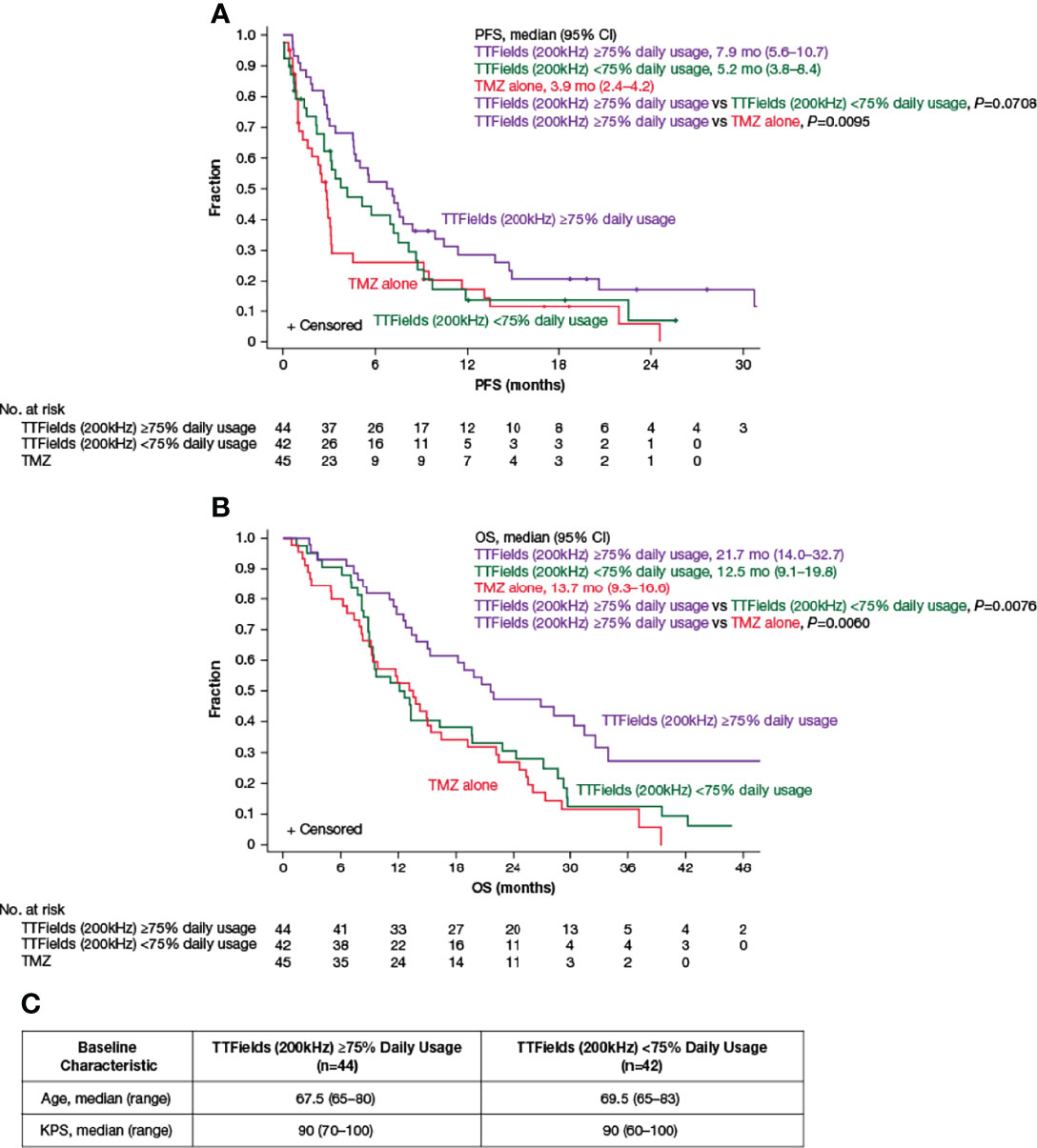
Figure 3 Kaplan–Meier curves of (A) median PFS and (B) median OS of TTFields plus TMZ combination with TTFields daily usage of ≥75% (purple lines) compared to TTFields daily usage of <75% (green lines) and TMZ alone (red lines). (C) Baseline age and KPS of patients ≥65 years of age with TTFields daily usage of ≥75% and <75%. CI, confidence interval; KPS, Karnofsky Performance Status; mo, month; no, number; OS, overall survival; PFS, progression-free survival; TMZ, temozolomide; TTFields, Tumor Treating Fields.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: elderly patients, newly diagnosed glioblastoma, TTFields, Tumor Treating Fields, phase 3 clinical trial, efficacy and safety, quality-of-life, temozolomide
Citation: Ram Z, Kim C-Y, Hottinger AF, Idbaih A, Nicholas G and Zhu J-J (2022) Corrigendum: Efficacy and Safety of Tumor Treating Fields (TTFields) in Elderly Patients With Newly Diagnosed Glioblastoma: Subgroup Analysis of the Phase 3 EF-14 Clinical Trial. Front. Oncol. 12:902929. doi: 10.3389/fonc.2022.902929
Received: 23 March 2022; Accepted: 25 March 2022;
Published: 12 April 2022.
Copyright © 2022 Ram, Kim, Hottinger, Idbaih, Nicholas and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zvi Ram, enZpcmFtQHRhc21jLmhlYWx0aC5nb3YuaWw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.