- 1Department of Oncology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Medical Center on Aging of Ruijin Hospital (MCARJH), Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Gastroenterology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 4Department of Geriatrics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5State Key Laboratory of Oncogenes and Related Genes, Shanghai Jiao Tong University, Shanghai, China
Aims: Although brain metastasis from gastric adenocarcinoma (GaC) is rare, it may significantly affect survival and quality of life. The aim of this large, comprehensive, population-based cohort investigation was to investigate factors that were associated with brain metastasis from GaC and to explore the prognostic factors and time-dependent cumulative mortalities among cases with GaC and brain involvement.
Methods: Population-based information on cases with GaC diagnosed from 2010 to 2016 was obtained from a large-scale database. Factors that were associated with brain metastasis were investigated utilizing multivariable logistic regression. Time-dependent tumor-specific mortalities of cases with GaC and brain involvement were then computed utilizing the cumulative incidence functions (CIFs), and mortalities were compared between subgroups utilizing Gray’s test. Factors that were associated with death were further evaluated utilizing multivariable Fine–Gray subdistribution hazard regression.
Results: Together, 28,736 eligible cases were included, which comprised 231 (1%) cases with brain metastasis and 10,801 (38%) with metastasis to other sites, encompassing a follow-up of 39,168 person-years. Brain metastasis occurred more often among younger patients (within overall cancers), in cases with stomach cardia tumors, within cases with signet-ring cell carcinoma (within overall cancers), and within cases with positive lymph nodes (within overall tumors); it was less often detected among black people. Brain involvement was associated with more lung and bone metastases. The median survival time of cases having brain metastasis was only 3 months; the 6- and 12-month tumor-specific cumulative mortalities were 57% and 71%, respectively. Among cases with GaC and brain metastasis, those with gastric cardia cancers (when receiving radiotherapy), those undergoing resection, and those receiving chemotherapy had lower mortality risks, while younger patients (when receiving chemotherapy or radiotherapy) and people with positive lymph nodes (when receiving radiotherapy) had higher death hazards.
Conclusion: Among patients with GaC, brain metastasis was correlated with several clinical and pathological variables, including ethnicity, age, cancer histology, location, lymph node involvement, and metastases to other sites. Cases having brain metastasis had poor survival that was correlated with age, cancer location, lymph node metastasis, and management. These findings offer vital clues for individualized patient care and future mechanistic explorations.
Background
Gastric cancer, most of which is gastric adenocarcinoma (GaC), is the fifth most common malignancy and the fourth most frequent cause of cancer-associated death worldwide, with approximately 1,100,000 new incident cases and about 800,000 related mortalities estimated in 2020 (1–4). Metastatic GaCs (mGaCs) with malignant involvement of distant sites take up about 1/3 of all GaCs on diagnosis and are associated with inferior survival because of aggressive progression (1, 5–7).
We previously reported that within cases with mGaC, the proportions of people having brain metastasis (BM) were 2% and 1% in the USA and the Netherlands, respectively, among overall patients with mGaC, and 1% and <1%, respectively, within those with resected mGaC (5). Although BM is rare among mGaCs, it may have been underestimated (8–11). BM from GaC was first reported in 1960 (12) and can occur synchronously with, or early, or even years after, curative resection for early GaCs because of residual micrometastases, and its incidence keeps increasing as disease-associated prognosis improves with the advancement of treatment (13–18). BM represents a severe condition and may cause early onset of neurological symptoms, markedly undermining prognosis, and deteriorating quality of life (QoL) (19–22), and it can even cause the initial symptoms of gastric cancer (23–26). The prognosis of patients with GaC involving the central nervous system remains very poor (27–29), and early identification of BM is vital for guiding clinical treatment and preventing related complications. People with GaC and BM represent a unique and largely heterogeneous population (30–33), and there have been few studies with a sufficient number of cases pertaining to the factors that were associated with BM and its prognostic significance within cases with GaC, which might be because of the underestimation of diagnosis and/or rarity of the disease.
In this comprehensive investigation, a large-scale population-based cohort was analyzed to delineate features of GaC cases having BM, who were compared with cases having distant metastasis that spared the brain and those without distant metastasis, and to reveal factors that were associated with BM in different subgroups of GaC cases. We further computed GaC-specific cumulative mortalities at various follow-up time-points with the cumulative incidence function (CIF) and explored prognostic factors with competing risk (CR) regression among patients with GaC and BM. The findings might help with individualized care for this special patient group.
Methods
Participants
After the corresponding agreement was signed and the data utilization permission was obtained, individual case-level data were obtained from the Surveillance, Epidemiology, and End Results-18 database of the National Cancer Institute, which collects data from several population-based cancer registries and is an authoritative source of data pertaining to the US cancer statistics. The National Cancer Institute staff works together with the North American Association of Central Cancer Registries to guide all of the state registries to provide compatible data allowable for pooling. The database is the only comprehensive source of US population-based cancer data that includes tumor stage on diagnosis and individual survival information (34).
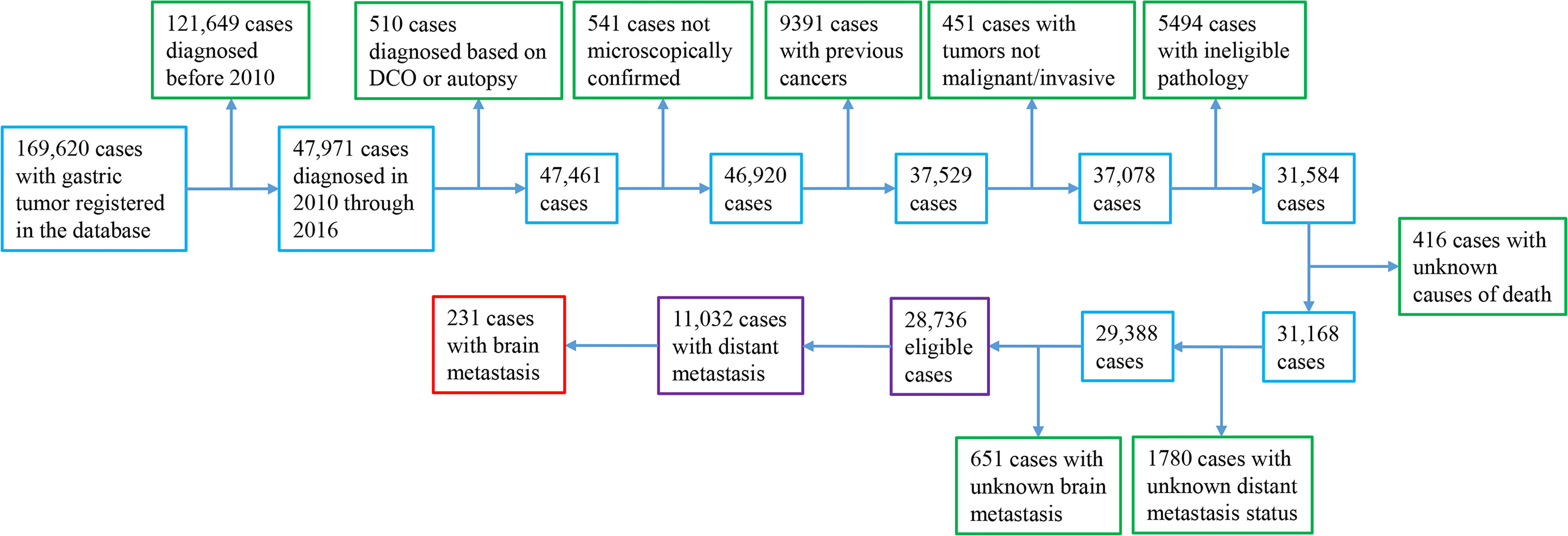
Patients with pathologically verified primary invasive stomach adenocarcinoma diagnosed in 2010–2016 were included (Supplementary Figure S1). We determined the enrollment time period by considering the availability of information on the metastatic site. We included signet-ring cell carcinoma (SRC), which is a special type of adenocarcinoma (3). We excluded patients with prior cancers, with a diagnosis based on autopsy or death certificate only, and/or with ineligible histology (neuroendocrine tumor/carcinoid, gastrointestinal stromal tumor (GIST)/sarcoma, squamous cell cancer, lymphoma, and germ-cell neoplasm; Supplementary Table S1). To enable analyses of CRs, cases with unclear causes of mortality were excluded. We excluded patients with unclear BM or distant involvement status, considering that they did not contribute to addressing the study focus-BM.
The investigated registry routinely registers information pertaining to case demographics (e.g., ethnicity, age, sex, and year of diagnosis), primary cancer location, stage on diagnosis, cancer morphology (from which differentiation and histology could be derived), first-course management (resection, chemotherapy, and radiation therapy), cause of mortality, and survival status and time, which were included for analyses in this investigation. The National Center for Health Statistics provided the survival information. Nonresection treatment information was under-ascertained with low sensitivity (35). We dichotomized the year of diagnosis into two time periods: 2014–2016 and 2010–2013, and categorized age into five subgroups: ≥80, 70–79, 60–69, 50–59, and <50 years. Cancer location contained four categories: gastric antrum/pylorus, fundus/body, cardio, and others.
Analyses
Continuous variables were presented as mean ± standard deviation, median (interquartile range), and categorical data were shown as count [percentage (%)]; the patient, cancer, management, and prognosis features for cases with GaC and BM, cases with distant involvement that spared the brain, and cases without distant involvement were described. We calculated survival time from initial diagnosis until mortality or last follow-up, whichever took place first, and estimated follow-up time utilizing the reverse Kaplan–Meier method (36).
We quantified the correlations of BM versus no brain and/or distant involvement with age, sex, ethnicity, year of diagnosis, cancer location, SRC histology, and liver, lung, and bone involvements with the multivariable logistic regression, mutually adjusting for these factors, among overall and metastatic tumors. For cancer differentiation and adjacent structure and lymph node (LN) involvements with missing data, we assessed the correlations via additionally adding these factors one by one into the abovementioned model.
Mortalities were calculated with the CIFs (37, 38), where, different from the routine Kaplan–Meier method, estimation of an event incidence for cancer-specific mortalities while taking into account CRs from other causes of mortality is allowable. We computed cumulative mortalities overall and categorized them by patient, cancer, and management factors at follow-ups of 6 and 12 months, and assessed differences in mortality between groups with Grays’ test for CIF equality.
Considering that incidence of death was focused on in this study rather than etiology, Fine–Gray subdistribution hazard regression (37, 38) was applied to investigate prognostic factors and to compare the cumulative hazards of tumor-related mortalities across subgroups with different individual prognostic and risk factors within overall GaC cases with BM, and the corresponding hazard ratios (HRSD) and 95% confidence intervals (CIs) were computed; we mutually adjusted the model for ethnicity, age group, sex, period of diagnosis, cancer location, SRC histology, liver, lung, and bone involvement, and surgical resection. For cancer differentiation, and adjacent structure and LN involvements with missing data, we assessed the correlations by additionally adding these factors one by one into the abovementioned model. We did not incorporate nonsurgical variables into the multivariable-adjusted models due to the low sensitivity and under-ascertainment (35) and did stratified analyses for cases undergoing chemotherapy or radiation therapy. Before doing modeling survival analyses, we confirmed the proportional hazard (PH) assumption both analytically utilizing the scaled Schoenfeld residuals test and graphically utilizing the log–log plot (39). The univariable survival curves for both overall cases categorized by the brain and distant involvement and cases having BM categorized by patient and cancer factors were plotted. We did analyses utilizing the R 4.1.2 software (http://cran.r-project.org) and considered the findings to be statistically significant with a two-sided p-value <0.05.
Results
Features of participants
Within 169,620 registered patients with gastric cancer, 11,032 cases with mGaC and clear BM status and 17,704 with nonmetastatic GaC who were diagnosed in 2010 through 2016 were eligible, together encompassing a follow-up of 39,168 person-years; within patients with metastatic disease, 231 (2%) had BM (Figure 1). When compared with cases having metastasis that spared the brain and those with nonmetastatic cancer, patients having BM were slightly more frequently diagnosed in 2014 or later (46% versus 45% and 43%), of younger age (mean age, 61 versus 63 and 68 years), and of male gender (73% versus 64% and 64%) (Table 1). The proportion of cases ≥80 years was smaller (8% versus 14% and 22%) in people with BM, while the proportion of cases <50 years was greater (19% versus 16% and 9%). Cases having BM were more frequently of white ethnicity (82% versus 71% and 69%) and had more frequently gastric cardia tumors (75% versus 52% and 49%) but less frequent gastric antrum/pylorus tumors (8% versus 27% and 34%). Tumors with BM often had more positive LNs (60% versus 57% and 47%). Within metastatic cancers, those with BM also more often had metastases to the lung (33% versus 14%) and bone (32% versus 13%), but less frequently to the liver (36% versus 43%). Resection was less frequently done for cases with BM (7% versus 11% and 64%). The median survival time was 3, 5, and 25 months for cases with BM, cases with nonbrain metastasis, and cases without distant metastasis, respectively, and 13%, 16%, and 48% of cases, respectively, survived in the three groups at the cutoff of follow-up (Supplementary Figure S1).
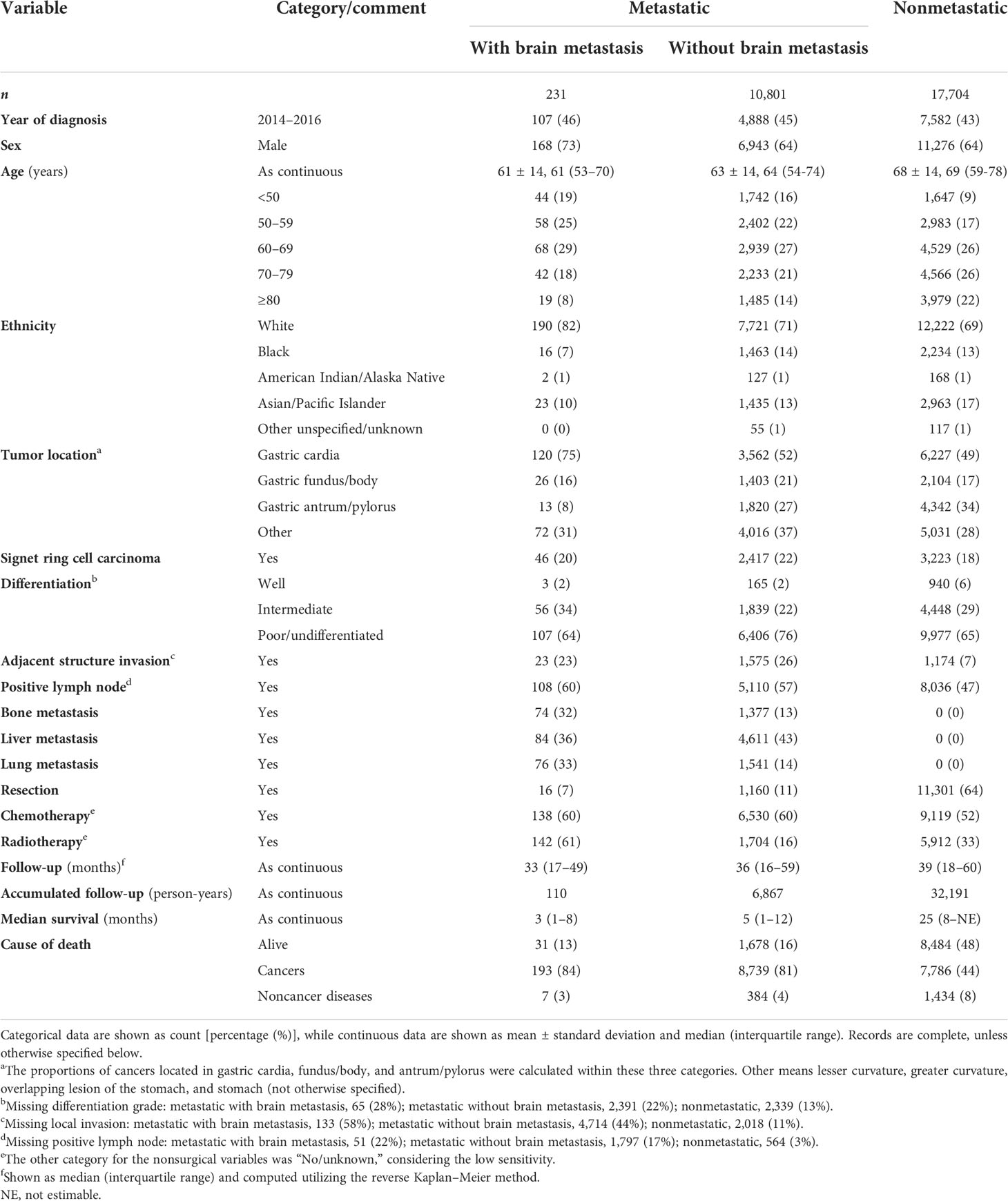
Table 1 Baseline features of cases with metastatic (with and without brain involvement) and nonmetastatic gastric adenocarcinoma, 2010 through 2016.
Brain metastasis-associated factors
Among cases with mGaC (Table 2), BM less often occurred in black people (OR = 0.57) and those with gastric antrum/pylorus tumors (OR = 0.28); it was more often correlated with bone (OR = 2.60) and lung involvements (OR = 2.56), but less frequently liver involvement (OR = 0.64). Within overall cancers, the patterns and strengths of associations were mostly similar with those in metastatic diseases, with some exceptions: The trend of the negative association between age and BM became significant, and octogenarians significantly had often less BM compared to patients aged 60–69 years (versus other nonbrain or no metastasis, OR = 0.55; versus no metastasis, OR = 0.33). The association with black ethnicity became insignificant when versus no metastasis. Neither SRC carcinoma nor LN metastasis was not significantly correlated with BM among metastatic tumors, while they became significantly associated with an increased risk of BM among overall cancers when versus no metastasis (OR = 1.94 and 1.69, respectively). When versus other nonbrain or no metastasis, the strength of the association with BM became stronger (OR = 4.87); BM also turned out to be positively associated with liver metastasis (OR = 1.44).
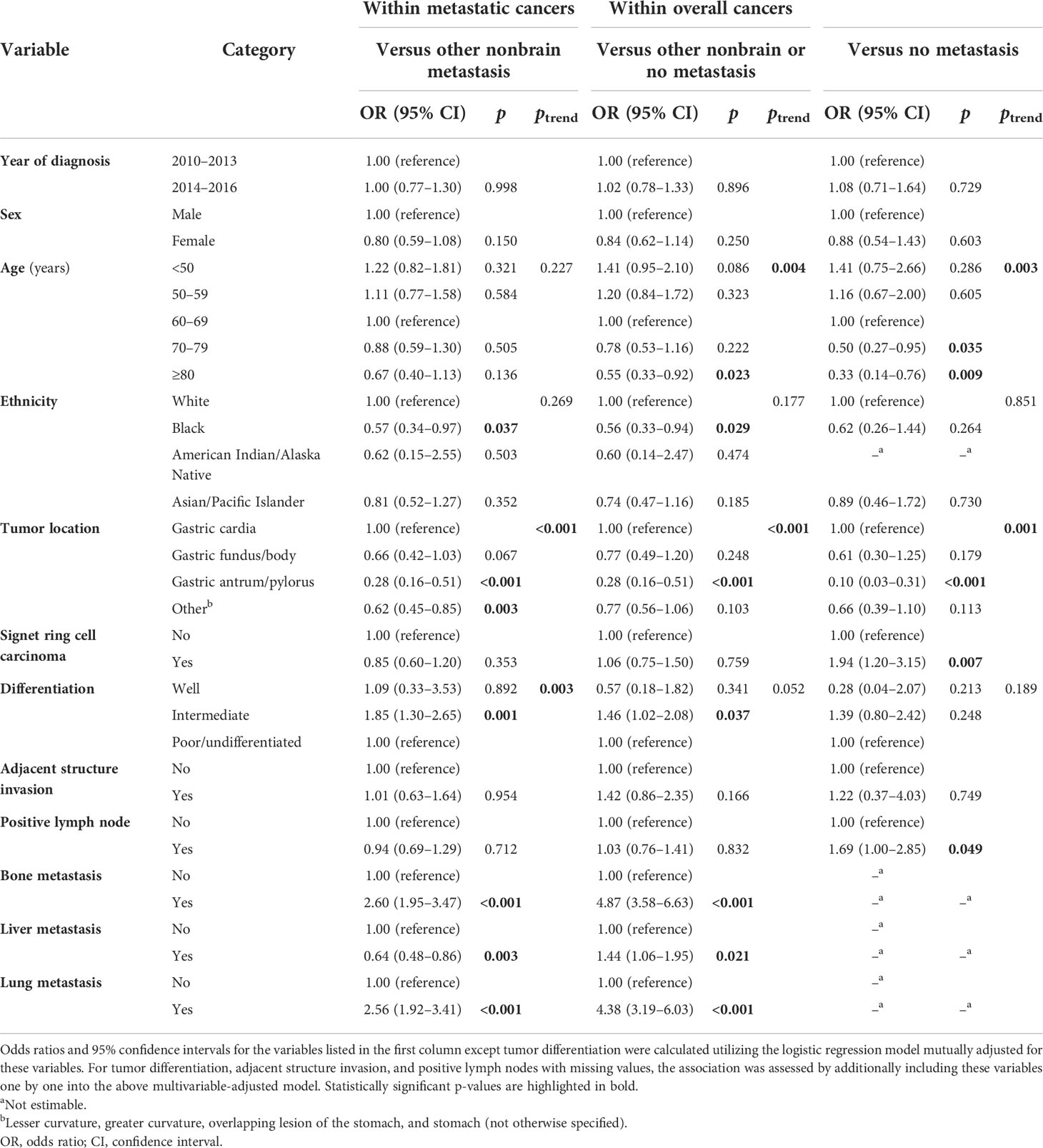
Table 2 Factors associated with brain metastasis versus no brain metastasis (nonbrain or no metastasis) in cases with metastatic or overall gastric adenocarcinoma, 2010–2016.
Overall and stratified survival of cases with GaC and brain involvement
The Kaplan–Meier survival of cases with BM stratified by age group, sex, ethnicity, period of diagnosis, cancer location, and liver, lung, and bone involvement is shown in Figure 2. Utilizing CIFs, overall and categorized tumor-specific mortalities were computed for cases with GaC and BM (Table 3). The speed of increment in death slowed down with a longer follow-up period. Within overall cases, the 6-month death rate already was as high as 57%, and the 12-month death rate was 71%. Death rates categorized by ethnicity, age group, sex, cancer location, SRC histology, differentiation grade, adjacent structure invasion, LN, bone, liver, and lung involvement, resection, or radiotherapy were not significantly different across subgroups, while cases receiving chemotherapy had lower death rates than their counterparts within the first year of follow-up (6 months, 45% versus 76%; 12 months, 65% versus 79%).
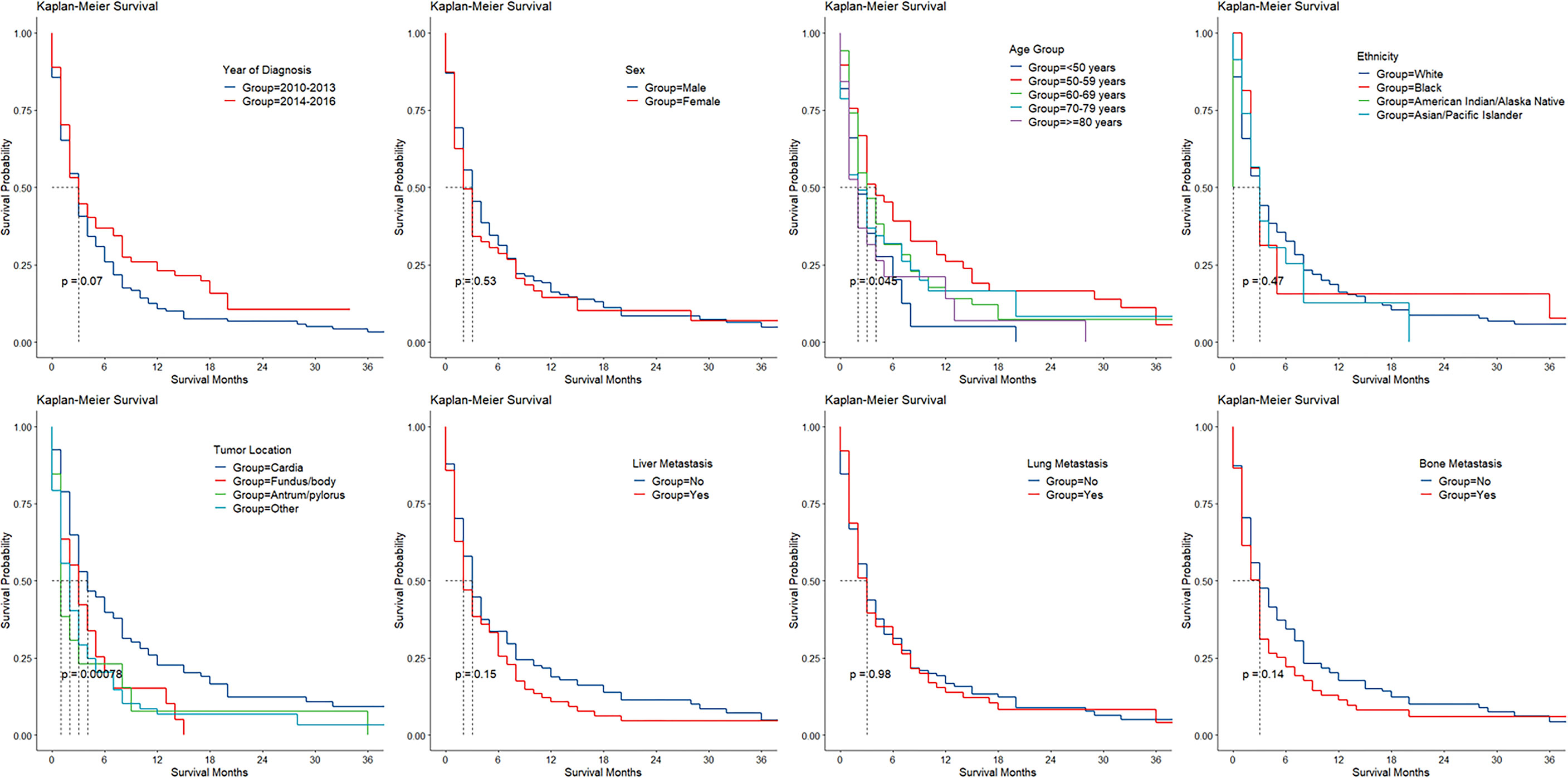
Figure 2 Kaplan–Meier survival by patient and cancer factors in gastric adenocarcinoma with brain involvement.
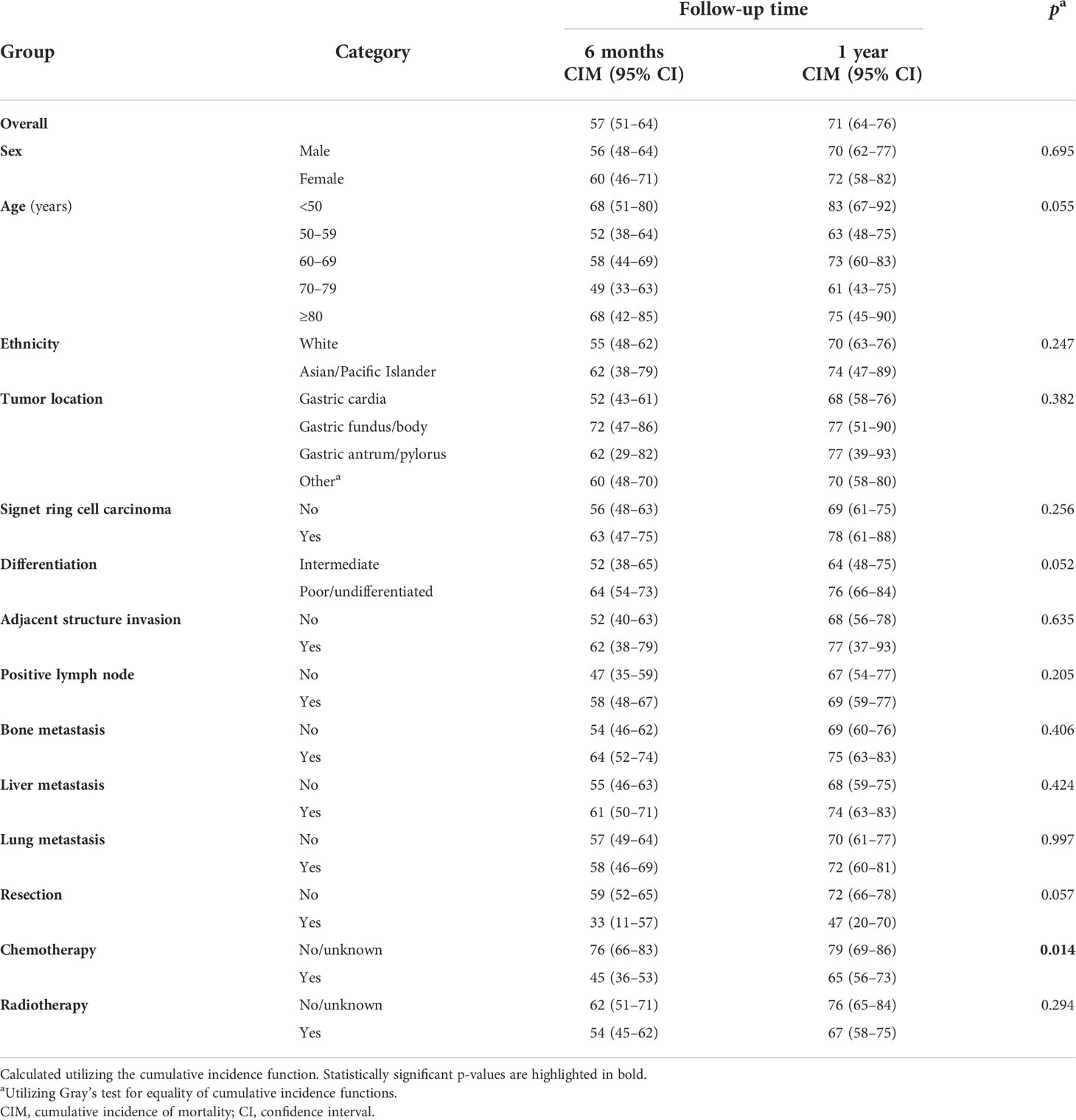
Table 3 Cumulative incidences of cancer-specific mortality (%) at various follow-up points in cases with gastric adenocarcinoma and brain metastasis, overall and categorized.
Prognostic factors in GaC cases with brain involvement
Utilizing the Fine–Gray subdistribution hazard function models, factors that were associated with tumor-specific death in GaC patients having BM are listed in Table 4. In overall patients, those who underwent resection had a 52% unit reduction in death risk. In subgroups of patients receiving chemotherapy, septuagenarians had a significantly lower mortality risk compared to patients aged 60–69 years (HRSD = 0.39). Among patients receiving radiotherapy, those <50 years had a significantly higher mortality risk compared to those aged 60–69 years (HRSD = 2.03), and individuals with gastric antrum/pylorus tumors had significantly a higher death hazard compared with those with gastric cardia tumors (HRSD = 4.63). LN involvement was associated with a 0.77-unit increase in death risk.
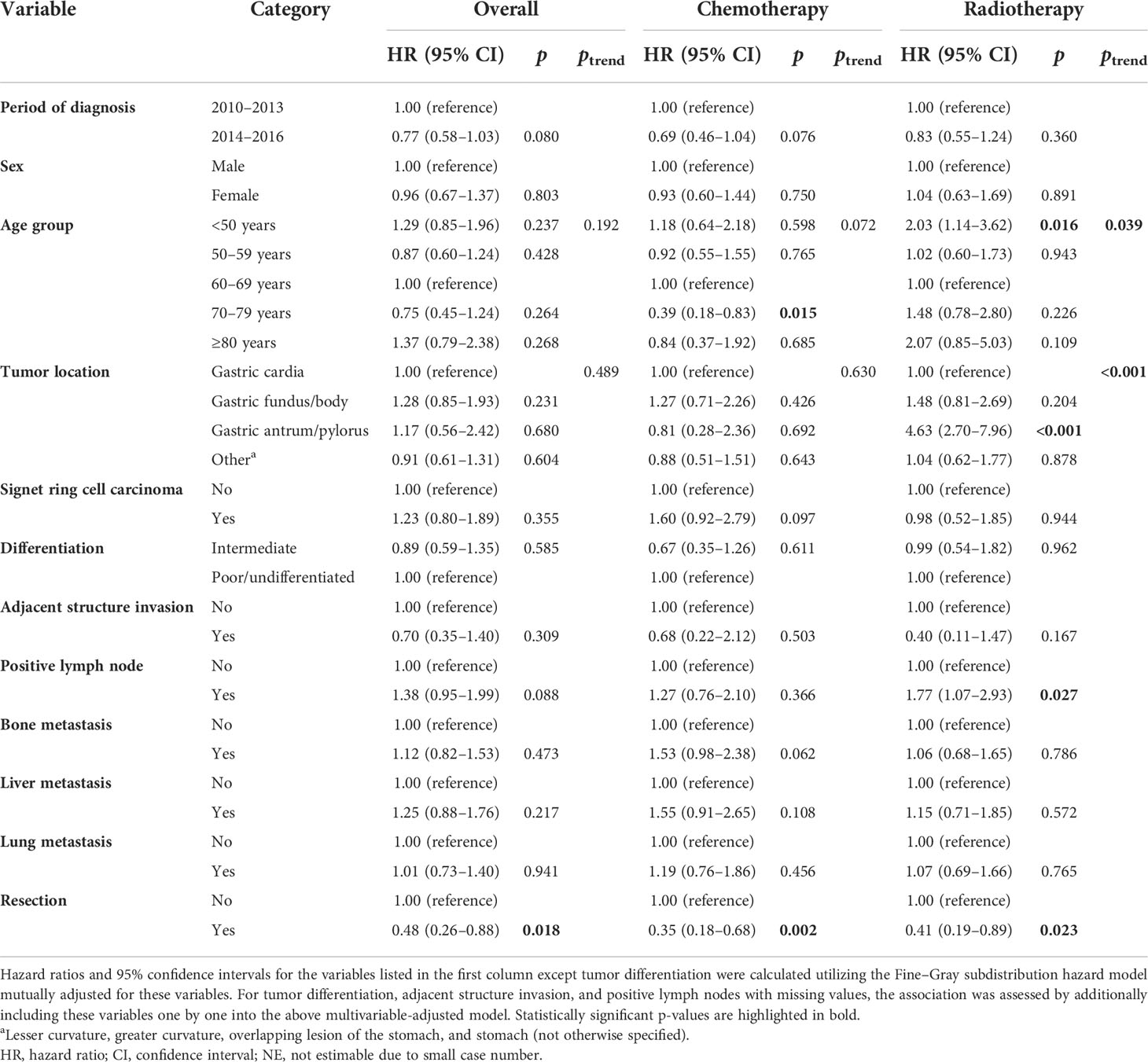
Table 4 Fine–Gray subdistribution hazard ratios for cancer-specific mortality among cases with gastric adenocarcinoma and brain metastasis in overall patients and those treated with chemotherapy or radiotherapy.
Discussion
In our large population-based cohort investigation, 231 patients with BM were identified within about 30,000 cases with GaC encompassing a follow-up of about 40,000 person-years, and their characteristics were described comprehensively, with comparison to those with distant involvement sparing brain or without distant metastasis. Unique risk characteristics specific to BM were identified and further verified with multivariable-adjusted analyses. Taking into account CRs, the overall and categorized tumor-specific death rates of cases with GaC and BM were further shown utilizing CIFs, representing a more trustable prognosis estimation. We further supported comparisons of cumulative death rates by utilizing multivariable-adjusted CR analyses. The findings provide vital hints for screening and treatment of BM from GaCs.
Patients having BM comprised 1% and 2% of cases with overall and metastatic GaC, respectively. While much rarer than metastases to other sites like the liver and lung and from other malignancies like melanoma, lung, and breast cancers, BM from GaC is quickly progressing and could markedly deteriorate survival and undermine QoL (40–42). The overall cumulative death rate increased quickly to 57% at 6 months and 71% at 12 months, with a median overall survival of merely 3 months. With the advancements in techniques of diagnosis and treatment, BM was more often detected and had an enhanced prognosis in more recent years. Long-term survival beyond 5 years may be possible in a small minority of selected brain-metastatic GI cancer patients with specific characteristics, including only one brain metastasis without metastasis to extracranial sides, age <60 years, Karnofsky performance status score of 100, surgical resection, brain metastasis-directed treatment, and additional systemic therapy (43).
There are some methods to identify BM from GaC. BM arising from gastric cancer is frequently revealed by hypointensity on T2-weighted MRI, which may be due to the accumulation of collagen in the tissues (44). FDG-PET can provide additional information on BM (45). Measurements of pre- and postoperative brain blood flow may be helpful in the case of chronic subdural hematoma after dural metastasis from gastric cancer (46). Cerebrospinal fluid cytology may also be used to diagnose leptomeningeal metastasis from HER2+ gastric cancer (47). The peroxidase-antiperoxidase method for carcinoembryonic antigen (CEA) may increase the diagnostic accuracy of cytology in the cerebrospinal fluid for BM from GaC (48). Despite these methods, it remains challenging to detect BM from GaC early, which could often be underestimated and/or misdiagnosed. There may be a great intrapatient heterogeneity in circulating cancer cells at the single-cell level in the cerebrospinal fluid of patients with gastric cancer and BM (49). The expressions of ZEB2 and miRNA-200 family members are correlated with BM from GaC (50). Nonetheless, the driving force behind BM remains largely unclear. It would be important to investigate risk factors associated with BM to facilitate early and efficient detection of this clinically significant but rare condition (51).
We found that BM from GaC occurred more often with younger age, nonblack ethnicities, gastric cardiac location, SRC histology, and LN involvement. Younger patients, cases having antrum/pylorus tumors, and cases having LN involvement had increased death hazards, while surgical resection was associated with a lower mortality hazard. Of note, cases younger than 50 years comprised 19% of all cases with GaC and BM compared with 8% for cases who were ≥80 years. Older cases had generally slower tumor progression, and the preference of BM for younger cases with longer anticipated survival is worthy of doctors’ attention. Cardiac tumors, which are different from noncardiac cancers in various aspects, are correlated with poorer prognosis (18), and we found them to be more frequently correlated with BM, which was supported by an institution-based study (52). Interestingly, they were correlated with higher survival rates among cases with GaC and BM who received radiotherapy. SRC, which is a special histology type, might have higher biologic aggressiveness and lower sensitivity to systemic therapy, making immediate upfront resectional surgery of critical value. While positive LN was more often correlated with BM, 40% of patients with GaC and BM had uninvolved LNs. LN involvement rather than adjacent structure involvement was associated with prognosis among patients with GaC and BM receiving radiotherapy. GaCs with multiple metastasis sites including the brain are noteworthy clinically, while bone, liver, or lung involvement was not correlated significantly with worse survival among patients with GaC and BM in this investigation.
At present, there exist no screening or treatment guidelines for GaCs with BM (8), and managing BM from GaC can be challenging. Multidisciplinary treatment may enhance long-term survival and QoL (53–55). We found that 7% of GaC cases with BM underwent resection, which may increase the overall survival time for this specific patient population (41, 56, 57). Of note, although surgical resection was correlated with better prognosis in GaC cases with BM, causality and definitive management benefits cannot be derived from this observational investigation. Tailored and timely management is vital, and notably, nowadays, radiotherapy and immune checkpoint inhibitor therapy may be particularly effective for cases with GaC affecting the brain (58). We found that within GaC patients with BM receiving radiotherapy, younger age, antrum/pylorus location, and LN metastasis were correlated with worse survival, which should call for clinicians’ attention.
Gamma-knife stereotactic radiosurgery seems a safe and effective treatment modality for the treatment of BM from GaC with good local control and few side effects, and dose-escalated approaches may improve local control rates (59–61). Radiosurgery appears a nice alternative to surgical resection for cases with BM from advanced gastric cancer (62); gamma-knife radiosurgery and recursive partitioning analysis class 2 may contribute to more favorable clinical outcomes (63). Resection and stereotactic radiosurgery for BM contributed to long-term survival in two Japanese people with BM identified more than 1 year after the removal of primary GaC, who remained alive after 6.5 years and died of non-GaC causes 4 years after surgery, respectively (64). Stereotactic radiotherapy is also associated with better outcomes and longer overall survival after brain relapse (65). Cumulative intracranial tumor volume is a vital prognostic factor in cases with stereotactic radiosurgery-managed gastrointestinal tract cancer BM (66). A case report described a patient with advanced gastric cancer and multiple brain and abdominal LN involvements who was successfully treated through an abscopal effect, which refers to the phenomenon where local radiation therapy is correlated with the regression of metastatic tumor, which is located distantly from the irradiated site, through brain radiation and anti-PD-1 therapies (67). Combined therapy with nivolumab and gamma-knife radiosurgery appeared effective for treating another 55-year-old male with gastric cancer and BM (68). A 68-year-old man with gastric cancer and BM was also reported to be successfully treated with nivolumab (69). Another 63-year-old man with gastroesophageal junction cancer and brain, bone, and gastric intramural metastases was reported to be responsive to combined modality therapy (70). Notably, acquired mutation of CTNNB1 may drive immune checkpoint inhibitor (ICI)-acquired resistance in microsatellite instability (MSI)-high esophagogastric adenocarcinoma with BM (71).
Agents targeting HER2, pSTAT3, EGFR, and angiogenesis-associated molecules may be feasible for the treatment of BM from gastric cancer (72–78). The combination of apatinib and continual nutritional support may be another option for the treatment of BM from gastric cancer, which may prolong survival (27). HER2+ patients with gastroesophageal adenocarcinoma may have an increased risk to develop brain metastasis (15), and patients with HER2+ gastric cancer may have a higher susceptibility to disease recurrence in the central nervous system (79). The proportion of HER2+ status in patients with gastroesophageal adenocarcinoma and brain involvement appeared higher than expected (80). In cases with gastrointestinal cancer and brain metastases, HER2+ status was more frequent intracranially compared to prior disease sites, suggesting that examining HER2 in brain metastases of those patients might offer additional treatment options, irrespective of HER2 status in previously examined tissues (81). Trastuzumab deruxtecan showed efficacy for the management of a 65-year-old man with BM originating from advanced HER2-positive gastric cancer (82). It would be desirable to investigate if other HER2-targeting antibody-drug conjugates (ADCs; e.g., GQ1001 and T-DM1 (7)) could show similar efficacies against BM from GaCs. BM from advanced gastric cancer was correlated with VEGF expression, and metformin therapy might be helpful for modulating the metastasis capacity via reducing the expression of VEGF and blocking epithelial-to-mesenchymal transition (EMT) (83). Arterial infusion chemotherapy with tegafur, epirubicin, and lobaplatin appeared effective for the management of a 73-year-old man with advanced gastric cancer affecting the brain (84). Irinotecan plus cisplatin and irradiation appeared effective for the management of another two Japanese cases with BM from gastric cancer (85). Paclitaxel may also be effective for GaC with BM (86, 87). Accordingly, we found that cases receiving chemotherapy had lower death rates.
The treatment scheme for this specific patient subpopulation should be individualized and based on expected survival, performance status, symptoms, the number, location, and size of metastases, the status of the primary tumor, and dissemination to other organs (61, 88–91). QoL should also be factored into management decisions, especially in the setting of aggressive treatment (17). Searching for ideal treatment pathways for this specific patient subgroup would be greatly desired.
This observational investigation shared some common limitations with other registry-based population-based studies. Information on some other variables that were potentially correlated with BM (e.g., genetic and environmental risk factors) and prognostic variables [e.g., health conditions and comorbidities (92–94)] were not available. HER2 status was not available in the analyzed database. Nonetheless, the majority of the common prognostic and risk factors had been included in multivariable-adjusted models. A few variables (e.g., cancer differentiation grade, local invasion, and LN metastasis) had missing data; they were not initially included in the main multivariable-adjusted analyses but were separately added into the main multivariable-adjusted models to assess the associations with them. Nonsurgical therapies were under-ascertained and registered with low sensitivity but high specificity (35), and detailed data on management [e.g., agents and courses (95)] were not available. Accordingly, they were not included in the main multivariable-adjusted survival analyses, and subgroup analyses for cases having chemotherapy or radiation therapy were specifically done. Furthermore, the findings were based on the US cases and might not be well generalizable to patients from other countries, particularly Asians, in which gastric cancer would be much more prevalent. We would strongly encourage analyses of databases from other nations.
To our knowledge, this report appears to be the largest real-world population-based investigation utilizing robust statistics and patient-level data to address BM in GaC patients. The strict and careful case selection, utilization of comprehensive multivariable-adjusted CR analysis methods, and meticulous stratified investigations enable this study to offer useful, valid, and robust references for personalized management of cases with GaC and BM.
Conclusions
Among patients with GaC, brain metastasis was correlated with several clinical and pathological factors, which included ethnicity, age, cancer histology, location, LN involvement, and metastases to other sites. Cases with brain involvement had poor survival, which was correlated with age, cancer location, LN metastasis, and management. The findings offer clinically relevant and helpful clues and evidence for individualized treatment of cases with GaC and brain metastasis and future mechanistic explorations.
Data availability statement
Publicly available datasets were analyzed in this study after signing the appropriate agreement and obtaining the data use approval. This data can be found here: Surveillance, Epidemiology, and End Results Program Research Data (www.seer.cancer.gov).
Ethics statement
Ethical review and approval was not required for this study using the Surveillance, Epidemiology, and End Results Program database after signing the appropriate agreement and obtaining the data use approval in accordance with the local legislation and institutional requirements. New written informed consent was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception or design: LH, WH, and JZ. Acquisition, analysis, or interpretation of data: LH, LW, YS, YZ, CX, WH, and JZ. Drafting of the manuscript: LH. Critical revision of the manuscript for important intellectual content: LW, YS, YZ, CX, WH, and JZ. Statistical analysis: LH. Administrative, technical, or material support: WH and JZ. All authors have given final approval of the manuscript for submission and publication.
Funding
This study was supported by the Fund for Medical Center on Aging (GB202103), and the Start-up Fund for the Introduction of High-Level Talents by Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The funders had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Acknowledgments
We are very grateful to the staff of the Surveillance, Epidemiology, and End Results Program for their great work in data collection and delivery and to the members of the Chinese Anti-Cancer Association (CACA), Chinese Society of Clinical Oncology (CSCO), and Chinese Medical Association for all their great support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.897681/full#supplementary-material
References
1. Huang L, Jansen L, Balavarca Y, Verhoeven RHA, Ruurda JP, Van Eycken L, et al. Decreasing resection rates for nonmetastatic gastric cancer in Europe and the united states. Clin Trans Med (2020) 10(6):e203. doi: 10.1002/ctm2.203
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Huang L, Zhang X, Wei Z, Xu A. Importance of examined lymph node number in accurate staging and enhanced survival in resected gastric adenocarcinoma-the more, the better? a cohort study of 8,696 cases from the US and China, 2010-2016. Front Oncol (2020) 10:539030. doi: 10.3389/fonc.2020.539030
4. Huang L, Jansen L, Verhoeven RHA, Ruurda JP, Van Eycken L, De Schutter H, et al. Survival trends of patients with non-metastatic gastric adenocarcinoma in the US and European countries: the impact of decreasing resection rates. Cancer Commun (London England) (2022) 42(7):648–62. doi: 10.1002/cac2.12318
5. Huang L, Jansen L, Verhoeven RHA, Ruurda JP, Van Eycken L, De Schutter H, et al. Largely varying patterns and trends of primary cancer-directed resection for gastric carcinoma with synchronous distant metastasis in Europe and the US: a population-based study calling for further standardization of care. Ther Adv Med Oncol (2021) 13:17588359211027837. doi: 10.1177/17588359211027837
6. Wei Z, Chen L, Meng L, Han W, Huang L, Xu A. LncRNA HOTAIR promotes the growth and metastasis of gastric cancer by sponging miR-1277-5p and upregulating COL5A1. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2020) 23(6):1018–32. doi: 10.1007/s10120-020-01091-3
7. Huang L, Shi Y. Editorial: The use of chemotherapy in treating gastric cancers. Front Oncol (2022) 12:974023. doi: 10.3389/fonc.2022.974023
8. Brunner M, Soll D, Adler K, Sasse A, König U, Mekolli A, et al. Brain metastases in gastroesophageal cancers-an underestimated complication. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2022) 25(1):161–9. doi: 10.1007/s10120-021-01219-z
9. Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Trans Res (2015) 7(11):2141–58.
10. Huang L, Xu A, Li T, Han W, Wu S, Wang Y. Detection of perioperative cancer antigen 72-4 in gastric juice pre- and post-distal gastrectomy and its significances. Med Oncol (Northwood London England) (2013) 30(3):651. doi: 10.1007/s12032-013-0651-3
11. Huang L, Liu H, Yu J, Lin T, Hu YF, Li TJ, et al. Long-term outcomes in laparoscopic D2 gastrectomy for gastric cancer: a Large comprehensive study proposing novel hypotheses. J Gastrointest Surg Off J Soc Surg Alimentary Tract (2019) 23(7):1349–61. doi: 10.1007/s11605-018-4008-2
12. Yoshida K, Matsuoka T. A case of cancer of the stomach with peculiar metastasis to the brain. Folia Psychiatr Neurol Japonica (1960) 14:81–94. doi: 10.1111/j.1440-1819.1960.tb02230.x
13. Ohki A, Koba T, Tsurumi M, Hashimoto Y, Nagao G, Takeuchi H, et al. Early-stage gastric cancer with solitary brain metastasis four years after curative surgery: a case report and literature review. Clin J Gastroenterol (2022) 15(1):90–4. doi: 10.1007/s12328-021-01564-7
14. Luo H, Peng L, Wang N, Zhang J, Zheng X, Sun Y, et al. Early brain metastasis of advanced gastric cancer with a pathological complete response to neoadjuvant chemotherapy followed by surgery: A case report and literature review. Cancer Biol Ther (2018) 19(10):875–8. doi: 10.1080/15384047.2018.1456600
15. Limon D, Gal O, Gordon N, Katz L, Perl G, Purim O, et al. Brain metastasis in gastroesophageal adenocarcinoma and HER2 status. J Neuro-Oncol (2018) 138(2):315–20. doi: 10.1007/s11060-018-2798-4
16. Philip AZ, Namakydoust A, Varilla VM, Macatangay C, Dowsett R, Tannenbaum SH. Late recurrence of gastric cancer with isolated brain metastasis. Trans Gastroenterol Hepatol (2016) 1:61. doi: 10.21037/tgh.2016.07.02
17. Sakurai K, Muguruma K, Murata A, Toyokawa T, Amano R, Kubo N, et al. Early gastric cancer with suspected brain metastasis arising eight years after curative resection: a case report. BMC Res Notes (2014) 7:818. doi: 10.1186/1756-0500-7-818
18. Huang L, Xu AM. Adenocarcinoma of esophagogastric junction: controversial classification, surgical management, and clinicopathology. Chin J Cancer Res = Chung-kuo Yen Cheng Yen Chiu (2014) 26(3):226–30. doi: 10.3978/j.issn.1000-9604.2014.06.14
19. Esmaeilzadeh M, Majlesara A, Faridar A, Hafezi M, Hong B, Esmaeilnia-Shirvani H, et al. Brain metastasis from gastrointestinal cancers: a systematic review. Int J Clin Pract (2014) 68(7):890–9. doi: 10.1111/ijcp.12395
20. Chen ZM, Huang L, Li MM, Meng L, Ying SC, Xu AM. Inhibitory effects of isocryptotanshinone on gastric cancer. Sci Rep (2018) 8(1):9307. doi: 10.1038/s41598-018-27638-0
21. Huang L, Li TJ. Laparoscopic surgery for gastric cancer: where are we now and where are we going? Expert Rev Anticancer Ther (2018) 18(11):1145–57. doi: 10.1080/14737140.2018.1520098
22. Huang L, Chen L, Gui ZX, Liu S, Wei ZJ, Xu AM. Preventable lifestyle and eating habits associated with gastric adenocarcinoma: A case-control study. J Cancer (2020) 11(5):1231–9. doi: 10.7150/jca.39023
23. Murawa D, Nowaczyk P, Szymkowiak M, Karaszewska B. Brain metastasis as the first symptom of gastric cancer–case report and literature review. Polski Przeglad Chir (2013) 85(7):401–6. doi: 10.2478/pjs-2013-0061
24. Joo HY, Chae MH, Lim JH, Yi HG, Lee MH, Kim CS, et al. A case of gastric cancer manifesting as a solitary brain metastasis in the cerebellopontine angle that mimicked acoustic neuroma. Chonnam Med J (2013) 49(3):133–5. doi: 10.4068/cmj.2013.49.3.133
25. Nomura T, Yoshikawa T, Kato H, Nikkuni K, Sasaki K, Shirai Y, et al. Early gastric cancer manifested as brain metastasis: report of a case. Surg Today (1997) 27(4):334–6. doi: 10.1007/bf00941808
26. Huang L, Xu AM, Li TJ, Han WX, Xu J. Should peri-gastrectomy gastric acidity be our focus among gastric cancer patients? World J Gastroenterol (2014) 20(22):6981–8. doi: 10.3748/wjg.v20.i22.6981
27. Yang Y, Pei X, Yang M. Combination of apatinib and continuous nutritional support for a gastric cancer patient with brain metastasis prolongs survival. J Clin Pharm Ther (2018) 43(5):726–9. doi: 10.1111/jcpt.12708
28. Huang L, Wei ZJ, Li TJ, Jiang YM, Xu AM. A prospective appraisal of preoperative body mass index in D2-resected patients with non-metastatic gastric carcinoma and siewert type II/III adenocarcinoma of esophagogastric junction: results from a large-scale cohort. Oncotarget (2017) 8(40):68165–79. doi: 10.18632/oncotarget.19251
29. Zhang JW, Huang L, Xu AM. Preoperative monocyte-lymphocyte and neutrophil-lymphocyte but not platelet-lymphocyte ratios are predictive of clinical outcomes in resected patients with non-metastatic siewert type II/III adenocarcinoma of esophagogastric junction: a prospective cohort study (the AMONP corhort). Oncotarget (2017) 8(34):57516–27. doi: 10.18632/oncotarget.15497
30. Sperduto PW, Fang P, Li J, Breen W, Brown PD, Cagney D, et al. Estimating survival in patients with gastrointestinal cancers and brain metastases: An update of the graded prognostic assessment for gastrointestinal cancers (GI-GPA). Clin Trans Radiat Oncol (2019) 18:39–45. doi: 10.1016/j.ctro.2019.06.007
31. Sperduto PW, Fang P, Li J, Breen W, Brown PD, Cagney D, et al. Survival and prognostic factors in patients with gastrointestinal cancers and brain metastases: have we made progress? Trans Res J Lab Clin Med (2019) 208:63–72. doi: 10.1016/j.trsl.2019.02.011
32. Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res (2014) 4(2):189–95.
33. Huang L, Qi DJ, He W, Xu AM. Omeprazole promotes carcinogenesis of fore-stomach in mice with co-stimulation of nitrosamine. Oncotarget (2017) 8(41):70332–44. doi: 10.18632/oncotarget.19696
34. SEER. Surveillance. Epidemiology, and End Results (SEER) Program Research Data. National Cancer Institute, DCCPS, Surveillance Research Program. Available at: www.seer.cancer.gov.
35. Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER treatment data with Medicare claims. Med Care (2016) 54(9):e55–64. doi: 10.1097/MLR.0000000000000073
36. Huang L, Jansen L, Balavarca Y, van der Geest L, Lemmens V, Groot Koerkamp B, et al. Significance of examined lymph node number in accurate staging and long-term survival in resected stage I-II pancreatic cancer-more is better? a Large international population-based cohort study. Ann Surg (2021) 274(6):e554–63. doi: 10.1097/sla.0000000000003558
37. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation (2016) 133(6):601–9. doi: 10.1161/CIRCULATIONAHA.115.017719
38. Huang L, Shi Y, Zhao YJ, Wang L, Hu WG, Zhu ZG, et al. Long-term cardiac disease- and cancer-associated mortalities in patients with non-metastatic stomach adenocarcinoma receiving resection and chemotherapy: A Large competing-risk population-based cohort study. World J Oncol (2022) 13(2):69–83. doi: 10.14740/wjon1445
39. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Stat Med (1995) 14(15):1707–23. doi: 10.1002/sim.4780141510
40. Shoji Y, Furuhashi S, Kelly DF, Bilchik AJ, Hoon DSB, Bustos MA. Current status of gastrointestinal tract cancer brain metastasis and the use of blood-based cancer biomarker biopsy. Clin Exp Metastasis (2022) 39(1):61–9. doi: 10.1007/s10585-021-10094-y
41. Lemke J, Scheele J, Kapapa T, von Karstedt S, Wirtz CR, Henne-Bruns D, et al. Brain metastases in gastrointestinal cancers: is there a role for surgery? Int J Mol Sci (2014) 15(9):16816–30. doi: 10.3390/ijms150916816
42. Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer (2011) 117(16):3630–40. doi: 10.1002/cncr.25940
43. Nieder C, Hintz M, Popp I, Bilger A, Grosu AL. Validation of the graded prognostic assessment for gastrointestinal cancers with brain metastases (GI-GPA). Radiat Oncol (London England) (2020) 15(1):35. doi: 10.1186/s13014-020-1484-9
44. Hirano H, Yokoyama S, Yunoue S, Yonezawa H, Yatsushiro K, Yoshioka T, et al. MRI T2 hypointensity of metastatic brain tumors from gastric and colonic cancers. Int J Clin Oncol (2014) 19(4):643–8. doi: 10.1007/s10147-013-0596-8
45. Mizumatsu S, Nishimura T, Sakai K, Goto M, Sugatani H, Higashi T. [A case of brain metastasis from gastric cancer involving bilateral middle cerebellar peduncles]. No Shinkei Geka Neurol Surg (2006) 34(9):955–60.
46. Fukino K, Terao T, Kojima T, Adachi K, Teramoto A. Chronic subdural hematoma following dural metastasis of gastric cancer: measurement of pre- and postoperative cerebral blood flow with N-isopropyl-p-[123I]iodoamphetamine–case report. Neurol Medico-Chir (2004) 44(12):646–9. doi: 10.2176/nmc.44.646
47. Cavanna L, Rocchi A, Gorgni S, Ambroggi M, Foroni RP, Ubbiali A, et al. Cerebrospinal fluid cytology diagnosis of HER2-positive leptomeningeal carcinomatosis from HER2-positive metastatic gastric cancer: case report. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29(13):e367–8. doi: 10.1200/jco.2010.33.2577
48. Iwa N, Yutani C, Kobayashi TK. Immunocytochemical localization of carcinoembryonic antigen in cerebrospinal fluid with metastatic carcinoma of the stomach: report of four cases. Diagn Cytopathol (1988) 4(4):312–5. doi: 10.1002/dc.2840040408
49. Cho JH, Sim MH, Kim SY, Kim K, Lee T, Lee J, et al. Analysis of intrapatient heterogeneity of circulating tumor cells at the single-cell level in the cerebrospinal fluid of a patient with metastatic gastric cancer. J Cancer Res Ther (2021) 17(4):1047–51. doi: 10.4103/jcrt.JCRT_108_19
50. Minn YK, Lee DH, Hyung WJ, Kim JE, Choi J, Yang SH, et al. MicroRNA-200 family members and ZEB2 are associated with brain metastasis in gastric adenocarcinoma. Int J Oncol (2014) 45(6):2403–10. doi: 10.3892/ijo.2014.2680
51. Mizokami Y, Mitsuya K, Hayashi N, Yasui H, Harada H, Nishimura T, et al. [Metastatic brain tumors from gastrointestinal cancer: an analysis of patient background and treatment results]. No Shinkei Geka Neurol Surg (2013) 41(8):669–77.
52. Harada K, Hwang H, Wang X, Abdelhakeem A, Iwatsuki M, Murphy MAB, et al. Brain metastases in patients with upper gastrointestinal cancer is associated with proximally located adenocarcinoma and lymph node metastases. Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2020) 23(5):904–12. doi: 10.1007/s10120-020-01075-3
53. Nakazawa K, Tanaka R, Kametani N, Hirakawa T, Kato Y, Komoto M, et al. [Multidisciplinary therapy for advanced gastric cancer with liver and brain metastases]. Gan To Kagaku Ryoho Cancer Chemother (2015) 42(12):2009–11.
54. Tamura S, Takeno A, Miki H, Uchiyama C, Kanemura T, Ono H, et al. Clinical outcomes in patients with brain metastasis from gastric cancer. Gan To Kagaku Ryoho Cancer Chemother (2011) 38(12):2093–6.
55. Kasakura Y, Fujii M, Mochizuki F, Suzuki T, Takahashi T. Clinicopathological study of brain metastasis in gastric cancer patients. Surg Today (2000) 30(6):485–90. doi: 10.1007/s005950070112
56. Karamchandani MM, Ganti T, Jaiswal S, Wu JK, Saif MW. A rare occurrence of isolated brain metastases from gastric cancer. Case Rep Med (2019) 2019:8075421. doi: 10.1155/2019/8075421
57. York JE, Stringer J, Ajani JA, Wildrick DM, Gokaslan ZL. Gastric cancer and metastasis to the brain. Ann Surg Oncol (1999) 6(8):771–6. doi: 10.1007/s10434-999-0771-3
58. Ahn MJ, Lee K, Lee KH, Kim JW, Kim IY, Bae WK. Combination of anti-PD-1 therapy and stereotactic radiosurgery for a gastric cancer patient with brain metastasis: a case report. BMC Cancer (2018) 18(1):173. doi: 10.1186/s12885-017-3906-0
59. Paudel N, Helenowski I, Kane L, Sachdev S, Bloch O, Tate M, et al. Stereotactic radiosurgery for the treatment of brain metastasis from gastrointestinal primary cancers. J Radiosurg SBRT (2019) 6(1):27–34.
60. Da Silva AN, Nagayama K, Schlesinger DJ, Sheehan JP. Gamma knife surgery for brain metastases from gastrointestinal cancer. J Neurosurg (2009) 111(3):423–30. doi: 10.3171/2008.9.jns08281
61. Hasegawa T, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brain metastases from gastrointestinal tract cancer. Surg Neurol (2003) 60(6):506–14. doi: 10.1016/s0090-3019(03)00356-2
62. Han JH, Kim DG, Chung HT, Kim CY, Park CK, Chung YS, et al. Radiosurgery for brain metastasis from advanced gastric cancer. Acta Neurochir (2010) 152(4):605–10. doi: 10.1007/s00701-009-0554-4
63. Park YS, Chang JH, Chang JW, Park YG. The efficacy of gamma knife radiosurgery for advanced gastric cancer with brain metastases. J Neuro-Oncol (2011) 103(3):513–21. doi: 10.1007/s11060-010-0405-4
64. Matsunaga M, Wada S, Daa T, Harada K, Okamura K, Noguchi T. Long-term survival after resection of brain metastases from esophagogastric junction adenocarcinoma: report of two cases and review of the literature. Clin J Gastroenterol (2014) 7(3):213–8. doi: 10.1007/s12328-014-0491-5
65. Ghidini M, Petrelli F, Hahne JC, De Giorgi A, Toppo L, Pizzo C, et al. Clinical outcome and molecular characterization of brain metastases from esophageal and gastric cancer: a systematic review. Med Oncol (Northwood London England) (2017) 34(4):62. doi: 10.1007/s12032-017-0919-0
66. Joshi RS, Hirshman BR, Ali MA, Alattar A, Carroll K, Nagano O, et al. Prognostic importance of cumulative intracranial tumor volume in patients with gastrointestinal brain metastasis treated with stereotactic radiosurgery. World Neurosurg (2019) 121:e747–e54. doi: 10.1016/j.wneu.2018.09.209
67. Muto M, Nakata H, Ishigaki K, Tachibana S, Yoshida M, Muto M, et al. Successful treatment of advanced gastric cancer with brain metastases through an abscopal effect by radiation and immune checkpoint inhibitor therapy. J Gastric Cancer (2021) 21(3):319–24. doi: 10.5230/jgc.2021.21.e24
68. Yamamoto H, Tanabe S, Ishida Y, Sato H, Takezawa A, Furukawa K, et al. [Nivolumab and gamma knife radiosurgery for a gastric cancer patient with brain metastasis-a case report]. Gan To Kagaku Ryoho Cancer Chemother (2021) 48(7):963–5.
69. Omoto K, Urakami H, Seki S, Tanizawa S, Isobe Y. [A case of brain metastasis of gastric cancer successfully treated with nivolumab]. Gan To Kagaku Ryoho Cancer Chemother (2021) 48(5):713–6.
70. Takagi T, Kobayashi S, Sekimura A, Komaya K, Yamauchi Y, Hori A. Advanced esophagogastric junction cancer with brain, bone and gastric intramural metastases responding to combined modality therapy. J Rural Med JRM (2021) 16(3):179–83. doi: 10.2185/jrm.2020-055
71. Klempner SJ, Schrock AB, Ali SM, Kubicky CD, Taylor MH. Acquired CTNNB1 mutation drives immune checkpoint inhibitor-acquired resistance in a microsatellite instability-high gastroesophageal adenocarcinoma with brain metastases. JCO Precis Oncol (2019) 3:1–6. doi: 10.1200/po.18.00208
72. Preusser M, Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Marosi C, et al. Brain metastases of gastro-oesophageal cancer: evaluation of molecules with relevance for targeted therapies. Anticancer Res (2013) 33(3):1065–71.
73. Wei Z, Huang L, Zhang X, Xu A. Expression and significance of Her2 and ki-67 in gastric adenocarcinoma without distant metastasis: a cohort study. BMC Gastroenterol (2020) 20(1):343. doi: 10.1186/s12876-020-01484-9
74. Meng L, Chen Z, Jiang Z, Huang T, Hu J, Luo P, et al. MiR-122-5p suppresses the proliferation, migration, and invasion of gastric cancer cells by targeting LYN. Acta Biochim Biophys Sin (2020) 52(1):49–57. doi: 10.1093/abbs/gmz141
75. Huang L, Xu AM, Peng Q. CD147 and MMP-9 expressions in type II/III adenocarcinoma of esophagogastric junction and their clinicopathological significances. Int J Clin Exp Pathol (2015) 8(2):1929–37.
76. Xu AM, Huang L, Han WX, Wei ZJ. Monitoring of peri-distal gastrectomy carbohydrate antigen 19-9 level in gastric juice and its significance. Int J Clin Exp Med (2014) 7(1):230–8.
77. Wu RL, Huang L, Zhao HC, Geng XP. Hyaluronic acid in digestive cancers. J Cancer Res Clin Oncol (2017) 143(1):1–16. doi: 10.1007/s00432-016-2213-5
78. Huang L, Li TJ, Zhang JW, Liu S, Fu BS, Liu W. Neoadjuvant chemotherapy followed by surgery versus surgery alone for colorectal cancer: meta-analysis of randomized controlled trials. Medicine (2014) 93(28):e231. doi: 10.1097/md.0000000000000231
79. Cavanna L, Seghini P, Di Nunzio C, Orlandi E, Michieletti E, Stroppa EM, et al. Gastric cancer with brain metastasis and the role of human epidermal growth factor 2 status. Oncol Lett (2018) 15(4):5787–91. doi: 10.3892/ol.2018.8054
80. Feilchenfeldt J, Varga Z, Siano M, Grabsch HI, Held U, Schuknecht B, et al. Brain metastases in gastro-oesophageal adenocarcinoma: insights into the role of the human epidermal growth factor receptor 2 (HER2). Br J Cancer (2015) 113(5):716–21. doi: 10.1038/bjc.2015.279
81. Mitra D, Clark JW, Shih HA, Oh KS, Brastianos PK, Wo JY, et al. Enrichment of HER2 amplification in brain metastases from primary gastrointestinal malignancies. Oncol (2019) 24(2):193–201. doi: 10.1634/theoncologist.2018-0152
82. Yoshida J, Sugiyama K, Satoh M, Shiraishi K, Nishibori R, Kitagawa C. Efficacy of trastuzumab deruxtecan in an advanced gastric cancer patient with brain metastasis. Curr Problems Cancer (2021) 45(6):100757. doi: 10.1016/j.currproblcancer.2021.100757
83. Jun KH, Lee JE, Kim SH, Jung JH, Choi HJ, Kim YI, et al. Clinicopathological significance of n-cadherin and VEGF in advanced gastric cancer brain metastasis and the effects of metformin in preclinical models. Oncol Rep (2015) 34(4):2047–53. doi: 10.3892/or.2015.4191
84. Peng Z, Xu S, Li H, Sun C, Fu M, Gao M. Advanced gastric cancer with brain metastasis effectively treated by arterial infusion chemotherapy: A case report. Oncol Lett (2014) 7(2):449–51. doi: 10.3892/ol.2013.1699
85. Rino Y, Sekino Y, Yamada T, Nakayama T, Arai H, Kanari M, et al. [Irinotecan+cisplatin and irradiation are effective for brain metastases of gastric cancer–two case reports]. Gan To Kagaku Ryoho Cancer Chemother (2007) 34(7):1095–8.
86. Kitayama Y, Yoden Y, Okamoto N. [A case of effective paclitaxel therapy for gastric cancer with brain metastasis]. Gan To Kagaku Ryoho Cancer Chemother (2006) 33(7):981–4.
87. Xu AM, Huang L, Liu W, Gao S, Han WX, Wei ZJ. Neoadjuvant chemotherapy followed by surgery versus surgery alone for gastric carcinoma: systematic review and meta-analysis of randomized controlled trials. PloS One (2014) 9(1):e86941. doi: 10.1371/journal.pone.0086941
88. Kraszkiewicz M, Wydmanski J. Brain metastases from stomach cancer - the role of different treatment modalities and efficacy of palliative radiotherapy. Rep Pract Oncol Radiother J Greatpoland Cancer Center Poznan Polish Soc Radiat Oncol (2015) 20(1):32–7. doi: 10.1016/j.rpor.2014.08.003
89. Menis J, Fontanella C, Follador A, Fasola G, Aprile G. Brain metastases from gastrointestinal tumours: tailoring the approach to maximize the outcome. Crit Rev Oncol/Hematol (2013) 85(1):32–44. doi: 10.1016/j.critrevonc.2012.04.001
90. Jia C, Wang G, Wang T, Fu B, Zhang Y, Huang L, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular carcinoma. Int J Biol Sci (2020) 16(14):2542–58. doi: 10.7150/ijbs.45446
91. Huang L, Balavarca Y, van der Geest L, Lemmens V, Van Eycken L, De Schutter H, et al. Development and validation of a prognostic model to predict the prognosis of patients who underwent chemotherapy and resection of pancreatic adenocarcinoma: a large international population-based cohort study. BMC Med (2019) 17(1):66. doi: 10.1186/s12916-019-1304-y
92. Rades D, Bartscht T, Schild SE. Predictors of survival in patients with brain metastases from gastric cancer. Neoplasma (2017) 64(1):136–9. doi: 10.4149/neo_2017_117
93. Bartelt S, Momm F, Weissenberger C, Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol (2004) 10(22):3345–8. doi: 10.3748/wjg.v10.i22.3345
94. Huang L, Jansen L, Balavarca Y, van der Geest L, Lemmens V, Koerkamp BG, et al. Significance of examined lymph node number in accurate staging and long-term survival in resected stage I-II pancreatic cancer-more is better? a Large international population-based cohort study. Ann Surg (2021) 274(6):e554–e63. doi: 10.1097/sla.0000000000003558
Keywords: gastric adenocarcinoma, brain metastasis, survival, cumulative incidence function, Fine-Gray sub-distribution hazard regression, competing risk analysis, large population-based cohort study
Citation: Huang L, Wang L, Shi Y, Zhao Y, Xu C, Zhang J and Hu W (2022) Brain metastasis from gastric adenocarcinoma: A large comprehensive population-based cohort study on risk factors and prognosis. Front. Oncol. 12:897681. doi: 10.3389/fonc.2022.897681
Received: 16 March 2022; Accepted: 14 September 2022;
Published: 18 October 2022.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Gianpiero Fasola, Azienda Sanitaria Universitaria Friuli Centrale (ASU FC), ItalyLuigi Cavanna, Ospedaliera di Piacenza, Italy
Copyright © 2022 Huang, Wang, Shi, Zhao, Xu, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Huang, bGVpLmh1YW5nQGFsdW1uaS5ka2Z6LmRl; Jun Zhang, anVuemhhbmcxMDk3N0BzanR1LmVkdS5jbg==; Weiguo Hu, d2dodUByamguY29tLmNu
†These authors have contributed equally to this work
‡Lead contact
§ORCID: Lei Huang, orcid.org/0000-0002-4225-9200
 Lei Huang
Lei Huang Lei Wang2,3†
Lei Wang2,3† Yan Shi
Yan Shi Jun Zhang
Jun Zhang