
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 07 October 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.899659
This article is part of the Research Topic Recurrence of Liver Tumors: The Issue of Iterative Approaches View all 17 articles
Background: The survival benefit of primary and metastatic resection for patients with colorectal cancer with liver metastasis (CRLM) has been observed, but methods for discriminating which individuals would benefit from surgery have been poorly defined. Herein, a predictive model was developed to stratify patients into sub-population based on their response to surgery.
Methods: We assessed the survival benefits for adults diagnosed with colorectal liver metastasis by comparing patients with curative surgery vs. those without surgery. CRLM patients enrolled in the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2015 were identified for model construction. Other data including CRLM patients from our center were obtained for external validation. Calibration plots, the area under the curve (AUC), and decision curve analysis (DCA) were used to evaluate the performance of the nomogram compared with the tumor–node–metastasis (TNM) classification. The Kaplan–Meier analysis was performed to examine whether this model would distinguish patients who could benefit from surgery.
Results: A total of 1,220 eligible patients were identified, and 881 (72.2%) underwent colorectal and liver resection. Cancer-specific survival (CSS) for the surgery group was significantly better than that for the no-surgery group (41 vs. 14 months, p < 0.001). Five factors were found associated with CSS and adopted to build the nomograms, i.e., age, T stage, N stage, neoadjuvant chemotherapy, and primary tumor position. The AUC of the CRLM nomogram showed a better performance in identifying patients who could obtain benefits in the surgical treatment, compared with TNM classification (training set, 0.826 [95% CI, 0.786–0.866] vs. 0.649 [95% CI, 0.598–0.701]; internal validation set, 0.820 [95% CI, 0.741–0.899] vs. 0.635 [95% CI, 0.539–0.731]; external validation set, 0.763 [95% CI, 0.691–0.836] vs. 0.626 [95% CI, 0.542–0.710]). The calibration curves revealed excellent agreement between the predicted and actual survival outcomes. The DCA showed that the nomogram exhibited more clinical benefits than the TNM staging system. The beneficial and surgery group survived longer significantly than the non-beneficial and surgery group (HR = 0.21, 95% CI, 0.17–0.27, p < 0.001), but no difference was observed between the non-beneficial and surgery and non-surgery groups (HR = 0.89, 95% CI, 0.71–1.13, p = 0.344).
Conclusions: An accurate and easy-to-use CRLM nomogram has been developed and can be applied to identify optimal candidates for the resection of primary and metastatic lesions among CRLM patients.
Colorectal cancer (CRC) is the third most common cancer worldwide, and 50% of patients develop liver metastasis during the course of the disease (1, 2). Among the potential curative therapies, primary and metastatic resection are the primary option to improve the prognosis of patients (3, 4). However, there remains substantial heterogeneity for some patients with resectable colorectal cancer with liver metastasis (CRLM). At present, the tumor–node–metastasis (TNM) staging classification is mainly considered in the prediction of CRLM prognosis (5). However, some studies demonstrates that CRLM patients with the same TNM classification have a different clinical outcome, and many valuable clinical factors are neglected, which are associated with the prognosis of CRLM patients undergoing primary and metastatic resection (6, 7).
More precise categorization is needed to identify those who may benefit more from surgery. Thus, it is necessary to stratify patients based on their preoperative features to provide more individualized treatments. All kinds of prediction models have been developed and validated to overcome the drawback of the TNM classification system (8). Among these models, the nomogram developed based on several independent prediction factors was widely considered an accurate and easy-to-use tool to visualize the prognosis of patients individually (9–12). It has been reported that the C-index of the nomogram predicting the risk of bone metastasis in colorectal cancer reached 0.929 (13).
Although some studies (14–16) have explored the nomogram to predict the prognosis of CRLM patients, they only predicted the overall survival, and they did not inform patients if they could live longer without the surgery. In this study, a new clinical outcome was established, which included a comparison with the median survival time of non-surgical patients. We assumed that patients receiving surgical treatment who lived longer than the median cancer-specific survival (CSS) time of those who did not undergo surgery could benefit from the operation. Based on this unusual clinical outcome, we aimed to investigate the preoperative prognostic factors, develop and validate an effective predictive model, and then make a reference standard based on the possibility of benefit to identify CRLM patients who would benefit from resection of primary and metastatic lesions.
For this study, the data we analyzed were extracted from the Surveillance, Epidemiology, and End Results (SEER) database (2000–2018, November 2020 submission), which covers approximately 28% of the US population (17). The SEER*Stat, Version 8.3.9, was applied to examine the data for research between 2004 and 2015.
The inclusion criteria for patients were as follows: 1) patients came from the database of “Incidence—SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018)”. 2) The International Classification of Diseases for Oncology (ICD-O-3) was used for the CRLM definition. “Site recode ICD-O-3/WHO 2008” was used to record tumor location information, including ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and cecum. 3) Liver metastasis. 4) Single primary site. 5) The TNM stage was stated as “M1a”. “M1a” was confined as “Metastasis limited to a single distant organ except for peritoneum” (6), and “Year of diagnosis” was set to 2004–2015. 7) According to “Histologic Type ICD-O-3”, the following pathological types were included in the following: adenocarcinoma (8140), adenocarcinoma arising in a polyp (8210), adenocarcinoma in tubulovillous adenoma (8263), mucinous/colloid adenocarcinoma (8480), and adenosquamous carcinoma (8560).
The exclusion criteria were as follows: 1) less than 20 years old; 2) diagnosed with no positive histology and not only from a death certificate or autopsy; 3) the information about the surgery to the primary site and metastatic lesion was missing; 4) clinical pathological information (tumor size, carcinoembryonic antigen (CEA), T stage, N stage, histologic type, and neoadjuvant chemotherapy) was missing; and 5) survival information (survival month and final cause of death) was missing.
CRLM patients treated in the Shanghai Cancer Center, Fudan University (FUSCC) from 2016 to 2017 were enrolled as an independent external validation set for this study. The included criteria are as follows: 1) over 18 years old; 2) single primary site; 3) diagnosed with positive histology; 4) the TNM stage was stated as “M1a”; and 4) complete demographic data, clinical parameters, TNM stage information, and full follow-up results.
The analyzed data included age (<50, 50 ≤ X < 70, and ≥70), sex (male and female), primary site (rectum, and left and right colon), tumor size (>5 and ≤5 cm), CEA (positive and negative/normal), T stage (T1, T2, T3, and T4), N stage (N0, N1, and N2), differentiation grade (Grade I, Grade II, Grade III, Grade IV, and unknown), histologic type (adenocarcinoma and others), neoadjuvant chemotherapy (yes and no), marital status (married, separated/divorced, single, widowed, and unknown), surgery to the primary site (yes and no), and surgery to the metastatic lesions (yes and no). Overall survival (OS), CSS, and survival month were extracted from the SEER database. OS time was defined as the time from diagnosis to death or to the time of data analysis. Living patients were excluded at the time of the last recording. The CSS duration can be calculated from the date of diagnosis to a documented CRC-related death. According to the published papers (18), we identified “Nonprimary surgical procedure to distant site” as the resection of metastatic lesions.
The research group was separated into two groups based on therapy, surgery versus non-surgery. Clinical differences (categorical variables) were represented as a number with percentage and compared by using the chi-square test and Fisher’s exact test. The Kaplan–Meier (K-M) method and the log-rank test were analyzed in two groups to confirm the influence of surgery on the survival of patients. To identify independent predictors, univariate and multivariate Cox proportional hazards regression analyses were performed. Hazard ratios (HRs) were calculated with 95% confidence intervals (CIs). Statistical data were analyzed with SPSS 24.0 (IBM Corp, Armonk, NY, USA). All statistical tests were two-sided, and only p < 0.05 could be regarded as statistically significant.
The eligible people receiving surgery were randomly divided into the training and validation cohorts. Patients who survived longer than the median CSS time of those who received no surgery were defined as benefiting from the surgical treatment. According to the univariate and multivariate Cox analyses, the factors independently affecting the CSS were indicated in the training cohort. Based on the multivariable logistic analysis, the nomogram to identify the patients who may obtain benefits from primary and metastasis resection was developed. The areas under the receiver operating characteristic (ROC) curve (AUCs) were applied to quantify ROC performance to assess the discriminative and calibration capacity of the nomograms. Calibration curves were utilized to demonstrate no deviations from the reference line, indicating a high degree of dependability. What is more, the decision curve analysis (DCA) was also used to evaluate the clinical application value and clinical practicability of the models. Overall, we used ROC, calibration plots, and DCA to graphically describe the performance of our model. What is more, we attempted to assign all CRLM patients undergoing surgery to two groups—beneficial and surgery group and non-beneficial and surgery group—in terms of probability of benefit of 50%. The Kaplan–Meier analyses and the log-rank test were employed to test whether this model could identify individuals who could indeed benefit from the resection of primary and metastatic lesions.
From the SEER database, 1,220 patients with M1a CRLM who met inclusion criteria were identified from 2004 to 2015. The flowchart is illustrated in Figure 1. Among them, 881 (72.2%) received primary and metastatic resection, while 339 (27.8%) had no surgical treatment. Men predominantly made up 53.4% of these cases, 20.7% of these patients were under the age of 50, and 15.1% of the tumors were in the rectum; 66.2% of tumors were classified as grade II in terms of the differentiation grade. In addition, 80.6% of patients were CEA-positive. Adenocarcinoma was found in 87.0% of the patients. Also, neoadjuvant chemotherapy was administered to 81.3% of patients. According to the TNM stage classification, 54.3% of the tumor were categorized as T3, and 27.8% of them were categorized as N2. The detailed clinical information of all patients is summarized in Table S1.
In total, 12 variables were incorporated in the least absolute shrinkage and selection operator (LASSO) regression, and all of them were included: age, sex, race, differentiation grade, histology type, T stage, N stage, tumor size, marital status, neoadjuvant chemotherapy, CEA, and primary tumor position (Figure 2).
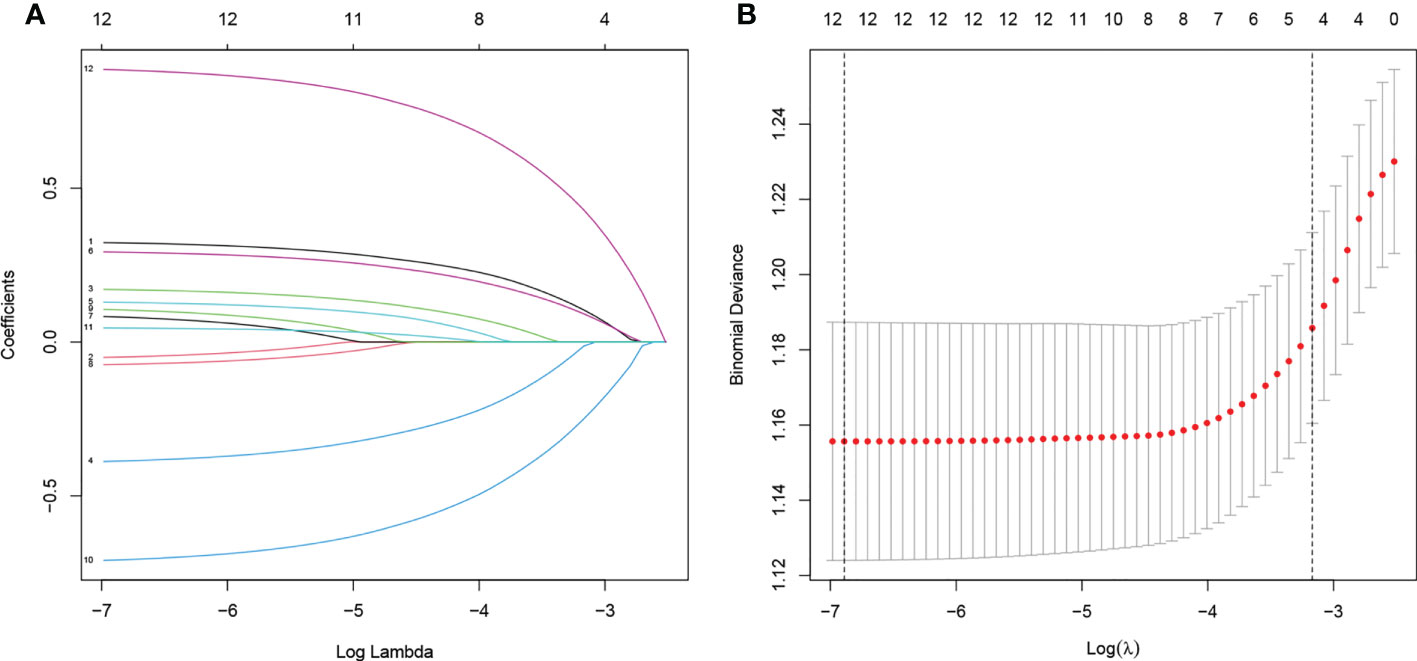
Figure 2 (A) Plot of partial likelihood deviance of CSS. (B) LASSO coefficient profile plot of CSS. CSS, cancer-specific survival; LASSO, least absolute shrinkage and selection operator.
According to the K-M analysis and log-rank test in the SEER cohort (Figure S1), patients who received excision of primary and metastatic tumors enjoyed a longer CSS. The median CSS time was 41 months (95% CI, 37.15–44.85) for individuals who underwent resection of primary and metastatic tumors, compared to 14 months (95% CI, 11.37–16.63) for patients with no surgery.
Compared with logistic regression, the Cox analysis focused more on the influence of variables on the survival of CRLM patients. In the univariate and multivariate Cox analyses, age, race, differentiation grade, primary site, T stage, N stage, neoadjuvant chemotherapy, marital status, and CEA were found to be independent predictors for the survival of patients with stage M1a CRLM. However, sex, tumor size, and histology were shown to have no significant impact on CSS (Table 1). Moreover, surgery was found to be independently linked with higher CSS (HR = 0.28, 95% CI, 0.24–0.33, p < 0.001), which further indicated the significance of surgery in the treatment.
The median CSS time (14 months) of non-surgical patients was considered as the reference line. Patients who underwent curative surgery yielded better CSS than this reference line and were identified to be beneficial in the operation. Conversely, surgical patients whose CSS was lower than 14 months were considered non-beneficial patients. A total of 708 (80.36%) patients were categorized as “beneficial”. The characteristics of patients are presented in Table 2.
We randomly assigned 881 patients who underwent curative surgery in a 4:1 ratio to the training cohort (n = 705) and the validation cohort (n = 176). The median CSS time of the training cohort and validation cohort was 41 [36.74–45.26] and 40 [31.34–48.66] months, respectively.
Nine independent predictors including age, race, differentiation grade, T stage, N stage, neoadjuvant chemotherapy, marital status, CEA, and primary tumor position were collected into the multivariate logistic regression. We identified five effective factors and developed a nomogram to predict the stage M1a CRLM patients who could benefit from the surgical treatment based on the training cohort (Figure 3; Table 3).
ROC analysis demonstrated the AUCs of the training and validation cohorts reached 0.826 [95% CI, 0.786–0.866] and 0.820 [95% CI, 0.741–0.899], respectively, outperforming the American Joint Committee on Cancer (AJCC)–TNM classification of 0.649 [95% CI, 0.598–0.701] and 0.635 [95% CI, 0.539–0.731], respectively (Figures 4A, B). In addition, the performance of the model was visualized by the calibration plots, and the calibration curves showed good agreement between prediction and observation (Figures 4C, D). Finally, DCA showed a higher clinical application value and better clinical practicability (Figure 5).
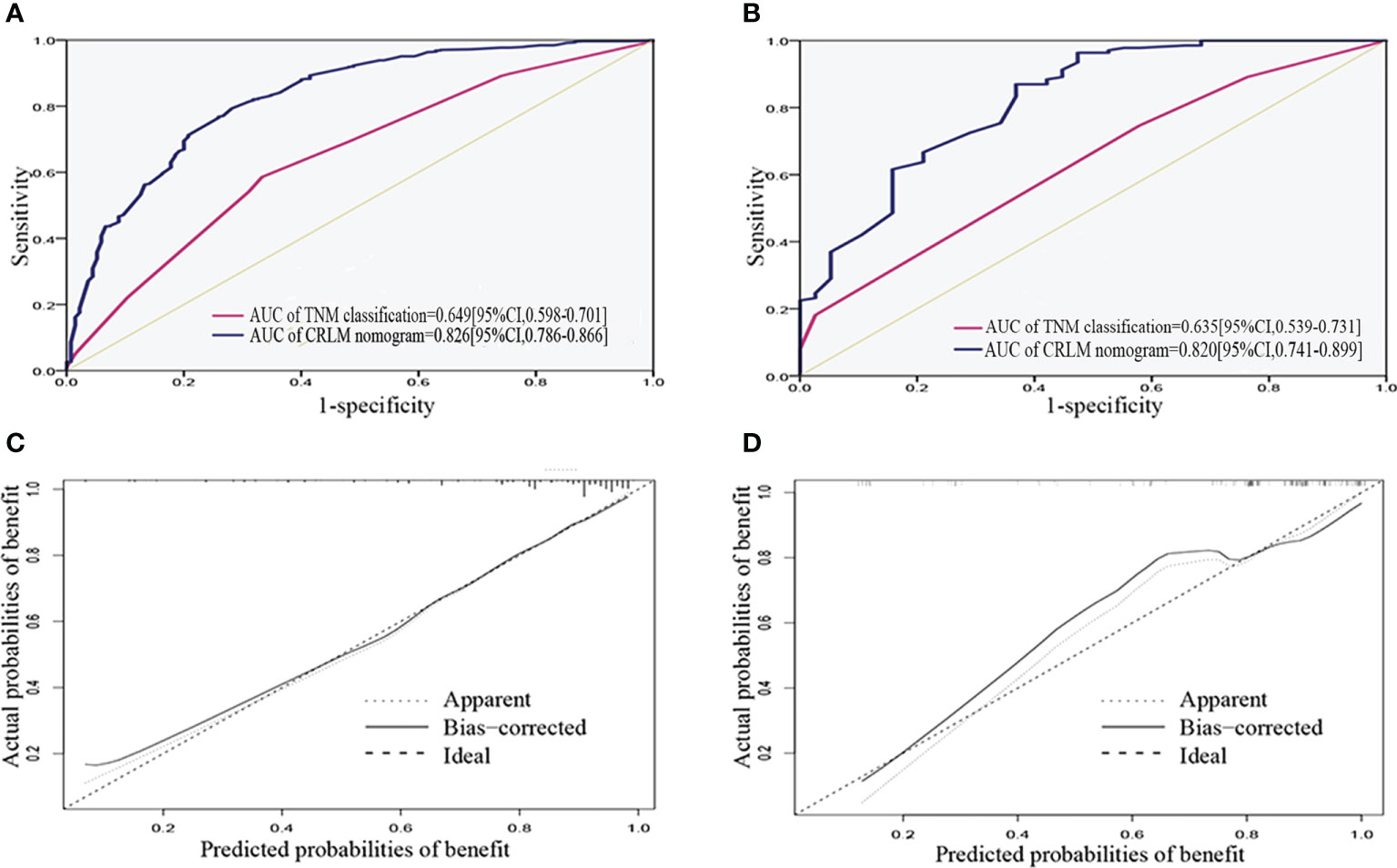
Figure 4 Validation of the nomogram. (A, B) ROC curve for discrimination in the training and validation cohorts. (C, D) Calibration plots for the actual (observed) and predicted probabilities of the nomograms in the training and validation cohorts. ROC, receiver operating characteristic curve.
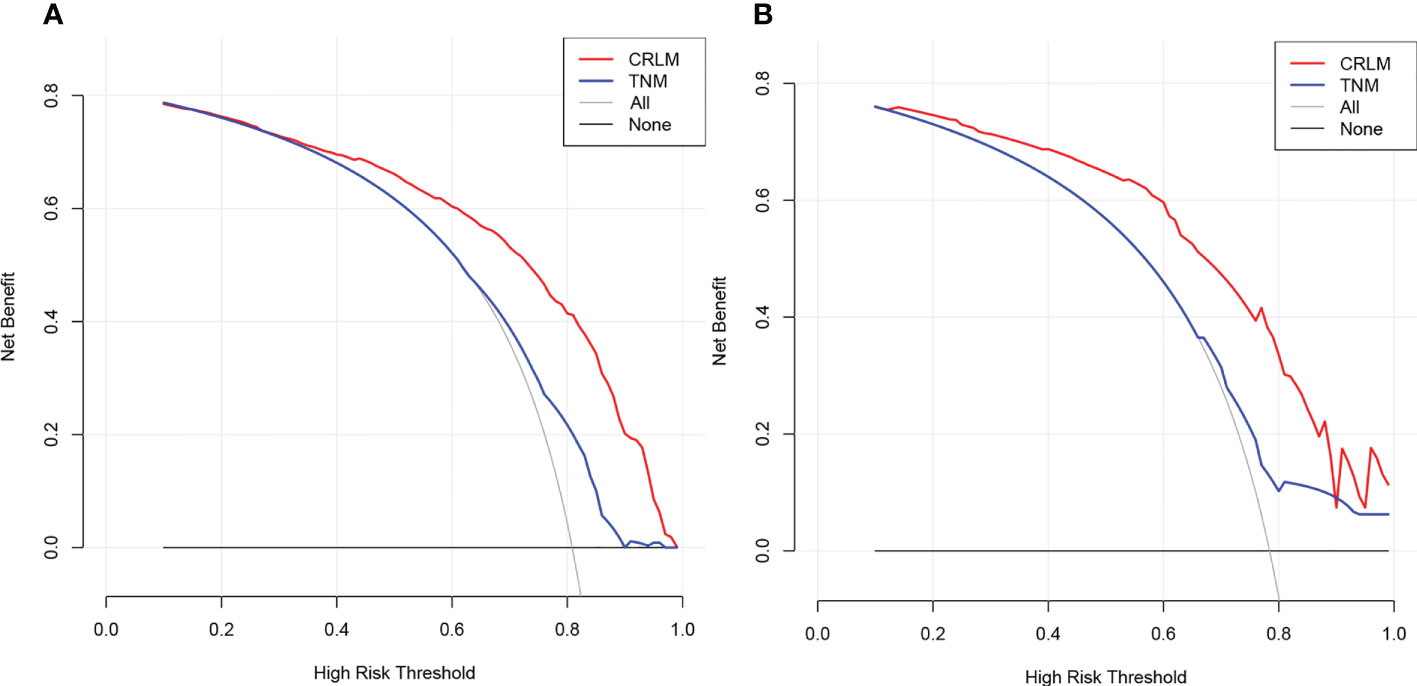
Figure 5 Decision curve analysis of CRLM nomogram in the training (A) and validation (B) cohorts. Horizontal lines indicate that no cases will experience the event. Gray lines indicate that all cases will experience the event. Red and blue lines represent the net benefits across threshold probabilities according to the CRLM nomogram and TNM classification, respectively. CRLM, colorectal cancer with liver metastasis..
In this study, external validation was performed in the FUSCC cohort. The AUC of the CRLM nomogram was 0.763 [95% CI, 0.691–0.836], outperforming the AJCC-TNM classification of 0.626 [95% CI, 0.542–0.710] (Figure S5A). Moreover, both DCA and the calibration curves of the CRLM nomogram demonstrated better performance compared with the TNM classification (Figures S5B, C).
According to the above results, a dynamic web-based probability calculator (Dynamic Nomogram (shinyapps.io)) was constructed at https://fusccliver.shinyapps.io/dynnomapp/ to identify optimal candidates for surgery based on the previous nomogram. The predicted probability of benefit in the surgery can be simply calculated by inputting clinical characteristics and viewing the output of the webserver’s output figures and tables.
According to the Kaplan–Meier analysis and log-rank test, the beneficial and surgery group had a considerably longer survival time than the non-beneficial and surgery group (HR = 0.21, 95% CI, 0.17–0.27, p < 0.001). However, no significant difference was found between the non-beneficial and surgery and non-surgery groups (Figure 6) (HR = 0.89, 95% CI, 0.71–1.13, p = 0.344).
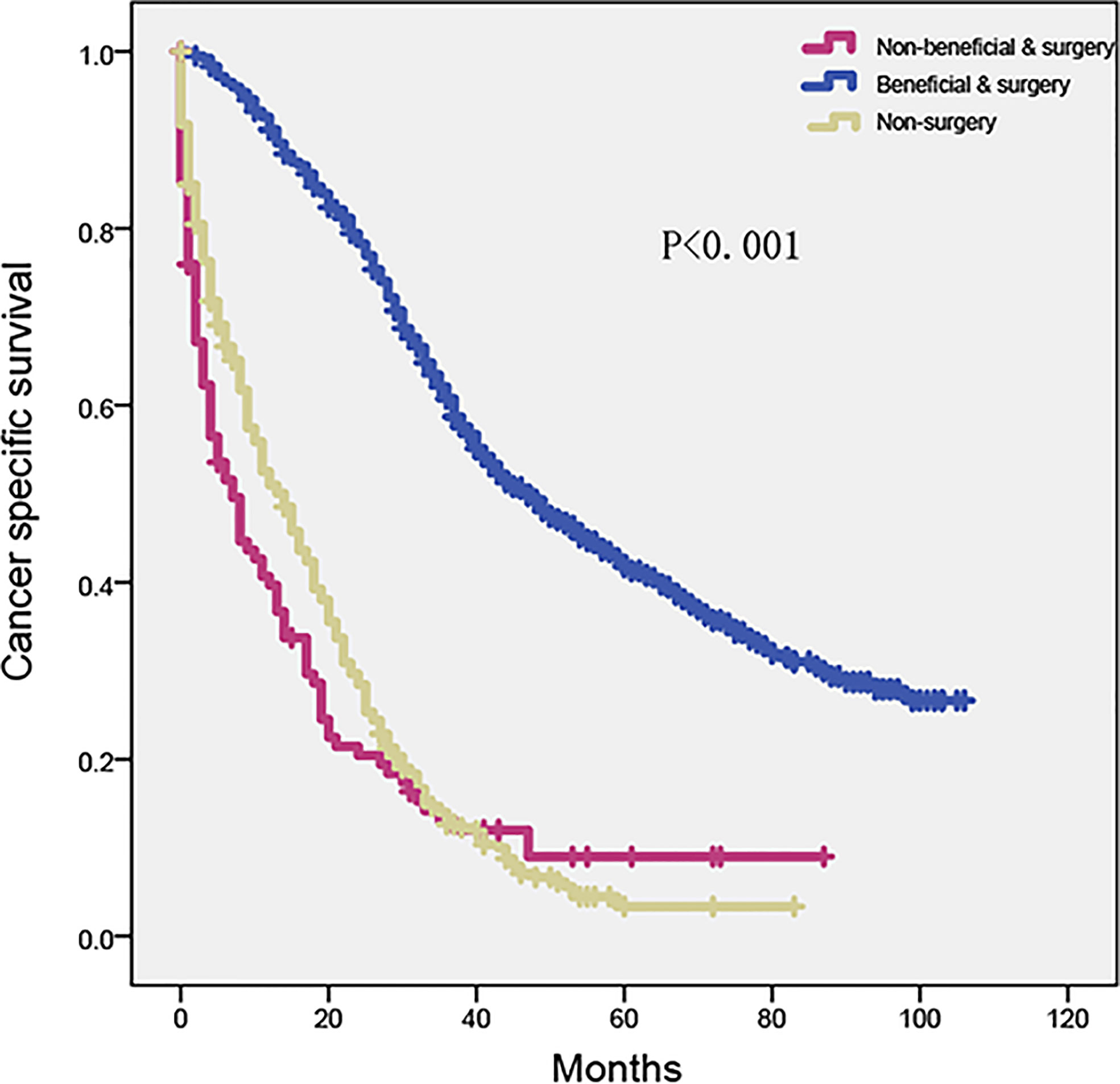
Figure 6 Kaplan–Meier plot to differentiate beneficial group according to CRLM nomogram in SEER cohort. SEER, Surveillance, Epidemiology, and End Results; CRLM, colorectal cancer with liver metastasis.
In the present study, a nomogram including age, T stage, N stage, neoadjuvant chemotherapy, and primary tumor position was constructed and validated to identify optimal candidates for the primary and metastatic resection. In clinical practice, our nomogram can aid clinicians in the process of making decisions as a convenient and accurate predictive model.
Five factors were considered into account in our model in this study and were attached to different risk scores, which could indicate the impact they did on the decision. Present results supported our hypothesis and revealed some significant discoveries. Our nomogram shared several parameters with previous studies on CRLM survival prediction. Some factors marked with a high-risk score in our model, like T stage, age, neoadjuvant chemotherapy, and primary tumor position, were also generally recognized in other studies (12, 15, 16, 19, 20).
For the first time, age was indicated to correlate with the potential of benefit strongly. A growing body of evidence suggests that the elderly have a poorer prognosis, which is consistent with our result (21–23). The elderlies undergoing surgery usually have poor physical and mental health, which has a bad effect on the subsequent adjuvant therapy. What is more, due to neglect of regular physical examination, the tumor is often at an advanced stage when discovered.
In our nomogram, the T1 stage had the least risk scores, indicating that patients with T1-stage tumors are unlikely to benefit from surgery. This distinct phenomenon went against common sense. However, the research of Lupo Wu also noticed this phenomenon and attributed it to the distinct genetic profile of the T1 stage tumors (24). This finding indicated that more attention should be paid to the surveillance and the screening of CRLM with early T stage.
The primary tumor sites served as a high-ranking risk factor, which could affect the potential surgery benefit in our models, and other studies have also confirmed this observation (20, 25, 26). Patients with left-sided tumors often had a better survival outcome than those with right-sided tumors, with a longer CSS of 89 vs. 78 months (p = 0.001) in a SEER cohort (27). Moreover, a national multi-center retrospective study launched by Shida demonstrated that right-sided CRC (RCRC) patients had worse OS than left-sided CRC (LCRC) patients (22). Some studies revealed that histological and molecular characteristics played an important role in this phenomenon (22, 28–30). The gene profile of RCRC and LCRC is completely different. RCRC was mostly diploid with high microsatellite instability, mucinous histology, CpG island methylation, and BRAF mutation, which made RCRC tend to have a more advanced clinical behavior than LCRC. Conversely, LCRC has frequent p53 and KRAS mutations (29, 31). Additionally, it is more difficult to detect RCRC at an early stage because of its flat morphology in the screening of colonoscopy (32, 33). Therefore, the primary lesion of RCRC is often discovered in more advanced stages than that of LCRC.
The tumor size and grade demonstrated a correlation to CSS based on LASSO regression. Nevertheless, the strength of correlation cannot meet the criteria for multiple variable Cox and logistic regression. Therefore, they were excluded from our prediction models. A similar outcome was found in other CRLM studies (12, 15, 16). Conversely, sex was proved that it had a certain effect on the outcome in Kattan’s research (19), and we attributed the cause to the difference between the data from different centers for this conflict.
Additionally, our research demonstrated that carcinoembryonic antigen (CEA) was associated with OS and CSS in LASSO regression. Some studies have indicated that preoperative serum CEA level plays a significant role in the prognosis of CRC patients as an independent risk factor for prognosis (34–37). However, CEA was not statistically meaningful for CSS while performing multi-logistic regression in our study. Hence, we made a new nomogram including CEA (Figure S2), of which AUC [training 0.829 95% CI, 0.790–0.869, validation 0.843 95% CI 0.772–0.913] and calibration plots (Figures S3C, D) show no significant difference between the new nomogram and the former. That is to say, the prediction efficiency can not be greatly improved by taking CEA into account. The ROC of the new nomogram is shown in Figures S3A, B.
During the study, we were puzzled by the differences between surgical and non-surgical patients (Table S1), which are mainly in the field of grade, T stage, and N stage. It was difficult to understand the fact that many patients with grade II, T1, or N0 staging were treated with no surgery, which is contrary to our previous perception. We believe that the reason for these incredible differences is the changes in the treatment modality for CRLM patients. With the development of medical technology, more and more CRLM patients were identified to be able to obtain survival benefits in surgical treatment, and surgery becomes the first choice for patients’ treatment. In our study, some people who lived in the time when the benefit of surgical treatment was not recognized were included. Although their condition was considered to meet the criteria for surgery now, they were not suggested to receive the surgery.
Compared with the nomogram currently published about predicting the prognosis of CRLM patients (5), our model performs better in terms of the accuracy of the nomogram.
What is more, CRLM patients diagnosed between 2004 and 2015 from the SEER database were collected for model construction and internal validation. An independent dataset was obtained from China for external validation. As a well-known database, the SEER database has larger and multi-center data compared with the limited data of our own center, which can improve the model’s predictive performance. However, these are two datasets covering highly different epidemiological, genetic, molecular, and cultural backgrounds, which are not free from potential selection bias.
However, there are still several limitations in the present study. Firstly, some factors reported to be significant for the prognosis of CRLM patients such as the number and size of liver metastasis were not investigated in this study because of the lack of relevant information (12, 15). Despite the lack of that information, our model still demonstrates a better performance for identifying CRLM patients than the TNM stage system, which encouraged us to continue this study. In the future, we are going to collect multi-center data to develop a modified CRLM (m-CRLM) nomogram that takes other significant indicators such as the size and number of liver metastases into account, in order to make our predicting tool more comprehensive and reliable. Secondly, the median CSS time is unable to reflect the overall survival characteristic of the non-surgical group, and some reference standards that could represent the prognostic status of the unoperated patient comprehensively are worthy of further research. Thirdly, due to the relatively limited amount of validation set, the performance of this model is still needed to be confirmed in a larger and prospective cohort.
In summary, we have provided a novel and simple model to identify stage M1a CRLM patients who could indeed benefit from surgery. This predicting model could output individualized results with good accuracy, availability, and applicability. This nomogram might influence the clinician’s decision making in the process of treatment.
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XJ and YW designed the study. XJ, YW, YF, and ZL collected the data and analyzed the data. XJ and YW drafted the manuscript. TZ, BY, and NZ reviewed the manuscript. WZ and LW were responsible for the whole project and supervised the study. All authors contributed to the article and approved the submitted version.
This work was funded by the National Natural Science Foundation of China (81874056, 81874182), Shanghai Natural Science Foundation Project (22ZR1413300), the National Key Project of China (2017ZX10203204-007-004), the Public Health Bureau Foundation of Shanghai (201840019, 201940043), and 2019 clinical science and technology innovation project (SHDC12019X19).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.899659/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier plot of CSS in stage M1a CRLM patients according to primary and metastatic resection. CSS, cancer specific survival; CRLM, colorectal cancer with liver metastasis.
Supplementary Figure 2 | Construction of the new CRLM nomogram with the addition of CEA. CRLM, colorectal cancer with liver metastasis; CEA, carcinoembryonic antigen.
Supplementary Figure 3 | Validation of the new CRLM nomogram. (A, B) ROC curve for discrimination in the training and validation cohorts. (C, D) Calibration plots for the actual (observed) and predicted probabilities of the new CRLM nomograms in the training and validation cohorts.
Supplementary Figure 4 | Decision curve analysis of new CRLM nomogram with the addition of CEA in the training and validation cohorts. Horizontal lines indicates that no cases will experience the event; Gray lines indicates that all cases will experience the event; Red and blue lines represent the net benefits across threshold probabilities according to the CRLM nomogram and TNM classification, respectively. CRLM, colorectal cancer with liver metastasis; TNM, tumor, node, and metastasis; CEA, carcinoembryonic antigen.
Supplementary Figure 5 | External validation of the nomogram. (A) ROC curve for discrimination in the FUSCC cohorts. (B) Calibration plots for the actual (observed) and predicted probabilities of the nomogram in the FUSCC cohorts. (C) Decision curve analysis of CRLM nomogram in the FUSCC cohorts. ROC, receiver operating characteristic curve; FUSCC, Shanghai Cancer Center, Fudan University.
Supplementary Table 1 | Characteristics of M1a CRLM patients.
Supplementary Table 2 | Characteristics of training set, internal and external validation set.
1. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg (2006) 244(2):254–9. doi: 10.1097/01.sla.0000217629.94941.cf
2. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer (2018) 18(1):78. doi: 10.1186/s12885-017-3925-x
3. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol (2016) 27(8):1386–422. doi: 10.1093/annonc/mdw235
4. Lupinacci RM, Coelho FF, Perini MV, Lobo EJ, Ferreira FG, Szutan LA, et al. [Current management of liver metastases from colorectal cancer: recommendations of the são paulo liver club]. Rev Col Bras Cir (2013) 40(3):251–60. doi: 10.1590/S0100-69912013000300016
5. Weiser MR. AJCC 8th edition: Colorectal cancer. Ann Surg Oncol (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
6. Nagtegaal ID, Quirke P, Schmoll HJ. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol (2011) 9(2):119–23. doi: 10.1038/nrclinonc.2011.157
7. Zhuo C, Wu X, Li J, Hu D, Jian J, Chen C, et al. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci Rep (2018) 38(4). doi: 10.1042/BSR20180580
8. Weiser MR, Gönen M, Chou JF, Kattan MW, Schrag D. Predicting survival after curative colectomy for cancer: individualizing colon cancer staging. J Clin Oncol (2011) 29(36):4796–802. doi: 10.1200/JCO.2011.36.5080
9. Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xiao G, et al. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac Oncol (2018) 13(9):1338–48. doi: 10.1016/j.jtho.2018.05.037
10. Hirabayashi S, Kosugi S, Isobe Y, Nashimoto A, Oda I, Hayashi K, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol (2014) 25(6):1179–84. doi: 10.1093/annonc/mdu125
11. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol (2015) 33(8):861–9. doi: 10.1200/JCO.2014.56.6661
12. Liu W, Wang K, Han Y, Liang JY, Li YH, Xing BC. Nomogram predicted disease free survival for colorectal liver metastasis patients with preoperative chemotherapy followed by hepatic resection. Eur J Surg Oncol (2019) 45(11):2070–7. doi: 10.1016/j.ejso.2019.06.033
13. Han L, Dai W, Mo S, Xiang W, Li Q, Xu Y, et al. Nomogram to predict the risk and survival of synchronous bone metastasis in colorectal cancer: a population-based real-world analysis. Int J Colorectal Dis (2020) 35(8):1575–85. doi: 10.1007/s00384-020-03612-z
14. Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a project study for hepatic surgery of the Japanese society of hepato-Biliary-Pancreatic surgery. J Hepatobiliary Pancreat Sci (2012) 19(1):72–84. doi: 10.1007/s00534-011-0460-z
15. Wang Y, Zheng J, Chen H, Hu C, Sun B, Wang H, et al. A prognostic nomogram for colorectal cancer liver metastases after percutaneous thermal ablation. Int J Hyperthermia (2018) 34(6):853–62. doi: 10.1080/02656736.2017.1368095
16. Wu Q, Wang WJ, Huang YQ, Fang SY, Guan YJ. Nomograms for estimating survival in patients with liver-only colorectal metastases: A retrospective study. Int J Surg (2018) 60:1–8. doi: 10.1016/j.ijsu.2018.10.032
17. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, epidemiology, and end results (SEER) database. JAMA Surg (2018) 153(6):588–9. doi: 10.1001/jamasurg.2018.0501
18. Guo X, Liu Y, Liu LJ, Li J, Zhao L, Jin XR, et al. Development and validation of survival nomograms in colorectal cancer patients with synchronous liver metastases underwent simultaneous surgical treatment of primary and metastatic lesions. Am J Cancer Res (2021) 11(6):2654–69.
19. Kattan MW, Gönen M, Jarnagin WR, DeMatteo R, D'Angelica M, Weiser M, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg (2008) 247(2):282–7. doi: 10.1097/SLA.0b013e31815ed67b
20. Luo Z, Fu Z, Li T, Zhang Y, Zhang J, Yang Y, et al. Development and validation of the individualized prognostic nomograms in patients with right- and left-sided colon cancer. Front Oncol (2021) 11:709835. doi: 10.3389/fonc.2021.709835
21. Ngu JC, Kuo LJ, Teo NZ. Minimally invasive surgery in the geriatric patient with colon cancer. J Gastroint Oncol (2020) 11(3):540–4. doi: 10.21037/jgo.2020.02.02
22. Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, et al. Prognostic impact of primary tumor location in stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol (2020) 55(10):958–68. doi: 10.1007/s00535-020-01706-7
23. Kuai L, Zhang Y, Luo Y, Li W, Li XD, Zhang HP, et al. Prognostic nomogram for liver metastatic colon cancer based on histological type, tumor differentiation, and tumor deposit: A TRIPOD compliant Large-scale survival study. Front Oncol (2021) 11:604882. doi: 10.3389/fonc.2021.604882
24. Wu L, Fu J, Chen Y, Wang L, Zheng S. Early T stage is associated with poor prognosis in patients with metastatic liver colorectal cancer. Front Oncol (2020) 10:716. doi: 10.3389/fonc.2020.00716
25. Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F, et al. The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol (2016) 114(7):803–9. doi: 10.1002/jso.24425
26. Elizabeth McCracken EK, Samsa GP, Fisher DA, Farrow NE, Landa K, Shah KN, et al. Prognostic significance of primary tumor sidedness in patients undergoing liver resection for metastatic colorectal cancer. HPB (Oxford) (2019) 21(12):1667–75. doi: 10.1016/j.hpb.2019.03.365
27. Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol (2008) 15(9):2388–94. doi: 10.1245/s10434-008-0015-y
28. Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol Res (2018) 11(4):264–73. doi: 10.14740/gr1062w
29. Imperial R, Ahmed Z, Toor OM, Erdoğan C, Khaliq A, Case P, et al. Comparative proteogenomic analysis of right-sided colon cancer, left-sided colon cancer and rectal cancer reveals distinct mutational profiles. Mol Cancer (2018) 17(1):177. doi: 10.1186/s12943-018-0923-9
30. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol (2017) 3(2):211–9. doi: 10.1001/jamaoncol.2016.4227
31. Mukund K, Syulyukina N, Ramamoorthy S, Subramaniam S. Right and left-sided colon cancers - specificity of molecular mechanisms in tumorigenesis and progression. BMC Cancer (2020) 20(1):317. doi: 10.1186/s12885-020-06784-7
32. Nitsche U, Stögbauer F, Späth C, Haller B, Wilhelm D, Friess H, et al. Right sided colon cancer as a distinct histopathological subtype with reduced prognosis. Dig Surg (2016) 33(2):157–63. doi: 10.1159/000443644
33. Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med (2011) 154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004
34. Stojkovic Lalosevic M, Stankovic S, Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, et al. Can preoperative CEA and CA19-9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hell J Nucl Med (2017) 20(1):41–5. doi: 10.1967/s002449910505
35. Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: Results from the national cancer database. Ann Surg Oncol (2016) 23(5):1554–61. doi: 10.1245/s10434-015-5014-1
36. Kim CG, Ahn JB, Jung M, Beom SH, Heo SJ, Kim JH, et al. Preoperative serum carcinoembryonic antigen level as a prognostic factor for recurrence and survival after curative resection followed by adjuvant chemotherapy in stage III colon cancer. Ann Surg Oncol (2017) 24(1):227–35. doi: 10.1245/s10434-016-5613-5
Keywords: stage M1a colorectal cancer, liver metastases, resection of primary and metastatic lesions, SEER database, nomogram
Citation: Jin X, Wu Y, Feng Y, Lin Z, Zhang N, Yu B, Mao A, Zhang T, Zhu W and Wang L (2022) A population-based predictive model identifying optimal candidates for primary and metastasis resection in patients with colorectal cancer with liver metastatic. Front. Oncol. 12:899659. doi: 10.3389/fonc.2022.899659
Received: 26 March 2022; Accepted: 13 September 2022;
Published: 07 October 2022.
Edited by:
Alessandra Bertacco, Padua University Hospital, ItalyReviewed by:
Matteo Donadon, Università degli Studi del Piemonte Orientale, ItalyCopyright © 2022 Jin, Wu, Feng, Lin, Zhang, Yu, Mao, Zhang, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Wang, d2FuZ2x1emxAZnVkYW4uZWR1LmNu; Weiping Zhu, d3B6aHVzaEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.