- 1Thomas Jefferson University Hospital, Jefferson University Hospitals, Philadelphia, PA, United States
- 2Department of Medical Oncology, Division of Hematologic Malignancy and Stem Cell Transplantation, Philadelphia, PA, United States
Allogeneic stem cell transplantation has improved survival for patients with acute myeloid leukemia (AML), especially for patients with disease at high risk of relapse. However, relapse remains the most common cause of treatment failure and death in the post-transplant period. Maintenance therapy, an extended course of treatment after achieving remission to reduce the rate of relapse, is an important component of the treatment of various hematologic malignancies; however, its role in the treatment of AML is far less well-defined. Recently, there has been significant interest in the use of novel therapeutic agents as maintenance therapy after allogeneic stem cell transplant, utilizing new mechanisms of treatment and more favorable toxicity profiles. In this review, we will discuss the mechanistic and clinical data for post-transplant maintenance therapies in AML. Then, we will review several emergent and current clinical trials which aim to incorporate novel agents into maintenance therapy regimens.
Introduction
Since its initial description in the 1950s, allogeneic stem cell transplantation has improved survival for patients with acute myeloid leukemia (AML), especially for patients with disease at high risk of relapse (1). Despite this life-saving advancement, relapse remains the most common cause of treatment failure and death in the post-transplant period, representing the primary cause of death for more than half of transplant recipients depending on the type of transplant received (2). Survival after relapse remains poor, with less than 25% of patients alive at 1 year post-relapse and less than 20% at 2 years (3, 4). These figures underscore the importance of identifying treatments to decrease rates of relapse and improve post-transplant survival.
Maintenance therapy, an extended course of treatment after achieving remission to reduce the rate of relapse, is an important component of the treatment of various hematologic malignancies including acute lymphoblastic leukemia; however, its role in the treatment of AML is far less well-defined (5). Post-transplant maintenance for AML dates back to the 1960s, when chemotherapeutic agents and/or early immunotherapies such as interferon were trialed (6, 7). The use of these agents was not broadly adopted due to both their high degree of toxicity and the unclear survival benefit (8). More recently, there has been a groundswell of interest in the use of novel therapeutic agents as maintenance therapy after allogeneic stem cell transplant, leveraging new understanding and identification of genetic mutations, epigenetic influences, and cell-signaling pathways which play critical roles in the behavior of leukemic cells combined with the more favorable toxicity profiles of these agents (7, 8).
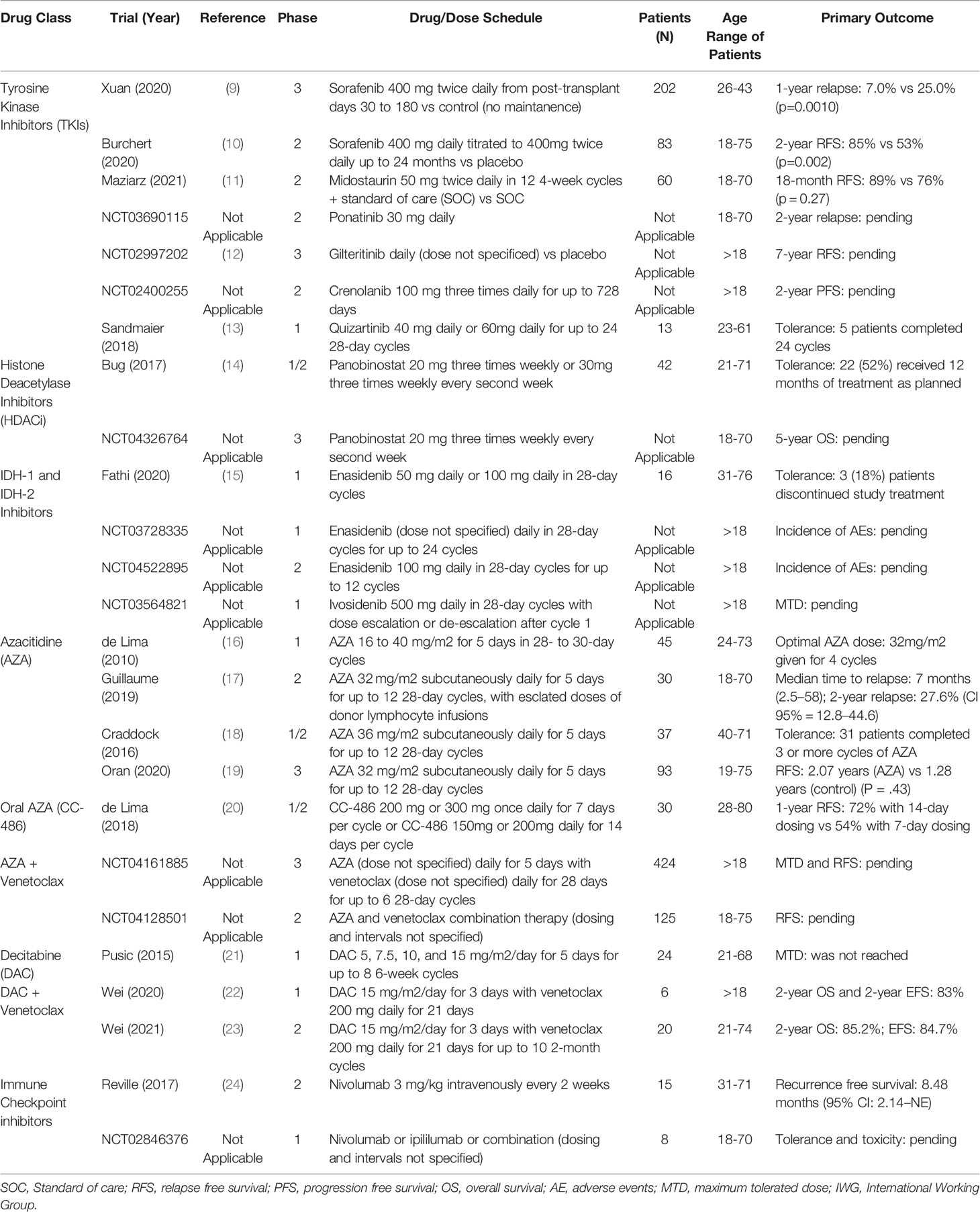
In this review, we will discuss the mechanistic and clinical data for post-transplant maintenance therapies in AML. Then, we will review several emergent and current clinical trials which aim to incorporate novel agents into maintenance therapy regimens. Many of these key studies are listed in Table 1.
FLT3 Inhibitors
FMS-like tyrosine kinase 3 (FLT3) is a transmembrane receptor expressed in CD34+ hematopoietic stem cells that plays a critical role in both proliferation and apoptosis through several key cellular signaling pathways including phosphatidylinositol-3-kinase and RAS (25). FLT3 internal tandem duplication mutation (FLT3-ITD) is found in approximately 25% of AML cases and is considered a high-risk feature (26, 27). Although allogeneic stem cell transplantation is utilized in the treatment of patients with FLT3-ITD AML, these patients have a higher incidence of relapse and decreased leukemia-free survival when compared to those with non-FLT3-ITD AML (28). With FLT3 mutation prevalence and prognostic impact in mind, several FLT3 inhibitors have been developed and utilized both pre- and post-transplant. These agents can be largely characterized in two categories: first generation/multi-targeted tyrosine kinase inhibitors (TKIs), and next generation/selective TKIs (27, 29).
Sorafenib is amongst the growing number of first generation TKIs with promising efficacy as post-transplant maintenance therapy in FLT3-ITD AML. To date, two large-scale randomized control trials have been published with data supportive of sorafenib use in this treatment setting (9, 10). As published by Xuan et al. in 2020, a phase III clinical trial recruited 202 patients with FLT3-ITD AML across seven hospitals in China and randomized patients at post-transplant day 30 to either placebo or sorafenib 400 mg twice per day until post-transplant day 180. They found that patients receiving sorafenib maintenance therapy had a cumulative 1-year incidence of relapse of 7.0% (95% CI 3.1%-13.1%) as compared to 25% (95% CI 16.6%-33.3%) in the placebo group (HR 0.25; 95% CI 0.11-0.57; p=0.0010) (9). There was no significant difference in the overall incidence of grade 3 or 4 adverse events between the sorafenib and placebo groups, though there was a relative increase in the incidence of grade 3 or 4 hematologic (15% sorafenib, 7% placebo) and dermatologic (7% sorafenib, 1% placebo) adverse events (9). Post-hoc multivariable analysis found that sorafenib maintenance therapy was the only protective factor in survival (9). Published within the same month, the SORMAIN trial recruited 83 patients with FLT3-ITD AML and randomized patients to 24 months of sorafenib maintenance therapy or placebo starting between post-transplant day 60 and 100 (10). Patients receiving sorafenib compared to placebo had a HR of relapse or death of 0.39 (95% CI 0.18-0.85; p=0.013), and 2-year relapse-free survival (RFS) of 85% (95% CI 0.70%-0.93%) compared to 53% in the placebo group (95% CI 0.36%-0.68%); the overall HR for relapse or death was 0.256 (95% CI 0.10-0.65; p=0.002) (10).
Midostaurin is another multi-targeted TKI with growing evidence in both the pre- and post-transplant treatment of FLT3-ITD AML (11, 30). The RADIUS trial was a phase II clinical trial randomizing 60 patients with FLT3-ITD AML after allogeneic stem cell transplant to up to 12 4-week cycles of standard of care treatment with or without midostaurin maintenance therapy (11). The 18-month RFS of patients with midostaurin was 89% (95% CI 69%-96%) as compared to 76% with standard of care alone (95% CI 54%-88%); rates of relapse were 11% and 24% respectively, resulting in a 46% relative reduction (11). It should be noted that the study was not powered to detect a statistically significant difference between the two arms of the trial, with the authors generating a sample size of 60 patients to detect a 50% reduction in the relative risk of relapse (11). Finally, multiple clinical trials are investigating the use of other broadly active TKIs in the post-transplant setting, such as ponatinib in the PONALLO trial (NCT03690115).
Numerous selective TKIs have been studied in the post-transplant maintenance of FLT3-ITD AML, though it remains too early for definitive conclusions regarding their efficacy in this treatment setting. Blood and Marrow Transplant Clinical Trials Network Protocol 1506 (NCT02997202) is a phase III randomized control trial with patients receiving gilteritinib or placebo after undergoing allogeneic stem cell transplant; the trial has completed patient accrual and is currently underway (12). Crenolanib is being evaluated in the single-arm phase II clinical trial NCT02400255 with two cohorts: patients in complete remission at time of transplant, and those that were not in complete remission at time of transplant. Quizartinib was the subject of a 2018 phase I clinical trial of 13 patients with FLT3-ITD AML which showed acceptable tolerability and only 1 patient experiencing relapse (13).
Histone Deacetylase Inhibitors
Histone deacetylase inhibitors (HDACi) are agents which enact epigenetic change on oncogenes or tumor suppressor genes to elicit cell cycle arrest, cessation of cellular differentiation, and apoptosis (31). HDACi have been shown to have a variety of other potentially therapeutic effects, including increased reactive oxidative species and regulation of death receptor expression (32). Panobinostat, a non-selective HDACi, was shown in phase I clinical trial to have antileukemic effect in patients with high risk refractory AML, acute lymphocytic leukemia (ALL), and myelodysplastic syndrome (MDS) (33). The PANOBEST trial published by Bug et al. in 2017 was a phase I/II clinical trial demonstrating 2-year overall survival (OS) of 88% and RFS of 74% in patients receiving panobinostat after allogeneic stem cell transplant, which compared favorably to similar cohorts (14). An active phase III randomized control trial (NCT04326764) is comparing the combination of panobinostat and donor lymphocyte infusions to standard of care (donor lymphocyte infusions alone) after allogeneic stem cell transplant and monitoring survival over 5 years; 52 patients have been enrolled since July 2018, and the study has an estimated primary completion date of June 2022.
IDH-1 and IDH-2 Inhibitors
Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) are enzymes involved in the conversion of isocitrate to 2-oxoglutarate. The accumulation of this product can result in inhibition of histone demethylases and the downstream modified expression of oncogenes and tumor suppressor genes (34, 35). Occurring in approximately 20% of patients with AML, these mutations are generally associated with adverse effects on RFS, especially mutations to IDH2 (36, 37). Ivosidenib and enasidenib are first-in-class oral therapies which inhibit IDH-1 and IDH-2 respectively and have a growing role in the treatment of IDH-mutated AML (38–41). The active phase I clinical trial NCT03515512 has enrolled 23 patients with IDH2-mutated AML who received enasidenib after allogeneic stem cell transplant. As reported by Fathi et al. in 2020, enasidenib was well-tolerated without a report of dose-limiting toxicity and a relapse rate of 13% (with note that longer follow up is necessary for further insight) (15). Additional active clinical trials NCT03728335 and NCT04522895 are evaluating the use of enasidenib in the post-transplant setting. The phase I trial NCT03564821 is evaluating the use of ivosidenib in the post-transplant setting for patients with IDH1-mutated AML; enrollment began in January 2019 and the estimated primary completion date is December 2022.
Hypomethylating Agents
Hypomethylating agents (HMAs) are another class of drugs that function through epigenetic manipulation. Azacitidine (AZA) and Decitabine (DAC) are nucleoside analogues that irreversibly bind to enzymes responsible for methylation and induce cellular degradation (42, 43). Given the overall suppressive effect of DNA methylation, the downregulation of tumor suppressor genes can have significant implications on apoptosis. As such, HMAs are used to enhance expression of these genes and induce cellular death. HMAs have become proven and effective components of the treatment of AML (26, 42, 44). Their use in the post-transplant treatment phase has been proposed due to induction of graft versus leukemia, increased NK cell activity, and accelerated reconstitution of Treg cells (45, 46). Additional studies have shown that they induce endogenous retroviral elements leading to interferon-mediated death of cancer cells (47). When first utilized in the 1960s and 1970s, HMAs were administered at high doses with unacceptable toxicities and insufficient anti-tumor effect; however, it was found that their anti-leukemic effect was not dose-dependent, and protocols incorporating lower doses at more frequent intervals both reduced toxicity and increased efficacy (48–50). Highlighting these promising effects, a large meta-analysis published by Bewersdorf et al. in 2021 studied 809 patients undergoing post-transplant maintenance therapy with either TKIs (for those with FLT-ITD AML) or HMAs with control groups receiving standard-of-care post-transplant therapy. 2-year OS rates were 81.7% (95% CI 73.8-87.7%) and 65.7% (95% CI 55.1-74.9%) among patients treated with TKIs and HMAs respectively (51).
Multiple studies have been conducted regarding the use of AZA in AML after allogeneic stem cell transplant, and several have shown efficacy as a salvage therapy (52, 53). As maintenance therapy, several observational and single arm trials have shown efficacy. Ali et al. performed a retrospective analysis from two separate institutions comparing AZA maintenance therapy post-transplant (n=59) with historical controls (n=90). Their data showed that AZA maintenance therapy improved event-free survival (EFS) (p=0.019) and OS (p=0.011) (54). Other studies show similar results, with tolerable toxicities and modest improvements in event free survival (EFS) and OS (16–18). Oran et al. recently published a phase III, open label-randomized trial with AZA maintenance therapy post-transplant in November 2020; unfortunately, this did not show any improvement in RFS for the 187 enrolled patients but did show a higher toxicity burden in the AZA maintenance arm (19).
Intravenous AZA maintenance therapy can be highly disruptive to post-treatment life due to the need for frequent infusion appointments as well as toxicities including cytopenias and diarrhea (18, 55). In recognition of these issues, an oral form of AZA (CC-486) has been developed. CC-486 has been able to limit toxicity while prolonging exposure to the drug and increasing its ability to amplify hypomethylation (20). The QUAZAR AML-001 phase III double blind, randomized control trial studied patients with AML who are in clinical remission, but not a candidate for transplant; it was published in December 2020 by Wei et al. and showed favorable results. The CC-486 treatment arm (n=238) had a significantly longer median OS of 24.7 months versus 14.8 months for placebo (p<0.001) (56). This was also demonstrated in RFS of 10.2 months with CC-486 versus 4.8 months with placebo (p<0.001) (56). Based on this trial, CC-468 has been FDA approved for maintenance for first remission after induction therapy in patients who were not candidates for transplant. This drug is being further investigated for its efficacy and tolerability in different populations with current clinical trials (NCT04887857, NCT04806906) both as monotherapy and in combination with other therapeutic agents. Excitingly, this drug has also been examined in the post-transplant maintenance setting. De Lima et al. recently published the first prospective phase I/II dose-finding study for CC-486 as post-transplant maintenance therapy in AML or MDS. Their trial studied 30 patients on 4 different CC-486 dosage schedules in repeated 28-day cycles: CC-486 200 mg daily for 7 days per cycle, 300 mg daily for 7 days per cycle, 150 mg daily for 14 days per cycle, or 200 mg daily for 14 days per cycle. The 1-year cumulative incidence of relapse was 43% in the combined 7-day dosing group versus 13% in the combined 14-day dosing group (20). Similar results were seen in the 1-year relapse and progression-free survival (PFS) rates, with 54% and 72% in the 7-day and 14-day dosing groups respectively (20). Treatment emergent adverse events were mostly gastrointestinal and hematologic events, with 22 patients (73%) experiencing grade 1-4 events, one of which (intracranial hemorrhage) resulted in death (20).
DAC is another HMA utilized for induction chemotherapy in AML that has growing evidence for its use as maintenance therapy (7, 21, 26, 57). A dose and frequency of DAC 10mg/m2/day for 5 days was proposed by Pusic et al. due to decreased hematologic toxicity compared to higher doses; however, relapse was seen in 6 of the 22 patients with this regimen (21). A later study by Ma et al. conducted between 2015 and 2018 had more favorable results with a regimen of DAC 20 mg/m2/day for 5 days every three months. The 3-year OS was 92.9% versus 51.8% (p=0.003) and the 3 year DFS was 94.1% versus 55% (p=0.002) comparing the DAC and control arms respectively (57).
Azacitidine + Gemtuzumab Ozogamicin
Gemtuzumab ozogamicin (GO), a recombinant humanized monoclonal antibody conjugated to the cytotoxic antibiotic calicheamicin, is another exciting novel therapeutic agent being tested as post-transplant maintenance therapy in combination with AZA. The antibody component targets the CD33+ cell surface marker that is expressed on cancerous cells in the majority of AML patients (58). Once the antibody locates the CD33+ leukemic cell, the antibiotic is internalized, ultimately leading to cell death (59). Interestingly, GO was initially approved for the treatment of CD33+ AML in 2000 but voluntarily removed from the market after fatal adverse events including hemorrhage, infection, and acute respiratory distress syndrome were observed; it was approved once again by the FDA in 2017 with dose adjustment.
In 2014, Oshikawa et al. published a small study of 10 post-transplant patients started on maintenance therapy with intravenous AZA 30 mg/m2 days 1–7, followed by GO at 3 mg/m2 on day 8. This was repeated every 4 weeks, or as soon as the patient’s hematologic counts recovered. The study ultimately was unable to contribute any statistically significant data, though it reported an OS of 70% versus 59.8% (p=0.138) and DFS 60% versus 42.8% at 1 year (p=0.222) when comparing the groups receiving AZA and GO to control (58). Additional significant study limitations included the control group composition (were randomly chosen from the institution’s database as the “no maintenance” arm) (58). Data unfortunately remains limited on the combination of AZA and GO, perhaps in recognition of the aforementioned toxicity of GO.
BCL2 Inhibitors
B Cell leukemia/lymphoma 2 (BCL2) is an oncoprotein that acts to promote cell survival and prevent apoptosis. Venetoclax is a BCL2 inhibitor which acts as a BCL2-homology 3 mimetic, binding the oncoprotein and allowing for appropriate cellular death (60, 61). It has been shown to be safe and very tolerable for patients with relapsed/refractory AML, or in patients who are not fit to receive intensive chemotherapy (61, 62).
Venetoclax has been used as monotherapy for maintenance post-transplant. In 2020, Kent et al. published the results of 23 post-transplant patients (22 AML and 1 MDS) who received venetoclax daily titrated to a final dose of 400 mg daily. 6-month OS and RFS were both 87% (63). The most commonly reported adverse effects were cytopenias and diarrhea, with 3 patients discontinuing the drug due to adverse events (63). Additional case series have demonstrated the reasonable tolerability and low toxicity of venetoclax both alone and in combination with additional agents (64, 65).
The combination of HMAs (namely AZA and DAC) and BLC2 inhibitors (venetoclax) is a treatment regimen of considerable recent interest and examination. Their use pre-transplant has grown in no small part due to their relative tolerance compared to traditional induction and consolidation regimens, prompting experimentation in the post-transplant setting (26, 66). A retrospective study from 11 German transplant centers evaluating 30 post-transplant MDS or AML patients with relapsed disease who received AZA or DAC with venetoclax showed an overall response rate (ORR) of 47%, with no significant difference seen when comparing those receiving AZA and venetoclax to those receiving DAC and venetoclax (67). Notably, 29 of the 30 patients had neutropenia and there was a 16% rate of fatal infections, highlighting the risks associated with these combinations (67).
The first prospective trial using DAC and venetoclax as post-transplant maintenance therapy was published in 2020 by Wei et al. 6 patients were studied, with 2 in partial remission prior to transplant and 4 with minimal residual disease (MRD). Approximately day 100 post-transplantation, all patients received DAC 15 mg/m2/day for 3 days followed by venetoclax 200 mg on days 1-21. Results were promising, with both 2-year OS and EFS of 83%; 33% of patients had grade 1-2 adverse events (most commonly neutropenia, thrombocytopenia, anemia, and neutropenic fever), and none experience grade 3 or 4 adverse events (22). Reporting again on this study in 2021, Wei et al. provided new data after recruiting 20 total patients (17 with AML, 3 with MDS). Incorporating these new patients, the 2-year OS and EFS were 85.2% and 84.7% respectively (23).
AZA and venetoclax is another combination being actively evaluated as post-transplant maintenance therapy. NCT04161885 is an active phase III randomized open-label trial evaluating AZA and venetoclax in post-transplant patients currently in clinical remission; enrollment began February 2020 with an estimated primary completion date of 2025. NCT04128501 is a phase II trial using AZA and venetoclax as maintenance therapy in patients with MRD after allogeneic stem cell transplant for AML, T-cell leukemia, and acute mixed type leukemia; this study is also actively recruiting and has an estimated primary completion date of October 2022.
ICP Inhibitors
Immune checkpoints (ICP) are proteins that function to identify healthy cells to T regulatory cells in normal immune function (68). There are numerous IPCs of therapeutic significance, including cytotoxic T-lymphocyte- associated protein 4 (CTLA-4) and programmed death 1 (PD-1) receptors and their respective ligands (B7-1/B7-2 and PD-L1/PD-L2). Tumor cells manipulate this system by altering their expression of ICPs to appear as normal cells to the immune system, allowing them to escape destruction (68). ICP inhibitors function to block this escape mechanism, allowing for proper immunologic function and cellular destruction; in this way, ICP inhibitors work to increase the graft versus leukemia effect. Examples of ICP inhibitors include the monoclonal antibodies nivolumab (anti-PD1), ipilimumab (anti-CTLA-4), and pidilizumab (anti-Delta-like 1).
Limited studies have been published regarding the use of ICP inhibitors in AML; however, there are numerous clinical trials underway evaluating their safety and efficacy in this application. Nivolumab has been shown to be a successful maintenance therapy in patients with high-risk AML in remission who are not candidates for allogeneic stem cell transplant (24). A 2021 phase II clinical trial of 6 measurable residual disease (MRD) negative patients and 9 MRD positive patients receiving nivolumab showed only 1 MRD negative patient experiencing relapse but only 2 patients with MRD positive AML achieving remission; this study was not in support of single use agent nivolumab but encouraged future directions (24). A 2016 phase I/Ib clinical trial by Davids et al. studied ipilimumab in AML patients with relapsed disease post-transplant, with 5 patients (22%) achieving a complete response (69). However, significant immune-related side effects were observed in 6 patients, with 4 developing graft versus host disease (GvHD) (69). The REMAIN trail (NCT02275533) seeks to assess single agent nivolumab as maintenance therapy post-induction and consolidation chemotherapy. NCT02846376 is an active trial investigating the role of nivolumab and ipilimumab as post-transplant maintenance therapy in 8 AML patients at high risk of relapse; it has an estimated completion date of December 2023.
In addition to ICP inhibitors alone as post-transplant maintenance therapy, there are trials underway to further assess the synergistic relationship between HMAs and ICP inhibitors. Treatment with HMAs have been shown to increase tumor cell expression of ICPs such as PD-1, PD-L1, PD-L2, and CTLA-4 (70). This has been seen in MDS, AML, and chronic myelogenous leukemia (CML), as demonstrated by Yang et al. in 2014 with the use of DAC (70, 71). Thus, the combination of HMAs and ICP inhibitors seeks to theoretically use HMAs to increase ICP expression which can be subsequently targeted with ICP inhibitors. This is a relatively new direction in AML treatment, and there is still much more to be studied regarding the combination of these drugs. One active but no longer recruiting clinical trial (NCT02775903) is utilizing AZA and durvalumab (anti-PDL1) in high-risk MDS and elderly patients with AML.
Conclusion
Despite the advent of allogeneic stem cell transplant and the monumental change it brought to the treatment of AML, mortality remains high even amongst patients well enough to undergo transplant. Relapse after stem cell transplant can be devastating and highlights the need for treatment modalities to increase disease-free survival. New and innovative advancements in maintenance therapies has the potential to improve both overall survival and disease-free survival in this patient population, with the added potential for toxicity. Continued evidence is arising for maintenance treatment with varying combinations of the previously discussed therapies, as their largely favorable side effect profiles make them desirable. Post-transplant maintenance therapy is a new frontier of AML treatment and will continue to expand with further research.
Author Contributions
SM and TR were responsible for the citation research, data analysis, and prose writing for the entirety of this review. NP and LW provided the topic of discussion, initial foundational research and guidance, extensive content review and editing, and final approval for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thomas ED, Lochte HL, Lu WC, Ferrebee JW. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and Chemotherapy. N Engl J Med (1957) 257(11):491–6. doi: 10.1056/NEJM195709122571102
2. Horowitz M, Schreiber H, Elder A, Heidenreich O, Vormoor J, Toffalori C, et al. Epidemiology and Biology of Relapse After Stem Cell Transplantation. Bone Marrow Transpl (2018) 53(11):1379–89. doi: 10.1038/s41409-018-0171-z
3. Bejanyan N, Weisdorf DJ, Logan BR, Hai-Lin W, Devine S, de Lima M, et al. Survival of Patients With Acute Myeloid Leukemia Relapsing After Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transpl (2015) 21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007
4. Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML After Hematopoietic Stem Cell Transplantation: Methods of Monitoring and Preventive Strategies. A Review From the ALWP of the EBMT. Bone Marrow Transplant (2016) 51(11):1431–8. doi: 10.1038/bmt.2016.167
5. National Comprehensive Cancer Network. Acute Lymphoblastic Leukemia (Version 4.2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/all.pdf (Accessed February 14, 2022).
6. Ellison RR, Holland JF, Weil M, Jacquillat C, Boiron M, Bernard J, et al. Arabinosyl Cytosine: A Useful Agent in the Treatment of Acute Leukemia in Adults. Blood (1968) 32(4):507–23. doi: 10.1182/blood.V32.4.507.507
7. Molica M, Breccia M, Foa R, Jabbour E, Kadia TM. Maintenance Therapy in AML: The Past, the Present and the Future. Am J Hematol (2019) 94(11):1254–65. doi: 10.1002/ajh.25620
8. Reville PK, Kadia TM. Maintenance Therapy in AML. Front Oncol (2020) 10:619085. doi: 10.3389/fonc.2020.619085
9. Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib Maintenance in Patients With FLT3-ITD Acute Myeloid Leukaemia Undergoing Allogeneic Haematopoietic Stem-Cell Transplantation: An Open-Label, Multicentre, Randomised Phase 3 Trial. Lancet Oncol (2020) 21(9):1201–12. doi: 10.1016/S1470-2045(20)30455-1
10. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Rollig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol (2020) 38(26):2993–3002. doi: 10.1200/JCO.19.03345
11. Maziarz RT, Levis M, Patnaik MM, Scott BL, Mohan SR, Deol A, et al. Midostaurin After Allogeneic Stem Cell Transplant in Patients With FLT3-Internal Tandem Duplication-Positive Acute Myeloid Leukemia. Bone Marrow Transpl (2021) 56(5):1180–9. doi: 10.1038/s41409-020-01153-1
12. Levis MJ, Hamadani M, Logan BR, Rosales M, Delgado D, Bahceci E, et al. BMT CTN Protocol 1506: A Phase 3 Trial of Gilteritinib As Maintenance Therapy After Allogeneic Hematopoietic Stem Cell Transplantation in Patients With FLT3-ITD+ AML. Blood (2019) 134(Supplement_1):4602–2. doi: 10.1182/blood-2019-124322
13. Sandmaier BM, Khaled S, Oran B, Gammon G, Trone D, Frankfurt O. Results of a Phase 1 Study of Quizartinib as Maintenance Therapy in Subjects With Acute Myeloid Leukemia in Remission Following Allogeneic Hematopoietic Stem Cell Transplant. Am J Hematol (2018) 93(2):222–31. doi: 10.1002/ajh.24959
14. Bug G, Burchert A, Wagner EM, Kroger N, Berg T, Guller S, et al. Phase I/II Study of the Deacetylase Inhibitor Panobinostat After Allogeneic Stem Cell Transplantation in Patients With High-Risk MDS or AML (PANOBEST Trial). Leukemia (2017) 31(11):2523–5. doi: 10.1038/leu.2017.242
15. Fathi AT, Li S, Soiffer RJ, Lewis M, Mims A, Devine SM, et al. A Phase I Study of the IDH2 Inhibitor Enasidenib As Maintenance Therapy for IDH2 -Mutant Myeloid Neoplasms Following Hematopoietic Cell Transplantation. Blood (2020) 136(Supplement 1):4–5. doi: 10.1182/blood-2020-140176
16. de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance Therapy With Low-Dose Azacitidine After Allogeneic Hematopoietic Stem Cell Transplantation for Recurrent Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Cancer (2010) 116:5420–31. doi: 10.1002/cncr.25500
17. Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective Phase II Study of Prophylactic Low-Dose Azacitidine and Donor Lymphocyte Infusions Following Allogeneic Hematopoietic Stem Cell Transplantation for High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Bone Marrow Transplant (2019) 54:1815–26. doi: 10.1038/s41409-019-0536-y
18. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transpl (2016) 22(2):385–90. doi: 10.1016/j.bbmt.2015.09.004
19. Oran BV, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A Phase 3 Randomized Study of 5-Azacitidine Maintenance vs Observation After Transplant in High-Risk AML and MDS Patients. Blood Adv (2020) 4(21):5580–8. doi: 10.1182/bloodadvances.2020002544
20. de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. CC-486 Maintenance After Stem Cell Transplantation in Patients With Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol Blood Marrow Transpl (2018) 24(10):2017–24. doi: 10.1016/j.bbmt.2018.06.016
21. Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF, et al. Maintenance Therapy With Decitabine After Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transpl (2015) 21(10):1761–9. doi: 10.1016/j.bbmt.2015.05.026
22. Wei Y, Cao Y, Jin X, He X, Sun R, Xiong X, et al. Low-Dose Decitabine Plus Venetoclax Maintenance Therapy Can Decrease the Relapse After Allogeneic Stem Cell Transplantation for MRD Positive High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood (2020) 136(Supplement 1):33. doi: 10.1182/blood-2020-140359
23. Wei Y, Xiong X, Li X, Lu W, He X, Jin X, et al. Low-Dose Decitabine Plus Venetoclax is Safe and Effective as Post-Transplant Maintenance Therapy for High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Cancer Sci (2021) 112(9):3636–44. doi: 10.1111/cas.15048
24. Reville PK, Kantarjian HM, Ravandi F, Jabbour E, DiNardo CD, Daver N, et al. Nivolumab Maintenance in High-Risk Acute Myeloid Leukemia Patients: A Single-Arm, Open-Label, Phase II Study. Blood Cancer J (2021) 11(3):60. doi: 10.1038/s41408-021-00453-z
25. Grafone T, Palmisano M, Nicci C, Storti S. An Overview on the Role of FLT3-Tyrosine Kinase Receptor in Acute Myeloid Leukemia: Biology and Treatment. Oncol Rev (2012) 6(1):e8. doi: 10.4081/oncol.2012.e8
26. National Comprehensive Cancer Network. Acute Myeloid Leukemia (Version 1.2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (Accessed February 14, 2022).
27. Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 Mutations in AML: Review of Current Knowledge and Evidence. Leukemia (2019) 33(2):299–312. doi: 10.1038/s41375-018-0357-9
28. Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 Internal Tandem Duplication on the Outcome of Related and Unrelated Hematopoietic Transplantation for Adult Acute Myeloid Leukemia in First Remission: A Retrospective Analysis. J Clin Oncol (2012) 30(7):735–41. doi: 10.1200/JCO.2011.36.9868
29. Wang Z, Cai J, Cheng J, Yang W, Zhu Y, Li H, et al. FLT3 Inhibitors in Acute Myeloid Leukemia: Challenges and Recent Developments in Overcoming Resistance. J Med Chem (2021) 64(6):2878–900. doi: 10.1021/acs.jmedchem.0c01851
30. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin Plus Chemotherapy for Acute Myeloid Leukemia With a FLT3 Mutation. N Engl J Med (2017) 377(5):454–64. doi: 10.1056/NEJMoa1614359
31. Kim HJ, Bae SC. Histone Deacetylase Inhibitors: Molecular Mechanisms of Action and Clinical Trials as Anti-Cancer Drugs. Am J Transl Res (2011) 3(2):166–79.
32. Rosato RR, Grant S. Histone Deacetylase Inhibitors: Insights Into Mechanisms of Lethality. Expert Opin Ther Targets (2005) 9(4):809–24. doi: 10.1517/14728222.9.4.809
33. Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, et al. A Phase I Study of Intravenous LBH589, a Novel Cinnamic Hydroxamic Acid Analogue Histone Deacetylase Inhibitor, in Patients With Refractory Hematologic Malignancies. Clin Cancer Res (2006) 12(15):4628–35. doi: 10.1158/1078-0432.CCR-06-0511
34. DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, Clinical Outcome, and Prognostic Significance of IDH Mutations in AML. Am J Hematol (2015) 90(8):732–6. doi: 10.1002/ajh.24072
35. Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH Mutation Impairs Histone Demethylation and Results in a Block to Cell Differentiation. Nature (2012) 483(7390):474–8. doi: 10.1038/nature10860
36. Paschka P, Schlenk RF, Gaidzik VI, Mabdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 Mutations are Frequent Genetic Alterations in Acute Myeloid Leukemia and Confer Adverse Prognosis in Cytogenetically Normal Acute Myeloid Leukemia With NPM1 Mutation Without FLT3 Internal Tandem Duplication. J Clin Oncol (2010) 28(22):3636–43. doi: 10.1200/JCO.2010.28.3762
37. Willander K, Falk IJ, Chaireti R, Paul E, Hermansson M, Green H, et al. Mutations in the Isocitrate Dehydrogenase 2 Gene and IDH1 SNP 105c > T Have a Prognostic Value in Acute Myeloid Leukemia. biomark Res (2014) 2:18. doi: 10.1186/2050-7771-2-18
38. Issa GC, DiNardo CD. Acute Myeloid Leukemia With IDH1 and IDH2 Mutations: 2021 Treatment Algorithm. Blood Cancer J (2021) 11(6):107. doi: 10.1038/s41408-021-00497-1
39. Stein EM, DiNardo CD, Fathi AT, Mims AS, Pratz KW, Savona MR, et al. Ivosidenib or Enasidenib Combined With Intensive Chemotherapy in Patients With Newly Diagnosed AML: A Phase 1 Study. Blood (2021) 137(13):1792–803. doi: 10.1182/blood.2020007233
40. Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an Inhibitor of Mutant IDH2 Proteins, Induces Durable Remissions in Older Patients With Newly Diagnosed Acute Myeloid Leukemia. Leukemia (2019) 33(11):2575–84. doi: 10.1038/s41375-019-0472-2
41. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altmann JK, Mims AS, et al. Durable Remissions With Ivosidenib in IDH1 -Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378(25):2386–98. doi: 10.1056/NEJMoa1716984
42. Stomper J, Rotondo JC, Greve G, Lubbert M. Hypomethylating Agents (HMA) for the Treatment of Acute Myeloid Leukemia and Myelodysplastic Syndromes: Mechanisms of Resistance and Novel HMA-Based Therapies. Leukemia (2021) 35:1873–89. doi: 10.1038/s41375-021-01218-0
43. Ghoshal K, Datta J, Majumder S, et al. 5-Aza-Deoxycytidine Induces Selective Degradation of DNA Methyltransferase 1 by a Proteasomal Pathway That Requires the KEN Box, Bromo-Adjacent Homology Domain, and Nuclear Localization Signal [Published Correction Appears in Mol Cell Biol. Mol Cell Biol (2005) 25(11):4727–41. doi: 10.1128/MCB.25.11.4727-4741.2005
44. Baylin SB, Jones PA. A Decade of Exploring the Cancer Epigenome - Biological and Translational Implications. Nat Rev Cancer (2011) 11(10):726–34. doi: 10.1038/nrc3130
45. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine Augments Expansion of Regulatory T Cells After Allogeneic Stem Cell Transplantation in Patients With Acute Myeloid Leukemia (AML). Blood (2012) 119(14):3361–9. doi: 10.1182/blood-2011-09-377044
46. Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, Caballero-Velazquez T, Blanco B, Herrero-Sánchez C, et al. Immunomodulatory Effect of 5-Azacytidine (5-Azac): Potential Role in the Transplantation Setting. Blood (2010) 115(1):107–21. doi: 10.1182/blood-2009-03-210393
47. Schroeder T, Rautenberg C, Haas R, Germing U, Kobbe G. Hypomethylating Agents for Treatment and Prevention of Relapse After Allogeneic Blood Stem Cell Transplantation. Int J Hematol (2018) 107:138–50. doi: 10.1007/s12185-017-2364-4
48. Karon M, Sieger L, Leimbrock S, Finklestein JZ, Nesbit ME, Swaney JJ. 5-Azacytidine: A New Active Agent for the Treatment of Acute Leukemia. Blood (1973) 42(3):359–65. doi: 10.1182/blood.V42.3.359.359
49. Saiki JH, Bodey GP, Hewlett JS, Amare M, Morrison FS, Wilson HE, et al. Effect of Schedule on Activity and Toxicity of 5-Azacytidine in Acute Leukemia: A Southwest Oncology Group Study. Cancer (1981) 47(7):1739–42. doi: 10.1002/1097-0142(19810401)47:7<1739::aid-cncr2820470702>3.0.co;2-2.
50. Hassan HE, Keita J-A, Narayan L, Brady SM, Frederick R, Carlson S, et al. Fandy. The Combination of Dimethoxycurcumin With DNA Methylation Inhibitor Enhances Gene Re-Expression of Promoter-Methylated Genes and Antagonizes Their Cytotoxic Effect. Epigenetics (2016) 11:10:740–9. doi: 10.1080/15592294.2016.1226452
51. Bewersdorf JP, Allen C, Mirza AS, Grimshaw AA, Giri S, Podoltsev NA, et al. Hypomethylating Agents and FLT3 Inhibitors As Maintenance Treatment for Acute Myeloid Leukemia and Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation-A Systematic Review and Meta-Analysis. Transplant Cell Ther (2021) 27(12):997.e1–997.e11. doi: 10.1016/j.jtct.2021.09.005
52. Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and Donor Lymphocyte Infusions as First Salvage Therapy for Relapse of AML or MDS After Allogeneic Stem Cell Transplantation. Leukemia (2013) 27(6):1229–35. doi: 10.1038/leu.2013.7
53. Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical Activity of Azacitidine in Patients Who Relapse After Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. Haematologica (2016) 101(7):879–83. doi: 10.3324/haematol.2015.140996
54. Ali N, Tomlinson B, Metheny L, Goldstein SC, Fu P, Cao S, et al. Conditioning Regimen Intensity and Low-Dose Azacitidine Maintenance After Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia. Leukemia Lymphoma (2020) 61:12:2839–49. doi: 10.1080/10428194.2020.1789630
55. de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance Therapy With Low-Dose Azacitidine After Allogeneic Hematopoietic Stem Cell Transplantation for Recurrent Acute Myelogenous Leukemia or Myelodysplastic Syndrome: A Dose and Schedule Finding Study. Cancer (2010) 116(23):5420–31. doi: 10.1002/cncr.25500
56. Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N Engl J Med (2020) 383(26):2526–37. doi: 10.1056/NEJMoa2004444
57. Ma Y, Qu C, Dai H, Yin J, Li Z, Chen J, et al. Maintenance Therapy With Decitabine After Allogeneic Hematopoietic Stem Cell Transplantation to Prevent Relapse of High-Risk Acute Myeloid Leukemia. Bone Marrow Transplant (2020) 55:1206–8. doi: 10.1038/s41409-019-0677-z
58. Oshikawa G, Kakihana K, Saito M, Aoki J, Najima Y, Kobayashi T, et al. Post-Transplant Maintenance Therapy With Azacitidine and Gemtuzumab Ozogamicin for High-Risk Acute Myeloid Leukaemia. Br J Haematol (2015) 169(5):756–9. doi: 10.1111/bjh.13248
59. Baron J, Wang ES. Gemtuzumab Ozogamicin for the Treatment of Acute Myeloid Leukemia. Expert Rev Clin Pharmacol (2018) 11(6):549–59. doi: 10.1080/17512433.2018.1478725
60. Adams JM, Cory S. The Bcl-2 Apoptotic Switch in Cancer Development and Therapy. Oncogene (2007) 26(9):1324–37. doi: 10.1038/sj.onc.1210220
61. Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria Primed by Death Signals Determine Cellular Addiction to Antiapoptotic BCL-2 Family Members. Cancer Cell (2006) 9(5):351–65. doi: 10.1016/j.ccr.2006.03.027
62. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383(7):617–29. doi: 10.1056/NEJMoa2012971
63. Kent A, Polleya DA, Winters A, Jordan CT, Smith C, Gutman JA, et al. "Venetoclax is Safe and Tolerable as Post-Transplant Maintenance Therapy for AML Patients at High Risk for Relapse.". Blood (2020) 136:11–125. doi: 10.1182/blood-2020-138832
64. Moukalled NM, El Darsa H, Haibe Y, Massoud R, Kanj SS, Mahfouz R, et al. Feasibility of Venetoclax-Based Combinations for Adult Patients With Acute Myeloid Leukemia Relapsing After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2019) 54:620–4. doi: 10.1038/s41409-018-0347-6
65. Byrne M, Danielson N, Sengsayadeth S, Rasche A, Culos K, Gatwood K, et al. The Use of Venetoclax-Based Salvage Therapy for Post-Hematopoietic Cell Transplantation Relapse of Acute Myeloid Leukemia. Am J Hematol (2020) 95(9):1006–14. doi: 10.1002/ajh.25859
66. Gaut D, Burkenroad A, Duong T, Feammelli J, Sasine J, Schiller G. Venetoclax Combination Therapy in Relapsed/Refractory Acute Myeloid Leukemia: A Single Institution Experience. Leuk Res (2020) 90:106314. doi: 10.1016/j.leukres.2020.106314
67. Schuler E, Wagner-Drouet EM, Ajib S, Bug G, Crysandt M, Dressler S, et al. “Treatment of Myeloid Malignancies Relapsing After Allogeneic Hematopoietic Stem Cell Transplantation With Venetoclax and Hypomethylating Agents-a Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann Hematol (2021) 100:959–68:4. doi: 10.1007/s00277-020-04321-x
68. Assi R, Masri N, Abou Dalle I, El-Cheikh J, Bazarbachi A. Post-Transplant Maintenance Therapy for Patients With Acute Myeloid Leukemia: Current Approaches and the Need for More Trials. J Blood Med (2021) 12:21–32. doi: 10.2147/JBM.S270015
69. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients With Relapse After Allogeneic Transplantation. N Engl J Med (2016) 375(2):143–53. doi: 10.1056/NEJMoa1601202
70. Héninger E, Krueger TE, Lang JM. Augmenting Antitumor Immune Responses With Epigenetic Modifying Agents. Front Immunol (2015) 6:29. doi: 10.3389/fimmu.2015.00029
Keywords: AML – acute myeloid leukaemia, maintanance, novel treatment, stem cell transplant (SCT), post-transplant
Citation: Manobianco SA, Rakiewicz T, Wilde L and Palmisiano ND (2022) Novel Mechanisms for Post-Transplant Maintenance Therapy in Acute Myeloid Leukemia. Front. Oncol. 12:892289. doi: 10.3389/fonc.2022.892289
Received: 08 March 2022; Accepted: 08 June 2022;
Published: 12 July 2022.
Edited by:
Alison Loren, University of Pennsylvania, United StatesReviewed by:
Michael Richard Grunwald, Levine Cancer Institute, United StatesFrancesco Onida, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright © 2022 Manobianco, Rakiewicz, Wilde and Palmisiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neil D. Palmisiano, TmVpbC5QYWxtaXNpYW5vQGplZmZlcnNvbi5lZHU=
 Steven A. Manobianco
Steven A. Manobianco Tara Rakiewicz
Tara Rakiewicz Lindsay Wilde
Lindsay Wilde Neil D. Palmisiano
Neil D. Palmisiano