
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 16 May 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.892539
Paraneoplastic neurological syndromes (PNSs) are a group of neurological disorders triggered by an underlying remote tumor. Ovarian teratoma (OT) is the most common histologic type of germ cell tumor in females. The most common PNSs associated with OT is anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. However, with the increasing number of new antibodies reported over the last decade, the clinical spectrum of OT-related PNSs is also expanding. Our knowledge of OT-related PNSs is still far from complete. Here, we provide a comprehensive review of the most recent findings in the field of OT-related PNSs, with a particular focus on their clinical and pathological characteristics. Overall, the description of neuronal antibodies in PNSs associated with OT strongly suggests that antibodies may be responsible for the clinical symptoms in some cases. OT-related PNSs are associated with various clinical manifestations, including anti-NMDAR encephalitis, limbic encephalitis, encephalomyelitis, progressive cerebellar syndrome and opsoclonus-myoclonus syndrome. The pathological characteristics of the OT suggest that the mechanism of PNSs is probably due to heteromorphic neurons in the tumor tissue, the ectopic expression of the antigens in neural tissue within the teratomas and patients’ unusual immune response. Despite the severity of the neurological syndromes, most patients with OT-related PNSs showed good neurologic response to early tumor resection combined with immunotherapy. To further advance the management of OT-related PNSs, additional studies are needed to explore this complex topic.
Paraneoplastic neurological syndromes (PNSs) are a group of neurological disorders triggered by an underlying remote tumor but not directly caused by cancer metastasis (1). PNSs usually develop before the diagnosis of the tumor. According to the recently updated diagnostic criteria for PNSs, PNSs mainly include the following clinical manifestations: encephalomyelitis, limbic encephalitis (LE), rapidly progressive cerebellar syndrome (PCD), opsoclonus-myoclonus syndrome (OMS), sensory neuronopathy, gastrointestinal pseudo-obstruction (enteric neuropathy), and Lambert-Eaton myasthenic syndrome (LEMS) (2). At present, small-cell lung cancer, breast cancer, testicular cancer, and lymphoma have all been reported as possible causes of PNSs. Ovarian teratoma (OT), which accounts for approximately 20% of all ovarian neoplasms, is one of the common tumor types causing PNSs (3, 4). Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a well-known PNSs associated with OT (5). However, with the increasing number of new antibodies reported over the last decade, the clinical spectrum of OT-related PNSs is expanding. For example, recent studies suggest that OT may trigger paraneoplastic neuromyelitis optica spectrum disorders (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated encephalomyelitis (MOG-EM), and anti-contactin-associated protein-like 2 (CASPR2) antibody-associated autoimmune encephalitis (6–9). Our knowledge of OT-related PNSs is still far from complete. The paucity of scientific understanding of OT-related PNSs results in delayed diagnosis and decision-making difficulties, creating a vicious cycle. In the present review, we describe OT-related PNSs as comprehensively as possible, focusing on the clinical spectrum and distinctive immunopathology of teratoma. Due to the low incidence of OT-associated PNSs, the current literatures are mostly case reports or case series studies and mainly focuses on anti-NMDAR encephalitis. These data will expand clinicians’ awareness about OT-related PNSs and allow them to perform the OT screening in appropriate patients, leading to early recognition of this condition, preventing more disability, and increasing further recovery.
The frequency of OT-related PNSs lacks objective and comprehensive documentation. To date, there have been only two reports showing the incidence rate of anti-NMDAR encephalitis in patients with ovarian teratoma. A Japanese single-center retrospective study, involving 343 patients from January 2008 to December 2016, found anti-NMDAR encephalitis in only 6 (1.17%) of all ovarian teratoma patients (10). The second one was retrospectively identified in a series of 233 Israeli patients, which found that anti-NMDAR encephalitis was diagnosed in 0.85% of women with mature teratomas (MTs) over 12 years (11). However, neither of the above publications analyzed PNSs other than anti-NMDAR encephalitis nor conducted comprehensive antibody screening.
Comparatively, three studies investigated whether neurologically asymptomatic individuals diagnosed with OT may have positive serum autoimmune antibodies. Two of the studies (recruited 10 German OT patients and 80 Chinese OT patients) aimed at detecting NMDAR antibodies showed that NMDAR antibodies were not detected in the serum of all neurologically asymptomatic patients with OT (12, 13). Another study recruiting 20 patients with OT conducted more extensive antibody testing (including (NMDAR-NR1a, NMDAR-NR1a/NR2b, a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor [AMPAR]-GluR1/GluR2, DPPX-IF1, DPPX-IF2, γ-aminobutyric acid receptor-B [GABAR]-B1/B2, leucine-rich glioma-inactivated protein 1 [LGI1], CASPR2, GLRA1b, GRM1, GRM5, MOG, Tr/DNER, anti-aquaporin-4 [AQP4], GAD65, GAD67, ZIC, ARHGAP26, Yo, amphiphysin, Hu, Ri, Ma1, Ma2, CV2, Sox-1, and recoverin) and found that none of the patients with OT had autoimmune antibodies (14). These data indicate that serum NMDAR antibody testing can be omitted in patients with OT unless complicating autoimmune encephalitis is suspected. However, due to the small samples included or the single type of antibodies tested in these studies, we need to be more cautious about these conclusions.
Based on previous studies, OT exhibited few gynecological symptoms (lower abdominal pain before the onset of acute torsion of ovarian teratoma) in patients with PNSs. The confirmation of OT is usually detected during further examination because of the combined PNSs.
The most common PNSs caused by OT is anti-NMDAR encephalitis (Table 1) (10, 15–39), which is an autoimmune neurological disease characterized by a clinical presentation of encephalitis and the presence of cerebrospinal fluid (CSF) antibodies against the GluN1 subunit of the NMDAR (15). The prevailing theory for the pathogenesis of anti-NMDAR encephalitis is that antibody-mediated injury to neurons is caused by antibodies generated against NMDARs on the teratoma that cross the blood brain barrier (Figure 1) (49, 50). It has been reported that OTs exist in almost 37.4% of cases with anti-NMDAR encephalitis, primarily in young females aged between 18 and 35 years (51). Some patients with anti-NMDAR encephalitis manifest prodromal symptoms, usually including fever and headache (28). In 2016, a team of experts proposed the following guidelines for the diagnosis of definite anti-NMDAR encephalitis. It can be retained when all three of the following criteria have been met (52): (1) rapid onset (less than 3 months) of at least four of the six major groups of symptoms, including (i) psychiatric symptoms or cognitive impairment, (ii) seizures, (iii) speech dysfunction, (iv) abnormal movements, (v) decreased consciousness, (vi) autonomic dysfunction or central hypoventilation; (2) positive CSF IgG anti-GluN1 antibodies; and (3) reasonable exclusion of other disorders. Importantly, anti-NMDAR encephalitis with OT had a more severe clinical presentation. Dai et al. reported that the incidence of fever, decreased consciousness, autonomic dysfunction, central hypoventilation and intensive unit care were significantly higher in patients with OT than in those without OT (53). Similarly, Jiang et al. observed that patients with OT required more frequently mechanical ventilation and intensive care, and had higher CSF antibody titers (54).
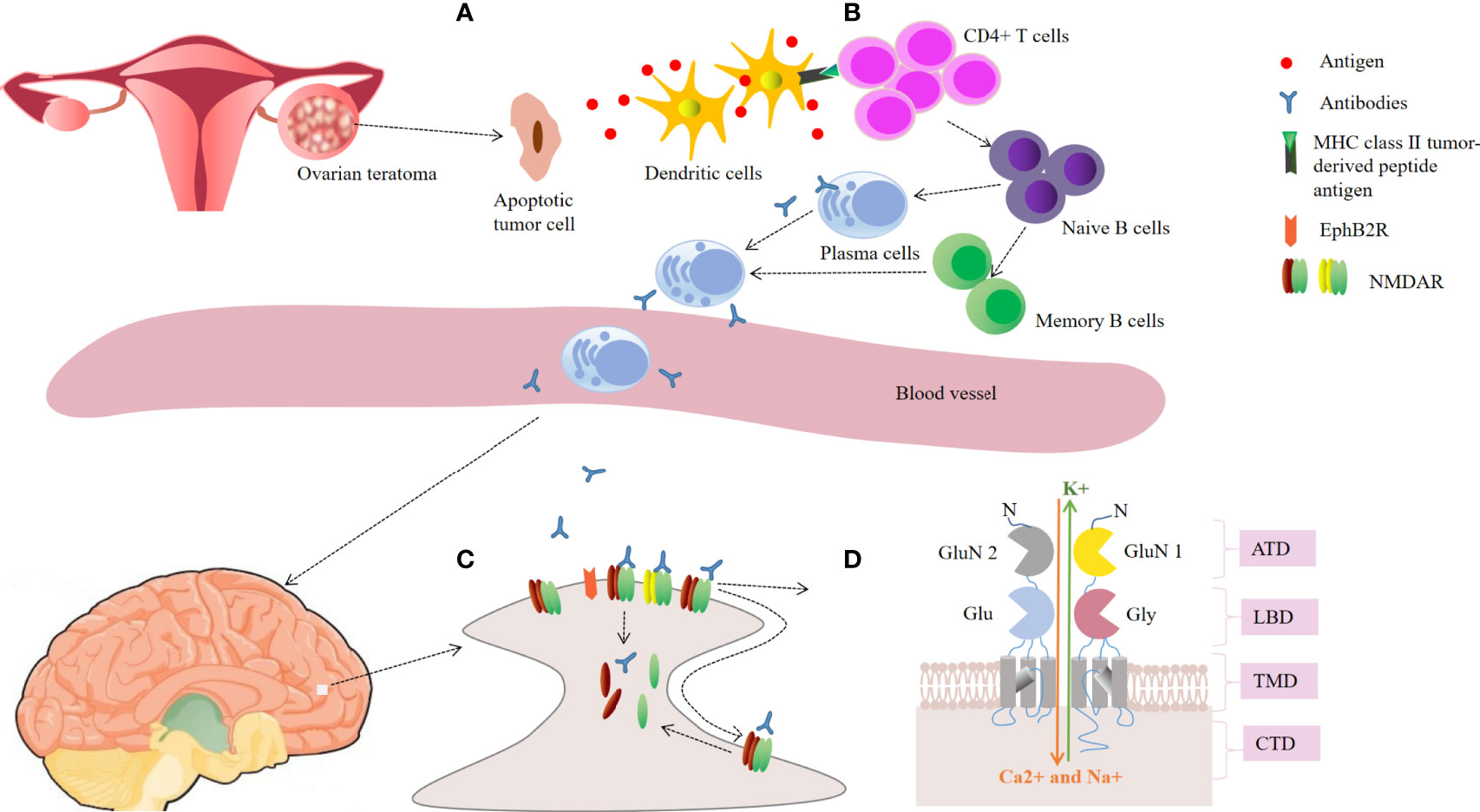
Figure 1 Schematic representation of the possible pathogenesis in ovarian teratoma-related anti-NMDAR encephalitis. (A) Ovarian teratoma contains apoptotic tumor cells, neuroglial cells and inflammatory infiltrates. Antigens of N-methyl-d-aspartate receptor (NMDAR) released by apoptotic tumor cells are loaded into dendritic cells; (B) The antigen is presented to naive B cells by mature dendritic cells in cooperation with CD4 T cells, leading to generation of plasma cells and memory B cells. Plasma cells migrate into the brain and generate large amounts of IgG autoantibodies; (C) The autoantibodies lead to receptor cross-linking and internalization, and disrupting the interaction between NMDAR and Ephrin-B2 receptor (EphB2R), thus reducing the number of NMDARs on the neuronal surface; (D) The GluN1 and GluN2 subunits of the NMDAR. Antibodies pre-dominantly bind to an epitope region in the amino-terminal domain (ATD) of GluN1. CTD, carboxyl-terminal domain; LBD, ligand binding domain; TMD, transmembrane domain.
Evidence of OT-related LE other than anti-NMDAR encephalitis is based on case series and reports in the context of the detection of a novel antibody. LE is characterized by the subacute progression (less than 3 months) of seizures, working memory deficits, or psychiatric symptoms (52). According to the updated diagnostic criteria of LE, antibody status is not needed, which could delay the diagnosis. However, the identification of autoimmune antibodies remains important, especially to identify the immunological subgroup of LE. Some of the onconeural and neuronal surface antibodies associated with OT-related LE, such as AMPAR, CASPR2 and Ri antibodies, have been reported recently (9, 40, 42).
The term encephalomyelitis should be used to describe those patients with clinical dysfunction at multiple sites of the nervous system, including LE, brainstem encephalitis, PCD, myelitis (anterior horn), sensory neuronopathy (dorsal root ganglia), and chronic gastrointestinal pseudo-obstruction (55). The evidence for paraneoplastic encephalomyelitis associated with OT is based on a small sample series or case reports. Some cases were combined with antineuronal antibodies (such as anti-Yo, anti-leucine zipper 4 [LUZP4], and anti-MOG antibodies) (6, 41, 44, 47, 48). some reports without screening antibodies, and in some cases, antibodies were not found, although extensive antibody screening was performed (56, 57).
PCD manifests clinically as incoordination of movements (ataxia), balance and gait disturbances, speech disorder (dysarthria), and altered ocular movements (nystagmus, often in a downbeat form) (55). In a large retrospective review of 249 patients with teratoma-related encephalitis, 22 (4%) developed PCD (58). Another type of antibody found in PCD was kelch-like protein 11 (KLHL11), which is a new antibody that has been described by Mandel-Brehm et al. (59). Anti-KLHL11 antibody seropositive status suggests testicular germ-cell tumors or breast cancer. Surprisingly, a recent study found that among 16 female patients with positive KLHL11 antibodies, 10 (62.5%) patients had OTs. Two patients had PCD, 5 had anti-NMDAR encephalitis (with concurrent anti-NMDAR antibodies), 1 (10%) had opsoclonus-myoclonus and chronic psychosis, and 1 (10%) had subacute encephalopathy (43). However, a recent study found discordant results: in a French nationwide study, patients with KLHL11 antibodies showed a homogeneous clinical syndrome. All the patients were male, with a predominantly cerebellar/brainstem syndrome. Most of them had an associated testicular “burned-out” seminoma, and a large group of anti-NMDAR patients with associated OT were tested for KLHL11, with negative results (60).
OMS is a rare syndrome characterized by opsoclonus, which is spontaneous, irregular, multivectorial saccadic eye movements, along with focal or diffuse myoclonus and sometimes ataxia (55). In addition to KLHL antibodies as described above, one case of anti-NMDAR antibody-positive OT-associated OMS has been reported (29). In a large series with OMS (n = 114), 39% (45/114) of patients had paraneoplastic OMS. Among the patients with paraneoplastic OMS, 18% (8/45) had OTs, but all of them were NMDA antibody-negative (61).
NMOSD is a relapsing inflammatory disease characterized by transverse myelitis and optic neuritis (ON) (52). The disease usually has seropositivity for AQP4-IgG in up to 80% of patients. In a recent review, Ikeguchi et al. analyzed 6 patients with AQP4-IgG–seropositive NMOSD associated with OT. All 6 patients presented with transverse myelitis, 2 with ON, and 5 with nausea and/or vomiting. In one case, anti-NMDAR encephalitis developed before NMOSD (7).
Autoimmune GFAP astrocytopathy is a new autoimmune CNS disease diagnosable by GFAP-IgG testing in CSF (62). It may affect broad CNS regions, including subcortical white matter, basal ganglia, hypothalamus, cerebellum, brainstem, and spinal cord, although linear enhancement oriented radially to the ventricles is typical. Primary clinical manifestations of autoimmune GFAP astrocytopathy include fever, headache, abnormal vision, myelitis, encephalopathy, involuntary movement, and autonomic dysfunction (63). Approximately one in four individuals has a coexisting neoplasm, most commonly OT (63). In addition to GFAP-antibodies, the coexistence of anti-NMDAR and/or AQP4 antibodies was observed in more than half of the autoimmune GFAP astrocytopathy patients with OT (serologic complexity) (64).
In most patients with encephalitis, EEG may show a nonspecific pattern, including slow background activity, bilateral or unilateral temporal lobe epileptic discharges, and periodic lateralized epileptiform discharge. However, EEG may be helpful in patients with anti-NMDAR-encephalitis. It has been reported that one-third of anti-NMDAR-encephalitis patients showed extreme delta brush on EEG, although these EEG abnormalities are not specific for anti-NMDAR encephalitis (65).
In most cases of OT-related PNSs associated with cell-surface abs (e.g., antibodies against NMDARs and AMPARs), inflammatory changes and positive antibodies in CSF were observed (28, 40). In addition, 67% of cases with OT-related NMOSD showed mild to moderate CSF pleocytosis (7). Only a few OT-related PNSs cases associated with onconeuronal antibodies reported CSF analysis, two cases described elevated CSF protein and mild to moderate pleocytosis, and one case showed CSF oligoclonal bands positivity (41).
Interestingly, Jiang et al. observed higher CSF NMDAR antibody titers and increasing trends of cytokine/chemokines (including interleukin [IL]-10, IL-1α, tumor necrosis factor-α, granulocyte-macrophage colony-stimulating factor, and vascular endothelial growth factor A) in anti-NMDAR encephalitis patients with OT than in patients without tumors (54), which indicated an inflammatory process in the pathogenesis of paraneoplastic anti-NMDAR encephalitis. Although some experts proposed that the immune response of OT may trigger BBB dysfunction in PNSs, studies that measure brain barrier dysfunction (CSF and serum oligoclonal band, CSF albumin quotient, or intrathecal IgG synthesis rate) specifically for patients with OT-related PNSs are limited.
Although neuroimaging studies are not key to establishing the diagnosis of PNS, they should be performed to exclude other diseases responsible for neurologic symptoms. In patients with OT-associated PNSs, MRI abnormalities are associated with PNSs type, e.g., PCD patients reveal cerebellar atrophy, anti-NMDAR encephalitis patients show abnormalities in various brain regions (such as the hippocampus, basal ganglia, brainstem, cerebral cortex, and insular regions), and NMOSD patients show characteristic dorsal brainstem lesions on MRI (7, 58, 66).
Importantly, if PNS is suspected, systemic imaging of the underlying tumor should be performed. Transvaginal ultrasound (may not be feasible in young patients) and/or MRI- elvis/abdomen is the investigation of choice for these patients. If negative, CT-chest searching for extra-pelvic teratomas is recommended. If initial examinations do not detect any neoplasm, it is recommended to repeat them every 6 months for 2 years (67, 68).
The histopathology of anti-NMDAR encephalitis associated OT has not been fully elucidated but limited studies have focused on alterations in immune cell populations and neuroglial tissues (Table 2) (16–26). In patients with anti-NMDAR encephalitis, the most common comorbid tumor type was unilateral MTs. Immature teratomas (ITs) represent only about 12-26% of all ovarian teratomas in patients with anti-NMDAR encephalitis (51). The diameter of the anti-NMDAR encephalitis associated OT is not very large (average 3.48 cm, ranging from 0.5 to 22 cm) when viewed grossly (16, 26). Anti-NMDAR encephalitis associated OT may not exhibit significant features after specimen dissection.
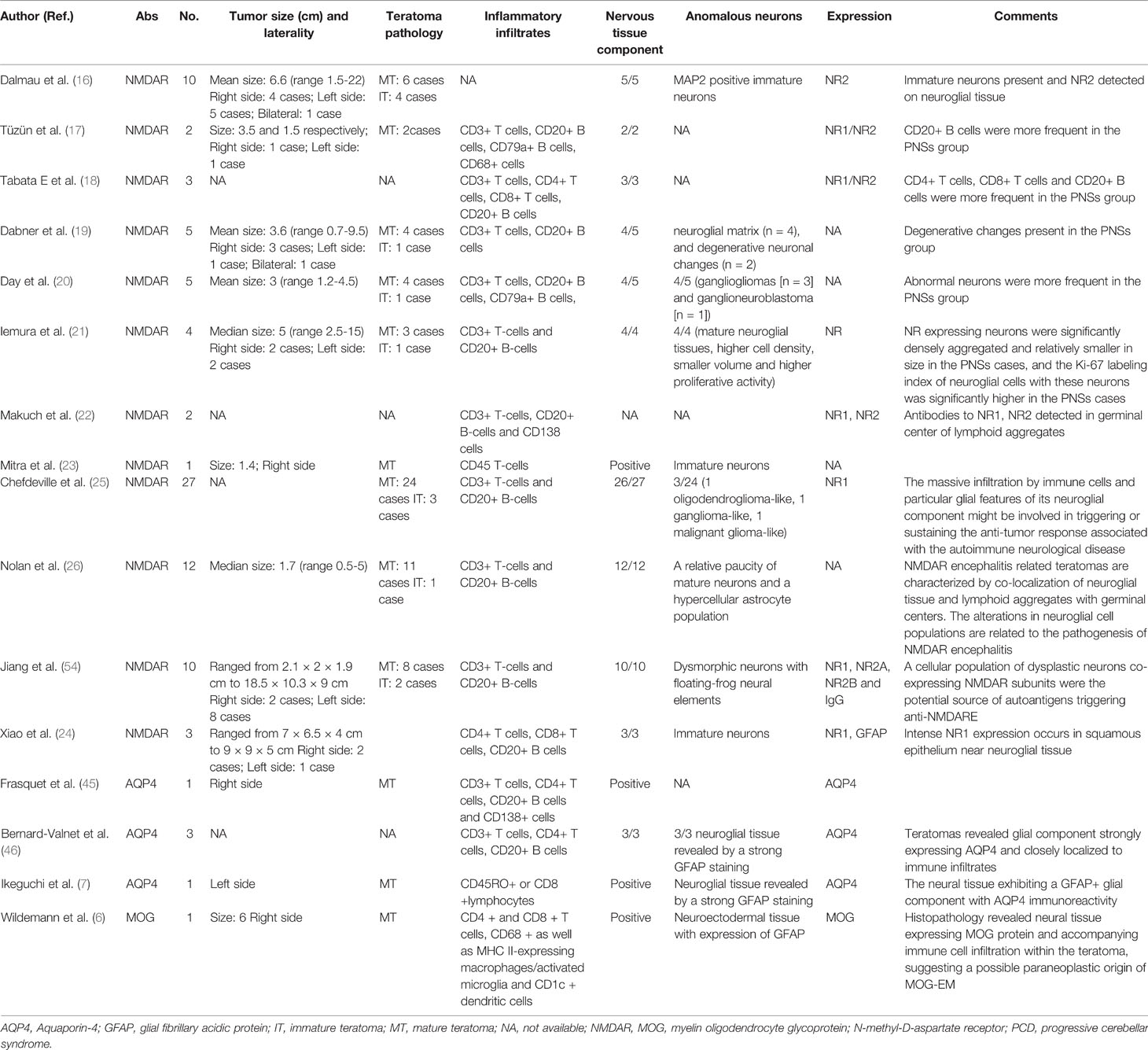
Table 2 Pathological characteristics in teratoma tissues in patients with paraneoplastic neurological syndromes.
There are two main features of basic tissue staining for anti-NMDAR encephalitis-associated OT. First, nervous tissues were observed with higher frequencies in teratomas resected from anti-NMDAR encephalitis cases than controls (25). Moreover, aggregates of dysmorphic neurons (for example, floating-frog like dysplastic neurons, gangliogliomas, and ganglioneuroblastoma) were observed more frequently in anti-NMDAR encephalitis associated teratomas than sporadic teratomas (20, 26, 54). The association of these abnormal neurons within teratomas in the pathogenesis of anti-NMDAR encephalitis is unknown; one potential explanation is that these dysmorphic neurons may provide the nidus for an immune response triggering NMDAR antibody formation against tumor antigens (26). Second, the reduced density of mature neurons and degenerative changes in the neurons have been described in previous studies (19, 26). Nolan et al. suggested that these changes represent the result of sustained autoimmune injury to the neurons, containing a spectrum of damage ranging from degenerative changes to cell loss (26). Regarding the immunopathology of anti-NMDAR encephalitis associated OT, the striking particularity was that lymphoid B cells, T cells, and mature dendritic cells infiltration were predominant in the tumor tissue of the anti-NMDAR encephalitis group, and the inflammatory infiltrate was mostly adjacent to the receptor-positive nervous tissue or squamous epithelium in the teratoma tissue (18, 20, 24, 25, 54). Remarkably, CD20+ B cells were more frequent in anti-NMDAR encephalitis associated OT than in control OT, while CD8+ T cells were less frequent in anti-NMDAR encephalitis associated OT (25, 54). These immune cell populations suggest that humoral immunity plays an important role in anti-NMDAR encephalitis associated OT. In addition, the expression of the NR1, NR2A, and NR2B subunits by the teratoma nervous tissue was significantly more often glial in anti-NMDAR encephalitis associated OT than in control OT (18, 21, 25, 54). Specifically, immunofluorescence showed consistent colocalization of these NMDAR subunits with IgG, supporting the notion that dysmorphic neurons with coexpression of NR1/NR2A/NR2B and IgG in OT play a significant role in the pathogenesis of anti-NMDAR encephalitis (25, 54). Elevated proliferative activity was found in anti-NMDAR encephalitis-associated OT by using Ki-67 immunohistochemistry (24). Unfortunately, to date, no tumor markers are available to differentiate teratomas that may cause anti-NMDAR encephalitis. Last, although a study found significantly elevated levels of HLA-A and HLA-DBR1 in teratoma tissues from patients with anti-NMDAR encephalitis (69), the association of both alleles with disease susceptibility was weak and needs confirmation.
Due to the low incidence of OT-associated NMOSD, to date, there have been 5 reported cases describing pathologic findings of AQP4-IgG–seropositive NMOSD associated with OT (7, 45, 46). In all of these patients, teratomas revealed the expression of AQP4 and GFAP in areas containing neuroglial tissue. These areas were surrounded by intense inflammatory infiltrates, mainly composed of CD20 B+ cells. Only a few CD8+ T cells were observed in reported cases (7, 45, 46). These findings suggest that teratomas featuring AQP4-positive neural tissue accompanied by infiltrating lymphocytes contribute to NMOSD development. To our knowledge, only Wildemann et al. have previously described the pathological characteristics of MOG-EM associated teratoma and indicated the presence of MOG protein within the tumor (6). Analysis of tumor tissue from one Caucasian patient revealed teratoma-contained neuroectodermal tissue with the expression of GFAP. A detailed examination of the neuroectodermal tissue showed oligodendrocytes and axons with intermittent myelination, as demonstrated by the expression of oligodendroglial markers, including MOG. In addition, inflammatory infiltrates with CD4+ and CD8+ T lymphocytes were found. Additionally, CD68+ and MHC II-expressing macrophages/activated microglia and CD1c+ dendritic cells were observed in and immediately adjacent to the neuroectodermal tissue. Interestingly, CD20+ B cells and CD138-positive plasma cells were not observed in the teratoma, which was different from AQP4-IgG–seropositive OT and anti-NMDAR encephalitis associated OT (6).
Regardless of the type of PNSs, pathological features of OT include the expression of specific epitopes for autoantibodies and inflammatory infiltrate in the CNS-like tissue component. Whether mechanisms of mimicry toward protein expression or breakdown of immunologic tolerance are involved in OT-related PNSs is unknown. Dysmorphic neurons are currently only reported in anti-NMDAR encephalitis, and whether this abnormality exists in other types of PNSs needs to be further explored. The immunopathological findings of AQP4-IgG–seropositive OT were similar to those of anti-NMDAR encephalitis-associated OT. However, although it is difficult to draw definitive conclusions with only one case, we cannot rule out that OT may have different mechanisms for MOG-EM and anti-NMDAR encephalitis based on immunopathological findings. Given the small number of reported cases, our understanding of the pathological features of OT in PNSs is still limited.
The therapeutic methods of OT-related PNSs consist of tumor resection, immunotherapy, and symptom control. First-line immunotherapy includes corticosteroids, intravenous immunoglobulins, and plasma exchange. Second-line immunotherapy involves cyclophosphamide, rituximab, and mycophenolate mofetil (28). In addition, the treatment of immature teratoma requires chemotherapy according to its stage and postoperative histopathological grading. It has been reported that early tumor resection combined with immunotherapy markedly improves outcomes and prevents more severe neurological deterioration (15). Importantly, tumor resection has been reported to be more beneficial than immunotherapy in stopping neurological progression (70). In several cases with OT, severe neurological symptoms are reversible if the neoplasm is removed, and some do not even require additional immunotherapy. Previous studies suggest that critical systemic and neurological complications should not be considered contraindications for surgery (53). If teratoma resection is delayed, continued antigen presentation induces the production of long-lived plasma cells and increased antibody affinity, rendering the patient ineffective for late resection of the tumor and unresponsive to immunotherapy. Female patients with PNSs symptoms, especially those who do not respond to immunosuppressive therapy, should be checked for OT immediately. Prophylactic oophorectomy is not recommended in anti-NMDAR encephalitis without detectable OT. However, in selected patients with severe neurological involvement and proven lack of response to first- and second- line immunotherapies for more than 2-3 months, blind oophorectomy might be considered after carefully weighing the benefit/risk ratio of the procedure (2, 71).
Generally, in patients with PNSs, the response to therapy and prognosis depended on the underlying immune mechanism. Patients with antibodies against intracellular (onconeural) antigens (mediated by T cells) are more likely to have poor responses than patients with antibodies against surface or synaptic antigens (B cell mechanism) (72). The subgroup of patients with OT-associated anti-NMDAR encephalitis seems to have a good prognosis with partial or full recovery in about 80% of patients (50). Of note, a few cases experienced severe disability or even death. These patients often had treatment delays and serious complications, or their tumor tissue proved to be ITs. In addition, most of the patients with PNSs (including AQP4-IgG+ NMOSD, MOG-EM) had full recovery or almost full recovery after removal of teratoma and immunotherapy (6, 7). Improvement was observed in cases with OT-related anti-Ri encephalitis or anti-Yo encephalitis (41, 42). Due to the small number of patients that have been identified to date, further reports may be helpful to understand the therapeutic effect of OT-related PNSs.
Several studies have shown that early teratoma resection helps to reduce the recurrence of anti-NMDAR encephalitis (28, 53), and interestingly, previously published cases suggest that this condition seems to also be applicable for AQP4-IgG+ NMOSD and MOG-EM (6, 7). For example, it has been reported that about 30% of patients with AQP4-IgG+ NMOSD experience relapse despite treatment. However, in patients with AQP4-IgG+ NMOSD associated with OT, only 16.7% (1/6) of patients experienced relapse after removal of teratoma. Similarly, approximately 50% and 80% of patients who developed MOG-EM relapsed within the first 2 years and 5 years, respectively (73). However, in patients with MOG-EM associated with OT, none of them experienced recurrence after teratoma resection.
Our knowledge of OT-related PNSs is still far from complete and remains to be investigated. First, studies have been conducted on large samples of patients with other tumors commonly associated with PNSs, such as small cell lung cancer and melanoma. However, there have been no large-scale prospective or retrospective studies investigating various autoantibodies in patients with OT. Our review suggests that it would be worthwhile to test for a panel of autoantibodies and not just specific autoantibodies against NMDAR-IgG in patients with OT presenting with PNSs. Second, there are few studies on the pathophysiology of OT-related PNSs. OT-related PNSs other than anti-NMDAR encephalitis are case reports. Therefore, whether there are pathophysiological differences in different types of OT-related PNSs is not yet known. Third, recent studies have reported that PNSs are not related to a particular histological tumor type but rather to a specific molecular signature of tumor cells. These studies suggested that tumor genetic alterations are the initial triggers. However, the genetic analysis in PNSs and OTs remains unknown.
Taken together, clinicians should be aware that in addition to anti-NMDAR encephalitis, OT may also be associated with other rare PNSs. These PNSs have to be recognized and managed effectively during the course of OT because they often respond well to tumor resection. Although significant progress has been made in understanding the pathogenesis and heterogeneity of diseases through molecular and immunopathology, further work is needed to investigate the pathophysiological process by which ovarian teratoma leads to PNSs.
JFL and MW drafted the manuscript. JW collected the data. JML conceptualized and designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Supported by the National Natural Science Foundation of China (grants 82071459) and the Institute of Brain science and Brain-inspired technology of West China Hospital, Sichuan University (grants ZYJC21001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Albert ML, Darnell RB. Paraneoplastic Neurological Degenerations: Keys to Tumour Immunity. Nat Rev Cancer (2004) 4(1):36–44. doi: 10.1038/nrc1255
2. Graus F, Vogrig A, Muniz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol Neuroimmunol Neuroinflamm (2021) 8(4):e1014. doi: 10.1212/NXI.0000000000001014
3. Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Munstedt K. Squamous-Cell Carcinoma in Mature Cystic Teratoma of the Ovary: Systematic Review and Analysis of Published Data. Lancet Oncol (2008) 9(12):1173–80. doi: 10.1016/S1470-2045(08)70306-1
4. Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, et al. Molecular Characteristics of Malignant Ovarian Germ Cell Tumors and Comparison With Testicular Counterparts: Implications for Pathogenesis. Endocr Rev (2013) 34(3):339–76. doi: 10.1210/er.2012-1045
5. Zaborowski MP, Spaczynski M, Nowak-Markwitz E, Michalak S. Paraneoplastic Neurological Syndromes Associated With Ovarian Tumors. J Cancer Res Clin Oncol (2015) 141(1):99–108. doi: 10.1007/s00432-014-1745-9
6. Wildemann B, Jarius S, Franz J, Ruprecht K, Reindl M, Stadelmann C. MOG-Expressing Teratoma Followed by MOG-IgG-Positive Optic Neuritis. Acta Neuropathol (2021) 141(1):127–31. doi: 10.1007/s00401-020-02236-5
7. Ikeguchi R, Shimizu Y, Shimomura A, Suzuki M, Shimoji K, Motohashi T, et al. Paraneoplastic AQP4-IgG-Seropositive Neuromyelitis Optica Spectrum Disorder Associated With Teratoma: A Case Report and Literature Review. Neurol Neuroimmunol Neuroinflamm (2021) 8(5):e1045. doi: 10.1212/NXI.0000000000001045
8. Tardo L, Wang C, Rajaram V, Greenberg BM. Pediatric Paraneoplastic Neuromyelitis Optica Spectrum Disorder Associated With Ovarian Teratoma. Mult Scler (2022) 28(1):160–3. doi: 10.1177/13524585211037582
9. Herrmann ML, Bruchner N, Martin P, Wildgruber D. Psychosis Associated to CASPR2 Autoantibodies and Ovarian Teratoma: A Case Report. Psychiatry Res (2020) 285:112725. doi: 10.1016/j.psychres.2019.112725
10. Yaguchi H, Tsuji T, Yabe I, Hirayama E, Nomura T, Ohashi I, et al. Incidence of Anti-NMDAR Encephalitis in Patients Undergoing Resection of Ovarian Teratoma in a Single Institution. J Neurol Sci (2020) 409:116608. doi: 10.1016/j.jns.2019.116608
11. Pekar-Zlotin M, Rabinovich I, Goldrat I, Vaknin Z, Gidoni Y, Zur-Naaman H, et al. Ovarian Dermoid Cysts Associated With Paraneoplastic Syndrome N-Methyl-D-Aspartic Acid Receptor Antibodies Encephalitis. J Minim Invasive Gynecol (2021) 28(6):1190–3. doi: 10.1016/j.jmig.2020.09.018
12. Trillsch F, Eichhorn P, Oliveira-Ferrer L, Kuempfel T, Burges A, Mahner S, et al. No Need for NMDA Receptor Antibody Screening in Neurologically Asymptomatic Patients With Ovarian Teratomas. J Neurol (2018) 265(2):431–2. doi: 10.1007/s00415-017-8717-3
13. Gong SY, Zhou MK, Shi G, Guo J, Chen N, Yang R, et al. Absence of Nmda Receptor Antibodies in Patients With Ovarian Teratoma Without Encephalitis. Neurol-Neuroimmunol (2017) 4(3):e344. doi: 10.1212/NXI.0000000000000344
14. Mangler M, Trebesch de Perez I, Teegen B, Stocker W, Pruss H, Meisel A, et al. Seroprevalence of Anti-N-Methyl-D-Aspartate Receptor Antibodies in Women With Ovarian Teratoma. J Neurol (2013) 260(11):2831–5. doi: 10.1007/s00415-013-7074-0
15. Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-Receptor Encephalitis: Case Series and Analysis of the Effects of Antibodies. Lancet Neurol (2008) 7(12):1091–8. doi: 10.1016/S1474-4422(08)70224-2
16. Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic Anti-N-Methyl-D-Aspartate Receptor Encephalitis Associated With Ovarian Teratoma. Ann Neurol (2007) 61(1):25–36. doi: 10.1002/ana.21050
17. Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for Antibody-Mediated Pathogenesis in Anti-NMDAR Encephalitis Associated With Ovarian Teratoma. Acta Neuropathol (2009) 118(6):737–43. doi: 10.1007/s00401-009-0582-4
18. Tabata E, Masuda M, Eriguchi M, Yokoyama M, Takahashi Y, Tanaka K, et al. Immunopathological Significance of Ovarian Teratoma in Patients With Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Eur Neurol (2014) 71(1-2):42–8. doi: 10.1159/000353982
19. Dabner M, McCluggage WG, Bundell C, Carr A, Leung Y, Sharma R, et al. Ovarian Teratoma Associated With Anti-N-Methyl D-Aspartate Receptor Encephalitis: A Report of 5 Cases Documenting Prominent Intratumoral Lymphoid Infiltrates. Int J Gynecol Pathol (2012) 31(5):429–37. doi: 10.1097/PGP.0b013e31824a1de2
20. Day GS, Laiq S, Tang-Wai DF, Munoz DG. Abnormal Neurons in Teratomas in NMDAR Encephalitis. JAMA Neurol (2014) 71(6):717–24. doi: 10.1001/jamaneurol.2014.488
21. Iemura Y, Yamada Y, Hirata M, Kataoka TR, Minamiguchi S, Haga H. Histopathological Characterization of the Neuroglial Tissue in Ovarian Teratoma Associated With Anti-N-Methyl-D-Aspartate (NMDA) Receptor Encephalitis. Pathol Int (2018) 68(12):677–84. doi: 10.1111/pin.12732
22. Makuch M, Wilson R, Al-Diwani A, Varley J, Kienzler AK, Taylor J, et al. N-Methyl-D-Aspartate Receptor Antibody Production From Germinal Center Reactions: Therapeutic Implications. Ann Neurol (2018) 83(3):553–61. doi: 10.1002/ana.25173
23. Mitra AD, Afify A. Ovarian Teratoma Associated Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Difficult Diagnosis With a Favorable Prognosis. Autops Case Rep (2018) 8(2):e2018019. doi: 10.4322/acr.2018.019
24. Xiao Y, Li J, Liu L, Xiong J, Xu J, Liang Q, et al. Clinicopathological Characteristics of Dysplastic Teratomous Neuroglia With Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Clin Immunol (2020) 210:108271. doi: 10.1016/j.clim.2019.108271
25. Chefdeville A, Treilleux I, Mayeur ME, Couillault C, Picard G, Bost C, et al. Immunopathological Characterization of Ovarian Teratomas Associated With Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Acta Neuropathol Commun (2019) 7(1):38. doi: 10.1186/s40478-019-0693-7
26. Nolan A, Buza N, Margeta M, Rabban JT. Ovarian Teratomas in Women With Anti-N-Methyl-D-Aspartate Receptor Encephalitis: Topography and Composition of Immune Cell and Neuroglial Populations Is Compatible With an Autoimmune Mechanism of Disease. Am J Surg Pathol (2019) 43(7):949–64. doi: 10.1097/PAS.0000000000001249
27. Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti-N-Methyl-D-Aspartate Receptor (NMDAR) Encephalitis in Children and Adolescents. Ann Neurol (2009) 66(1):11–8. doi: 10.1002/ana.21756
28. Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and Prognostic Factors for Long-Term Outcome in Patients With Anti-NMDA Receptor Encephalitis: An Observational Cohort Study. Lancet Neurol (2013) 12(2):157–65. doi: 10.1016/S1474-4422(12)70310-1
29. Urriola NX, Helou J, Maamary J, Pogson J, Lee F, Parratt K, et al. NMDA Receptor Antibody in Teratoma-Related Opsoclonus-Myoclonus Syndrome. J Clin Neurosci (2018) 58:203–4. doi: 10.1016/j.jocn.2018.10.011
30. Bost C, Chanson E, Picard G, Meyronet D, Mayeur ME, Ducray F, et al. Malignant Tumors in Autoimmune Encephalitis With Anti-NMDA Receptor Antibodies. J Neurol (2018) 265(10):2190–200. doi: 10.1007/s00415-018-8970-0
31. Zhang L, Lu Y, Xu L, Liu L, Wu X, Zhang Y, et al. Anti-N-Methyl-D-Aspartate Receptor Encephalitis With Accompanying Ovarian Teratoma in Female Patients From East China: Clinical Features, Treatment, and Prognostic Outcomes. Seizure (2020) 75:55–62. doi: 10.1016/j.seizure.2019.12.016
32. Xu X, Lu Q, Huang Y, Fan S, Zhou L, Yuan J, et al. Anti-NMDAR Encephalitis: A Single-Center, Longitudinal Study in China. Neurol Neuroimmunol Neuroinflamm (2020) 7(1):e633. doi: 10.1212/NXI.0000000000000633
33. Chiu HC, Su YC, Huang SC, Chiang HL, Huang PS. Anti-NMDAR Encephalitis With Ovarian Teratomas: Review of the Literature and Two Case Reports. Taiwan J Obstet Gyne (2019) 58(3):313–7. doi: 10.1016/j.tjog.2019.03.004
34. Yu M, Li S, Cheng J, Zhou L, Jiang Z, Di W. Ovarian Teratoma-Associated Anti-NMDAR Encephalitis: A Single-Institute Series of Six Patients From China. Arch Gynecol Obstet (2021) 303(5):1283–94. doi: 10.1007/s00404-020-05861-3
35. Ahmad J, Sohail MS, Khan A, Qavi AH, Gaudel P, Zahid M, et al. Anti-N-Methyl-D-Aspartate-Receptor (NMDAR) Encephalitis in Association With Ovarian Teratoma. Cureus (2017) 9(7):e1425. doi: 10.7759/cureus.1425
36. Lwin S, San Yi M, Mardiana K, Woon SY, Nwe TM. Ovarian Teratoma-Associated Anti-NMDAR Encephalitis in a 12-Year-Old Girl. Med J Malaysia (2020) 75(6):731–3.
37. Lee CH, Kim EJ, Lee MH, Yim GW, Kim KJ, Kim KK, et al. Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Rare Complication of Ovarian Teratoma. J Korean Med Sci (2020) 35(24):e207. doi: 10.3346/jkms.2020.35.e207
38. Chernyshkova I, Estefan B, Hoque MR, Lee A. Neurologic Presentation of Probable Seronegative Paraneoplastic Encephalitis in a Woman With an Ovarian Teratoma. Cureus (2020) 12(6):e8485. doi: 10.7759/cureus.8485
39. Li W, Jia D, Tong L, Lun Z, Li H. Anti-N-Methyl-D-Aspartate Receptor Encephalitis Induced by Bilateral Ovarian Teratomas With Distinct Histopathologic Types: A Case Report and Brief Literature Review. Med (Baltimore) (2019) 98(48):e18148. doi: 10.1097/MD.0000000000018148
40. Hoftberger R, van Sonderen A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, et al. Encephalitis and AMPA Receptor Antibodies: Novel Findings in a Case Series of 22 Patients. Neurology (2015) 84(24):2403–12. doi: 10.1212/WNL.0000000000001682
41. Jurkiewicz E, Kotulska K, Nowak K, Malczyk K, Borkowska J, Bilska M. Severe Central and Peripheral Paraneoplastic Demyelination Associated With Tumours of the Ovaries. Childs Nerv Syst (2015) 31(9):1601–6. doi: 10.1007/s00381-015-2731-5
42. Fadare O, Hart HJ. Anti-Ri Antibodies Associated With Short-Term Memory Deficits and a Mature Cystic Teratoma of the Ovary. Int Semin Surg Oncol (2004) 1(1):11. doi: 10.1186/1477-7800-1-11
43. Maudes E, Landa J, Munoz-Lopetegi A, Armangue T, Alba M, Saiz A, et al. Clinical Significance of Kelch-Like Protein 11 Antibodies. Neurol-Neuroimmunol (2020) 7(3):e666. doi: 10.1212/NXI.0000000000000666
44. Dubey D, Kryzer T, Guo Y, Clarkson B, Cheville JC, Costello BA, et al. Leucine Zipper 4 Autoantibody: A Novel Germ Cell Tumor and Paraneoplastic Biomarker. Ann Neurol (2021) 89(5):1001–10. doi: 10.1002/ana.26050
45. Frasquet M, Bataller L, Torres-Vega E, Duran-Moreno M, Garcia-Verdugo JM, Sevilla T, et al. Longitudinally Extensive Transverse Myelitis With AQP4 Antibodies Revealing Ovarian Teratoma. J Neuroimmunol (2013) 263(1-2):145–7. doi: 10.1016/j.jneuroim.2013.07.003
46. Bernard-Valnet R, Cobo-Calvo A, Siegfried A, Marasescu R, Bonnan M, Ballan G, et al. Paraneoplastic Neuromyelitis Optica and Ovarian Teratoma: A Case Series. Mult Scler Relat Disord (2019) 31:97–100. doi: 10.1016/j.msard.2019.03.031
47. Jarius S, Kleiter I, Ruprecht K, Asgari N, Pitarokoili K, Borisow N, et al. MOG-IgG in NMO and Related Disorders: A Multicenter Study of 50 Patients. Part 3: Brainstem Involvement - Frequency, Presentation and Outcome. J Neuroinflamm (2016) 13(1):281. doi: 10.1186/s12974-016-0719-z
48. Cobo-Calvo A, Ruiz A, D'Indy H, Poulat AL, Carneiro M, Philippe N, et al. MOG Antibody-Related Disorders: Common Features and Uncommon Presentations. J Neurol (2017) 264(9):1945–55. doi: 10.1007/s00415-017-8583-z
49. Dalmau J. NMDA Receptor Encephalitis and Other Antibody-Mediated Disorders of the Synapse The 2016 Cotzias Lecture. Neurology (2016) 87(23):2471–82. doi: 10.1212/WNL.0000000000003414
50. Dalmau J, Armangue T, Planaguma J, Radosevic M, Mannara F, Leypoldt F, et al. An Update on Anti-NMDA Receptor Encephalitis for Neurologists and Psychiatrists: Mechanisms and Models. Lancet Neurol (2019) 18(11):1045–57. doi: 10.1016/S1474-4422(19)30244-3
51. Wu CY, Wu JD, Chen CC. The Association of Ovarian Teratoma and Anti-N-Methyl-D-Aspartate Receptor Encephalitis: An Updated Integrative Review. Int J Mol Sci (2021) 22(20):10911. doi: 10.3390/ijms222010911
52. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A Clinical Approach to Diagnosis of Autoimmune Encephalitis. Lancet Neurol (2016) 15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9
53. Dai Y, Zhang J, Ren H, Zhou X, Chen J, Cui L, et al. Surgical Outcomes in Patients With Anti-N-Methyl D-Aspartate Receptor Encephalitis With Ovarian Teratoma. Am J Obstet Gynecol (2019) 221(5):485.e481–485.e410. doi: 10.1016/j.ajog.2019.05.026
54. Jiang XY, Lei S, Zhang L, Liu X, Lin MT, Blumcke I, et al. Co-Expression of NMDA-Receptor Subunits NR1, NR2A, and NR2B in Dysplastic Neurons of Teratomas in Patients With Paraneoplastic NMDA-Receptor-Encephalitis: A Retrospective Clinico-Pathology Study of 159 Patients. Acta Neuropathol Commun (2020) 8(1):130. doi: 10.1186/s40478-020-00999-2
55. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended Diagnostic Criteria for Paraneoplastic Neurological Syndromes. J Neurol Neurosurg Psychiatry (2004) 75(8):1135–40. doi: 10.1136/jnnp.2003.034447
56. Muni RH, Wennberg R, Mikulis DJ, Wong AM. Bilateral Horizontal Gaze Palsy in Presumed Paraneoplastic Brainstem Encephalitis Associated With a Benign Ovarian Teratoma. J Neuroophthalmol (2004) 24(2):114–8. doi: 10.1097/00041327-200406000-00004
57. van Altena AM, Wijnberg GJ, Kolwijck E, de Hullu JA, Massuger LF. A Patient With Bilateral Immature Ovarian Teratoma Presenting With Paraneoplastic Encephalitis. Gynecol Oncol (2008) 108(2):445–8. doi: 10.1016/j.ygyno.2007.10.005
58. Armangue T, Titulaer MJ, Sabater L, Pardo-Moreno J, Gresa-Arribas N, Barbero-Bordallo N, et al. A Novel Treatment-Responsive Encephalitis With Frequent Opsoclonus and Teratoma. Ann Neurol (2014) 75(3):435–41. doi: 10.1002/ana.23917
59. Mandel-Brehm C, Dubey D, Kryzer TJ, O'Donovan BD, Tran B, Vazquez SE, et al. Kelch-Like Protein 11 Antibodies in Seminoma-Associated Paraneoplastic Encephalitis. N Engl J Med (2019) 381(1):47–54. doi: 10.1056/NEJMoa1816721
60. Vogrig A, Pericart S, Pinto AL, Rogemond V, Muniz-Castrillo S, Picard G, et al. Immunopathogenesis and Proposed Clinical Score for Identifying Kelch-Like Protein-11 Encephalitis. Brain Commun (2021) 3(3):fcab185. doi: 10.1093/braincomms/fcab185
61. Armangue T, Sabater L, Torres-Vega E, Martinez-Hernandez E, Arino H, Petit-Pedrol M, et al. Clinical and Immunological Features of Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies. JAMA Neurol (2016) 73(4):417–24. doi: 10.1001/jamaneurol.2015.4607
62. Fang BY, McKeon A, Hinson SR, Kryzer TJ, Pittock SJ, Aksamit AJ, et al. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy A Novel Meningoencephalomyelitis. JAMA Neurol (2016) 73(11):1297–307. doi: 10.1001/jamaneurol.2016.2549
63. Kunchok A, Zekeridou A, McKeon A. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy. Curr Opin Neurol (2019) 32(3):452–8. doi: 10.1097/WCO.0000000000000676
64. Flanagan EP, Hinson SR, Lennon VA, Fang BY, Aksamit AJ, Morris PP, et al. Glial Fibrillary Acidic Protein Immunoglobulin G as Biomarker of Autoimmune Astrocytopathy: Analysis of 102 Patients. Ann Neurol (2017) 81(2):298–309. doi: 10.1002/ana.24881
65. Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme Delta Brush: A Unique EEG Pattern in Adults With Anti-NMDA Receptor Encephalitis. Neurology (2012) 79(11):1094–100. doi: 10.1212/WNL.0b013e3182698cd8
66. Wang R, Lai XH, Liu X, Li YJ, Chen C, Li C, et al. Brain Magnetic Resonance-Imaging Findings of Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Cohort Follow-Up Study in Chinese Patients. J Neurol (2018) 265(2):362–9. doi: 10.1007/s00415-017-8707-5
67. Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for Tumours in Paraneoplastic Syndromes: Report of an EFNS Task Force. Eur J Neurol (2011) 18(1):19–e13. doi: 10.1111/j.1468-1331.2010.03220.x
68. Pattanayak P, Solnes LB. Paraneoplastic Syndrome With Anti-NMDAR Encephalitis Associated With Ovarian Teratomas. Clin Nucl Med (2017) 42(2):e128–9. doi: 10.1097/RLU.0000000000001471
69. Zhao XY, Li J, Zhu Q, Liang GL, Xia W, He XQ, et al. HLA-A and HLA-DRB1 May Play a Unique Role in Ovarian Teratoma-Associated Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Reprod Biol Endocrin (2020) 18(1):107. doi: 10.1186/s12958-020-00661-5
70. Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, van der Holt B, et al. Paraneoplastic Cerebellar Degeneration Associated With Antineuronal Antibodies: Analysis of 50 Patients. Brain (2003) 126(Pt 6):1409–18. doi: 10.1093/brain/awg133
71. Johnson N, Henry C, Fessler AJ, Dalmau J. Anti-NMDA Receptor Encephalitis Causing Prolonged Nonconvulsive Status Epilepticus. Neurology (2010) 75(16):1480–2. doi: 10.1212/WNL.0b013e3181f8831a
72. Bien CG, Vincent A, Barnett MH, Becker AJ, Blumcke I, Graus F, et al. Immunopathology of Autoantibody-Associated Encephalitides: Clues for Pathogenesis. Brain (2012) 135:1622–38. doi: 10.1093/brain/aws082
Keywords: ovarian teratoma, paraneoplastic neurological syndromes, pathological findings, antibodies, anti-N-methyl-D-aspartate receptor encephalitis
Citation: Lin J, Wang M, Wang J and Li J (2022) Ovarian Teratoma-Related Paraneoplastic Neurological Syndromes. Front. Oncol. 12:892539. doi: 10.3389/fonc.2022.892539
Received: 09 March 2022; Accepted: 11 April 2022;
Published: 16 May 2022.
Edited by:
Virginie Desestret, Université Claude Bernard Lyon 1, FranceReviewed by:
Alberto Vogrig, Azienda Sanitaria Universitaria Friuli Centrale (ASU FC), ItalyCopyright © 2022 Lin, Wang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinmei Li, bGlqaW5tZWlAd2Noc2N1LmNu
†ORCID: Jinmei Li, orcid.org/0000-0002-7411-8269
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.