
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 30 June 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.896939
 Georgina L. Jones1*
Georgina L. Jones1* Rachael H. Moss2
Rachael H. Moss2 Frances Darby1
Frances Darby1 Neda Mahmoodi1
Neda Mahmoodi1 Bob Phillips3
Bob Phillips3 Jane Hughes4
Jane Hughes4 Katharina S. Vogt2
Katharina S. Vogt2 Diana M. Greenfield5
Diana M. Greenfield5 Grete Brauten-Smith6
Grete Brauten-Smith6 Jacqui Gath7
Jacqui Gath7 Tonia Campbell8
Tonia Campbell8 Daniel Stark9
Daniel Stark9 Galina Velikova9
Galina Velikova9 John A. Snowden5
John A. Snowden5 Ellissa Baskind10
Ellissa Baskind10 Mariano Mascerenhas10
Mariano Mascerenhas10 Daniel Yeomanson11
Daniel Yeomanson11 Jonathan Skull5
Jonathan Skull5 Sheila Lane12
Sheila Lane12 Hilary L. Bekker13,14
Hilary L. Bekker13,14 Richard A. Anderson15
Richard A. Anderson15Background: Women with a new cancer diagnosis face complex decisions about interventions aiming to preserve their fertility. Decision aids are more effective in supporting decision making than traditional information provision. We describe the development and field testing of a novel patient decision aid designed to support women to make fertility preservation treatment decisions around cancer diagnosis.
Methods: A prospective, mixed-method, three stage study involving: 1) co-development of the resource in collaboration with a multi-disciplinary group of key stakeholders including oncology and fertility healthcare professionals and patient partners (n=24), 2) alpha testing with a group of cancer patients who had faced a fertility preservation treatment decision in the past (n=11), and oncology and fertility healthcare professionals and stakeholders (n=14) and, 3) beta testing with women in routine care who had received a recent diagnosis of cancer and were facing a fertility preservation treatment decision (n=41) and their oncology and fertility healthcare professionals (n=3). Ten service users recruited from a closed Breast Cancer Now Facebook group and the support group Cancer and Fertility UK also provided feedback on CFM via an online survey.
Results: A 60-page paper prototype of the Cancer, Fertility and Me patient decision aid was initially developed. Alpha testing of the resource found that overall, it was acceptable to cancer patients, healthcare professionals and key stakeholders and it was considered a useful resource to support fertility preservation treatment decision-making. However, the healthcare professionals felt that the length of the patient decision aid, and elements of its content may be a barrier to its use. Subsequently, the prototype was reduced to 40 pages. During beta testing of the shortened version in routine care, women who received the resource described its positive impact on their ability to make fertility preservation decisions and support them at a stressful time. However, practical difficulties emerged which impacted upon its wider dissemination in clinical practice and limited some elements of the evaluation planned.
Discussion: Women receiving the decision aid within the cancer treatment pathway found it helped them engage with decisions about fertility preservation, and make better informed, values-based care plans with oncology and fertility teams. More work is needed to address access and implementation of this resource as part of routine oncology care pathways.
Worldwide, cancer patient survival rates are rising, resulting in an increased focus on helping people adjust to life after cancer treatment (1). For women of fertile years, one of the most distressing outcomes of some cancer treatments is its impact on fertility, potentially denying them the opportunity to have their own biological child (2, 3).
The impact of cancer treatment upon fertility may vary because of age, and how treatments inconsistently affect gonadal and uterine function. A Scottish population-based analysis showed a 38% reduction in the likelihood of a pregnancy rate after cancer treatments in girls and women aged <40 years across all diagnoses compared with the general population (4). It is recommended that fertility preservation (FP) treatments for women (such as egg, embryo, and ovarian tissue cryopreservation) should be discussed before cancer treatment starts, enabling women to consider their options, supported by the provision of written information/resources where possible (5–9). However, evidence suggests that many women are either not considered, or referred, for FP, are inappropriately referred (10, 11), or are poorly supported in making these complex decisions.
Patient decision aids are evidence-based resources supporting patients to make informed, values-based decisions between treatments (12, 13). Patient decision aids present information about the condition under focus and should provide the reader with unbiased information regarding their options/treatments and the associated benefits and risks (e.g., side effects) in a neutral way (14). They are intended to support but not replace good quality patient-doctor communication (15). A systematic review of published studies which had evaluated a patient decision aid in a randomised controlled trial compared to usual care (i.e., no intervention, usual care, placebo interventions, guidelines or a combination of these) concluded there was moderate to high quality evidence that patient decision aids enabled patients to be significantly more active in their treatment decision-making and become more knowledgeable, informed, and clear about their values compared to usual care with less subsequent decisional regret (15). Similar findings were also observed in a review of cancer treatment and screening specific patient decision aids which had also been evaluated in the context of a randomised controlled trial. The authors found that patients exposed to the patient decision aid had higher average knowledge scores, accurate risk perceptions and were more likely to be active in their decision-making compared to those exposed to usual care (16).
This paper describes the development and evaluation of a novel patient decision aid to support women of reproductive age at risk of losing their fertility because of cancer treatment to make FP treatment decisions entitled ‘Cancer, Fertility and Me’ (CFM).
The current study used a prospective mixed-method observational study design and is reported following the Standards for UNiversal reporting of Decision Aid Evaluations (SUNDAE) guidelines (17).
CFM was initially designed in a print-based booklet form. It was developed and evaluated in three stages in line with best practice methodological recommendations from the International Patient Decision Aid Standards (IPDAS) (18), and consideration of other best practice decision science guidance (19, 20), past decision aid development and evaluation studies (21) and frameworks for assessing complex interventions (22). The three stages included the development of CFM with key stakeholders (stage 1), alpha testing the first prototype using past patients and healthcare professionals (HCPs) (stage 2) and beta (field) testing with patients and HCPs as part of routine clinical practice (stage 3). A protocol outlining these stages is described in-depth elsewhere (23) but summarised in Supplementary Figure 1 and reported briefly below to meet the SUNDAE standards of reporting required and to highlight the places where the protocol changed from the initial version.
This stage identified the theoretical framework to guide development of CFM and the ‘active’ components the resource needed.
The decision theory-centred, Ottawa Decision Support Framework (ODSF) (24, 25) was chosen as it is particularly suitable when the decision in question is preference sensitive and it provides systematic methods to identify the needs of different stakeholders when developing patient resources.
The scope, purpose and target audience of CFM was defined following a consideration of the evidence gathered from i) a systematic review of women’s values, treatment preferences and decision-making experiences (26), a longitudinal, mixed-methods study which explored the needs and experiences of women with cancer as they contemplated FP treatment decisions (the PreFer Study) (27) a mixed-method evaluation of a local FP service (28). In 2005, Thewes et al. (29) reported on the key fertility-related questions that needed answering from the perspective of young women with early-stage breast cancer – the largest cancer group facing a fertility preservation discussion which were also considered.
The results of the systematic review highlighted that FP decisions are preference-sensitive, time-sensitive, and typically stressful. They often occur around the time of cancer diagnosis, with FP interventions enacted before cancer treatment is initiated. External and internal factors can affect FP decision-making for women around cancer diagnosis in oncology services (26). External factors included barriers outside of the patient’s control (e.g., lack of/poor information provision, lack of knowledge and referral to fertility services amongst others). Internal factors highlighted the role of individual differences and subjective emotions as a barrier to FP decision-making including the fear associated with delaying cancer treatment, fear of aggravating a hormone positive cancer, and the fear associated with the consequences of a future pregnancy. These emotions can cause conflict for the patient about whether cancer or FP treatment should be prioritised (27), something that has been identified as a key factor in other areas of cancer-related decision-making (30).
The resource aims to better support women at risk of losing their fertility because of cancer treatment, to make the most appropriate FP treatment decision for them. This evidence suggested that a resource, administered around the time of cancer diagnosis in oncology services was most needed and desired. At the time the review was undertaken, it identified only two FP patient decision aids for women of reproductive age, both designed for women with breast cancer specifically (31, 32). It therefore highlighted how women with other cancer diagnoses (e.g., lymphoma) did not have a resource available to them to better support them with the FP treatment decision and that CFM should be relevant to women with cancer diagnoses beyond breast cancer.
To confirm the need, format and distribution plan, a PPI focus group (consisting of three women and a partner who had either faced the fertility preservation decision or missed out on such an opportunity) was also undertaken by GJ and JH to further confirm our CFM development plans.
To guide CFM decision aid development, a multi-disciplinary group (n=24) comprising key stakeholders was convened, growing over time to consist of senior clinicians including paediatric, teenage and adult oncologists (n=6), a haematologist (n=1), fertility specialists (n= 4), oncology nurses (n=2), health psychologists/decision scientists (n=4), health service researcher (n=2), representatives from relevant charity organisations (n=3), and patient representatives (n=2). To further inform the content of CFM, we reviewed clinical guidelines on fertility preservation and cancer in females (5, 6), undertook an environmental scan to appraise the quality of existing fertility preservation resources (33) and undertook informal observations of local service delivery across sites in Yorkshire, UK undertaken as part of study set-up.
The key stakeholder group considered all the information referenced previously to identify key components to guide the design and content of a draft (e.g., via the discussion of potential CFM content during face-to-face meetings, and via email) until a consensus was reached. A design team (Making Sense Ltd) developed the illustrations and design of the resource.
Women with experience of FP decision-making in the context of a cancer diagnosis, HCPs and key stakeholders completed the Preparation for Decision-making Questionnaire (34), four items taken from the QQ-10 (designed to measure the face validity of a questionnaire) (35), and three open-ended questions relating to the acceptability and utility of CFM. Questionnaire data were analysed using SPSS Software (Version 24). Interviews were anonymised during transcription, uploaded onto NVivo and analysed using thematic analysis (36). An initial coding framework based on two interviews, was developed by four researchers (KSV, FD, NM and GJ) and was extended as appropriate. One researcher coded all interviews (KSV) and a second researcher (DM) coded two randomly selected interviews to check for differences. The generated themes were discussed between KSV, FD and GJ, until consensus was reached.
In stage 3 we aimed to field test CFM as part of routine practice with 78 new patients recruited from oncology clinics and oncology HCPs (23). We had planned to use the referral model of recruitment, whereby women would be informed and offered the opportunity to take part in the study by their oncologist around cancer diagnosis. However, due to problems encountered with recruitment of women in oncology services, patient recruitment was further widened and extended from this initial protocol (23) (Table 1) to recruit women with cancer who were contemplating FP and had been referred to fertility services as well. Patients were recruited before starting cancer treatment (baseline). Baseline measures completed included the EQ-5D three level version (EQ-5D-3L) (37) and the traditional version of the Decisional Conflict Scale (DCS) (38). After women had completed at least their first round of chemotherapy (time 2) both patients and HCPs were asked to complete the EQ-5D-3L and the Decisional Regret Scale (DRS) (39) and invited to take part in a qualitative interview to explore their experiences of using CFM (time 2b).
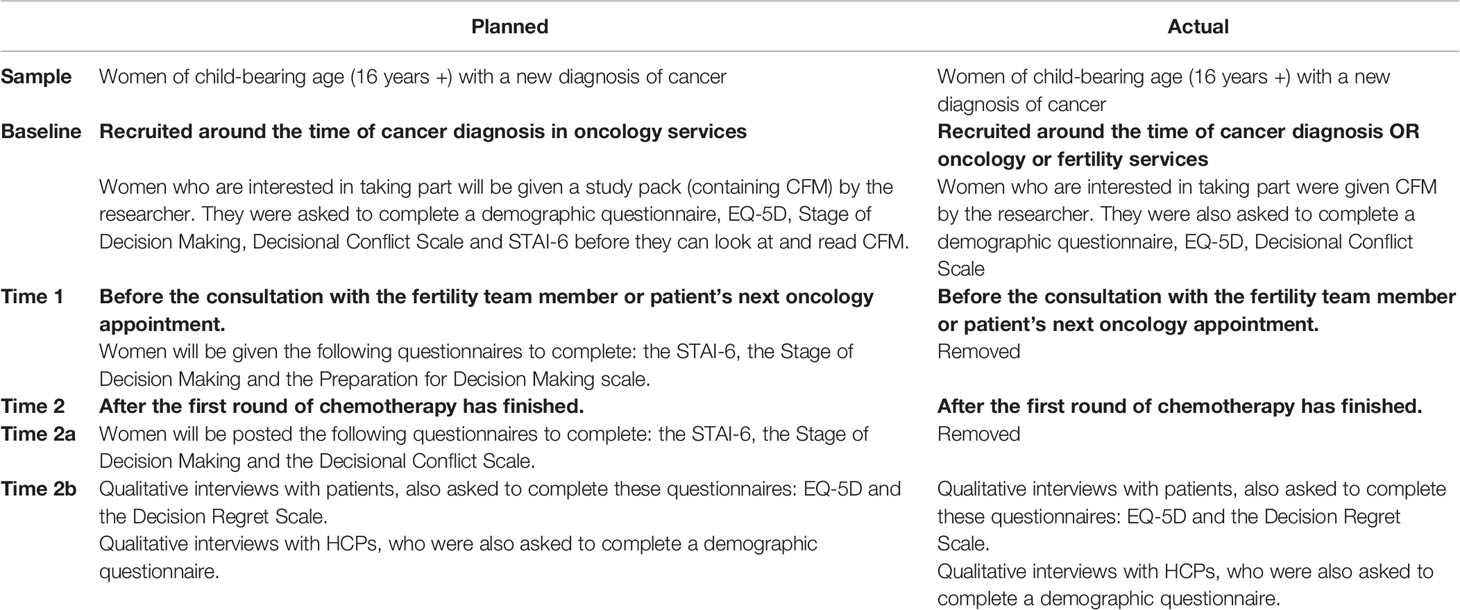
Table 1 Changes made to the methodology used during the beta testing of the CFM patient decision aid.
The same analysis methods were applied as in Stage 2, with the exception that a lay advocate (JG) independently coded three interview transcripts. We used the criteria from the SUNDAE Checklist (17) seeking to understand: i) How much and which components were used, ii) the degree to which it was delivered and used as intended (“fidelity”), and iii) any anticipated and unanticipated consequences. Count data was gathered to measure the number of CFM patient decision aids given to women and HCPs, website views, and the downloads of the PDF version.
A subset of women were also recruited in an online survey via social media to a closed Breast Cancer Now Facebook group (formerly Breast Cancer Care) and the support group Cancer and Fertility UK to give feedback on the CFM patient decision aid.
The development of CFM was an iterative process, with over 100 versions created before the first 60-page prototype (version 1.0) was produced.
The key components in the patient decision aid include:
* Explicit information about, and description of, the decision to be made (i.e., helping women with cancer to make decisions about FP treatment before starting cancer therapy),
* Describing the health problems (i.e., cancer, fertility and the reproductive system, potential impact of cancer treatments on fertility),
* Providing information, in visual, text, numerical (%) and table formats, to describe treatment options (including benefits/harm/consequences), which also included avoiding or postponing treatment (i.e., no FP, egg, embryo or ovarian tissue freezing, with or without ovarian suppression),
* Tailoring this information for each of the following factors (e.g., relevant patient group, features of the intervention - including where the FP treatment option may be considered a newer treatment method, implications for achieving a successful pregnancy, and chances of cancer recurrence),
* Providing guidance for communication and deliberation about the FP decision with HCPs and important others (e.g., via suggested questions to use, spaces to write and list what they like and dislike about each option, exercises to think about the importance of referral, and help the women clarify their own values) (Figure 1),
* Information on the other fertility decisions to consider during and after cancer treatment, represented by a decision map (30) to each stage of the cancer pathway before, during and after cancer treatment,
* Other components (i.e., information about useful contacts, sources evidence, glossary, the team),
* A one-page summary table, for potential use as an option grid. Columns are the fertility preservation options and the rows the frequently asked questions.
To support health literacy, reading levels were obtained for each page. The resource had an overall Flesch-Kincaid reading score of 52.14, which is at an average level to easily be understood by 15- to 16-year-olds.
Eleven women, 10 HCPs and four other key stakeholders from Yorkshire Cancer Research, and the British Fertility Society, were recruited to the alpha testing of CFM (Table 2). From the 10 HCPs in clinical roles, there were five medical consultants and one junior doctor, three nurses and a social worker (mean age = 45.2 years old, range = 30-58 years). Their clinical specialities included breast cancer, teenage and young adult oncology, reproductive medicine, late effects, paediatric oncology, haematology and bone marrow transplantation. QQ-10 questionnaires showed both patients (mean = 4.3, range = 3.3 – 4.7), and HCPs (mean = 4.2, range = 2.55 – 4.91) found the resource acceptable (Table 3). The resource also appeared to be considered useful in preparing women for FP treatment decisions (mean = 4.3, range = 3.9 – 4.5) and HCPs (mean = 4.2, range = 4.0 – 4.6). When converted out of 100, this resulted in a score of 82.3 for the women and 81.0 for the HCPs (Table 3).
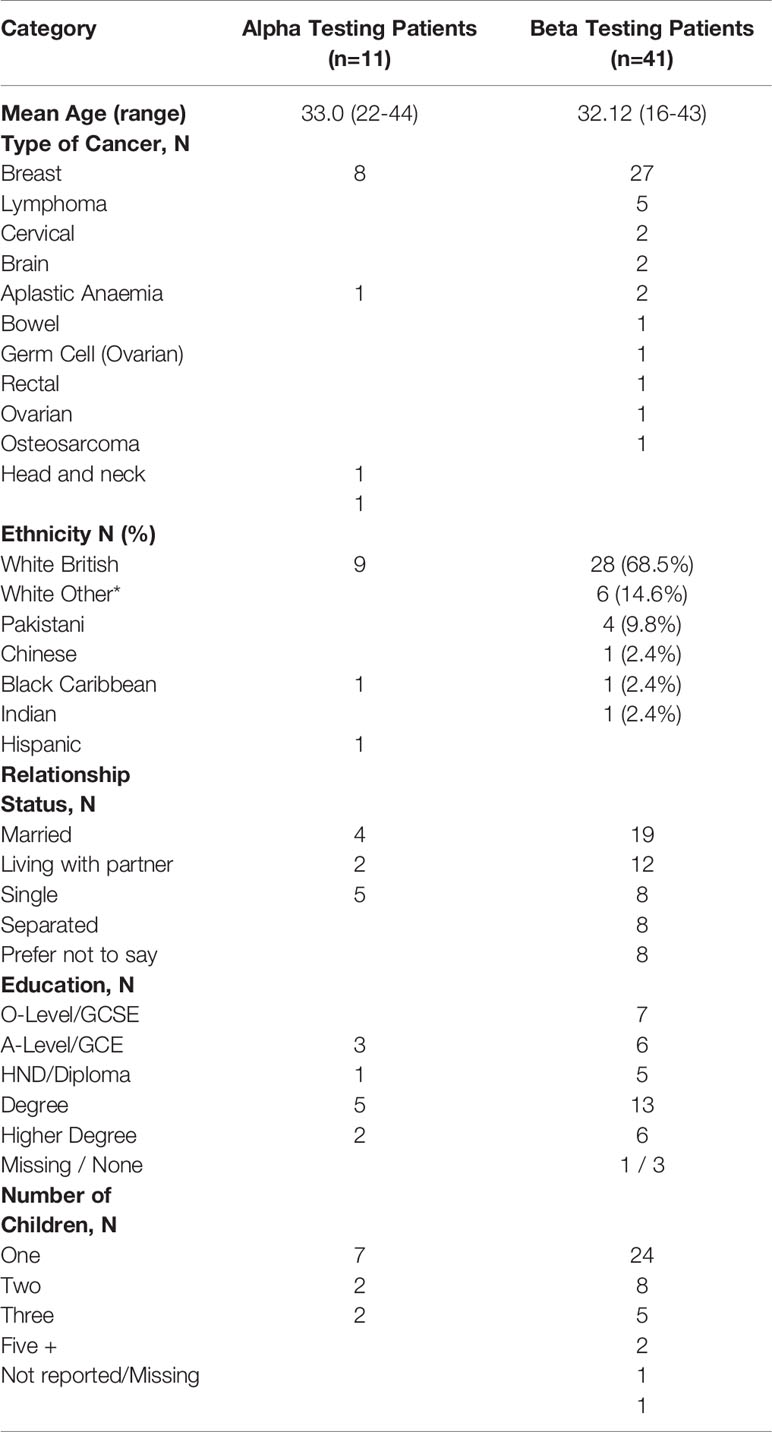
Table 2 Demographic and clinical characteristics of the patient and HCPs in the alpha and beta testing stages of the study.
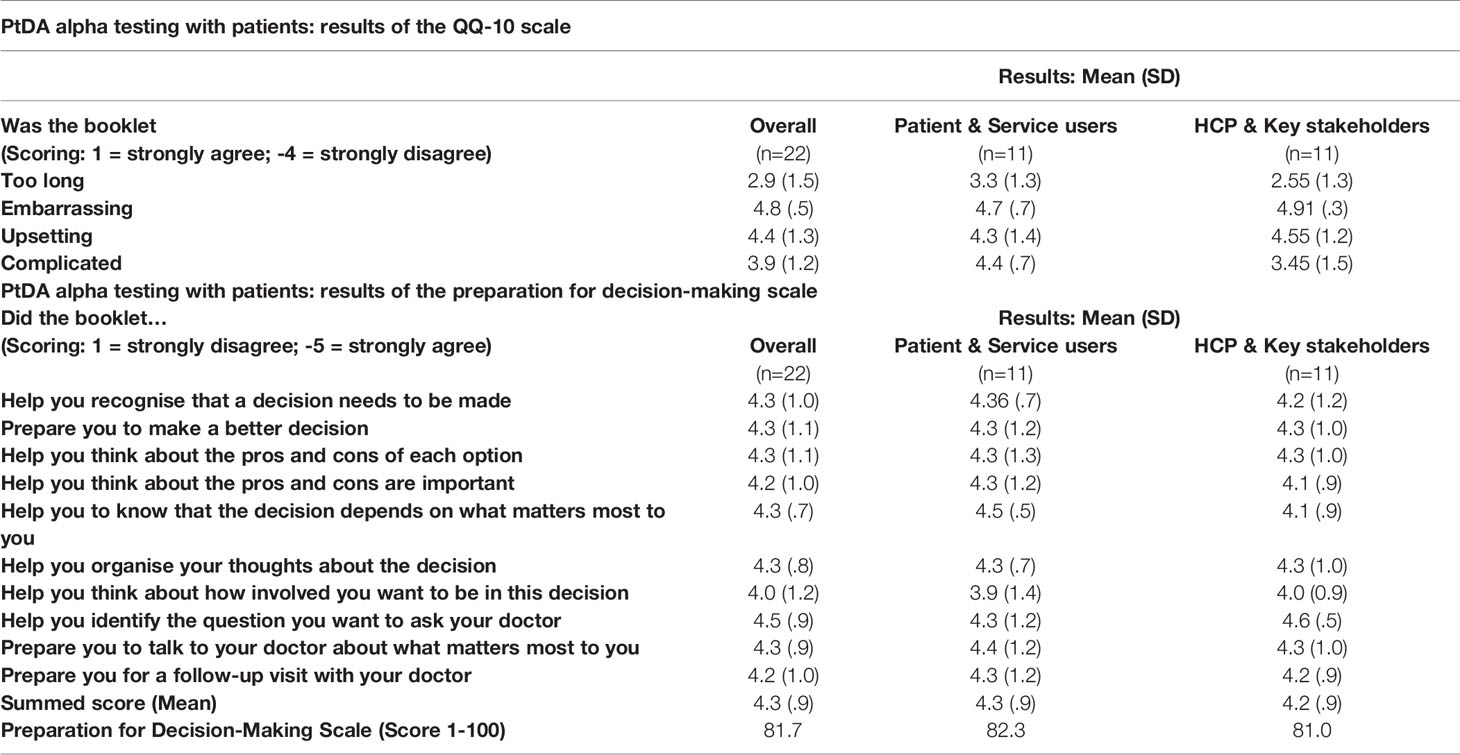
Table 3 Results of the QQ-10 scale and the preparation for decision-making scale during alpha testing.
Thematic analysis generated three key themes from the patient and HCP interview data. These related to resource design and content, its use in supporting FP decision-making and, its use in practice. The feedback on the resource design and content including the colours, paper, font size, general layout, order of content, and infographics were very positive. However, the length of the resource was raised as a concern by some HCPs. All patients and most HCPs also felt the resource supported decision-making, although some of the text was considered too complex by a few HCPs, and therefore they questioned whether CFM would be helpful in supporting patients to make FP decisions. With regards to the usability of the resource, most HCPs felt that the length, its complex content and the need to maximise survival over fertility would be a barrier to use at the time of a stressful cancer diagnosis. In contrast, only four women expressed concerns about complexity or length.
The practical recommendations for changes that were raised by patients and HCPs are shown in Tables 4, 5 respectively. Following a discussion and consideration of these findings with the steering group, the CFM decision aid underwent a set of revisions and amendments before being used in stage 3. These revisions shortened the resource by 20 pages.
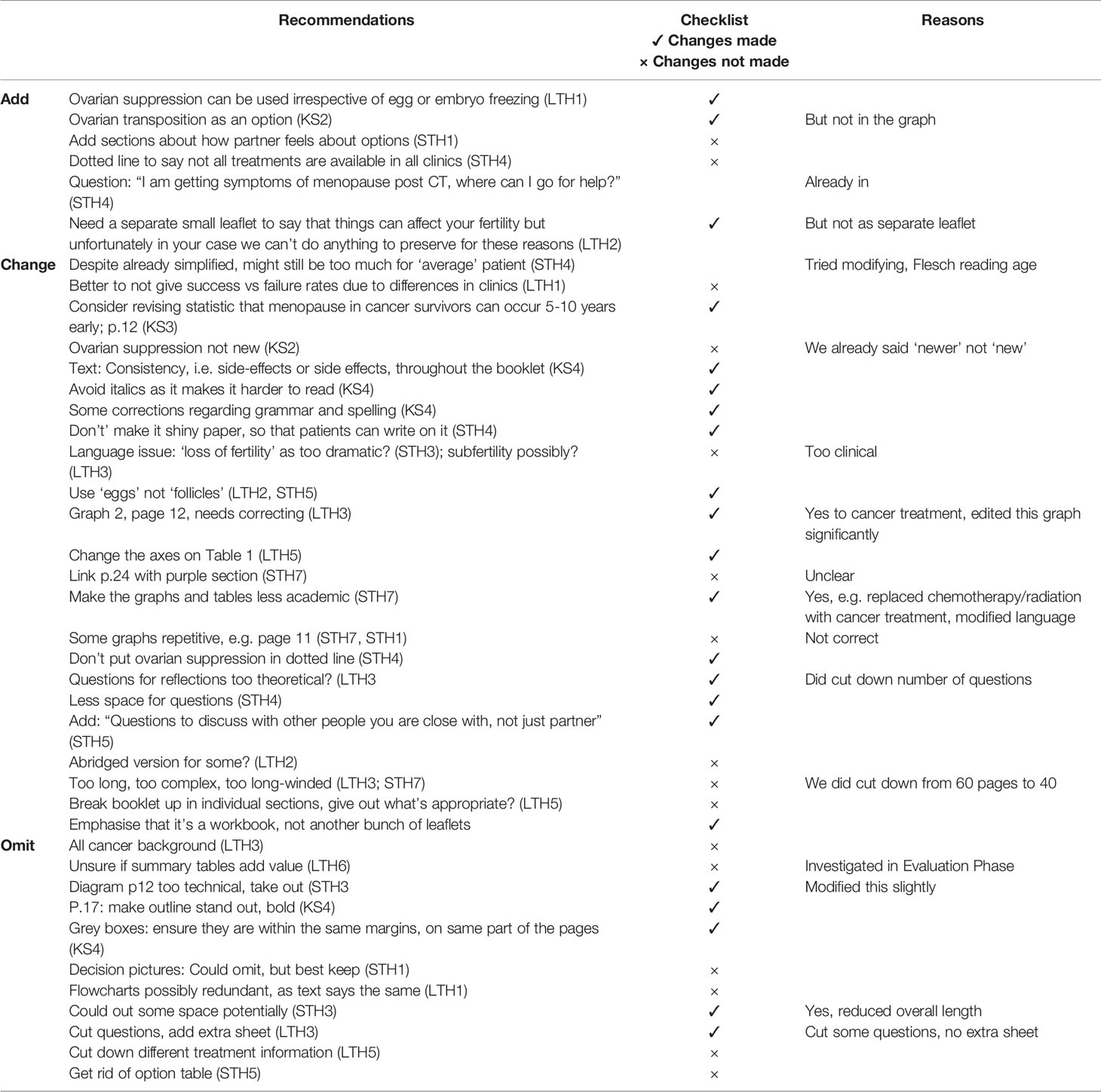
Table 5 Practical recommendations made by the healthcare professionals and key stakeholders following alpha testing.
Forty-one women were recruited (13 from oncology clinics and 26 from fertility services) (Table 2).
In consideration of FP options at baseline and before using CFM, 17 women (41%) felt they were considering their options right now, with 12.8% who had not yet begun to think about their options. Thirty-seven (90.2%), completed all 16 items of the decisional conflict scale at baseline (Supplementary Figure 2A). The total mean score was 30.7 (SD: 21.4; range: 0 - 76.6 on a scale of 0-100) indicating lower than average levels of decisional conflict, although the uncertainty sub-scale exceeded the threshold of ‘higher’ decisional conflict. Baseline EQ-5D-3L mean scores revealed low levels of problems in the five areas of quality of life. With the exception of usual activities (p = 0.018), there were no other significant differences in quality-of-life scores based upon the EQ-5D data pre and post receipt of CFM.
Thirty-one women and three HCPs were subsequently interviewed, with 10 women declining to take part at this stage primarily being too ill to be interviewed. Recruitment/administration of the resource in the beta stage appeared to fall to the same small number of HCPs across the two centres. Due to work commitments of the staff, we were only able to approach three - all of whom accepted. Prior to completing the interview, 29 women (70.7%) completed the decisional regret scale (DRS). The mean DRS score was 42.6 (SD = 7.7; range = 25-65 of a scale of 0-100) (Supplementary Figure 2B). There were no significant differences in total DCS scores between those women who subsequently took part in the qualitative interview and those who did not.
Overall, CFM was highly regarded and the majority of the components of CFM were used (Table 6). Women highly regarded the colours, graphs, statistics presented, tables and flow charts, expressing that they were clear and focused and provided a helpful summary of the information in order to assist in decision making:
“The colours I think is a really good choice. They’re kind of calming colours.” (Patient, age 20, lymphoma, fertility clinic)
“That [summary table] was really helpful. You go through all of the information and you’re desperately trying to absorb it all…’’ (Patient, age 38, breast cancer, oncology clinic)
“A lot of time and effort I think has been taken and that makes me feel better when I’m reading it because it makes me feel like it’s come from a source that actually cares and is reputable.” (Patient, age 29, breast cancer, oncology clinic)
“I actually liked the fact it was A4 cos everything else is A5. It somehow separated it out and made it not about cancer.” (Patient, age 40, breast cancer, oncology clinic)
These components helped to facilitate realisation of information and evidence, as well as increase knowledge awareness:
“I just kept going over the sections where it gives you the options and I just kept reading the statistics. I thought having the statistics was really helpful.” (Patient, age 40, breast cancer, oncology clinic)
“This is a lot better, I can read through it myself in my own time and it is not biased, it is telling me facts and options.” (Patient, age 29, lymphoma, fertility clinic)
“I think it strengthened my decision, it was something I might not have been clear on without the booklet.” (Patient, age 30, lymphoma, fertility clinic)
“It affirmed it. What it meant is I understood what I was doing. It meant that I understood the other options properly and I knew what I was turning down essentially.” (Patient, age 20 lymphoma, fertility clinic)
However, not all women used all components of CFM, for example, some women stated that they did not always use the spaces provided in the booklet to make notes, or they did not complete the decision-making values exercise in the booklet. It appeared that the majority of women may not put pen to paper and would refer to these prompts when thinking through the process of decision-making. For the few women who did write in the booklet, they described the value of being able to take it to the consultation as an aide-memoire for discussion:
‘‘I just kind of answered what it said so it takes my opinion on things which I thought was quite a helpful way to organise my thoughts and balance up my opinions really. It also makes like a note of what you want to ask a nurse or a doctor.’’ (Patient, age 20, lymphoma, fertility clinic)
Table 7 shows the anticipated consequences of using CFM. No unanticipated consequences arose. For some, it helped to reduce feelings of denial, highlighting the reality of their situation and the decision faced. It also helped to convey information to friends and family, and facilitated conversations about cancer and fertility to take place with the support of others:
“… mum helped me with making the decisions cos she’s been through it [fertility treatment]. She sat with me, and we talked about it for a good two days straight.”
(Patient, age 25, ovarian cancer, oncology clinic)
The process of sharing the information with friends and family also worked as a tool to help inform others:
“she’s [patient’s mother] read more of the booklets……. whereas because it happened so quick, and the actual treatment was so quick I think I was just in a whirlwind of getting from A to B whereas she took more information on than I did.” (Patient, age 28, breast cancer, fertility clinic)
Some women regarded the content of CFM upsetting – particularly how cancer and its associated treatment can affect fertility, the consequence being they chose to avoid reading those sections:
“I know there’s the bit about how the cancer can affect it, how the treatments can affect it … it brings me down a little bit so I try and avoid sections like that.” (Patient, age 29, brain cancer, fertility clinic)
Following the reading of CFM content guilt was expressed by some women who were contemplating undertaking FP at a time when their body was having to undergo challenging cancer treatment:
“Why am I doing this when I’ve got something else that I’m going to have inject into me body? ”(Patient, age 30, Lymphoma, fertility clinic)
Our intention for recruitment was that all women of reproductive age at risk of losing their fertility because of cancer treatment, would be offered the opportunity to take part in the study and read the resource around the time of their cancer diagnosis in oncology.
In practice, it was the fertility clinics which became the primary source of delivery. Operational pressures, significant clinical workloads (as a result of staff shortages) and competing clinical priorities (urgency of the cancer treatment) at a particularly stressful point in the pathway for patients (i.e., diagnosis) particularly within the cancer setting, perhaps meant the study could not always be prioritised:
“You’ve got a short window of opportunity to take this opportunity and in that sense there’s pressure, there’s a lot of pressure. It’s a pressured decision-making process………. we have 650 new cancers a year and 300 women living with secondary breast cancer and there’s seven of us and we’re never all here.” (HCP, oncology clinic)
“The more there is to read when people are in a state of distress, I think the harder it is to concentrate and focus.” (HCP, oncology clinic)
“I did not [read CFM]. If I would have got the information given to me in hospital by [oncologist], definitely I would read it.” (Patient, age 36, breast cancer, fertility clinic)
However, some HCPs expressed that the low recruitment rate was simply because:
“The majority of women that I gave it to have a clear vision in their mind as to what they wanted.” (HCP, oncology clinic)
This was echoed in some women’s responses to us:
“The doctor that told me I’d got cancer, he must have spoken to me for a good hour and I literally couldn’t tell you a single thing he said to me. It’s like white noise, you just don’t hear anything.” (Patient, age 29, breast cancer, oncology clinic)
“When in the midst of being told that I’m about to have chemo I was given it and told that it’s to help make my decision on fertility.” (Patient, age 23, ovarian cancer, oncology clinic)
“I think I already knew before I read the book.” (Patient, age 29, bowel cancer, fertility clinic)
Thus, the delivery of the resource, particularly in the cancer setting, was often not as had been intended with the responsibility often falling to the oncology nurses instead. There was limited (or no) chance for women to sit and discuss the contents of the resource with patients describing that their HCP did not take the opportunity to signpost their patients to useful information that would help them to consider FP decisions,
“I do wonder if there was a part my age played in that a little bit, potentially, I’m not sure. I think if maybe I was under thirty, in that twenty to thirties bracket, there may have been more of a ‘you really ought to be taking a while to think about this … I think, somebody younger, there may have been more of a push.” (Patient, age 34, breast cancer, oncology clinic).”
“So the booklet was really helpful…. because the consultant himself was very much just trying to push me to just not even think about that, but we wanted to think about that – being referred to the fertility clinic, and the booklet did help us know we’re making the right choice.” (Patient, age 38, breast cancer, fertility clinic)
“I weren’t given any option by my oncologist, so he never went through any of this with me. So obviously that booklet [CFM] was very informative.” (Patient, age 23, cervical cancer, oncology clinic)
For some women this meant that they were not aware of the information in CFM, until it was too late in their cancer treatment process. Women often turned to the resource in retrospect with the realisation of how crucial it could have been to them when making their FP decisions:
“I wish I’d read it beforehand so I would have actually known what to ask him [the oncologist] you know? Cos you don’t actually realise what questions you want to ask at that time. Cos obviously everything was just done so quickly, and you can’t think at the time of what you need to do, what you shouldn’t do and stuff like that.” (Patient, age 34, breast cancer, fertility clinic)
Finally, of the 29 participants who consented to take part in the social media study, 10 completed the online survey. Women were diagnosed with breast cancer (n=4), bowel cancer (n=2), childhood pelvic rhabdomyosarcoma (n=1), lymphoma (n=1) and cancer of the uterus (n=1) (missing n=1). The mean age was 36.8 (SD = 5.29; range = 28 – 43). Five were currently undergoing cancer treatment. The responses further confirmed the acceptability and value of CFM as a resource to support the FP decisional needs of this patient group:
“I think this is brilliant and much needed. I feel it’s essential ALL girls/women with cancer are aware of ALL options prior to starting treatment.” (Participant, age 41, childhood pelvic rhabdomyosarcoma, Facebook survey)
“I think it is really important for women to have access to this information. The thought of losing my fertility was the most upsetting part of my cancer experience.” (Participant, age 31, breast cancer, Facebook survey).
The aim of this study was to develop, and field test a novel patient decision aid to support women, aged 16 years and older, diagnosed with any cancer, to make FP treatment decisions before the start of their cancer treatment. It was well received during both the alpha and beta testing stages, with women describing how it helped them engage with decisions about FP, and make better informed, values-based care plans with their oncology and fertility care team.
Of the 41 women taking part in the beta testing study, only a quarter felt they had already made their decision and were unlikely to change their mind. With the majority still uncertain, the need for using such a resource very early in the cancer pathway is thus indicated. Low levels of fertility-related knowledge have been linked to increased decisional conflict in young patients with breast cancer (40) and a perceived lack of overall support for women (41).
Whilst the quality of the tool was acknowledged, there were difficulties in wider dissemination in clinical practice thus limiting extensive evaluation, such that CFM was not delivered in the same way for all participants. This concerns the ‘fidelity’ of a decision aids use and implementation (17). Our intention was that the tool would be administered in cancer services around the time of diagnosis. Instead, most women were recruited in fertility services. Discussions with cancer HCPs provided several explanations for the low recruitment figures, including the priority to decide and deliver the cancer treatment, demanding workloads, the need to protect women from further stress and the perception or assumption that many women had already made their decision beforehand. It is undoubted that some patients may have been unsuitable to partake in this research study. However, several women who missed recruitment to the study in cancer services then welcomed the opportunity to take part in the study once approached in fertility services.
It can be very difficult for oncology healthcare professionals to have fertility discussions in the context of a recent cancer diagnosis (42). It depends upon members of the oncology team using their best judgements to communicate information related to prognosis and treatment options which are complex and frightening (43). They have also described lacking the knowledge to advise appropriately during their fertility discussions with women and have requested more specialised resources to support them during these consultations. For example, one study highlighted that 87% of oncologists expressed a need for more specialist FP information, and that only 38% of oncologists routinely provided patients with written information (44). Similarly, a survey of 273 physicians involved in the care of breast cancer patients was conducted to explore fertility and pregnancy issues (pre and post cancer diagnosis) in young women with breast cancer. Between 17.6% and 48.4% reported having inadequate knowledge about the FP treatment options and it was concluded that further educational initiatives are needed in the future to better inform and support these physicians (45). In our study, one healthcare professional we interviewed described how lack of data on the ways that socio-demographic factors such as body mass index affected FP treatment outcomes made it difficult to know what best to do clinically in certain situations.
Whilst we attempted to prepare the clinical staff at several points throughout the study by targeting communication skills training on how to incorporate the utility of CFM in their doctor-patient communication/relationships (e.g., by providing scripts) more needs to be done to prepare, support and upskill staff in this new area of practice, especially regarding the ‘referral model’ of recruitment (46) and use of CFM in routine practice as a tool to support shared-decision-making. In relation to recruitment and administration of CFM, we found this was often delegated to the oncology nurses (particularly the breast cancer nurses). Therefore, one possible solution to addressing low recruitment rates in these types of studies may be to design patient recruitment around oncology nurses rather than the oncologists/surgeons. Implementation of FP patient decision aids in routine clinical care may also be improved if delivered by nurses. However, one possible explanation for why the resource was not given out as intended was because of a possible lack of clarity from within the multidisciplinary clinical team regarding whose role it was to have the FP consultation with patients and at what time point in the patients care pathway. All HCPs, including nursing staff will only be able to undertake this work, if it becomes clearly defined within their clinical roles, responsibilities and expectations. For this reason, we are currently in the process of developing a more comprehensive training package that may better support HCPs (including nurses) working across cancer services to use CFM in consultations. We also plan to undertake future work to identify and evaluate where in the clinical pathway the resource may work best; the findings also suggest that CFM may work better if it is administered to women in advance of seeing the oncologist. The use of the one-page summary table as an option grid on its own also needs evaluating.
Planned analyses were not possible with a small sample size and as such our focus shifted more to interpreting the qualitative interview data. As such interrogating quantitatively whether patient demographic characteristics which are known to influence FP treatment decision-making such as relationship status, age and parity (26) was not possible. We also did not achieve the diversity in the sample we had hoped for in terms of ethnicity for example to explore these issues. Despite this, from the qualitative interview data overall, it appeared that very similar experiences of FP decision-making arose, regardless of personal characteristics. With the exception that some women considered that their age and financial situation had influenced the FP treatment options discussed with them, and the decisions they made. Furthermore, those with dyslexia, poor eyesight or who did not speak English as a first language described how reading CFM was affected by these personal characteristics. This requires further detailed analysis and future studies should be undertaken to explore these issues fully.
Caution in interpreting some of the descriptive statistical findings is needed. For example, our mean DRS was 42.6, which is lower (better) than the average DRS scores found following the use of a FP decision aid in other studies (31). However, as some women reported using CFM after they had been referred to fertility services, it cannot be assumed that decisional regret outcomes can be solely attributed to our resource and adjustments to the woman’s diagnosis and its implications may have also influenced DRS scores.
There was little or no evidence in the individual patient case notes of women that may have been eligible to receive CFM, that a discussion regarding FP had occurred. We recommend that not only the FP discussion is documented but the quality and outcomes of any FP consultation should be recorded in the notes of cancer patients and reiterated in a summary clinical letter. Breast Cancer Now have developed a Fertility Toolkit for HCPs in breast cancer services which supports them to initiate and document a FP discussion with patients (47) but more work needs to be done to change practice and raise adoption of this tool or a local variation.
We found that CFM was generally used in the way it was intended by women, although some avoided reading some parts of the resource because of the emotions it invoked. This has been highlighted as a risk associated with FP patient decision aids (6), and other similar resources previously (48). It suggests that, given the benefits of such tools, patients should be encouraged to express any anxieties, concerns or other types of emotions e.g., guilt, during their clinical consultations that may arise from such interventions being used in routine care or research.
Despite the limitations and issues reported above, overall, our evidence suggests that CFM is a valid and acceptable resource to women with cancer facing the FP treatment decision. It better informed them about their FP options, enabling them to reason about the FP treatment decision in the context of their cancer treatment. It also supported conversations with others e.g., family members.
Our resource is intended to be used by women at risk of losing their fertility because of cancer treatment. The process of FP (information and possibilities) is deeply influenced by the disease site and its prognosis. In recognition of this, the resource reiterates the message that certain FP options may not be suitable or available to all women based upon their individual circumstances. But for many women, being fully informed about the full range of options and then understanding why some might not be suitable for them, we consider is an important part of the FP discussion. Our finding that all women found CFM better informed and supported them to make FP treatment decisions, regardless of the cancer type, supported our approach.
Since its development, it has also been converted into an online format, with both the print and digital formats freely available at https://cancerfertilityandme.org.uk. Its views online, adoption across a range of academic, clinical, policy and third-party sectors further demonstrates its value. At the time of writing there have been 20,902 page views of CFM online. Interestingly 60.4% are from the UK but the remainder from overseas visitors which may also suggest that the resource is having wider reach and interest internationally than previously anticipated. The resource has been endorsed by IPDAS, the UK National Institute for Health and Clinical Excellence (July 2020) and the UK government fertility regulator, the Human Fertilisation and Embryology Authority. A number of leading national cancer charities including Breast Cancer Now, Lymphoma Action, Brainstrust and Cancer Research UK all signpost patients to the CFM resource from their websites. Whilst the booklet version does not have to be read cover to cover, the one-page summary table, and the online version provide alternative versions of the resource for those concerned that the length of the booklet may limit its utilisation in clinical practice.
Our resource was initially informed by a systematic review of women’s values, treatment preferences and decision-making experiences (26), consideration of the evidence gathered from patients’ and clinician’s views on patient’s FP decision-making needs in the PreFer Study (27), a FP service evaluation of a local service (28) and an environmental scan and review of clinical guidelines (5, 6). Since our study started, more fertility preservation patient decision aids are now available to support women diagnosed with cancer around the globe (49). ESHRE have also recently produced a comprehensive guidance document which details and critiques the fertility preservation patient decision aids available (6). At least six are now intended for use by women without a certain cancer type (49). More recently, 24 innovative cancer-specific Dutch tailored patient decision aids have been developed to provide patients with personalised information which can be tailored to their cancer diagnosis and treatment (50). However, none of the existing tools have been co-developed and tested with women residing in the UK, and therefore CFM provides a valuable resource and addresses an unmet need for this patient group.
Our next goals are to evaluate the online version of CFM and undertake a randomised study to explore the effectiveness of the resource. Some effectiveness studies of the existing international FP patient decision aids are pending but the results from three (6), suggest that overall, they may better support women to make FP decisions. Except for one study finding slightly higher decisional conflict scores for those women that used their decision aid compared to usual care (32), the effectiveness studies found these interventions increased knowledge (31, 44), lowered decisional conflict (31, 32, 51), lowered decisional regret at 12 months (but only after adjusting for education) (31), improved patient satisfaction (31, 51) and reduced the time needed to make decision (51).
Despite the need for an effectiveness study, a recent study revealed that only 44% of patient decision aids were used in some capacity following their trial as a tool to support shared decision-making – key reasons for this were lack of funding, and disagreements between clinicians and patients over its use (52). A key goal for our team is to undertake an evaluation of the implementation and effectiveness of the resource, particularly amongst the breast and haematological cancer care pathways. Our findings show that the fertility HCPs seemed more comfortable in approaching patients and administering the resource compared with HCPs in cancer services. A new training package may prove helpful as part of a larger problem-solving approach to better support members of the cancer multidisciplinary teams to adopt CFM in their clinical practice and to have FP discussions that supports shared decision-making. Other approaches and models of care, such as multidisciplinary clinics integrating cancer and fertility joint working and service improvement developments to reduce the workload burden of cancer staff in busy clinics to meet patient need could also be considered. These issues were not the focus of our planned study but impacted and warrant further consideration for optimal patient care.
In the context of a cancer diagnosis, the provision of our evidence-based CFM resource was successful in helping and better supporting women of reproductive age, to make FP decisions. However, whilst we addressed an unmet need for female cancer patients, at risk of losing their fertility, the research also highlighted some barriers which prevented access to use for these women at the time in the cancer pathway when they would have benefitted from it the most. Thus, using it as a tool to facilitate shared decision-making in oncology services requires further work. Given the challenges associated with patient decision aid recruitment, integration and adoption in routine clinical practice, existing frameworks could be modified to place a greater emphasis on identifying and addressing these issues during any need’s assessment work.
The anonymised raw data supporting the conclusions of this article may be made available by the authors upon reasonable request.
NHS Ethics Approval was obtained from the Health Research Authority (Reference: 16/EM/0122) and we also obtained National Institute of Health Research (Clinical Research Network) portfolio status (CPMS ID 30522). All patients/ participants provided their written informed consent to participate in this study.
Principal investigator, conceived the idea (GJ), contributed to grant development and protocol (GJ, JH, DG, JG, GB-S, JS, and HB), contributed to the CFM steering group (GJ, RM, FD, NM, BP, JH, KV, DG, GB-S, JG, TC, DS, GV, JAS, EB, MM, DY, JS, SL, HB, RA), contributed to CFM patient decision aid development (GJ, RM, FD, NM, BP, JH, KV, DG, GB-S, JG, TC, DS, GV, JAS, EB, MM, JS, SL, HB, RA), assisted with patient and service user recruitment (RM, FD, NM, BP, JH, KV, DG, GB-S, DS, GV, JAS, EB, MM, DY, JS, RA), assisted with data collection and analysis (GJ, RM, FD, NM, JH, KV, RA), undertook the environmental scan (NM, HB, JH, GJ), assisted with the development of the online version of CFM, and the IPDAS and NICE endorsement submissions (GJ, RM, FD, RA), wrote the first draft of the manuscript (GJ, RM, FD, NM, KV and BP). All authors contributed to the subsequent drafts of the article and approved the submitted version.
The study was funded by Yorkshire Cancer Research (S391).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Cancer, Fertility and Me team are incredibly grateful to all the women and members of the oncology and fertility multi-disciplinary teams who gave their time to help us with this research. We are also extremely grateful to Professor Karen Collins from Sheffield Hallam University who contributed to the grant application and early stages of the study before retiring; Making Sense Ltd and Lampson Designs for their support and assistance in the design of the print-based version and creation of the online version of the resource; Dr Sophia McDougall for assisting with two interviews and Danielle Musson for her assistance in coding interview data at stage 2; Becki McGuinness from Cancer and Fertility UK for supporting us with recruitment to stage 3 of our research and to Dr Melanie Davis at the University College London for providing additional feedback and comments on the resource.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.896939/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Peate M, Meiser B, Hickey M, Friedlander M. The Fertility-Related Concerns, Needs and Preferences of Younger Women With Breast Cancer: A Systematic Review. Breast Cancer Res Treat (2009) 116(2):215–23. doi: 10.1007/s10549-009-0401-6
3. Blumenfeld Z, Haim N. Prevention of Gonadal Damage During Cytotoxic Therapy. Ann Med (1997) 29(3):199–206. doi: 10.3109/07853899708999337
4. Anderson RA, Brewster DH, Wood R, Nowell S, Fischbacher C, Kelsey TW, et al. The Impact of Cancer on Subsequent Chance of Pregnancy: A Population-Based Analysis. Hum Reprod (2018) 33(7):1281–90. doi: 10.1093/humrep/dey216
5. National Institute for Health and Care Excellence (NICE). Cryopreservation to Preserve Fertility in People Diagnosed With Cancer. Available at: http://pathways.nice.org.uk/pathways/fertility#path=view%3A/pathways/fertility/cryopreservation-to-preserve-fertility-in-people-diagnosed-withcancer.xml&content=view-index (Accessed 3rd February 2022).
6. ESHRE. Guideline of the European Society for Human Reproduction and Embryology Female Fertility Preservation (2020). Available at: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Female-fertility-preservation (Accessed 3rd February 2022).
7. Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility Preservation for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol (2013) 31:2500–10. doi: 10.1200/JCO.2013.49.2678
8. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility Preservation and Post-Treatment Pregnancies in Post-Pubertal Cancer Patients: ESMO Clinical Practice Guidelines. Ann Oncol (2020) 31:1664–78. doi: 10.1016/j.annonc.2020.09.006
9. Yasmin E, Balachandren N, Davies MC, Jones GL, Lane S, Mathur R, et al. Fertility Preservation for Medical Reasons in Girls and Women: British Fertility Society Policy and Practice Guideline. Hum Fertil (Camb) (2018) 21(1):3–26. doi: 10.1080/14647273.2017.1422297
10. Breast Cancer Care: Standards of Care for Younger Women. Results From the Survey of Health Care Professionals . Available at: https://www.bbc.co.uk/news/health-30129324 (Accessed 3rd February 2022).
11. Kirkman M, Winship I, Stern C, Neil S, Mann GB, Fisher JRW. Women’s Reflections on Fertility and Motherhood After Breast Cancer and its Treatment. Eur J Cancer Care (Engl) (2014) 23:502–13. doi: 10.1111/ecc.12163
12. Stacey D, Volk RJ. The International Patient Decision Aid Standards (IPDAS) Collaboration: Evidence Update 2.0. Med Decis Making (2021) 41(7):729–33. doi: 10.1177/0272989X211035681
13. Standards Framework for Shared-Decision-Making Support Tools, Including Patient Decision Aids (NICE) (2021). Available at: https://www.nice.org.uk/corporate/ecd8/chapter/patient-decision-aid-development-process (Accessed 3rd February 2022).
14. An introduction to patient decision aids. (2012) Drug and therapeutics bulletin. 50(8):90–92. doi: 10.1136/dtb.2012.08.0121
15. Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision Aids for People Facing Health Treatment or Screening Decisions. Cochrane Database. Syst Rev (2017) 4(4):CD001431. doi: 10.1002/14651858.CD001431.pub5
16. Stacey D, Samant R, Bennett C. Decision Making in Oncology: A Review of Patient Decision Aids to Support Patient Participation. CA: Cancer J Clin (2008) 58:293–304. doi: 10.3322/CA.2008.0006
17. Sepucha KR, Abhyankar P, Hoffman AS, Bekker HL, LeBlanc A, Levin CA, et al. Standards for UNiversal Reporting of Patient Decision Aid Evaluation Studies: The Development of SUNDAE Checklist. BMJ Qual Saf (2018) 27:380–8. doi: 10.1136/bmjqs-2017-006986
18. International Patient Decision Aids Standards Collaboration. Criteria for Judging the Quality of Patient Decision Aids (2005). Available at: http://www.ipdas.ohri.ca/IPDAS_checklist.pdf (Accessed 3rd February 2022).
19. Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, Weijden T. A Systematic Development Process for Patient Decision Aids. BMC Med Inform Decis Mak (2013) 13(2):S2. doi: 10.1186/1472-6947-13-S2-S2
20. Ottawa Hospital Research Institute Patient Decision Aids Research Group. (2014). Available at: https://decisionaid.ohri.ca/eval.html (Accessed 3rd February 2022).
21. Winterbottom AE, Gavaruzzi T, Mooney A, Wilkie M, Davies SJ, Crane D, et al. Patient Acceptability of the Yorkshire Dialysis Decision AID (YODDA) Booklet: A Prospective Non-Randomized Comparison Study Across 6 Predialysis Services. Peritoneal Dialysis Int (2016) 36(4):374–81. doi: 10.3747/pdi.2014.00274
22. Medical Research Council. Developing and Evaluating Complex Interventions (2006). Available at: https://mrc.ukri.org/documents/pdf/complex-interventions-guidance (Accessed 3rd February 2022).
23. Jones GL, Hughes J, Mahmoodi N, Greenfield D, Brauten-Smith G, Skull J, et al. Observational Study of the Development and Evaluation of a FP Patient Decision Aid for Teenage and Adult Women Diagnosed With Cancer: The Cancer, Fertility and Me Research Protocol. BMJ Open (2017) 7(3):e013219. doi. 10.1136/bmjopen-2016-013219
24. O’Connor AM, Tugwell P, Wells G, Elmslie T, Jolly E, Hollingworth G. A Decision Aid for Women Considering Hormone Therapy After Menopause: Decision Support Framework and Evaluation. Patient Educ Couns (1998) 33(3):267–79. doi: 10.1016/S0738-3991(98)00026-3
25. Stacey D, Légaré F, Boland L, Lewis KB, Loiselle MC, Hoefel L, et al. 20th Anniversary Ottawa Decision Support Framework: Part 3 Overview of Systematic Reviews and Updated Framework. Med Decis Making (2020) 40(3):379–98. doi: 10.1177/0272989X20911870
26. Jones GL, Hughes J, Mahmoodi N, Smith E, Skull J, Ledger W. What Factors Hinder the Decision-Making Process for Women With Cancer Contemplating FP Treatment? Hum Reprod Update (2017) 23(4):433–57. doi: 10.1093/humupd/dmx009
27. Vogt KS, Hughes J, Wilkinson A, Mahmoodi N, Skull J, Wood H, et al. Preserving Fertility in Women With Cancer (PreFer): Decision-Making and Patient-Reported Outcomes in Women Offered Egg and Embryo Freezing Prior to Cancer Treatment. Psychooncology (2018) 27(12):2725–32. doi: 10.1002/pon.4866
28. McDougall S, Vogt KS, Wilkinson A, Skull J, Jones GL. Outcomes of Delivering a FP Service for Women With Cancer Over a 12-Year Period at a UK Assisted Conception Unit. J Obstet Gynaecol (2020) 40(2):252–9. doi: 10.1080/01443615.2019.1621823
29. Thewes B, Meiser B, Taylor A, Phillips KA, Pendlebury S, Capp A, et al. Fertility- and Menopause-Related Information Needs of Younger Women With a Diagnosis of Early Breast Cancer. J Clin Oncol (2005) 23(22):5155–65. doi: 10.1200/JCO.2005.07.773
30. Mazzocco K, Masiero M, Carriero Maria C, Pravettoni G. The Role of Emotions in Cancer Patients’ Decision-Making. Ecancermedicalscience(2019)13 914. doi: 10.3332/ecancer.2019.914
31. Peate M, Meiser B, Cheah B, Saunders C, Butow P, Thewes B, et al. Making Hard Choices Easier: A Prospective, Multicentre Study to Assess the Efficacy of a Fertility-Related Decision Aid in Young Women With Early-Stage Breast Cancer. Br J Cancer (2012) 106:1053–61. doi: 10.1038/bjc.2012.61
32. Garvelink MM, ter Kuile MM, Fischer MJ, Louwé LA, Hilders CG, Kroep JR, et al. Development of a Decision Aid About Fertility Preservation for Women With Breast Cancer in the Netherlands. J Psychosom Obstet Gynecol (2013) 34:170–8. doi: 10.3109/0167482X.2013.851663
33. Mahmoodi N, Bekker HL, King NV, Hughes J, Jones GL, Cancer, Fertility and Me research team. Are Publicly Available Internet Resources Enabling Women to Make Informed FP Decisions Before Starting Cancer Treatment: An Environmental Scan? BMC Med Inform Decis Mak (2018) 18(1):104. doi: 10.1186/s12911-018-0698-3
34. Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O'Connor MM. Validation of a Preparation for Decision Making Scale. Patient Educ Couns (2010) 78:130–3. doi: 10.1016/j.pec.2009.05.012
35. Moores KL, Jones GL, Radley SC. Development of an Instrument to Measure Face Validity, Feasibility and Utility of Patient Questionnaire Use During Health Care: The QQ-10. Int J Qual Health Care (2012) 24(5):517–24. doi: 10.1093/intqhc/mzs051
36. Braun V, Clarke V. Using Thematic Analysis in Psychology. Qual Res Psychol (2016) 3(2):77–101. doi: 10.1191/1478088706qp063oa
37. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and Preliminary Testing of the New Five-Level Version of EQ-5d (EQ-5d-5l). Qual Life Res (2011) 20(10):1727–36. doi: 10.1007/s11136-011-9903-x
38. O’Connor AM. Validation of a Decisional Conflict Scale. Med Decis Making (1995) 15(1):25–30. doi: 10.1177/0272989X9501500105
39. Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a Decision Regret Scale. Med Decis Making (2013) 23(4):281–92. 10.1177/0272989X03256005
40. Bastings L, Baysal O, Beerendonk CCM, IntHout J, Traas MAF, Verhaak CM, et al. Deciding About FP After Specialist Counselling. Hum Reprod (2014) 29:1721–9. doi: 10.1093/humrep/deu136
41. Peate M, Meiser B, Friedlander M, Zorbas H, Rovelli S, Sansom-Daly U, et al. It’s Now or Never: Fertility-Related Knowledge, Decision-Making Preferences, and Treatment Intentions in Young Women With Breast Cancer-an Australian Fertility Decision Aid Collaborative Group Study. J Clin Oncol (2011) 29(13):1670–7. doi: 10.1200/JCO.2010.31.2462
42. Mersereau J. Communication Between Oncofertility Providers and Patients. In: Gracia C, Woodruff TK, editors. Oncofertility Medical Practice. New York: Springer (2012).
43. Hollen P. J., Gralla R. J., Jones R. A., Thomas C. Y., Brenin D. R., Weiss G. R., et al. A Theory-Based Decision Aid for Patients With Cancer: Results of Feasibility and Acceptability Testing of DecisionKEYS for Cancer. Support Care Cancer (2013) 21(3):889–99. doi: 10.1007/s00520-012-1603-8
44. Adams E, Hill E, Watson E. Fertility Preservation in Cancer Survivors: A National Survey of Oncologists’ Current Knowledge, Practice and Attitudes. Br J Cancer (2012) 108:1602–15. doi: 10.1038/bjc.2013.139
45. Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, et al. The BCY3/BCC 2017 Survey on Physicians' Knowledge, Attitudes and Practice Towards Fertility and Pregnancy-Related Issues in Young Breast Cancer Patients. Breast (2018) 42:41–9. doi: 10.1016/j.breast.2018.08.099
46. Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C, et al. “Many Miles to Go …": A Systematic Review of the Implementation of Patient Decision Support Interventions Into Routine Clinical Practice. BMC Med Inform Decis Mak (2013) 13 Suppl 2:S14. doi: 10.1186/1472-6947-13-S2-S14
47. Breast Cancer Now. Younger Women Resources for Healthcare Professionals . Available at: https://breastcancernow.org/information-support/healthcare-professionals/younger-women-resources-healthcare-professionals (Accessed 3rd February 2022).
48. Bekker HL, Hewison J, Thornton JG. Understanding Why Decision Aids Work: Linking Process With Outcome. Patient Educ Couns (2003) 50(3):323–9. doi: 10.1016/S0738-3991(03)00056-9
49. Wang Y, Anazodo A, Logan S. Systematic Review of Fertility Preservation Patient Decision Aids for Cancer Patients. Psychooncology (2019) 28(3):459–67. doi: 10.1002/pon.4961
50. van den Berg M, van der Meij E, Bos A, Boshuizen M, Determann D, van Eekeren R, et al. Development and Testing of a Tailored Online Fertility Preservation Decision Aid for Female Cancer Patients. Cancer Med (2021) 10(5):1576–88. doi: 10.1002/cam4.3711
51. Ehrbar V, Urech C, Rochlitz C, Zanetti Dällenbach R, Moffat R, Stiller R, et al. Randomised Controlled Trial on the Effect of an Online Decision Aid for Young Female Cancer Patients Regarding Fertility Preservation. Hum Reprod (2019) 34(9):1726–34. doi: 10.1093/humrep/dez136
Keywords: patient decision aid, fertility preservation, cancer, women, mixed-method study, survivorship, gonadotoxic treatment
Citation: Jones GL, Moss RH, Darby F, Mahmoodi N, Phillips B, Hughes J, Vogt KS, Greenfield DM, Brauten-Smith G, Gath J, Campbell T, Stark D, Velikova G, Snowden JA, Baskind E, Mascerenhas M, Yeomanson D, Skull J, Lane S, Bekker HL and Anderson RA (2022) Cancer, Fertility and Me: Developing and Testing a Novel Fertility Preservation Patient Decision Aid to Support Women at Risk of Losing Their Fertility Because of Cancer Treatment. Front. Oncol. 12:896939. doi: 10.3389/fonc.2022.896939
Received: 15 March 2022; Accepted: 26 May 2022;
Published: 30 June 2022.
Edited by:
Yawei Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Enes Taylan, Mount Sinai Hospital, United StatesCopyright © 2022 Jones, Moss, Darby, Mahmoodi, Phillips, Hughes, Vogt, Greenfield, Brauten-Smith, Gath, Campbell, Stark, Velikova, Snowden, Baskind, Mascerenhas, Yeomanson, Skull, Lane, Bekker and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgina L. Jones, Zy5sLmpvbmVzQGxlZWRzYmVja2V0dC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.