- 1Centre for Health Management and Policy Research, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2National Health Commission (NHC) Key Laboratory of Health Economics and Policy Research, Shandong University, Jinan, China
- 3Center for Health Preference Research, Shandong University, Jinan, China
Objective: We aimed to investigate the cost-effectiveness of nivolumab plus chemotherapy and nivolumab plus ipilimumab versus chemotherapy in the first-line treatment for advanced esophageal squamous-cell carcinoma (ESCC) patients from a healthcare system perspective in China.
Methods: On the basis of the CheckMate 648 trial, a partitioned survival model was constructed to estimate economic costs and health outcomes among overall and PD-L1-positive advanced ESCC patients over a 10-year lifetime horizon. The health-related costs and utilities were obtained from the local charges and published literature. The lifetime costs, life-years, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) were measured. One-way and probabilistic sensitivity analyses (PSA) were performed to assess the robustness of the model.
Results: In the base-case analysis, in overall and PD-L1-positive advanced ESCC patients, the ICERs were $415,163.81/QALY and $216,628.00/QALY for nivolumab plus chemotherapy, and$430,704.11/QALY and $185,483.94/QALY for nivolumab plus ipilimumab, respectively, compared with chemotherapy. One-way sensitivity analyses revealed that patients’ weight was the most influential parameter on ICER. The PSA demonstrated that the probability of nivolumab combination therapy being cost-effective was 0% over chemotherapy at the current price and willingness-to-pay threshold ($38,351.20/QALY). When the price of nivolumab and ipilimumab decreased 80%, the cost-effective probability of nivolumab plus ipilimumab increased to 40.44% and 86.38% in overall and PD-L1-positive advanced ESCC patients, respectively.
Conclusion: Nivolumab combination therapy could improve survival time and health benefits over chemotherapy for advanced ESCC patients, but it is unlikely to be a cost-effective treatment option in China.
Introduction
Esophageal cancer ranks seventh in terms of incidence (604, 000 new cases) and sixth in mortality (544, 000 deaths) worldwide, and East Asian countries were with the highest incidence rates, in part because of the enormous burden in China (1). Nearly half of the esophageal cancer across the world were in China, and the prevention of esophageal cancer has become an important goal for the Chinese government (2). Esophageal squamous-cell carcinoma (ESCC) and esophageal adenocarcinoma are the two major histological types of esophageal cancer, the former accounts for approximately 85% of the cases (3). Standard platinum plus fluorouracil or paclitaxel-based chemotherapy are the recommended first-line treatment option for patients with unresectable advanced, recurrent or metastatic ESCC (4, 5). Although chemotherapy has been widely used as first-line treatment for decades, survival improvement in these patients remains poor (median survival, <1 year) (6, 7), and novel treatment strategies are urgently needed.
Nivolumab, a human monoclonal anti-PD-1 antibody, has been demonstrated to improve the survival benefits for the treatment of several solid tumors in previously published studies (8–10). Programmed death ligand 1 (PD-L1) expression is enriched in ESCC, with expression ranging from 15% to 83% in tumor cells, and from 13% to 31% in immune cells (11). Recently, the results of CheckMate 648 trial, which compared nivolumab plus chemotherapy, nivolumab plus the monoclonal antibody ipilimumab, and chemotherapy in patients with unresectable advanced, recurrent, or metastatic ESCC, have revealed that overall survival (OS) was significantly longer with nivolumab plus chemotherapy than with chemotherapy alone in the overall population (median, 13.2 vs. 10.7 months; hazard ratio [HR], 0.74; 99.1% confidence interval [CI], 0.58-0.96; P=0.002) and also among patients with tumor-cell PD-L1 expression of 1% or greater (median, 15.4 vs. 9.1 months, HR, 0.54; 99.5% CI, 0.37-0.80; P<0.001) (12). A significant OS benefit was also seen with nivolumab plus ipilimumab over chemotherapy alone in overall and PD-L1-positive advanced ESCC patients (12). The CheckMate 648 trial indicated that nivolumab combination therapy could be considered as novel standard first-line treatment options to clinicians and decision-makers for the treatment of advanced ESCC patients, and these treatments has been recommended by the Chinese Society of Clinical Oncology (CSCO) Guidelines of Esophageal Cancer (13).
Significant costs always accompany the research and development of innovative drugs (14). The high cost of nivolumab and ipilimumab may limit its availability and impose a substantial financial burden on the national healthcare system. Although previous economic evidence demonstrated that nivolumab was unlikely to be cost-effective compared with chemotherapy in the second-line treatment of advanced ESCC patients from the perspective of Chinese society (15), the cost-effectiveness of nivolumab plus chemotherapy and nivolumab plus ipilimumab was not clear yet. Therefore, the objective of this study was to assess the cost-effectiveness of nivolumab combination therapy as first-line management for advanced ESCC patients in China. Such evidence may better inform clinical practice and reimbursement policy to optimize resource utilization.
Methods
Patients and intervention
This economic evaluation study was based on the CheckMate 648 trial (12), and the ethical approval of the institutional review board was exempted because no real human participants were involved. This study followed the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) reporting guidelines (Supplementary Table 1) (16). The target patient population was kept with the cohort included in the CheckMate 648 trial, an open-label, phase 3 trial conducted at 182 sites in 26 countries. Eligible patients were at least 18 years of age and had been confirmed unresectable advanced, recurrent, or metastatic ESCC, regardless of PD-L1 expression status, according to Response Evaluation Criteria in Solid Tumors (12).
Included patients were randomly assigned in a 1:1:1 ratio to receive nivolumab (240 mg intravenously on day 1 and day 15 every 4 weeks) plus chemotherapy (consisting of fluorouracil at a dose of 800 mg per square meter of the body-surface area on days 1 through 5 and cisplatin at a dose of 80 mg per square meter on day 1 each 4-week); nivolumab (3 mg per kilogram of body weight every 2 weeks) plus ipilimumab (1 mg per kilogram every 6 weeks); or chemotherapy alone until disease progression, unacceptable toxicity or other reasons (12). Patients were permitted to receive nivolumab or nivolumab plus ipilimumab up to a maximum of 2 years in line with package insert information and published resource. Subsequently, patients were managed with chemotherapy until progression.
Model structure
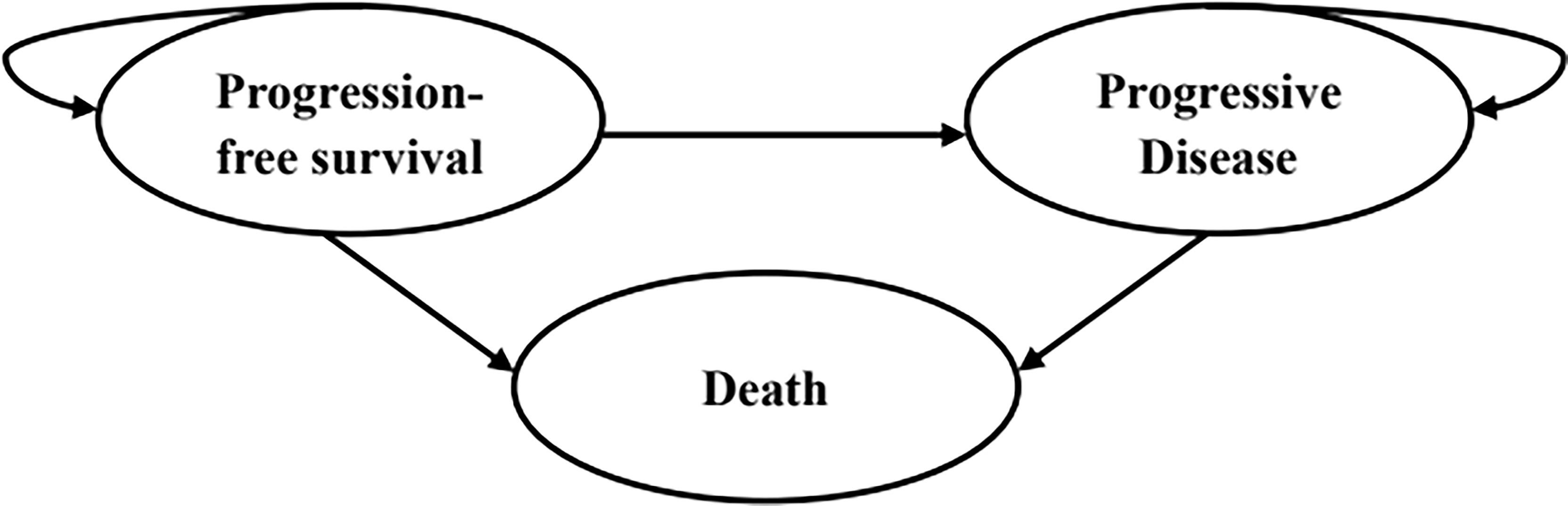
A partitioned survival model was developed using Microsoft Excel 2019 to compare the cost and effectiveness of the three competing regimens mentioned above among patients with advanced ESCC. The model was composed of three mutually exclusive health states: progression-free survival (PFS), progressed disease (PD) and death (Figure 1). The initial health state of all patients was PFS state, and that they could maintain their assigned health state or redistribute to another health state during each cycle. The proportion of patients in the PFS state at each time point was estimated as the area under the curve (AUC) for the PFS, while the proportion of patients in the death state was calculated by 1 minus the OS curve. The AUC between the PFS and OS curves was the PD state. The cycle length of the model was set at 4 weeks to facilitate parameter calculation. The time horizon was ten years to ensure that ESCC patients fully entered the terminal state.
This study was conducted from a Chinese healthcare system perspective. The primary outcomes of the model were total cost, life-years, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) between the treatment strategies. ICER was described as the additional cost required for each additional QALY. A half-cycle correction was implemented to improve the accuracy of the results. According to China Guidelines for Pharmacoeconomic Evaluations, a 5% annual discount rate was applied for all costs and QALYs (17). Based on the local Consumer Price Index, all costs were adjusted to 2022 prices and converted into US dollars (1$=6.33 CNY). As recommended by the World Health Organization, we used three times of the per capita gross domestic product (GDP) of China in 2021 ($38,351.20) as the willingness-to-pay (WTP) threshold to determine the cost-effectiveness of treatment regimens (18–21). Treatment options were considered highly cost-effective when the ICER was less than 1 times GDP per capita, while treatment options were considered cost-effective when the ICER was less than 3 times the GDP per capita (18). This WTP threshold has been widely employed in health technology assessment within low- and middle-income countries (20).
Clinical data
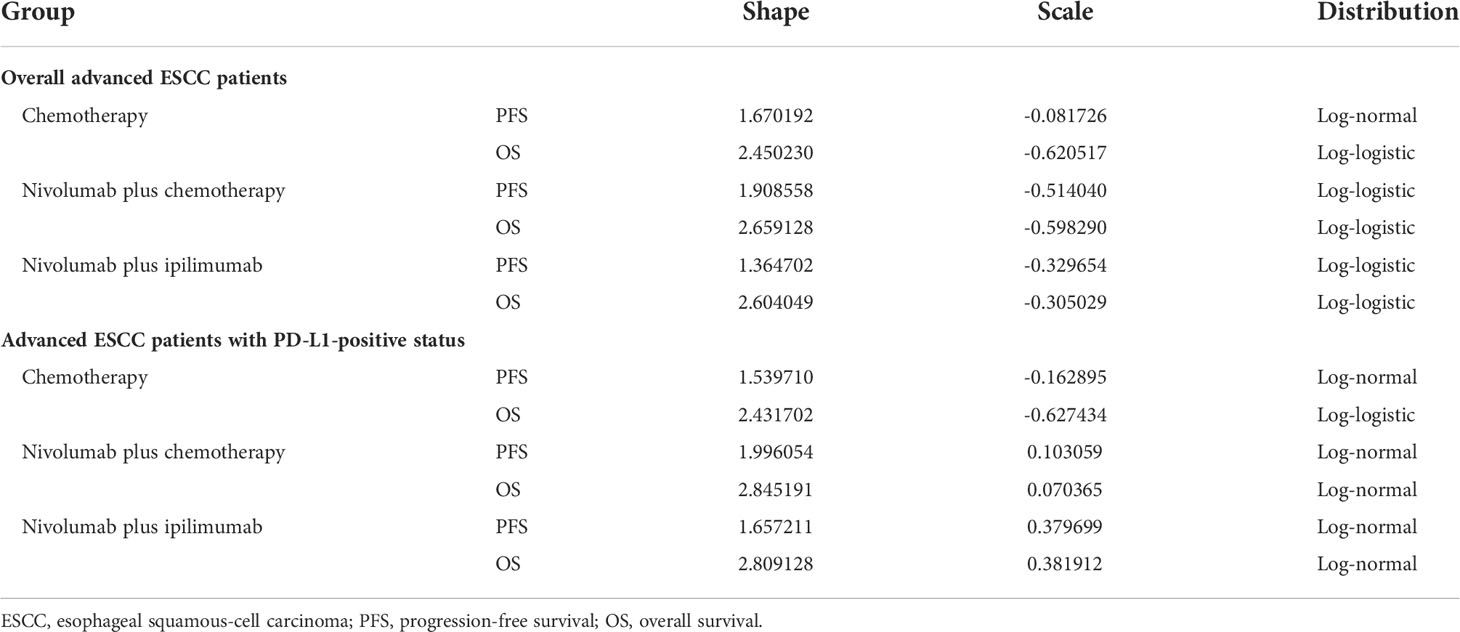
The clinical efficacy and safety data were derived from the CheckMate 648 trial (12). As individual patient data (IPD) was not available, the GetData Graph Digitizer 2.26 (http://www.getdata-graph-digitizer.com/) was used to extract PFS and OS data points from the corresponding Kaplan–Meier survival curves. Different parametric distributions, including Exponential, Weibull, Log-logistic, Log-normal, and Gompertz, were fitted to extrapolate the survival curves beyond the follow-up duration of the clinical trials (22). The distribution with the best fit was evaluated based on graphical validation, Akaike information criterion (AIC) and Bayesian information criterion (BIC) (Supplementary Tables 2, 3) (23). The AIC and BIC were calculated using survival analyses with Stata 15.1. As for the long-tail curve, we used the sub-optimal or Weibull distribution for extrapolation to avoid overestimating the survival time (24). A total of 12 parametric survival curves were modeled, including the PFS and OS of overall and PD-L1-positive advanced ESCC patients (Supplementary Figures 1–12). The estimated scale (λ) and shape (γ) parameters of the fitting model are presented in Table 1.
Costs
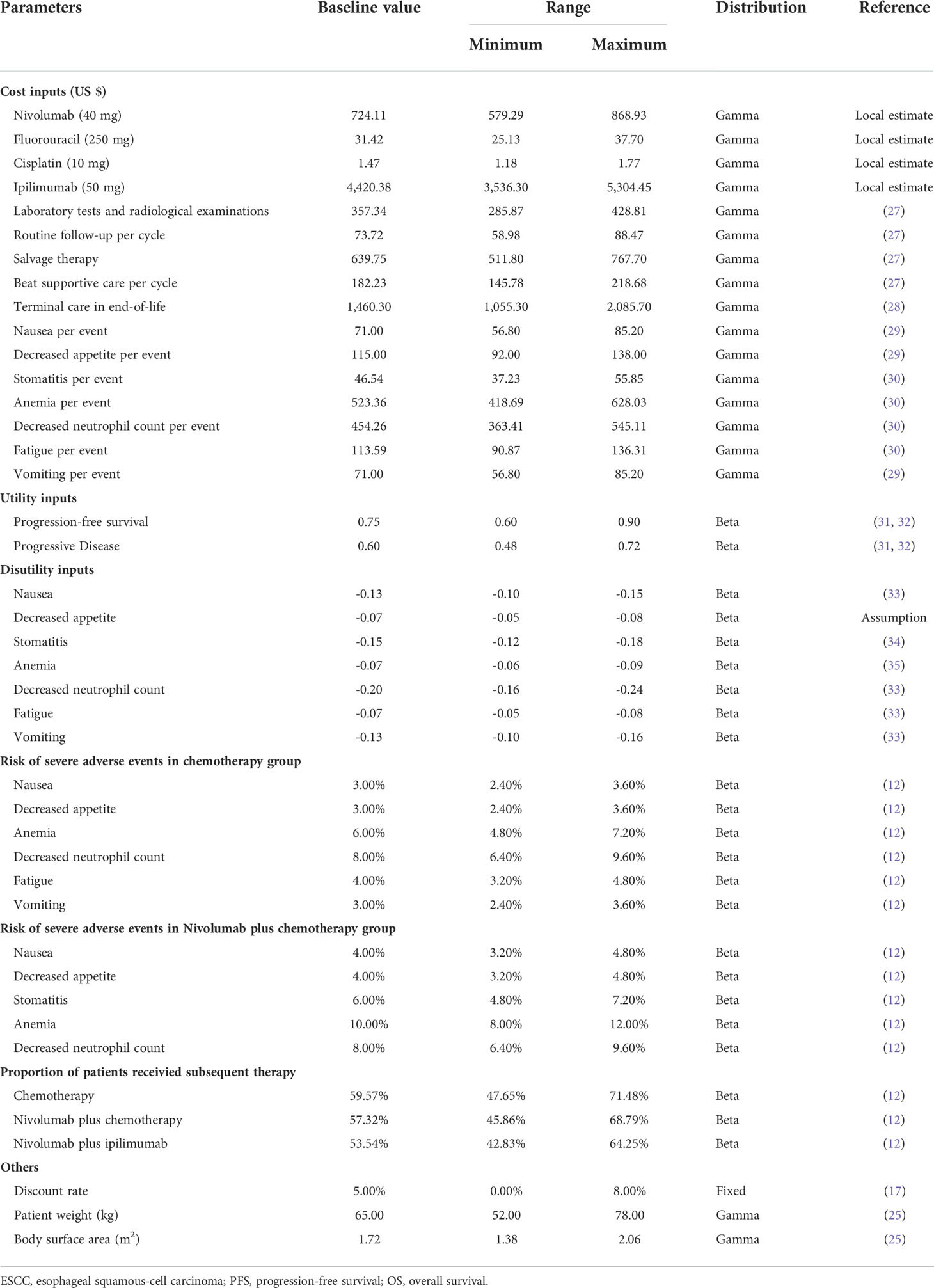
Only direct medical costs were considered, including costs for drugs, laboratory tests and radiological examinations, routine follow-up, management of treatment-related severe adverse events (AEs), salvage therapy, best supportive care, and terminal care in end-of-life. The drug administration schedules were in accordance with the CheckMate 648 trial. To estimate the dosage of chemotherapy agents, a typical patient weighed 65 kg and had a height of 1.64 m was assumed, resulting in a body surface area of 1.72 m2 (25). The model included management costs associated with grade 3-4 AEs that occurred in 3% or greater of patients as they have a substantial effect on the survival and costs. In this condition, our analysis calculated the costs of nausea, decreased appetite, stomatitis, anemia, neutropenia, fatigue, and vomiting. The treatment of neutropenia covered that of leukopenia, so that the cost of leukopenia was not included based on expert consensus (26). Furthermore, owing to the unavailability of cost and disutility values, mucosal inflammation was not considered either. All costs were acquired from local hospitals or previously published literature (27–30). The nivolumab patient assistance program (PAP) was currently implemented in patients with advanced or recurrent gastric or gastro-oesophageal junction adenocarcinoma, so we only considered the effection of price reductions for nivolumab and ipilimumab.
Utilities
Each health state was assigned a utility value anchored in 0 (death) and 1 (perfect health) in this partitioned survival model. QALYs were measured to determine health outcomes, namely, the utility values in a particular health state multiplied by the years of the corresponding state lasted. As the CheckMate 648 trial did not report the utility values of different health states, we obtained from another published study, a global, randomized, double-blind phase III trial, in which the utility values were measured by the EuroQol five dimensions health status questionnaire (EQ-5D-3L) and the UK-specific value algorithm (31, 32). In addition, we considered the disutility values caused by grade 3-4 AEs according to the relevant literature (33–35). All costs and utilities are shown in Table 2.
Scenario analysis
Our analyses covered two scenarios. In the first scenario, we assumed that nivolumab and ipilimumab were reduced to 80%, 60%, 40% or 20% of the current price to explore the cost-effectiveness of nivolumab combination therapy, respectively. In addition, we evaluated the impact of a longer or shorter time horizon of simulation on ICERs.
Sensitivity analyses
In order to evaluate the robustness of the model and identify the variables that have considerable impacts on the analysis results, we performed one-way and probabilistic sensitivity analyses (PSA) for input parameters. In the one-way sensitivity analysis, input parameters were adjusted one-by-one to their respective minimum and maximum values, with a range of the 95% confidence intervals reported in the referenced literature or a ± 20% change from the base-case value, in order to ascertain the variables that significantly influenced the economic outcomes. The range of discount rate was 0%-8%. Tornado diagram was used to present the results. A Monte Carlo simulation of 10,000 iterations was conducted for PSA by simultaneously sampling all input parameters from the pre-specified distributions. All the costs were sampled from Gamma distribution. The utility values and probabilities were sampled from Beta distribution. Cost-effectiveness acceptability curves (CEAC) were plotted based on the outcomes from 10,000 iterations to illustrate the probability of cost-effectiveness of nivolumab plus chemotherapy and nivolumab plus ipilimumab against chemotherapy alone at various WTP thresholds.
Results
Base-case results
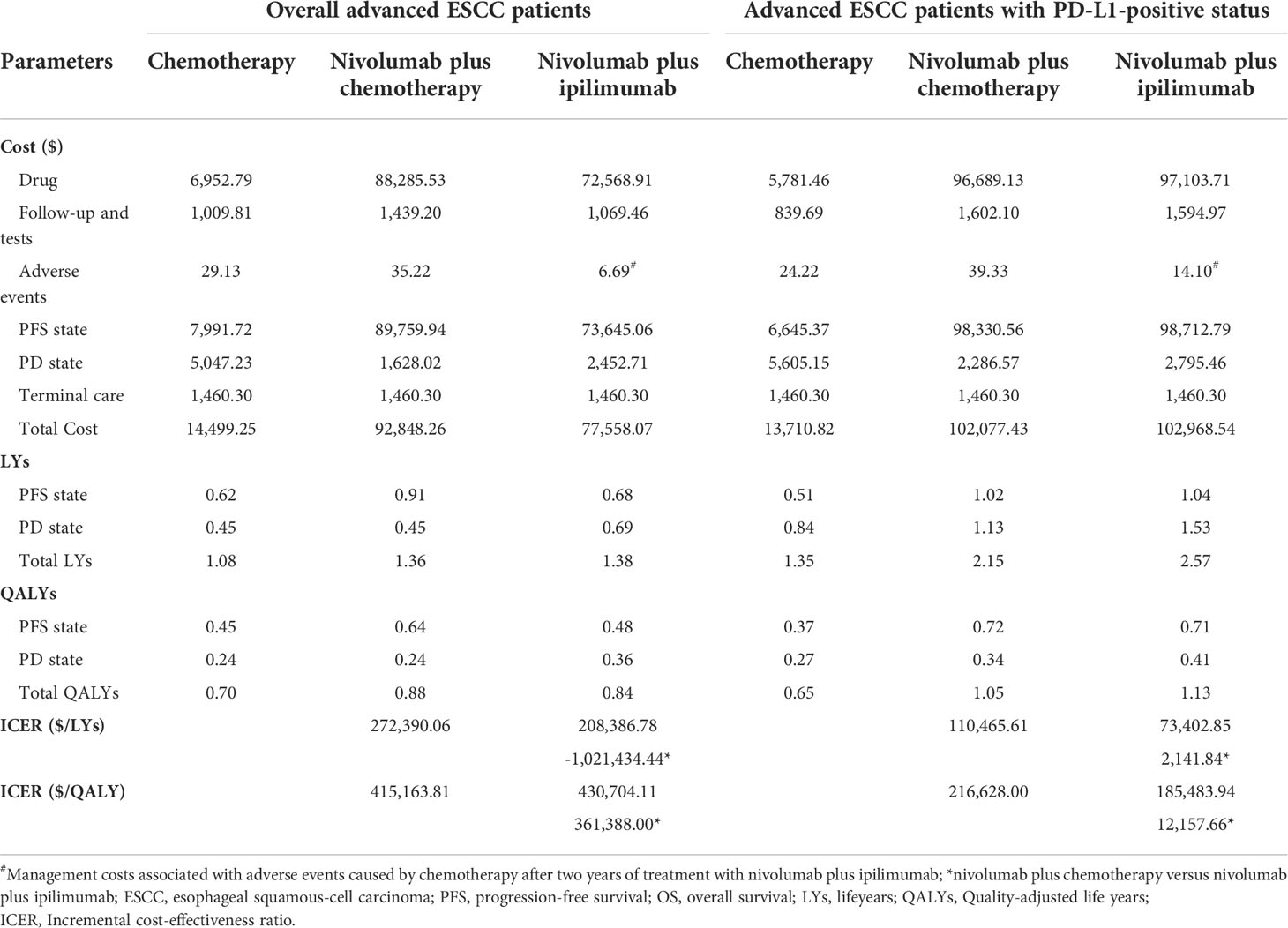
The base-case results are presented in Table 3. Over the lifetime horizon of 10 years, compared with chemotherapy, nivolumab plus chemotherapy or ipilimumab as first-line therapy for overall advanced ESCC patients provided an incremental cost of $78,349.01 and $63,058.82 with additional 0.19 QALYs and 0.15 QALYs, respectively, resulting in an ICER of $415,163.81/QALY and $430,704.11/QALY. Compared with chemotherapy, nivolumab plus chemotherapy or ipilimumab as first-line therapy for PD-L1-positive advanced ESCC patients generated an incremental cost of $ 88,366.61 and $ 89,257.72 with additional 0.41 QALYs and 0.48 QALYs, respectively, resulting in an ICER of $216,628.00/QALY and $185,483.94/QALY. In the pairwise comparison between the two nivolumab combination therapies, nivolumab plus ipilimumab increased the cost by $891.12 with the augments of 0.07 QALYs against nivolumab plus chemotherapy for PD-L1-positive advanced ESCC patients, and the ICER ($12,157.66/QALY) was lower than the WTP threshold.
Scenario analysis results
The results of the scenario analysis are shown in Supplementary Tables 4, 5. As the price of nivolumab and ipilimumab decreased or the time horizon of simulation increased, the ICER of nivolumab combination therapy over chemotherapy gradually decreased. With 80% price reduction of nivolumab and ipilimumab, the ICER ($29,649.50/QALY) of nivolumab plus ipilimumab versus chemotherapy was below the WTP threshold in the treatment of PD-L1-positive advanced ESCC patients.
One-way sensitivity analysis
The top 10 parameters that most influenced the base-case analysis of overall advanced ESCC patients are presented in Tornado diagrams (Figures 2–4). Patients’ weight, utility values, and the prices of nivolumab and ipilimumab greatly influenced the model results. Similar results were obtained in PD-L1-positive advanced ESCC patients (Supplementary Figures 13–15).
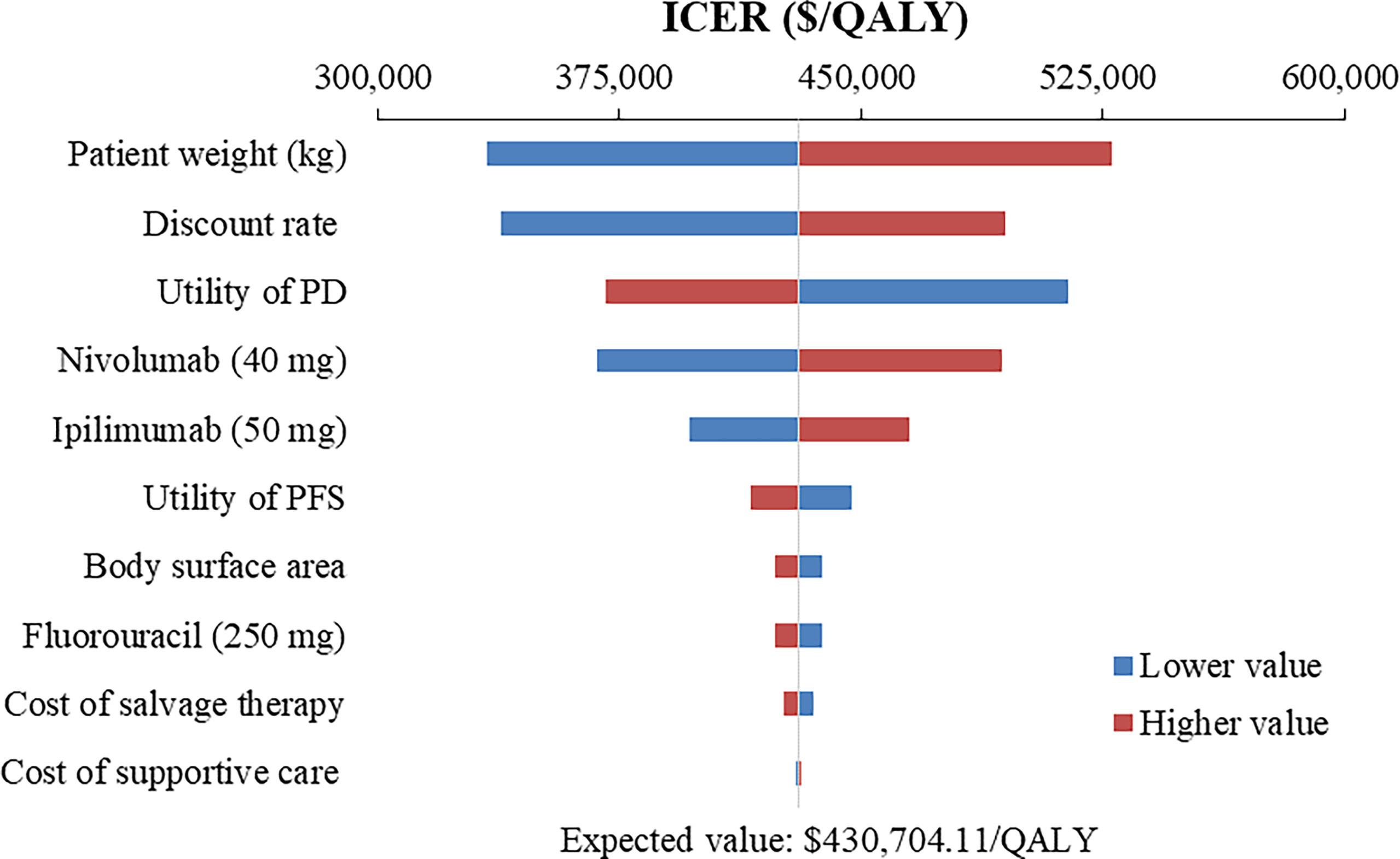
Figure 2 Tornado diagram of one-way sensitivity analysis of Nivolumab plus ipilimumab versus Chemotherapy in the treatment of overall advanced ESCC patients. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PFS, progression-free survival; PD, progressive disease.
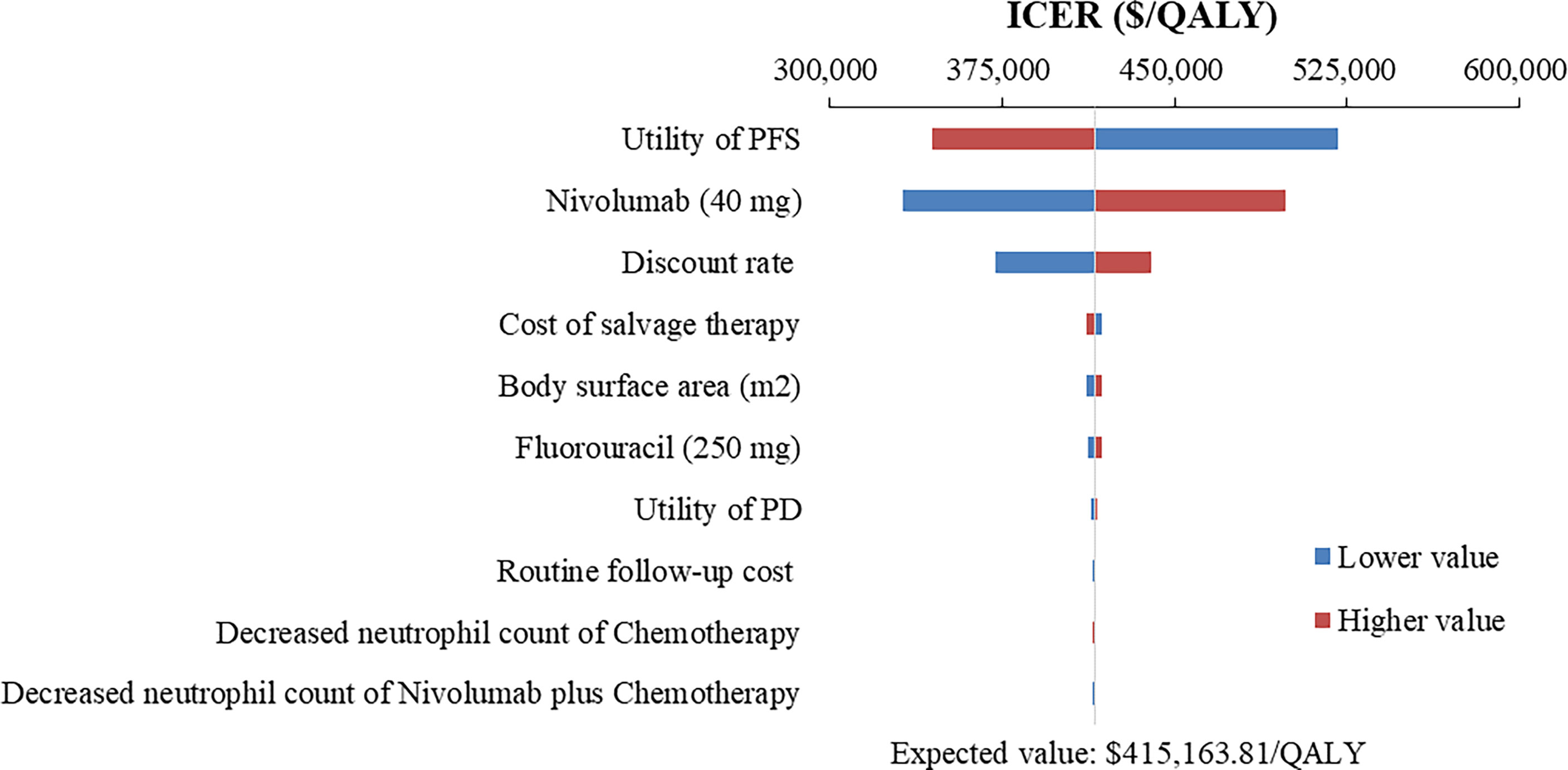
Figure 3 Tornado diagram of one-way sensitivity analysis of Nivolumab plus chemotherapy versus Chemotherapy in the treatment of overall advanced ESCC patients. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PFS, progression-free survival; PD, progressive disease.
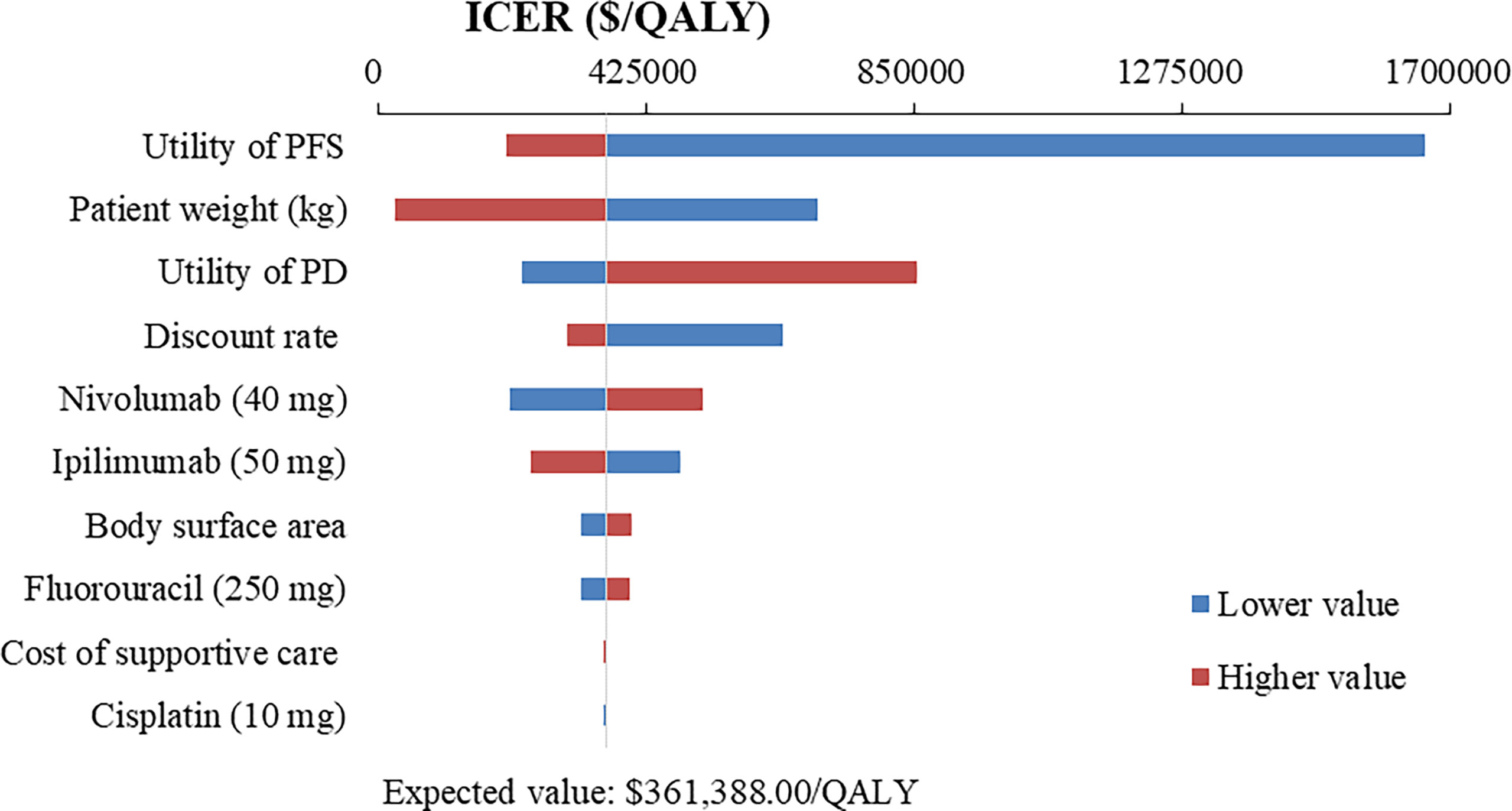
Figure 4 Tornado diagram of one-way sensitivity analysis of Nivolumab plus chemotherapy versus Nivolumab plus ipilimumab in the treatment of overall advanced ESCC patients. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PFS, progression-free survival; PD, progressive disease.
Probabilistic sensitivity analysis
At the base-case WTP threshold and current price, the CEAC demonstrated that the probability of nivolumab combination therapy strategies being cost-effective was 0% in overall and PD-L1-positive ESCC patients (Figures 5, 6). As the price of nivolumab and ipilimumab decreased, the results of the PSA have changed. When the price of nivolumab and ipilimumab reduced 80%, the probability of being cost-effective increased to 0% and 7.85% for nivolumab plus chemotherapy and 40.44% and 86.38% for nivolumab plus ipilimumab in overall and PD-L1-positive advanced ESCC patients, respectively. In the pairwise comparison between the two nivolumab combination therapies, the probability of nivolumab plus chemotherapy being cost-effectiveness was 4.72% and 41.26% in overall and PD-L1-positive advanced ESCC patients at the WTP threshold of $38,351.20 per QALY, respectively, compared with nivolumab plus ipilimumab (Supplementary Figures 16, 17).
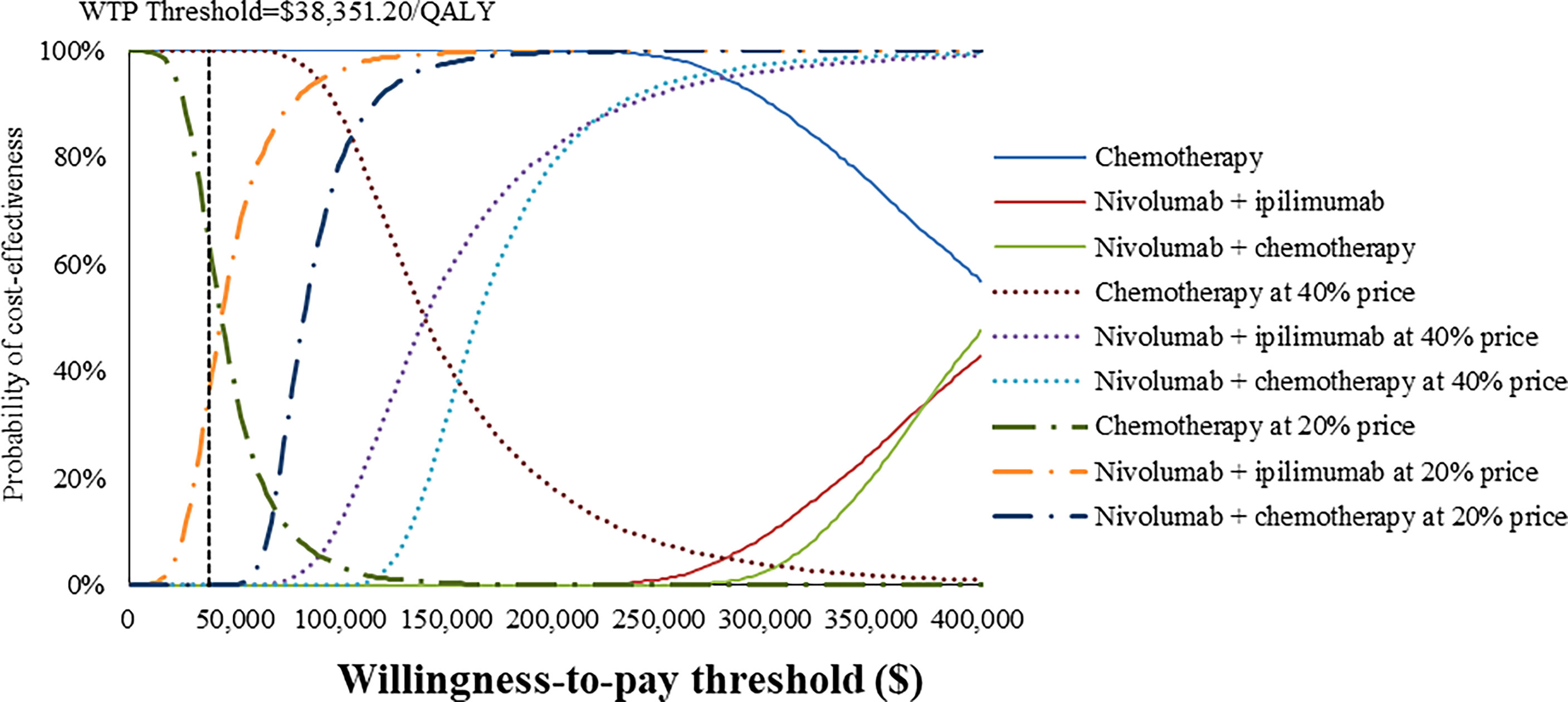
Figure 5 Cost-effectiveness acceptability curves of nivolumab plus ipilimumab and nivolumab plus chemotherapy versus chemotherapy in the treatment of overall advanced ESCC patients from the Chinese healthcare perspective.
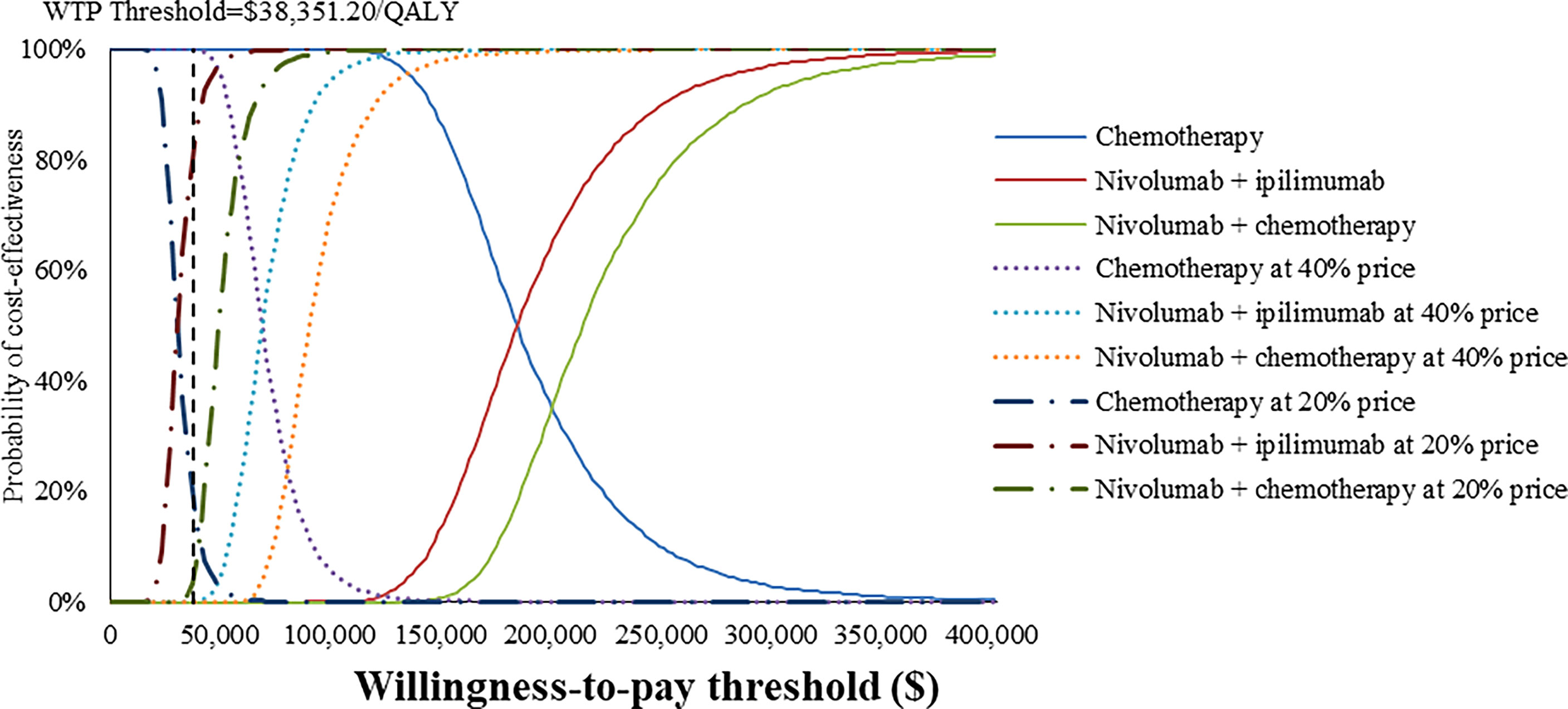
Figure 6 Cost-effectiveness acceptability curves of nivolumab plus ipilimumab and nivolumab plus chemotherapy versus chemotherapy in the treatment of PD-L1-positive advanced ESCC patients from the Chinese healthcare perspective.
Discussion
To our knowledge, this study was the first modeling analysis to examine the cost-effectiveness of nivolumab combination therapy in the treatment of advanced ESCC patients by incorporating the latest evidence from a Chinese healthcare system perspective. The results revealed that nivolumab combination therapy could provide higher health outcomes with higher cost expenditures, the ICER well above the WTP threshold based on the latest GDP. Sensitivity analyses confirmed that the model results were robust. Considering the implementation of the national price negotiation policy in China (36, 37), we assumed that nivolumab and ipilimumab were reduced to 40% or 20% of the current price, respectively, to explore the optimal treatment options. The results of PSA indicated that when the price of nivolumab and ipilimumab at 20% price, the cost-effective probability of nivolumab plus ipilimumab improved from 0% to 40.44% and 86.38% in overall and PD-L1-positive advanced ESCC patients, respectively, otherwise chemotherapy was dominant at a WTP threshold of $38,351.20/QALY.
Regardless of the overall or PD-L1-positive advanced ESCC patients, nivolumab combined with chemotherapy or ipilimumab yielded near-equal health outcomes over a 10-year lifetime horizon estimation. In the PFS state, the QALYs produced by nivolumab plus ipilimumab were much lower than that of nivolumab plus chemotherapy for overall advanced ESCC patients, while there was almost identity between the two treatment regiments for PD-L1 positive ESCC patients. In the PD state, with the increase of time horizon, nivolumab plus ipilimumab could accumulate more QALYs than nivolumab plus chemotherapy, which benefited from the improvement of overall survival time. As such, the cost-effectiveness advantage of nivolumab plus ipilimumab compared with nivolumab plus chemotherapy progressively emerged as the simulation time increased. It was worth mentioning that these results should be interpreted with caution, due to the lack of sufficient data on the cost and disutility values of treatment-related AEs in the nivolumab plus ipilimumab group.
Due to the dramatically increasing cost and the uncertainty of survival benefits, innovative drugs combined with existing treatment schemes often have lower cost-effective probabilities than standard treatment regimens (14). Although the survival benefits of nivolumab combination therapy were superior to chemotherapy in the treatment of advanced ESCC, the higher expenditures and limited improvement in health outcomes were such that substantial price reductions still could not salvage its cost-effectiveness. Previous economic evidence suggested that nivolumab was not a cost-effective treatment option compared with chemotherapy in the second-line treatment of advanced ESCC patients from the perspective of Chinese society (15, 38). Our findings were consistent with those of previous economic evaluations, and the total cost and QALYs were different, which might be caused by various treatment schedules, modeling techniques, and cost measurements used in the two studies.
Among patients with advanced ESCC, the addition of camrelizumab (an anti-PD-1 antibody) to chemotherapy also significantly improved PFS (6.9 vs. 5.6 months; HR for progression or death, 0.56; 95% CI, 0.46-0.68; P<0.001) and OS (15.3 vs. 12.0 months; HR for death, 0.70; 95% CI, 0.56-0.88; P=0.001) in comparison with single-agent chemotherapy (39). Similarly, the latest cost-effectiveness analysis has shown that camrelizumab plus chemotherapy was unlikely to be cost-effective versus chemotherapy in patients with advanced or metastatic ESCC over a 5-year lifetime horizon estimation in China (27). However, after a price reduction of 85.2% through China’s drug price negotiation mechanism, camrelizumab was a cost-effective treatment regimen against chemotherapy for advanced or metastatic ESCC patients (35). Consequently, in the absence of further breakthroughs in efficacy at this time, a substantial price reduction is the key to ensuring cost-effectiveness and affordability of treatment options, especially in countries with a huge cancer burden and limited medical resources (40). Our sensitivity analyses also indicated that drug price was an important variable affecting ICER, and price reduction could improve the cost-effective probability of nivolumab combination therapy. In addition, equitable and niche-targeting PAP can yet be regarded as a shortcut to improve affordability.
In addition to the price of nivolumab and ipilimumab, sensitivity analyses demonstrated that patients’ weight and utility values for PFS and PD state were the most influential parameter within the model. We used the default body weight to estimate the dosage of the therapeutic agents in the base-case analysis, which limited the transferability and representativeness of specific population, such as the over-weight (41, 42). Therefore, weight-specific economic evaluations warranted further studies to best inform cancer precision medicine and reimbursement policy (43). Furthermore, the quality of life research of esophageal neoplasms has been available in China (44, 45), but these still cannot meet the urgent needs of health technology assessment, especially the lack of utility and disutility values associated with various health states and treatment regimens. Hence, developing health utility values based on realistic modeling needs remains a priority.
As model assumptions and limited data, several potential limitations should be considered in the current economic evaluation. First, we reconstructed IPD rather than actual data from the CheckMate 648 trial because the original data were unavailable from the published literature. Although this approach was not perfect, it approximately reflected the actual survival data observed in the clinical trials so as to guarantee the credibility of this simulation. Second, since the quality of life was not reported in the CheckMate 648 trial, we obtained utility values from the published literature. That might lead to some deviations between the simulation results and actual health outcomes. Therefore, we used a wide range (± 20%) of utility values to examine the effect of changes on outcomes in the sensitivity analysis, which did not substantially impact the base-case results. Third, we only considered disutility values and costs related to grade 3-4 AEs of chemotherapy and nivolumab plus chemotherapy group, as these were difficult to define and obtain in the nivolumab plus ipilimumab group. Fourth, some important cost variables were derived from published economic evaluations rather than the real-world medical data, although one-way sensitivity analysis proved that these costs exerted minimal influence on the model results, except for the costs of nivolumab and ipilimumab. Fifth, we assumed that the best supportive care was administrated after the progression of nivolumab combination therapy, which might differ from the actual treatment options.
Conclusion
In summary, nivolumab combination therapy was unlikely to be a cost-effective treatment regimen compared with chemotherapy in the first-line treatment of patients with advanced ESCC in China. When the price of nivolumab and ipilimumab decreased 80%, nivolumab plus ipilimumab was the optimal treatment option among PD-L1-positive advanced ESCC patients in China.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
SXL, SPL, and LD were responsible for study design, model building and statistical analysis. SXL prepared the manuscript. KW, ZS, RW, XZ, and ZHS searched literatures and collected data. All authors critically reviewed the model structure, verified results and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.899966/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Li SB, Chen H, Man JY, Zhang TC, Yin XL, He QF, et al. Changing trends in the disease burden of esophageal cancer in China from 1990 to 2017 and its predicted level in 25 years. Cancer Med (2021) 10(5):1889–99. doi: 10.1002/cam4.3775
3. Arnold M, Ferlay J, Henegouwen MIV, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut (2020) 69(9):1564–71. doi: 10.1136/gutjnl-2020-321600
4. Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D, Comm EG. Oesophageal cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27(suppl 5):v50–7. doi: 10.1093/annonc/mdw329
5. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus (2019) 16(1):25–43. doi: 10.1007/s10388-018-0642-8
6. Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, et al. Randomised phase ii study of cisplatin and 5-fluorouracil (5-fu) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer (1997) 33(8):1216–20. doi: 10.1016/s0959-8049(97)00088-9
7. Lee SJ, Kim S, Kim M, Lee J, Park YH, Im YH, et al. Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: A randomized phase ii study. BMC Cancer (2015) 15:693. doi: 10.1186/s12885-015-1716-9
8. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-Small-Cell lung cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
9. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/s0140-6736(21)00797-2
10. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol (2022) 40(2):127–37. doi: 10.1200/jco.21.02229
11. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (Attraction-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/s1470-2045(19)30626-6
12. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med (2022) 386(5):449–62. doi: 10.1056/NEJMoa2111380
13. CSCO. Guidelines of Chinese society of clinical Oncologhy(Csco) esophageal cancer. Beijing: People's Medical Publishing House (2022).
14. Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol (2011) 12(10):933–80. doi: 10.1016/s1470-2045(11)70141-3
15. Zhang P-F, Xie D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol (2020) 16(17):1189–98. doi: 10.2217/fon-2019-0821
16. Husereau D, Drummond M, Augustovski F, De Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards (Cheers) 2022 explanation and elaboration: A report of the ispor cheers ii good practices task force. Value Health (2022) 25(1):10–31. doi: 10.1016/j.jval.2021.10.008
17. Liu G, Hu S, Wu J, Wu J. China guidelines for pharmacoeconomic evaluations. Beijing: China Market Press (2020) 1–8.
18. Ochalek J, Wang H, Gu Y, Lomas J, Cutler H, Jin C. Informing a cost-effectiveness threshold for health technology assessment in China: A marginal productivity approach. Pharmacoeconomics (2020) 38(12):1319–31. doi: 10.1007/s40273-020-00954-y
19. Cameron D, Ubels J, Norstrom F. On what basis are medical cost-effectiveness thresholds set? clashing opinions and an absence of data: A systematic review. Glob Health Action (2018) 11(1):1447828. doi: 10.1080/16549716.2018.1447828
20. Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: The past, the present and the future. Pharmacoeconomics (2018) 36(5):509–22. doi: 10.1007/s40273-017-0606-1
21. Tzanetakos C, Stefanou G, Gourzoulidis G. Does a standard willingness-To-Pay threshold exist in Greece? Value Health (2019) 22:S772–S3. doi: 10.1016/j.jval.2019.09.1963
22. Ishak KJ, Kreif N, Benedict A, Muszbek N. Overview of parametric survival analysis for health-economic applications. Pharmacoeconomics (2013) 31(8):663–75. doi: 10.1007/s40273-013-0064-3
23. Latimer NR. Survival analysis for economic evaluations alongside clinical trials-extrapolation with patient-level data: Inconsistencies, limitations, and a practical guide. Med Decis Making (2013) 33(6):743–54. doi: 10.1177/0272989x12472398
24. Ball G, Levine M, Thabane L, Tarride J-E. Onwards and upwards: A systematic survey of economic evaluation methods in oncology. Pharmacoecon Open (2021) 5(3):397–410. doi: 10.1007/s41669-021-00263-w
25. Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: A cost-effectiveness analysis in a health resource-limited setting. PloS One (2012) 7(3):e32530. doi: 10.1371/journal.pone.0032530
26. Zhang Y, Zeng X, Deng H, Ma F, Peng Y, Yi L, et al. Cost-effectiveness analysis of adding palbociclib as a second-line endocrine therapy for Hr+/Her2(-) metastatic breast cancer from the us and Chinese perspectives. Clin Ther (2019) 41(6):1175–85. doi: 10.1016/j.clinthera.2019.04.033
27. Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-effectiveness analysis of camrelizumab vs. placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol (2021) 11:790373(1). doi: 10.3389/fonc.2021.790373
28. Wu B, Li T, Cai J, Xu Y, Zhao G. Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer (2014) 14(1):984. doi: 10.1186/1471-2407-14-984
29. Yang F, Fu Y, Kumar A, Chen M, Si L, Rojanasarot S. Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Ann Transl Med (2021) 9(15):1226. doi: 10.21037/atm-21-1803
30. Shi G, Park SH, Ren H, Xue M, Lu X, Dong P, et al. Cost analysis for different sequential treatment regimens for metastatic renal cell carcinoma in China. J Med Econ (2018) 21(12):1150–8. doi: 10.1080/13696998.2018.1515769
31. Al-Batran SE, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. Quality-Of-Life and performance status results from the phase iii rainbow study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol (2016) 27(4):673–9. doi: 10.1093/annonc/mdv625
32. Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (Rainbow): A double-blind, randomised phase 3 trial. Lancet Oncol (2014) 15(11):1224–35. doi: 10.1016/s1470-2045(14)70420-6
33. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol (2017) 13(5):E195–203. doi: 10.1111/ajco.12477
34. Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer (2006) 95(6):683–90. doi: 10.1038/sj.bjc.6603326
35. Cai H, Xu B, Li N, Zheng B, Zheng Z, Liu M. Cost-effectiveness analysis of camrelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma. Front Pharmacol (2021) 12:732912(1) (2021) in press. doi: 10.3389/fphar.2021.732912
36. Huang C, Ung COL, Wushouer H, Bai L, Li XY, Guan XD, et al. Trends of negotiated targeted anticancer medicines use in China: An interrupted time series analysis. Int J Health Policy Manage. doi: 10.34172/ijhpm.2021.47
37. Li H, Liu GG, Wu J, Wu J-H, Dong C-H, Hu S-L. Recent pricing negotiations on innovative medicines pilot in China: Experiences, implications, and suggestions. Value Health Reg Issues (2018) 15(1):133–7. doi: 10.1016/j.vhri.2018.01.009
38. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: An open-label, multicentre, phase 2 trial. Lancet Oncol (2017) 18(5):631–9. doi: 10.1016/s1470-2045(17)30181-x
39. Luo HY, Lu J, Bai YX, Mao T, Wang J, Fan QX, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The escort-1st randomized clinical trial. JAMA (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836
40. Wu Q, Qin Y, Liao W, Zhang M, Yang Y, Zhang P, et al. Cost-effectiveness of enfortumab vedotin in previously treated advanced urothelial carcinoma. Ther Adv Med Oncol (2022) 14(1):17588359211068733. doi: 10.1177/17588359211068733
41. Steffen A, Huerta JM, Weiderpass E, Bueno-de-Mesquita HB, May AM, Siersema PD, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European prospective investigation into cancer and nutrition. Int J Cancer (2015) 137(3):646–57. doi: 10.1002/ijc.29432
42. Cho JH, Shin CM, Han KD, Yoon H, Park YS, Kim N, et al. Abdominal obesity increases risk for esophageal cancer: A nationwide population-based cohort study of south Korea. J Gastroenterol (2020) 55(3):307–16. doi: 10.1007/s00535-019-01648-9
43. Li H. Cancer precision medicine in China. Genomics Proteomics Bioinf (2016) 14(5):325–8. doi: 10.1016/j.gpb.2016.10.002
44. Liu Q, Zeng H, Xia R, Chen G, Liu S, Zhang Z, et al. Health-related quality of life of esophageal cancer patients in daily life after treatment: A multicenter cross-sectional study in China. Cancer Med (2018) 7(11):5803–11. doi: 10.1002/cam4.1817
45. Wang H, Pan Y, Guo C, Li F, Xu R, Liu M, et al. Health-related quality of life among rural residents aged 45-69 years in hua county, henan province, China: Results of esecc trial for esophageal cancer screening with endoscopy. Chin J Cancer Res (2018) 30(2):240–53. doi: 10.21147/j.issn.1000-9604.2018.02.07
Keywords: nivolumab, ipilimumab, chemotherapy, cost-effectiveness, esophageal squamous-cell carcinoma, first-line treatment
Citation: Liu S, Dou L, Wang K, Shi Z, Wang R, Zhu X, Song Z and Li S (2022) Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front. Oncol. 12:899966. doi: 10.3389/fonc.2022.899966
Received: 19 March 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Luigi Marano, University of Siena, ItalyReviewed by:
Xiaoyu Xi, China Pharmaceutical University, ChinaLongjiang She, First People’s Hospital of Foshan, China
George Gourzoulidis, Health Through Evidence, Greece
Copyright © 2022 Liu, Dou, Wang, Shi, Wang, Zhu, Song and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunping Li, bGlzaHVucGluZ0BzZHUuZWR1LmNu
 Shixian Liu
Shixian Liu Lei Dou
Lei Dou Kaixuan Wang
Kaixuan Wang Zhao Shi
Zhao Shi Ruixue Wang
Ruixue Wang Xiaohong Zhu
Xiaohong Zhu Zehua Song
Zehua Song Shunping Li
Shunping Li